A robust auxin network controls reprogramming toward embryogenesis in suspensor cells via a basic Helix-Loop-Helix transcriptional module, a process involving large transcriptomic changes before anatomical changes.
Abstract
Land plants reproduce sexually by developing an embryo from a fertilized egg cell. However, embryos can also be formed from other cell types in many plant species. Thus, a key question is how embryo identity in plants is controlled, and how this process is modified during nonzygotic embryogenesis. The Arabidopsis (Arabidopsis thaliana) zygote divides to produce an embryonic lineage and an extra-embryonic suspensor. Yet, normally quiescent suspensor cells can develop a second embryo when the initial embryo is damaged, or when response to the signaling molecule auxin is locally blocked. Here we used auxin-dependent suspensor embryogenesis as a model to determine transcriptome changes during embryonic reprogramming. We found that reprogramming is complex and accompanied by large transcriptomic changes before anatomical changes. This analysis revealed a strong enrichment for genes encoding components of auxin homeostasis and response among misregulated genes. Strikingly, deregulation among multiple auxin-related gene families converged upon the re-establishment of cellular auxin levels or response. This finding points to a remarkable degree of feedback regulation to create resilience in the auxin response during embryo development. Starting from the transcriptome of auxin-deregulated embryos, we identified an auxin-dependent basic Helix Loop Helix transcription factor network that mediates the activity of this hormone in suppressing embryo development from the suspensor.
INTRODUCTION
In many land plants, including Arabidopsis (Arabidopsis thaliana), zygotic embryogenesis begins with an asymmetric cell division, generating two cells with distinct fates. The small apical cell is the founder of the pro-embryo and will form most of the plant body. The larger basal cell divides several times and gives rise to the suspensor, a filamentous support structure of which the topmost cell generates part of the seedling root (Laux and Jurgens, 1997; Mayer and Jürgens, 1998). Hence, the suspensor is largely an extra-embryonic, yet zygote-derived structure. In contrast with the pro-embryo, the suspensor is already fully developed at the globular stage and plays an important role in embryo development (Schwartz et al., 1997). The suspensor provides mechanistic and nutritional support required for the growing embryo as well as a connection between the pro-embryo and the maternal endosperm (Raghavan, 2006).
Despite their mitotic quiescence under normal conditions, suspensor cells in several species have the potential to generate a new embryo (Lakshmanan and Ambegaokar, 1984). In Arabidopsis, mutations that impair growth of the pro-embryo (raspberry [rsy], suspensor [sus], and twin [twn]) can cause suspensor proliferation, eventually recapitulating embryogenesis and generating a new pro-embryo (Schwartz et al., 1994; Vernon and Meinke, 1994). Suspensor-derived embryogenesis can also be induced by experimental ablation of the pro-embryo through radiation or chemicals, or through genetic ablation (Haccius, 1955; Weijers et al., 2003) or laser irradiation (Gooh et al., 2015; Liu et al., 2015). These observations reveal the developmental potential of the suspensor to undergo embryonic transformation and proves that its potential is limited by normal growth of the embryo proper. Although regulatory mechanisms underlying this switch in fate are largely unknown, we reported that this process involves the signaling molecule auxin (Rademacher et al., 2012). Components of the auxin response are expressed in suspensor cells (Rademacher et al., 2011, 2012), and when these are inhibited, suspensor cells proliferate, express embryo markers, and develop a second embryo, which can ultimately give rise to twin-like seedlings (Rademacher et al., 2012). At present, it is unclear how direct the involvement of auxin response in embryonic fate conversion is, but it provides a good entry point into studying the regulatory mechanisms underlying suspensor-to-embryo transformation (abbreviated S>E henceforth).
The auxin response is mediated by transcription factors of the AUXIN RESPONSE FACTOR (ARF) family, which are inhibited by interacting Auxin/indole-3-Acetic Acid (Aux/IAA) proteins. In the presence of auxin, Aux/IAA proteins are ubiquitinated by the SKP1-CULLIN1-F-BOX -TRANSPORT INHIBITOR RESISTANT1/AUXIN F-BOX auxin receptor complex (Wang and Estelle, 2014) and marked for degradation. This releases ARFs to regulate the transcription of primary target genes (Lokerse and Weijers, 2009; Wang and Estelle, 2014). Although several target genes mediating the auxin response during embryonic root initiation (Schlereth et al., 2010; Crawford et al., 2015), lateral root development (Okushima et al., 2007; De Rybel et al., 2010), and flower development (Zhao et al., 2010; Yamaguchi et al., 2013) have been isolated, their role in maintaining suspensor identity is not yet clear. Here, we used auxin-dependent, suspensor-derived embryogenesis (Radoeva and Weijers, 2014) to identify molecular components that mediate this important cell fate transformation.
Through genome-wide transcriptional profiling upon local inhibition of the auxin response, we discovered a convergent misregulation of 39 genes involved in auxin homeostasis and response that collectively re-establish auxin activity. Following an expression pattern analysis during embryogenesis of a selected subset of differentially expressed genes, we identified a set of basic Helix Loop Helix (bHLH) genes that is regulated during suspensor-derived embryogenesis. Previously, bHLH transcription factors have been identified as direct ARF target genes (Schlereth et al., 2010; De Rybel et al., 2013;). Our work suggests a role for bHLH49 as a key mediator in controlling the developmental potential of the suspensor.
RESULTS
Transcriptional Analysis of Suspensor Reprogramming
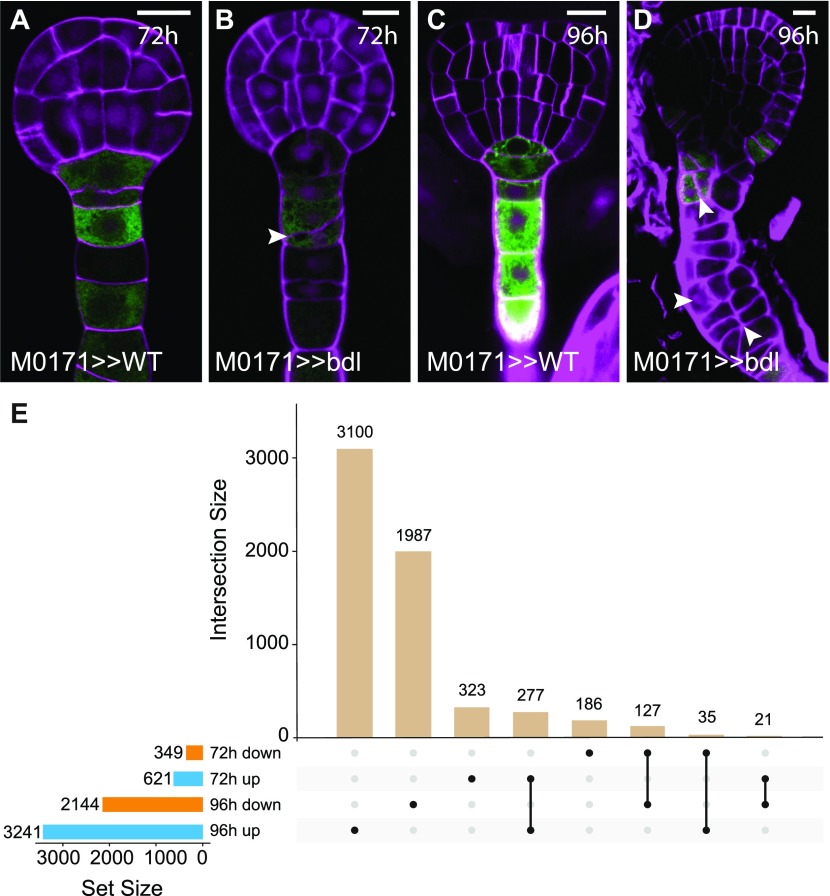
Expression of the mutated, stabilized transcriptional auxin response inhibitor protein iaa12/bodenlos (bdl) in suspensor cells efficiently induces a switch from extra-embryonic to embryonic identity (Rademacher et al., 2012). We used this predictable, uniform response to identify genes whose expression changes during this fate transition. IAA12/BDL protein is normally degraded in response to auxin (Dharmasiri et al., 2005), but a Pro74Ser mutation in the BDL protein prevents degradation and leads to accumulation of this ARF inhibitor (Hamann et al., 2002; Dharmasiri et al., 2005). We expressed mutant bdl protein using the two-component Galactose-induced gene 4/Upstream Activation Sequence (GAL4/UAS) system (Weijers et al., 2006). The GAL4 driver line M0171 is active in suspensor cells (Figure 1) until the heart stage (Figure 1A), after which GAL4 expression expands to include cells in the pro-embryo (Rademacher et al., 2012; Radoeva et al., 2016). By crossing homozygous M0171 and UAS-bdl plants, suspensor proliferation could be induced in most embryos at the heart stage (83%; n = 126). For whole-genome transcriptomic analysis, a time point after pollination should be selected, which is after the onset of M0171 expression and before the appearance of phenotypic abnormalities. We found that by 72 h, about one-third of suspensors showed a first aberrant division (Figures 1A and 1B; 31%; n = 149) and selected this as the first time point for transcriptomic profiling. We also included a 96-h time point because by this time, most suspensors vigorously proliferated (Figures 1C and 1D; 86%; n = 109).
Figure 1.
Selection of Time Points for Transcriptional Analysis of M0171>>bdl Embryos.
(A) to (D) Embryos from crosses between M0171 and wild type [(A) and (C)] or M0171 and UAS-bdl [(B) and (D)] prepared 72 h [(A) and (B)] or 96 h after pollination [(C) and (D)]. GFP expression reflects the activity of the M0171 enhancer trap, which drives the expression of GAL4:VP16, which in turn activates the linked UAS-erGFP gene. Images show overlay of GFP and cell wall (counterstained with Renaissance RS2200-magenta) signals. Scale bar represents 10 μm in all panels. Arrowheads in (B) and (D) indicate aberrant cell divisions in suspensor cells.
