In rice, ABA induces the production of H2O2 by NADPH oxidase, which inhibits the activity of a type 2C protein phosphatase and activates a calcium/calmodulin-dependent protein kinase.
Abstract
In plants, Ca2+/calmodulin-dependent protein kinase (CCaMK) is a positive regulator of abscisic acid (ABA) responses, including root growth, antioxidant defense, and tolerance of both water stress and oxidative stress. However, the underlying molecular mechanisms are poorly understood. Here, we show a direct interaction between DMI3 (Doesn't Make Infections 3), a rice (Oryza sativa) CCaMK and PP45, a type 2C protein phosphatase in rice (PP2C). This interaction involves the CaM binding domain of DMI3 and the PP2C domain of PP45. In the absence of ABA, PP45 directly inactivates DMI3 by dephosphorylating Thr-263 in DMI3. However, in the presence of ABA, ABA-induced H2O2 production by the NADPH oxidases RbohB/E inhibits the activity of PP45 not only by inhibiting the expression of PP45 but also by oxidizing Cys-350 and Cys-428 residues to form PP45 intermolecular dimers. ABA-induced oxidation of Cys-350 and Cys-428 in PP45 blocked the interaction between PP45 and DMI3 and substantially prevented PP45-mediated inhibition in DMI3 activity. Genetic analysis indicated that PP45 is an important negative regulator of ABA signaling. These results reveal important pathways for the inhibition of DMI3 under the basal state and for its ABA-induced activation in rice.
INTRODUCTION
In plants, cytosolic Ca2+ is a ubiquitous second messenger and mediates stimulus response coupling to regulate plant growth, development, and responses to environmental stresses. Various abiotic and biotic stresses cause changes in the cytosolic Ca2+ concentration (Yang and Poovaiah, 2003; Lecourieux et al., 2006; DeFalco et al., 2009; Reddy et al., 2011; Batistič and Kudla, 2012), and these Ca2+ signals are detected, decoded, and transmitted to produce downstream responses by a variety of Ca2+ binding proteins that function as Ca2+ sensors. The main types of Ca2+ sensors include calmodulin (CaM) and CaM-like protein, calcium-dependent protein kinase, calcineurin B-like protein, and Ca2+/CaM-dependent protein kinase (CCaMK; Yang and Poovaiah, 2003; Harper et al., 2004; DeFalco et al., 2009; Batistič and Kudla, 2012).
CCaMK is a plant-specific protein kinase, consisting of a Ser/Thr kinase domain, a CaM binding domain overlapping an autoinhibitory domain, and a visinin-like domain containing three EF (helix-loop-helix) hands (Singh and Parniske, 2012; Poovaiah et al., 2013). CCaMK was first identified and cloned in lily (Lilium longiflorum), and early biochemical studies on lily CCaMK showed that its kinase activities are regulated by both Ca2+ and Ca2+/CaM (Patil et al., 1995; Takezawa et al., 1996). CCaMK is a key regulator of nodule organogenesis and rhizobial infection (Lévy et al., 2004; Mitra et al., 2004; Gleason et al., 2006; Tirichine et al., 2006; Hayashi et al., 2010; Madsen et al., 2010; Shimoda et al., 2012; Takeda et al., 2012; Miller et al., 2013). Interacting Protein of DMI3 (IPD3)/CYCLOPS and CCaMK-Interacting Protein of Approximately 73 kD (CIP73) were identified to interact with and to be phosphorylated by CCaMK (Messinese et al., 2007; Yano et al., 2008; Kang et al., 2011). CCaMK-IPD3/CYCLOPS complex has been shown to be essential for rhizobial and mycorrhizal colonization (Horváth et al., 2011; Singh et al., 2014; Jin et al., 2016; Pimprikar et al., 2016).
In addition to regulating rhizobial and mycorrhizal symbioses, CCaMK is involved in the responses of plants to abiotic stresses (Ma et al., 2012; Shi et al., 2012, 2014; Zhu et al., 2016) and biotic stresses (Wang et al., 2015). Genetic evidence indicates that the rice (Oryza sativa) CCaMK DMI3 is a positive regulator of a variety of abscisic acid (ABA) responses, including root growth, antioxidant defense, and tolerance to both water stress and oxidative stress (Shi et al., 2012, 2014). DMI3-mediated activation of mitogen-activated protein kinase 1 (MPK1), a major ABA-activated mitogen-activated protein kinase, regulates the activities of the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) in ABA signaling (Shi et al., 2014). A recent study showed that Zm-NAC84, a NAC (NAM, ATAF1/2, and CUC2) transcription factor in maize (Zea mays), interacted with and was phosphorylated by Zm-CCaMK, and Zm-NAC84 is essential for ABA-induced antioxidant defense in a Zm-CCaMK-dependent manner (Zhu et al., 2016). However, the molecular mechanisms by which ABA induces the activation of CCaMK and how the activated CCaMK regulates ABA responses remain to be determined.
Here, we identify Protein Phosphatase 2C 45 (PP45,also called BIPP2C1 [Benzothiadiazole-Induced Protein Phosphatase 2C1]) as an interacting protein of DMI3. PP45 belongs to a member of group K in the family of type 2C protein phosphatases (PP2Cs; Singh et al., 2010) and was shown to be involved in the response of rice to pathogen infection, ABA, and various environmental stresses (Hu et al., 2006). Here, we demonstrate that PP45 is an important negative regulator of ABA signaling and elucidate key molecular mechanisms by which PP45 inactivates DMI3 in the basal state and by which ABA induces the activation of DMI3 in rice.
RESULTS
DMI3 Interacts with PP45
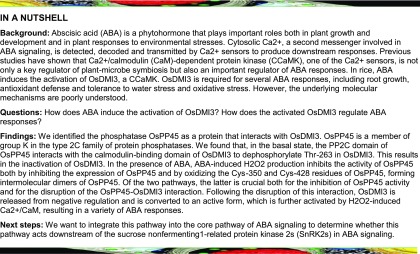
To isolate proteins that interact with DMI3 in rice leaves, the full-length DMI3 protein was used as bait to screen a rice leaf complementary DNA (cDNA) library. Several independent positive clones were isolated, and three of these clones were identified as encoding PP45. The interaction of DMI3 with PP45 using the yeast two-hybrid (Y2H) system is shown in Figure 1A.
Figure 1.
PP45 Interacts with DMI3 Both In Vitro and In Vivo.
(A) Y2H assay. AD vector and BD vector are Y2H vectors with no insert. SD-Trp-Leu-His-Ade/AbA/X-α-gal medium was used for testing the interaction. The combination of BD-P53 plus AD-SV40 was used as a positive control, and BD-Lam/AD-SV40 was used as a negative control.
(B) Pull-down assay. PP45-His and DMI3-GST proteins were expressed in Escherichia coli. For IB, PP45-His was detected with an anti-His antibody, and DMI3-GST was detected with an anti-GST antibody.
(C) BiFC analysis. Using the particle bombardment method, the constructs indicated were transiently expressed in onion epidermis cells. Coexpression of SYFPN-PP45 plus SYFPC or SYFPC-DMI3 plus SYFPN were used as negative controls. Scale bars, 90 μm.
(D) Co-IP test. After PP45-His and DMI3-Myc were cotransformed into rice protoplasts, total proteins of protoplasts were immunoprecipitated (IP) using an anti-Myc antibody and were detected with anti-His and anti-Myc antibodies. Protein input is shown by IB analysis of protein extracts before immunoprecipitation and antibodies against the respective tags. Molecular mass markers in kD are shown on the left. All the experiments were repeated at least three times with similar results.
To confirm the interaction between DMI3 and PP45 both in vitro and in vivo, glutathione S-transferase (GST) pull-down assays, bimolecular fluorescence complementation (BiFC) analyses, and co-immunoprecipitation (Co-IP) assays were performed. In vitro pull-down assays showed that PP45 interacted with GST-DMI3 but not with GST alone (Figure 1B). When split yellow florescent protein (SYFP)N-PP45 was co-transformed with SYFPC-DMI3 into onion epidermis cells, BiFC analyses showed a strong YFP fluorescence signal in the nucleus, the cytosol, and the plasma membrane, indicating physical interaction of PP45 and DIM3 (Figure 1C). In Co-IP assays, immunoblot (IB) analyses using an anti-His antibody revealed an interaction between DMI3-Myc and PP45-His (Figure 1D). In support of the above observations, subcellular localization analysis by confocal laser scanning microscopy showed that, as seen in the BiFC analyses, both DMI3 and PP45 were localized in the nucleus, the cytosol, and the plasma membrane (Supplemental Figure 1).
To determine which region(s) of DMI3 mediates its binding to PP45, a series of deletion constructs of DMI3 were made and then tested for interaction with PP45 using the Y2H assay. As shown in Supplemental Figure 2A, PP45 interacted with the CaM-binding domain (301 to 336 amino acids) of DMI3. A firefly luciferase complementation imaging (LCI) assay confirmed the in vivo interaction between PP45 and the CaM-binding domain of DMI3 (Supplemental Figure 2B). A similar strategy was used to identify which domain of PP45 was necessary for its interaction with DMI3. Y2H assay showed that the PP2C domain (343 to 569 amino acids) of PP45 was sufficient for interaction with DMI3 (Supplemental Figure 2C). A LCI assay confirmed the in vivo interaction (Supplemental Figure 2D). Taken together, these results indicate that DMI3-PP45 interaction involves the CaM-binding domain of DMI3 and the PP2C domain of PP45.
The rice genome encodes six members in group K of the PP2C family, and PP111, PP57, and PP1 have the highest homology with PP45 (Singh et al., 2010). To determine whether other members of group K would interact with DMI3, both Y2H and BiFC analysis were conducted. PP111, PP57, and PP1 did not interact with DMI3 either in yeast cells (Supplemental Figure 3A) or in onion epidermis cells (Supplemental Figure 3C), suggesting that only DMI3 specifically interacts with PP45. In addition, Y2H assays (Supplemental Figure 3B) and BiFC analyses (Supplemental Figure 3D) showed that PP45 did not interact with the rice sucrose nonfermenting1-related protein kinase 2s (SnRK2s) SAPK8/9/10, which are homologs of Arabidopsis (Arabidopsis thaliana) SnRK2.6/Open Stomata1 (OST1) and were shown to be activated by ABA (Kobayashi et al., 2004). Previous studies have shown that SnRK2.6/OST1, a global positive regulator of ABA signaling, interacts with the group A PP2Cs in Arabidopsis (Umezawa et al., 2009; Vlad et al., 2009).
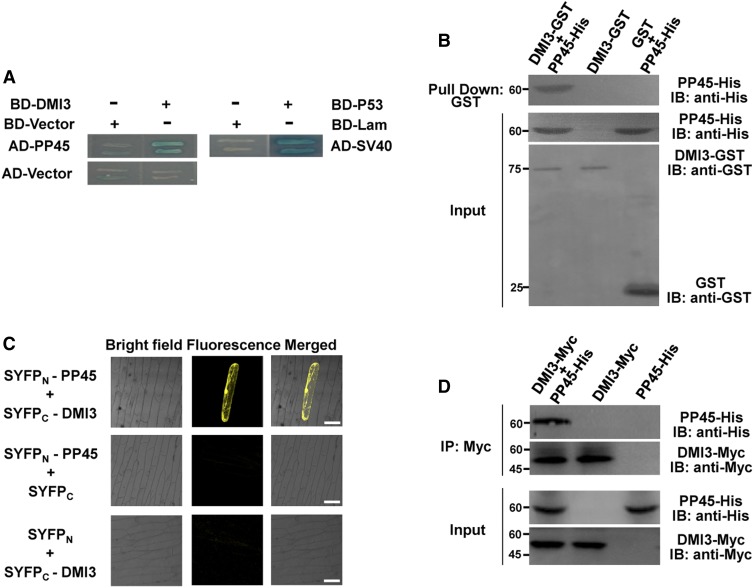
ABA and H2O2 Inhibit the Interaction of DMI3 and PP45
To investigate whether ABA or H2O2 treatment affects the interaction between DMI3 and PP45, both in vitro and in vivo experiments were performed. Y2H (Figure 2A) and pull-down assays (Figure 2B) showed that H2O2 completely blocked the interaction between DMI3 and PP45. However, ABA treatment (30 to 100 μM) did not inhibit the interaction in pull-down assays (Figure 2B). By contrast, IB analyses using specific anti-PP45 and anti-DMI3 antibodies (Supplemental Figure 4) revealed that PP45 did not interact with DMI3 in rice leaves treated with either 100 μM ABA or 10 mM H2O2 (Figure 2C). In addition, LCI analysis showed that the same ABA or H2O2 treatment strongly reduced the in vivo interaction between DMI3 and PP45 (Figure 2D). These results demonstrate that both ABA and H2O2 can block the interaction of DMI3 and PP45 in rice cells.
Figure 2.
ABA and H2O2 Inhibit the Interaction of PP45 and DMI3.
(A) H2O2 inhibits the interaction between PP45 and DMI3. SD-Trp-Leu-His-Ade/X-α-gal medium with various concentrations of H2O2 was used in a Y2H assay for testing the interaction. BD-P53 plus AD-SV40 was used as a positive control.
(B) In vitro pull-down assay showing that H2O2, but not ABA, directly inhibits the DMI3-PP45 interaction. PP45-His was incubated with GST-DMI3 conjugated to magnetic beads in the pull-down binding buffer plus various concentrations of either H2O2 (left) or ABA (right). Protein input was shown by IB analysis.
(C) A Co-IP assay showing that both ABA and H2O2 block the DMI3-PP45 interaction in rice leaves. The proteins extracted from rice leaves treated with 100 μM ABA for 90 min or 10 mM H2O2 for 45 min were immunoprecipitated (IP) with anti-DMI3 antibody and were detected by IB with anti-PP45 antibody and anti-DMI3 antibody. The control lanes (left, no ABA and no H2O2) are from samples not treated with either ABA or H2O2. Input protein is shown by IB analysis of protein extracts before IP. β-actin (bottom) was used as a loading control.
(D) Luciferase complementation imaging assay showing that both ABA and H2O2 block the interaction between PP45 and DMI3 in tobacco leaves. The indicated constructs were expressed in Nicotiana benthamiana leaves, and the leaves were treated either with 100 μM ABA for 90 min or with 10 mM H2O2 for 45 min. Luciferase signals were captured using the Tanon-5200 image system. Scale bars, 1.5 cm. Proteins (right) were detected by IB analysis of the leaf protein extracts after treatment. The Rubisco large subunit was used as a loading control visualized by staining with Coomassie Brilliant Blue. Molecular mass markers in kD are shown on the left.
(E) ABA-mediated inhibition in the interaction of PP45 and DMI3 is blocked in the rbohB/E mutant. The proteins extracted from the leaves of rbohB/E or wild-type (WT) plants treated with 100 μM ABA for 90 min were immunoprecipitated (IP) using an anti-DMI3 antibody and were detected by IB with either an anti-PP45 antibody or an anti-DMI3 antibody. Protein input is shown by IB analysis of protein extracts before IP. The levels of β-actin indicate equal protein loading. Molecular mass markers in kD were indicated on the left. All experiments were repeated at least three times with similar results.
