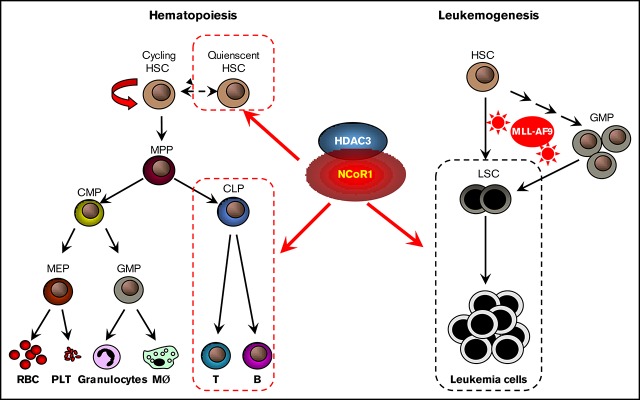
Key Points
NCoR1 plays a crucial role in murine adult hematopoiesis.
NCoR1 contributes to MLL-AF9–induced leukemogenesis in vivo.
Abstract
Enhanced understanding of normal and malignant hematopoiesis pathways should facilitate the development of effective clinical treatment strategies for hematopoietic malignancies. Nuclear receptor corepressor 1 (NCoR1) has been implicated in transcriptional repression and embryonic organ development, but its role in hematopoiesis is yet to be fully elucidated. Here, we showed that hematopoietic-specific loss of NCoR1 leads to expansion of the hematopoietic stem cell (HSC) pool due to aberrant cell cycle entry of long-term HSCs under steady-state conditions. Moreover, NCoR1-deficient HSCs exhibited normal self-renewal capacity but severely impaired lymphoid-differentiation potential in competitive hematopoietic-reconstitution assays. Transcriptome analysis further revealed that several hematopoiesis-associated genes are regulated by NCoR1. In addition, NCoR1 deficiency in hematopoietic cells delayed the course of leukemia and promoted leukemia cell differentiation in an MLL-AF9–induced mouse model. NCoR1 and its partner, histone deacetylase 3, can modulate histone acetylation and gene transcription through binding the promoter regions of myeloid-differentiation genes. Our collective results support the critical involvement of NCoR1 in normal and malignant hematopoiesis in vivo.
Visual Abstract
Introduction
Hematopoiesis, the process of mature blood cell lineage production, is supported by rare hematopoietic stem cells (HSCs) throughout the lifetime. HSCs reside at the apex of the hematopoietic hierarchy and possess self-renewal and differentiation capabilities.1 Long-term HSCs (LT-HSCs) are mostly quiescent and seldom enter the cell cycle in the steady-state; however, under stress conditions, such as inflammation, DNA damage, hemorrhage, and anemia, LT-HSCs adapt and enter the cell cycle to replenish the HSC pool, ultimately differentiating into hematopoietic progenitor and mature hematopoietic cells.2-4 In adult mammals, HSCs are predominantly located in the bone marrow (BM) in a specialized environment (designated “niche”), which is important for their development, maintenance, and regeneration.5 In addition to extrinsic factors, a variety of intrinsic transcription factors, signaling molecules, and epigenetic regulators regulate HSC identity, fate, and function.6-8 Characterization of the plethora of factors controlling multilineage differentiation and self-renewal of HSCs is one of the major challenges in HSC biology.
Nuclear receptor corepressor 1 (NCoR1) and its paralog, NCoR2 (SMRT), were initially found to interact with nuclear receptors and mediate transcriptional repression of target genes.9 Previous biochemical studies have reported that NCoR1 and NCoR2 exist in a large protein complex comprising histone deacetylase 3 (HDAC3), transducin β-like 1/transducin β-like related protein 1, and G-protein pathway suppressor 2.10-12 NCoR1 and NCoR2 are proposed to play essential, but nonredundant, roles in mouse embryonic development, in view of the finding that whole-body knockout of either gene results in embryonic lethality.13-15 NCoR1-null mice exhibit multiple defects in central nervous system, erythrocyte, and thymocyte development. 13 NCoR1 was further shown to be essential for erythroid differentiation in the K562 cell line16 and heme synthesis.17 However, the precise in vivo role of NCoR1 in hematopoiesis has not been established. Moreover, the NCoR1/HDAC complex is implicated in the pathogenesis of promyelocytic leukemia–retinoic acid receptor α (PML-RARα), promyelocytic leukemia zinc finger–retinoic acid receptor α (PLZF-RARα), and acute myeloid leukemia 1 (AML1)–ETO translocation-induced leukemia through repression of target gene transcription.18 Because the role of the NCoR1/HDAC complex in different types of leukemia appears to be context dependent, the potential function of NCoR1 in other types of leukemia remains unclear.
In this study, we used Vav-iCre–transgenic mice for conditional deletion of NCoR1 in hematopoietic cells and examined the effects of NCoR1 ablation on HSC self-renewal and differentiation capacities. NCoR1-deficient HSCs exhibited significantly enhanced proliferation activity in the steady-state, but their repopulation ability after transplant remained unchanged in the competitive transplantation assays. Moreover, NCoR1-deficient HSCs exhibited impaired lymphoid-differentiation potential in competitive hematopoietic-reconstitution assays. In addition, NCoR1 facilitated MLL-AF9–induced leukemogenesis in a leukemia disease model. Chromatin immunoprecipitation (ChIP)–quantitative polymerase chain reaction (qPCR) assays revealed that NCoR1 represses transcription of myeloid-differentiation genes by recruiting HDAC3 and promoting histone deacetylation in leukemia cells. Data from our investigation support crucial roles for NCoR1 in normal and malignant hematopoiesis in vivo.
Materials and methods
Mice
Mice strain carrying flox alleles of NCoR1 were backcrossed to the C57BL/6 background for ≥6 generations.19 NCoR1f/f mice were further crossed with Vav-iCre– or Mx1-Cre–transgenic mice. In all experiments, sex-matched Vav-iCre−, NCoR1f/f littermate mice were used as controls. All mice were bred in a pathogen-free animal facility. All experimental procedures were performed in accordance with the Institutional Animal Care and Use Committee at Institut Pasteur of Shanghai, Chinese Academy of Sciences.
Flow cytometry
Prepared samples of peripheral blood (PB), thymus, spleen, and BM cells were analyzed on an LSR II flow cytometer or an LSRFortessa cell analyzer (BD Biosciences). Cell sorting was performed on a FACSAria II (BD Biosciences). Detailed methods and antibodies were described previously.20 A BrdU Flow Kit (559619; BD Biosciences) and Ki-67 antibody (14-5698-80; eBioscience) were used to detect cell proliferation, according to the manufacturers’ instructions. Mice were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU) for 2 hours. Then BM cells were isolated and stained with antibodies, followed by fixation, permeabilization, and staining with anti-BrdU antibody and Hoechst 33342, according to the manufacturer’s instructions (561908; BD Biosciences). An Annexin V Apoptosis Detection Kit (556547; BD Biosciences) was used for the apoptosis assay, according to the manufacturer’s instructions. Data were analyzed by FlowJo software (TreeStar, Ashland, OR).