(E) UpSet plot showing overlap between differentially expressed genes (up- or downregulated relative to wild type) in M0171>>bdl embryos at 72 h and 96 h after pollination. Numbers indicate the number of common genes in each comparison.
M0171 plants were pollinated with UAS-bdl pollen (M0171>>bdl), embryos were manually dissected after 72 and 96 h, and 300 to 400 individuals per biological replicate were pooled for RNA extraction. In parallel, M0171 plants were pollinated with Columbia-0 (Col-0) wild-type pollen to serve as isogenic wild-type controls (M0171>>Col-0) in whole-genome transcript profiling. After statistical analysis, we identified 621 and 349 genes that were robustly up- or downregulated in M0171>>bdl 72 h embryos compared with the control, respectively (>2-fold change in gene expression; significance at false discovery rate [FDR] < 0.05; Figure 1E; Supplemental Data Set 1). By contrast, in M0171>>bdl 96-h embryos, 3421 genes were upregulated and 2144 genes were downregulated (Figure 1E). Because the number of misregulated genes in the M0171>>bdl 96-h embryos equals one-quarter of the Arabidopsis genome and probably includes secondary and tertiary transcriptional responses, our analysis was focused on the 72-h M0171>>bdl transcriptome.
Due to the large number of misregulated genes, we did not identify obvious enrichment of functional categories or known regulators in Gene Ontology analysis. Nonetheless, this transcriptome analysis revealed massive genome-wide transcriptional reprogramming associated with the induction of proliferation and embryogenesis in the suspensor well before its morphological manifestation.
Convergent Regulation of Auxin Homeostasis
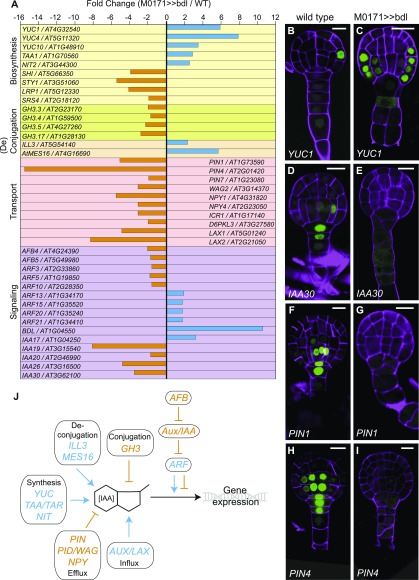
Initial inspection of the M0171>>bdl transcriptome confirmed an expected (10.6-fold) upregulation of BDL/IAA12. Conversely IAA30, whose suspensor-specific expression (at earlier stages of embryogenesis) is lost in the pARF13:iaa10 background (Rademacher et al., 2012), is 3.5-fold downregulated, confirming the validity of the M0171>>bdl data set. Further analysis revealed that many other genes involved in auxin homeostasis and signaling were misregulated because of the targeted inhibition of the auxin response in the suspensor. In addition to BDL and IAA30, several other members of the Aux/IAA family (IAA19, 20, 26) were also strongly downregulated in the M0171>>bdl data set (Figure 2). A strong enrichment for genes encoding core factors and regulators of auxin biosynthesis, transport, (de)conjugation pathways, and response was observed as well (Figure 2A). Interestingly, the expected effect of expression changes of single genes would be an increase in intracellular auxin levels. Specifically, family members of three of the four Trp-dependent auxin biosynthesis pathways (YUCCA1 [YUC1], 4, 10; TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1; NITRILASE2) were strongly upregulated in the M0171>>bdl data set (Figure 2A), suggesting an increase in auxin concentration as a result of the inhibited auxin response. Furthermore, four GRETCHEN HAGEN3 (GH3) family members (GH3.3, 3.4, 3.5, 3.17) encoding auxin-conjugating enzymes were downregulated, whereas two genes that encoding hydrolases involved in auxin deconjugation (IAA-amino acid hydrolase-IRL1-like 3 and ARABIDOPSIS THALIANA METHYL ESTERASE16) were upregulated. The net effect of the latter would also likely be elevated free auxin levels. This interrelationship between positive and negative misregulation is further toned up by the downregulation of 10 auxin transport-associated genes found in our data set (Figure 2A).
Figure 2.
Convergent Regulation of Auxin Homeostasis Genes.
(A) Misregulated auxin homeostasis and core signaling genes in the M0171>>bdl data sets (in fold change M0171>>bdl/M0171>>wild type). Genes are grouped per category [auxin biosynthesis, (de)conjugation, transport, and signaling].
(B) to (I) Expression of promoter-n3GFP reporters for YUC1 [(B) and (C)], IAA30 [(D) and (E)], PIN1 [(F) and (G)], and PIN4 [(H) and (I)] in wild type [(B), (D), (G), (H)] and M0171>>bdl [(C), (E), (G), (I)] embryos ∼72 h after pollination. Cell walls in all images are counterstained with Renaissance RS2200 (magenta signal). Scale bar represents 10 μm in all panels.
(J) Schematic overview of convergent regulation of auxin homeostasis genes. Blue arrows represent upregulation in the M0171>>bdl data set and positive effect on IAA concentration or the auxin response; orange bars represent downregulation in the M0171>>bdl data set and negative effect on IAA concentration or the auxin response.
To address the spatial aspects of the apparent convergent regulation of auxin homeostasis and response upon targeted inhibition of the auxin response, we investigated the expression patterns of genes encoding the auxin biosynthesis enzyme YUC1, the Aux/IAA family member IAA30, and two auxin efflux carriers (PIN-FORMED1 [PIN1] and PIN4) in the M0171>>bdl background. Normally, YUC1 is expressed in the protoderm at the globular stage of embryogenesis, but in M0171>>bdl embryos, it was more broadly expressed, also including the inner cells of the proembryo (Figures 2B and 2C). These results indicate that auxin biosynthesis increases after the perceived depletion of auxin, and they confirm the upregulation of YUC1 in the M0171>>bdl data set. During embryogenesis, as previously described (Rademacher et al., 2012), IAA30 is expressed in the suspensor cells as well as the lower tier of the pro-embryo, which are known sites of the auxin response. In M0171>>bdl embryos, no IAA30 expression could be detected (Figures 2D and 2E), which confirms that the transcriptional auxin response is indeed inhibited in these embryos. Similarly, the expression of the two auxin efflux carrier genes PIN1 and PIN4, which is normally found in both the suspensor and the lower tier of the pro-embryo, was also lost from both embryonic expression domains (Figures 2F and 2I). These results suggest that an inhibited auxin response in the suspensor results in impaired auxin transport as well as an impaired auxin response throughout the embryo.
Collectively, the misregulation of more than 10 gene families involved in auxin homeostasis occurs in such a way that it appears that the system is responding to inhibition of the auxin response by triggering increased auxin biosynthesis, decreased auxin transport, and altered auxin responsiveness (Figure 2J). Based on the misregulation of auxin homeostasis and transport genes, we predicted that auxin levels should be higher in M0171>>bdl embryos versus the wild type. We tested this prediction experimentally using the ratiometric auxin sensor R2D2 (Ratiometric version of two Domain II’s; Liao et al., 2015). This sensor combines auxin-sensitive pRPS5A-driven DII-n3Venus and auxin-insensitive pRPS5A-driven mDII-ntdTomato (nuclear tandem dimeric Tomato) as an internal control (Figure 3; Liao et al., 2015). The mDII/DII ratio provides a measure of cellular auxin levels irrespective of whether auxin triggers gene expression changes. As predicted, the R2D2-derived auxin levels strongly increased in M0171>>bdl embryos compared with wild type (Figures 3A, 3B, 3G, and 3H). We next tested whether the excess auxin leads to an increased auxin response, which would be counterintuitive. For this, we used the dual auxin-responsive reporter DR5/DR5v2. This reporter harbors the classical DR5 and highly sensitive DR5v2 reporters, each driving a different fluorescent protein, on the same transgene locus (Liao et al., 2015). Normally, DR5v2 is expressed in the first suspensor cells at the globular stage of embryogenesis and is activated in the cotyledon tips and the vasculature later on at the heart stage (Figures 3C to 3F). Instead, DR5v2 expression was completely absent from M0171>>bdl embryos up to the heart stage (Figures 3C to 3E and 3I to 3K), confirming that the phenotypic embryos lacked an auxin response. Strikingly, later on during embryogenesis, at the torpedo stage, DR5v2 expression was re-established and observed throughout the embryo (Figures 3F and 3L). Thus, local suppression of the auxin response causes systemic changes in auxin homeostasis at many levels and involving many gene families. This systemic change induces a strong increase in cellular auxin levels, but not in the auxin response. These results point to genetic wiring of the auxin network aimed at high resilience to perturbation. The results also indicate that inhibiting the auxin response in the context of the embryo has strong noncell autonomous effects.
Figure 3.
Auxin Levels and Response upon Inhibition of the Suspensor-Specific Response.
(A), (B), (G), and (H) Ratio of ntdTomato / n3xVenus fluorescence of the R2D2 reporter in wild type [(A) and (B)] and M0171>>bdl embryos [(G) and (H)]. This ratio is displayed as false color according to scale in the bottom, reflecting low to high auxin levels.
(C) to (F) and (I) to (L) Expression of DR5-n3GFP/DR5v2-ntdTomato reporter in wild type [(C) to (F)] and M0171>>bdl embryos [(I) to (L)]. Cell walls in all images are counterstained with Renaissance RS2200 (gray signal). Scale bar represents 10 μm in all panels.
