Discrete regulatory DNA elements, including far upstream located ones, regulate a proper spatiotemporal expression of NIN, a key transcription factor required during symbiotic root nodule development.
Abstract
The legume-rhizobium symbiosis results in nitrogen-fixing root nodules, and their formation involves both intracellular infection initiated in the epidermis and nodule organogenesis initiated in inner root cell layers. NODULE INCEPTION (NIN) is a nodule-specific transcription factor essential for both processes. These NIN-regulated processes occur at different times and locations in the root, demonstrating a complex pattern of spatiotemporal regulation. We show that regulatory sequences sufficient for the epidermal infection process are located within a 5 kb region directly upstream of the NIN start codon in Medicago truncatula. Furthermore, we identify a remote upstream cis-regulatory region required for the expression of NIN in the pericycle, and we show that this region is essential for nodule organogenesis. This region contains putative cytokinin response elements and is conserved in eight more legume species. Both the cytokinin receptor 1, which is essential for nodule primordium formation, and the B-type response regulator RR1 are expressed in the pericycle in the susceptible zone of the uninoculated root. This, together with the identification of the cytokinin-responsive elements in the NIN promoter, strongly suggests that NIN expression is initially triggered by cytokinin signaling in the pericycle to initiate nodule primordium formation.
INTRODUCTION
The formation of nitrogen-fixing nodules is induced by rhizobium bacteria on the roots of legumes. It involves several processes: the induction of intracellular infection by rhizobia; nodule organogenesis; and a negative feedback loop that determines the number of nodules (Kosslak and Bohlool, 1984; Downie, 2014). Strikingly, the transcription factor NODULE INCEPTION (NIN), which is specifically expressed during nodulation, plays a key role in all of these processes (Schauser et al., 1999; Marsh et al., 2007; Soyano et al., 2014). These processes occur at different time points and locations, suggesting that NIN has a complex spatiotemporal regulation of expression that is regulated by distinct cis-regulatory sequences in its promoter. However, although NIN was identified almost two decades ago in Lotus japonicus (Lotus; Schauser et al., 1999) and more than 10 years ago in Medicago truncatula (Medicago; Marsh et al., 2007), the promoter regions required for full complementation of nin knockout mutants have not been identified. Currently, it is unclear how NIN is involved in the multiple steps of the nodulation process.
In Medicago, nodule organogenesis starts with the local mitotic activation of pericycle cells, and subsequently cell division extends to the more outward-located endodermis and cortex (Xiao et al., 2014). Sinorhizobium meliloti bacteria invade roots through tube-like structures called infection threads. Formation of infection threads in root hairs requires the prior induction of root hair curling. A tight curl is formed when the tip of the curling root hair touches the shank of the hair and the root hair stops growing and forms an infection chamber. Microcolonies of rhizobia then develop within these chambers, in which the rhizobia can induce formation of the infection thread.
In nin null mutants, extensive root hair curling and deformation are induced by bacteria, a proper infection chamber fails to be established, and only a few bacteria are present within curled root hairs (Schauser et al., 1999; Marsh et al., 2007; Fournier et al., 2015). Wild-type NIN induces infection thread formation by triggering expression of genes required for infection thread formation such as Nuclear transcription factor Y subunit A-1 (NF-YA1), and nodulation pectate lyase (Xie et al., 2012; Soyano et al., 2013; Laporte et al., 2014). Subsequently, infection threads grow toward the nodule primordia. There, rhizobia are released into nodule primordium cells derived from the cortex. These cells become infected cells that host thousands of nitrogen-fixing bacteria. NIN is also required for autoregulation of nodulation, a negative feedback system involving root-to-shoot communication to determine the optimal number of nodules. This autoregulation mechanism includes the induction of CLAVATA3/ESR-RELATED genes by NIN, and the CLAVATA3/ESR-RELATED-encoded peptides induce systemic signaling between root and shoot, suppressing the formation of new nodule primordia (Soyano et al., 2014).
Expression of NIN is induced in the epidermis upon perception of nodulation (Nod) factors, which are lipochitooligosaccharides secreted by rhizobia (van Zeijl et al., 2015; Vernié et al., 2015). Nod factor signaling induces Ca2+ spiking, which activates the nucleus-localized calcium and calmodulin-dependent kinase (CCaMK; Ehrhardt et al., 1996; Mitra et al., 2004). CCaMK phosphorylates CYCLOPS, a transcription factor that activates NIN expression (Yano et al., 2008; Singh et al., 2014). At ∼24 h post inoculation, formation of both infection threads and nodule primordium is initiated in Medicago roots (Xiao et al., 2014). At this developmental stage, Nod factor signaling occurs exclusively in the epidermis because rhizobia are present only there and Nod factors are immobile molecules (Goedhart et al., 2000). Therefore, NIN can induce infection thread formation in a cell-autonomous way in the epidermis, but it remains unclear how NIN can induce nodule primordium formation in inner root cell layers. It has been postulated that NIN expression can be induced in these root layers by a mobile signal that is generated upon Nod factor signaling in the epidermis (Hayashi et al., 2014). Alternatively, NIN proteins produced in the epidermis may be transported to the inner root layers (Vernié et al., 2015; Jardinaud et al., 2016).
In addition to regulation of NIN by CYCLOPS, NIN expression depends on cytokinin signaling. Exogenous application of cytokinin is sufficient to trigger NIN expression and also formation of structures resembling nodules (Gonzalez-Rizzo et al., 2006; Heckmann et al., 2011; Plet et al., 2011). Notably, Nod factor application results in the accumulation of cytokinin (van Zeijl et al., 2015). Furthermore, the induction of NIN expression by either Nod factors or cytokinin requires the cytokinin receptor 1 (CRE1), which plays a key role in nodule organogenesis (Gonzalez-Rizzo et al., 2006; Plet et al., 2011; van Zeijl et al., 2015).
Studies of the weak nin allele of the Lotus daphne mutant (Yoro et al., 2014) have provided valuable insight into the involvement of different cis-regulatory sequences in the NIN promoter that regulate infection and nodule organogenesis. In daphne roots, rhizobium infection and primordium formation are uncoupled. Formation of nodule primordia is completely absent, and increased numbers of infection threads are formed in the epidermis. The daphne mutation is caused by a large insertion that is ∼7 kb upstream of the NIN start codon. This suggests that this 7 kb region includes essential cis-regulatory regions that are required for infection thread formation in the epidermis but are insufficient for the activation of cortical cell divisions. To determine how NIN induces these processes, we must identify the precise cis-regulatory regions in the NIN promoter that drive proper spatiotemporal NIN transcription.
Here, we have identified a conserved NIN promoter region that is essential for nodule organogenesis in Medicago. This region contains several putative cytokinin response elements and regulates NIN expression in the pericycle, where CRE1 and a B-type response regulator (RR1) are constitutively expressed. This reveals a key role for the pericycle in formation of nodule primordia, in which NIN expression is most likely activated by cytokinin signaling.
RESULTS
Isolation of a Medicago nin Mutant in Which Infection and Nodule Organogenesis Are Uncoupled
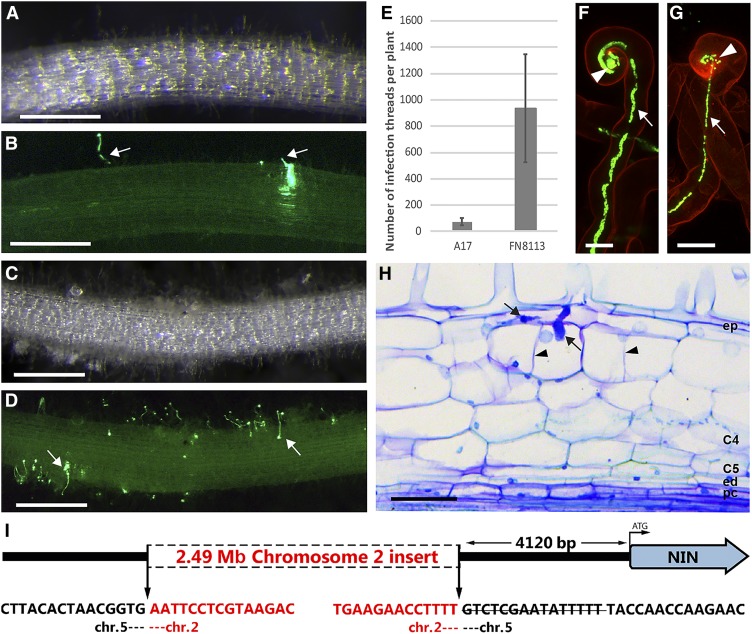
By screening a plant population obtained from Medicago seeds that were mutagenized by fast neutron bombardment (Noble Research Institute), we identified a Nod− mutant that we have named FN8113. Three weeks post inoculation (wpi) with S. meliloti, the FN8113 mutant formed excessive numbers of infection threads, but nodulation was strongly impaired (compare Figures 1A and 1B with Figures 1C and 1D). We quantified the infection thread number in FN8113 and wild-type roots at 2 wpi. The number of infection threads in FN8113 roots was more than 10-fold the number in wild-type roots (Figure 1E). Root hair curling in FN8113 resembled that of the wild type, as entrapped bacteria formed colonies and infection threads were formed (Figures 1F and 1G). The majority of infection threads were arrested in root hairs, but longitudinal sections of roots showed that a few infection threads could reach cortical cell layers (Figure 1H). Occasionally, some cortical cells had divided locally around infection threads. However, cell divisions were not induced in the inner root cell layers, where nodule primordia are initiated in wild-type Medicago plants.
Figure 1.
In the Medicago nin Mutant daphne-like, Infection and Nodule Organogenesis Are Uncoupled.
(A) to (D) Images of wild-type and mutant roots. These transmitted light stereomacroscopy images (A) and (C) and corresponding green fluorescence stereomacroscopy images (B) and (D) were taken at 3 wpi. Roots of daphne-like (FN8113) mutant plants ([C] and [D]) have an excessive number of infection threads in comparison with wild-type A17 roots ([A] and [B]). Bars = 2 mm.
(E) Quantification of infection thread number. The number of infection threads per root was counted at 2 wpi in both A17 roots (n = 12) and FN8113 roots (n = 12). Data are means ± sd.
(F) and (G) Infection thread formation in mutant and wild-type roots. These confocal images of roots stained with propidium iodide at 1 wpi show that a bacterial colony (arrowheads) is formed inside a daphne-like curled root hair and an infection thread (arrows) is initiated (F) like in a wild-type root hair (G). S. meliloti containing constitutively expressed GFP was used as inoculum. Bars = 10 µm.
