Abstract
Context
Roux-en-Y gastric bypass (RYGB) is associated with postprandial hyperinsulinemia.
Objective
This study assessed whether increased blood insulin levels may be due to an increase in maximal β-cell function.
Design, Setting, and Participants
We performed a cross-sectional study at Columbia University Medical Center, New York, New York. Subjects without a history of diabetes were studied after surgery (n = 12) and were compared with nonsurgical controls (n = 10) who were mean matched for body mass index, insulin sensitivity, and hemoglobin A1c and with nonobese controls (n = 8).
Methods
Subjects underwent a mixed-meal tolerance test and on a separate day an intravenous glucose tolerance test followed by a hyperglycemic clamp (450 mg/dL; 25 mM blood glucose) and arginine stimulation. The main outcome measure was maximal insulin secretion quantified after arginine stimulation (AinsRmax).
Results
The RYGB group exhibited greater peak postprandial glucose levels and fourfold greater peak insulin levels than control groups; however, there were no significant differences in insulinogenic index or AinsRmax. Another finding was significantly greater postprandial glucagon levels in the RYGB group compared with controls.
Conclusions
Our results suggest that after RYGB, the increase in postprandial levels of insulin are not due to changes in maximal β-cell function but appear to be an appropriate response to altered nutrient flow and absorption.
Keywords: Roux-en-Y gastric bypass, bariatric surgery, obesity, β-cell function, hyperinsulinemia, arginine stimulation
Roux-en-Y gastric bypass (RYGB) is associated with exaggerated blood levels of insulin following meal ingestion [1, 2]. This increased β-cell response occurs shortly after surgery and, in addition to decreased caloric intake and weight loss, likely contributes to drastic improvements in glucose homeostasis. Increased postprandial insulin levels have also been associated with morbidity starting at least 1 year or more after surgery in patients who experience symptomatic hyperinsulinemic hypoglycemic episodes [3, 4]. Several hypotheses have been proposed for the underlying cause of postprandial hyperinsulinemia after RYGB [5–7]. Higher glucagon-like peptide-1 (GLP-1) levels after surgery detected in both gut tissue and blood samples indicate that excessive GLP-1 secretion may be responsible for the enhanced insulin levels. Alternatively, there is evidence that an increase in intestinal glucose transporters and increased gut absorption of glucose after RYGB may then drive increased insulin secretion [8–10]. Another possible etiology is that before RYGB, patients develop increased β-cell mass to compensate for insulin resistance, which then results in hyperinsulinemic hypoglycemia when insulin sensitivity (SI) improves after weight loss. Furthermore, chronic exposure to increased levels of GLP-1 may inhibit β-cell apoptosis, resulting in increased β-cell mass [11].
The aim of this study was to investigate whether patients who have undergone RYGB have evidence of increased β-cell function when assessed independently of gut stimulation. Maximal islet cell function can be elicited by administration of intravenous arginine in the setting of high blood glucose levels [12]. Although there are other methods for eliciting maximal islet cell response, arginine stimulation after a glucose ramp was found to be better than either glucose alone or glucagon [13, 14]. Our study used arginine stimulation after a hyperglycemic glucose ramp to determine if there is evidence for increased β-cell function after RYGB.
1. Materials and Methods
A. Study Participants
Post-RYGB subjects (n = 12) were at least 18 months postsurgery, had a weight loss of ≥15% of presurgery total body weight, and had been weight stable for at least 6 months. Two control groups consisted of healthy patients with obesity who were mean matched for body mass index (BMI), SI evaluated by intravenous glucose tolerance testing (IVGTT), and hemoglobin A1c (HbA1c) to post-RYGB subjects (n = 10) and nonobese (BMI, 18 to 25.5 kg/m2) individuals (n = 8). Main exclusion criteria included diabetes or a history of diabetes before surgery (to lessen baseline impairment of β-cell function as a confounding variable), use of diabetes medication, use of medications with known effects on glucose homeostasis, and current serious illness. The study protocol was approved by the Columbia University Internal Review Board, and all subjects gave informed written consent.
