Key Points
Both aGVHD and cGVHD protect against ALL relapse.
Grades I and II aGHVD favorably affect survival in ALL in CR1/CR2 and cGVHD favorably affects survival in advanced ALL.
Abstract
Allogeneic hematopoietic cell transplant is a potential curative therapy for acute lymphoblastic leukemia (ALL). Delineating the graft-versus-leukemia (GVL) effect as a function of graft-versus-host disease (GVHD) offers the potential to improve survival. We examined 5215 transplant recipients with ALL reported to the Center for International Blood and Marrow Transplant Research registry. Overall survival (OS) was compared according to the presence and severity of GVHD and evaluated in 3 cohorts: 2593 adults in first or second complete remission (CR1/CR2), 1619 pediatric patients in CR1/CR2, and 1003 patients with advanced (CR ≥3 or active disease) ALL. For patients in CR1/CR2, development of acute GVHD (aGVHD) or chronic GVHD (cGVHD) was associated with lower risk of relapse than no GVHD (hazard ratio [HR], 0.49-0.69). Patients with advanced ALL developing grades III and IV aGVHD or cGVHD were also at lower risk of relapse (HRs varied from 0.52 to 0.67). Importantly, adult and children in CR1/CR2 with grades I and II aGVHD without cGVHD experienced the best OS compared with no GVHD (reduction of mortality with HR, 0.83-0.76). Increased nonrelapse mortality accompanied grades III and IV aGVHD (HRs varied from 2.69 to 3.91) in all 3 cohorts and abrogated any protection from relapse, resulting in inferior OS. Patients with advanced ALL had better OS (reduction in mortality; HR, 0.69-0.73) when they developed cGVHD with or without grades I and II aGVHD. In conclusion, GVHD was associated with an increased GVL effect in ALL. GVL exerted a net beneficial effect on OS only if associated with low-grade aGVHD in CR1/CR2 or with cGVHD in advanced ALL.
Visual Abstract
Introduction
The graft-versus-leukemia (GVL) effect associated with allogeneic hematopoietic cell transplant (alloHCT) provides potent antileukemic therapy for patients with acute lymphoblastic leukemia (ALL) as reflected by a significantly reduced relapse rate compared with standard chemotherapy or autologous HCT.1 Although the GVL effect may occur in the absence of clinical graft-versus-host disease (GVHD), data suggest that acute GVHD (aGVHD) and chronic GVHD (cGVHD) are associated with an augmented GVL effect.2-13 Nevertheless, GVHD affecting >50% of patients remains a major cause of mortality after alloHCT. Hence, the increased nonrelapse mortality (NRM) associated with GVHD may abrogate the favorable GVL effect on disease relapse. The strength of the GVL effect has been shown to differ between hematological malignancies.14 As shown in a large registry study, acute myeloid leukemia (AML) was relatively insensitive to aGVHD and limited cGVHD; nevertheless, reductions in relapse risk have been reported in patients experiencing extensive cGVHD. Conversely, ALL was sensitive to both aGVHD and cGVHD, with reduced relapse risks comparable to chronic myeloid leukemia. In fact, the higher sensitivity of ALL to GVHD compared with AML was first described in 1979 in a cohort of allogeneic and syngeneic marrow transplants.15 Accordingly, the net impact of aGVHD and cGVHD on survival may differ considerably between patients with AML and ALL.10,14,16 Although the net impact of GVHD on transplant outcomes has been explored in AML, robust studies in the modern era are lacking in ALL.12,13 Thus, the aim of the present Center for International Blood and Marrow Transplant Research (CIBMTR) registry-led study was to explore the impact of aGVHD and cGVHD of varying severity on transplant outcomes in a large cohort of patients with ALL treated with alloHCT.
Methods
Data source
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. CIBMTR comprises a voluntary network of >450 transplantation centers worldwide that contribute detailed data on consecutive alloHCT and autologous HCT to a centralized statistical center. Observational studies conducted by CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants.17
Study design
This retrospective study was designed to explore the GVL effect in ALL and the impact of aGVHD and cGVHD on transplant-related outcomes, including NRM, relapse, disease-free survival (DFS), and overall survival (OS). The study population consisted of patients with ALL who underwent alloHCT and who met all the following criteria: (1) age >1 year at HCT; (2) first alloHCT; (3) adult (aged >18 years) patients in first or second complete remission (CR1/CR2) with any conditioning regimen intensity or pediatric (≤18 years) patients in CR1/CR2 with myeloablative (MAC) regimens only or patients of all ages in CR ≥3 or with active ALL with MAC regimens only; (4) recipients of grafts from matched sibling (MSD), unrelated (MUD), single or double umbilical cord blood (UCB) donors; (5) transplanted between the years 2000 and 2014; and (6) reported to the CIBMTR. Patients were excluded if they met one or more of the following criteria: (1) haploidentical grafts (as too few were available for inclusion); (2) donor-recipient HLA disparity unknown; (3) ex vivo T-cell–depleted grafts; (4) failure to engraft; (5) recipients of planned or preemptive donor lymphocyte infusion; (6) no 100-day comprehensive research form available; and (7) no signed informed consent available for data inclusion.
The following GVHD patient groups were compared: no GVHD vs grades I and II aGVHD or grades III and IV aGVHD; and no cGVHD vs cGVHD with or without preceding grades I and II or grades III and IV aGVHD.