(H) Longitudinal plastic section of daphne-like root at 3 wpi. The section stained with toluidine blue displays an infection thread (arrows). The infection threads in a mutant can occasionally reach cortical cell layers and induce some cell divisions (arrowheads). C4 and C5, cortical cell layers 4 and 5; ed, endodermis; ep, epidermis; pc, pericycle. Bar = 50 μm.
(I) Schematic representation of the chromosome translocation at the NIN locus in the daphne-like mutant. The strikethrough indicates a 15 bp deleted sequence.
A segregating F2 population resulting from a cross between FN8113 (cv Jemalong A17) and cv Jemalong A20 showed an ∼3:1 ratio of Nod+:Nod− plants (118 F2 plants; 84 Nod+:34 Nod−). This indicates that FN8113 has a single recessive mutation that is responsible for its Nod− phenotype. Simple sequence repeat markers were used to determine the position of the mutation, which was localized to the end of chromosome 5, where NIN is located. Next, whole-genome sequencing was used to identify the mutation in this region, and this revealed a translocation of an ∼2.49 Mb region from chromosome 2 into chromosome 5. This was inserted 4120 bp upstream of the NIN start codon (–4120). In addition, a small deletion of 15 bp between –4121 and –4135 was detected (Figure 1I). No mutations were found in the NIN coding sequence. FN8113 was shown to be a nin mutant because its Nod− phenotype could be complemented with a biologically functional NIN promoter driving NIN (described below). Because the phenotype of FN8113, as well as the nature of its mutation, are strikingly similar to Lotus daphne, we named the FN8113 mutant daphne-like.
The 5 kb Upstream Region of Medicago NIN Contains Discrete Regulatory Sequences That Affect Root Hair Curling and Infection
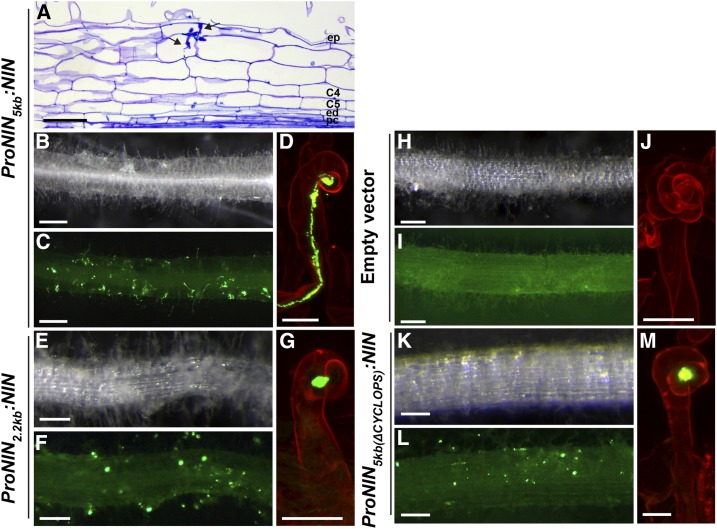
The phenotype of daphne-like strongly suggests that NIN regulatory sequences required for primordium formation are located more than 4120 bp upstream of the NIN start codon. In addition, this phenotype indicates that the regulatory sequences located within this 4120 bp region are sufficient for proper root hair curling and infection thread formation. We tested this by using a construct containing the 5 kb region upstream of the start codon to drive expression of NIN. We introduced this construct, ProNIN5kb:NIN, into Medicago nin-1 (null mutant; Marsh et al., 2007) roots by Agrobacterium rhizogenes-mediated root transformation (Limpens et al., 2004). At 4 wpi, 41 of 44 analyzed transgenic roots showed excessive infection thread formation (Figures 2B to 2D). Despite the numerous infections, these roots did not form nodules, except for one root on which four nodules were observed. As other transgenic roots of this composite plant have no nodules, we assume that this is caused by transgene insertion. Longitudinal sections of infected transgenic roots confirmed that cell divisions were not induced in the pericycle, endodermis, and inner cortical cell layers (Figure 2A). Infection threads were arrested in the epidermis, but occasionally some of these reached the cortex. Thus, the 5 kb promoter region is sufficient for infection thread formation, but it lacks regulatory sequences for primordium formation.
Figure 2.
The Infection Process in Medicago nin-1 Roots Is Partially Rescued by Introducing ProNIN5kb:NIN or ProNIN2.2kb:NIN.
(A) to (D) Phenotype of nin-1 roots transformed with ProNIN5kb:NIN at 4 wpi with S. meliloti constitutively expressing GFP. The transmitted light microscopy image of a longitudinal plastic section of a transgenic root stained with toluidine blue displays infection threads (arrow) that occasionally can reach cortical cell layers (A). The transmitted light stereomacroscopy image (B) and corresponding green fluorescence stereomacroscopy image (C) show excessive infection thread formation in transgenic roots. The confocal image of a transgenic root stained with propidium iodide shows an infection thread initiated in the curled root hair (D). Bars = 50 µm (A), 2 mm ([B] and [C]), and 10 µm (D).
(E) to (G) Phenotype of nin-1 roots transformed with ProNIN2.2kb:NIN at 4 wpi with S. meliloti constitutively expressing GFP. The transmitted light stereomacroscopy image (E) and corresponding green fluorescence stereomacroscopy image (F) display numerous curled root hairs with bacterial colonies in transgenic roots. In the confocal image of a transgenic root stained with propidium iodide (G), colonies are formed inside the chamber of the root hair curl, but an infection thread does not develop. Bars = 2 mm ([E] and [F]) and 10 µm (G).
(H) to (J) Phenotype of nin-1 roots transformed with empty vector at 4 wpi with S. meliloti constitutively expressing GFP. The transmitted light stereomacroscopy image (H) and corresponding green fluorescence stereomacroscopy image (I) show that the transgenic root forms neither infection threads nor bacterial colonies. A transgenic root stained with propidium iodide shows excessive root hair curling (J). Bars = 2 mm ([H] and [I]) and 10 µm (J).
(K) to (M) Phenotype of nin-1 roots transformed with ProNIN5kb(Δcyclops):NIN at 4 wpi with S. meliloti constitutively expressing GFP. The transmitted light stereomacroscopy image (K) and corresponding green fluorescence stereomacroscopy image (L) display many bacterial colonies in transgenic root hairs. The confocal image of a transgenic root stained with propidium iodide shows that a colony is formed inside the chamber of the root hair curl but an infection thread is not initiated (M). Bars = 2 mm ([K] and [L]) and 10 µm (M).
Interestingly, a single putative CYCLOPS/IPD3 binding site is located about 3 kb upstream of the start codon (Figures 3 and 4A; Supplemental Figure 1, Supplemental Table 1; Singh et al., 2014). We therefore checked whether the function of NIN in the epidermis fully depends on this putative CYCLOPS binding site by using the –2.2 kb region (Figure 4A) to drive NIN expression. The ProNIN2.2kb:NIN construct was introduced into nin-1 by A. rhizogenes-mediated root transformation. The nin-1 null mutant has excessive root hair curling but fails to form infection threads (Figures 2H to 2J). Although all 37 analyzed transgenic roots at 4 wpi showed tight root hair curls that enclosed bacterial colonies, infection threads were rare (Figures 2E to 2G). Of 298 curled root hairs in ProNIN2.2kb:NIN transgenic roots containing a bacterial colony, only ∼3% had an infection thread. This shows that root hair curling and establishment of infection chambers do not rely on the putative CYCLOPS binding site. By contrast, ∼70% of these curled root hairs (n = 324) formed infection threads in ProNIN5kb:NIN transgenic roots.
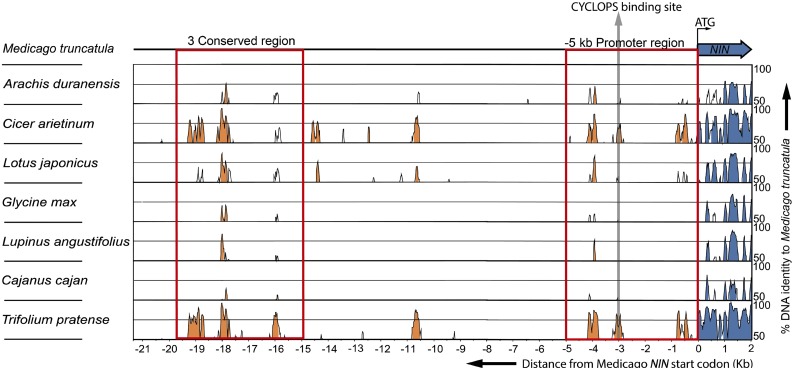
Figure 3.
mVISTA Alignment of Genomic DNA Sequences Including 2 kb from the Start Codon of NIN and 5′-Upstream Regions from Eight Legume Species.
Peaks indicate the level of identity with Medicago on a scale of 50 to 100%. Identities lower than 50% were not scored. The figure shows 2 kb of each NIN sequence downstream of the start codon (blue) and the 5′ upstream DNA sequence (orange). The red rectangle on the right next to the NIN coding sequence indicates the –5 kb promoter region. The red rectangle on the left indicates the 3C. The gray arrow shows the putative CYCLOPS binding site.
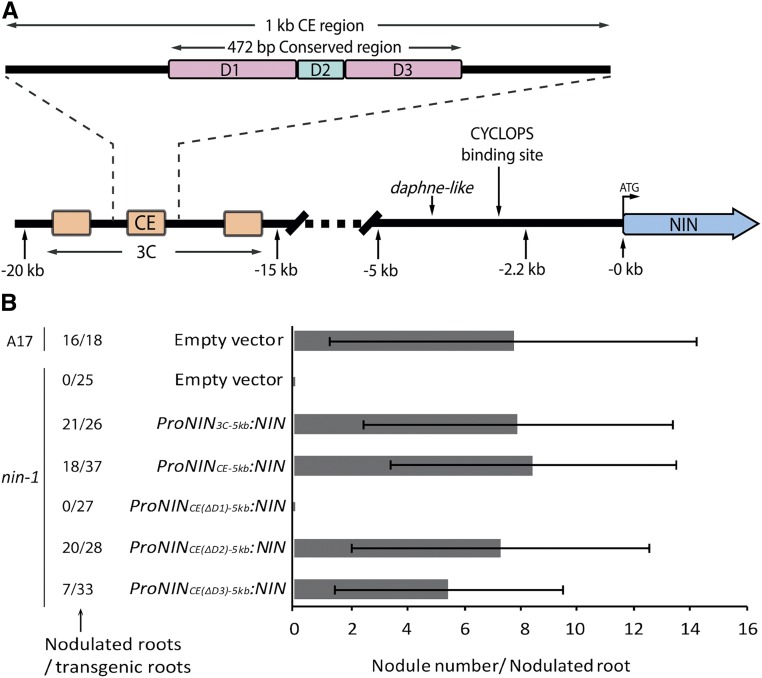
Figure 4.