B. Interventions
All subjects underwent frequently sampled IVGTT as previously described [15], followed by an arginine stimulation test [13]. After a 10-hour fast, glucose (0.3 g/kg body weight as dextrose 50 g/dL) was administered intravenously within 2 minutes at t = 0, and subsequent samples were obtained at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 24, 25, 27, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160, and 180 minutes. At 20 minutes, an intravenous injection of regular insulin (0.03 U/kg body weight) was administered to increase the accuracy of measuring SI [16]. One hour after completion of the IVGTT (4 hours after initial administration of glucose), subjects received a progressively increasing infusion of a 20% dextrose solution at a rate of increase of ~0.3 mM/min over 60 minutes to increase blood glucose levels to approximately 25 mM (450 mg/dL). A supplemental 25-mL bolus of D50% as used in other studies [17] was required to achieve this blood glucose goal in 10 subjects: two lean controls, six controls with obesity, and two RYGB patients. After a 5-minute hyperglycemic clamp, an intravenous bolus injection of 5 g of 10% arginine was administered over 30 seconds. Blood was sampled at 2-minute intervals for 10 minutes. No less than 1 week after the arginine stimulation, fasting subjects were given a liquid test meal of 320 kcal of Optifast (Nestle Healthcare Nutrition, Inc., Bridgewater, NJ) consisting of 50% carbohydrate (40 g total carbohydrate; 34 g sugar), 35% protein, and 15% fat. Venous blood was drawn from an indwelling catheter. Test meal data were unavailable from one subject in the nonobese group.
C. Assays
Assays for determination of hormone concentrations were as follows: (i) insulin [18] and C-peptide [19] were measured using the Immulite Analyzer (Siemens, Los Angeles, CA); (ii) total GLP-1, (7-36) and (9-36), was measured by ELISA (Millipore, Billerica, MA; assay sensitivity 1.5 pM; intra-assay and interassay coefficients of variation of 2% and <12%, respectively; no significant cross-reactivity to GLP-2, gastric inhibitory polypeptide, glucagon, and oxyntomodulin) [20]; and (iii) glucagon was measured by RIA (Millipore; assay sensitivity 18 pg/mL; intra-assay and interassay coefficients of variation of <7% and <14%, respectively; cross-reactivity to oxyntomodulin <0.1%) [21]. All samples analyzed by ELISA or RIA were run in duplicate, and an internal standard from pooled plasma was included in each assay to ensure consistency between assays.
D. Calculations and Statistical Analysis
Acute insulin response to glucose, SI, and the disposition index (DI) were calculated using the Bergman minimal model (MINMOD Millennium 6.02 software) [22]. The potentiation of glucose-stimulated β-cell secretion by arginine at 25 mM glucose [maximal insulin secretion quantified after arginine stimulation (AinsRmax)] was computed as the mean of the three peak insulin values after arginine was injected, with subtraction of the insulin value at maximal blood glucose before arginine administration [17]. Parameters of maximal α-cell secretion using plasma glucagon measurements were computed in a similar fashion (AglucRmax). β-Cell sensitivity to glucose was calculated as the slope of plasma insulin or C-peptide vs plasma glucose during the 60-minute glucose ramp. The insulinogenic index was calculated as the change in insulin level 15 minutes after the test meal compared with the fasting level divided by the change in blood glucose value from fasting over the same time period. The C-peptide index was similarly calculated. Data are presented as mean ± SEM. Area under the curve (AUC) was calculated using the trapezoidal rule. The primary endpoint was AinsRmax. ANOVA with the post hoc Tukey test was used to determine P values, which were considered statistically significant if <0.05 using GraphPad Prism version 6.0f for Mac OS X.
2. Results
The pre-RYGB BMI was 46.3 ± 6.3 kg/m2. Subjects had experienced a mean loss of 32.7 ± 2.7% total body weight over a duration of 5.1 ± 0.9 years after surgery. By study design, the mean BMI was similar between post-RYGB subjects and controls with obesity, with both groups having greater BMI than the nonobese control group (Table 1). All three groups had similar fasting levels of glucose, insulin, HbA1c, and GLP-1. Fasting glucagon levels were greater in the RYGB and obese control groups than in nonobese controls.
Table 1.