Previous studies have shown that RbohB and RbohE, which encode NADPH oxidase in rice, are involved in ABA-induced H2O2 production (Shi et al., 2012; Zhang et al., 2014). To determine whether ABA-induced inhibition of the interaction between DMI3 and PP45 is related to the ABA-induced production of H2O2, an rbohB/E double mutant knockout (KO) line (rbohB/E-KO1) was generated by CRISPR/Cas9 (Supplemental Figure 5A). ABA-induced H2O2 production, as detected by 3,3-diaminobenzidine staining (Supplemental Figure 5B) and by confocal microscopy (Supplemental Figure 5C), was impaired in rbohB/E-KO1. This indicates that RbohB and RbohE are required for ABA-induced H2O2 production in rice. In rbohB/E-KO1, ABA treatment failed to inhibit the interaction between DMI3 and PP45 detected by Co-IP (Figure 2E), suggesting that H2O2 is required for ABA-induced inhibition in the interaction of DMI3 and PP45.
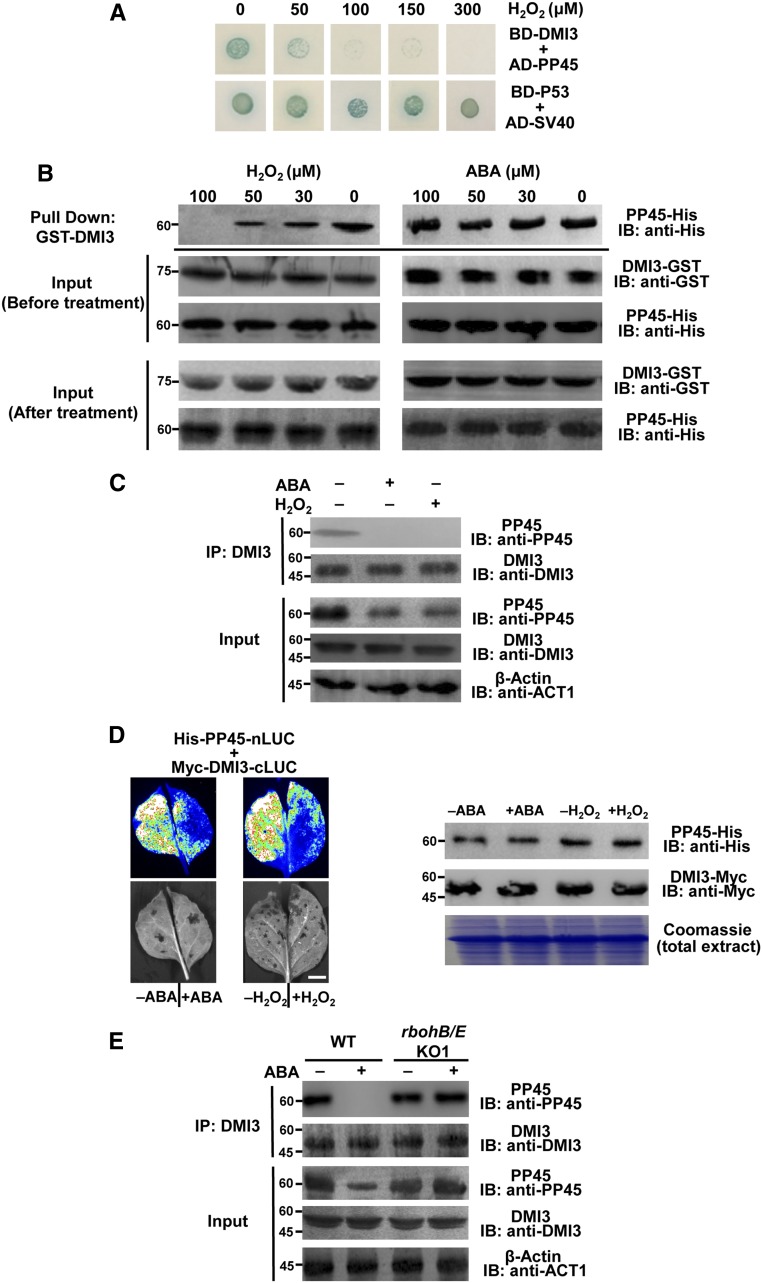
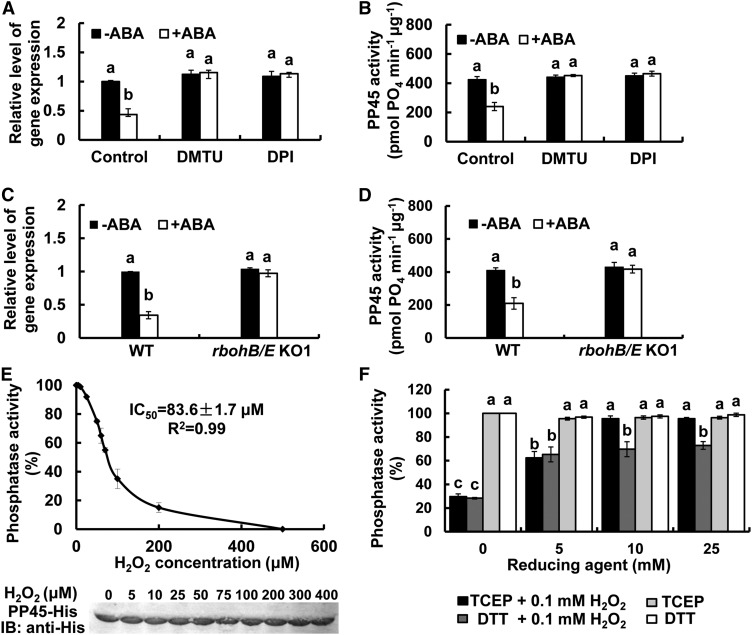
ABA and H2O2 Transiently Downregulate PP45
A previous study showed that both ABA and H2O2 induced the expression of DMI3 and the activity of DMI3 in the leaves of rice plants (Shi et al., 2012). To investigate the effects of ABA and H2O2 on the expression of PP45, the PP45 protein level, and the activity of PP45 in leaves of rice seedlings, we performed relative quantitative PCR analysis and IB analysis and Ser/Thr phosphatase activity assays. The expression of PP45 (Figure 3A), the level of PP45 protein (Figure 3B), and the activity of PP45 (Figure 3C) in the leaves of rice plants exposed to 100 μM ABA or 10 mM H2O2 were first downregulated and then upregulated. For ABA treatment, the maximum decrease in PP45 expression, the PP45 level, and PP45 activity occurred at 30, 60, and 90 min after ABA treatment, respectively, and the maximum increase appeared at 2, 6, and 6 h after ABA treatment, respectively. For H2O2 treatment, the maximum decreases occurred sooner—at 15, 45, and 45 min after H2O2 treatment, respectively—and the maximum increase appeared at 2, 4, and 4 h after H2O2 treatment, respectively. To compare the degree of change between PP45 protein level and PP45 activity with ABA and H2O2 treatment, the protein level of PP45 was quantified and the treatment/control ratios of the protein level and the activity of PP45 were calculated, respectively. The results showed that, for either ABA or H2O2 treatment, the decrease in enzyme activity was much greater than the decrease in protein level (Figure 3D), showing that PP45 activity is more sensitive to oxidative stress than is the PP45 protein level.
Figure 3.
ABA and H2O2 Regulate the Expression of PP45, the Level of PP45 Protein, and the Activity of PP45.
(A) to (C) Changes in the expression (A), the protein level (B), and the activity (C) of PP45 in leaves of rice plants exposed to ABA (100 μM) or H2O2 (10 mM) treatment for various times as indicated. Relative expression level of PP45 was analyzed by real-time quantitative PCR (A). The level of PP45 protein was analyzed by IB with an anti-PP45 antibody, and β-actin was used as a loading control. Molecular mass markers in kilodaltons are shown on the left (B). The activity of PP45 was measured by the serine/threonine phosphatase assay (C).
(D) The treatment/control ratio of the protein level in (B) and the activity in (C) of PP45. For quantitative analysis of band intensity in (B), the starting point (0 h) was set to 1, and the other points were compared with it. In (A), (C), and (D), values are means ± sem of three independent experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan’s multiple range test. In (B), experiments were repeated at least three times with similar results.
H2O2 Is Required for the ABA-Induced Inhibition of PP45
To determine whether ABA-induced inhibition of PP45 is due to the action of endogenous H2O2, we used dimethylthiourea (DMTU), a trap for H2O2, and diphenyleneiodonium chloride (DPI), an inhibitor of NADPH oxidase, to reduce the level of H2O2. Pretreatments with DMTU or DPI completely relieved the ABA-induced inhibition of both the expression of PP45 (Figure 4A) and the activity of PP45 (Figure 4B), suggesting that H2O2 is required for the ABA-induced inhibition of PP45. We next used the rbohB/E double mutant to show that knockout of these two genes removed the ABA-induced inhibition of PP45 expression (Figure 4C) and the its activity (Figure 4D), although it did not affect the expression and the activity of PP45 under the control conditions. Finally, we tested for a direct effect of H2O2 on the phosphatase activity of PP45. PP45 was highly sensitive to H2O2. Following treatment with H2O2, PP45 lost its activity, with an IC50 of 84 μM (Figure 4E). The addition of the reducing agents Tris (2-carboxyethyl) phosphine (TCEP) or dithiothreitol (DTT) at high levels prevented the inactivation of PP45 by H2O2 (Figure 4F), and these reducing agents themselves did not interfere with the sensitivity of the assay (Supplemental Figure 6), demonstrating a sensitive and reversible response of PP45 activity to changes in redox conditions.
Figure 4.
H2O2 Is Required for ABA-Induced Inhibition of PP45.
(A) and (B) Effects of pretreatments with DMTU and DPI on the expression of PP45 (A) and on the activity of PP45 (B) in rice leaves exposed to ABA. The rice seedlings were either untreated (Control) or pretreated with 5 mM DMTU or 100 μM DPI for 4 h and then exposed to 100 μM ABA for 30 min (A) or 90 min (B).
(C) and (D) The expression of PP45 (C) and the activity of PP45 (D) in rbohB/E mutant. The rice seedings were treated with 100 μM ABA for 90 min, and the relative expression levels of PP45 and the activity of PP45 were analyzed by real-time quantitative PCR and by Ser/Thr phosphatase assay, respectively.
(E) H2O2 directly inhibits the activity of PP45 in vitro. The in vitro-expressed PP45-His protein was incubated with various concentrations of H2O2. The graph shown the activity of PP45 as measured by the Ser/Thr phosphatase assay. The protein level of OPP45 after treatment was determined by IB analysis (bottom). The phosphatase activity of PP45 without H2O2 treatment was set to 100%.
(F) The inactivation of PP45 by H2O2 is reversed by TCEP and DTT. Recombinant PP45 protein was incubated with 0.1 mM H2O2 for 30 min, followed by different concentrations of the reducing agents TCEP or DTT for another 30 min. The activity of PP45 was measured by the Ser/Thr phosphatase assay. All experiments were repeated at least three times with similar results. Values are means ± sem of three independent experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan’s multiple range test.
PP45 Negatively Regulates and DMI3 Positively Regulates ABA Responses in Rice Plants
Using a dmi3 mutant, previous studies have revealed that DMI3 is a positive regulator of ABA responses, including root growth, antioxidant defense, and tolerance to water stress and oxidative stress (Shi et al., 2012, 2014). To investigate the role of PP45 in the regulation of ABA responses, two independent PP45-overexpressing (OE) lines (PP45-OE1, PP45-OE2) and two independent PP45-knockout lines (pp45-KO1, pp45-KO2) were generated. For comparison, two independent DMI3-overexpressing lines (DMI3-OE1, DMI3-OE2) and two independent DMI3-knockout lines (dmi3-KO1, dmi3-KO2) were also generated. Moreover, two independent PP45-knockdown lines (pp45-RNAi1, pp45-RNAi2) and two independent DMI3-knockdown lines (dmi3-RNAi1, dmi3-RNAi2) were also generated.
In the dmi3-KO lines (Supplemental Figure 7A) and the pp45-KO lines (Supplemental Figure 7C) generated by CRISPR/Cas9 system, the activities of both DMI3 (Supplemental Figure 7B) and PP45 (Supplemental Figure 7D) were undetectable. The PP45-OE lines and the DMI3-OE lines exhibited high expression levels of PP45 and DMI3 (Supplemental Figure 8A) and high activities of PP45 and DMI3 (Supplemental Figures 7B and 7D). In pp45-RNAi lines, PP45 mRNA was reduced by ∼80% in comparison with the wild type, but the expression of PP1, PP57, and PP111 was not affected (Supplemental Figure 8A). This shows that PP45 expression was specifically suppressed by RNA interference (RNAi). The expression of DMI3 in dmi3-RNAi lines was also suppressed (Supplemental Figure 8A).
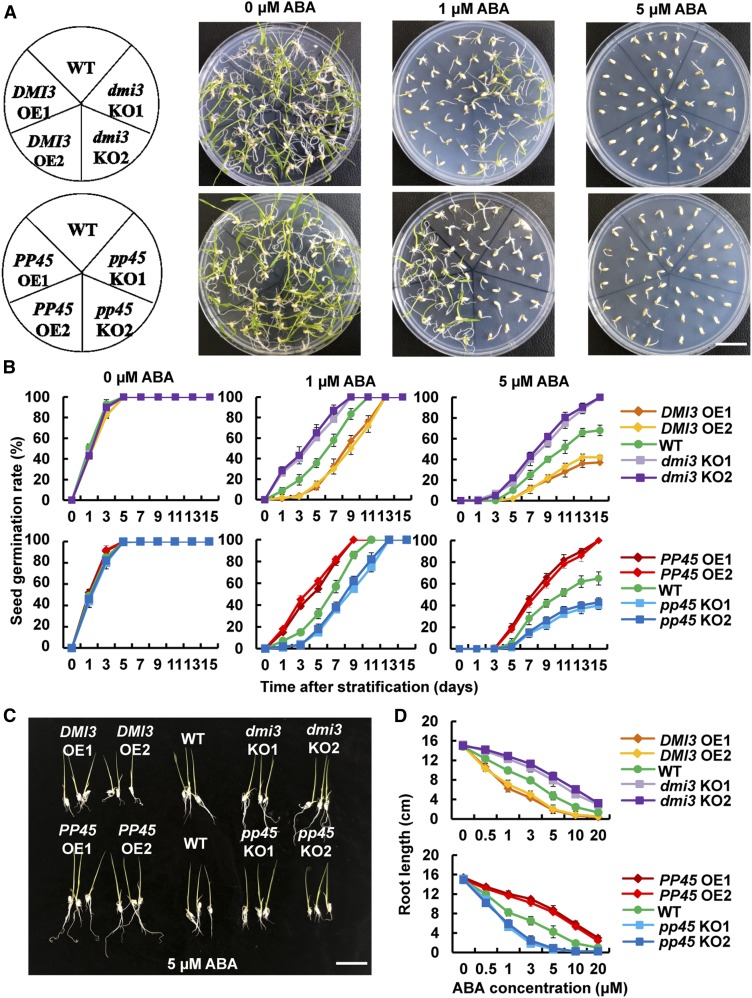
In the absence of ABA, there were no obvious differences between these transgenic lines and the wild type in rates of seed germination (Figures 5A and 5B; Supplemental Figures 8B and 8C). ABA treatment significantly inhibited seed germination (Figures 5A and 5B; Supplemental Figures 8B and 8C) and primary root growth (Figures 5C and 5D; Supplemental Figures 8D and 8E) in wild type. The ABA sensitivity of both seed germination and primary root growth was enhanced in pp45-KO lines, pp45-RNAi lines, and DMI3-OE lines, and it was reduced in PP45-OE lines, dmi3-KO lines, and dmi3-RNAi lines. These results indicate that PP45 reduces and DMI3 enhances ABA sensitivity in seed germination and root growth.