Competitive transplantation assay
The competitive transplantation assay was performed as previously described.20 Briefly, 1 × 106 donor cells (CD45.2+) were mixed with 1 × 106 competitive cells (CD45.1+) prior to injection into lethally irradiated (9.5 Gy, X-ray) recipient mice (CD45.1+CD45.2+). When using the Mx1-Cre strain, recipient mice were given 4 intraperitoneal injections of 0.2 mg polyinosinic-polycytidylic acid (Poly(I:C); 27-4110-01; GE Healthcare) to induce NCoR1 deletion at 6 weeks posttransplantation.
ChIP-qPCR
ChIP experiments were performed as previously described.21 Protein–DNA complexes were precipitated with anti-H3K27ac (ab4729; Abcam), anti-H4ac (06-598; Millipore), anti-Hdac3 (113301; GeneTex), and anti-NCoR1 (5948; Cell Signaling Technology). ChIP DNA was amplified for Cebpe using forward (5′-AGAAGCTACTCTGGGCAAGG-3′) and reverse (5′-CTTGGGGTGACTAAGGGAGG-3′) primers. ChIP DNA was amplified for Mpo using forward (5′-TTGTCCACCCCTCCTTCTTC-3′) and reverse (5′-ACTCCTCTCACTGACAACGG-3′) primers.
Retroviral production, cell transduction, and leukemia mouse model
The murine stem cell virus retroviral construct MLL-AF9-IRES-GFP has been described.22 Retroviral supernatants were harvested from HEK293T cells and then used to transduce Lin− BM cells. A total of 10 000 GFP+ cells was injected into lethally irradiated recipient mice. For the secondary transplantation, 1 × 106 GFP+ spleen cells from the sick primary recipients were injected into sublethally irradiated (5 Gy, X-ray) recipients.
RNA-Seq and real-time qPCR
RNA sequencing (RNA-Seq) experiments were performed as previously described.23 Briefly, total RNA was isolated from flow-sorted Lin−Sca-1+c-Kit+ (LSK) cells or using an RNeasy Mini Kit (74104; Qiagen, Valencia, CA), and RNA-Seq analysis was performed at The Beijing Genomics Institute using an Illumina HiSeq 2000. Raw data were deposited in the Sequence Read Archive database (accession number SRP133801). Primers for polymerase chain reaction (PCR) were designed using Primer3 software or selected from the PrimerBank database. The primer sequences are listed in supplemental Table 1. All reactions were performed on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) using SYBR Premix Ex Taq II (RR820A; TaKaRa).
Statistical analysis
The statistical significance of differences between groups was calculated using the unpaired 2-tailed Student t test. Survival curves were compared using the log-rank test. Statistical significance is indicated by *P < .05, **P < .01, and ***P < .001 in the figures.
Results
Hematopoietic-specific loss of NCoR1 leads to reduction of peripheral lymphocytes
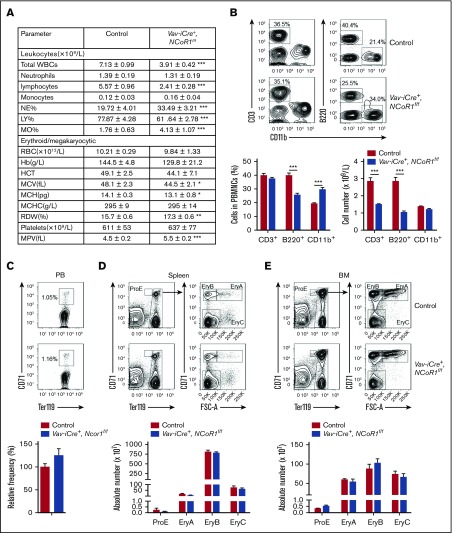
To define the role of NCoR1 in hematopoiesis, we examined NCoR1 messenger RNA (mRNA) levels in representative populations of mouse BM via real-time qPCR. NCoR1 expression was relatively higher in immature hematopoietic progenitor cells in BM, including LSK cells, multipotent progenitors (MPPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs) and was lower in the differentiated fraction (Lin+), as well as in monocytes (CD11b+Gr-1−), granulocytes (CD11b+Gr-1+), and erythrocytes (Ter119+) (supplemental Figure 1). An earlier study reported that NCoR1-null mice died by embryonic day (E)15.5 as a result of developmental defects in multiple organs.13 For systematic analysis of NCoR1 function in a hematopoietic system, we used a conditional knockout approach in Vav-iCre–transgenic mice, which produced Cre recombinase in hematopoietic cells after HSC formation. Efficient hematopoietic-specific deletion of NCoR1 was confirmed via PCR using genomic DNA from BM cells (supplemental Figure 2A) and real-time qPCR in LSK cells in the BM (supplemental Figure 2B). The mRNA level of NCoR2 in LSK cells from Vav-iCre+, NCoR1f/f mice was comparable to that of control mice (supplemental Figure 2C). Vav-iCre+, NCoR1f/f mice were born at the expected Mendelian ratios and were healthy and fertile, with no apparent differences compared with control littermates. Analysis of PB from Vav-iCre+, NCoR1f/f mice revealed that the absolute numbers of total white blood cells (WBCs) and lymphocytes decreased by nearly half, whereas the absolute numbers of other cell lineages, such as monocytes and neutrophils, remained unchanged (Figure 1A). Flow cytometry analysis showed that the percentage of B lymphocytes was significantly decreased, whereas the percentage of myeloid cells was increased, in PB samples from NCoR1-knockout mice (Figure 1B).
Figure 1.
NCoR1 is essential for lymphoid differentiation but dispensable for erythropoiesis. (A) PB cell counts in 8-week-old control mice (n = 6) and Vav-iCre+, NCoR1f/f mice (n = 5) determined using a Hemavet 950FS analyzer (Drew Scientific). (B) Fluorescence-activated cell sorting (FACS) analysis of T cells (CD3+), B cells (B220+), and myeloid cells (CD11b+) in PB samples from control mice (n = 6) and Vav-iCre+, NCoR1f/f mice (n = 5). Representative FACS profiles (upper panel) and the relative frequency (lower left panel) and cell number (lower right panel) are shown. (C) FACS analysis of immature erythrocytes (CD71+Ter119+) in control and Vav-iCre+, NCoR1f/f (n = 6 per group) PB samples. Representative FACS profiles (upper panel) and the relative frequency (lower panel) are shown. FACS analysis of CD71/Ter119 erythroid subsets in spleen (D) and BM (E) from control and Vav-iCre+, NCoR1f/f mice (n = 5 per genotype). Representative FACS profiles (upper panels) and the absolute cell numbers of ProE (CD71highTer119intermediate), EryA (CD71highTer119highFSChigh), EryB (CD71highTer119highFSClow), and EryC (CD71lowTer119highFSClow) (lower panels) are shown. Data are mean ± standard error of the mean from 3 independent experiments. *P < .05, **P < .01, ***P < .001. Hb, hemoglobin; HCT, hematocrit; LY, lymphocyte; MCH, mean corpuscular hemoglobin; MCHC, mean cell hemoglobin concentration; MCV, mean corpuscular volume; MO, monocyte; MPV, mean platelet volume; NE, neutrophil; RDW, red cell distribution width.