The CE Region in the NIN Promoter Is Essential for Nodule Organogenesis.
(A) Schematic illustration of the Medicago NIN promoter. The 3C region (orange) was identified among the eight legume species studied here. The second 3C region is most conserved, and it includes about 10 putative B-type cytokinin signaling RR binding sites and is named the CE region. The CE region in turn contains a highly conserved 472 bp sequence that was divided into three parts named D1, D2, and D3. D1 and D3 (purple) contain six and three putative cytokinin response elements, respectively, whereas D2 (green) contains a putative AP2 binding site and a single putative cytokinin response element.
(B) The number of nodules formed on wild-type (A17) roots transformed with empty vector and nin-1 roots transformed with the constructs carrying NIN driven by different parts of the NIN promoter as indicated. S. meliloti containing constitutively expressed GFP was used as inoculum. Nodule numbers per nodulated root were counted at 4 wpi. Data are means ± sd.
These results indicate that the –5 to –2.2 kb region contains regulatory sequences that are critical for infection thread formation. The observed phenotype is reminiscent of that of Lotus and Medicago cyclops-3/ipd3-2 mutants (Yano et al., 2008; Horváth et al., 2011), which do not form infection threads but show formation of bacterial colonies in tightly curled root hairs. Therefore, the –2.2 kb region can activate NIN expression in the epidermis, and the expression level is sufficient for tight root hair curling, allowing rhizobia to form a colony inside the curl. However, additional regulatory sequences located between –5 and –2.2 kb, probably involving the putative CYCLOPS binding site, are required for efficient infection thread formation. To test this, we analyzed nin-1 roots transformed with NIN driven by the –5 kb promoter in which the putative CYCLOPS binding site was deleted (ProNIN5kb(Δcyclops):NIN) (Figures 2K to 2M). Due to this mutation, the number of curled root hairs with a colony (similar in size to the one formed in wild-type roots) that initiated an infection thread dropped from 70 to 7% (n = 434). This shows that the putative CYCLOPS binding site within the NIN promoter is essential for efficient infection thread formation.
A Conserved Region with Putative Cytokinin Response Elements is Located ∼18 kb Upstream of the NIN Coding Region in Medicago
The daphne-like mutant, as well as nin-1 transformed with ProNIN5kb:NIN, can induce formation of infection threads but not nodule primordia. Based on this, we hypothesized that the regulatory elements required for NIN-induced nodule primordium formation are located upstream of –5 kb. This resembles the Lotus daphne mutant, which contains a chromosomal insertion at ∼7 kb upstream of the NIN start codon. Therefore, we expected that such remote regulatory regions would be conserved in Lotus and Medicago and probably in other legumes. To test this, we compared the genomic DNA sequences spanning from the NIN coding region to the first upstream gene in eight legume species (Medicago, Lotus, Arachis duranensis, Cicer arietinum, Glycine max, Lupinus angustifolius, Cajanus cajan, and Trifolium pratense).
Based on the high level of identity (50–100%) among all these species, we identified DNA sequences with three conserved regions (3C) far upstream of the NIN start codon (Figure 3; Supplemental Table 1). In Medicago, 3C is located 15 to 20 kb upstream of the NIN start codon, and in Lotus, it is located 42 to 49 kb upstream (Supplemental Table 1). The levels of identity in conserved regions of 3C are similar to that of the NIN coding region (Figure 3). The second region in 3C is most conserved and includes about 10 putative B-type cytokinin signaling RR binding sites (Figure 4; Supplemental Figure 2; Hosoda et al., 2002; Sheen, 2002; Heyl and Schmülling, 2003; Imamura et al., 2003). Therefore, we named this middle region the CE region (for cytokinin response elements-containing). Because cytokinin signaling is essential for nodule organogenesis and induction of NIN expression, the CE region may be involved in regulation of NIN expression during initiation of nodule primordium formation.
The CE Region Contains Regulatory Elements Required for Nodule Organogenesis
To determine whether 3C (∼4 kb) contains regulatory sequences necessary for nodule primordium formation, we fused it to the (upstream) –5 kb region (ProNIN3C-5kb:NIN), as the latter is sufficient for infection. ProNIN3C-5kb:NIN was introduced into nin-1 by A. rhizogenes-mediated root transformation. Twenty-one of 26 analyzed transgenic roots (at 4 wpi) formed, on average, eight nodules per root (Figure 4B). As the CE region (∼1 kb) contains several putative cytokinin response elements, we tested whether this region is sufficient to trigger primordium formation. To this end, we transformed nin-1 with the CE region fused to the –5 kb region driving NIN (ProNINCE-5kb:NIN). This resulted in 18 out of 37 transgenic roots forming on average eight nodules per root (Figure 3B). This demonstrates that the CE region contains regulatory sequences that are required for primordium formation. Furthermore, the number of nodules formed on ProNINCE-5kb:NIN-expressing roots was similar to the number on wild-type roots transformed with an empty vector control (Figure 3B). This suggests that the autoregulation of nodulation mechanism is also activated (Soyano et al., 2014). In addition, 12 out of 22 nin-1 roots transformed with ProNINCE-5kb:NIN displayed wild-type-like infection thread numbers, indicating that the excessive infection phenotype can be partially rescued by including the CE region in the NIN promoter.
Normal nodules are pink due to the presence of leghemoglobin, which keeps the oxygen level low in the infected cells of the fixation zone so that the anaerobic process of nitrogen fixation can proceed. Pink nodules were formed on nin-1 roots transformed with either ProNIN3C-5kb:NIN or ProNINCE-5kb:NIN. Longitudinal sections of these nodules showed a zonation similar to wild-type nodules: a meristem at the apex; the infection zone, where rhizobia are released from the infection thread and subsequently divide and begin to enlarge; and the fixation zone, where rhizobia have reached their fully enlarged shape and are able to fix nitrogen (Figures 5A to 5D). Nodules formed by inoculation with S. meliloti carrying the PronifH:GFP (green florescent protein) reporter showed that nitrogenase nifH was expressed in the fixation zone, confirming that these nodules are functional (Supplemental Figure 3). Thus, CE in combination with the –5 kb region is sufficient to induce wild-type-like nodule organogenesis.
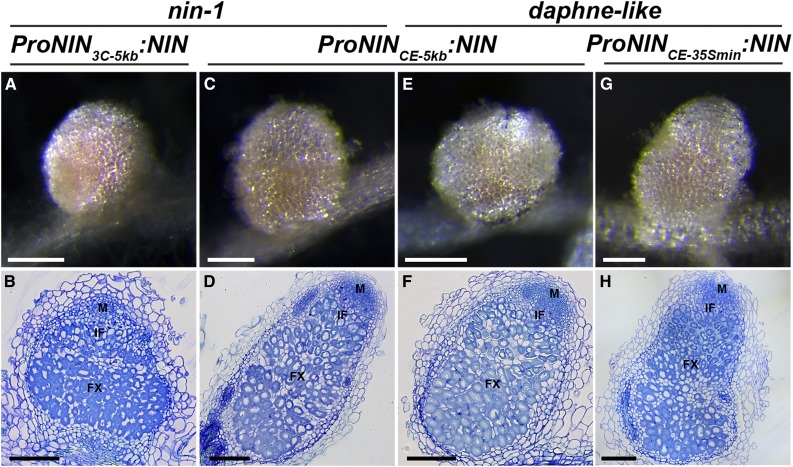
Figure 5.
Nonnodulating Phenotypes of nin-1 and daphne-like Are Rescued by A. rhizogenes-Mediated Transformation with ProNIN3C-5kb:NIN, ProNINCE-5kb:NIN, and ProNINCE-35Smin:NIN.
(A) to (D) Nodules formed on nin-1 roots transformed with the indicated constructs at 4 wpi with S. meliloti. Transmitted light macroscopy images of nodules are shown ([A] and [C]). Nodules are pink due to the presence of leghemoglobin. Longitudinal plastic sections of these nodules stained with toluidine blue display normal zonation ([B] and [D]). FX, fixation zone; IF, infection zone; M, meristem. Bars = 2 mm ([A] and [C]) and 200 µm ([B] and [D]).
(E) to (H) Nodules formed on daphne-like roots transformed with the indicated constructs at 4 wpi with S. meliloti. Transmitted light macroscopy images of nodules are shown ([E] and [G]). Longitudinal plastic sections of these nodules stained with toluidine blue display normal zonation ([F] and [H]). Bars = 2 mm ([E] and [G]) and 200 µm ([F] and [H]).
NIN Expression Cannot be Induced by Cytokinin in the daphne-like Mutant
Because the ProNINCE-5kb:NIN construct can fully restore nodulation ability in nin-1 roots, we used it to verify that daphne-like is indeed a nin allele. Therefore, daphne-like was transformed with ProNINCE-5kb:NIN (Figures 5E and 5F), and 15 of 17 transgenic roots analyzed at 4 wpi formed on average about seven nodules per root. The excessive infection phenotype in the daphne-like background was rescued by ProNINCE-5kb:NIN in 11 of these 17 transgenic roots. This result shows that daphne-like is a nin mutant. Its phenotype is most likely caused by the ∼2.49 Mb insertion by which the CE region is positioned too far away from the transcription start to contribute to the correct expression of NIN for nodule primordium formation.
Furthermore, to test whether the CE region is sufficient to complement nodule organogenesis in daphne-like, we used a minimal –46 bp CaMV 35S promoter (Benfey and Chua, 1990) fused to the CE region (ProNINCE-35Smin:NIN; Figures 5G and 5H). We found that 37 out of 45 transgenic daphne-like roots had formed on average four nodules per root at 4 wpi. This indicates not only that the CE region is sufficient to induce nodule organogenesis but also that, in combination with the –5 kb region, more nodules (about seven per root) can be formed. The ability to form nodules can be rescued in daphne-like by the CE region driving NIN expression. Therefore, it is likely that the CE region in daphne-like cannot regulate the expression of NIN.