Figure 5.
PP45 Reduces and DMI3 Enhances ABA Sensitivity in Seed Germination and Root Growth.
(A) Photographs of seed germination in DMI3-OE, dmi3-KO, PP45-OE, pp45-KO, and the wild type (WT). The seeds of transgenic lines and the wild type were germinated and grown in 1/2 MS medium supplemented with different concentrations of ABA (0, 1, 5 μM) for 9 d after stratification. Scale bar, 3.5 cm.
(B) The germination rates of seeds under ABA treatments during 15 d after stratification.
(C) Phenotypes of rice seedlings grown in 5 μM ABA for 14 d. Scale bar, 4 cm.
(D) Primary root lengths of rice seedlings grown for 14 d in the different concentrations of ABA indicated. Approximately 50 seeds of each transgenic line were analyzed per replicate for each concentration of ABA in (A) to (D). In (A) and (C), experiments were repeated at least three times with similar results. In (B) and (D), values are means ± sem of three independent experiments.
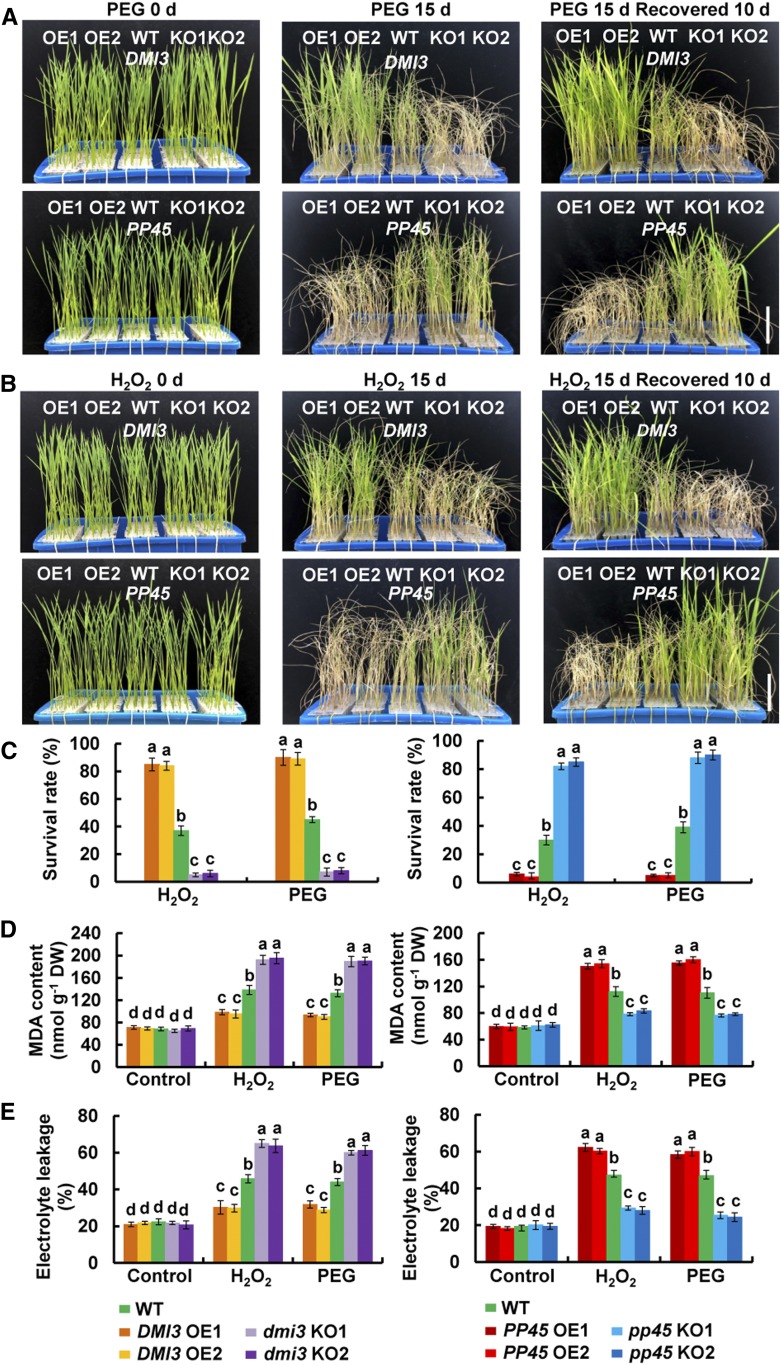
To test the role of PP45 in the tolerance of water stress and oxidative stress in plants, wild-type rice seedlings and the transgenic lines were treated with either polyethylene glycol (PEG) to simulate water stress or H2O2 to produce oxidative stress. Under the control conditions, there were no significant differences in the growth of rice seedlings between the transgenic plants and the wild-type plants (Figures 6A and 6B; Supplemental Figures 9A and 9B). When treated with 20% PEG (Figure 6A; Supplemental Figure 9A) or with 100 mM H2O2 (Figure 6B; Supplemental Figure 9B), PP45-OE, dmi3-KO, and dmi3-RNAi plants exhibited more severe wilting and chlorosis than wild-type plants (Figures 6A and 6B; Supplemental Figures 9A and 9B) and had lower survival rates after recovery by re-watering (Figure 6C; Supplemental Figure 9C). In contrast, DMI3-OE, pp45-KO, and pp45-RNAi plants exhibited less severe wilting and chlorosis than the wild-type plants after PEG and H2O2 treatments and had higher survival rates after recovery by rewatering. Moreover, the content of malondialdehyde (Figure 6D; Supplemental Figure 9D) and the percentage of electrolyte leakage (Figure 6E; Supplemental Figure 9E), which are indicators of oxidative damage, were higher in the leaves of the PP45-OE, dmi3-KO, and dmi3-RNAi plants exposed to PEG and H2O2 treatments than those in the wild-type plants, and they were lower in the leaves of the DMI3-OE, pp45-KO, and pp45-RNAi plants. Moreover, PP45 downregulated ABA-induced increases in the activities of MPK1 (Supplemental Figure 10A), SOD, and CAT (Supplemental Figure 10B), while DMI3 upregulated the ABA-induced increases in the activities of these enzymes (Supplemental Figures 10A and 10C). These results indicate that PP45 negatively and DMI3 positively regulate the tolerance of rice plants to water stress and oxidative stress.
Figure 6.
PP45 Negatively and DMI3 Positively Regulate the Tolerance of Rice Plants to Water Stress and Oxidative Stress.
(A) and (B) Photographs of DMI3-OE, dmi3-KO, PP45-OE, pp45-KO, and wild-type (WT) plants exposed to water stress (A) or oxidative stress (B). Ten-day-old seedlings were treated with 20% PEG 4000 (A) or 100 mM H2O2 (B) for 15 d and then allowed to recover for 10 d. Approximately 30 seedlings of each transgenic line were used per replicate. Scale bars, 5 cm.
(C) The survival rate (%) of the rice plants after recovery by rewatering for 10 d shown in (A) and (B).
(D) and (E) Malondialdehyde (MDA) content (D) and the percent leakage of electrolyte (E) in the leaves of DMI3-OE, dmi3-KO, PP45-OE, pp45-KO, and wild-type plants exposed to water stress or oxidative stress. Ten-day-old seedlings were treated with 20% PEG 4000 or 100 mM H2O2 for 2 d, and then leaves were sampled for the determination of MDA content and electrolyte leakage (%). Approximately 50 seedlings of each transgenic line were used per replicate. In (A) and (B), experiments were repeated at least three times with similar results. In (C) to (E), values are means ± sem of three independent experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
PP45 Inactivates DMI3 by Dephosphorylating Thr-263 in DMI3
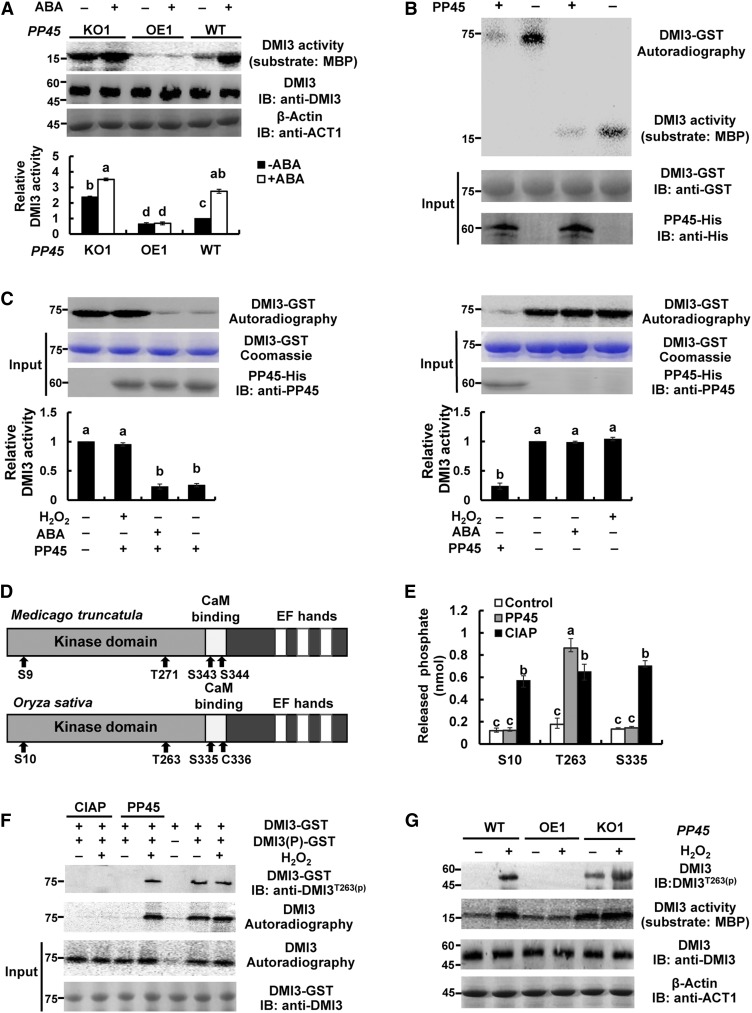
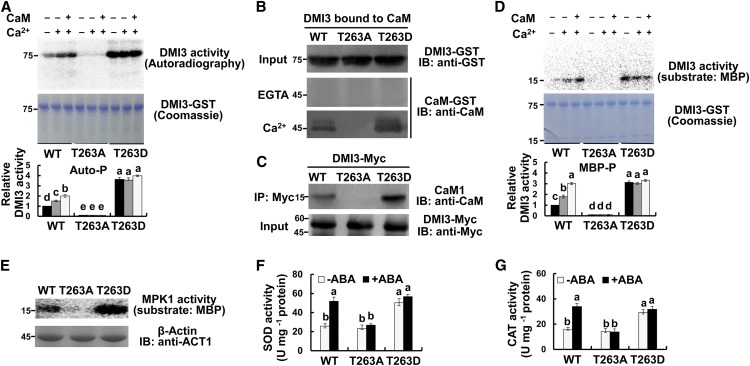
Under the control (nontreated) conditions, the PP45-OE1 plants showed reduced activity of DMI3 and the pp45-KO1 plants showed increased DMI3 activity compared with wild-type plants (Figure 7A). ABA treatment induced a significant increase in the activity of DMI3 in the leaves of wild-type plants. This ABA-induced increase was blocked in PP45-OE1 plants and was further enhanced in pp45-KO1 plants. Furthermore, in vitro experiments showed that PP45 directly inhibited the autophosphorylation and substrate phosphorylation activities of DMI3 (Figure 7B). The PP45-mediated inhibition in DMI3 autophosphorylation was released by the addition of H2O2 but not by ABA addition (Figure 7C). ABA and H2O2 themselves did not affect the autophosphorylation and substrate phosphorylation activities of DMI3 in vitro (Figure 7C; Supplemental Figure 11A), and Ca2+ was required for H2O2-induced activation of DMI3 in rice plants (Supplemental Figure 11B). On the other hand, DMI3 did not regulate the expression of PP45 and the activity of PP45, regardless of the presence or absence of ABA (Supplemental Figure 12). In Medicago truncatula CCaMK (Mt-CCaMK), four autophosphorylation sites (Ser-9, Thr-271, Ser-343, and Ser-344) were identified, and Thr-271 was found to be a major autophosphorylation site in the kinase (Poovaiah et al., 2013; Routray et al., 2013). In Os-DMI3, the corresponding sites were Ser-10, Thr-263, and Ser-335, respectively (note that the amino acid residue at position 336 of Os-DMI3 is a Cys; Figure 7D). To determine whether PP45 can dephosphorylate these sites in DMI3, three synthetic phosphopeptides, corresponding to the three phosphorylation sites of DMI3, were used. As shown in Figure 7E, PP45 dephosphorylated only the synthetic phosphopeptide corresponding to residues 258 to 271 of DMI3, suggesting that PP45 specifically dephosphorylates Thr-263 in DMI3. By contrast, calf intestine alkaline phosphatase dephosphorylated all the synthetic phosphopeptides. Furthermore, in vitro assays using an anti-phospho-Thr-263 antibody showed that PP45-His inhibited Thr-263 phosphorylation in the phosphorylated DMI3-GST, and the PP45-mediated inhibition was reversed by the addition of H2O2 (Figure 7F). Similar changes in the autophosphorylation activity of DMI3 were also observed. In vivo assays also showed that Thr-263 in DMI3 was phosphorylated in the leaves of pp45-KO1 plants, and H2O2 treatment induced Thr-263 phosphorylation in wild-type and enhanced Thr-263 phosphorylation in pp45-KO1 plants (Figure 7G). Similar changes in the substrate phosphorylation of DMI3 were also observed. Together, these results support that PP45 directly inactivates DMI3 by dephosphorylating Thr-263 in DMI3.
Figure 7.
PP45 Directly Inactivates DMI3 by Dephosphorylating Thr-263 in DMI3.
(A) The activity of DMI3 in PP45-OE, pp45-KO, and the wild type (WT). Rice seedlings were treated with 100 μM ABA for 90 min, and the activity of DMI3 was analyzed by immunoprecipitation kinase assay using MBP as substrate (top). Kinase activities were quantitated by Quantity One software (bottom). The activity of DMI3 in the wild type in the control was set to 1. DMI3 input was analyzed by IB using an anti-DMI3 antibody. β-actin was used as the total protein loading control.