NCoR1 is dispensable for erythropoiesis in vivo
Previous studies indicate that definitive erythropoiesis is blocked in conventional NCoR1-knockout embryos.13 However, in our experiments, the number of red blood cells (RBCs) in the PB of Vav-iCre+, NCoR1f/f mice was comparable to that of control mice (Figure 1A). Moreover, hemoglobin concentrations and hematocrit were similar in PB samples from Vav-iCre+, NCoR1f/f mice and control mice (Figure 1A). However, red cell distribution width and mean platelet volume were increased slightly in PB from Vav-iCre+, NCoR1f/f mice (Figure 1A). Flow cytometry analysis showed no alterations in immature erythrocytes, defined as CD71+Ter119+, in the PB of Vav-iCre+, NCoR1f/f mice (Figure 1C). Erythrocyte progenitors in the spleen and BM were divided into 4 subsets (ProE, EryA, EryB, and EryC) based on CD71 and Ter119 staining. The ProE subset contained CD71highTer119intermediate cells. Ter119high cells were subdivided into less mature large EryA erythroblasts (CD71highTer119highFSChigh), smaller more mature EryB erythroblasts (CD71highTer119highFSClow), and most mature EryC erythroblasts (CD71lowTer119highFSClow). NCoR1 was efficiently deleted in CD71/Ter119 erythroid subsets in spleen and BM from Vav-iCre+, NCoR1f/f mice (supplemental Figure 2D). The frequency of immature erythrocytes in Vav-iCre+, NCoR1f/f mice was examined further. The absolute cell numbers of ProE, EryA, EryB, and EryC were not changed in the spleen or BM from Vav-iCre+, NCoR1f/f mice (Figure 1D-E). Our results suggest that NCoR1 is largely dispensable for erythropoiesis in vivo.
NCoR1 deficiency alters the HSC compartment in vivo
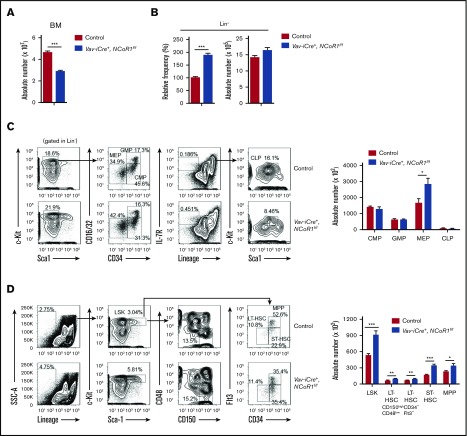
Next, we investigated the effects of NCoR1 loss on hematopoietic cells in the BM. The absolute number of BM cells in Vav-iCre+, NCoR1f/f mice was significantly reduced relative to that of the controls (Figure 2A). Flow cytometry experiments showed that the absolute number of Lin− cells in the BM of Vav-iCre+, NCoR1f/f mice remained largely unchanged, whereas the relative frequency of the Lin− fraction was significantly increased, compared with that of controls (Figure 2B). Our results suggest that the hypocellularity of BM cells is primarily attributable to a reduction in the differentiated Lin+ fraction in Vav-iCre+, NCoR1f/f mice.
Figure 2.
NCoR1 deletion causes HSCs expansion. (A) Absolute number of BM nucleated cells in control and Vav-iCre+, NCoR1f/f mice (n = 6 per genotype). (B) Relative frequency (left) and absolute number (right) of immature cells (Lin−) in BM from control and Vav-iCre+, NCoR1f/f mice (n = 6 per genotype). (C) Fluorescence-activated cell sorting (FACS) analysis of common myeloid progenitors (CMPs), GMPs, MEPs, and CLPs in control and Vav-iCre+, NCoR1f/f BM cells (n = 6 per genotype). Representative FACS profiles (left panels) and absolute cell numbers (right panel) are shown. (D) FACS analysis of LSK cells, LT-HSCs (CD150highCD48low LSK cells and CD34−Flt3− LSK cells), ST-HSCs, and MPPs in control and Vav-iCre+, NCoR1f/f BM cells (n = 6 per genotype). Representative FACS profiles (left profiles) and absolute cell numbers (right) are shown. Data are mean ± standard error of the mean from 3 independent experiments. *P < .05, **P < .01, ***P < .001.
We further analyzed the immature hematopoietic cell population in the BM of Vav-iCre+, NCoR1f/f mice. The numbers of common myeloid progenitors, GMPs, and common lymphoid progenitors (CLPs) remained largely unchanged, whereas MEP number was significantly increased (Figure 2C). Assessment of B-cell differentiation in the BM of Vav-iCre+, NCoR1f/f mice revealed considerable blockage of differentiation in B-cell progenitors (supplemental Figure 3A). An in vitro methylcellulose colony-forming assay disclosed a significant decrease in the number of pre-B colonies in BM cells in Vav-iCre+, NCoR1f/f mice (supplemental Figure 3B). Interestingly, a nearly twofold increase in the absolute number of LSK cells was detected in the BM of Vav-iCre+, NCoR1f/f mice via fluorescence-activated cell sorting (FACS) analysis (Figure 2D). Moreover, the numbers of LT-HSCs (CD34−Flt3− LSK cells or CD150highCD48low LSK cells), short-term HSCs (ST-HSCs; CD34+Flt3− LSK cells), and MPPs (CD34+Flt3+ LSK cells) were increased in NCoR1-depleted mice (Figure 2D). We used another broadly applicable strategy to separate HSCs from MPP1 (Flt3−CD150+CD48− LSK cells) and divided MPPs into 3 distinct subsets: erythroid-primed progenitor MPP2 (Flt3−CD150+CD48+ LSK cells), myeloid-primed progenitor MPP3 (Flt3−CD150−CD48+ LSK cells), and lymphoid-primed progenitor MPP4 (Flt3+CD150− LSK cells) (supplemental Figure 4A). Consistently, absolute cell numbers for the LSK cell–defined fraction MPP1 and erythroid-primed progenitor MPP2 were significantly increased in the BM of Vav-iCre+, NCoR1f/f mice, whereas the cell numbers of MPP3 and MPP4 remained unchanged. These results suggest that NCoR1 loss leads to alterations in the HSC compartment in vivo.