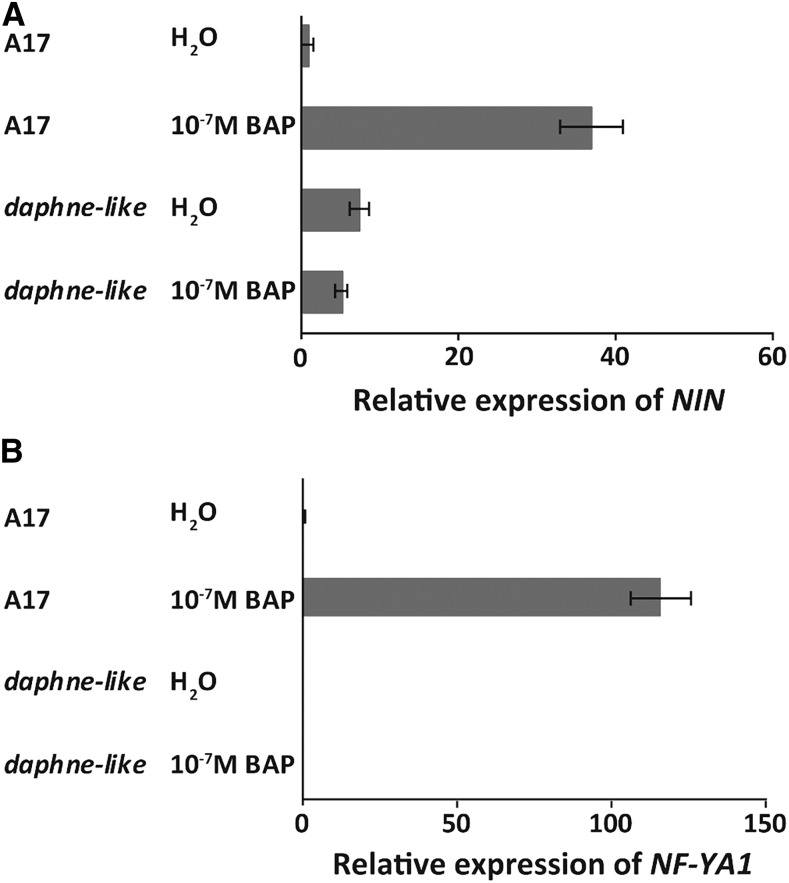
Because the CE region contains several putative response elements, we hypothesized that NIN expression would not be induced by cytokinin in daphne-like. To test this, we compared the induction of NIN expression by cytokinin versus water (as a control) in the wild type (A17) and daphne-like. We found that 16 h after 10−7 M benzylaminopurine application, NIN expression level increased 37-fold compared with the control and NF-YA1 expression level increased over 100-fold in the wild type, while both NIN and NF-YA1 expression levels in daphne-like were not changed (Figures 6A and 6B). This suggests that the CE region is required for the induction of NIN expression by cytokinin.
Figure 6.
NIN and NF-YA1 Expression Cannot Be Induced by Cytokinin Application in daphne-like Mutants.
Quantitative real-time RT-PCR shows NIN (A) and NF-YA1 (B) expression in wild-type (A17) and daphne-like roots 16 h after application of 10−7 M benzylaminopurine (BAP) or water. Data are means ± se of three biological replicates.
A Domain with Six Putative Cytokinin Response Elements Is Essential for Nodule Primordium Formation
Because cytokinin is known to be a positive regulator of nodule primordium formation (Suzaki et al., 2013), we tested whether the putative cytokinin response elements within the CE region are essential for primordium formation. To this end, we made several deletions in the CE region, which contains a 472 bp region that is highly conserved in all eight legume species studied here (Figures 3 and 4A; Supplemental Figure 2). We divided this 472 bp region into three parts named domains 1 to 3 (D1 to D3). D1 and D3 contain six and three putative cytokinin response elements, respectively, whereas D2 contains a putative AP2 binding site as well as a single cytokinin response element (Figure 4A; Supplemental Figure 2).
Several studies have shown that transcription factors of the AP2 family, including ERN (an ethylene response factor required for nodulation), are involved in regulating nodulation (Andriankaja et al., 2007; Middleton et al., 2007; Wang et al., 2014). To investigate their respective contributions to nodule primordium formation, the D1, D2, or D3 regions were separately deleted from the 1 kb CE region (Figure 4A; Supplemental Figure 2), and the modified CE regions were fused to the –5 kb region to drive NIN expression. These three constructs were introduced into nin-1 by A. rhizogenes-mediated root transformation. Our results show that deletion of D1 eliminated nodulation ability (Figure 4B; Supplemental Figure 4A), whereas deletion of D2 had no significant effect on nodulation (Figure 4B; Supplemental Figure 4B). Deletion of D3 caused a reduction of the relative number of roots with nodules from 49 to 21% and also reduced the average nodule number per root from eight to five (Figure 4B; Supplemental Figure 4C). These results show that regulatory sequences in D1 are essential for NIN-regulated nodule primordium formation and suggest that the putative cytokinin response elements within D1 are likely responsible. In contrast, the putative AP2 binding site in D2 is not essential for nodule organogenesis.
Induction of NIN Expression in Inner Root Cell Layers Occurs in a Non-Cell-Autonomous Manner
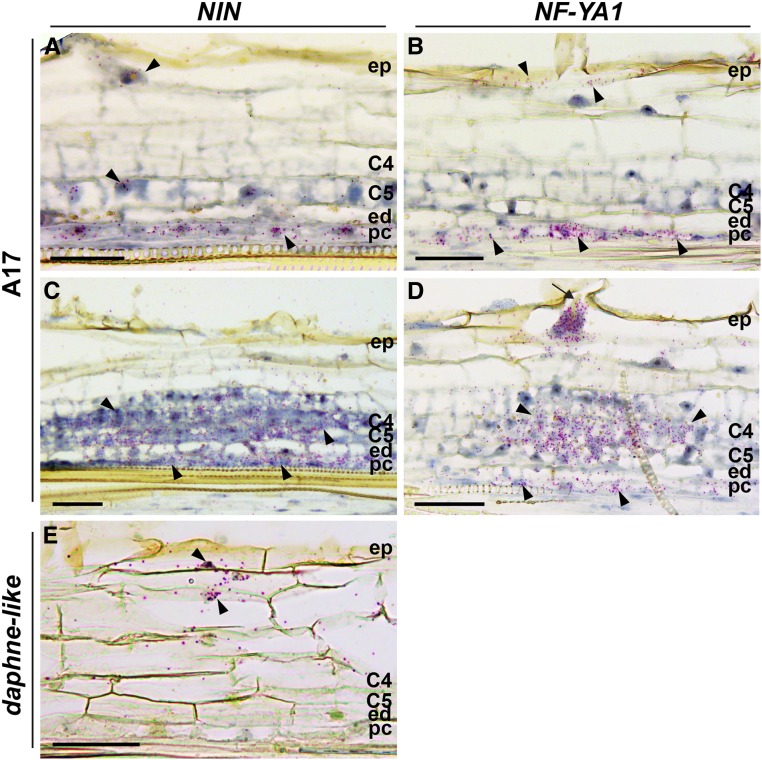
It was shown that the 2.2 kb upstream region of Medicago NIN is activated in the epidermis, 24 h after Nod factor application (Vernié et al., 2015). Because this promoter region lacks the regulatory sequences required for nodule organogenesis, we wanted to determine whether expression of NIN is induced in inner root cell layers during primordium formation. We studied the localization pattern of NIN mRNA in nodule primordia via in situ hybridization. Plants were grown on plates and spot inoculated with S. meliloti. We analyzed a primordial stage at 2 d post inoculation (dpi) in which the pericycle cells have divided and some anticlinal divisions have occurred in the inner cortical cell layers C4 and C5 (Figures 7A and 7B). We also analyzed cells at a slightly later stage at 3 dpi when cortical cells have divided more extensively (Figures 7C and 7D). At both stages, the infection thread had not yet reached the primordia. At the younger stage, NIN mRNA occurred in pericycle and epidermis, but it was hardly detectable in the divided cortical cells (Figure 7A). The highest expression level occurred in the pericycle-derived cells. At the stage when cortical cells have divided more extensively, the expression level of NIN in cortex-derived cells was similar to that in the pericycle (Figure 7C). This shows that expression of NIN was first strongly induced in the pericycle after which it extended to the other inner cell layers.
Figure 7.
NIN and NF-YA1 Expression Patterns in Medicago Wild-Type (A17) Nodule Primordia and daphne-like Mutant.
(A) to (D) RNA in situ localization of NIN ([A] and [C]) and NF-YA1 ([B] and [D]) in nodule primordia at 2 dpi ([A] and [B]) and at 3 dpi ([C] and [D]). The arrow indicates an infection thread.
(E) RNA in situ localization of NIN in roots of the daphne-like mutant at 2 dpi.
Hybridization signals are visible as red dots (arrowheads). C4 and C5, cortical cell layers 4 and 5; ed, endodermis; ep, epidermis; pc, pericycle. Bars = 50 µm.
NF-YA1 is a known direct target of NIN (Soyano et al., 2013). Like NIN, it is expressed in the epidermis, where it regulates rhizobial infection (Laporte et al., 2014). To test whether NIN might also regulate NF-YA1 expression in the primordia, we performed RNA in situ hybridization using NF-YA1 as a probe. This analysis showed that NF-YA1 expression is similar to NIN expression, as it is also first induced in pericycle and most likely cortical cell division precedes NIN and NF-YA1 expression (Figures 7B and 7D). This suggests that NF-YA1 is regulated by NIN in both pericycle and other nodule primordium cells.
Therefore, rhizobia present in the epidermis induce NIN and NF-YA1 expression in the pericycle-derived cells. Furthermore, because Nod factors are immobile molecules (Goedhart et al., 2000) that do not diffuse to the inner cell layers, NIN and NF-YA expression in the inner cell layer is most likely induced by a mobile signal generated in the epidermis where Nod factor signaling takes place.