Main Characteristics of Study Groups and Mixed Meal Tests
| Characteristics |
Groups
|
P Values
|
||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | RYGB | Nonobese vs Obese | RYGB vs Nonobese | RYGB vs Obese | |
| n (female/male) | 8 (5/3) | 10 (7/3) | 12 (11/1) | |||
| Age, y | 32.8 ± 5.0 | 41.7 ± 3.9 | 46.9 ± 3.7 | 0.33 | 0.06 | 0.64 |
| Weight, kg | 63.1 ± 4.3 | 91.9 ± 7.4 | 82.2 ± 4.1 | 0.005 | 0.06 | 0.41 |
| BMI, kg/m2 | 23.3 ± 0.9 | 32.7 ± 1.9 | 31.9 ± 1.7 | 0.002 | 0.003 | 0.94 |
| Hemoglobin A1c, % | 5.1 ± 0.1 | 5.2 ± 0.1 | 5.2 ± 0.1 | 0.85 | 0.69 | 0.92 |
| Hemoglobin A1c, mmol/mol | 31.7 ± 1.4 | 32.9 ± 1.0 | 33.4 ± 1.2 | 0.86 | 0.66 | 0.92 |
| Glucose fasting, mg × dL−1 | 83.0 ± 2.2 | 90.7 ± 4.2 | 85.4 ± 0.9 | 0.14 | 0.81 | 0.32 |
| Glucose peak, mg × dL−1 | 96.1 ± 3.7 | 95.0 ± 3.9 | 122.0 ± 6.7 | 0.95 | 0.02 | 0.004 |
| Glucose nadir, mg × dL−1 | 68.5 ± 5.9 | 71.1 ± 3.5 | 63.1 ± 3.7 | 0.45 | 0.99 | 0.29 |
| Insulin fasting, μIU × mL−1 | 4.26 ± 0.78 | 8.53 ± 2.44 | 4.34 ± 0.89 | 0.16 | >0.99 | 0.14 |
| Insulin peak, μIU × mL−1 | 57.4 ± 11.0 | 51.4 ± 9.6 | 220.1 ± 43.1 | 0.99 | 0.006 | 0.002 |
| C-peptide fasting, ng × mL−1 | 1.6 ± 0.2 | 1.9 ± 0.4 | 1.6 ± 0.2 | 0.81 | 0.99 | 0.73 |
| C-peptide peak, ng × mL−1 | 7.9 ± 1.2 | 5.7 ± 1.1 | 15.2 ± 2.0 | 0.67 | 0.02 | 0.001 |
| GLP-1 fasting, pmol × L−1 | 31.1 ± 5.5 | 39.1 ± 7.6 | 34.0 ± 4.9 | 0.65 | 0.94 | 0.82 |
| GLP-1 peak, pmol × L−1 | 49.9 ± 10.5 | 66.4 ± 11.2 | 125.7 ± 15.5 | 0.70 | 0.002 | 0.007 |
| Glucagon fasting, pg × mL−1 | 39.6 ± 4.4 | 74.7 ± 6.8 | 72.4 ± 6.8 | 0.001 | 0.002 | 0.96 |
| Glucagon peak, pg × mL−1 | 74.6 ± 7.9 | 96.0 ± 6.7 | 154.1 ± 11.2 | 0.39 | <0.0001 | 0.0008 |
| Insulinogenic index0–15 | 2.67 ± 2.69 | 2.09 ± 1.06 | 3.70 ± 1.26 | 0.97 | 0.90 | 0.72 |
| C-peptide index0–15 | 1.04 ± 0.68 | 0.21 ± 0.10 | 0.23 ± 0.10 | 0.20 | 0.18 | 0.99 |
Data are presented as mean ± SE. P values are shown for between-group comparisons. For the nonobese group, n = 7 for meal test data.