NCoR1 regulates HSC quiescence and proliferation
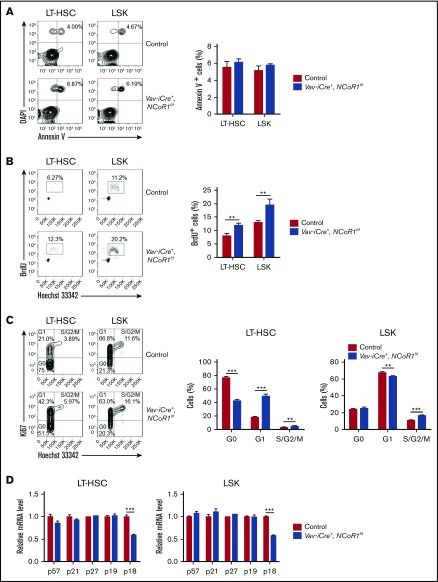
The next step was to determine whether the expansion of LT-HSC and LSK cell populations in the BM of Vav-iCre+, NCoR1f/f mice was due to changes in apoptosis or proliferation of HSCs. To this end, LT-HSC and LSK cell populations were subjected to Annexin V, BrdU-incorporation, and Ki-67 staining assays. No significant differences were evident between the frequencies of Annexin V+ cell populations in LT-HSCs and LSK cells of Vav-iCre+, NCoR1f/f and control mice (Figure 3A). However, the BrdU-incorporation assay disclosed a significant increase in the frequency of BrdU+ (S phase) LT-HSCs and LSK cells in the BM of Vav-iCre+, NCoR1f/f mice relative to control mice (Figure 3B). Ki-67 staining assays further confirmed an increase in the S2/G/M phases of LT-HSCs and LSK cells in the BM of NCoR1-deficient mice (Figure 3C). Remarkably, a significant reduction in G0 stage cells in the LT-HSC compartment was detected in the BM of Vav-iCre+, NCoR1f/f mice (Figure 3C). Consistently, expression of the cyclin-dependent kinase inhibitor, p18INC4C, was markedly downregulated in sorted LT-HSC and LSK cells of Vav-iCre+, NCoR1f/f mice, as determined via real-time qPCR (Figure 3D). These findings clearly support a crucial role for NCoR1 in the maintenance of HSC quiescence, possibly through regulation of p18INC4C expression.
Figure 3.
NCoR1 deficiency promotes HSC proliferation. (A) Apoptosis analysis of LT-HSCs and LSK cells in control and Vav-iCre+, NCoR1f/f mice (n = 6 per genotype). Representative FACS profiles (left panel) and the frequency of Annexin V+ cells (right panel) are shown. (B) Cell cycle analysis of LT-HSCs and LSK cells in control and Vav-iCre+, NCoR1f/f mice (n = 5 per genotype). Representative FACS profiles (left panel) and the frequency of BrdU+ cells (right panel) are shown. (C) Cell cycle analysis of LT-HSCs and LSK cells in control and Vav-iCre+, NCoR1f/f mice (n = 5 per genotype). Representative FACS profiles (left panel) and the frequency of cell cycle distribution (left and middle panels) are shown. (D) Real-time qPCR analysis of the expression of cycling-dependent kinase inhibitors genes (n = 3). mRNA levels were normalized to Gapdh expression. Data are mean ± standard error of the mean from 3 independent experiments. **P < .01, ***P < .001.
To investigate the potential roles of NCoR1 in niches within the BM, we transplanted 1 × 106 BM cells from Vav-iCre+, NCoR1f/f or control mice into lethally irradiated wild-type recipients. At 12 weeks after transplantation, LSK cells in recipients were monitored via flow cytometry (supplemental Figure 5A). A nearly twofold increase in the absolute numbers of LSK cells was detected in the BM of the recipients transplanted with Vav-iCre+, NCoR1f/f BM cells compared with those transplanted with control BM cells (supplemental Figure 5B). No significant differences were observed in the frequency of Annexin V+ cell populations in LSK cells between recipients transplanted with Vav-iCre+, NCoR1f/f or control BM cells (supplemental Figure 5C). In contrast, the frequency of BrdU+ (S phase) LSK cells was significantly increased in BM of Vav-iCre+, NCoR1f/f mice relative to control chimeric mice (supplemental Figure 5D). Furthermore, expression of p18INC4C was markedly downregulated in LSK cells of Vav-iCre+, NCoR1f/f chimeric mice, as determined via real-time qPCR (supplemental Figure 5E). Therefore, these results suggest that there is indeed a cell-intrinsic requirement for NCoR1 in HSC quiescence and proliferation.
NCoR1 is dispensable for HSC self-renewal but essential for lymphoid reconstitution
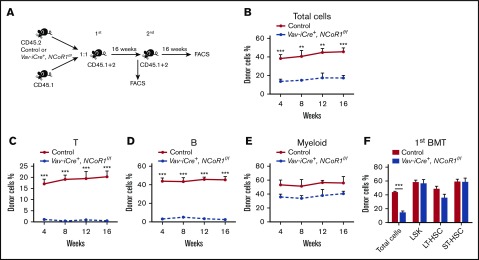
We next assessed the capacity of NCoR1-deficient HSCs to reconstitute the hematopoietic system after BM transplantation. To examine the homing capacity of NCoR1-deficient BM cells, we transplanted control and Vav-iCre+, NCoR1f/f BM cells into lethally irradiated recipients. At 16 hours after transplantation, homing capacity was evaluated using the flow cytometry assay. Vav-iCre+, NCoR1f/f BM cells were able to migrate into the BM of recipients as efficiently as control BM cells (supplemental Figure 6A). One million BM cells from Vav-iCre+, NCoR1f/f mice or control mice, together with an equal number of wild-type competitor BM cells, were cotransplanted into lethally irradiated recipients, and hematopoietic regeneration was monitored via flow cytometry (Figure 4A). At 16 weeks posttransplantation, recipients of NCoR1-deficient donor cells exhibited a significant decrease in PB chimerism compared with controls (Figure 4B; supplemental Figure 7A). Detailed analysis revealed that NCoR1 deficiency causes selective defects in lymphoid lineage reconstitution in recipients of the first and second rounds of transplantation (Figure 4C-E; supplemental Figure 8A-H). LSK cell, LT-HSC, and ST-HSC populations in the BM of recipients were characterized. NCoR1-deficient donor cells could effectively reconstitute the HSC compartment (LSK cells, LT-HSCs, and ST-HSCs) in the recipients of first and second rounds of transplantation (Figure 4F; supplemental Figures 7B and 8I). We transplanted 3000 LSK cells from Vav-iCre+, NCoR1f/f mice or control mice together with 1 × 106 competitor BM cells into lethally irradiated recipients (supplemental Figure 9A). At 16 weeks posttransplantation, Vav-iCre+, NCoR1f/f LSK cells effectively reconstituted the HSC compartments, but not the lymphoid lineage, in the recipients (supplemental Figure 9B-C). These results clearly indicated that NCoR1 is essential for lymphoid lineage differentiation but not for self-renewal of HSCs.
Figure 4.
NCoR1 deficiency compromises the long-term lymphoid-repopulation capacity of HSCs. (A) Schematic diagram for serial competitive transplantation assay with control and Vav-iCre+, NCoR1f/f BM cells. (B) Percentage of donor-derived cells (total cells) in PB of primary recipient mice (n = 5 per group) at the indicated time points. Percentage of donor-derived T (CD3+) cells (C), B (B220+) cells (D), and myeloid (CD11b+) cells (E) in the PB of recipient animals (n = 5 per group) at the indicated time points. (F) Percentage of donor-derived cells (total cells, LSK cells, LT-HSCs, and ST-HSCs) in BM of primary recipient mice (n = 5 per group) 16 weeks after transplantation. Data are mean ± standard error of the mean from 3 independent experiments. **P < .01, ***P < .001. 1st, first competitive transplantation; 2nd, second competitive transplantation.