The CE Region Is Required for Induction of NIN Expression in the Pericycle
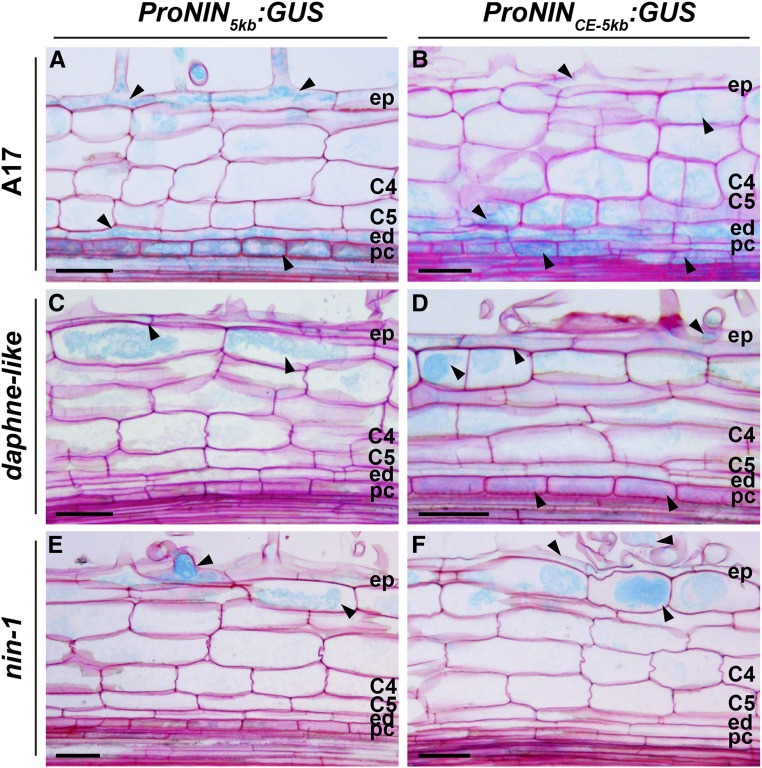
We wanted to test whether the CE region is required for NIN expression in the inner cell layers, so we compared expression patterns of ProNINCE-5kb:GUS (beta-glucuronidase) and ProNIN5kb:GUS in roots. We first introduced these constructs into wild-type Medicago (A17) roots by A. rhizogenes-mediated transformation. We analyzed an early stage of primordium development when pericycle cells have divided and some anticlinal divisions have occurred in the inner cortical cell layers similar to the early stage tested for in situ. Both constructs were expressed in epidermis, pericycle, and endodermis, and a lower signal was detected in some cortical cells (Figures 8A and 8B). This result is surprising, considering that ProNIN5kb:NIN is not sufficient for primordium formation in the nin-1 background. Therefore, we hypothesized that expression of ProNIN5kb:GUS in inner cell layers is induced by endogenous NIN that is produced in the wild-type background. This implies that NIN expression in the inner layers is regulated by a positive feedback loop involving NIN itself and that the essential cis-regulatory elements required for this are located in the –5 kb promoter region.
Figure 8.
The CE Region Is Required for Rhizobium-Induced NIN Expression in the Pericycle.
Tissue-specific ProNIN5kb:GUS and ProNINCE-5kb:GUS expression patterns are shown in the wild type and nin mutants at 2 dpi. Arrowheads indicate GUS expression (light blue) in wild-type ([A] and [B]), in daphne-like ([C] and [D]), and in nin-1 ([E] and [F]) roots. C4 and C5, cortical cell layers 4 and 5; ed, endodermis; ep, epidermis; pc, pericycle. Bars = 50 µm.
To test this hypothesis, we introduced ProNINCE-5kb:GUS and ProNIN5kb:GUS into daphne-like by A. rhizogenes-mediated transformation. In daphne-like, infection threads can be formed indicating that NIN is induced in the epidermis and the production of the mobile signal might not be affected. However, nodule primordium formation is impaired, indicating that there is no NIN production in the inner cell layers. Indeed, ProNIN5kb:GUS transgenic roots showed GUS expression only in epidermis and outer cortex (Figure 8C), whereas no expression was observed in the pericycle cells. In contrast, ProNINCE-5kb:GUS transgenic roots showed GUS expression in epidermis, outer cortex, and the pericycle (Figure 8D). In this case, cell division was not induced in the pericycle, due to the absence of NIN. Taken together, these results demonstrate that the CE region regulates NIN expression in the pericycle prior to cell division in wild-type roots. This means that the CE region is required for the initial induction of NIN expression in the pericycle. In addition, the expression of ProNINCE-5kb:GUS in the pericycle of daphne-like is weak, which is consistent with the involvement of NIN in a feedback loop by (directly or indirectly) positively regulating its own expression.
To further demonstrate that the CE region is required for NIN expression in the pericycle, we studied NIN expression in daphne-like roots using RNA in situ hybridization at 2 dpi with rhizobia. In contrast to the wild type (Figure 7A), NIN is expressed in the epidermis and outer cortex but not in the pericycle (Figure 7E). This result supports the idea that the CE region is required for NIN expression in the pericycle.
Induction of NIN in the Pericycle Depends on NIN Expression in the Epidermis
It is likely that a mobile signal generated by Nod factor signaling in the epidermis induces NIN expression in the pericycle. If true, NIN expression in the pericycle would depend on NIN induction in the epidermis. To test this, we introduced ProNINCE-5kb:GUS and ProNIN5kb:GUS into nin-1 by hairy root transformation. In both cases, GUS was present only in the epidermis and outer cortex, and not in the pericycle, at 3 dpi (Figures 8E and 8F). This suggests that NIN is required in the epidermis, probably for the generation of the mobile signal, in order to induce NIN expression in pericycle cells.
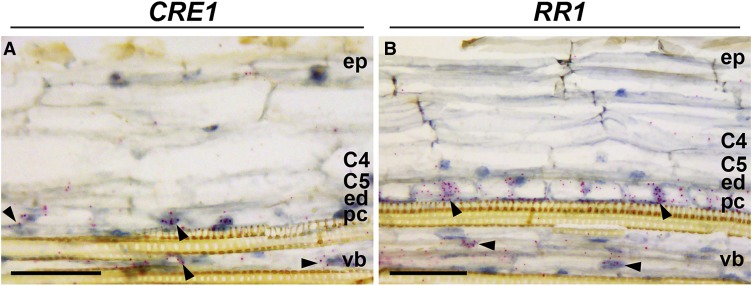
CRE1 and RR1 Are Expressed in the Pericycle of Uninoculated Roots
Rhizobium-induced NIN expression in the pericycle is dependent on the CE region, and it precedes formation of nodule primordia. The occurrence of multiple B-type RR response regulatory elements in the CE region suggests that the cytokinin signaling machinery is important for NIN transcriptional activation in the pericycle. To examine this, we determined the expression pattern of the cytokinin receptor CRE1 and its putative target the B-type response regulator RR1, which is known to be expressed during nodule formation (Gonzalez-Rizzo et al., 2006). Using RNA in situ hybridization, we found that CRE1 is actively transcribed in pericycle and vasculature cells of uninoculated roots, but not in endodermal or cortical cells (Figure 9A). Also, messenger RNA (mRNA) of the B-type RR1 was present at the highest level in pericycle cells and to a lower extent in root vasculature cells (Figure 9B). Therefore, both CRE1 and RR1 have been already expressed in the pericycle by the time rhizobial signaling starts, suggesting that, initially, only this layer is responsive to cytokinin.
Figure 9.
CRE1 and RR1 Are Expressed in the Pericycle of Uninoculated Roots.
RNA in situ localization of CRE1 (A) and RR1 (B) is shown in the susceptible zone of uninoculated roots. For in situ hybridization, root tips of ∼1 cm of 4-d-old seedlings were used. Hybridization signals are visible as red dots (arrowheads). C4 and C5, cortical cell layers 4 and 5; ed, endodermis; ep, epidermis; pc, pericycle; vb, vascular bundle. Bars = 50 µm.
DISCUSSION
In this study, we show that remote upstream regulatory sequences (the CE region) are required for proper regulation of NIN expression and Medicago nodule organogenesis. By contrast, regulatory sequences required for the infection process are located within a 5 kb region directly upstream of the start codon. The CE region contains several putative cytokinin response elements, and D1, which contains six of these elements, is essential for nodule primordia formation. The CE region appears to be important for cytokinin-induced expression of NIN, as daphne-like has lost this ability. Formation of nodule primordium initiates with NIN induction in the pericycle, and subsequently it extends to the cortical cells. The fact that CRE1 and RR1 are expressed in the pericycle supports the idea that cytokinin perception is necessary for the induction of NIN at the start of primordium formation.
In animals, many genes have been identified that are regulated by remote cis-regulatory elements that can be megabases away from the transcription start site. By contrast, in plants, only a few remote cis-regulatory sequences are known (Symmons and Spitz, 2013; Shlyueva et al., 2014; Weber et al., 2016). One of the best characterized remote cis-regulatory sequences is the enhancer of booster1 in Zea mays, which is located 100 kb upstream of the gene (Stam et al., 2002). It has been shown that gene activation by remote enhancers can be associated with chromatin loop formation that brings the enhancer in close proximity to the promoter, a process that can facilitate assembly of transcription complexes (Cook, 2003; Nolis et al., 2009; Deng et al., 2012). The distance between the CE region and the transcription start site varies in the legume species studied here. In L. angustifolius it is about –7 kb, whereas in Lotus it is about –45 kb. We demonstrated that the CE region fused to the –5 kb promoter can rescue nodule organogenesis in Medicago. This shows that the sequences between the CE region and the –5 kb region are not essential for nodule organogenesis. However, we cannot exclude the possibility that in this region there are regulatory sequences required for fine-tuning NIN expression.
During the infection process, NIN participates in a mechanism wherein root hair growth stops when a proper curl is formed. Regulatory sequences required for this process are located within the –2.2 kb promoter region. The fact that this region lacks the putative CYCLOPS binding site implies that, in addition to CYCLOPS (IPD3 in Medicago), another transcription factor or factors is involved in regulating NIN expression in the epidermis. Because this –2.2 kb region is not sufficient for efficient infection thread formation, we assume that the expression level of NIN in the epidermis remains below the threshold level required for infection thread formation, whereas this level can be reached by the –5 kb promoter region that includes the putative CYCLOPS binding site (Figure 10).