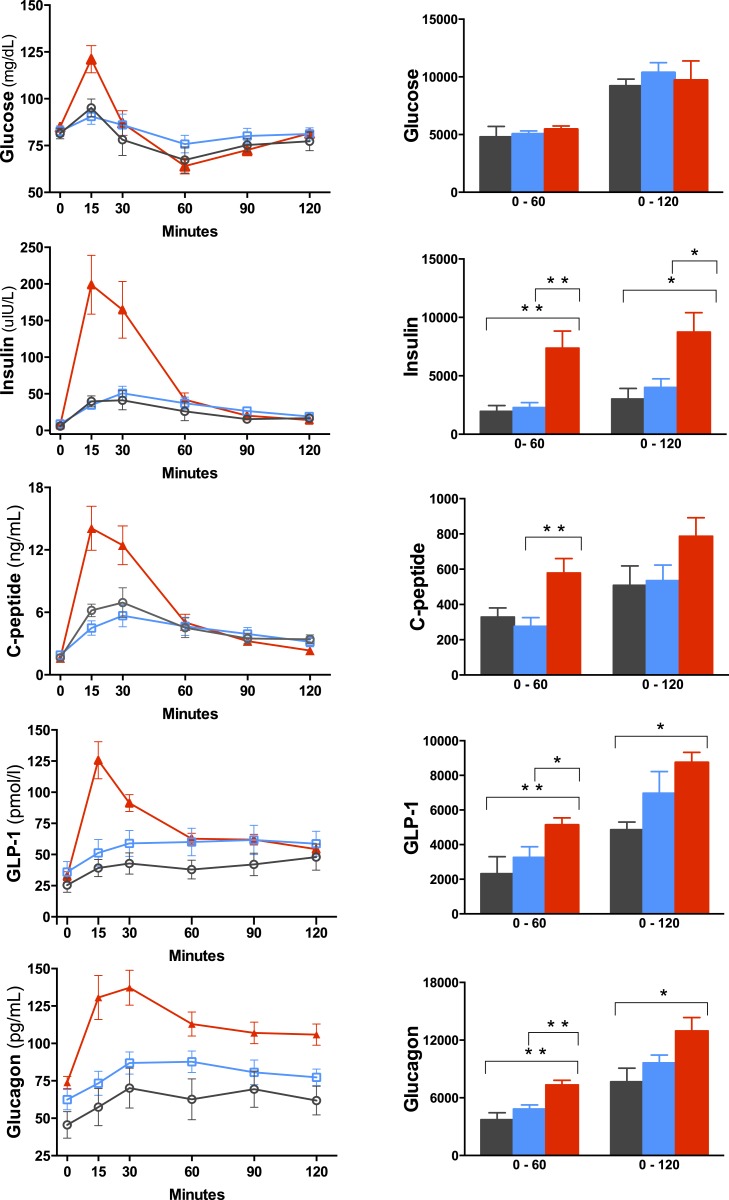
Because of the marked early postprandial changes in circulating glucose and hormone levels, test meal data are presented as peak values and AUC values from 0 to 60 minutes and from 0 to 120 minutes (Fig. 1; Table 1). Peak postprandial values of glucose, insulin, C-peptide, GLP-1, and glucagon were all greatest in the RYGB group. Of note, glucose, insulin, C-peptide, and GLP-1 values returned to fasting levels 2 hours after meal test ingestion, whereas glucagon level remained significantly higher in the RYGB group than in the controls with obesity (P = 0.02) and nonobese controls (P = 0.001). AUC0–60 values for insulin, C-peptide, GLP-1, and glucagon were significantly greater in the RYGB group than in both control groups expect for C-peptide between RYGB and nonobese controls (P = 0.054). Although peak values of insulin and C-peptide were higher in the RYGB group, when adjusted for change in glucose levels, the insulinogenic and C-peptide indices were not different from those of the control groups. Although no subject reported symptoms of hypoglycemia during the meal test, there were instances in all three groups of glucose levels dropping below 70 mg/dL, a widely accepted cutoff level for hypoglycemia. The numbers of subjects reaching below 70 mg/dL were four (57%), three (30%), and seven (58%) in the nonobese, obese, and RYGB groups, respectively.
Figure 1.
Changes during a test meal in blood glucose and hormone levels in nonobese controls (black circles and black bars), controls with obesity (blue squares and blue bars), and RYGB subjects (red triangles and red bars). Data are presented as mean concentration ± SEM. Bars represent AUC measurements calculated from 0 to 60 min and 0 to 120 min. *P < 0.05; **P < 0.01.
Results from the IVGTT and arginine stimulation after the glucose ramp are presented in Table 2. As calculated from the IVGTT, SI, acute insulin response to glucose, and DI were not significantly different between groups. The glucose ramp β-cell sensitivity calculated from insulin and C-peptide values during the glucose ramp was also similar between groups (Table 2). All groups reached similar peak glucose levels during the hyperglycemic clamp. Levels of glucose, insulin, and glucagon during the glucose ramp and after arginine stimulation are depicted in Fig. 2. Although postprandial insulin and glucagon levels were increased after RYGB compared with levels in the control groups, the responses to arginine (AinsRmax and AglucRmax) were not different between groups.