Identification of Transcriptional Regulators during Suspensor Reprogramming
Suspensor-specific inhibition of the auxin response in M0171>>bdl embryos induces transcriptional reprogramming that leads to systemic rewiring of auxin homeostasis, eventually leading to the proliferation of suspensors and the development of embryo-like characteristics in proliferated suspensors. To address whether the initiation of these embryo-like properties is also detectable at the transcriptional level, we surveyed the expression of genes known to promote the acquisition of embryonic identity in other systems (Radoeva and Weijers, 2014). Of these, two are differentially expressed in the M0171>>bdl data set. LEAFY COTYLEDON1-LIKE, also known as NUCLEAR FACTOR-Y B6, encoding a subunit of the NUCLEAR FACTOR-Y transcription factor complex, is able to induce embryo development in somatic cells when overexpressed (Lotan et al., 1998; Kwong et al., 2003). LEAFY COTYLEDON1-LIKE is 2.5-fold upregulated in our data set. Another factor implicated in somatic embryogenesis that is 1.9-fold upregulated in our data set is FUSCA3, encoding a B3 domain transcription factor that induces late embryo properties in somatic cells after ectopic expression (Gaj et al., 2005). Moreover, SHOOT MERISTEMLESS, an important embryo marker (Long et al., 1996) that is expressed in proliferating suspensor cells (Rademacher et al., 2012), is 2.3-fold upregulated in the M0171>>bdl data set, whereas the CLAVATA3 gene, which is expressed in the shoot apical meristem (Fletcher et al., 1999), was 45-fold upregulated. Hence, even before morphological changes are evident, the M0171>>bdl transcriptome reveals aspects of reprogramming toward embryo identity.
To identify new components in the control of suspensor proliferation and/or the induction of embryo identity, we selected 68 genes that were robustly misregulated in M0171>>bdl embryos (Supplemental Table). Selection was based on (1) the amplitude of misregulation (Fold-change M0171>>bdl versus M0171>>Col-0), (2) functional annotation (transcription factors, signaling components), and (3) known auxin-responsiveness (using public microarray data). All genes were at least twofold misregulated, and 19 genes were among the 10% most strongly misregulated genes. Twenty-two of the selected genes were identified as auxin-responsive in the Arabidopsis Hormone Database 2.0 (including one Aux/IAA), and 33 encode transcription factors (Plant Transcription Factor Database v2.0; Zhang et al., 2011), 20 of which belong to auxin-responsive transcription factor families (Paponov et al., 2008).
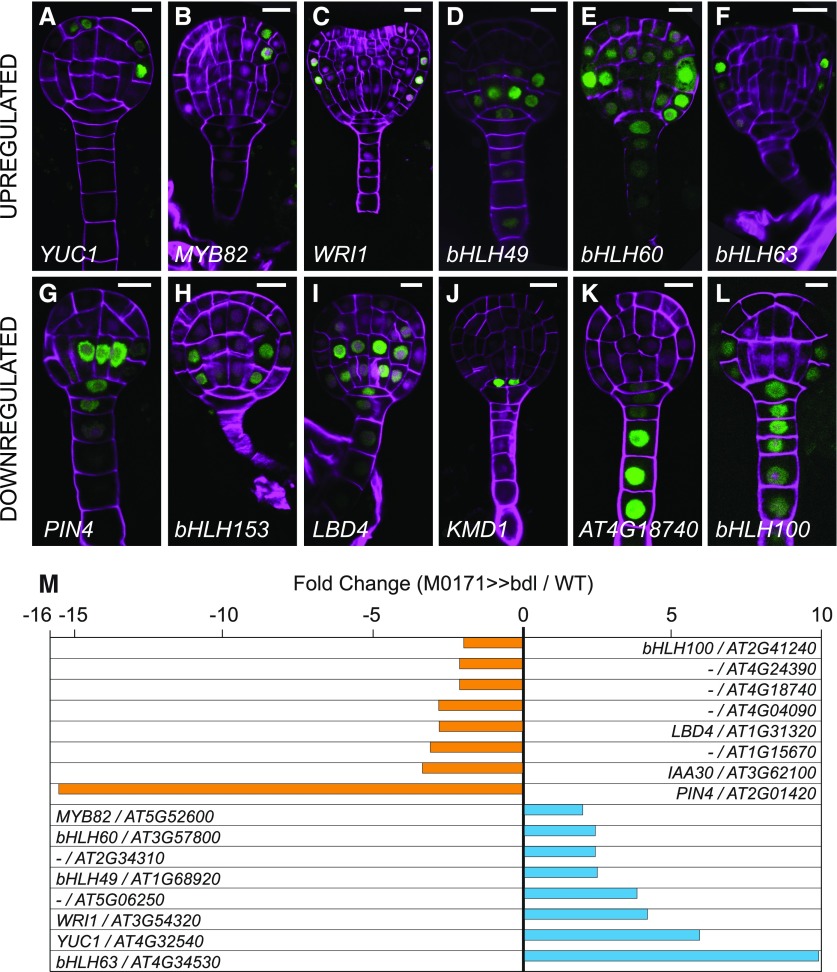
We generated promoter-reporter lines for these 68 genes using a sensitive nuclear-localized 3x green florescent protein (n3GFP) to assess their embryonic expression domains. We analyzed the patterns of expression in T2 generation wild-type embryos in up to eight independent primary transformants for all genes, but expression in the embryo and/or suspensor was only detected for 40 genes. The expression pattern of these 40 genes was further confirmed in the T3 generation in at least two representative lines. Because our microarray analysis was aimed at identifying genes that are misregulated as part of suspensor proliferation and/or embryo identity induction, we predict that, ideally, upregulated genes are normally expressed in embryo cells and downregulated genes in suspensor cells. Of the 40 genes that were expressed during embryogenesis, 24 had broader expression domains, including both the pro-embryo and suspensor, which were inconsistent with our predictions (Figures 4H and 4J; Supplemental Table). The 16 remaining genes conformed to the aforementioned criteria (Figure 4). Of these, eight were upregulated and expressed in the pro-embryo (Figures 4A to 4F and 4M), whereas eight were downregulated and expressed in the suspensor (Figures 4G, 4I, and 4K to 4M). Notably, this subset of validated genes contained four genes encoding bHLH transcription factors (Figures 4D to 4F and 4L). bHLHs are well-known regulators of cell identity in multicellular organisms (Murre et al., 1994), including plants (Feller et al., 2011). Importantly, other bHLHs were previously shown to mediate auxin-dependent development (Chandler et al., 2009; Schlereth et al., 2010; De Rybel et al., 2013). In particular, the bHLH genes TARGET OF MONOPTEROS5 (TMO5) and TMO7 are both activated by auxin in an ARF5-dependent manner and contribute to auxin-dependent vascular tissue and embryonic root development (Schlereth et al., 2010; De Rybel et al., 2013). We therefore focused our analysis on these genes as potential regulators of suspensor proliferation.
Figure 4.
Expression Patterns of Genes Misregulated in M0171>>bdl Embryos.
(A) to (L) Expression of promoter-n3GFP reporters for YUC1 (A), MYB82 (B), WRI1 (C), bHLH49 (D), bHLH60 (E), bHLH63 (F), PIN4 (G), bHLH153 (H), LBD4 (I), KMD1 (J), AT4G18740 (K), and bHLH100 (L). All images show expression in the globular stage, except (C), which shows expression in the heart stage. Images (A) to (F) show expression of genes upregulated, whereas images (G) to (L) show expression of genes downregulated in the M0171>>bdl data set. Cell walls in all images are counterstained with Renaissance RS 2200 (magenta signal). Scale bar represents 10 μm in all panels.
(M) Differential expression (in fold change M0171>>bdl/M0171>>wild type) of 16 genes conforming to the selection criteria discussed in the text.
bHLH Genes Are an Output of the Embryonic Auxin Response
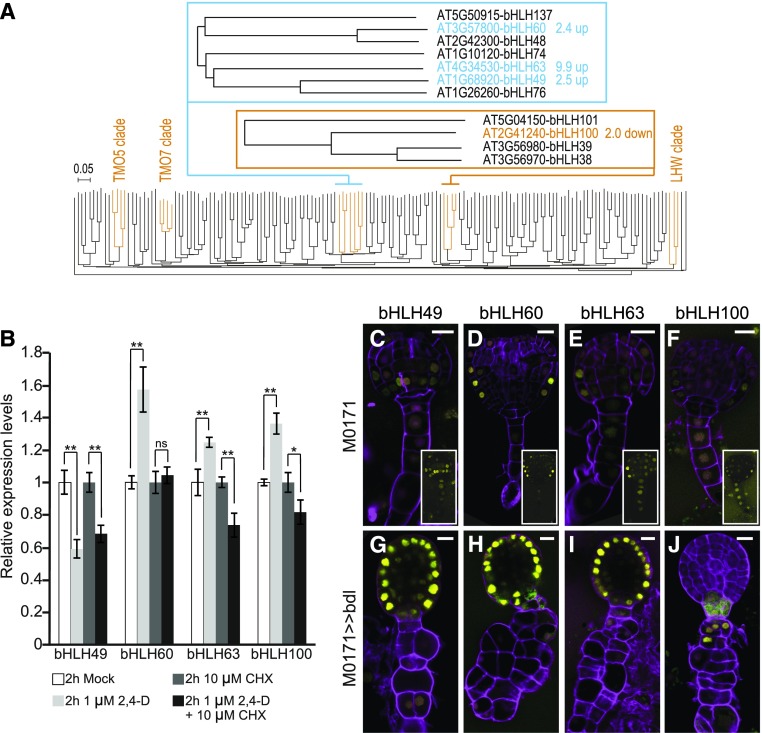
The bHLH genes identified here (bHLH49, 60, 63, and 100; Figure 5) belong to two different clades, 12 and 25 (Figure 5A; Supplemental File). Although a postembryonic function has been described for bHLH63, also known as CRYPTOCHROME-INTERACTING bHLH1 (Liu et al., 2008) and bHLH100 (Wang et al., 2007; Sivitz et al., 2012; Andriankaja et al., 2014), no embryonic function has been reported for any of these genes. bHLH49 was expressed in the basal tier of the embryo, and weak expression was also detected in the suspensor (Figure 4D). bHLH60 was expressed in the outer cells of the pro-embryo, and weak transient expression was detected in the inner cells of the pro-embryo and suspensor (Figure 4E). bHLH63 had a very specific expression pattern, limited to the protoderm in the junction between the apical and basal tier of the pro-embryo (Figure 4F). These largely pro-embryo–enriched expression patterns are consistent with their upregulation in the microarray data set. Consistent with its downregulation in the M0171>>bdl microarray, bHLH100 showed suspensor-specific expression until the globular stage, after which the domain extended to protodermal cells in the basal tier (Figure 4L).