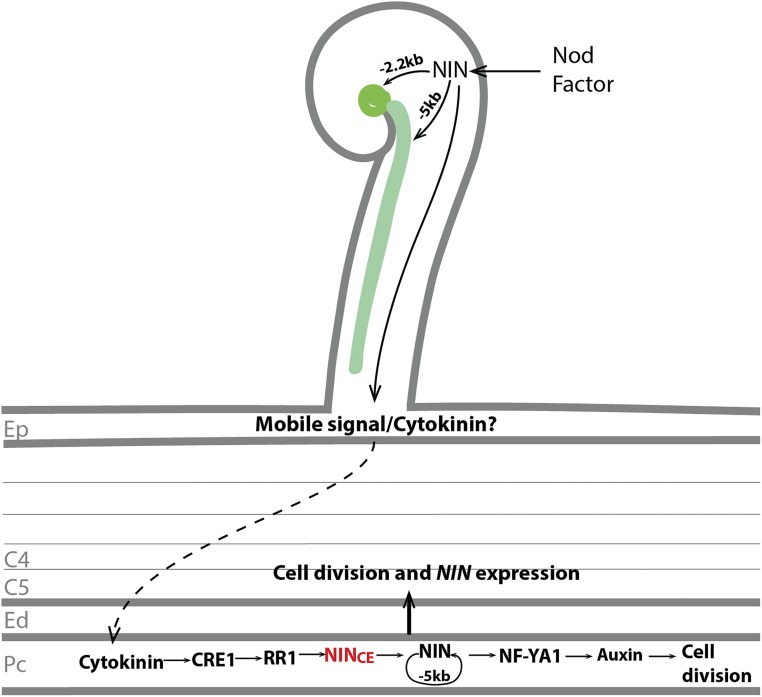
Figure 10.
Proposed Model of NIN Function during Initiation of Nodule Primordia.
After perception of the Nod factor, NIN expression is induced in the epidermis. The –5 kb regulatory region of the NIN promoter is sufficient for both tight root hair curling and infection thread formation. By contrast, expression driven by the –2.2 kb region is sufficient only for the tight root hair curling and formation of bacterial colonies inside the curl. A mobile signal is generated in the epidermis in a NIN-dependent manner, and this signal translocates to the pericycle. Whether or not this mobile signal is cytokinin or an unknown signal, it causes cytokinin accumulation in the inner root cell layers. The CRE1 receptor in the pericycle perceives cytokinin and activates the B-type RR1, which further activates NIN expression. NIN directly or indirectly regulates its own expression via a positive feedback loop, and the –5 kb promoter region is sufficient for this feedback regulation. NIN directly activates NF-YA1 expression and stimulates further cell divisions. Later, the NIN-induced response in pericycle contributes to cell division and NIN expression in the endodermis and cortical cells. C4 and C5, cortical cell layers 4 and 5; Ed, endodermis; Ep, epidermis; Pc, pericycle.
We present a model for the regulation of expression of NIN in Figure 10. After the rapid induction of NIN in the epidermis, NIN is subsequently induced in the pericycle. The latter most likely precedes the mitotic activation of pericycle cells. The induction of NIN in the pericycle requires the presence of the CE region and involves a positive feedback loop including NIN itself. The proposed feedback loop was based on our observation that expression of ProNIN5kb:GUS in the Medicago wild-type background was induced in nodule primordia, despite the fact that this promoter region is not sufficient to trigger primordium formation. This result is similar to what was found in Lotus, where a promoter region of NIN that does not trigger primordium formation was sufficient to drive expression of GUS in primordia (Heckmann et al., 2011; Kosuta et al., 2011; Yoro et al., 2014).
Our conclusion that nodule primordium formation requires the induction of NIN expression in inner root layers is consistent with the observation that nodule organogenesis is restored in the Lotus daphne mutant by NIN driven by a heterologous Arabidopsis (Arabidopsis thaliana) enhancer that is active in endodermis and cortex (Yoro et al., 2014). When we transformed the Medicago nin null mutant with the ProNIN2.2kb:NIN construct, nodule organogenesis was not restored. By contrast, Vernié et al. (2015) reported the formation of nodules on a Medicago nin null mutant transformed with a similar construct. However, the nodule number was very low and nodules were observed a long time (50 d) after inoculation. To determine whether these structures are indeed genuine nodules and not simply modified lateral roots, analysis of sections is required.
Deletion of sequences within the CE region, which contains six putative cytokinin response elements, blocks primordium formation. We hypothesize that cytokinin signaling in the pericycle induces NIN expression. This hypothesis is supported by the fact that the expression of CRE1 and RR1 is observed in the pericycle before rhizobial signaling is initiated. This agrees with a previous study showing that a CRE1 promoter region driving GUS expression is specifically expressed in endodermis/pericycle cells opposite the protoxylem poles (Boivin et al., 2016), the sites where nodule primordia are formed (Heidstra et al., 1997). The involvement of the CE region in cytokinin-induced NIN expression is indicated by the daphne-like mutant, which has lost this ability. However, it remains to be proven that the CE region is sufficient for the cytokinin-induced NIN expression. Our attempts to show this in A. rhizogenes-mediated hairy root transformation were inconclusive due to high basal levels of NIN expression in the absence of applied cytokinin in hairy roots.
The CE region is conserved in the eight legume species that we studied. They belong to different clades of the legume Papilionoideae subfamily, representing the Genistoids, Inverted Repeat-lacking clade, Robinioids, Milletioids, and Dalbergioids clades. This suggests that the regulation of NIN expression by cytokinin is conserved in this subfamily. After the induction of NIN in the pericycle, NIN expression extends to the endodermis and inner cortex. In young nodule primordia in which cortical cells have divided anticlinally (Figures 7A and 7B), expression of both NIN and NF-YA1 are highest in pericycle, and it is hardly detectable in the divided cortical and endodermal cells. This suggests that NIN-induced responses in the pericycle contribute to cell division in endodermis and cortical cells (Figure 10). At a later stage of development, NIN is expressed in the dividing cortical cells (Figures 7C and 7D). How NIN expression is regulated in these cells remains to be studied.
Expression of cytokinin biosynthesis genes as well as bioactive cytokinin accumulation are induced by Nod factor signaling (van Zeijl et al., 2015). As it is a mobile molecule, it is likely that this results in the accumulation of cytokinin in (at least) the pericycle. Whether cytokinin biosynthesis in the pericycle is triggered by an as yet unknown mobile signal generated in epidermis or whether cytokinin itself is this mobile signal that accumulates in the pericycle is not known.
Cell division in nodule primordia correlates with auxin accumulation, and this occurs before the first cell division (Mathesius et al., 1998; Suzaki et al., 2012). Auxin accumulation (DR5 expression) depends on NIN, as it does not occur in a nin null mutant (Suzaki et al., 2012). Furthermore, ectopic expression of both NIN and NF-YA1 is sufficient to induce abnormal cell division during lateral root development (Soyano et al., 2013), suggesting that their expression causes the local accumulation of auxin. Therefore, we hypothesize that cytokinin signaling in the pericycle triggers NIN expression and that this results in the local accumulation of auxin, which subsequently triggers mitotic activity (Figure 10). This is supported by a previous study showing that STY genes are targets of NF-YA1 (Hossain et al., 2016). STY genes encode transcription factors that have been shown to regulate YUCCA auxin biosynthesis genes in Arabidopsis (Sohlberg et al., 2006; Eklund et al., 2010a, 2010b). If this is the case, then during nodule primordium formation, NIN-induced NF-YA expression in the pericycle might induce the local production of auxin, which subsequently induces cell division in pericycle, endodermis, and cortex.
METHODS
Plant Material and Growth, Hairy Root Transformation, and Inoculation with Rhizobia
Medicago (Medicago truncatula) cv Jemalong A17 was used as the wild type. Agrobacterium rhizogenes msu 440-mediated hairy root transformation was performed according to Limpens et al. (2004). Medicago plants were grown in perlite saturated with low-nitrate [0.25 mM Ca(NO3)2] Färhaeus medium (Catoira et al., 2000) at 21°C under a 16-h-light/8-h-dark regime. After 1 week of growth, plants were inoculated with Sinorhizobium meliloti 2011 constitutively expressing GFP or carrying the PronifH:GFP reporter (OD600 = 0.1, 1 mL per plant). Plants growing on Färhaeus plates were spot inoculated with 0.5 μL of rhizobium suspension per root.
DNA Constructs
DNA fragments of NIN including the 3′ untranslated region and promoter regions were generated by PCR using Medicago genomic DNA as a template and Phusion high-fidelity DNA polymerase (Finnzymes) with the specific primers listed in Supplemental Table 2. The DNA fragments used for pENTR-D-TOPO cloning (Invitrogen) were amplified with forward primers containing an extra 5′-CACC sequence. Forward primers containing an attB4 site (5′-GGGGACAACTTTGTATAGAAAAGTTGNN-3′) and reverse primers with an attB1 site (5′-GGGGACTGCTTTTTTGTACAAACTTGN-3′) were used to generate DNA fragments for cloning into pDONOR P4-P1 by BP recombination (Invitrogen). The forward primers with attB2 (5′-GGGGACAGCTTTCTTGTACAAAGTGGAA-3′) and reverse primers with attB3 (5′-GGGGACAACTTTGTATAATAAAGTTGC-3′) were used to amplify DNA fragments for cloning into pDONOR P2-P3. To generate deletions (D1/D2/D3) in the CE region and deletion of the putative CYCLOPS binding site in the –5 kb region, two rounds of PCR were performed. In the first round, two DNA fragments that are separated by the deletion were amplified with specific primers to introduce a 15 bp overhang (Supplemental Table 2). Subsequently, the PCR products were purified and mixed and 5 μL of this mixture was used as a template in a second round of PCR with ProNINCE-F and ProNINCE-R or ProNIN5kb-F and ProNIN5kb-R primers (Supplemental Table 2). This allowed creation of a single amplicon with a deletion in either the CE or the –5 kb region. The entry vectors were recombined into the modified Gateway binary vector pKGW-RR-MGW (Ovchinnikova et al., 2011) using Multisite LR recombination (Invitrogen).
Histological Analysis and Microscopy
Transgenic roots carrying the ProNIN:GUS constructs were incubated in GUS buffer (3% [w/v] sucrose, 10 mM EDTA, 2 mM k-ferrocyanide, 2 mM k-ferricyanide, and 0.5 mg/mL 5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid, cyclohexylammonium salt [X-Gluc] in 0.1 M phosphate buffer [pH = 7]) at 37°C for 1 to 2 h. Embedding of plant tissue in plastic, sectioning, and tissue staining were performed as described by Xiao et al. (2014). Sections were analyzed using a DM5500B microscope equipped with a DFC425C camera (Leica). Bright-field and fluorescence images of transgenic roots and nodules were taken using a stereomacroscope (M165 FC, Leica). Confocal images were taken with an SP8 (Leica) microscope, using excitation wavelengths of 488 and 543 nm for GFP and propidium iodide, respectively.