To further investigate the cell-autonomous role of NCoR1 in hematopoiesis, we used Mx1-Cre–transgenic mice expressing interferon-inducible Cre recombinase induced by Poly(I:C). Untreated Mx1-Cre+, NCoR1f/f and control BM cells were transplanted into lethally irradiated recipients (supplemental Figure 10A). Six weeks after transplantation, cells from both groups had comparable repopulation capacities. Recipient mice received Poly(I:C) 4 times to induce NCoR1 deletion and were analyzed 16 weeks later. Similarly, the percentage of Mx1-Cre+, NCoR1f/f–derived cells was significantly decreased in PB chimerism compared with control groups (supplemental Figure 10B). However, the percentages of Mx1-Cre+, NCoR1f/f–derived LSK cells, LT-HSCs, and ST-HSCs were not altered in BM of recipient mice (supplemental Figure 10C), indicating that intrinsic NCoR1 is required for differentiation rather than self-renewal of HSCs.
NCoR1 deletion alters the transcriptional signature of HSCs
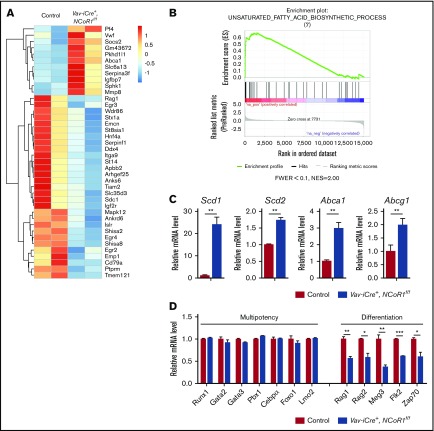
To further explore the molecular mechanisms underlying NCoR1-mediated HSC homeostasis and function, we performed genome-wide expression profiling on isolated LSK cells. Using standard criteria (fold change > 2; false discovery rate < 0.05), we identified 57 significantly upregulated and 102 significantly downregulated genes in Vav-iCre+, NCoR1f/f LSK cells (Figure 5A; supplemental Table 1). Several genes affected by NCoR1 loss, including Pf4,24 Vwf,25 Socs2,26 Abca1,27 Igf2r,28 Sphk1,29 and Emcn,30 have previously been shown to participate in HSC activity. Gene set enrichment analysis31 revealed that genes upregulated in Vav-iCre+, NCoR1f/f LSK cells are enriched in unsaturated fatty acid biosynthetic processes (Figure 5B). Real-time qPCR was applied to confirm the RNA-Seq data showing upregulation of mRNA levels of stearoyl-CoA desaturase 1 (SCD1), stearoyl-CoA desaturase 2 (SCD2), and cholesterol ATP-binding cassette (ABC) transporters A1 (Abca1) and G1 (Abcg1) after NCoR1 deletion (Figure 5C). Stearoyl-CoA desaturase is the rate-limiting enzyme that catalyzes the synthesis of monounsaturated fatty acids. NCoR1 was previously shown to interact with liver X receptor or thyroid hormone receptor, followed by recruitment to the Scd1 promoter to repress its expression in hepatocytes.32 Moreover, expression patterns of HSC multipotency genes (Runx1, Gata2, Gata3, Pbx1, Cebpa, Foxo1, and Lmo2) remained unchanged in Vav-iCre+, NCoR1f/f HSCs. In contrast, NCoR1-null HSCs showed lower expression of factors crucial for differentiation, such as Rag1, Rag2, Flk2, Meg3, and Zap70 (Figure 5D). Accordingly, we propose that downregulation of differentiation genes after NCoR1 depletion may contribute to the lymphoid-differentiation defects in NCoR1-deficient HSCs.
Figure 5.
NCoR1 deletion changes the gene-expression profile. (A) Heat map depicting a portion of significantly dysregulated genes in Vav-iCre+, NCoR1f/f LSK cells compared with control LSK cells (fold change > 2; false discovery rate < 0.05). See also supplemental Table 1. (B) Gene set enrichment analysis of control and Vav-iCre+, NCoR1f/f LSK cells. (C) Relative expression levels of Scd1, Scd2, Abca1, and Abcg1 in control and Vav-iCre+, NCoR1f/f LSK cells, as measured by real-time PCR (n = 3). mRNA levels were normalized to Gapdh expression. (D) Relative expression levels of select multipotency and differentiation genes in control and Vav-iCre+, NCoR1f/f LSK cells measured by real-time PCR analysis (n = 3). mRNA levels were normalized to Gapdh expression. *P < .05, **P < .01, ***P < .001. FWER, family wise error rate; NES, normalized enrichment score.
NCoR1 deficiency impedes MLL-AF9–induced leukemic transformation in vivo
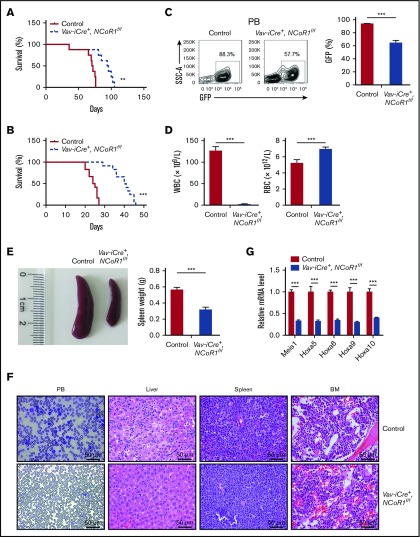
NCoR1 plays essential roles in PML-RARα–induced acute promyelocytic leukemia (APL), PLZF-RARα–induced APL, and AML1-ETO–induced AML. Here, we examined whether NCoR1 plays a role in the initiation and progression of MLL-AF9–induced AML. To this end, Vav-iCre+, NCoR1f/f and control Lin− BM cells were transduced with retrovirus expressing MLL-AF9 fusion protein and transplanted into lethally irradiated recipients (supplemental Figure 11A). NCoR1 deficiency led to a significant delay in mortality following primary transplantation (Figure 6A). Furthermore, a significant survival benefit was again observed upon secondary transplantation with decreased WBC number, along with increased RBC number in PB (Figure 6B-D). However, flow cytometry experiments showed a mild decrease in the percentage of leukemia cells in spleen and BM (supplemental Figure 11B). We also observed less profound splenomegaly in Vav-iCre+, NCoR1f/f mice (Figure 6E). Histological assays revealed the presence of fewer leukemia blasts in the PB and liver of NcoR1-deficient mice (Figure 6F). Consistently, aberrant activation of Meis1 and Hox genes (Hoxa5, Hoxa6, Hoxa9, and Hoxa10), a key feature of MLL-AF9 pathogenesis,33-35 was significantly blocked in sorted GFP+ leukemia cells in Vav-iCre+, NCoR1f/f mice (Figure 6G). Accordingly, we propose that NCoR1 plays an important role in progression of MLL-AF9–induced leukemia.
Figure 6.