Figure 5.
Auxin-Dependent Expression of bHLH Genes.
(A) Phylogenetic tree of Arabidopsis bHLH proteins, indicating the divergent positions of the TMO5, TMO7, and LHW clades relative to the misregulated bHLH genes. The misregulation of genes in M0171>>bdl embryos is indicated in the top panels.
(B) Relative expression levels of bHLH49, 60, 63, and 100 in roots upon treatment with 1 μM 2,4-D, 10 μM CHX or both, for 2 h. Expression levels in untreated wild-type (or mock treated samples) were set to 1. Error bars indicate se; t test: *P < 0.05, **P < 0.001. Reactions were done in triplicate, with three biological replicates (representing separate experiments).
(C) to (J) Expression of promoter-nVenus reporters for bHLH49 [(C) and (G)], bHLH60 [(D) and (H)], bHLH63 [(E) and (I)], and bHLH100 [(F) and (J)] in wild type [(C) to (F)] and M0171>>bdl embryos [(G) to (J)]. Insets in (C) to (F) show the Venus signal at increased brightness. Magenta counterstaining in all images is Renaissance RS2200. Scale bar indicates 10 μm in all panels.
Although some bHLH proteins act cell autonomously in the cells where their underlying gene is transcribed (e.g., TMO5; Schlereth et al., 2010), others act non cell autonomously by moving to adjacent cells (e.g., TMO7; Schlereth et al., 2010; UPBEAT1; Tsukagoshi et al., 2010). To determine whether the bHLH proteins encoded by the genes identified here are mobile, we generated translational fusions of genomic fragments fused to sensitive sYFP protein. Consistent with their function as transcription factors, all four bHLH proteins localized to the nucleus. Protein localization domains exactly matched the promoter expression domains (Supplemental Figure 1), demonstrating that these proteins likely do not move and that protein accumulation is transcriptionally controlled.
These bHLH genes were identified based on their misexpression upon inhibition of the auxin response and were expressed in the relevant cell types within the embryo. We next examined whether the bHLH genes were indeed rapidly regulated by auxin. We first tested the effect of exogenous auxin on transcript levels in seedling roots. bHLH60, 63, and 100 were upregulated after 2 h of auxin treatment (Figure 5B). By contrast, bHLH49 was downregulated, suggesting that all four bHLHs are indeed regulated by auxin. In addition, to test whether this auxin-dependent bHLH regulation is direct, we performed a 2 h auxin treatment in the presence of cycloheximide (CHX). CHX is an inhibitor of protein biosynthesis and in its presence, only direct transcriptional regulation should occur (Franco et al., 1990). Auxin-dependent repression of bHLH49 expression was still observed in the presence of CHX, which strongly suggests that bHLH49 is directly regulated by auxin (Figure 5B). By contrast, the activation of bHLH60, 63, and 100 was suppressed by CHX, suggesting that the auxin-induced regulation of these genes does not represent a direct transcriptional response (Figure 5B).
The auxin-dependent expression of these four bHLH genes and their misregulation in M0171>>bdl embryos suggested that these bHLH proteins might play a role in actual cell fate transformation. To determine whether this is indeed the case, we analyzed the expression of all four bHLHs in M0171>>bdl embryos. The expression of the downregulated genes in M0171>>bdl can only be affected within their normal expression domain. Consistent with its 2.0-fold downregulation in the microarray, the expression of bHLH100 was absent from almost all excessively dividing suspensor cells (Figures 5F and 5J), suggesting that these cells have lost their original identity. Upregulation in the M0171>>bdl transcriptome could either reflect enhanced or ectopic expression. Consistent with the transcriptome data, the expression of bHLH49, bHLH60, and bHLH63 was strongly enhanced in M0171>>bdl embryos (Figures 5G to 5I). Strikingly, however, their expression was not activated in the suspensor, but it was instead enhanced in the pro-embryo, notably in the L1 layer. Therefore, in contrast with the simple inference that suppression of auxin response in suspensor cells cell autonomously de-regulates bHLH49, 60, and 63, their misregulation in M0171>>bdl embryos appears to be noncell autonomous and likely the consequence of systemic effects on auxin homeostasis and response. In the case of bHLH60 and 63, the regulatory effect of auxin appears to be indirect, because no regulation was observed in roots treated with auxin in the presence of CHX. bHLH49, however, is a direct auxin response gene, because it was downregulated upon auxin treatment to roots. Therefore, rather than being locally regulated by auxin in suspensor cells, bHLH49 is likely upregulated as a consequence of systemic suppression of the auxin response in M0171>>bdl embryos. Thus, the S>E transformation is a multi-step process that involves intensive communication between the suspensor and pro-embryo and includes an auxin response in both cell types.
bHLH Genes Help Establish the Suspensor-Embryo Junction
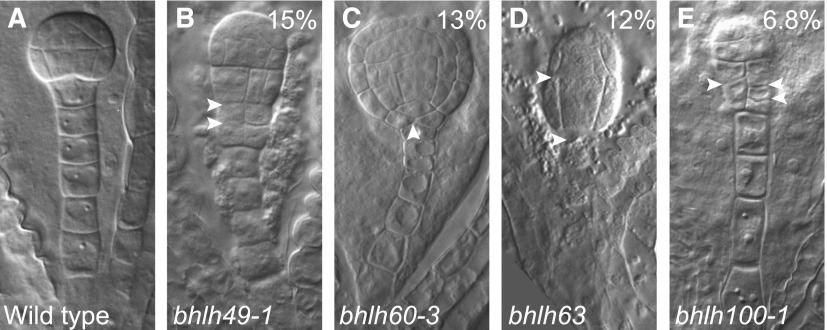
To determine whether bHLH49, 60, 63, and 100 contribute to zygote-derived embryogenesis, we identified and characterized insertion mutants (Figure 6) with strongly reduced transcript levels (Supplemental Figure 2). Strikingly, all single mutants showed embryo defects, with frequencies ranging from 7% to 15% (n > 100; Figures 6A to 6E) that were distinguishable from the globular stage onward. Although these defects were incompletely penetrant, these rates are highly significant given the 1.7% (n = 120) of comparable defects observed in the wild type. These phenotypes appear to be the consequence of bHLH mutations, rather than unlinked mutations, because the same phenotypes were found in multiple independent alleles for bHLH49 and bHLH100 (bhlh49-1: 15%, n = 137; bhlh49-2: 25%, n = 83; bhlh100-1: 6.8%, n = 118; bhlh100-2: 22%, n = 101), and the mutant phenotype of the bhlh49 alleles was rescued by introducing pbHLH49:bHLH49-tdTomato (bhlh49-1: 5.5%, n = 199; bhlh49-2: 4.95%, n = 202). Regardless of the rather broad expression domains of the genes, the phenotype of the loss-of-function mutants was specific to the suspensor and/or the hypophysis (topmost cell of the suspensor), suggesting these genes contribute to normal suspensor and/or hypophysis development. In the bhlh49-1 and bhlh100-1 mutants, proliferative divisions were observed at the suspensor-embryo junction. It could not be unequivocally determined whether the primary defect originated in the pro-embryo or the suspensor (Figures 6A, 6B, and 6E). bhlh60-3 showed abnormal hypophyseal cell divisions, whereas marginal proembryo defects were observed in bhlh63 (Figures 6A, 6C, and 6D). Nonetheless, these results suggest that these bHLH genes, especially bHLH49 and bHLH100, are required for normal embryo development, particularly for the embryo-suspensor junction.
Figure 6.
bHLH Genes Help Establish the Suspensor-Embryo Junction.
(A) to (E) Embryo phenotypes of wild-type (A) and mutants in bHLH49 (B), bHLH60 (C), bHLH63 (D), or bHLH100 (E). Percentages indicate penetrance of phenotypes in homozygous mutants (bhlh49-1: 15%, n = 137; bhlh60-3: 13%, n = 265; bhlh63: 12%, n = 115; bhlh100-1: 6.8%, n = 118). Arrowheads indicate abnormal cell divisions.
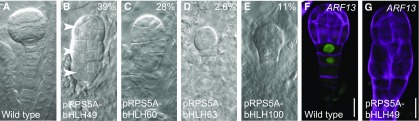
Although the loss-of-function phenotype suggests these genes are required for normal embryo development, it is unclear whether their upregulation contributes to suspensor proliferation and/or embryo transformation in M0171>>bdl embryos. To determine whether their upregulation contributes to abnormal suspensor development, we misexpressed each gene individually (Supplemental Figure 2B) using the RPS5A promoter (Figure 7), which drives strong gene expression throughout the embryo, including the suspensor (Weijers et al., 2001). Analysis of up to four independent lines per construct revealed that bHLH49 and bHLH60 were able to induce severe suspensor phenotypes, whereas bHLH63 and 100 were less able to do so (Figures 7A to 7E). In particular, pRPS5A-bHLH49 embryos showed proliferated suspensors with an embryo-like appearance (39%, n = 185); in some cases, three pro-embryo–like structures were stacked on top of each other (arrowheads, Figure 7B). Consistent with the phenotypic appearance, the suspensor-specific expression of pARF13-n3GFP (Rademacher et al., 2011) was lost in the proliferating suspensor cells (Figures 7F and 7G). It is therefore conceivable that bHLH49 is by itself sufficient to induce the loss of suspensor identity along with proliferative cell divisions in the suspensor. Thus, limiting the expression of bHLH49 is important for extra-embryonic cell identity specification.
Figure 7.
Overexpression of bHLH49 Affects Suspensor and Embryo Development.
(A) to (E) Embryo phenotypes of wild-type (A), pRPS5A-bHLH49 (B), pRPS5A-bHLH60 (C), pRPS5A-bHLH63 (D), and PRPS5A-bHLH100 (E).