Table 2.
Results From IVGTT and Arginine Stimulation
|
Groups
|
P Values
|
|||||
|---|---|---|---|---|---|---|
| Nonobese (n = 8) | Obese (n = 10) | RYGB (n = 12) | Obese vs Nonobese | RYGB vs Nonobese | RYGB vs Obese | |
| IVGTT | ||||||
| AIRg, mL−1 × μU × min | 356 ± 85 | 445 ± 60 | 492 ± 87 | 0.73 | 0.46 | 0.90 |
| SI, mL × μU−1 × min−1 | 8.0 ± 1.6 | 6.6 ± 1.4 | 5.4 ± 0.6 | 0.70 | 0.29 | 0.75 |
| DI | 2016 ± 175 | 2684 ± 716 | 2685 ± 577 | 0.74 | 0.80 | 0.99 |
| β-cell glucose sensitivity | ||||||
| Insulin | 0.167 ± 0.051 | 0.198 ± 0.035 | 0.097 ± 0.018 | 0.80 | 0.34 | 0.09 |
| C-peptide | 0.017 ± 0.003 | 0.020 ± 0.002 | 0.013 ± 0.002 | 0.69 | 0.51 | 0.11 |
| Arginine stimulation | ||||||
| Peak glucose, mg × dL−1 | 512 ± 23 | 478 ± 17 | 474 ± 13 | 0.38 | 0.29 | 0.99 |
| AinsRmax, μU × mL−1 | 146 ± 41 | 202 ± 31 | 191 ± 32 | 0.52 | 0.64 | 0.97 |
| AglucRmax, pg × mL−1 | 34 ± 6 | 37 ± 5 | 37 ± 7 | 0.95 | 0.93 | 0.99 |
Data are presented as mean ± SE. P values are shown for between-group comparisons.
Abbreviation: AglucRmax, maximal glucagon secretion quantified after arginine stimulation; AIRg, acute insulin response to glucose.
Figure 2.
Concentrations of glucose, insulin, and glucagon starting 240 min after IVGTT during a graded glucose infusion from 240 to 300 min and after administration of an arginine bolus (dotted vertical line) in nonobese controls (black circles), controls with obesity (blue squares), and RYGB subjects (red triangles). Data are presented as mean concentration ± SEM.
2. Discussion
In this study, we demonstrated, as expected, that marked postprandial hyperinsulinemia was uniquely associated with the RYGB group compared with two control groups matched for HbA1c and SI as determined by IVGTT. Similarly, the peak postprandial level of GLP-1 in the surgical group was twofold greater than in the control groups. We investigated whether postprandial hyperinsulinemia after RYGB may be due to increased β-cell function by using a hyperglycemic clamp and arginine stimulation but found similar levels of insulin secretion after arginine stimulation. Because the maximal insulin level after RYGB was similar to that in controls, it appears that the meal-induced hyperinsulinemia was not likely the result of “hyperfunctioning” islets in our subjects and that other mechanisms likely underlie increased postprandial blood insulin levels after surgery.
Consistent with our finding, another study showed a similar insulin response to arginine in post-RYGB subjects compared with BMI-matched controls [23], although maximal stimulation was not tested because the hyperglycemic clamp reached only 9 mM glucose as opposed to 25 mM, the greater concentration required to elicit maximal potentiation of insulin secretion [12]. More important, it should be noted that none of our study patients reported a history of neuroglycopenia or experienced symptomatic hypoglycemia during the meal test, even though nadir glucose values were as low as 45, 50, and 42 mg/dL in the nonobese, BMI-matched, and RYGB groups, respectively. Patients with neuroglycopenia have exhibited exaggerated incretin and insulin secretory response to a mixed meal [24], lower postprandial insulin clearance rates [25], or increased insulin-independent glucose disposal [6] compared with asymptomatic post-RYGB patients. It would be interesting to determine whether arginine stimulation results in higher insulin secretion in patients who experience neuroglycopenia.