Loss of NCoR1 delays leukemogenesis in MLL-AF9–induced AML. (A) Kaplan-Meier survival curve of recipient mice transplanted with 104 GFP+ cells of the indicated genotypes in the primary transplantation assay (n = 8 per group). (B) Kaplan-Meier survival curve of recipient mice transplanted with 1 million GFP+ cells from primary recipients in the secondary transplantation assay (n = 12 per group). (C) FACS analysis of GFP+ cells in PB of secondary recipients (n = 5 per group) 20 days after transplantation. Representative FACS profiles (upper panel) and the percentage of GFP+ cells (lower panel) are shown. (D) WBC and RBC counts, using a Hemavet 950FS analyzer, in PB of secondary recipients (n = 5 per group) 20 days after transplantation. (E) Spleen weight of secondary recipients (n = 5 per group) 20 days after transplantation (right panel). A representative photograph is shown (left panel). (F) Wright-Giemsa staining of blood smear, and hematoxylin and eosin staining of liver, spleen, and BM from secondary recipients 20 days after transplantation. (G) Real-time qPCR analysis of Meis1, Hoxa5, Hoxa6, Hoxa9, and Hoxa10 in sorted BM leukemia cells (GFP+) of secondary recipients (n = 5 per group) 20 days after transplantation. mRNA levels were normalized to Gapdh expression. Data are mean ± standard error of the mean from 3 independent experiments. **P < .01, ***P < .001.
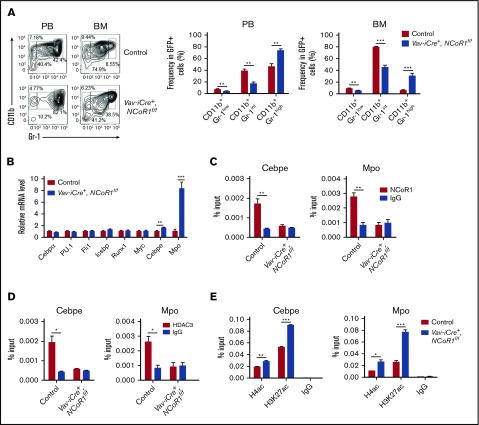
To elucidate the mechanisms underlying NCoR1 activity in MLL-AF9–induced AML, we determined the homing capacity, as well as the apoptosis and cell-cycle state, of leukemia cells. MLL-AF9–expressing Vav-iCre+, NCoR1f/f leukemia cells migrated into the BM of recipients as efficiently as did MLL-AF9–expressing control leukemia cells (supplemental Figure 11C). No significant differences in apoptosis or cell cycle were detected between GFP+ leukemic cells from Vav-iCre+, NCoR1f/f mice and control mice (supplemental Figure 11D-E). FACS analysis revealed a significant decrease in the frequency of immature neutrophils (CD11b+Gr-1intermediate) and an increase in the frequency of mature neutrophils (CD11b+Gr-1high) in PB and BM of Vav-iCre+, NCoR1f/f mice compared with control mice (Figure 7A). Morphological analysis was performed, which revealed the presence of fewer immature neutrophils in the PB, spleen, and BM of Vav-iCre+, NCoR1f/f mice (supplemental Figure 12A). Moreover, expression of Cebpe and Mpo, 2 myeloid-differentiation factors, was significantly upregulated in leukemia cells of Vav-iCre+, NCoR1f/f mice (Figure 7B). These results indicate that NCoR1 deficiency promotes differentiation of MLL-AF9–induced leukemia cells into mature myeloid cells.
Figure 7.
NCoR1/HDAC3 corepressor complex could block myeloid differentiation in MLL-AF9–induced AML. (A) FACS analysis of CD11b+Gr-1low, CD11b+Gr-1intermediate, and CD11b+Gr-1high populations in GFP+ leukemia cells from PB and BM of secondary recipients (n = 5 per group) 20 days after transplantation. Representative FACS profiles are shown (left panel). (B) Real-time qPCR analysis of myeloid differentiation factors in BM leukemia cells of secondary recipients (n = 5 per group) 20 days after transplantation. mRNA levels were normalized to Gapdh expression. ChIP analysis of the Cebpe (left panels) and Mpo (right panels) promoters using anti-NCoR1 (C), anti-HDAC3 (D), and the indicated antibodies (E) in BM leukemia cells of secondary recipients 20 days after transplantation. IgG served as a negative control. Data are mean ± standard error of the mean from 3 independent experiments. *P < .05, **P < .01, ***P < .001.
To determine whether NCoR1 protein binds directly to the promoter regions of myeloid-specific factors to suppress their expression, we performed a ChIP-qPCR assay using an anti-NCoR1 antibody in MLL-AF9–induced leukemia cells. Enrichment of NCoR1 was detected at Cebpe and Mpo promoters, clearly indicating localization of the protein to promoters of myeloid-specific factors (Figure 7C). Because HDAC3 could be recruited by NCoR1 to mediate target gene repression through promoting histone deacetylation,36 we determined whether the protein directly binds myeloid-specific factor promoters and examined the corresponding acetylation status of their promoters after NCoR1 deletion. In the ChIP-qPCR assay, HDAC3 bound Cebpe and Mpo promoters, which was abolished upon NCoR1 deletion (Figure 7D). Moreover, NCoR1 deletion induced a marked increase in the acetylation levels of histone 4 (H4) and histone 3 lysine 27 (H3K27) at the Cebpe and Mpo promoters, indicating that the protein is required for transcription-repressing H4ac and H3K27ac marks at these regions (Figure 7E). Our results support a crucial role for the NCoR1/HDAC3 complex in removing H4ac and H3K27ac at promoters of myeloid-specific factors and blocking myeloid differentiation in MLL-AF9–induced leukemia cells.
Discussion
The nuclear receptor corepressor, NCoR1, plays a range of roles in embryonic development, as well as homeostatic processes, including circadian rhythms, metabolism, inflammation, regeneration, and carcinogenesis.37 In the current study, we have provided evidence that NCoR1 regulates HSC function and facilitates MLL-AF9–mediated leukemogenesis in mice. In our experiments, NCoR1 deletion in mouse hematopoietic cells led to enhanced proliferation and an increased pool of HSCs and decreased numbers of terminally differentiated lymphocytes. Moreover, NCoR1 appeared essential for long-term lymphoid reconstitution in competitive transplantation assays but dispensable for the long-term repopulation capacity of HSCs and facilitated leukemogenesis in an MLL-AF9–mediated leukemia model. In MLL-AF9–transformed cells, NCoR1 bound the promoter regions of several myeloid-specific genes and suppressed their expression. Our current findings provide novel evidence for the significance of NCoR1 in normal hematopoiesis and leukemogenesis.
In an earlier study, NCoR1-null embryos exhibited impaired definitive erythropoiesis and underwent death by day E15.5.13 However, we showed that NCoR1 deletion in the hematopoietic system does not affect in vivo erythropoiesis in a Vav-iCre–mediated NCoR1 conditional knockout mouse model. Therefore, a hematopoiesis-independent function of NCoR1 may be required for definitive erythropoiesis in mouse embryos. In addition, studies using a human chronic myelogenous leukemia cell line have demonstrated a critical role for NCoR1 in erythroid differentiation.16 In contrast to these findings, we detected no obvious defects in erythropoiesis in hematopoietic-specific NCoR1-knockout mice. This discrepancy may be due to the distinct roles of NCoR1 in leukemic and normal hematopoietic cells.