These three distinct patient cohorts were separately analyzed: (1) adult patients in CR1/CR2 with MAC or reduced intensity conditioning (RIC) regimens; (2) pediatric patients in CR1/CR2 with MAC regimens; and (3) patients of all ages with advanced disease with MAC regimens.
Definitions
Advanced disease was defined as ALL in CR ≥3 and any patient with active disease (primary induction failure or resistant relapse). Cytogenetic abnormalities considered as adverse included t(9;22), t(4;11), t(8;14), t(14;18), hypodiploid, and complex karyotype. Conditioning regimen intensity was defined according to CIBMTR criteria,18 and aGVHD and cGVHD were diagnosed and graded according to consensus criteria.19,20 NRM was defined as death during continuous CR after alloHCT. Relapse was defined as clinical or hematological leukemia recurrence. For analyses of DFS, failures were clinical or hematological relapses or deaths from any cause. For OS analyses, failure was death from any cause.
Statistical analysis
Cumulative incidence curves were used for relapse incidence and NRM considering each other as competing risk. Probabilities of DFS and OS were calculated by using Kaplan-Meier estimates.
Time-dependent analysis was performed for each of the 3 distinct cohorts examining the main GVHD patient groups: no GVHD vs grades I and II aGVHD; no GVHD vs grades III and IV aGVHD; no GVHD vs cGVHD; no GVHD vs grades I and II aGVHD plus cGVHD; and no GVHD vs grades III and IV aGVHD plus cGVHD. Assessment of GVHD and GVL effects were evaluated in multivariate analysis (MVA) by using Cox models for treatment failure (1-DFS) and overall mortality (1-OS), in which GVHD of the specified grade or onset type was treated as a time-dependent covariate21,22 and in cumulative incidence competing hazards modeling for relapse and NRM. Multivariate models considered the following additional factors (Table 1): patient age, sex, Karnofsky performance status (KPS) scores (<90% vs ≥90%), HCT–comorbidity index (0 vs 1 vs 2 vs ≥3), leukocyte count at diagnosis according to age group and cell lineage (T-cell, ≤100 × 109/L vs >100 × 109/L; B-cell pediatric, ≤30 × 109/L vs 30 to 50 × 109/L vs >50 × 109/L; and B-cell adults, ≤50 × 109/L vs >50 × 109/L), extramedullary disease at diagnosis, cytogenetic risk group (normal/standard [SR] vs Philadelphia-positive vs other poor risk), time to achieve CR1 (<4 weeks vs 4-8 weeks vs >8 weeks), time from diagnosis to HCT for patients in CR1 (<6 months vs 6-12 months vs >12 months), conditioning regimen (total body irradiation [TBI]–based MAC vs non-TBI–based MAC vs RIC), graft type (bone marrow vs peripheral blood vs UCB), donor type and HLA match (MSD vs MUD [8/8] vs 1 antigen or allele mismatch unrelated donor [URD] [7/8] vs >1 antigen or allele mismatch URD [<7/8] vs UCB), donor–recipient sex combination, donor–recipient cytomegalovirus (CMV) serostatus, URD age (18-29 years vs 30-44 years vs ≥45 years), GVHD prophylaxis (calcineurin inhibitor [CNI] plus methotrexate vs CNI plus mycophenolate mofetil vs other), in vivo T-cell depletion (using antithymocyte globulin or alemtuzumab), and year of transplantation (2000-2003 vs 2004-2007 vs 2008-2011 vs 2012-2014).
Table 1.
Characteristics of study population
| Variable | Adults, CR1/CR2, MAC/RIC | Pediatrics, CR1/CR2, MAC | All ages PIF/relapse/CR ≥3, MAC |
|---|---|---|---|
| No. of patients | 2593 | 1619 | 1003 |
| No. of centers | 232 | 165 | 205 |
| Patient age, y | |||
| 1-9 | 0 | 941 (58) | 187 (19) |
| 10-18 | 0 | 678 (42) | 225 (22) |
| 19-29 | 928 (36) | 0 | 281 (28) |
| 30-39 | 568 (22) | 0 | 139 (14) |
| 40-49 | 558 (22) | 0 | 107 (11) |
| 50-59 | 402 (16) | 0 | 57 (6) |
| ≥60 | 137 (5) | 0 | 7 (<1) |
| Median (range) | 36 (18-75) | 9 (1-18) | 21 (1-67) |
| Sex | |||
| Male | 1537 (59) | 1018 (63) | 651 (65) |
| Female | 1056 (41) | 601 (37) | 352 (35) |
| KPS score | |||
| <90% | 733 (28) | 205 (13) | 293 (29) |
| ≥90% | 1774 (68) | 1345 (83) | 648 (65) |
| Missing | 86 (3) | 69 (4) | 62 (6) |
| HCT–comorbidity index | |||
| 0 | 518 (20) | 449 (28) | 157 (16) |