(F) and (G) Expression of pARF13-n3GFP in wild-type (F) and pRPS5A-bHLH49 (G). Arrowheads in (B) indicate the pro-embryo and pro-embryo–like structures. Percentages indicate penetrance of phenotypes in overexpression lines (pRPS5A-BHLH49: 39%, n = 185; pRPS5A-bHLH60: 28%, n = 128; pRPS5A-bHLH63: 2.6%, n = 117; pRPS5A-bHLH100: 11%, n = 175). Magenta counterstaining in (F) and (G) is Renaissance RS2200. Scale bar = 10 μm in all panels.
The analysis of bHLH49 expression in M0171>>bdl embryos showed that its upregulation was restricted to pro-embryo cells (Figure 5G). We therefore addressed the spatial requirements of bHLH49 misexpession for the induction of the misexpression phenotype. Because of the lack of a reliable promoter that is solely expressed in the entire pro-embryo, yet absent from the suspensor, our analysis was limited to suspensor-specific misexpression. We used the suspensor-specific driver line M0171 and another suspensor-specific promoter (pARF13; Figure 7F; Rademacher et al., 2011) to express bHLH49. We found that suspensor-specific expression of bHLH49 did not lead to excessive divisions in the suspensor cells, as observed in pRPS5A-bHLH49 (M0171>>bHLH49: 0%, n = 181; pARF13-bHLH49: 0%, n = 150). This result suggests that local regulation of bHLH49 in the suspensor does not contribute to its misexpression phenotype and is consistent with its upregulation in M0171>>bdl pro-embryos to be causal to altered development.
bHLH49 Mediates Postembryonic Auxin-Dependent Growth
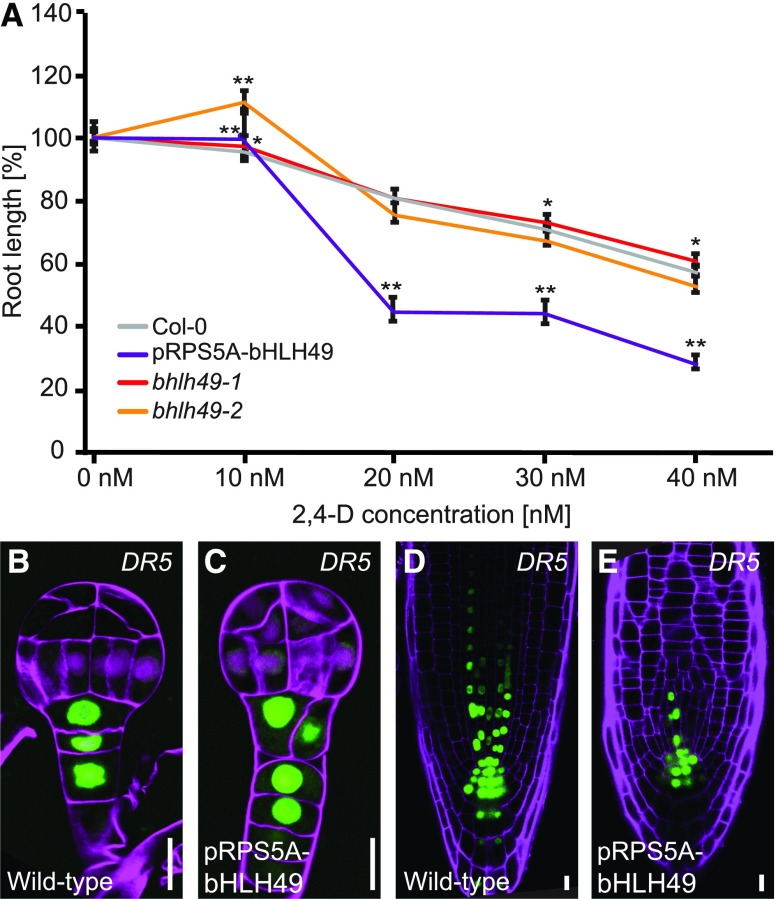
The auxin-dependent bHLH49 gene is required for normal embryo development, and its misexpression results in the loss of suspensor identity and the formation of embryo-like structures in the suspensor. In contrast with the other three bHLHs, post-embryonic developmental phenotypes of plants overexpressing bHLH49 were also observed (Supplemental Figure 3). The defects observed include short, almost empty siliques (Supplemental Figures 3A and 3B), roots with abnormal cell division, and distorted root apical meristems (Supplemental Figures 3C and 3D). Rare defects included the absence of flowers (Supplemental Figure 3E) and defective cotyledon development phenotypes such as cup-shaped or monocotyledonous seedlings or very distorted seedlings with no cotyledons and a rudimentary root (Supplemental Figure 3F to 3H) reminiscent of auxin-related phenotypes (Hardtke and Berleth, 1998; Hamann et al., 2002). To determine whether bHLH49 function contributes to the developmental output of auxin signaling, we first examined auxin sensitivity in bhlh49 mutants and in the pRPS5A-bHLH49 misexpression lines (Figure 8). Because no facile auxin sensitivity assay for embryos is currently available, this was performed in postembryonic roots, in which bHLH49 is expressed in an auxin-dependent manner (Figure 5B). Changes in root growth inhibition by the synthetic auxin 2,4-D can be quantified and used to determine the auxin sensitivity (Lincoln et al., 1990). Our results show that auxin sensitivity was greatly enhanced in pRPS5A-bHLH49 roots (Figure 8A), whereas bhlh49 mutant roots did not show a clear change in sensitivity (Figure 8A). Hence, bHLH49 is not only regulated by auxin, but its repression is also required for a normal auxin response during root growth. To determine whether auxin treatment affects the bHLH49 misexpression phenotype, we observed root tips of the RPS5A-bHLH49 line grown on various auxin concentrations. We found that auxin treatment did not obviously modify the disorganized primary roots in this line (Supplemental Figures 4A to 4E, 4H, and 4I).
Figure 8.
bHLH49 Mediates Postembryonic Auxin-Dependent Growth.
(A) Root length of wild type (Col-0), pRPS5A-bHLH49, bhlh49-1, and bhlh49-2 seedlings upon treatment with 10 nM, 20 nM, 30 nM, and 40 nM 2,4-D, compared with untreated control (t test: *P < 0.05; **P < 0.001). Error bars indicate sd in three separate experiments where at least 30 and up to 50 seedlings per genotype were used for the measurement.
(B) to (E) Expression of DR5-n3GFP in wild-type [(B) and (D)] and pRPS5A-bHLH49 [(C) and (E)] globular stage embryos [(B) and (C)] and roots [(D) and (E)]. Magenta counterstaining in (B) and (C) is Renaissance RS2200 and in (D) and (E) it is Propidium iodide. Scale bar = 10 μm in all panels.
Finally, because auxin activity is characterized by many feedback loops (Figure 2J; Benjamins and Scheres, 2008; Jones et al., 2010; Leyser, 2010), we determined whether bHLH49 expression is only an output of auxin-induced regulation, or whether it feeds back into auxin activity. We introduced the pDR5-GFP auxin response reporter (Ulmasov et al., 1997; Friml et al., 2003) into pRPS5A-bHLH49 misexpression lines and found no disruption in the DR5-GFP expression pattern (Figures 8B to 8E), even after auxin treatment (Supplemental Figures 4F, 4G, 4M, and 4N), despite the clear developmental defects in this line, implying that there is no feedback from bHLH49 activity to the auxin response.
bHLH49 Mediates Auxin-Dependent Regulation of Gene Expression in Suspensors
When overexpressed, bHLH49 is able to induce proliferative cell divisions and (subsequent) suspensor identity loss, resembling the suspensor-specific bdl misexpression. To determine whether increased bHLH49 expression contributes to the gene expression program mediating embryo initiation, we performed whole-genome transcript profiling on bhlh49 mutant and pRPS5A-bHLH49 overexpression lines. Ideally, this should be done on embryonic tissue; however, given the severe embryo defects of pRPS5A-bHLH49 (Supplemental Figure 3), we could not collect enough embryos and used root tips instead. We found 237 genes to be differentially expressed when comparing bhlh49 versus wild type, and 1216 gene when comparing pRPS5A-bHLH49 versus wild type (>1.3-fold misregulated; FDR < 0.05).
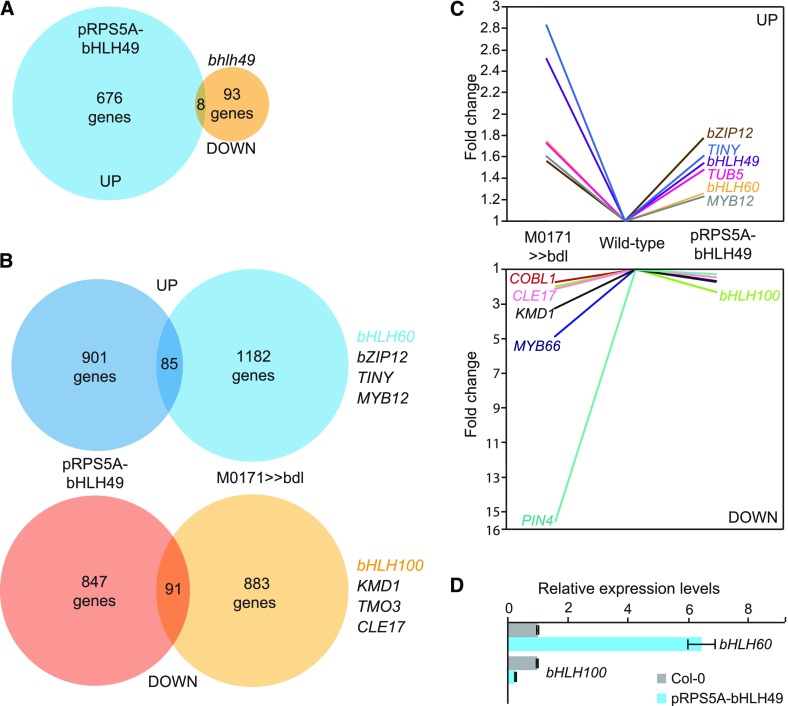
We then performed a meta-analysis to determine whether the upregulation of bHLH49 contributes significantly to auxin-dependent suspensor proliferation (Figure 9). This meta-analysis revealed a significant overlap between the genes that were differentially expressed in either RPS5A-bHLH49 roots or M0171>>bdl embryos. We found 85 and 91 genes to be up- and downregulated, respectively, in both array experiments (>1.3-fold; P < 3.5e-06 for upregulated genes and P < 4.0e-14 for downregulated genes; Figures 9B and 9C). Interestingly, bHLH60 and bHLH100 were 1.3-fold up- and 2.3-fold downregulated, respectively, in the RPS5A-bHLH49 data set. This recapitulates their misregulation in M0171>>bdl embryos, and together with the finding that bHLH60 and bHLH100 were not immediate auxin targets (Figure 5B) implies that auxin-dependent regulation of bHLH60 and 100 is mediated by bHLH49. The latter was also confirmed by quantitative RT-PCR (qRT-PCR; Figure 9D). Thus, bHLH49 appears to be an important mediator of the auxin-dependent suppression of embryo identity in suspensor cells.