Another unique aspect of the RYGB group is the postprandial trajectory of blood glucose levels, which had an early and approximately 28% greater peak compared with that of controls. A study comparing food passing through the post-RYGB gut suggests that decreased gut transit time, which is a factor in increasing glucose absorption and the release of intestinal hormones, is related to an increase in circulating insulin level [8]. Several studies using a glucose tracer demonstrated changes in glucose kinetics after RYGB in response to meal ingestion [10, 26–28]. Coincident with the glucose peak we observed increased levels of GLP-1 and insulin. When we examined the insulinogenic index, the meal test results showed that increased insulin levels were proportionate responses to the increased glucose levels given that there were not significant differences in comparison with the control groups. In fact, the C-peptide index was nearly identical between RYGB and obese controls (P = 0.99) even though peak C-peptide level was almost threefold greater after RYGB (P = 0.001). Our results suggest that the exaggerated insulin secretion after RYGP may be related to accelerated transit and absorption of glucose [8, 9] and is an appropriate response to the higher blood postprandial glucose levels in surgical subjects.
It has been suggested that increased β-cell mass may underlie excessive insulin secretion after RYGB. Currently, no procedures can directly measure or image β-cell mass in humans, although maximal islet cell function has been shown in some circumstances to be a good proxy measurement [29]. Measurement of maximal insulin secretion after arginine stimulation under hyperglycemic conditions has correlated with β-cell mass after autologous β-cell transplants in baboons [30]. In a study of patients with chronic pancreatitis who underwent autologous islet cell transplantation, arginine stimulation also correlated well with the number of transplanted islets [13]. However, no studies have indicated that this methodology can be used as a surrogate measure for β-cell mass in humans after RYGB, so our results must be interpreted with caution when considering any relationship with β-cell mass. In animal models of bariatric surgery, variable results have been obtained regarding the effect on β-cell mass. Increased β-cell mass was observed in a porcine model of nonobese nondiabetic animals [31] and in obese rats after RYGB [32]. A strength of these studies was the ability to use direct measurements of β-cell mass and islet cell number. However, a small study involving Goto-Kakizaki rats showed no increase in β-cell mass after sleeve gastrectomy and duodenal-jejunal bypass [33]. In a human study, pancreatic tissue obtained from patients with symptomatic postprandial hypoglycemia after RYGB did not show increased β-cell mass [34], although others have reported opposite results [3, 4, 35]. Given these discrepant results, the important question of whether RYGB causes changes in β-cell mass is still open.
An interesting finding in this and other studies is that both the RYGB and the obese control groups had higher fasting levels of glucagon than the nonobese controls; however, unique to the RYGB group was a sustained postprandial elevation of glucagon levels that was greater than in BMI-matched controls [25, 27, 28, 36, 37]. Although seemingly paradoxical, this hyperglucagonemia may be necessary to prevent further hypoglycemia in the presence of postprandial insulin levels, which were fourfold higher in the RYGB group. Increased glucagon levels may also be beneficial for the maintenance of weight loss through the well-documented effects of glucagon on satiety and energy expenditure [38–40]. It is unclear why there was sustained elevation of glucagon even 2 hours after meal ingestion. Possibilities include aberrant cleavage of preproglucagon within the intestinal L cells after RYGB and/or lack of α-cell suppression [41]. It is unlikely these results are due to lack of assay specificity, as we ran duplicates of samples across a range of concentrations in an ELISA from Mercodia (Uppsala, Sweden) that has been tested for specificity [42] and found that the correlation between the RIA used in this study and the Mercodia ELISA was r = 0.779, P < 0.0001 (data not shown). Perhaps the most likely explanation is stimulation of glucagon secretion by GLP-2, which has been shown to increase in the postprandial state after RYGB [43, 44].
Another intriguing finding is a trend toward decreased β-cell sensitivity in the RYGB group compared with controls with obesity. This unexpected observation is consistent with a recent cross-sectional study by Salehi et al. [45] that reported more than 50% less sensitivity in a post-RYGB group than in controls matched for age, BMI, and fat mass. A previous study, however, did not show a difference between RYGB and controls [46]. These observations deserve further attention given the cross-sectional nature of the studies and differences in results.
A limitation of our study is that subjects were not studied before surgery; however, we have shown that postprandial hyperinsulinemia is uniquely observed after RYGB, as it was not present in nonsurgical controls matched for BMI, SI, and DI. Our results suggest that the mechanism underlying postprandial hyperinsulinemia is likely an appropriate response to rapid nutrient transit and increased glucose absorption through the surgically altered gut rather than changes in intrinsic β-cell function.