| 1 | 165 (6) | 46 (3) | 30 (3) |
| 2 | 129 (5) | 14 (<1) | 18 (2) |
| ≥3 | 283 (11) | 34 (2) | 45 (4) |
| NA | 1498 (58) | 1076 (66) | 753 (75) |
| Philadelphia positive | |||
| No | 1574 (61) | 1315 (81) | 813 (81) |
| Yes | 957 (37) | 249 (15) | 150 (15) |
| Missing or untested | 62 (2) | 55 (3) | 40 (4) |
| Immunophenotype | |||
| T cell | 381 (15) | 262 (16) | 128 (13) |
| B cell | 2119 (82) | 1248 (77) | 804 (80) |
| Missing | 93 (4) | 109 (7) | 71 (7) |
| WBC at diagnosis by age group and lineage, × 109/L | |||
| T, ≤100 | 214 (8) | 105 (6) | 70 (7) |
| T, >100 | 66 (3) | 112 (7) | 30 (3) |
| Adult B, ≤30 | 1200 (46) | 0 | 216 (22) |
| Adult B, >30 | 531 (20) | 0 | 124 (12) |
| Pediatric B, ≤30 | 0 | 646 (40) | 157 (16) |
| Pediatric B, 30-50 | 0 | 86 (5) | 31 (3) |
| Pediatric B, >50 | 0 | 276 (17) | 54 (5) |
| Missing (WBC or lineage) | 582 (22) | 394 (24) | 321 (32) |
| Extramedullary disease at diagnosis | |||
| No | 2171 (84) | 1301 (80) | 819 (82) |
| Yes | 328 (13) | 270 (17) | 146 (15) |
| Missing | 94 (4) | 48 (3) | 38 (4) |
| Previous CNS disease | |||
| No | 2330 (90) | 1360 (84) | 871 (87) |
| Yes | 169 (7) | 211 (13) | 94 (9) |
| Missing | 94 (4) | 48 (3) | 38 (4) |
| Disease status before HCT | |||
| Primary induction failure | 0 | 0 | 145 (14) |
| CR1 | 1815 (70) | 644 (40) | 0 |
| CR2 | 778 (30) | 975 (60) | 0 |
| CR ≥3 | 0 | 0 | 403 (40) |
| Relapse | 0 | 0 | 455 (45) |
| Cytogenetics scoring | |||
| Normal karyotype | 768 (30) | 400 (25) | 301 (30) |
| Philadelphia chromosome | 957 (37) | 249 (15) | 150 (15) |
| Other poor risk | 230 (9) | 281 (17) | 95 (9) |
| SR | 428 (17) | 460 (28) | 212 (21) |
| Not tested/missing | 210 (8) | 229 (14) | 245 (24) |
| MRD at time of HCT | |||
| No | 183 (7) | 109 (7) | 25 (2) |
| Yes | 326 (13) | 134 (8) | 56 (6) |
| Missing | 2084 (80) | 1376 (85) | 922 (92) |
| Time from diagnosis to HCT (CR1 cases) | |||
| <6 mo | 1120 (43) | 378 (23) | 0 |
| 6-12 mo | 602 (23) | 217 (13) | 0 |
| >12 mo | 93 (4) | 49 (3) | 0 |
| Not applicable (CR2+/PIF/relapse) | 778 (30) | 975 (60) | 1003 |
| Median (range) | 7 (<1-21) | 17 (<1-44) | |
| Time to achieve CR1, median (range), wk | 7 (<1-113) | 5 (<1-167) | 5 (<1-252) |
| Conditioning regimen | |||
| MAC with TBI | 1917 (74) | 1478 (91) | 874 (87) |
| MAC without TBI | 317 (12) | 141 (9) | 129 (13) |
| RIC | 359 (14) | 0 | 0 |
| Graft type | |||
| Bone marrow | 659 (25) | 687 (42) | 331 (33) |
| Peripheral blood | 1611 (62) | 239 (15) | 431 (43) |
| Single cord | 87 (3) | 591 (37) | 168 (17) |
| Double cords | 236 (9) | 102 (6) | 73 (7) |
| Donor type | |||
| HLA-identical sibling | 903 (35) | 367 (23) | 197 (20) |
| Well-matched unrelated or 8/8 | 934 (36) | 328 (20) | 334 (33) |
| Partially/mismatched unrelated or ≤7/8 | 433 (17) | 231 (14) | 231 (23) |
| Cord blood | 323 (12) | 693 (43) | 241 (24) |
| Donor–recipient sex match | |||
| Male–male | 951 (37) | 548 (34) | 382 (38) |
| Male–female | 562 (22) | 330 (20) | 191 (19) |
| Female–male | 559 (22) | 424 (26) | 248 (25) |
| Female–female | 473 (18) | 246 (15) | 147 (15) |
| Missing | 48 (2) | 71 (4) | 35 (3) |
| Donor–recipient CMV serology | |||
| +/+ | 763 (29) | 249 (15) | 227 (23) |
| +/− | 255 (10) | 115 (7) | 80 (8) |
| −/+ | 781 (30) | 538 (33) | 346 (34) |
| −/− | 676 (26) | 665 (41) | 302 (30) |
| Missing | 118 (5) | 52 (3) | 48 (5) |
| URD age, median (range), y | 33 (19-57) | 33 (18-57) | 34 (19-57) |
| GVHD prophylaxis | |||
| CNI + MTX | 1684 (65) | 882 (54) | 621 (62) |
| CNI + MMF | 549 (21) | 327 (20) | 207 (21) |
| Other | 357 (14) | 410 (25) | 172 (17) |
| Missing | 3 (<1) | 0 | 3 (<1) |
| In vivo TCD (ATG or alemtuzumab) | |||
| No | 2048 (79) | 991 (61) | 712 (71) |
| Yes | 544 (21) | 628 (39) | 290 (29) |
| Missing | 1 (<1) | 0 | 1 (<1) |
| Year of HCT | |||
| 2000-2003 | 597 (23) | 494 (31) | 376 (37) |
| 2004-2007 | 897 (35) | 580 (36) | 372 (37) |
| 2008-2011 | 619 (24) | 337 (21) | 175 (17) |
| 2012-2014 | 480 (19) | 208 (13) | 80 (8) |
| Median follow-up of survivors (range), mo | 70 (2-184) | 72 (2-191) | 86 (3-176) |
Data are n (%) unless otherwise indicated.