RNA Isolation and qRT-PCR
RNA was isolated from 1-week-old A17 and daphne-like roots using the EZNA Plant RNA mini kit (Omega Bio-tek). For cDNA synthesis, 1 µg of this RNA was used with the iScript cDNA synthesis kit (Bio-Rad). Real-time qPCR was performed in 10-μL reactions using SYBR Green Supermix (Bio-Rad) and a CFX real-time system (Bio-Rad). Gene expression levels were determined using the primers listed in Supplemental Table 2. The gene expression was normalized using ACTIN2 as a reference gene.
Quantification of Colonies, Infection Threads, and Nodules
To quantify the number of curled root hairs containing colonies or infection threads, more than 20 transgenic roots (5–10 cm long) were cut into fragments of ∼1 cm and randomly selected for counting. To quantify the nodule number per root, 5- to 10-cm-long transgenic roots were selected.
RNA in Situ Hybridization
Medicago roots were fixed with 4% paraformaldehyde mixed with 3% glutaraldehyde in 50 mM phosphate buffer (pH 7.4) and were then embedded in paraffin (Paraplast X-tra, McCormick Scientific). Root sections (7 μm) were prepared using an RJ2035 microtome (Leica). RNA in situ hybridization was conducted using Invitrogen ViewRNA ISH Tissue 1-Plex Assay kits (Thermo Fisher Scientific) and was performed according to the user manual available at https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets/LSG/manuals/UM17400-ViewRNA-ISH-Tissue-1-Plex-Assay.pdf. RNA ISH probe sets were designed and synthesized by request at Thermo Fisher Scientific. Catalogue numbers of probes for Medicago genes are as follows: VF1-20312 for NIN, VF1-6000865 for CRE1, VF1-6000866 for RR1, and VF-20311 for NF-YA1. A typical probe set consisted of ∼20 pairs of oligonucleotide probes (20 nucleotides long) that hybridize to specific regions across the target mRNA. Each probe was composed of a region of ∼20 nucleotides, a short linker region, and a tail sequence. The two tail sequences (double Z) together form a site for signal amplification. This design controls increased background by reducing the chance of a nonspecific hybridization event being amplified. For the nodulation-specific genes, we used uninoculated roots as a negative control. For ISH with CRE1 and RR1 performed on noninoculated roots of 4-d-old seedlings, we used ENOD2 (nodule-specific gene) probe set as a negative control. Images were taken with an AU5500B microscope equipped with a DFC425c camera (Leica).
Map-Based Cloning of daphne-like
A segregating F2 population resulting from a cross between FN8113 (cv Jemalong A17) and cv Jemalong A20 (118 plants) was made. DNA was extracted using a standard cetyl-trimethyl-ammonium bromide DNA miniprep method (Taylor and Powell, 1982). Initially, simple sequence repeat markers based on Mun et al. (2006) were used to determine the global chromosomal location of the FN8113 locus. Subsequently, additional simple sequence repeat markers were developed for the FN8113 locus on chromosome 5 and were used for chromosome walking. PCR was performed using 100 ng of genomic DNA and was analyzed on 2.5% agarose gels. The simple sequence repeat marker JH5.17 (Supplemental Table 2) on bacterial artificial chromosome clone CU424494 showed the closest linkage to the FN8113 locus. No crossovers were found at the distal end of chromosome 5. Next, whole-genome sequencing (Illumina Hiseq2000, paired-end) was used to identify mutations in the genomic region identified from the genetic mapping. The genomic sequence of the mutated region is provided in Supplemental Data Set. Cleaned DNA sequence reads were mapped against the Medicago genome (Young et al., 2011) using the bwa_mem algorithm (Li and Durbin, 2010). Clipped reads and mismapped mate pairs revealed an interchromosomal translocation, and this was further confirmed by aligning reads spanning the mutation to the genome using BLASTN (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Alignment of Upstream Regions of NIN
Most of the alignment work used Geneious v8.1.9 (https://www.geneious.com) (Kearse et al., 2012). The Medicago NIN protein sequence was analyzed using custom BLAST databases and Geneious v8.1.9 (Altschul et al., 1990; Kearse et al., 2012). A diverse selection of legume species with a good quality of publicly available genomic sequences were used: Medicago (Young et al., 2011; Tang et al., 2014), Lotus japonicus (Sato et al., 2008), Arachis duranensis (Bertioli et al., 2016), Cicer arietinum (Varshney et al., 2013), Glycine max (Schmutz et al., 2010), Lupinus angustifolius (Hane et al., 2017), Cajanus cajan (Varshney et al., 2011), and Trifolium pratense (De Vega et al., 2015). Selected NIN scaffolds (Supplemental Table 1) and up to 80 kb of upstream sequence and 10 kb of downstream sequence of NIN were extracted. Selected sequences were custom aligned using the mVISTAs web-based alignment tool (http://genome.lbl.gov/vista/mvista) (Frazer et al., 2004). The alignment program selected was the shuffle-lagan global alignment program, which detects rearrangements (Brudno et al., 2003). In addition to this larger scale alignment, individual alignments were made using MAUVE as a Geneious plugin (Darling et al., 2004). This better allowed for more precise determination of conserved sequences relative to the NIN start codon in all species. A complete overview of detected conserved regions can be found in Supplemental Table 1.
Alignment of CE Regions and Prediction of Binding Sites
Detected conserved sequences of CE regions for selected scaffolds (Supplemental Table 1) were aligned using MAFFTv7.017 as a Geneious plugin (Katoh et al., 2002). Conserved binding sites were predicted by using PlantPAN2.0 (Chow et al., 2016). Some sites were manually added based on homology with known putative B-type RR binding sequences (Hosoda et al., 2002; Heyl and Schmülling, 2003; Imamura et al., 2003).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL or Mt4.0v1 databases under the following accession numbers: NIN (Medtr5g099060), CRE1 (Medtr8g106150), NF-YA1 (Medtr1g056530), and RR1 (Medtr3g102600).
Supplemental Data
Supplemental Figure 1. MAFFT alignment of the putative CYCLOPS binding site of eight legume species.
Supplemental Figure 2. MAFFT alignment of the 472 bp conserved region of eight legume species.
Supplemental Figure 3. nifH expression is induced in ProNINCE-5kb:NIN transgenic nin-1 root nodules.
Supplemental Figure 4. Phenotype of nin-1 transformed with ProNINCE(ΔD1/D2/D3)-5kb:NIN constructs.
Supplemental Table 1. Sequence information of aligned species.
Supplemental Table 2. Primers used in this study.
Supplemental Data Set. Genomic sequence of the mutated region in FN8113.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This research was supported by the EC European Research Council (ERC-2011-AdG-294790), the Netherlands Organization for Scientific Research (863.15.010 and 865.13.001), and the China Scholarship Council (201506300062 to J.L.).
AUTHOR CONTRIBUTIONS
J.L., L.R., O.K., R.G., and T.B. designed the research. J.L., L.R., O.K., E.L., T.v.d.M., R.v.V., and W.K. performed research and analyzed data. E.L., R.v.V., R.C., and Y.C. identified the daphne-like mutant. J.L., T.B., and O.K. wrote the article.