Figure 9.
bHLH49 Mediates Auxin-Dependent Regulation of Gene Expression in Suspensors.
(A) Venn diagram depicting the number of genes upregulated in pRPS5A-bHLH49 and downregulated in bhlh49 root tips.
(B) Venn diagrams showing the overlap in genes either up- or downregulated in pRPS5A-bHLH49 seedling roots and M0171>>bdl embryos. Examples of genes present in the overlap are listed on the right.
(C) Expression levels of selected genes in M0171>>bdl embryos and pRPS5A-bHLH49 seedling roots in micro-array experiments.
(D) qRT-PCR validation of the expression levels of bHLH60 and bHLH100 in wild-type (Col-0) and pRPS5A-bHLH49 seedling roots. Expression levels in wild-type samples were set to 1. Error bars indicate se. Three technical and three biological replicates (representing separate experiments) were performed.
DISCUSSION
The plant suspensor plays a supportive role during embryogenesis and is mitotically quiescent after three initial rounds of cell division in Arabidopsis (Laux and Jurgens, 1997; Mayer and Jürgens, 1998). This pattern of suspensor development is representative for a large number of plant species, although significant divergence is observed in the plant kingdom (Yeung and Meinke, 1993; Yeung and Clutter, 2011). The quiescence of suspensor cells does not reflect its developmental potential. In several species, suspensor cells can be “reprogrammed” to form a second embryo (Yeung and Meinke, 1993). Although the mechanisms of this conversion and its regulators are largely unknown, we previously identified the transcriptional auxin response as a key pathway suppressing embryo identity in suspensor cells (Rademacher et al., 2012). Here, we further explored this system as a model for investigating the suspensor-to-embryo transformation. Our study identified a large degree of convergent homeostatic control of many genes involved in auxin homeostasis, which cooperatively work to restore the auxin response. We also identified a number of genes whose misregulation upon inhibition of the auxin response correlates with embryo identity specification. Importantly, we identified a genetic network involving several bHLH transcription factors that mediates auxin action in controlling suspensor development and/or suspensor cell identity maintenance.
Many genes are misregulated in the 72-h M0171>>bdl data sets even though the first visible morphological defects occurred at very low frequency, and there was a relatively narrow window of auxin response inhibition. This reflects the major role of auxin in these cells and suggests that reprogramming is a complex genetic response, and thus it may be difficult to isolate the first events. A possible explanation for the latter is that although the pro-embryo and suspensor domains are well defined, they remain physically and symplastically connected. In fact, the suspensor serves as a conduit for delivering nutrients and growth regulators to the pro-embryo (Kawashima and Goldberg, 2010). Besides, impairment of the pro-embryo triggers developmental changes in the suspensor (Schwartz et al., 1994; Weijers et al., 2003; Liu et al., 2015). Therefore, changes in suspensor cells could also be sensed by the pro-embryo and are expected to induce transcriptional changes and secondary consequences in the pro-embryo. The continuity between the pro-embryo and suspensor cells could also explain the broader expression domains of 24 out of 40 tested genes, which is not in line with the maintenance of auxin-mediated suspensor cell identity and/or embryo transformation.
Two readily apparent features of the transcriptomic and expression analysis data sets are (1) auxin homeostasis components and (2) bHLH transcription factors involved in the suspensor auxin response. Our initial global analysis of M0171>>bdl data sets revealed enrichment for genes involved in auxin homeostasis. In particular, genes that belong to more than 10 families representing each of the main facets of auxin homeostasis were misregulated in such a way that the end result will be an increase in free cellular auxin levels or an increased auxin response. The level of free auxin in the cell is cooperatively defined by biosynthesis, (de)conjugation, and transport and has a direct output, i.e., auxin signaling. Thus, inhibition of the auxin response might be sensed by the system as an auxin minimum. As a response to this process and to efficiently re-establish the auxin response, genes that will increase intracellular auxin levels are upregulated and genes involved in auxin transport are downregulated (Figures 2A to 2J), resulting in increased auxin levels in the embryo (Figures 3A, 3B, 3G, and 3H). Self-regulatory feedback loops from auxin signaling to the expression of auxin homeostatic genes are well known. For instance, the transcription of GH3 family members and most Aux/IAA inhibitors is highly auxin inducible (Liu et al., 1994). Similarly, the expression of both auxin efflux and influx carrier genes is upregulated by auxin (Vieten et al., 2005; Swarup et al., 2008). However, all of these feedback loops have been independently observed upon exogenous auxin treatment, and it is rather obscure to what degree they functionally converge. Our results demonstrate a feedback loop represented by the misregulation of genes involved in auxin homeostasis and responses linked to a biologically relevant output: suspensor proliferation and/or a subsequent switch of cell identity. The high degree of convergent regulation of 40 genes from 15 families shows how robust to perturbation the auxin system is.
Because many bHLH superfamily members were misregulated upon suspensor-specific inhibition of the auxin response, and because several bHLH transcription factors were previously shown to play a role in Arabidopsis embryo development, particularly in cell specification events downstream of auxin, we decided to further investigate a set of four bHLH genes in detail. Our results show that all are indeed regulated by auxin in an ARF-dependent manner. Their misregulation upon inhibition of the auxin response in the suspensor implies that the S>E transformation might not be a cell autonomous process involving a single-step identity switch. Our results suggest that instead, the S>E transformation might be a multi-step process, where upon inhibition of the auxin response, excessive cell division is turned on, followed by the loss of suspensor identity (cells undergo dedifferentiation) and subsequently (upon trigger) an installment of embryo identity.
Based on phenotypic data, as well as the dependence of auxin-regulation on de novo translation, bHLH49 appears to be a more direct and biologically more important regulator than the other bHLH proteins. Indeed, when probing the bHLH49-dependent transcriptome, we found that the other bHLH genes are among the targets. Thus, the auxin-repressed bHLH49 gene appears to be an important mediator of auxin-dependent suppression of proliferation in suspensor cells. Indeed, the misexpression of bHLH49 alone induced excess divisions and even the formation of multiple embryo-like structures in suspensors (Figure 7B), similar to the effect of inhibition of the auxin response.
An important open question is what primary cellular process bHLH49 targets to bring about suspensor proliferation. Identifying the identity of the bHLH49-dependent genes did not directly reveal a key cellular process that can explain its ability to trigger proliferative divisions in the suspensor. Nonetheless, the significant overlap with the genes misregulated in M0171>>bdl embryos hints toward the need for a defined set of genetic regulators required to induce the switch from suspensor to embryonic cell fate. Interestingly, however, we detected an overlap between M0171>>bdl responsive and bHLH49-dependent genes, even though one process is performed in embryos and the other in roots. Thus, it is quite possible that bHLH49 regulates a rather generic cellular process, such as cell division. However, the postembryonic misexpression phenotypes are not indicative of excess cell division. Postembryonic misexpression of bHLH49 induces very strong defects (Supplemental Figures 3A to 3H), but these do not include the induction of ectopic embryo formation. Thus, unlike “embryo inducers” such as LEAFY COTYLEDON1(Lotan et al., 1998) or BABY BOOM (Boutilier et al., 2002), bHLH49 does not appear to directly promote embryogenesis. Its action is instead associated with the dedifferentiation of suspensor cells (erasure of their initial transcriptional program) into cells that can divide but cannot be transformed completely into functional embryo structures (installation of the embryonic program). In this regard, it will be interesting to test the potential of bHLH49, using gain- and loss-of-function mutants, to induce embryogenesis in the context of somatic embryogenesis or during plant regeneration from hypocotyl explants (Iwase et al., 2011). Furthermore, the suspensor cells of several plant species undergo many more divisions, yet they do not show features of embryo identity (Yeung and Meinke, 1993; Kawashima and Goldberg, 2010; Yeung and Clutter, 2011). Hence, unless Arabidopsis, with its minimal number or suspensor cells, represents an exceptional situation, triggering cell division in suspensor cells may not be sufficient to induce embryogenesis.
METHODS
Plant Material
All Arabidopsis (Arabidopsis thaliana) plants used in this study were of the Col-0 ecotype except the M0171-GAL4 enhancer trap line, which is in the C24 background (made available by Dr. Jim Haseloff, Cambridge, UK). T-DNA insertion lines bhlh49-1 (SALK_135188C), bhlh49-2 (SALK_087424C), bhlh60-3 (SAIL_1219_E01), bhlh63-2 (SAIL_1211_F11), bhlh100-1 (SALK_150637C), and bhlh100-2 (SALK_074568C) and the M0171-GAL4 (Rademacher et al., 2012) enhancer trap line were obtained from Arabidopsis Stock Centers (NASC-ARBC) and genotyped using the primers listed in Supplemental Data Set 2. The pARF13-n3GFP and DR5/DR5v2 and Ratiometric version of 2 D2’s (R2D2) lines were described (Rademacher et al., 2012; Liao et al., 2015).