(B) PP45 inhibits both autophosphorylation and substrate phosphorylation of DMI3 in vitro. PP45-His and DMI3-GST proteins were expressed in Escherichia coli. Images show the activities of autophosphorylation (top) and substrate phosphorylation (bottom) of DMI3 in the presence or absence of PP45.
(C) H2O2 prevents the PP45-mediated inhibition of DMI3 in vitro. The gels show the autophosphorylation activity of DMI3 (top), the amounts of DMI3 (middle), and PP45 (bottom), respectively. The autophosphorylation activity of DMI3 was also quantified by Quantity One software (bottom; the activity of DMI3 without any treatment was set to 1).
(D) Schematic diagrams of Mt-CCaMK and Os-DMI3 showing domains and autophosphorylation sites. Note that the amino acid at position 336 of Os-DMI3 is Cys.
(E) Release of phosphate from the synthetic peptides. S10, synthetic peptide MSKTESRKLpSDDYEVVD, corresponding to residues 1 to 17 of DMI3; T263, synthetic peptide SFQDHpTWKTISSSA corresponding to residues 258 to 271 of DMI3; S335, synthetic peptide LRAAAIASVLpSCKVAL corresponding to residues 325 to 340 of DMI3.
(F) Dephosphorylation of Thr-263 in DMI3 by PP45 in vitro. Thr-263 phosphorylation of DMI3 was analyzed by IB using an anti-pT263 DMI3 antibody. The activity of DMI3 was analyzed using an autophosphorylation assay. DMI3(P)-GST indicates phosphorylated DMI3.
(G) Dephosphorylating Thr-263 in DMI3 in PP45-OE, pp45-KO, and wild-type plants exposed to H2O2 treatment. The rice seedling were treated with 10 mM H2O2 for 45 min and Thr-263 phosphorylation was analyzed by IB with anti-pT263 DMI3 antibody, and the activity of DMI3 was measured by immunoprecipitation kinase assay using MBP as substrate. DMI3 input was analyzed by IB with DMI3 antibody. β-actin was used as total protein loading control. All experiments were repeated at least three times with similar results. In (A), (C), and (E), values are means ± sem of three independent experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan’s multiple range test. Molecular mass markers in kD were shown on the left.
Thr-263 Autophosphorylation Is Required for the Activation of DMI3 and the Response Mediated by DMI3
To further determine the role of Thr-263 phosphorylation in the autophosphorylation activity of DMI3, Thr-263 of DMI3 was mutated either to Ala (DMI3T263A) to create a non-phosphorylatable mutant or to Asp (DMI3T263D) to create a phosphomimetic mutant. In the absence of Ca2+ and CaM, DMI3 was weakly autophosphorylated, and its autophosphorylation not only was significantly enhanced in DMI3T263D but also was almost abolished in DMI3T263A (Figure 8A). In the presence of Ca2+ and CaM, autophosphorylation of DMI3 was significantly increased. However, autophosphorylation in both DMI3T263A and DMI3T263D was unresponsive to addition of Ca2+ or Ca2+/CaM. These results indicate that Thr-263 phosphorylation is critical for the autophosphorylation activity of DMI3. Then, we tested whether Thr-263 phosphorylation affects CaM binding to DMI3. In the presence of Ca2+, DMI3 bound to CaM, and binding was markedly increased in DMI3T263D and completely blocked in DMI3T263A (Figure 8B). In vivo analysis of rice protoplasts also showed that CaM1 binding affinity of DMI3 was enhanced in the protoplasts with transiently expressed DMI3T263D and was lost in the protoplasts with transiently expressed DMI3T263A (Figure 8C). These results indicate that Thr-263 phosphorylation positively regulates CaM binding of DMI3. Further, the effect of Thr-263 phosphorylation on the substrate phosphorylation of DMI3 was analyzed. The changes in the substrate phosphorylation activity in DMI3, DMI3T263A, and DMI3T263D were similar to the changes in autophosphorylation activity in the presence or absence of Ca2+ and Ca2+/CaM (Figure 8D). These results indicate a positive effect of Thr-263 phosphorylation on the substrate phosphorylation activity of DMI3.
Figure 8.
Thr-263 Autophosphorylation Is Necessary for the Activation of DMI3 and the Response It Mediates.
(A) Autophosphorylation of DMI3, DMI3T263A, and DMI3T263D in vitro. Autophosphorylation activity in the presence of either 5 mM EGTA (−), 0.1 mM CaCl2, or 0.1 mM CaCl2 and 1 μM CaM was analyzed by in vitro kinase assay (top) and quantified by Quantity One software (bottom; the activity of DMI3 wild type [WT] without any treatment was set to 1). Corresponding Coomassie staining was also shown (middle).
(B) CaM binding of DMI3, DMI3T263A, and DMI3T263D in vitro. The CaM binding affinities of the mutant and wild-type protein extracts were tested in the presence of 1 mM CaCl2 (bottom) or 5 mM EGTA (middle).
(C) CaM binding of DMI3, DMI3T263A, and DMI3T263D in rice protoplasts. The indicated constructs were transfected into rice protoplasts. Total isolated proteins were incubated with an anti-Myc antibody and were detected by IB using an anti-CaM antibody (top). The input protein is shown by IB analysis using an anti-Myc antibody (bottom).
(D) Substrate phosphorylation of DMI3, DMI3T263A, and DMI3T263D in vitro. Images show substrate phosphorylation (top, MBP as substrate), the corresponding Coomassie staining (middle), and the relative activity of substrate phosphorylation (bottom). The activity of untreated DMI3 wild type was set to 1.
(E) The activity of MPK1 in the protoplasts with transiently expressed DMI3 or DMI3T263A or DMI3T263D. The activity of MPK1 in the protoplasts was analyzed by immunoprecipitation kinase assay (top). β-actin was used as a loading control (bottom).
(F) and (G) The activities of SOD (F) and CAT (G) in protoplasts transiently expressing DMI3 or DMI3T263A or DMI3T263D. Protoplasts were treated with 10 μM ABA for 10 min. All experiments were repeated at least three times with similar results. In (A), (D), (F), and (G), values are means ± sem of three independent experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan’s multiple range test. Molecular mass markers in kilodaltons were shown on the left.
Finally, the role of Thr-263 phosphorylation in the antioxidant defense response in rice protoplasts was tested. Transient expression of DMI3T263D significantly increased the activities of MPK1 (Figure 8E), SOD (Figure 8F), and CAT (Figure 8G). Transient expression of DMI3T263A markedly reduced the activity of MPK1, but did not affect the activities of SOD and CAT. However, the ABA-induced increase in the activities of SOD and CAT was blocked in the protoplasts with transiently expressed DMI3T263A. These results suggest that Thr-263 phosphorylation upregulates antioxidant defense in plant cells.
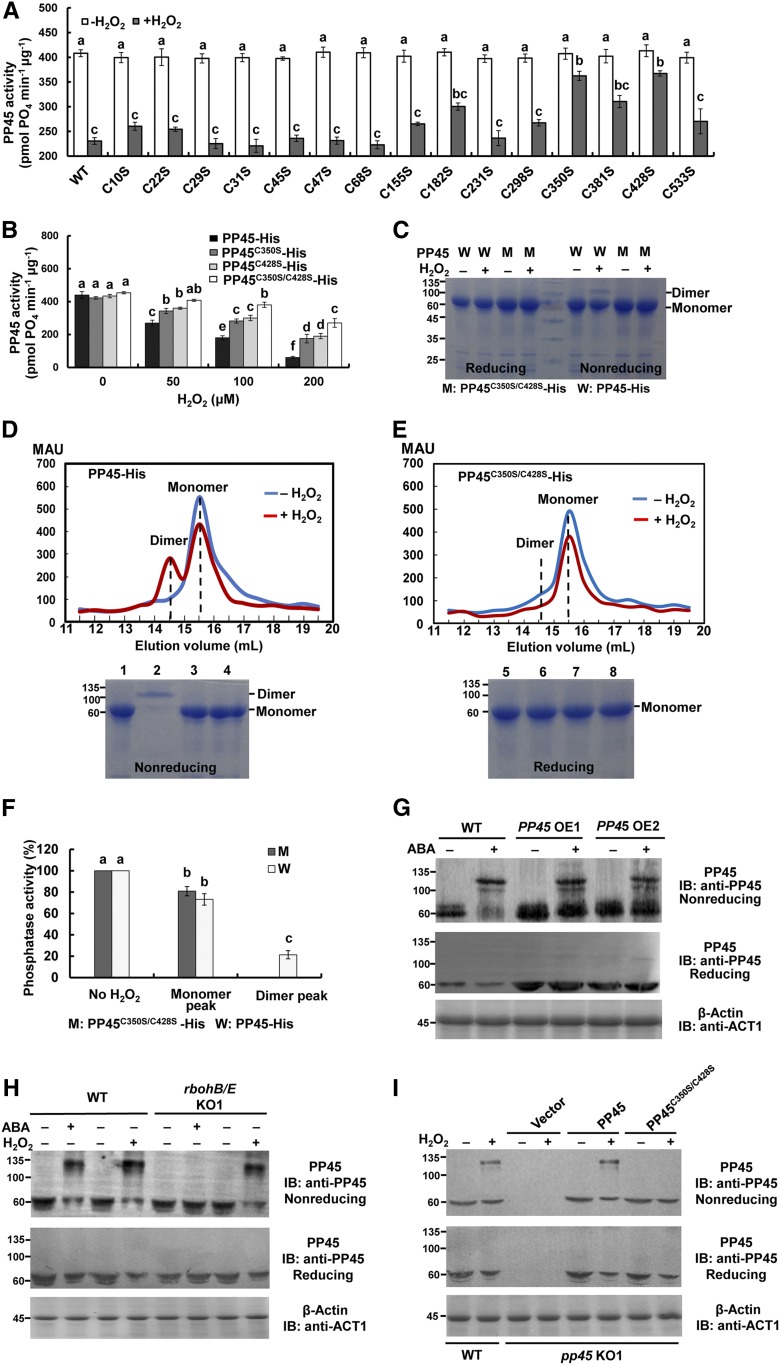
Cys-350 and Cys-428 Are the Key Cysteine Residues Responsible for H2O2-Mediated Inactivation of PP45
Cys residues in proteins can be alternatively oxidized and reduced to form and break disulfide linkages, respectively, and thus are one of the most sensitive targets for reactive oxygen species (ROS; Akter et al., 2015; Waszczak et al., 2015). In PP45, there are 15 Cys residues. Each of these Cys residues was mutated to Ser in order to test whether substitution of any of the 15 Cys residues would affect the inhibition of PP45 by H2O2. As shown in Figure 9A, two of the mutant proteins, PP45C350S and PP45C428S, showed significant protection against inactivation by H2O2, suggesting the importance of Cys-350 and Cys-428. Treatment with 50 μM H2O2 led to a significant decrease in the activity of PP45 but did not affect the phosphatase activity in the PP45C350S/C428S double mutant (Figure 9B). Following treatment with 0.2 mM H2O2, PP45C350S/C428S retained 60% of its phosphatase activity compared with PP45, which only had 13% of its original activity. These results indicate that Cys-350 and Cys-428 are the key Cys residues that mediate oxidative inactivation of PP45.
Figure 9.
H2O2 Directly Inhibits the Activity of PP45 by Oxidizing Cys-350 and Cys-428 Residues to Form PP45 Intermolecular Dimers.
(A) The activities of PP45-His wild-type and Cys point-mutant proteins exposed to 0.1 mM H2O2.
(B) The effects of H2O2 at different concentrations on the activities of PP45-His wild-type and C350S and C428S single- and double-mutant proteins.
(C) Reducing and nonreducing PAGE analysis of protein extracts from PP45 wild type (W) and OsPP45C350S/C428S double mutant (M) in the presence and absence of 0.1 mM H2O2 in vitro.
(D) and (E) Top shows the gel filtration profiles of PP45-His wild type (D) and PP45C350S/C428S-His mutant (E) in the presence and absence of 0.1 mM H2O2. PP45-His elutes primarily as monomers in the absence of H2O2 (blue chromatogram) and as a mixture of dimers and monomers following treatment with 0.1 mM H2O2 (red chromatogram). By contrast, PP45C350S/C428S-His eluted exclusively as monomers. mAU, absorbance at 280 nm × 1000. Bottom shows reducing and nonreducing PAGE analyses of gel filtration fractions of PP45-His (D) and PP45C350S/C428S -His (E) in the presence and absence of H2O2, respectively. Lanes 1 and 5, PP45-His monomer peak (15.5-mL fraction); 2 and 6, PP45-His dimer peak (14.5-mL fraction); 3 and 7, PP45C350S/C428S-His monomer peak (15.5-mL fraction); 4 and 8, PP45C350S/C428S-His dimer peak (14.7-mL fraction).
(F) The relative activity of PP45 protein from the untreated monomer fraction (no H2O2), from the dimer and monomer fractions of PP45-His treated with 0.1 mM H2O2 and from the monomer fraction of PP45C350S/C428S-His treated with 0.1 mM H2O2.
(G) and (H) ABA and H2O2 induce the formation of PP45 dimers in rice plants. PP45-OE (G), rbohB/E-KO (H), and wild-type plants were treated with 100 μM ABA for 90 min or with 10 mM H2O2 for 45 min, and both nonreducing (top) and reducing (middle) IB of PP45 were performed. β-actin recognized by an anti-β-actin antibody was used as a protein loading control (bottom).
(I) H2O2 induces the formation of PP45 dimers in the pp45-KO1 protoplasts transiently expressing PP45, but not in protoplasts transiently expressing PP45C350S/C428S. The indicated constructs were expressed in pp45-KO1 protoplasts, and the protoplasts were treated with 1 mM H2O2 for 10 min. The levels of β-actin indicate equal protein loading. All experiments were repeated at least three times with similar results. In (A), (B), and (F), values are means ± sem of three independent experiments. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan’s multiple range test. Molecular mass markers in kD are shown on the left.
To determine whether PP45 oxidation leads to the formation of intramolecular or intermolecular disulfide bonds, PP45 and PP45C350S/C428S produced in Escherichia coli were treated with 0.1 mM H2O2 and then analyzed by reducing and nonreducing PAGE (Figure 9C). Under nonreducing conditions, a band corresponding to twice the apparent molecular mass of PP45 (∼124 kD) was observed in H2O2-treated PP45 but not in PP45C350S/C428S, suggesting the formation of an intermolecular disulfide bond in oxidized PP45. However, after H2O2 treatment, no change of mobility was observed in the monomer (∼62 kD) of PP45 and PP45C350S/C428S under the nonreducing conditions (Figure 9C; Supplemental Figure 13). This is consistent with that of the Arabidopsis HAB1 (Supplemental Figure 13), which was shown to form only intermolecular disulfide bonds after H2O2 treatment (Sridharamurthy et al., 2014). By contrast, the oxidized TGA1, which was shown to form an intramolecular disulfide bridge (Després et al., 2003), had a slightly slower mobility than the reduced form (Supplemental Figure 13). These results suggest that no intramolecular disulfide bonds are formed in oxidized PP45.