Acknowledgments
The authors acknowledge Irene M. Conwell for excellent technical assistance and Donald J. McMahon for statistical analysis.
Financial Support: This publication was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant no. RO1 DK072011 (to J.K.); National Institutes of Health grant no. T32 DK007271 (to M.C.C.A.); UL1 TR000040 (to Columbia University); Endocrine Fellows Foundation (to M.C.C.A.); and National Center for Research Resources, UL1 RR024156.
Clinical Trial Information: ClinicalTrials.gov Identifier: NCT00627315 (registered 3 March 2008).
Disclosure Summary: M.B. receives research support from Endostim and is founder of EndObetes. J.K. has stock options in Digma Medical and is on the scientific advisory board. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AinsRmax
maximal insulin secretion quantified after arginine stimulation
- AUC
area under the curve
- BMI
body mass index
- DI
disposition index
- GLP-1
glucagon-like peptide-1
- HbA1c
hemoglobin A1c
- IVGTT
intravenous glucose tolerance testing
- RYGB
Roux-en-Y gastric bypass
- SI
insulin sensitivity
References and Notes
- 1. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236–2240. [DOI] [PubMed] [Google Scholar]
- 4. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. [DOI] [PubMed] [Google Scholar]
- 5. Cummings DE. Gastric bypass and nesidioblastosis: too much of a good thing for islets? N Engl J Med. 2005;353(3):300–302. [DOI] [PubMed] [Google Scholar]
- 6. Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver Spring). 2015;23(4):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patti ME, Goldfine AB. Hypoglycemia after gastric bypass: the dark side of GLP-1. Gastroenterology. 2014;146(3):605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–2009. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen NQ, Debreceni TL, Bambrick JE, Chia B, Deane AM, Wittert G, Rayner CK, Horowitz M, Young RL. Upregulation of intestinal glucose transporters after Roux-en-Y gastric bypass to prevent carbohydrate malabsorption. Obesity (Silver Spring). 2014;22(10):2164–2171. [DOI] [PubMed] [Google Scholar]
- 10. Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. List JF, Habener JF. Glucagon-like peptide 1 agonists and the development and growth of pancreatic β-cells. Am J Physiol Endocrinol Metab. 2004;286(6):E875–E881. [DOI] [PubMed] [Google Scholar]
- 12. Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74(4):1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robertson RP, Bogachus LD, Oseid E, Parazzoli S, Patti ME, Rickels MR, Schuetz C, Dunn T, Pruett T, Balamurugan AN, Sutherland DE, Beilman G, Bellin MD. Assessment of β-cell mass and α- and β-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes. 2015;64(2):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robertson RP, Raymond RH, Lee DS, Calle RA, Ghosh A, Savage PJ, Shankar SS, Vassileva MT, Weir GC, Fryburg DA; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium. Arginine is preferred to glucagon for stimulation testing of β-cell function. Am J Physiol Endocrinol Metab. 2014;307(8):E720–E727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell Function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990;71(6):1508–1518. [DOI] [PubMed] [Google Scholar]
- 17. Elder DA, Woo JG, D’Alessio DA. Impaired β-cell sensitivity to glucose and maximal insulin secretory capacity in adolescents with type 2 diabetes. Pediatr Diabetes. 2010;11(5):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RRID:AB_2750939.
- 19.RRID:AB_2757817.
- 20.RRID:AB_2757816.
- 21.RRID:AB_2757819.