ATG, antithymocyte globulin; CNS, central nervous system; NA, not applicable (before the year 2007); PIF, primary induction failure; TCD, T-cell depletion; TKI, tyrosine kinase inhibitor; WBC, white blood cell.
A stepwise model selection approach was used, and each step contained the main effect for GVHD. Factors that were significant at a 5% level were kept in the final model. The proportionality assumption was tested by adding a time-dependent covariate for each factor. When tests indicated differential effects over time (nonproportional hazards), models were constructed breaking the posttransplant course into 2 time periods, using the maximized partial likelihood method to find the most appropriate breakpoint. After the aforementioned modeling of time-varying effects, the final multivariate model was built.
Results
Patient, disease, and transplant characteristics
Overall, 5215 patients from 287 centers were eligible for analysis. Cohort 1 included 2593 adult patients (median age, 36 years; range, 18-75 years) in CR1/CR2 with MAC or RIC regimens; cohort 2 included 1619 pediatric patients (median age, 9 years; range, 1-18 years) in CR1/CR2 with MAC regimens; and cohort 3 included 1003 adult and pediatric patients (median age, 21 years; range, 1-67 years) in CR ≥3 (n = 403) and patients with active disease (primary induction failure, n = 145; relapse, n = 455) treated with MAC regimens.
Patient, disease, and treatment characteristics of the 3 cohorts are summarized in Table 1. Most (88%) patients were aged <50 years, 61.5% were male, and 26% had Philadelphia-positive ALL. In cohorts 1 and 2, 70% of adults and 40% of pediatric-aged patients were in CR1. Data on minimal residual disease (MRD) were available for only 16% of patients and thus were not considered in any analyses. MAC regimens were used in 86% of adults in CR1/CR2, and 28% received in vivo T-cell depletion.
Transplant outcomes
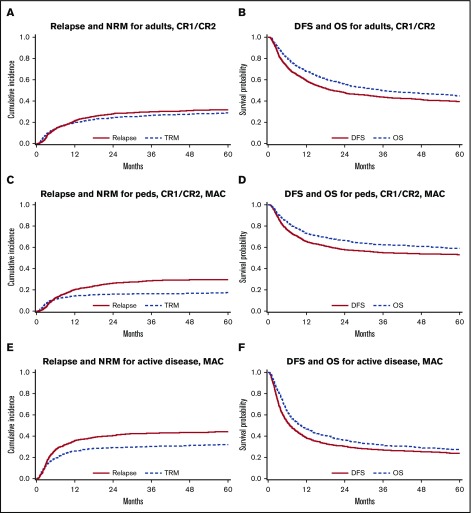
The median follow-up of survivors was 72 months (range, 3-191 months). At the time of analysis, 48% (2519 of 5215) of patients were alive. The 1- and 5-year cumulative incidence rates of relapse were 24% and 33%, respectively, and the corresponding NRM rates were 19% and 26%. The cumulative incidences of grades I to IV aGVHD by day 100 in the 3 cohorts were 59%, 61%, and 65%, and the cumulative incidences of cGVHD at 1 year were 46%, 29%, and 29%. The cumulative incidences of relapse and NRM and survival curves of DFS and OS in the 3 cohorts are shown in Figure 1.
Figure 1.
NRM and relapse, DFS, and OS in 3 cohorts. Relapse and NRM for adults (CR1/CR2) (A), pediatric patients (CR1/CR2, MAC) (C), and all patients with active disease (MAC) (E). DFS and OS for adults (CR1/CR2) (B), pediatric patients (CR1/CR2, MAC) (D), and all patients with active disease (MAC) (F).
Adult patients in CR1/CR2
Influence of aGVHD and cGVHD on transplant outcomes
The median follow-up of survivors was 70 months (range, 2-184 months). At the time of analysis, 48% (1250 of 2593) of adult patients in CR1/CR2 were alive, and 451 (17%), 640 (25%), 358 (14%), and 146 (6%) patients developed grades I, II, III, and IV aGVHD, respectively. The cumulative incidences of grades I and II and grades III and IV aGVHD by day 100 were 41% and 18%. The cumulative incidence of cGVHD at 1 year was 46%, and 22% never developed GVHD. The 1- and 5-year cumulative incidence rates of relapse were 21% and 32%, and the corresponding NRM rates were 20% and 29%. The 1- and 5-year OS rates were 68% and 45%.