Furthermore, H2O2-treated and untreated protein extracts from PP45 and PP45C350S/C428S were fractionated by size exclusion chromatography to distinguish monomers from dimers. In the absence of H2O2, His-PP45 eluted as monomers, but in the presence of H2O2, His-PP45 eluted as dimers and monomers (Figures 9D and 9E). By contrast, His-PP45C350S/C428S eluted only as monomers regardless of the presence or absence of H2O2. These results were confirmed using reducing and nonreducing PAGE (Figures 9D and 9E). In addition, the phosphatase activity in the fractions containing the dimers and the monomers was analyzed. H2O2-treated monomeric PP45 retained greater than 80% of its phosphatase activity compared to untreated protein, but dimeric PP45 only had 20% of its phosphatase activity (Figure 9F).
We also analyzed the formation of PP45 dimers in rice plants exposed to ABA or H2O2 treatment. ABA treatment induced the formation of PP45 dimers in wild-type and PP45-OE plants, but not in rbohB/E-KO plants under the nonreducing conditions (Figures 9G and 9H). Under nonreducing conditions, H2O2 induced the formation of PP45 dimers not only in wild-type plants but also in rbohB/E-KO plants (Figure 9H). The formation of PP45 dimers was accompanied by a decrease in PP45 monomers under these conditions. Under reducing conditions, the PP45 dimers disappeared, and the PP45 monomers increased, suggesting that the PP45 dimers are reduced to PP45 monomers under these conditions. Finally, PP45 and PP45C350S/C428S were constructed and expressed in pp45-KO1 protoplasts, and the protoplasts were treated with H2O2. The H2O2 treatment induced the formation of PP45 dimers in the pp45-KO1 protoplasts transiently expressing PP45, but not those expressing PP45C350S/C428S (Figure 9I). This demonstrates that the oxidation of Cys-350 and Cys-428 in PP45 is necessary for the formation of PP45 dimers in rice protoplasts under oxidative stress.
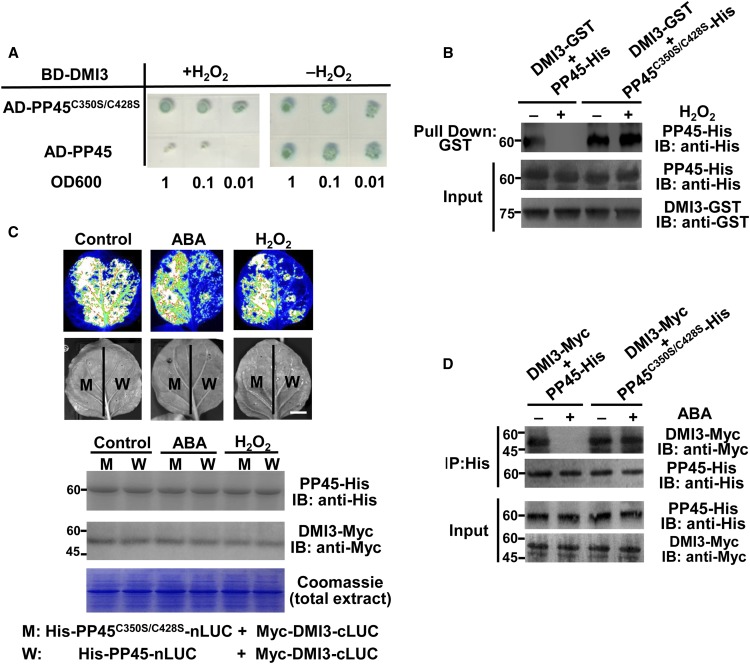
PP45C350S/C428S Abolishes the Effect of H2O2 on the DMI3-PP45 Interaction and on PP45-Mediated Inactivation of DMI3
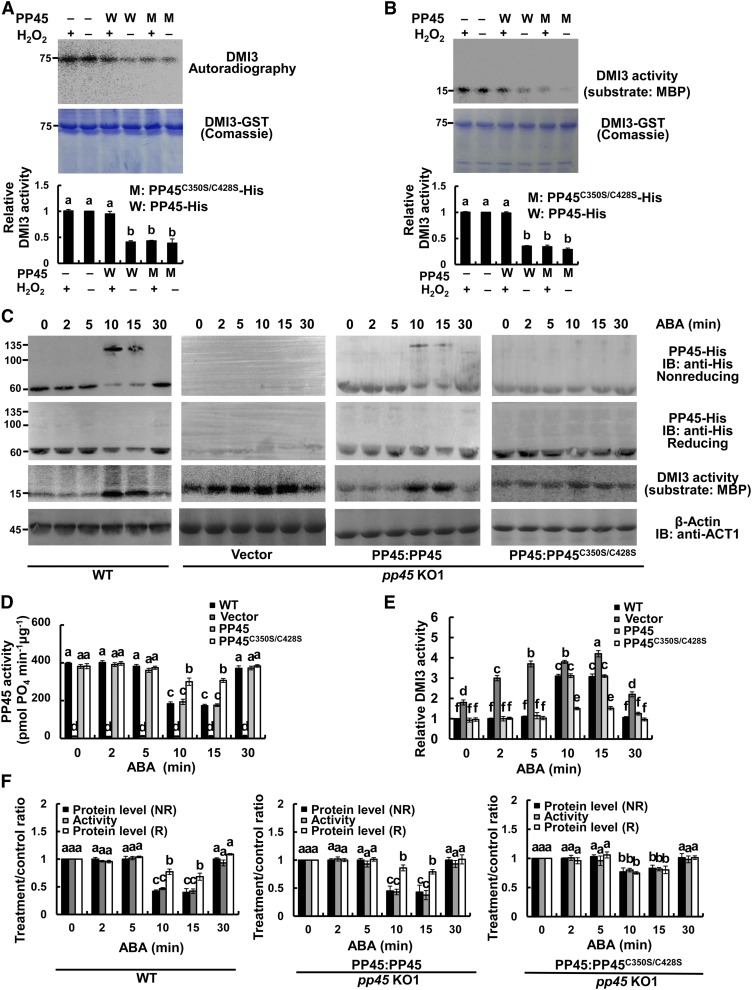
In the absence of H2O2, both PP45 and PP45C350S/C428S interacted with DMI3, as shown by both Y2H assays (Figure 10A) and pull-down assays (Figure 10B). H2O2 treatment strongly inhibited the interaction between DMI3 and PP45 but had no effect on the interaction between DMI3 and PP45C350S/C428S. LCI assays in tobacco leaves (Figure 10C) and Co-IP assays in rice protoplasts (Figure 10D) also showed that either ABA or H2O2 treatment inhibited the DMI3-PP45 interaction, but not the DMI3-PP45C350S/C428S interaction. These results indicate that the oxidation of Cys-350 and Cys-428 in PP45 is necessary to disrupt the DMI3-PP45 interaction under oxidative stress. Accordingly, in the absence of H2O2, both PP45 and PP45C350S/C428S significantly inhibited the activities of autophosphorylation (Figure 11A) and substrate phosphorylation of DMI3 (Figure 11B). H2O2 treatment released the inhibition by PP45 but not by PP45C350S/C428S.
Figure 10.
The Oxidation of Cys-350 and Cys-428 in PP45 Is Necessary to Relieve DMI3-PP45 Interaction.
(A) Y2H assay in the absence or presence of 0.1 mM H2O2. SD-Trp-Leu-His-Ade/AbA/X-α-gal medium was used for testing the interactions.
(B) In vitro pull-down assay in the absence or presence of 0.1 mM H2O2.
(C) LCI assay in tobacco leaves. The indicated constructs were expressed in Nicotiana benthamiana leaves, and the leaves were treated with 100 μM ABA for 90 min or 10 mM H2O2 for 45 min. Scale bar, 2 cm. The IB shows the expression levels of Myc-DMI3-cLUC and His-PP45-nLUC in tobacco leaves after treatment. The Rubisco large subunit visualized by staining with Coomassie Brilliant Blue was used as a loading control.
(D) Co-IP assay in rice protoplasts. The indicated constructs were expressed in rice protoplasts, and the protoplasts were treated with 10 μM ABA for 10 min. Total protoplast proteins were immunoprecipitated (IP) using an anti-His antibody and were then detected with an anti-Myc antibody or an anti-His antibody. Protein input is shown by IB analysis of protein extracts before IP and antibodies against the respective tags. All the experiments were repeated at least three times with similar results. Molecular mass markers in kD are indicated on the left.
Figure 11.
The Oxidation of Cys-350 and Cys-428 in PP45 Prevents the PP45-Mediated Inhibition of DMI3 Activity.
(A) and (B) Autophosphorylation (A) and substrate phosphorylation (B) by DMI3 along with PP45-His or PP45C350S/C428S-His in the absence or presence of 0.1 mM H2O2. Top shows autophosphorylation (A) and substrate phosphorylation (B) by DMI3, middle shows the corresponding Coomassie staining, and bottom shows the relative activity of phosphorylation. The activity of DMI3 without any treatment was set to 1.
(C) The oxidation of Cys-350 and Cys-428 in PP45 induced by ABA relieves the PP45-mediated inhibition of DMI3 activity in rice protoplasts. The indicated constructs were expressed in pp45-KO1 protoplasts, and the protoplasts were treated with 10 μM ABA for various times as indicated. PP45 was detected using nonreducing or reducing IB using an anti-PP45 antibody. The activity of DMI3 was measured by immunoprecipitation kinase assay using MBP as a substrate. The levels of β-actin indicate equal protein loading.
(D) The activity of PP45 in wild-type (WT) protoplasts or the transfected protoplasts of pp45-KO1 exposed to 10 μM ABA treatment. The activity of PP45 was measured using the Ser/Thr phosphatase assay.
(E) Quantitative analysis of DMI3 activity in (C). The relative activity of DMI3 was quantified by Quantity One software, and the activity of DMI3 in wild-type protoplasts treated with ABA for 0 min was set to 1.
(F) The treatment/control ratio of the protein level in (C) and the activity in (D) of PP45. For quantitative analysis of band intensity, the starting point (0 h) was set to 1. NR, nonreducing; R, reducing. All experiments were repeated at least three times with similar results. In (A), (B), (D), (E), and (F), values are means ± sem of three independent experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test. Molecular mass markers in kD are indicated on the left.
Furthermore, in rice protoplasts, ABA treatment for 10 to 15 min induced the formation of PP45 dimers, significantly decreased the activity of PP45, and increased the activity of DMI3 both in wild-type protoplasts and in pp45-KO1 protoplasts transiently expressing PP45 (Figures 11C to 11E). However, in the pp45-KO1 protoplasts transiently expressing PP45C350S/C428S, ABA did not induce the formation of PP45 dimers and led to only a slight decrease in PP45 activity and a slight increase in DMI3 activity. Quantitative analyses in pp45-KO1 protoplasts transiently expressing PP45 treated with ABA for 10 min showed that the treatment/control ratios of PP45 level (nonreducing) and PP45 activity were 0.47 and 0.44, respectively. The activity of DMI3 increased by 3.1-fold, but in the pp45-KO1 protoplasts transiently expressing PP45C350S/C428S treated with ABA for 10 min, the ratios of PP45 protein level (nonreducing) and PP45 activity were 0.81 and 0.81, respectively, and the activity of DMI3 only increased by 1.5-fold (Figures 11E and 11F). Moreover, ABA-induced oxidation of Cys-350 and Cys-428 in PP45 in the rice protoplasts was also shown to be required for ABA-induced increase in the activities of SOD and CAT (Supplemental Figure 14). These results further indicate that Cys-350 and Cys-428 residues are essential for redox sensing.
DISCUSSION
The activation mechanisms of CCaMKs have been widely studied in both animals and plants. Mammalian CaMKII, which contains an N-terminal kinase domain (catalytic), an autoinhibitory domain overlapping with a CaM-binding domain (regulatory), and a C-terminal association domain, has been used as a model for CCaMK due to its high sequence homology with CCaMK in both the kinase and CaM-binding domains. Under basal conditions, CaMKII is autoinhibited due to physical interaction between the regulatory and catalytic domains, blocking catalytic function. A rise in intracellular Ca2+ stimulates binding of Ca2+/CaM to the regulatory domain, releasing the catalytic domain and activating the enzyme. The activation leads to autophosphorylation of the kinase at Thr-287. CaMKII autophosphorylation markedly enhances the binding affinity of CaM and blocks the regulatory domain from inhibiting catalysis, thereby generating autonomous kinase activity (Erickson, 2014; Luczak and Anderson, 2014). This autonomous activity persists until the phosphate group is removed by a protein phosphatase (PP1 or PP2A; Erickson, 2014). In addition, CaMKII can be directly modified by ROS, resulting in autonomous activation (Erickson, 2014; Luczak and Anderson, 2014).
In plants, CCaMK can bind Ca2+ either directly through EF hand domains or indirectly through a CaM-binding domain. The activation mechanisms of CCaMK have been studied in detail in Lilium longiflorum, Medicago truncatula, and Lotus japonicas (Singh and Parniske, 2012; Poovaiah et al., 2013; Gobbato, 2015). Under basal conditions (not treated or stimulated), CCaMK is autoinhibited, and basal Ca2+-induced autophosphorylation at Thr-271 in Mt-CCaMK (or T265 in Lj-CCaMK or T267 in Li-CCaMK) plays a significant but complex role in the activation of CCaMK. It has been hypothesized that Thr-271/Thr-265 phosphorylation stimulates CCaMK activation by promoting interaction with CaM (Poovaiah et al., 2013) via destabilization of intramolecular hydrogen bonds (Shimoda et al., 2012). However, these models cannot explain why both phosphoablative and phosphomimetic mutations of this residue led to spontaneous nodulation (Gleason et al., 2006; Tirichine et al., 2006). By investigating the phosphorylation dynamics of the Thr-271 residue in Mt-CCaMK, a more recent model suggests that the autophosphorylation at Thr-271 negatively regulates CCaMK by stabilizing intramolecular hydrogen bonds, while interaction with Ca2+/CaM during Ca2+ spiking releases the auto-inhibition and promotes CCaMK activation (Miller et al., 2013). This model is more consistent with the experimental results regarding autophosphorylation of CCaMK. Further phosphorylation of Ser-343/Ser-344 in the CaM-binding domain disrupts CaM binding and inhibits CCaMK activity (Liao et al., 2012; Routray et al., 2013). However, it is unknown whether a phosphatase is involved in the regulation of CCaMK.