- 22. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236(6):E667–E677. [DOI] [PubMed] [Google Scholar]
- 23. Dirksen C, Eiken A, Bojsen-Møller KN, Svane MS, Martinussen C, Jørgensen NB, Holst JJ, Madsbad S. No islet cell hyperfunction, but altered gut-islet regulation and postprandial hypoglycemia in glucose-tolerant patients 3 years after gastric bypass surgery. Obes Surg. 2016;26(9):2263–2267. [DOI] [PubMed] [Google Scholar]
- 24. Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685. [DOI] [PubMed] [Google Scholar]
- 25. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, Patterson BW, Klein S. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S, Nannipieri M, Ciociaro D, Anselmino M, Mari A, Ferrannini E. Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes. 2013;62(11):3709–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobsen SH, Bojsen-Møller KN, Dirksen C, Jørgensen NB, Clausen TR, Wulff BS, Kristiansen VB, Worm D, Hansen DL, Holst JJ, van Hall G, Madsbad S. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56(10):2250–2254. [DOI] [PubMed] [Google Scholar]
- 29. Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest. 1992;89(6):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCulloch DK, Koerker DJ, Kahn SE, Bonner-Weir S, Palmer JP. Correlations of in vivo beta-cell function tests with β-cell mass and pancreatic insulin content in streptozocin-administered baboons. Diabetes. 1991;40(6):673–679. [DOI] [PubMed] [Google Scholar]
- 31. Lindqvist A, Spégel P, Ekelund M, Garcia Vaz E, Pierzynowski S, Gomez MF, Mulder H, Hedenbro J, Groop L, Wierup N. Gastric bypass improves β-cell function and increases β-cell mass in a porcine model. Diabetes. 2014;63(5):1665–1671. [DOI] [PubMed] [Google Scholar]
- 32. Zhang S, Guo W, Wu J, Gong L, Li Q, Xiao X, Zhang J, Wang Z. Increased β-cell mass in obese rats after gastric bypass: a potential mechanism for improving glycemic control. Med Sci Monit. 2017;23:2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inabnet WB, Milone L, Harris P, Durak E, Freeby MJ, Ahmed L, Sebastian M, Lifante JC, Bessler M, Korner J. The utility of [11C] dihydrotetrabenazine positron emission tomography scanning in assessing β-cell performance after sleeve gastrectomy and duodenal-jejunal bypass. Surgery. 2010;147(2):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased β-cell turnover. Diabetes Care. 2006;29(7):1554–1559. [DOI] [PubMed] [Google Scholar]
- 35. Patti ME, Goldfine AB, Hu J, Hoem D, Molven A, Goldsmith J, Schwesinger WH, La Rosa S, Folli F, Kulkarni RN. Heterogeneity of proliferative markers in pancreatic β-cells of patients with severe hypoglycemia following Roux-en-Y gastric bypass. Acta Diabetol. 2017;54(8):737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jørgensen NB, Jacobsen SH, Dirksen C, Bojsen-Møller KN, Naver L, Hvolris L, Clausen TR, Wulff BS, Worm D, Lindqvist Hansen D, Madsbad S, Holst JJ. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–E131. [DOI] [PubMed] [Google Scholar]
- 38. Geary N. Pancreatic glucagon signals postprandial satiety. Neurosci Biobehav Rev. 1990;14(3):323–338. [DOI] [PubMed] [Google Scholar]
- 39. Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, Chabenne J, Zhang L, Habegger KM, Fischer K, Campbell JE, Sandoval D, Seeley RJ, Bleicher K, Uhles S, Riboulet W, Funk J, Hertel C, Belli S, Sebokova E, Conde-Knape K, Konkar A, Drucker DJ, Gelfanov V, Pfluger PT, Müller TD, Perez-Tilve D, DiMarchi RD, Tschöp MH. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. [DOI] [PubMed] [Google Scholar]
- 41. Holst JJ, Pedersen JH, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia. 1983;25(5):396–399. [DOI] [PubMed] [Google Scholar]
- 42. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windeløv JA, Plamboeck A, Bojsen-Møller KN, Idorn T, Feldt-Rasmussen B, Knop FK, Vilsbøll T, Madsbad S, Deacon CF, Holst JJ. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57(9):1919–1926. [DOI] [PubMed] [Google Scholar]
- 43. Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;300(6):E1038–E1046. [DOI] [PubMed] [Google Scholar]
- 44. Nguyen KT, Billington CJ, Vella A, Wang Q, Ahmed L, Bantle JP, Bessler M, Connett JE, Inabnet WB, Thomas A, Ikramuddin S, Korner J. Preserved insulin secretory capacity and weight loss are the predominant predictors of glycemic control in patients with type 2 diabetes randomized to Roux-en-Y gastric bypass. Diabetes. 2015;64(9):3104–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salehi M, Gastaldelli A, D’Alessio DA. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obes Metab. 2018;20(4):872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim SH, Abbasi F, Lamendola C, Reaven GM, McLaughlin T. Glucose-stimulated insulin secretion in gastric bypass patients with hypoglycemic syndrome: no evidence for inappropriate pancreatic β-cell function. Obes Surg. 2010;20(8):1110–1116. [DOI] [PubMed] [Google Scholar]