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Andriankaja A., Boisson-Dernier A., Frances L., Sauviac L., Jauneau A., Barker D.G., de Carvalho-Niebel F. (2007). AP2-ERF transcription factors mediate Nod factor dependent Mt ENOD11 activation in root hairs via a novel cis-regulatory motif. Plant Cell 19: 2866–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P.N., Chua N.-H. (1990). The cauliflower mosaic virus 35S promoter: Combinatorial regulation of transcription in plants. Science 250: 959–966. [DOI] [PubMed] [Google Scholar]
- Bertioli D.J., et al. (2016). The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48: 438–446. [DOI] [PubMed] [Google Scholar]
- Boivin S., Kazmierczak T., Brault M., Wen J., Gamas P., Mysore K.S., Frugier F. (2016). Different cytokinin histidine kinase receptors regulate nodule initiation as well as later nodule developmental stages in Medicago truncatula. Plant Cell Environ. 39: 2198–2209. [DOI] [PubMed] [Google Scholar]
- Brudno M., Malde S., Poliakov A., Do C.B., Couronne O., Dubchak I., Batzoglou S. (2003). Glocal alignment: Finding rearrangements during alignment. Bioinformatics 19 (suppl. 1): i54–i62. [DOI] [PubMed] [Google Scholar]
- Catoira R., Galera C., de Billy F., Penmetsa R.V., Journet E., Maillet F., Rosenberg C., Cook D., Gough C., Dénarié J. (2000). Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell 12: 1647–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.N., Zheng H.Q., Wu N.Y., Chien C.H., Huang H.D., Lee T.Y., Chiang-Hsieh Y.F., Hou P.F., Yang T.Y., Chang W.C. (2016). PlantPAN 2.0: An update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 44: D1154–D1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P.R. (2003). Nongenic transcription, gene regulation and action at a distance. J. Cell Sci. 116: 4483–4491. [DOI] [PubMed] [Google Scholar]
- Darling A.C.E., Mau B., Blattner F.R., Perna N.T. (2004). Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14: 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Lee J., Wang H., Miller J., Reik A., Gregory P.D., Dean A., Blobel G.A. (2012). Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149: 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vega J.J., et al. (2015). Red clover (Trifolium pratense L.) draft genome provides a platform for trait improvement. Sci. Rep. 5: 17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J.A. (2014). Legume nodulation. Curr. Biol. 24: R184–R190. [DOI] [PubMed] [Google Scholar]
- Ehrhardt D.W., Wais R., Long S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85: 673–681. [DOI] [PubMed] [Google Scholar]
- Eklund D.M., Ståldal V., Valsecchi I., Cierlik I., Eriksson C., Hiratsu K., Ohme-Takagi M., Sundström J.F., Thelander M., Ezcurra I., Sundberg E. (2010a). The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. Plant Cell 22: 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund D.M., Thelander M., Landberg K., Ståldal V., Nilsson A., Johansson M., Valsecchi I., Pederson E.R.A., Kowalczyk M., Ljung K., Ronne H., Sundberg E. (2010b). Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens. Development 137: 1275–1284. [DOI] [PubMed] [Google Scholar]
- Fournier J., Teillet A., Chabaud M., Ivanov S., Genre A., Limpens E., de Carvalho-Niebel F., Barker D.G. (2015). Remodeling of the infection chamber before infection thread formation reveals a two-step mechanism for rhizobial entry into the host legume root hair. Plant Physiol. 167: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer K.A., Pachter L., Poliakov A., Rubin E.M., Dubchak I. (2004). VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 32: W273–W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedhart J., Hink M.A., Visser A.J.W.G., Bisseling T., Gadella T.W.J. Jr (2000). In vivo fluorescence correlation microscopy (FCM) reveals accumulation and immobilization of Nod factors in root hair cell walls. Plant J. 21: 109–119. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S., Crespi M., Frugier F. (2006). The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18: 2680–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane J.K., et al. (2017). A comprehensive draft genome sequence for lupin (Lupinus angustifolius), an emerging health food: Insights into plant-microbe interactions and legume evolution. Plant Biotechnol. J. 15: 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Shimoda Y., Sato S., Tabata S., Imaizumi-Anraku H., Hayashi M. (2014). Rhizobial infection does not require cortical expression of upstream common symbiosis genes responsible for the induction of Ca2+ spiking. Plant J. 77: 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann A.B., Sandal N., Bek A.S., Madsen L.H., Jurkiewicz A., Nielsen M.W., Tirichine L., Stougaard J. (2011). Cytokinin induction of root nodule primordia in Lotus japonicus is regulated by a mechanism operating in the root cortex. Mol. Plant Microbe Interact. 24: 1385–1395. [DOI] [PubMed] [Google Scholar]
- Heidstra R., Yang W.C., Yalcin Y., Peck S., Emons A.M., van Kammen A., Bisseling T. (1997). Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 124: 1781–1787. [DOI] [PubMed] [Google Scholar]
- Heyl A., Schmülling T. (2003). Cytokinin signal perception and transduction. Curr. Opin. Plant Biol. 6: 480–488. [DOI] [PubMed] [Google Scholar]
- Horváth B., et al. (2011). Medicago truncatula IPD3 is a member of the common symbiotic signaling pathway required for rhizobial and mycorrhizal symbioses. Mol. Plant Microbe Interact. 24: 1345–1358. [DOI] [PubMed] [Google Scholar]
- Hosoda K., Imamura A., Katoh E., Hatta T., Tachiki M., Yamada H., Mizuno T., Yamazaki T. (2002). Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 14: 2015–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., et al. (2016). Lotus japonicus NF-YA1 plays an essential role during nodule differentiation and targets members of the SHI/STY gene family. Mol. Plant Microbe Interact. 29: 950–964. [DOI] [PubMed] [Google Scholar]
- Imamura A., Kiba T., Tajima Y., Yamashino T., Mizuno T. (2003). In vivo and in vitro characterization of the ARR11 response regulator implicated in the His-to-Asp phosphorelay signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 44: 122–131. [DOI] [PubMed] [Google Scholar]
- Jardinaud M.-F., Boivin S., Rodde N., Catrice O., Kisiala A., Lepage A., Moreau S., Roux B., Cottret L., Sallet E., Brault M., Emery R.J., et al. (2016). A laser dissection-RNAseq analysis highlights the activation of cytokinin pathways by Nod factors in the Medicago truncatula root epidermis. Plant Physiol. 171: 2256–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., et al. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R.M., Bohlool B.B. (1984). Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 75: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S., Held M., Hossain M.S., Morieri G., Macgillivary A., Johansen C., Antolín-Llovera M., Parniske M., Oldroyd G.E.D., Downie A.J., Karas B., Szczyglowski K. (2011). Lotus japonicus symRK-14 uncouples the cortical and epidermal symbiotic program. Plant J. 67: 929–940. [DOI] [PubMed] [Google Scholar]
- Laporte P., Lepage A., Fournier J., Catrice O., Moreau S., Jardinaud M.-F., Mun J.-H., Larrainzar E., Cook D.R., Gamas P., Niebel A. (2014). The CCAAT box-binding transcription factor NF-YA1 controls rhizobial infection. J. Exp. Bot. 65: 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. (2010). Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens E., Ramos J., Franken C., Raz V., Compaan B., Franssen H., Bisseling T., Geurts R. (2004). RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J. Exp. Bot. 55: 983–992. [DOI] [PubMed] [Google Scholar]
- Marsh J.F., Rakocevic A., Mitra R.M., Brocard L., Sun J., Eschstruth A., Long S.R., Schultze M., Ratet P., Oldroyd G.E.D. (2007). Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol. 144: 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U., Schlaman H.R.M., Spaink H.P., Of Sautter C., Rolfe B.G., Djordjevic M.A. (1998). Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 14: 23–34. [DOI] [PubMed] [Google Scholar]
- Middleton P.H., et al. (2007). An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell 19: 1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R.M., Gleason C.A., Edwards A., Hadfield J., Downie J.A., Oldroyd G.E., Long S.R. (2004). A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc. Natl. Acad. Sci. USA 101: 4701–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun J.H., et al. (2006). Distribution of microsatellites in the genome of Medicago truncatula: A resource of genetic markers that integrate genetic and physical maps. Genetics 172: 2541–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolis I.K., McKay D.J., Mantouvalou E., Lomvardas S., Merika M., Thanos D. (2009). Transcription factors mediate long-range enhancer-promoter interactions. Proc. Natl. Acad. Sci. USA 106: 20222–20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikova E., et al. (2011). IPD3 controls the formation of nitrogen-fixing symbiosomes in pea and Medicago spp. Mol. Plant Microbe Interact. 24: 1333–1344. [DOI] [PubMed] [Google Scholar]
- Plet J., Wasson A., Ariel F., Le Signor C., Baker D., Mathesius U., Crespi M., Frugier F. (2011). MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 65: 622–633. [DOI] [PubMed] [Google Scholar]
- Sato S., et al. (2008). Genome structure of the legume, Lotus japonicus. DNA Res. 15: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauser L., Roussis A., Stiller J., Stougaard J. (1999). A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195. [DOI] [PubMed] [Google Scholar]
- Schmutz J., et al. (2010). Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- Sheen J. (2002). Phosphorelay and transcription control in cytokinin signal transduction. Science 296: 1650–1652. [DOI] [PubMed] [Google Scholar]
- Shlyueva D., Stampfel G., Stark A. (2014). Transcriptional enhancers: From properties to genome-wide predictions. Nat. Rev. Genet. 15: 272–286. [DOI] [PubMed] [Google Scholar]
- Singh S., Katzer K., Lambert J., Cerri M., Parniske M. (2014). CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15: 139–152. [DOI] [PubMed] [Google Scholar]
- Sohlberg J.J., Myrenås M., Kuusk S., Lagercrantz U., Kowalczyk M., Sandberg G., Sundberg E. (2006). STY1 regulates auxin homeostasis and affects apical-basal patterning of the Arabidopsis gynoecium. Plant J. 47: 112–123. [DOI] [PubMed] [Google Scholar]
- Soyano T., Kouchi H., Hirota A., Hayashi M. (2013). Nodule inception directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genet. 9: e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T., Hirakawa H., Sato S., Hayashi M., Kawaguchi M. (2014). Nodule Inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc. Natl. Acad. Sci. USA 111: 14607–14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam M., Belele C., Dorweiler J.E., Chandler V.L. (2002). Differential chromatin structure within a tandem array 100 kb upstream of the maize b1 locus is associated with paramutation. Genes Dev. 16: 1906–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T., Yano K., Ito M., Umehara Y., Suganuma N., Kawaguchi M. (2012). Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006. [DOI] [PubMed] [Google Scholar]
- Suzaki T., Ito M., Kawaguchi M. (2013). Genetic basis of cytokinin and auxin functions during root nodule development. Front. Plant Sci. 4: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmons O., Spitz F. (2013). From remote enhancers to gene regulation: Charting the genome’s regulatory landscapes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368: 20120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., et al. (2014). An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genomics 15: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B., Powell A. (1982). Isolation of plant DNA and RNA. Focus (Madison) 4: 4–6. [Google Scholar]
- van Zeijl A., Op den Camp R.H.M., Deinum E.E., Charnikhova T., Franssen H., Op den Camp H.J.M., Bouwmeester H., Kohlen W., Bisseling T., Geurts R. (2015). Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol. Plant 8: 1213–1226. [DOI] [PubMed] [Google Scholar]
- Varshney R.K., et al. (2011). Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat. Biotechnol. 30: 83–89. [DOI] [PubMed] [Google Scholar]
- Varshney R.K., et al. (2013). Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 31: 240–246. [DOI] [PubMed] [Google Scholar]
- Vernié T., Kim J., Frances L., Ding Y., Sun J., Guan D., Niebel A., Gifford M.L., de Carvalho-Niebel F., Oldroyd G.E.D. (2015). The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 27: 3410–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang L., Zou Y., Chen L., Cai Z., Zhang S., Zhao F., Tian Y., Jiang Q., Ferguson B.J., Gresshoff P.M., Li X. (2014). Soybean miR172c targets the repressive AP2 transcription factor NNC1 to activate ENOD40 expression and regulate nodule initiation. Plant Cell 26: 4782–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B., Zicola J., Oka R., Stam M. (2016). Plant enhancers: A call for discovery. Trends Plant Sci. 21: 974–987. [DOI] [PubMed] [Google Scholar]
- Xiao T.T., Schilderink S., Moling S., Deinum E.E., Kondorosi E., Franssen H., Kulikova O., Niebel A., Bisseling T. (2014). Fate map of Medicago truncatula root nodules. Development 141: 3517–3528. [DOI] [PubMed] [Google Scholar]
- Xie F., Murray J.D., Kim J., Heckmann A.B., Edwards A., Oldroyd G.E.D., Downie J.A. (2012). Legume pectate lyase required for root infection by rhizobia. Proc. Natl. Acad. Sci. USA 109: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., et al. (2008). CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA 105: 20540–20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoro E., Suzaki T., Toyokura K., Miyazawa H., Fukaki H., Kawaguchi M. (2014). A positive regulator of nodule organogenesis, NODULE INCEPTION, acts as a negative regulator of rhizobial infection in Lotus japonicus. Plant Physiol. 165: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N.D., et al. (2011). The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]