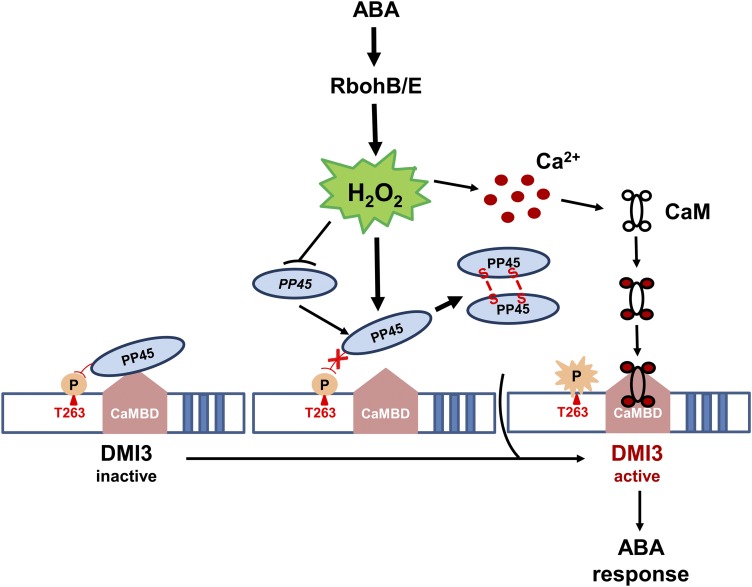
Here, we show a direct physical interaction between DMI3, a rice CCaMK, and PP45, a PP2C-type phosphatase (Figure 1). This interaction is between the CaM-binding domain of DMI3 and the PP2C domain of PP45 (Supplemental Figure 2). We found that PP45 directly inactivates DMI3 by dephosphorylating Thr-263 in DMI3 (Figure 7) and that Thr-263 autophosphorylation is required for the activation of DMI3, CaM binding to DMI3, and DMI3-mediated antioxidant defense (Figure 8). We propose a model for the inhibition of DMI3 by PP45 under the basal state and its ABA-induced activation (Figure 12).
Figure 12.
Proposed Model for the Activation of DMI3 in ABA Signaling.
In the basal state, the PP2C domain of PP45 interacts with the CaM-binding domain (CaMBD) of DMI3 and then dephosphorylates Thr-263 in DMI3, inactivating DMI3 (left). In the presence of ABA, ABA-induced H2O2 production by RbohB/E inhibits the activity of PP45 in two ways: both by inhibiting PP45 and production of its protein and by directly oxidizing the Cys-350 and Cys-428 residues of PP45, forming intermolecular dimers of PP45 (middle). The oxidation of Cys-350 and Cys-428 in PP45 induced by ABA is crucial both for the inhibition of PP45 activity and for the release of PP45-DMI3 interaction. DMI3 is thus released from the negative regulation and converted to an active form and is further activated by H2O2-induced Ca2+/CaM (right), thus resulting in ABA responses.
PP2Cs are a class of evolutionarily conserved Ser/Thr protein phosphatases and have been shown to be involved in a wide variety of plant processes, including ABA signaling, biotic and abiotic stress responses, plant immunity, K+ nutrient signaling, and plant development (Schweighofer et al., 2004; Singh et al., 2010, 2016; Fuchs et al., 2013). In the genomes of Arabidopsis and rice, there are 80 and 90 members, respectively, and these are divided into 11 subgroups (Singh et al., 2010; Fuchs et al., 2013). In Arabidopsis, the best studied PP2Cs belong to group A, which has nine members, including ABI1, ABI2, and HAB1. The members in group A are negative regulators of ABA responses (Schweighofer et al., 2004; Umezawa et al., 2010; Fuchs et al., 2013; Singh et al., 2016). In early ABA signal transduction, there are three major components: pyrabactin resistance (PYR)/PYR1-like/regulatory components of ABA receptors (ABA receptors), group A PP2Cs (negative regulators), and subclass III SnRK2s (positive regulators; Fujii et al., 2009; Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009; Vlad et al., 2009). In the absence of ABA, PP2Cs inactivate SnRK2s by direct dephosphorylation. In the presence of ABA, PYR/PYR1-like/regulatory components of ABA receptors interact with PP2Cs and inhibit phosphatase activity, allowing SnRK2 activation and phosphorylation of target proteins.
In this study, however, PP45 did not interact with rice SnRK2s SAPK8/9/10 (Supplemental Figures 3B and 3D), suggesting that PP45 is not a coreceptor for ABA signaling. However, we found that ABA treatment leads to a transient and significant decrease in the expression of PP45, the PP45 protein level, and its activity in leaves of rice plants (Figure 3). ABA also inhibited the in vivo interaction between PP45 and DMI3 (Figure 2). The ABA-induced increase in the activity of DMI3 was blocked in the PP45-OE plants, and it was further enhanced in the pp45-KO plants (Figure 7A). Our genetic data further show that PP45 negatively regulates ABA responses, including seed germination, root growth, antioxidant defense, and tolerance to water stress and oxidative stress (Figures 5 and 6; Supplemental Figures 8 to 10). Overall, our results clearly indicate that PP45, a member of group K PP2Cs, is also an important negative regulator of ABA signaling.
How does ABA relieve the PP45-mediated inhibition in the activity of DMI3? Previous studies have shown that some PP2Cs, such as ABI1, ABI2, and HAB1, are redox-sensitive proteins (Meinhard and Grill, 2001; Meinhard et al., 2002; Sridharamurthy et al., 2014; Zhu et al., 2014) and that the Cys residues in these proteins are the main targets of ROS (Akter et al., 2015; Waszczak et al., 2015). It was suggested that H2O2 reversibly inhibited the activities of ABI1 and ABI2 probably via oxidation of critical Cys residues (Meinhard and Grill, 2001; Meinhard et al., 2002). A recent study identified HAB1 Cys-186 and Cys-274 as H2O2-sensitive thiols and demonstrated that their oxidation inhibits both HAB1 catalytic activity and its ability to physically associate with SnRK2.6 via formation of intermolecular dimers (Sridharamurthy et al., 2014). In Arabidopsis, it has been demonstrated that ROS are rate-limiting second messengers in ABA signaling, and RBOH subunits D and F are major NADPH oxidase catalytic subunits that mediate ABA-induced ROS production (Kwak et al., 2003). In rice, it has been shown that RbohB and RbohE are involved in ABA-induced H2O2 production (Shi et al., 2012; Zhang et al., 2014). However, it is not clear whether ABA inhibits the activity of PP45 via the production of H2O2 induced by ABA.
In this study, we provide evidence to show that ABA-induced H2O2 by RbohB/E is required for ABA-induced inhibition in PP45 activity (Figure 4) and in the interaction of PP45 and DMI3 (Figure 2E). We identified Cys-350 and Cys-428 in PP45 as the key Cys residues that mediate oxidative inactivation of PP45 and showed that their oxidation resulted in formation of catalytically inactive PP45 intermolecular dimers (Figures 9A to 9F). ABA treatment induced the formation of PP45 dimers in wild-type, but not in rbohB/E-KO1 plants (Figure 9H), indicating that RbohB/E-mediated H2O2 production is required for ABA-induced formation of PP45 dimers. The oxidation of Cys-350 and Cys-428 in PP45 induced by ABA was also necessary to disrupt the DMI3-PP45 interaction (Figure 10) and PP45-mediated inhibition in DMI3 activity (Figure 11).
Taken together, our results suggest that, during ABA signaling, RbohB/E-mediated oxidation of Cys-350 and Cys-428 in PP45 plays a crucial role in the regulation of both the interaction of DMI3-PP45 and the activity of DMI3 (Figure 12). However, our results also showed that ABA and H2O2 induced a transient downregulation in the expression of PP45 (Figure 3A) and the level of PP45 (Figure 3B) in rice leaves. In the pp45-KO1 protoplasts transiently expressing PP45C350S/C428S treated with ABA, the amount of PP45 protein and the activity of PP45 did not completely return to the control levels (Figure 11F), and the activity of DMI3 still had a slight but significant increase (Figure 11E). These results suggest that the decrease in the expression of PP45 also contributes to the inhibition of PP45 and the activation of DMI3 in ABA signaling (Figure 12).
In this study, however, the expression of PP45, the level of PP45 protein, and the activity of PP45 in leaves of rice plants were also shown to be upregulated by the ABA and H2O2 following a transient downregulation (Figure 3). A previous study also showed that treatments with ABA and H2O2 induced the expression of PP45 in leaves of rice seedlings (Hu et al., 2006). But why can ABA and H2O2 also induce the activation of PP45? This may be associated with the upregulation of antioxidant defense by DMI3 in ABA and H2O2 signaling. It was shown that ABA-induced H2O2 production activates DMI3 and that the activation of DMI3 is required for ABA-induced antioxidant defense in rice (Shi et al., 2012). The upregulation in the activities of antioxidant enzymes enhances the ability of cells to scavenge H2O2. This, in turn, relieves the inhibition of PP45 by H2O2, thus resulting in the upregulation of PP45. The decrease in H2O2 was shown to remove ABA-induced inhibition in the expression of PP45 and the activity of PP45 in rice leaves (Figures 4A to 4D). In addition, the catalytically inactive PP45 intermolecular disulfide bonds induced by ABA might be reduced by thioredoxins, which have been shown to control the reduction of ROS-mediated intra- or intermolecular disulfide bonds in plant cells (Waszczak et al., 2015), thus resulting in the up-regulation of PP45 activity. However, the exact mechanism for the up-regulation of PP45 in ABA signaling remains to be determined.
METHODS
Plant Materials and Growth Conditions
All rice (Oryza sativa) plants used in this study were the rice cultivar Nipponbare or were derived from this cultivar. Rice seedlings were grown under the conditions as described previously (Zhang et al., 2014). When the second leaves were fully expanded, they were collected and used for investigations.
Y2H Screening
The full-length DMI3 was fused into the pGBKT7 vector (Clontech) as the bait. Total RNA was isolated from rice leaves and transcribed into single-stranded cDNAs. cDNA fragments longer than 500 bp were transformed with linearized pGADT7 vector (Clontech) to construct a rice cDNA library. Using the lithium acetate method described in Clontech’s yeast protocols handbook, the bait construct was transformed into yeast strain Y2HGold, and the library was transformed into yeast strain Y187. Screening of interaction clones was performed via mating according to the manufacturer’s instructions (Clontech). A total of 106 transformants from the cDNA library were screened for growth on the stringent SD-Leu-Trp-His-Ade dropout medium. Positive clones were able to activate the transcription of four independent reporter genes (AUR1-C, ADE2, HIS3, and MEL1). The library plasmid responsible for the activation of reporters was rescued, analyzed by restriction digestion, and transformed again into Y187. Yeast cells with preys were mated one-on-one in parallel against the yeast Y2HGold expressing the bait or the empty vector pGBKT7 as the negative control.
Y2H Assay
PP45 was amplified by PCR and was cloned into pGADT7. Prey constructs were transformed into yeast strain Y187, and bait vectors were transformed into yeast strain Y2HGold by the lithium acetate method noted above. The prey strain and the bait strain were mated, and the mating cultures were spread on stringent selective medium plates containing X-α-gal (40 μg mL−1). The plates were incubated at 30°C for 4 to 6 d. Plates were checked every 12 h for the development of blue color. Expressed proteins were tested for interaction by the quantitative interaction assay (β-galactosidase assay) described in Clontech’s yeast protocols handbook.
Expression of DMI3 and PP45
The DMI3 cDNA fragment was PCR amplified and cloned into pGEX-4T-1 (Amersham Pharmacia Biotech) using the SmaI and XhoI sites for GST fusion. To express the fusion protein, Escherichia coli Rosetta (DE3; Novagen) harboring the plasmids was induced with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside in Luria-Bertani broth for 6 h at 24°C. DMI3-GST was purified using the MagnetGST protein purification system (Promega). The PP45 cDNA fragment was PCR amplified and inserted in frame at the KpnI and BamHI sites of pET30a (Novagen) for fusion to a His tag. To express the fusion protein, Escherichia coli Rosetta (DE3) harboring the plasmids was induced with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside in Luria-Bertani broth for 8 h at 20°C. PP45-His was purified using MagnetHis protein purification system (Promega).
Pull-Down Assay
To detect interaction between DMI3 and PP45 in vitro, GST-tagged DMI3 or GST alone was immobilized on the Magnet GST Particles (Promega). The particles were incubated with PP45-His in pull-down binding buffer (4.2 mM Na2HPO4, 2 mM KH2PO4, 140 mM NaCl, 10 mM KCl, 10% bovine serum albumin [BSA], pH 7.2) with gentle shaking at 4°C for 2 h. The particles were washed at least three times in wash buffer (interaction buffer without BSA) and boiled in 1× SDS loading buffer (50 mM Tris-HCl, 2% SDS, 0.1% bromophenol blue, 10% glycerol, 10 mM DTT). Samples were separated on 12% SDS-PAGE gels and analyzed by IB using an anti-GST antibody (Abmart, lot 264160; 1:1000, v/v) or anti-His antibody (Abmart, lot 283874, 1:1000, v/v).
BiFC Assay
cDNA of DMI3 without its stop codon was amplified using PCR and cloned into the KpnI site of pSPYCE155 (Waadt et al., 2008) to construct SYFPC-DMI3. The cDNA of PP45 without its stop codon was cloned into pSPYCE173 (Waadt et al., 2008) using the KpnI and XhoI sites to construct SYFPN-PP45. Using the particle bombardment (Bio-Rad) method, the constructs were transiently expressed in onion epidermis cells as described previously (Lee et al., 2008). Fluorescence was detected 16 h following transfection using a laser confocal microscope (TCS-SP2, Leica), with excitation at 530 nm and emission at 525 nm.
Luciferase Complementation Imaging Assay
Full-length and truncated sequences of PP45 with His tags were fused to the pC1300-nLUC (luciferase complementation) vector using KpnI and SalI sites. Full-length and truncated sequences of DMI3 with Myc-tags sequences were fused to the pC1300-cLUC vector using KpnI and PstI sites. The constructed plasmid vectors were transformed into Agrobacterium strain GV3101 (Nova Lifetech). The positive clone was incubated in yeast extract broth liquid medium at 28°C for 16 h (Sparkes et al., 2006). The bacteria were mixed to a final OD600 of 0.5 and then were collected and resuspended in 2 mL of activity buffer (10 mM MES, pH 5.7, 10 mM MgCl2, 150 mM acetosyringone). After 2 to 5 h, the activity bacteria were injected into young Nicotiana benthamiana leaves. After 3 to 5 d, the abaxial sides of leaves were treated with 100 μM ABA for 90 min or 10 mM H2O2 for 45 min and sprayed with 1 mM luciferin (Thermo Fisher Scientific) and then kept in the dark for 1 h. A camera (Tanon 5200 Multi, Tanon Biomart) was used to capture the LUC signal.
Co-IP Assay
The coding sequences of DMI3 or PP45 were fused with sequences encoding Myc- or His-tags and cloned into pXZP008 vector at KpnI or XhoI-KpnI sites, respectively. Rice protoplasts were transfected with 35S: DMI3-Myc and 35S: PP45-His by PEG-calcium-mediated transformation (Yoo et al., 2007). After incubation for 16 h, the proteins from protoplasts were extracted with buffer (20 mM Tris-HCl, pH 7.5, 20 mM KCl, 1 mM EDTA, 10 mM Na3VO4, 0.5% (v/v) Triton X-100, 10% glycerol, 5 μg mL−1 leupeptin, 5 μg mL−1 aprotinin) and centrifuged at 12,000g for 30 min at 4°C. The supernatants were incubated with an anti-Myc antibody (Abmart, lot 294166; 1:1000, v/v) bound to protein A beads in IP buffer (20 mM Tris-HCl, pH 7.5, 20 mM KCl, 150 mM NaCl, 10 mM MgCl2, 10 mM Na3VO4, 5 μg mL−1 leupeptin, 5 μg mL−1 aprotinin) at 4°C for 6 h. The beads were washed three times with IP buffer, and the proteins were eluted by boiling in 1× SDS sample buffer for 5 min. After centrifugation, the supernatant fraction was analyzed by 12% SDS-PAGE, followed by IB with an anti-His antibody.