Here, we detected a significant increase in the number of LSK cells and LT-HSCs in the BM of Vav-iCre+, NCoR1f/f mice. Further analyses revealed that NCoR1-deficient HSCs display markedly decreased quiescence and increased proliferation, which may explain the enlarged pool of HSCs in Vav-iCre+, NCoR1f/f mice under steady-state conditions. Consistently, p18INC4C, a cyclin-dependent kinase inhibitor previously reported to be critical for self-renewing division,38-40 was significantly downregulated in NCoR1-deficient HSCs. Accordingly, we propose that NCoR1 limits the size of the HSC pool, at least in part by maintaining p18INC4C expression.
NCoR1 appears to be essential for maintenance of the HSC pool but dispensable for HSC self-renewal. NCoR1-deficient HSCs could replenish the HSC-enriched LSK cell population in the BM of recipients as efficiently as their control counterparts after transplantation. However, although myeloid cells could be reconstituted in the recipients transplanted with NCoR1-deficient HSCs, the lymphoid-differentiation potential of NCoR1-deficient HSCs was severely impaired. Meanwhile, differentiation of lymphocytes, in particular B lymphocytes, was additionally impaired in Vav-iCre+, NCoR1f/f mice. Several lymphoid-differentiation–related genes were significantly downregulated in NCoR1-deficient HSCs, indicating that NCoR1 controls lymphoid lineage differentiation through regulating the transcriptional activation of specific genes. Two recent reports have shown that thymocyte-specific deletion of NCoR1 causes a block in positive selection (CD4 single-positive and CD8 single-positive thymocytes)41 and promotes thymocyte apoptosis through increasing Bim expression.42 Thus, NCoR1 appears to exert its activity at different stages of development to regulate hematopoiesis and lymphogenesis.
NCoR1 functions in a large repression complex including HDAC3.11 HDAC3 recruitment is essential for the repressive activity of NCoR1.43-45 The involvement of HDAC3 in normal and malignant hematopoiesis has been documented.46-50 Interestingly, mice deficient in NCoR1 and Hdac3 share similar phenotypes, including hypocellular BM, increased Lin− fraction, and cycling HSCs in the BM. However, Hdac3 deficiency leads to a more severe phenotype than NCoR1 deficiency in mice. For example, T-lymphocyte development within the thymus is severely impaired in Hdac3-deficient mice,46 but it is impaired to a lesser extent in NCoR1-deficient mice. Moreover, Hdac3-deficient HSCs have impaired self-renewal ability and are unable to reconstitute the hematopoietic system in recipients,46 whereas the self-renewal ability of NCoR1-deficient HSCs appears normal. These results suggest that, although NCoR1 and HDAC3 coexist within a large protein complex and control a broad range of biological processes, HDAC3 plays a more important regulatory role in gene transcription in an NCoR1-independent manner.
In PML-RARα–induced APL, PLZF-RARα–induced APL, and AML1-ETO-induced AML, the NCoR1/HDAC3 corepressor complex is recruited to target promoters by RARα and ETO proteins to suppress transcription.51 In the present study, NCoR1 depletion delayed progression of MLL-AF9–induced AML. Interestingly, knockdown of HDAC3 or inhibition of its activity was recently shown to provide a significant survival benefit in an MLL-AF9–mediated AML mouse model,47 suggesting that the NCoR1/HDAC3 complex plays critical roles in diverse AML subtypes. Clarification of the molecular mechanisms underlying the involvement of the NCoR1/HDAC3 complex in leukemogenesis should aid in the development of effective alternative therapeutic strategies for AML.
In summary, we have provided direct evidence of the crucial involvement of the nuclear receptor corepressor NCoR1 in normal and malignant hematopoiesis. In view of its essential roles in diverse AML subtypes, NCoR1 presents a potential therapeutic target for leukemia.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank So Chi Wai Eric for providing the murine stem cell virus–MLL-AF9 plasmid; Jianmin Wang, Jinyong Wang, and Lijian Hui for providing the CD45.1+ mice, Vav-iCre mice, and Mx1-Cre mice, respectively; Tao Cheng for help with the leukemia mouse model; and Xiaoming Zhang, Ping Hu, Bo Zhou, Guangxun Meng, and Haibing Zhang for valuable discussions.
This work was supported by the National Basic Research Program of China (2015CB964900) and the National Natural Science Foundation of China (31670906, 31471207, 81270618, and 81370601).
Authorship
Contribution: X.W. and Y. Zhang designed the experiments, analyzed the data, and wrote the manuscript; X.W. and L.L. performed the experiments; P.Z., X.H., Q.H., F.Y., W.Z., X.D., X.Y., N.Z., Y. Zhao, Y.L., and P.H. analyzed the data; R.Z. contributed to the flow cytometry experiments; J.A. provided the NCoR1f/f mice; and X.S. and Q.L. analyzed the data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yan Zhang, Department of Hematology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 201620, China; e-mail: yan_zhang@sibs.ac.cn; Qibin Leng, Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 320 Yue-yang Rd, Shanghai, China 200031; e-mail: qbleng@sibs.ac.cn; and Xianmin Song, Department of Hematology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 201620, China; e-mail: shongxm@shjt.edu.cn.