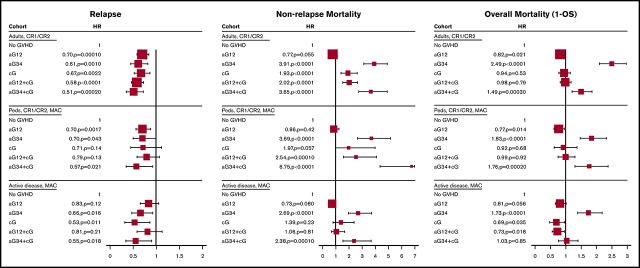
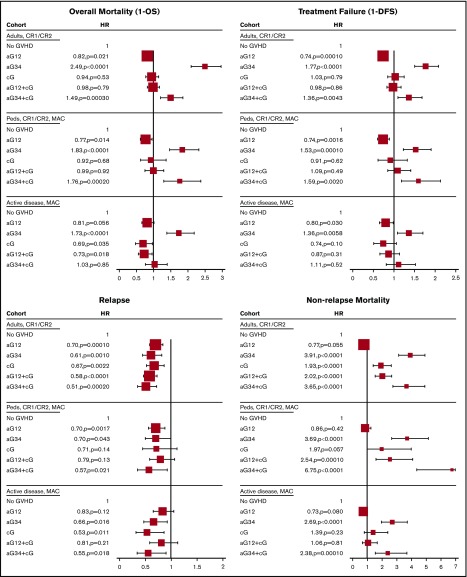
In MVA, using no GVHD as the reference category, all forms of GVHD were protective against leukemia relapse (hazard ratio [HR], 0.49-0.70). Conversely, grades III and IV aGVHD and cGVHD were each associated with increased risk of NRM (HR, 3.91 [95% CI, 3.12-4.91]; HR,1.93 [95% CI, 1.42-2.63], respectively). Compared with patients without GVHD, these differential effects translated into the following: (1) significantly improved DFS (HR, 0.74; 95% CI, 0.63-0.86) and OS (HR, 0.83; 95% CI, 0.69-0.97) with grades I and II aGVHD; (2) significantly worse DFS and OS with grades III and IV aGVHD with or without cGVHD (HR, 1.36 [95% CI, 1.10-1.68] and HR, 1.49 [95% CI, 1.20-1.85], with cGVHD; HR, 1.77 [95% CI, 1.50-2.08] and HR, 2.49 [95% CI, 2.09-2.96], without cGVHD); and (3) similar DFS and OS with cGVHD, with or without grades I and II aGVHD (Figure 2).
Figure 2.
Forest plot of main effect in time-dependent MVA models. The larger size of box indicates larger cohort.
MVA of other factors affecting OS
In the adult cohort, overall mortality increased with patient age ≥40 years and KPS scores <90%. Patients with Philadelphia-positive ALL had better OS than patients with SR-cytogenetics, whereas patients with poor and SR-cytogenetics had similar outcomes. TBI-based MAC compared favorably with non-TBI–based MAC; however, the number of patients receiving non-TBI–based MAC was disproportionally smaller (12%). Survival was similar among patients who received TBI-based MAC regimens and RIC/nonmyeloablative regimens. Survival was similar using either MSD, MUD, or UCB but significantly worse using mismatched URD. Other favorable factors included CMV-seronegative patients with CMV-seropositive donors, GVHD prophylaxis comprising a CNI plus methotrexate, and transplants in the more recent era (Table 2). Subgroup analyses yielded similar results within the CR1 and CR2 cohorts (data not shown).
Table 2.
Multivariate analyses of factors affecting OS
| Variable | Adults, CR1/CR2, MAC/RIC (n = 2593) | Pediatrics, CR1/CR2, MAC (n = 1619) | Advanced disease, MAC (n = 1003) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | P | n | HR (95% CI) | P | n | HR (95% CI) | P | ||
| Age, y | ||||||||||
| 1-9 | 941 | 1 | 187 | 1 | ||||||
| 10-18 | 678 | 1.43 (1.22-1.68) | <.0001 | 225 | 0.97 (0.75-1.25) | .80 | ||||
| 19-29 | 928 | 1 | 281 | 1.39 (1.07-1.79) | .012 | |||||
| 30-39 | 568 | 1.03 (0.89-1.20) | .68 | 139 | 1.26 (0.93-1.70) | .13 | ||||
| 40-49 | 558 | 1.28 (1.10-1.49) | .0015 | 107 | 2.10 (1.53-2.89) | <.0001 | ||||
| 50-59 | 402 | 1.66 (1.40-1.97) | <.0001 | 67 | 1.58 (1.10-2.29) | .015 | ||||
| ≥60 | 137 | 1.61 (1.20-2.15) | .0013 | 7 | 3.68 (1.40-9.68) | .0084 | ||||
| KPS score | ||||||||||
| <90% | 733 | 1 | 293 | 1 | ||||||
| 90%-100% | 1774 | 0.78 (0.69-0.88) | <.0001 | 648 | 0.81 (0.68-0.95) | .011 | ||||
| Missing | 86 | 0.90 (0.66-1.21) | .47 | 62 | 0.78 (0.56-1.09) | .15 | ||||
| Cytogenetics | ||||||||||
| Normal/standard | 1196 | 1 | 513 | 1 | ||||||
| Poor, Philadelphia positive | 957 | 0.83 (0.73-0.94) | .0029 | 150 | 0.77 (0.60-0.97) | .028 | ||||
| Poor, Philadelphia negative | 230 | 1.09 (0.89-1.32) | .41 | 95 | 1.41 (1.09-1.84) | .010 | ||||
| Missing | 210 | 1.18 (0.97-1.44) | .092 | 245 | 0.91 (0.75-1.09) | .30 | ||||
| Conditioning | ||||||||||
| MAC with TBI | 1917 | 1 | ||||||||
| MAC without TBI | 317 | 1.29 (1.09-1.53) | .0026 | |||||||
| RIC | 359 | 1.06 (0.88-1.27) | .57 | |||||||
| Donor source | ||||||||||
| Matched sibling | 903 | 1 | 197 | 1 | ||||||
| Matched URD | 934 | 1.08 (0.95-1.24) | .25 | 334 | 1.40 (1.11-1.76) | .0044 | ||||
| Mismatched URD | 433 | 1.39 (1.18-1.64) | <.0001 | 231 | 1.84 (1.44-2.35) | <.0001 | ||||
| UCB | 323 | 1.23 (0.98-1.53) | .077 | 241 | 1.37 (1.00-1.87) | .049 | ||||
| D/R CMV serology | ||||||||||