Immunocomplex Kinase Activity Assay for DMI3 or MPK1
Protein was extracted from rice leaves or protoplasts with buffer (20 mM Tris-HCl, pH 7.5, 20 mM KCl, 1 mM EDTA, 10 mM Na3VO4, 0.5% [v/v] Triton X-100, 10% glycerol, 5 μg mL−1 leupeptin, 5 μg mL−1 aprotinin) and was centrifuged at 12,000g for 30 min at 4°C. Protein content was determined according to the method of Bradford (1976) with BSA as the standard. Protein extracts (100 μg) were incubated with an anti-DMI3 antibody (2 μg, Abmart) or anti-MPK1 antibody (2 μg, Bejing Protein Innovation) bound to protein A beads. Kinase activity in the immunocomplex was determined by an in-gel kinase assay using myelin basic protein (MBP) as substrate as described previously (Shi et al., 2012, 2014). In brief, 1 μg MBP (Sigma-Aldrich) was incubated either with DMI3 in reaction buffer (25 mM Tris-HCl, pH7.5, 5 mM MgCl2, 0.5 mM CaCl2, 2 μM CaM [Sigma-Aldrich]) or with MPK1 in reaction buffer (25 mM Tris, pH 7.5, 5 mM MgCl2, 1 mM ethylene glycol tetraacetic acid [EGTA], 0.1 mM Na3VO4) with 10 μCi [γ32P]-ATP (3000 Ci mM−1) at 30°C for 30 min. The reaction products were separated by 12% SDS-PAGE and analyzed by autoradiography using X-ray film or a phosphostorage screen (Typhoon TRIO, Amersham Biosciences).
In the experiments for detecting the effect of PP45 on the activity of DMI3, the sample was divided into two parts: one with PP45-His and the other without PP45-His. The two parts were incubated in buffer (20 mM Tris-HCl, pH 7.5, 20 mM MgCl2, 1 mM EDTA, 100 mM NaCl) at 30°C for 30 min. Relative levels of DMI3 or MPK1 activity, detected by immunocomplex kinase activity assay and quantified by Quantity One software (Bio-Rad), are presented as values relative to those of the corresponding controls.
SDS-PAGE and IB Analysis
Protein extracts (20 μg) were separated by 12% SDS-PAGE. For nonreducing PAGE, the Native PAGE Bis-Tris Gel System (Thermo Fisher Scientific) was used. After electrophoresis, the gel was transferred to a polyvinylidene difluoride membrane at 110 V for 60 min at 4°C in a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad). The membrane was incubated in blocking solution containing PBS/Tween (140 mM NaCl, 10 mM KCl, 2 mM KH2PO4, 8 mM Na2HPO4, 0.1% Tween-20 [v/w], pH 7.6) that was supplemented with 5% (w/v) nonfat dry milk for 2 h at room temperature. The membrane was then washed with PBS/Tween buffer three times for 10 min. The blots were probed with anti-PP45 antibody (ABclonal), anti-DMI3 antibody (ABclonal), anti-ACT1 antibody (Beijing Protein Innovation), anti-DMI3T263(P) antibody (ABclonal), anti-CaM antibody (Sigma-Aldrich), anti-Myc antibody, anti-His antibody, and anti-GST antibody. Information about the anti-DMI3 antibody was described previously (Shi et al., 2012). The anti-DMI3 antibody was raised against peptides of DMI3. The anti-PP45 antibody was raised against full length of PP45. The anti-DMI3T263(p) antibody was raised against peptide (SFGDHTWKTISSSA) of DMI3. The anti-GST antibody, anti-Myc antibody, and anti-His antibody were obtained from immunized mice, and the other antibodies were obtained from immunized rabbits. The primary antibody was used at 1:1000 dilution and secondary antibody was used at 1:4000 dilution. Chemiluminescence was detected with the enhanced chemiluminescence immnoublotting detection system (GE Healthcare) and Kodak XJ300 film or a camera (Tanon 5200 Multi). Quantification was done using Quantity One software (Bio-Rad). For quantitative analysis, the data were normalized by dividing the band intensity of PP45 by the band intensity of β-actin in each lane. The starting point (0 min) was set to 1; other points were compared with it.
Autophosphorylation Assay
GST-tagged DMI3 was immobilized on Magnet GST particles and eluted with buffer (50 mM Tris-HCl, 50 mM glutathione, pH 8.0). Ten micrograms DMI3-GST fusion protein was added in a total 50-μL reaction buffer for 10 min at 30°C in 25 mM Tris-HCl (pH 7.5) containing 5 mM MgCl2,100 mM NaCl,10 μCi of [γ32P]-ATP (3000 Ci mM−1) with 0.5 mM CaCl2 or 5 mM EGTA. The reaction mixtures were incubated with or without PP45-His protein at 30°C for 30 min. The kinase reaction was stopped by mixing with SDS-PAGE sample buffer and boiling for 5 min. Samples were separated on 12% SDS-PAGE gels, and the phosphorylated DMI3 was visualized by autoradiography.
In Vitro Kinase Assay
An in vitro kinase assay was performed using 10 μg of the DMI3-GST fusion protein in reaction buffer (25 mM Tris, pH 7.5, 5 mM MgCl2, 0.5 mM CaCl2, 2 μM CaM) at 30°C for 30 min in a final volume of 50 μL that also contained 1 μg MBP, 10 μM ATP, 10 μCi [γ32P]-ATP. The reaction mixtures were incubated with or without PP45-His at 30°C for another 30 min. The reaction was stopped by adding SDS sample buffer and the reaction mix was separated on 12% SDS-PAGE. The phosphorylated substrates were visualized by autoradiography.
Site-Directed Mutagenesis
To mutate PP45 and DMI3, the Multi-Directed Mutagenesis Kit (Agilent Technologies) was used according to the manufacturer’s instructions. The DNA oligonucleotides used in mutagenesis were synthesized, and their sequences are listed in Supplemental Table 1. After mutagenesis, all of the mutated plasmids were confirmed by Sanger sequencing.
In Vitro Phosphate Measurement
Based on DMI3 phosphorylation sites, phosphorylated polypeptides were synthetized from ABclonal Biotech. To measure phosphate release from the synthetic phosphopeptides, the phosphopeptides (10 nM) were incubated for 15 min with 1 μg of OsPP45 or calf intestine alkaline phosphatase in phosphatase buffer (40 μL) at 30°C. Released phosphate was measured according to the method described previously (Van Veldhoven and Mannaerts, 1987).
Ser/Thr Phosphatase Assay System for PP45 Activity
Protein was extracted from rice leaves with a phosphatase storage buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 0.02% [w/v] sodium azide). After centrifugation at 100,000g for 1 h at 4°C, the supernatants were transferred into spin columns (Promega), followed by resuspending with Sephadex G-25 resin (Promega) and centrifugation at 600g for 5 min. The sample flow through in the tube contained the total PP2Cs. After extraction of total PP2C protein, the protein concentration was determined by Coomassie Brilliant Blue method (Braford, 1976). Each time point sample used the same amount of anti-PP45 antibody (ABclonal) and protein A-sepharose beads. According to the instructions of Serine/Threonine Phosphatase Assay System (Promega), a standard curve is required before every PP45 activity assay. Samples containing 0, 100, 200, 500, 1000, and 2000 pmol free phosphate in reaction buffer were used to prepare a standard curve. PP45 was added into the reaction buffer (250 mM imidazole, pH 7.2, 1 mM EGTA, 25 mM MgCl2, 0.1% [w/v] β-mercaptoethanol, 0.5 mg mL−1 BSA) with 1 mM phosphopeptide (Promega) at room temperature for 15 to 30 min. After reaction, the optical density of the samples was determined using a plate reader with a 630-nm filter. The phosphatase activity was calculated by comparing with the phosphate curve and was given in pmol PO4 per minute per μg of protein.
The purified PP45-His mutant and wild-type protein expressed from E. coli were incubated in the presence of various concentrations of H2O2 in the reaction buffer with 1 mM phosphopeptide at room temperature for 15 to 30 min. The PP45 activity in the reaction mixture was then measured.
To test whether reducing agents affect the activity of H2O2-treated PP45, PP45 extracts were preincubated with 0.1 mM H2O2 for 30 min and then treated with the different concentrations of TCEP (Sigma-Aldrich) or DTT for 30 min. The activity of PP45 was measured by the serine/threonine phosphatase assay noted above.
Size Exclusion Chromatography
The purified wild type and C350S/C428S mutant PP45-His proteins expressed from E. coli were treated with 0.1 mM H2O2 for 30 min at 30°C. The proteins were loaded onto Superdex 200 Increase 10/300 gel columns (Amersham Bioscience) connected on an AKTA explorer box-900 PH/C-900 (Amersham Bioscience) liquid chromatography system equipped with the UV detector set to detect absorbance at 280 nm. Samples were eluted with PBS to 0.7 mL min−1 flow rate and collected into 2 mL fractions. The fractions were confirmed by reducing and nonreducing PAGE. The void volume of the size exclusion chromatography column is ∼8 mL.
CaM Binding Assay In Vitro and In Vivo
For in vitro assays, the full-length CaM1 cDNA fragment was PCR amplified and cloned into pGEX-4T-1 using the EcoRI and XhoI sites for GST fusion. To express the fusion protein, Escherichia coli Rosetta (DE3) harboring the plasmids was induced with 0.5 mM isopropyl-1-thio-β-d-galactopyranoside in Luria-Bertani broth for 4 h at 24°C. Purified wild-type and mutant DMI3 proteins were separated by SDS-PAGE and electrophoretically transferred onto a polyvinylidene difluoride membrane. The membrane was subsequently incubated with binding buffer (10 mM Tris/HCl, 150 mM NaCl, and 5% [w/v] nonfat milk) containing CaM1 with 1 mM CaCl2 or 5 mM EGTA for 24 h at 4°C. The membrane was then washed three times. The bound CaM signal was detected by IB using an anti-CaM antibody.
For in vivo assays, the construct 35S:DMI3-Myc was delivered into rice protoplasts by PEG-calcium-mediated transformation. The total proteins extracted from protoplasts were incubated with anti-Myc antibody bound to protein A beads for 6 h. The beads were collected, washed, and boiled for 5 min with 1× SDS sample buffer. Samples were analyzed by 12% SDS-PAGE followed by IB with an anti-CaM antibody.
Quantitative RT-PCR Analyses
Total RNA was isolated from rice leaves using RNAiso Plus (TaKaRa) according to the manufacturer’s instructions. DNase treatment was included in the isolation step using RNase-free DNase. Approximately 2 μg of total RNA was reverse transcribed using an oligo d(T)16 primer and M-MLV reverse transcriptase (TaKaRa). Real-time quantitative RT-PCR was performed on a 7500 real-time PCR system (Applied Biosystems) using SYBR Premix Ex TaqTM (TaKaRa) according to the manufacturer’s instructions. cDNA was amplified by PCR using the primers shown in Supplemental Table 2. The expression level was normalized against that of rice glyceraldehyde-3-phosphate dehydrogenase gene. The relative expression levels of the target genes were calculated as x-fold changes relative to the appropriate control experiment for the different chemical treatments.
Transfection of Protoplasts with Plasmid DNAs
Rice protoplasts were isolated according to the method described previously (Zhang et al., 2012). The plasmids were delivered into protoplasts using a PEG-calcium-mediated method (Yoo et al., 2007). About 10 μg plasmid DNAs per 100 μL × 106 protoplasts were used for transient expression analysis.
Generation of PP45, DMI3, and RbohB/E Transgenic Rice Lines
Transgenic lines (PP45-OE and DMI3-OE) were generated by Biorun Biotechnology. To obtain the transgenic plants overexpressing PP45 or DMI3, the full-length open reading frame of either PP45 or DMI3 was inserted into the plant binary vector pBWA(V)HS. Then, the PP45 gene or DMI3 gene under the control of CaMV 35S promoter was transformed into rice (O. sativa sub. japonica cv Nipponbare) by the Agrobacterium-mediated transformation method (Hiei et al., 1994). Homozygous T3 seeds of the transgenic plants were used for further analysis.
The dmi3-KO, pp45-KO, and rbohB/E-KO plants were generated using the CRISPR/Cas9 system (Biogle). The sgRNAs of DMI3, PP45, RbohB, and RbohE are shown in Supplemental Figures 5 and 7. The single sgRNA was created in the BGK03 vector containing Cas9, which was introduced into Agrobacterium strain EHA105 and transformed into rice (O. sativa sub. japonica cv Nipponbare). To examine the function of CRISPR/Cas9 in vivo, genomic DNA was extracted from transgenic plants and primer pairs flanking the designed target site were used for PCR amplification (Supplemental Table 2). Sequence alignment revealed that two or one independent mutants of each gene, such as dmi3-KO1 and dmi3-KO2, pp45-KO1 and pp45-KO2, and rbohB/E-KO1 were obtained (Supplemental Figures 5 and 7).
Phenotype Analysis
For seed germination assays, seeds of the wild type, PP45-OE, pp45-KO, DMI3-OE, and dmi3-KO were plated in triplicate on 1/2× Murashige and Skoog medium plates. The medium contained 0.8% agar and was supplemented with different concentrations of ABA (0, 1, and 5 μM). The seeds were incubated at 4°C for 2 d before being placed in a growth chamber to germinate (16 h light/8 h dark; 200 μmol m−2 s−1 light intensity; 26°C), and germination (emergence of radicals) was scored at the indicated times. For the analysis of root growth, 4-d-old seedlings were individually transferred to the 1/2× Murashige and Skoog medium with different concentrations of ABA (0, 0.5, l, 3, 5, 10, and 20 μM). Seedlings were further incubated in the growth chamber for another 14 d, and then the length of primary roots was measured using a ruler. To evaluate the performance of the transgenic rice plants under water stress and oxidative stress, the seeds of PP45-OE, pp45-KO, DMI3-OE, dmi3-KO, and the wild type were germinated and grown in the growth chamber as described above. For the analysis of survival rate, rice seedlings were treated with 20% PEG 4000, 100 mM H2O2 for 15 d, and the survival rates were counted after recovery by rewatering for 10 d. For the analysis of oxidative damage to lipids and plasma membranes, the rice seedlings were treated with 20% PEG 4000, 100 mM H2O2 for 2 d, and the content of malondialdehyde and the percentage of electrolyte leakage were determined according to the methods described previously (Shi et al., 2012).