References
- 1.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169(2):338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118-1129. [DOI] [PubMed] [Google Scholar]
- 3.Zhao JL, Baltimore D. Regulation of stress-induced hematopoiesis. Curr Opin Hematol. 2015;22(4):286-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanpain C, Mohrin M, Sotiropoulou PA, Passegué E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8(1):16-29. [DOI] [PubMed] [Google Scholar]
- 5.Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017;17(9):573-590. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Ema H. Mechanisms of self-renewal in hematopoietic stem cells. Int J Hematol. 2016;103(5):498-509. [DOI] [PubMed] [Google Scholar]
- 7.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyama S, Kitamura T. Epigenetics in normal and malignant hematopoiesis: an overview and update 2017. Cancer Sci. 2017;108(4):553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377(6548):454-457. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Kalkum M, Chait BT, Roeder RG. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol Cell. 2002;9(3):611-623. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wang J, Wang J, et al. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 2000;19(16):4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000;14(9):1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 13.Jepsen K, Hermanson O, Onami TM, et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102(6):753-763. [DOI] [PubMed] [Google Scholar]
- 14.Jepsen K, Solum D, Zhou T, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450(7168):415-419. [DOI] [PubMed] [Google Scholar]
- 15.Jepsen K, Gleiberman AS, Shi C, Simon DI, Rosenfeld MG. Cooperative regulation in development by SMRT and FOXP1. Genes Dev. 2008;22(6):740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long MD, van den Berg PR, Russell JL, Singh PK, Battaglia S, Campbell MJ. Integrative genomic analysis in K562 chronic myelogenous leukemia cells reveals that proximal NCOR1 binding positively regulates genes that govern erythroid differentiation and Imatinib sensitivity. Nucleic Acids Res. 2015;43(15):7330-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Cho E, Wong J. A critical role for the co-repressor N-CoR in erythroid differentiation and heme synthesis. Cell Res. 2007;17(9):804-814. [DOI] [PubMed] [Google Scholar]
- 18.Wong MM, Guo C, Zhang J. Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation. Am J Clin Exp Urol. 2014;2(3):169-187. [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto H, Williams EG, Mouchiroud L, et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147(4):827-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Wong J, Klinger M, Tran MT, Shannon KM, Killeen N. MLL5 contributes to hematopoietic stem cell fitness and homeostasis. Blood. 2009;113(7):1455-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X, Liu L, Ding X, et al. Mll2 controls cardiac lineage differentiation of mouse embryonic stem cells by promoting H3K4me3 deposition at cardiac-specific genes. Stem Cell Rev. 2014;10(5):643-652. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Hao S, Liu Y, et al. Leukemic marrow infiltration reveals a novel role for Egr3 as a potent inhibitor of normal hematopoietic stem cell proliferation. Blood. 2015;126(11):1302-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Wan X, Zhou P, et al. The chromatin remodeling subunit Baf200 promotes normal hematopoiesis and inhibits leukemogenesis. J Hematol Oncol. 2018;11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudek AZ, Nesmelova I, Mayo K, Verfaillie CM, Pitchford S, Slungaard A. Platelet factor 4 promotes adhesion of hematopoietic progenitor cells and binds IL-8: novel mechanisms for modulation of hematopoiesis. Blood. 2003;101(12):4687-4694. [DOI] [PubMed] [Google Scholar]
- 25.Rhieu BH, Epperly MW, Cao S, et al. Increased hematopoiesis in long-term bone marrow cultures and reduced irradiation-induced pulmonary fibrosis in von Willebrand factor homologous deletion recombinant mice. In Vivo. 2014;28(4):449-456. [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali C, Bassani C, Chiodoni C, et al. SOCS2 controls proliferation and stemness of hematopoietic cells under stress conditions and its deregulation marks unfavorable acute leukemias. Cancer Res. 2015;75(11):2387-2399. [DOI] [PubMed] [Google Scholar]
- 27.Savage-Rumbaugh ES. Language acquisition in a nonhuman species: implications for the innateness debate. Dev Psychobiol. 1990;23(7):599-620. [DOI] [PubMed] [Google Scholar]
- 28.Heo K, Jariwala U, Woo J, et al. Involvement of Niemann-Pick type C2 protein in hematopoiesis regulation. Stem Cells. 2006;24(6):1549-1555. [DOI] [PubMed] [Google Scholar]
- 29.Adamiak M, Chelvarajan L, Lynch KR, Santos WL, Abdel-Latif A, Ratajczak MZ. Mobilization studies in mice deficient in sphingosine kinase 2 support a crucial role of the plasma level of sphingosine-1-phosphate in the egress of hematopoietic stem progenitor cells. Oncotarget. 2017;8(39):65588-65600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmfeldt P, Ganuza M, Marathe H, et al. Functional screen identifies regulators of murine hematopoietic stem cell repopulation [published correction appears in J Exp Med. 2016;213(11):2525]. J Exp Med. 2016;213(3):433-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545-15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha RA, Singh BK, Zhou J, et al. Loss of ULK1 increases RPS6KB1-NCOR1 repression of NR1H/LXR-mediated Scd1 transcription and augments lipotoxicity in hepatic cells. Autophagy. 2017;13(1):169-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milne TA. Mouse models of MLL leukemia: recapitulating the human disease. Blood. 2017;129(16):2217-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winters AC, Bernt KM. MLL-rearranged leukemias-an update on science and clinical approaches. Front Pediatr. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10(4):257-268. [DOI] [PubMed] [Google Scholar]
- 36.Atsumi A, Tomita A, Kiyoi H, Naoe T. Histone deacetylase 3 (HDAC3) is recruited to target promoters by PML-RARalpha as a component of the N-CoR co-repressor complex to repress transcription in vivo. Biochem Biophys Res Commun. 2006;345(4):1471-1480. [DOI] [PubMed] [Google Scholar]
- 37.Mottis A, Mouchiroud L, Auwerx J. Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev. 2013;27(8):819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Yuan Y, Shen H, Cheng T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood. 2006;107(3):1200-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6(5):436-442. [DOI] [PubMed] [Google Scholar]
- 40.Schwaller J, Pabst T, Koeffler HP, et al. Expression and regulation of G1 cell-cycle inhibitors (p16INK4A, p15INK4B, p18INK4C, p19INK4D) in human acute myeloid leukemia and normal myeloid cells. Leukemia. 1997;11(1):54-63. [DOI] [PubMed] [Google Scholar]
- 41.Müller L, Hainberger D, Stolz V, et al. The corepressor NCOR1 regulates the survival of single-positive thymocytes. Sci Rep. 2017;7(1):15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, He N, Zhang N, et al. NCoR1 restrains thymic negative selection by repressing Bim expression to spare thymocytes undergoing positive selection. Nat Commun. 2017;8(1):959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Codina A, Love JD, Li Y, Lazar MA, Neuhaus D, Schwabe JW. Structural insights into the interaction and activation of histone deacetylase 3 by nuclear receptor corepressors. Proc Natl Acad Sci USA. 2005;102(17):6009-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua G, Ganti KP, Chambon P. Glucocorticoid-induced tethered transrepression requires SUMOylation of GR and formation of a SUMO-SMRT/NCoR1-HDAC3 repressing complex. Proc Natl Acad Sci USA. 2016;113(5):E635-E643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Z, Feng D, Fang B, et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell. 2013;52(6):769-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Summers AR, Fischer MA, Stengel KR, et al. HDAC3 is essential for DNA replication in hematopoietic progenitor cells. J Clin Invest. 2013;123(7):3112-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long J, Fang WY, Chang L, et al. Targeting HDAC3, a new partner protein of AKT in the reversal of chemoresistance in acute myeloid leukemia via DNA damage response. Leukemia. 2017;31(12):2761-2770. [DOI] [PubMed] [Google Scholar]
- 48.Mehdipour P, Santoro F, Botrugno OA, et al. HDAC3 activity is required for initiation of leukemogenesis in acute promyelocytic leukemia. Leukemia. 2017;31(4):995-997. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Hong Z, Zhang RR et al. Bakkenolide A inhibits leukemia by regulation of HDAC3 and PI3K/Akt-related signaling pathways. Biomed Pharmacother. 2016;83:958-966. [DOI] [PubMed] [Google Scholar]
- 50.Su R, Gong JN, Chen MT, et al. c-Myc suppresses miR-451⊣YWTAZ/AKT axis via recruiting HDAC3 in acute myeloid leukemia. Oncotarget. 2016;7(47):77430-77443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Racanicchi S, Maccherani C, Liberatore C, et al. Targeting fusion protein/corepressor contact restores differentiation response in leukemia cells. EMBO J. 2005;24(6):1232-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.