All seeds were sterilized in 25% bleach/75% ethanol (v/v)solution for 10 min and washed twice with 70% ethanol and once with 100% ethanol. Dried seeds were plated on half-strength Murashige and Skoog (MS) medium and the appropriate antibiotic (50 mg/l kanamycin or 15 mg/l phosphinothricin) for the selection of transgenic seeds. After 24 h incubation at 4°C, the plants were cultured under long-day conditions (16 h light 110 µE m−2 s−1 [Philips Master TL-D HF 50W/840] and 8 h dark) at 22°C.
Plant transformation was performed using the floral dip method, as described in De Rybel et al. (2011).
Cloning
All cloning was performed using the LIC cloning system and previously described vectors (De Rybel et al., 2011). For transcriptional fusions, fragments up to 3 kb upstream of the ATG codon including the 5′-UTR were amplified from genomic DNA using Phusion Flash polymerase (Thermo Scientific). For translational fusions of bHLH genes, the same promoter fragments were amplified along with the genomic coding sequences excluding the stop codon. To generate constructs for pRPS5A-driven misexpression, the coding sequences were amplified from complementary DNA (cDNA) clones. All constructs were completely sequenced. The primers used for cloning are listed in Supplemental Data Set 2. At least three independent lines were analyzed per construct.
Microscopy
Differential interference contrast and confocal microscopy were performed as described previously (Llavata-Peris et al., 2013). Cleared embryos were observed under a Leica DMR microscope equipped with differential interference contrast optics, and confocal imaging was performed using a Leica SP5 II system (Hybrid detector). Cell outlines were generated by counterstaining with SCRI Renaissance Stain 2200 (Renaissance Chemicals).
Phylogenetic Analysis
The sequences of all predicted ArabidopsisbHLH proteins (https://www.arabidopsis.org/browse/genefamily/blhm.jsp) were retrieved from TAIR (https://www.arabidopsis.org). Subsequently, multiple sequence alignment was performed in ClustalW. An unrooted phylogenetic tree was constructed with TreeView (http://en.bio-soft.net/tree/TreeView.html).
Quantitative RT-PCR Analysis
qRT-PCR analysis was performed as described previously (De Rybel et al., 2010). RNA was isolated using TRIzol reagent (Invitrogen) and RNeasy kit (Qiagen). cDNA was prepared from 0.5 μg of total RNA with an iScript cDNA Synthesis Kit (BioRad). qRT-PCR was performed with iQ SYBR Green Supermix (BioRad) and analyzed on a CFX384 Real-Time PCR detection system (BioRad). The qRT-PCR cycling conditions were 95°C for 10 min; 45 cycles of 95°C for 10 s, 55°C for 20 s, 72°C for 20 s; 95°C for 10 s; 65°C for 5 s, followed by dissociation curve analysis. Reactions were done in triplicate, with three biological replicates (representing separate experiments). Data were analyzed with qBase as described in (Hellemans et al., 2007). Primers were designed with Beacon Designer 8 (Premier Biosoft International). Gene expression levels were normalized relative to CDKA1;1, EEFα4, and GAPC. Primers for qRT-PCR are listed in Supplemental Data Set 2.
Auxin Sensitivity Assay
The auxin sensitivity assay was performed according to Lincoln et al. (1990). Seeds were first germinated on standard half-strength MS medium. Six-day-old seedlings were transferred to fresh MS medium supplemented with 10 nM, 20 nM, 30 nM, and 40 nM 2,4-D or lacking 2,4-D. After two days, the plates were scanned, and the length of the newly grown roots was measured using ImageJ software. The percentage of root growth relative to root growth on MS without auxin was calculated.
Microarray Experiments
M0171>>bdl:
After crossing, embryos were isolated in 5% Suc solution containing 0.1% RNALater as described in (Xiang et al., 2011), and the isolated embryos were pooled in a 1.5 mL Eppendorf tube on dry ice (300-400 embryos for each biological replicate). Total RNA was extracted according to the protocol of the RNAqueous-micro kit and amplified before labeling following the protocol provided in the MessageAmp aRNA kit with minor modifications.
The Arabidopsis 70-mer oligo array slides prepared by University of Arizona were used in all the microarray experiments (version ATV 3.7.2; http://ag.arizona.edu/microarray). Antisense RNA was labeled according to the protocol of (Wellmer et al., 2004). The antisense RNA samples representing four biological replicates from experimental and control samples were labeled (two with Cy3 and two with Cy5) and hybridized to the slides following the protocol from http://eg.arizona.edu/microarray. Subsequently, the hybridized slides were scanned for Cy3- and Cy5-labeled mRNA targets with a ScanArray 4000 laser scanner (at a resolution of 10 μm). The QuantArray program (GSI Lumonics) was used for image analysis and signal quantification. Limma Software (Smyth, 2004) was used for normalization and to identify genes with modulated expression from the microarray data. To generate the plot in Figure 1E showing overlap between differentially expressed genes, UpSet plot was used (https://asntech.shinyapps.io/intervene/; Khan and Mathelier, 2017).
pRPS5A-bHLH49/bhlh49:
RNA from root tips was isolated as described above (see Quantitative RT-PCR), and total RNA (100 ng) was labeled using an Ambion Wild Type Expression kit (Life Technologies). The RNA was then hybridized to Arabidopsis gene ST arrays (Affimetrix), which probe the expression of 27,827 unique genes. Sample labeling and hybridization were performed according to manufacturer’s instructions. Microarray analysis was performed as previously described (De Rybel et al., 2014).
Global Analysis of M0171>>bdl Data Sets
The data sets were initially subjected to the Biological Networks Gene Ontology Tool to assess overrepresentation of gene ontology terms. Default settings were used (hypergeometric test, Benjamini and Hochberg FDR for correction of multiple testing, significance level of 0.05), and the whole genome annotation was used as a reference set together with gene ontology-SLIM ontology terms for Arabidopsis. No strong over- or underrepresentation of specific functions was found.
Accession Numbers
The accession numbers of the major genes described in this study are shown in Supplemental Table.
All microarray data have been deposited in the Gene Expression Omnibus at the National Center for Biotechnology Information (M0171>>bdl: GSE69854; bHLH49 mutant and overexpression: GSE69700).
Supplemental Data
Supplemental Figure 1. Localization of bHLH proteins.
Supplemental Figure 2. Expression of bHLH genes in insertion and misexpression lines.
Supplemental Figure 3. Post-embryonic phenotypes in pRPS5A-bHLH49 plants.
Supplemental Figure 4. Auxin treatment of pRPS5A-bHLH49 roots.
Supplemental Table. Sixty-eight genes selected for validation of M0171>>bdl data set.
Supplemental Data Set 1. M0171>>bdl data set.
Supplemental Data Set 2. Primers used in this study.
Supplemental File. Alignment used to produce the phylogenetic tree shown in Figure 5.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
The authors thank Dr. Jim Haseloff and the Nottingham Arabidopsis Stock Center for distributing the seeds and Bert De Rybel and Maritza van Dop for comments on the manuscript. This work was funded by grants from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research) (ALW-NSFC grant 846.11.001; ALW Open Competition grant 816.02.014) and the European Union ITN network SIREN (contract no.214788 to D.W.).
AUTHOR CONTRIBUTIONS
T.R., A.S.L., C.I.L.-P., and D.W. designed the research; T.R., A.S.L., C.I.L.-P., J.R.W., D.X., C.-Y.L., and L.V. performed research; all authors contributed to data analysis; T.R. and D.W. wrote the paper with contributions from A.S.L. and C.I.L.-P. and with input from all other authors.
Footnotes
Articles can be viewed without a subscription.