Antioxidant Enzyme Assays
Rice protoplasts were homogenized in a solution of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone. The homogenate was centrifuged at 12,000g for 20 min at 4°C, and the supernatant was used immediately for the antioxidant enzyme assays. The total activities of SOD and CAT were determined as described previously (Jiang and Zhang, 2001).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: DMI3, LOC_Os05g41090; PP45, LOC_Os03g09220; PP1, LOC_Os01g07090; PP57, LOC_Os03g59470; PP111, LOC_Os10g22460; RbohB, LOC_Os01g25820; RbohE, LOC_Os01g61880; SAPK8, LOC_Os03g55600; SAPK9, LOC_Os12g39630; SAPK10, LOC_Os03g41460; MPK1, LOC_Os06g06090; glyceraldehyde-3-phosphate dehydrogenase, LOC_Os02g38920; ACT1, LOC_Os03g50885.
Supplemental Data
Supplemental Figure 1. Subcellular localization of PP45 and DMI3.
Supplemental Figure 2. The CaM-binding domain of DMI3 interacts with the PP2C domain of PP45.
Supplemental Figure 3. DMI3 specifically interacts with PP45.
Supplemental Figure 4. The specificity of the anti-PP45 antibody and the anti-DMI3 antibodies.
Supplemental Figure 5. Identification of the rbohB/E-KO1 mutant.
Supplemental Figure 6. Standard curves of absorbance at 630 nm versus concentration of free phosphate with and without reducing agents.
Supplemental Figure 7. Identification of dmi3-KO and pp45-KO mutants.
Supplemental Figure 8. PP45 decreases and DMI3 increases ABA sensitivity of seed germination and root growth.
Supplemental Figure 9. PP45 reduces and DMI3 enhances the tolerance of rice plants to water stress and oxidative stress.
Supplemental Figure 10. PP45 negatively regulates and DMI3 positively regulates the activities of MPK1, SOD, and CAT.
Supplemental Figure 11. H2O2 does not directly affect the activity of DMI3 in vitro and Ca2+ is required for H2O2-induced activation of DMI3 in rice plants.
Supplemental Figure 12. DMI3 does not regulate the expression of PP45 and the activity of PP45.
Supplemental Figure 13. The oxidation of PP45 does not form an intramolecular disulfide bridge.
Supplemental Figure 14. The oxidation of Cys-350 and Cys-428 in PP45 is required for ABA-induced activities of SOD and CAT.
Supplemental Table 1. DNA oligonucleotides used for site-directed mutagenesis.
Supplemental Table 2. PCR primers used in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 31471427, 31671606, and 31601234), the National Basic Research Program of China (grant no. 2012CB114306), the Fundamental Research Funds for the Central Universities (grant no. KYTZ201402 and KJQN201736), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the China Postdoctoral Science Foundation (grant no. 2016M590464).
AUTHOR CONTRIBUTIONS
M.J. and L.N. conceived the project and designed the experiments. L.N. performed most of the experiments. X.F., H.Z., X.L., X.C., P.Z., L.L., Q.W., M.S., and Q.-W.W performed some of the experiments. M.J., A.Z., and Z.Z. analyzed data. M.J. and L.N. wrote the article.
References
- Akter S., Huang J., Waszczak C., Jacques S., Gevaert K., Van Breusegem F., Messens J. (2015). Cysteines under ROS attack in plants: a proteomics view. J. Exp. Bot. 66: 2935–2944. [DOI] [PubMed] [Google Scholar]
- Batistič O., Kudla J. (2012). Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta 1820: 1283–1293. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- DeFalco T.A., Bender K.W., Snedden W.A. (2009). Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 425: 27–40. [DOI] [PubMed] [Google Scholar]
- Després C., Chubak C., Rochon A., Clark R., Bethune T., Desveaux D., Fobert P.R. (2003). The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.R. (2014). Mechanisms of CaMKII activation in the heart. Front. Pharmacol. 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S., Grill E., Meskiene I., Schweighofer A. (2013). Type 2C protein phosphatases in plants. FEBS J. 280: 681–693. [DOI] [PubMed] [Google Scholar]
- Fujii H., Chinnusamy V., Rodrigues A., Rubio S., Antoni R., Park S.Y., Cutler S.R., Sheen J., Rodriguez P.L., Zhu J.K. (2009). In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason C., Chaudhuri S., Yang T., Muñoz A., Poovaiah B.W., Oldroyd G.E. (2006). Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441: 1149–1152. [DOI] [PubMed] [Google Scholar]
- Gobbato E. (2015). Recent developments in arbuscular mycorrhizal signaling. Curr. Opin. Plant Biol. 26: 1–7. [DOI] [PubMed] [Google Scholar]
- Harper J.F., Breton G., Harmon A. (2004). Decoding Ca(2+) signals through plant protein kinases. Annu. Rev. Plant Biol. 55: 263–288. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Banba M., Shimoda Y., Kouchi H., Hayashi M., Imaizumi-Anraku H. (2010). A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 63: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y., Ohta S., Komari T., Kumashiro T. (1994). Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6: 271–282. [DOI] [PubMed] [Google Scholar]
- Horváth B., et al. (2011). Medicago truncatula IPD3 is a member of the common symbiotic signaling pathway required for rhizobial and mycorrhizal symbioses. Mol. Plant Microbe Interact. 24: 1345–1358. [DOI] [PubMed] [Google Scholar]
- Hu X., Song F., Zheng Z. (2006). Molecular characterization and expression analysis of a rice protein phosphatase 2C gene, OsBIPP2C1, and overexpression in transgenic tobacco conferred enhanced disease resistance and abiotic tolerance. Physiol. Plant. 127: 225–236. [Google Scholar]
- Jiang M., Zhang J. (2001). Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 42: 1265–1273. [DOI] [PubMed] [Google Scholar]
- Jin Y., Liu H., Luo D., Yu N., Dong W., Wang C., Zhang X., Dai H., Yang J., Wang E. (2016). DELLA proteins are common components of symbiotic rhizobial and mycorrhizal signalling pathways. Nat. Commun. 7: 12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Zhu H., Chu X., Yang Z., Yuan S., Yu D., Wang C., Hong Z., Zhang Z. (2011). A novel interaction between CCaMK and a protein containing the Scythe_N ubiquitin-like domain in Lotus japonicus. Plant Physiol. 155: 1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Yamamoto S., Minami H., Kagaya Y., Hattori T. (2004). Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.-M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D.G., Schroeder J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux D., Ranjeva R., Pugin A. (2006). Calcium in plant defence-signalling pathways. New Phytol. 171: 249–269. [DOI] [PubMed] [Google Scholar]
- Lee M.O., et al. (2008). Novel rice OsSIPK is a multiple stress responsive MAPK family member showing rhythmic expression at mRNA level. Planta 227: 981–990. [DOI] [PubMed] [Google Scholar]
- Lévy J., et al. (2004). A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364. [DOI] [PubMed] [Google Scholar]
- Liao J., Singh S., Hossain M.S., Andersen S.U., Ross L., Bonetta D., Zhou Y., Sato S., Tabata S., Stougaard J., Szczyglowski K., Parniske M. (2012). Negative regulation of CCaMK is essential for symbiotic infection. Plant J. 72: 572–584. [DOI] [PubMed] [Google Scholar]
- Luczak E.D., Anderson M.E. (2014). CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 73: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F., Lu R., Liu H., Shi B., Zhang J., Tan M., Zhang A., Jiang M. (2012). Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. J. Exp. Bot. 63: 4835–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., Grill E. (2009). Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068. [DOI] [PubMed] [Google Scholar]
- Madsen L.H., Tirichine L., Jurkiewicz A., Sullivan J.T., Heckmann A.B., Bek A.S., Ronson C.W., James E.K., Stougaard J. (2010). The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard M., Grill E. (2001). Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 508: 443–446. [DOI] [PubMed] [Google Scholar]
- Meinhard M., Rodriguez P.L., Grill E. (2002). The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214: 775–782. [DOI] [PubMed] [Google Scholar]
- Messinese E., Mun J.H., Yeun L.H., Jayaraman D., Rougé P., Barre A., Lougnon G., Schornack S., Bono J.J., Cook D.R., Ané J.M. (2007). A novel nuclear protein interacts with the symbiotic DMI3 calcium- and calmodulin-dependent protein kinase of Medicago truncatula. Mol. Plant Microbe Interact. 20: 912–921. [DOI] [PubMed] [Google Scholar]
- Miller J.B., Pratap A., Miyahara A., Zhou L., Bornemann S., Morris R.J., Oldroyd G.E.D. (2013). Calcium/calmodulin-dependent protein kinase is negatively and positively regulated by calcium, providing a mechanism for decoding calcium responses during symbiosis signaling. Plant Cell 25: 5053–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R.M., Gleason C.A., Edwards A., Hadfield J., Downie J.A., Oldroyd G.E.D., Long S.R. (2004). A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc. Natl. Acad. Sci. USA 101: 4701–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S., Takezawa D., Poovaiah B.W. (1995). Chimeric plant calcium/calmodulin-dependent protein kinase gene with a neural visinin-like calcium-binding domain. Proc. Natl. Acad. Sci. USA 92: 4897–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimprikar P., Carbonnel S., Paries M., Katzer K., Klingl V., Bohmer M.J., Karl L., Floss D.S., Harrison M.J., Parniske M., Gutjahr C. (2016). A CCaMK-CYCLOPS-DELLA complex activates transcription of RAM1 to regulate Arbuscule branching. Curr. Biol. 26: 987–998. [DOI] [PubMed] [Google Scholar]
- Poovaiah B.W., Du L., Wang H., Yang T. (2013). Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant Physiol. 163: 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Ali G.S., Celesnik H., Day I.S. (2011). Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23: 2010–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routray P., Miller J.B., Du L., Oldroyd G., Poovaiah B.W. (2013). Phosphorylation of S344 in the calmodulin-binding domain negatively affects CCaMK function during bacterial and fungal symbioses. Plant J. 76: 287–296. [DOI] [PubMed] [Google Scholar]
- Schweighofer A., Hirt H., Meskiene I. (2004). Plant PP2C phosphatases: Emerging functions in stress signaling. Trends Plant Sci. 9: 236–243. [DOI] [PubMed] [Google Scholar]
- Shi B., Ni L., Zhang A., Cao J., Zhang H., Qin T., Tan M., Zhang J., Jiang M. (2012). OsDMI3 is a novel component of abscisic acid signaling in the induction of antioxidant defense in leaves of rice. Mol. Plant 5: 1359–1374. [DOI] [PubMed] [Google Scholar]
- Shi B., Ni L., Liu Y., Zhang A., Tan M., Jiang M. (2014). OsDMI3-mediated activation of OsMPK1 regulates the activities of antioxidant enzymes in abscisic acid signalling in rice. Plant Cell Environ. 37: 341–352. [DOI] [PubMed] [Google Scholar]
- Shimoda Y., Han L., Yamazaki T., Suzuki R., Hayashi M., Imaizumi-Anraku H. (2012). Rhizobial and fungal symbioses show different requirements for calmodulin binding to calcium calmodulin-dependent protein kinase in Lotus japonicus. Plant Cell 24: 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Parniske M. (2012). Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr. Opin. Plant Biol. 15: 444–453. [DOI] [PubMed] [Google Scholar]
- Singh A., Giri J., Kapoor S., Tyagi A.K., Pandey G.K. (2010). Protein phosphatase complement in rice: genome-wide identification and transcriptional analysis under abiotic stress conditions and reproductive development. BMC Genomics 11: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Pandey A., Srivastava A.K., Tran L.S., Pandey G.K. (2016). Plant protein phosphatases 2C: from genomic diversity to functional multiplicity and importance in stress management. Crit. Rev. Biotechnol. 36: 1023–1035. [DOI] [PubMed] [Google Scholar]
- Singh S., Katzer K., Lambert J., Cerri M., Parniske M. (2014). CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15: 139–152. [DOI] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Sridharamurthy M., Kovach A., Zhao Y., Zhu J.K., Xu H.E., Swaminathan K., Melcher K. (2014). H2O2 inhibits ABA-signaling protein phosphatase HAB1. PLoS One 9: e113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Maekawa T., Hayashi M. (2012). Nuclear-localized and deregulated calcium- and calmodulin-dependent protein kinase activates rhizobial and mycorrhizal responses in Lotus japonicus. Plant Cell 24: 810–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa D., Ramachandiran S., Paranjape V., Poovaiah B.W. (1996). Dual regulation of a chimeric plant serine/threonine kinase by calcium and calcium/calmodulin. J. Biol. Chem. 271: 8126–8132. [DOI] [PubMed] [Google Scholar]
- Tirichine L., et al. (2006). Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441: 1153–1156. [DOI] [PubMed] [Google Scholar]
- Umezawa T., Sugiyama N., Mizoguchi M., Hayashi S., Myouga F., Yamaguchi-Shinozaki K., Ishihama Y., Hirayama T., Shinozaki K. (2009). Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 17588–17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T., Nakashima K., Miyakawa T., Kuromori T., Tanokura M., Shinozaki K., Yamaguchi-Shinozaki K. (2010). Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 51: 1821–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P.P., Mannaerts G.P. (1987). Inorganic and organic phosphate measurements in the nanomolar range. Anal. Biochem. 161: 45–48. [DOI] [PubMed] [Google Scholar]
- Vlad F., Rubio S., Rodrigues A., Sirichandra C., Belin C., Robert N., Leung J., Rodriguez P.L., Laurière C., Merlot S. (2009). Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R., Schmidt L.K., Lohse M., Hashimoto K., Bock R., Kudla J. (2008). Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 56: 505–516. [DOI] [PubMed] [Google Scholar]
- Wang J.P., Munyampundu J.P., Xu Y.P., Cai X.Z. (2015). Phylogeny of plant calcium and calmodulin-dependent protein kinases (CCaMKs) and functional analyses of tomato CCaMK in disease resistance. Front. Plant Sci. 6: 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak C., Akter S., Jacques S., Huang J., Messens J., Van Breusegem F. (2015). Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 66: 2923–2934. [DOI] [PubMed] [Google Scholar]
- Yang T., Poovaiah B.W. (2003). Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 8: 505–512. [DOI] [PubMed] [Google Scholar]
- Yano K., et al. (2008). CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA 105: 20540–20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zhang H., Ni L., Liu Y., Wang Y., Zhang A., Tan M., Jiang M. (2012). The C2H2-type zinc finger protein ZFP182 is involved in abscisic acid-induced antioxidant defense in rice. J. Integr. Plant Biol. 54: 500–510. [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu Y., Wen F., Yao D., Wang L., Guo J., Ni L., Zhang A., Tan M., Jiang M. (2014). A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defence and oxidative stress tolerance in rice. J. Exp. Bot. 65: 5795–5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Zhu N., Song W.Y., Harmon A.C., Assmann S.M., Chen S. (2014). Thiol-based redox proteins in abscisic acid and methyl jasmonate signaling in Brassica napus guard cells. Plant J. 78: 491–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Yan J., Liu W., Liu L., Sheng Y., Sun Y., Li Y., Scheller H.V., Jiang M., Hou X., Ni L., Zhang A. (2016). Phosphorylation of a NAC transcription factor by a calcium/calmodulin-dependent protein kinase regulates abscisic acid-induced antioxidant defense in maize. Plant Physiol. 171: 1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]