| +/+ | 763 | 1 | 249 | 1 | 227 | 1 | ||||
| +/− | 255 | 0.91 (0.75-1.11) | .36 | 115 | 1.08 (0.75-1.54) | .69 | 80 | 0.66 (0.48-0.89) | .0074 | |
| −/+ | 781 | 0.86 (0.74-1.00) | .050 | 538 | 1.35 (1.06-1.72) | .017 | 346 | 0.82 (0.66-1.02) | .069 | |
| −/− | 767 | 0.83 (0.71-0.97) | .018 | 665 | 0.94 (0.73-1.20) | .60 | 302 | 0.59 (0.47-0.74) | <.0001 | |
| Missing | 118 | 1.19 (0.92-1.53) | .18 | 52 | 0.94 (0.58-1.51) | .79 | 48 | 1.06 (0.73-1.53) | .77 | |
| GVHD prophylaxis | ||||||||||
| CNI + MTX | 1684 | 1 | 621 | 1 | ||||||
| CNI + MMF | 549 | 1.23 (1.06-1.45) | .0087 | 207 | 1.30 (1.03-1.64) | .026 | ||||
| Other | 357 | 1.10 (0.93-1.30) | .24 | 172 | 0.97 (0.77-1.23) | .82 | ||||
| Year of transplantation | ||||||||||
| 2000-2003 | 597 | 1 | 494 | 1 | 376 | 1 | ||||
| 2004-2007 | 897 | 0.90 (0.79-1.04) | .16 | 580 | 0.74 (0.61-0.89) | .0013 | 372 | 0.78 (0.65-0.93) | .0059 | |
| 2008-2011 | 619 | 0.80 (0.68-0.95) | .0086 | 337 | 0.66 (0.53-0.83) | .00040 | 175 | 0.62 (0.49-0.80) | .00020 | |
| 2012-2014 | 480 | 0.64 (0.52-0.78) | <.0001 | 208 | 0.66 (0.50-0.88) | .0050 | 80 | 0.54 (0.38-0.78) | ||
GVHD is shown separately in Figure 2.
D/R, donor/recipient; MMF, mycophenolate mofetil; MTX, methotrexate; PIF, primary induction failure.
Pediatric patients in CR1/CR2
Influence of aGVHD and cGVHD on transplant outcomes
The median follow-up of survivors was 72 months (range, 2-191 months). At the time of analysis, 60% (976 of 1619) of pediatric patients in CR1/CR2 were alive, and 357 (22%), 344 (21%), 210 (13%), and 99 (6%) patients developed grades I, II, III, and IV aGVHD, respectively. The cumulative incidences of grades I and II and grades III and IV aGVHD by day 100 were 42% and 19%. The cumulative incidence of cGVHD at 1 year was 29%, and 30% never developed GVHD. The 1- and 5-year cumulative incidence rates of relapse were 20% and 30%, and the corresponding NRM rates were 14% and 17%. The 1- and 5-year OS rates were 73% and 59%.
In MVA, using no GVHD as the reference category, grades I and II aGVHD and grades III and IV aGVHD were protective against relapse (HR, 0.70 [95% CI, 0.56-0.88]; and HR, 0.70 [95% CI, 0.50-0.99], respectively). Conversely, grades III and IV aGVHD and cGVHD with any grade of aGVHD were each associated with increased risk of NRM (HR, 2.54-6.75). Compared with patients without GVHD, these differential effects translated into the following: (1) significantly improved DFS (HR, 0.74; 95% CI, 0.61-0.89) and OS (HR, 0.77; 95% CI, 0.62-0.95) with isolated grades I and II aGVHD; (2) significantly worse DFS and OS with grades III and IV aGVHD with or without cGVHD (HR, 1.59 [95% CI, 1.18-2.13] and HR, 1.76 [95% CI, 1.30-2.37], with cGVHD; HR, 1.53 [95% CI, 1.23-1.90] and HR, 1.83 [95% CI, 1.46-2.31], without cGVHD); and (3) similar DFS and OS with cGVHD, with or without grades I and II aGVHD.
MVA of other factors affecting OS
In the pediatric cohort, overall mortality increased with older patient age (10-18 years) and in CMV-seropositive patients with a CMV-seronegative donor. OS improved in the more recent transplant era. Subgroup analyses yielded similar results within the CR1 and CR2 cohorts (data not shown).
Adult and pediatric patients with advanced disease
Influence of aGVHD and cGVHD on transplant outcomes
The median follow-up of survivors was 86 months (range, 3-176 months). At the time of analysis, 29% (293 of 1003) of patients with advanced disease were alive, and 181 (18%), 239 (24%), 163 (16%), and 78 (8%) patients developed grades I, II, III and IV aGVHD, respectively. The cumulative incidences of grades I and II and grades III and IV aGVHD by day 100 were 42% and 23%. The cumulative incidence of cGVHD at 1 year was 29%, and 27% never developed GVHD. The 1- and 5-year cumulative incidence rates of relapse were 36% and 44%, and the corresponding NRM rates were 26% and 32%. The 1- and 5-year survival rates were 47% and 27%.