References
- Andriankaja M.E., et al. (2014). Transcriptional coordination between leaf cell differentiation and chloroplast development established by TCP20 and the subgroup Ib bHLH transcription factors. Plant Mol. Biol. 85: 233–245. [DOI] [PubMed] [Google Scholar]
- Benjamins R., Scheres B. (2008). Auxin: The looping star in plant development. Annu. Rev. Plant Biol. 59: 443–465. [DOI] [PubMed] [Google Scholar]
- Boutilier K., Offringa R., Sharma V.K., Kieft H., Ouellet T., Zhang L., Hattori J., Liu C.M., van Lammeren A.A., Miki B.L., Custers J.B., van Lookeren Campagne M.M. (2002). Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J.W., Cole M., Flier A., Werr W. (2009). BIM1, a bHLH protein involved in brassinosteroid signalling, controls Arabidopsis embryonic patterning via interaction with DORNROSCHEN and DORNROSCHEN-LIKE. Plant Mol. Biol. 69: 57–68. [DOI] [PubMed] [Google Scholar]
- Crawford B.C., Sewell J., Golembeski G., Roshan C., Long J.A., Yanofsky M.F. (2015). Plant development. Genetic control of distal stem cell fate within root and embryonic meristems. Science 347: 655–659. [DOI] [PubMed] [Google Scholar]
- De Rybel B., et al. (2010). A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20: 1697–1706. [DOI] [PubMed] [Google Scholar]
- De Rybel B., et al. (2014). Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 345: 1255215. [DOI] [PubMed] [Google Scholar]
- De Rybel B., van den Berg W., Lokerse A., Liao C.Y., van Mourik H., Möller B., Peris C.L., Weijers D. (2011). A versatile set of ligation-independent cloning vectors for functional studies in plants. Plant Physiol. 156: 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B., Möller B., Yoshida S., Grabowicz I., Barbier de Reuille P., Boeren S., Smith R.S., Borst J.W., Weijers D. (2013). A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Dev. Cell 24: 426–437. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Weijers D., Lechner E., Yamada M., Hobbie L., Ehrismann J.S., Jürgens G., Estelle M. (2005). Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9: 109–119. [DOI] [PubMed] [Google Scholar]
- Feller A., Machemer K., Braun E.L., Grotewold E. (2011). Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 66: 94–116. [DOI] [PubMed] [Google Scholar]
- Fletcher J.C., Brand U., Running M.P., Simon R., Meyerowitz E.M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914. [DOI] [PubMed] [Google Scholar]
- Franco A.R., Gee M.A., Guilfoyle T.J. (1990). Induction and superinduction of auxin-responsive mRNAs with auxin and protein synthesis inhibitors. J. Biol. Chem. 265: 15845–15849. [PubMed] [Google Scholar]
- Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jürgens G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153. [DOI] [PubMed] [Google Scholar]
- Gaj M.D., Zhang S., Harada J.J., Lemaux P.G. (2005). Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222: 977–988. [DOI] [PubMed] [Google Scholar]
- Gooh K., Ueda M., Aruga K., Park J., Arata H., Higashiyama T., Kurihara D. (2015). Live-cell imaging and optical manipulation of Arabidopsis early embryogenesis. Dev. Cell 34: 242–251. [DOI] [PubMed] [Google Scholar]
- Haccius B. (1955). Experimentally induced twinning in plants. Nature 176: 355–356. [Google Scholar]
- Hamann T., Benkova E., Bäurle I., Kientz M., Jürgens G. (2002). The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 16: 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke C.S., Berleth T. (1998). The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 17: 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A., Mitsuda N., Koyama T., Hiratsu K., Kojima M., Arai T., Inoue Y., Seki M., Sakakibara H., Sugimoto K., Ohme-Takagi M. (2011). The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 21: 508–514. [DOI] [PubMed] [Google Scholar]
- Jones B., Gunnerås S.A., Petersson S.V., Tarkowski P., Graham N., May S., Dolezal K., Sandberg G., Ljung K. (2010). Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22: 2956–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T., Goldberg R.B. (2010). The suspensor: Not just suspending the embryo. Trends Plant Sci. 15: 23–30. [DOI] [PubMed] [Google Scholar]
- Khan A., Mathelier A. (2017). Intervene: A tool for intersection and visualization of multiple gene or genomic region sets. BMC Bioinformatics 18: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong R.W., Bui A.Q., Lee H., Kwong L.W., Fischer R.L., Goldberg R.B., Harada J.J. (2003). LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell 15: 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan K.K., Ambegaokar K.B. (1984). Polyembryony. In Johri BM, ed, Embryology of Angiosperms. Springer, Heidelberg, Germany, pp 445–474. [Google Scholar]
- Laux T., Jurgens G. (1997). Embryogenesis: A new start in life. Plant Cell 9: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. (2010). The power of auxin in plants. Plant Physiol. 154: 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.Y., Smet W., Brunoud G., Yoshida S., Vernoux T., Weijers D. (2015). Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 12: 207–210, 2, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C., Britton J.H., Estelle M. (1990). Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yu X., Li K., Klejnot J., Yang H., Lisiero D., Lin C. (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322: 1535–1539. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li X., Zhao J., Tang X., Tian S., Chen J., Shi C., Wang W., Zhang L., Feng X., Sun M.X. (2015). Direct evidence that suspensor cells have embryogenic potential that is suppressed by the embryo proper during normal embryogenesis. Proc. Natl. Acad. Sci. USA 112: 12432–12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.B., Ulmasov T., Shi X., Hagen G., Guilfoyle T.J. (1994). Soybean GH3 promoter contains multiple auxin-inducible elements. Plant Cell 6: 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llavata-Peris C., Lokerse A., Möller B., De Rybel B., Weijers D. (2013). Imaging of phenotypes, gene expression, and protein localization during embryonic root formation in Arabidopsis. Methods Mol. Biol. 959: 137–148. [DOI] [PubMed] [Google Scholar]
- Lokerse A.S., Weijers D. (2009). Auxin enters the matrix--assembly of response machineries for specific outputs. Curr. Opin. Plant Biol. 12: 520–526. [DOI] [PubMed] [Google Scholar]
- Long J.A., Moan E.I., Medford J.I., Barton M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69. [DOI] [PubMed] [Google Scholar]
- Lotan T., Ohto M., Yee K.M., West M.A., Lo R., Kwong R.W., Yamagishi K., Fischer R.L., Goldberg R.B., Harada J.J. (1998). Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Mayer U., Jürgens G. (1998). Pattern formation in plant embryogenesis: A reassessment. Semin. Cell Dev. Biol. 9: 187–193. [DOI] [PubMed] [Google Scholar]
- Murre C., Bain G., van Dijk M.A., Engel I., Furnari B.A., Massari M.E., Matthews J.R., Quong M.W., Rivera R.R., Stuiver M.H. (1994). Structure and function of helix-loop-helix proteins. Biochim. Biophys. Acta 1218: 129–135. [DOI] [PubMed] [Google Scholar]
- Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M. (2007). ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov I.A., Paponov M., Teale W., Menges M., Chakrabortee S., Murray J.A., Palme K. (2008). Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol. Plant 1: 321–337. [DOI] [PubMed] [Google Scholar]
- Rademacher E.H., Möller B., Lokerse A.S., Llavata-Peris C.I., van den Berg W., Weijers D. (2011). A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J. 68: 597–606. [DOI] [PubMed] [Google Scholar]
- Rademacher E.H., Lokerse A.S., Schlereth A., Llavata-Peris C.I., Bayer M., Kientz M., Freire Rios A., Borst J.W., Lukowitz W., Jürgens G., Weijers D. (2012). Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell 22: 211–222. [DOI] [PubMed] [Google Scholar]
- Radoeva T., Weijers D. (2014). A roadmap to embryo identity in plants. Trends Plant Sci. 19: 709–716. [DOI] [PubMed] [Google Scholar]
- Radoeva T., Ten Hove C.A., Saiga S., Weijers D. (2016). Molecular characterization of Arabidopsis GAL4/UAS enhancer trap lines identifies novel cell-type-specific promoters. Plant Physiol. 171: 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V. (2006). Life and times of the suspensor cell signaling between the embryo and suspensor. In Double Fertilization: Embryo and Endosperm Development in Flowering Plants. (Heidelberg, Germany; Springer; ), pp. 81–100. [Google Scholar]
- Schlereth A., Möller B., Liu W., Kientz M., Flipse J., Rademacher E.H., Schmid M., Jürgens G., Weijers D. (2010). MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916. [DOI] [PubMed] [Google Scholar]
- Schwartz B., Yeung C., Meinke W. (1994). Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development 120: 3235–3245. [DOI] [PubMed] [Google Scholar]
- Schwartz B.W., Vernon D.M., Meinke D. (1997). Development of the suspensor: Differentiation, communication, and programmed cell death during plant embryogenesis. In Cellular and Molecular Biology of Plant Seed Development: Advances in Cellular and Molecular Biology of Plants.Vol. 4Larkins BA, Vasil IK, eds. (Dordrecht, Netherlands: Springer; ),pp. 53–72. [Google Scholar]
- Sivitz A.B., Hermand V., Curie C., Vert G. (2012). Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS One 7: e44843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G.K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3: e3. [DOI] [PubMed] [Google Scholar]
- Swarup K., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10: 946–954. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616. [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon D.M., Meinke D.W. (1994). Embryogenic transformation of the suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev. Biol. 165: 566–573. [DOI] [PubMed] [Google Scholar]
- Vieten A., Vanneste S., Wisniewska J., Benková E., Benjamins R., Beeckman T., Luschnig C., Friml J. (2005). Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531. [DOI] [PubMed] [Google Scholar]
- Wang R., Estelle M. (2014). Diversity and specificity: Auxin perception and signaling through the TIR1/AFB pathway. Curr. Opin. Plant Biol. 21: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.Y., Klatte M., Jakoby M., Bäumlein H., Weisshaar B., Bauer P. (2007). Iron deficiency-mediated stress regulation of four subgroup Ib BHLH genes in Arabidopsis thaliana. Planta 226: 897–908. [DOI] [PubMed] [Google Scholar]
- Weijers D., Franke-van Dijk M., Vencken R.J., Quint A., Hooykaas P., Offringa R. (2001). An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128: 4289–4299. [DOI] [PubMed] [Google Scholar]
- Weijers D., Van Hamburg J.P., Van Rijn E., Hooykaas P.J., Offringa R. (2003). Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol. 133: 1882–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D., Schlereth A., Ehrismann J.S., Schwank G., Kientz M., Jürgens G. (2006). Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev. Cell 10: 265–270. [DOI] [PubMed] [Google Scholar]
- Wellmer F., Riechmann J.L., Alves-Ferreira M., Meyerowitz E.M. (2004). Genome-wide analysis of spatial gene expression in Arabidopsis flowers. Plant Cell 16: 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang D., Venglat P., Tibiche C., Yang H., Risseeuw E., Cao Y., Babic V., Cloutier M., Keller W., Wang E., Selvaraj G., Datla R. (2011). Genome-wide analysis reveals gene expression and metabolic network dynamics during embryo development in Arabidopsis. Plant Physiol. 156: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Wu M.F., Winter C.M., Berns M.C., Nole-Wilson S., Yamaguchi A., Coupland G., Krizek B.A., Wagner D. (2013). A molecular framework for auxin-mediated initiation of flower primordia. Dev. Cell 24: 271–282. [DOI] [PubMed] [Google Scholar]
- Yeung E.C., Clutter M.E. (2011). Embryogeny of Phaseolus coccineus: The ultrastructure and development of the suspensor. Can. J. Bot. 57: 120–136. [Google Scholar]
- Yeung E.C., Meinke D.W. (1993). Embryogenesis in angiosperms: Development of the suspensor. Plant Cell 5: 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Jin J., Tang L., Zhao Y., Gu X., Gao G., Luo J. (2011). PlantTFDB 2.0: update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res. 39 (suppl_1): D1114–D1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Andersen S.U., Ljung K., Dolezal K., Miotk A., Schultheiss S.J., Lohmann J.U. (2010). Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092. [DOI] [PubMed] [Google Scholar]