In MVA, using no GVHD as the reference category, only grades III and IV aGVHD and de novo cGVHD were protective against leukemia relapse (HR, 0.66 [95% CI, 0.47-0.92]; and HR, 0.53 [95% CI, 0.32-0.86], respectively). Conversely, patients with grades III and IV aGVHD with or without cGVHD were at increased risk of NRM (HR, 2.38 [95% CI, 1.54-3.68]; and HR, 2.69 [95% CI, 1.95-3.70]) (Figure 2). Compared with patients without GVHD, these differential effects translated into the following: (1) significantly improved DFS and a trend to better OS among patients with isolated grades I and II aGVHD (HR, 0.80 [95% CI, 0.65-0.98]; and HR, 0.81 [95% CI, 0.66-1.01]); (2) significantly worse DFS among patients with isolated grades III and IV aGVHD (HR, 1.36; 95% CI, 1.09-1.70); (3) significantly improved OS among patients with cGVHD with or without grades I and II aGVHD (HR, 0.73 [95% CI, 0.56-0.95]; and HR, 0.69 [95% CI, 0.48-0.97]); and (4) significantly worse OS among patients with grades III and IV aGVHD without cGVHD (HR, 1.73; 95% CI, 1.38-2.18).
MVA of other factors affecting OS
Overall mortality increased with older age and KPS scores <90%. Patients with Philadelphia-positive ALL had better OS than patients with SR-cytogenetics, whereas patients with poor risk cytogenetics had worse OS compared with patients with SR-cytogenetics. OS was worse using matched/mismatched URD or UCB compared with MSD. CMV-seronegative patients with CMV-seronegative or positive donors had better OS than CMV-seropositive patients with CMV-seropositive donors. Other favorable factors included GVHD prophylaxis using a CNI plus methotrexate and transplants in the more recent era.
Discussion
The differential GVL effect in ALL based on aGVHD severity and disease status was examined in a large cohort of transplanted patients facilitated by the CIBMTR. These data indicate that severe aGVHD is no more protective against relapse than mild aGVHD. Most importantly, the net survival advantage of GVL in ALL is confined to adult and pediatric patients in CR1/CR2 experiencing maximum grades I and II aGVHD without cGVHD. Because grades I and II aGVHD do not increase NRM while they are associated with significant protection against relapse, they consequently yield the best OS (17%-24% lower risk of overall mortality than patients without GVHD); cGVHD is associated with increased NRM, which abrogates any relapse-protective effect of GVHD. In addition, the poor survival in patients with advanced ALL, which is mainly attributed to relapse/progression, is nevertheless significantly improved in the presence of cGVHD (27%-32% lower risk of overall mortality) and has a trend toward improved survival with grades I and II aGVHD (P = .056). Most importantly, in all disease cohorts, grades III and IV aGVHD with their two- to sixfold increase in NRM substantially decrease survival despite their favorable effect on disease relapse.23
In an attempt to determine any favorable net impact of mild aGVHD (grades I and II; 42% of recipients) on survival, we separately compared grades I and II aGVHD and grades III and IV aGVHD vs no GVHD. Indeed, more conventional comparisons of grades 0 and I vs grades II to IV aGVHD12,13,15 failed to uncover our findings (data not shown). Thus, we suggest that clinical discrimination and separate analyses of survivable grades I and II acute GVHD and severe grades III and IV aGVHD should be applied in future similar studies.
By examining 3 different cohorts of patients, our analysis revealed that GVHD-associated GVL and GVHD-associated NRM translate differently according to leukemia remission status but are remarkably similar in adult and pediatric patients with ALL in CR1/CR2. It is important to notice that subgroup analyses within CR1 and CR2 showed similar HR in the same direction, justifying the CR1/CR2 joined analysis as presented here. Although it was not the focus of our analyses, comparing survival among the 3 cohorts supports the prior observation that CR1/CR2 at the time of transplantation is a strong determinant of favorable posttransplant survival. One of the study limitations is a lack of data on pretransplant MRD in 84% of the patients. MRD is a major factor for posttransplant survival,24 and whether the GVL effect differs in MRD-positive vs MRD-negative settings deserves further investigation.
We compared our findings in ALL vs those of a similar retrospective CIBMTR study conducted in AML.12 In contrast to our findings in ALL, AML patients with aGVHD after MAC alloHCT had a worse OS compared with patients without GVHD, as aGVHD in AML lacked any protective effect against relapse while it was associated with increased NRM. However, AML patients with cGVHD only after RIC alloHCT had a better OS compared with patients without GVHD as the cGVHD-associated protective effect against relapse was only partially offset by increased NRM.
Transplant pioneers described the GVL effect in ALL 40 years ago.15 Nevertheless, previous studies did not show survival benefit of GVHD-associated GVL due to increased NRM. In contrast, the present study provides detailed evidence that different grades of GVHD are associated with different levels of protection against relapse. However, until we are better able to ameliorate the NRM in severe GVHD, the clinical benefit and best survival are confined to grades I and II aGVHD. Consequently, our findings may potentially be used to time the immunosuppression taper in those patients with high-risk ALL and no GVHD. We also highlight the improvement in survival over time: among many factors, we noted better KPS scores in recipients over time and more common use of TKI, RIC, and UCB grafts in the past decade.
Although our ability to tailor the most appropriate GVHD management for each transplanted individual remains a challenge, our findings support this nonstandard GVHD grouping for future studies. Better methods to truncate the GVHD progression and strategies to improve quality of life in cGVHD must be developed to better harness the powerful GVL effect in ALL.
Acknowledgments
The CIBMTR is supported primarily by public health service grant/cooperative agreement 5U24CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; a grant/cooperative agreement (4U10HL069294) from the National Heart, Lung and Blood Institute and the National Cancer Institute; a contract (HHSH250201200016C) with Health Resources & Services Administration (Department of Health and Human Services); and 2 grants (N00014-17-1-2388 and N0014-17-1-2850) from the Office of Naval Research. The CIBMTR is also supported by grants from the following companies (italics indicates corporate members): Actinium Pharmaceuticals, Inc.; Amgen, Inc.; Amneal Biosciences; Angiocrine Bioscience, Inc.; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; bluebird bio, Inc.; Bristol-Myers Squibb Oncology; Celgene Corporation; Cerus Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; Incyte Corporation; Janssen Scientific Affairs, LLC; Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; Mediware; Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd.–Japan; PCORI; Pfizer, Inc.; Pharmacyclics, LLC; PIRCHE AG; Sanofi Genzyme; Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources & Services Administration, or any other agency of the US Government.
Authorship
Contribution: M.Y., V.B., D.W., J.M.R., and M.S.T. designed the study; M.Y., V.B., and D.W. analyzed results and wrote the manuscript; M.-J.Z. and H.-L.W. prepared the data and performed statistical analysis; J.M.R., M.S.T., W.S., M.d.L., B.M.S., G.U., R.T.K., M.S.C., B.W.C., J.-Y.C., S.G., B.C., L.F.V., C.D., M.A.D., B.N.S., B.G., J.L., J.M., M.B., M.R.G., W.R.D., M.A.P., H.A.-A., T.P., M.J.W., R.M., M.N., A. Beitinjaneh, S.S., T.N., B.W., H.F., A. Bashey, S.M., and D.I.M. interpreted data and critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Moshe Yeshurun, Institute of Haematology, Davidoff Cancer Centre, Rabin Medical Centre, 39 Jabotinsky St, Petah Tikva 49141, Israel; e-mail: moshey@clalit.org.il.
References
- 1.Goldstone AH, Richards SM, Lazarus HM, et al. . In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, et al. . Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555-562. [PubMed] [Google Scholar]
- 3.Passweg JR, Tiberghien P, Cahn JY, et al. . Graft-versus-leukemia effects in T lineage and B lineage acute lymphoblastic leukemia. Bone Marrow Transplant. 1998;21(2):153-158. [DOI] [PubMed] [Google Scholar]
- 4.Ringdén O, Sundberg B, Lönnqvist B, Tollemar J, Gahrton G, Nilsson B. Allogeneic bone marrow transplantation for leukemia: factors of importance for long-term survival and relapse. Bone Marrow Transplant. 1988;3(4):281-290. [PubMed] [Google Scholar]
- 5.Ringdén O, Karlsson H, Olsson R, Omazic B, Uhlin M. The allogeneic graft-versus-cancer effect. Br J Haematol. 2009;147(5):614-633. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan KM, Weiden PL, Storb R, et al. . Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73(6):1720-1728. [PubMed] [Google Scholar]
- 7.Weisdorf DJ, Nesbit ME, Ramsay NK, et al. . Allogeneic bone marrow transplantation for acute lymphoblastic leukemia in remission: prolonged survival associated with acute graft-versus-host disease. J Clin Oncol. 1987;5(9):1348-1355. [DOI] [PubMed] [Google Scholar]
- 8.Gratwohl A, Brand R, Apperley J, et al. ; Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (CLWP-EBMT). Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukemia. Blood. 2002;100(12):3877-3886. [DOI] [PubMed] [Google Scholar]
- 9.Barrett AJ, Horowitz MM, Gale RP, et al. . Marrow transplantation for acute lymphoblastic leukemia: factors affecting relapse and survival. Blood. 1989;74(2):862-871. [PubMed] [Google Scholar]
- 10.Grigg AP, Szer J, Beresford J, et al. . Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukaemia. Br J Haematol. 1999;107(2):409-418. [DOI] [PubMed] [Google Scholar]
- 11.Rowe JM. Graft-versus-disease effect following allogeneic transplantation for acute leukaemia. Best Pract Res Clin Haematol. 2008;21(3):485-502. [DOI] [PubMed] [Google Scholar]
- 12.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18(11):1727-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storb R, Gyurkocza B, Storer BE, et al. . Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern M, de Wreede LC, Brand R, et al. . Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28(11):2235-2240. [DOI] [PubMed] [Google Scholar]
- 15.Weiden PL, Flournoy N, Thomas ED, et al. . Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068-1073. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka I, Kami M, Takahashi S, et al. ; Japan Society for Hematopoietic Cell Transplantation Working Party. Clinical impact of graft-versus-host disease against leukemias not in remission at the time of allogeneic hematopoietic stem cell transplantation from related donors. The Japan Society for Hematopoietic Cell Transplantation Working Party. Bone Marrow Transplant. 2004;34(8):711-719 [DOI] [PubMed] [Google Scholar]
- 17.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42(suppl 1):S1-S2. [DOI] [PubMed] [Google Scholar]
- 18.Giralt S, Ballen K, Rizzo D, et al. . Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. . 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 20.Sullivan KM, Shulman HM, Storb R, et al. . Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267-276. [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187-220. [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695-706. [DOI] [PubMed] [Google Scholar]
- 23.Ringdén O, Labopin M, Sadeghi B, et al. . What is the outcome in patients with acute leukaemia who survive severe acute graft-versus-host disease? J Intern Med. 2018;283(2):166-177. [DOI] [PubMed] [Google Scholar]
- 24.Pulsipher MA, Carlson C, Langholz B, et al. . IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125(22):3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]