Abstract
Purpose:
Daytime sleepiness, a frequent symptom of obstructive sleep apnea (OSA), can impact functional status. In patients with coronary artery disease (CAD) and concomitant OSA, the distinction between sleep-related functional impairment from underlying CAD versus OSA is unclear. This study evaluated the impact of OSA on sleep-related functional impairment in patients with CAD and compared the effect of 1-year continuous positive airway pressure (CPAP) use on change in impairment between those with and without excessive daytime sleepiness (EDS) and OSA. We hypothesized that sleep-related functional impairment is impacted by EDS independent of OSA in patients with CAD.
Methods:
105 CAD patients without OSA and 105 with moderate-to-severe OSA from the RICCADSA trial were matched on disease severity and included in the current substudy. Of those with OSA, 80 were allocated to CPAP. Functional Outcomes of Sleep Questionnaire (FOSQ) score <17.9 corresponded to sleep-related functional impairment.
Results:
Following revascularization, CAD patients with and without OSA frequently report sleep-related functional impairment (35% and 27.3%, respectively; p= .29). Moderate-to-severe OSA was not related to baseline FOSQ scores <17.9 in regression analyses; EDS was (OR 4.82, 95% CI 2.12–11.0; p< .001). CPAP use significantly improved FOSQ scores from baseline to 1-year follow-up in OSA patients with EDS (17.2±2.0 to 18.15±1.7, p= .002) despite suboptimal adherance.
Conclusions:
Sleep-related functional impairment may be reflective of persistent EDS, independent of OSA. Diagnosing OSA and initiating treatment is worthwhile in individuals with CAD and EDS, as both are important to guide appropriate therapy in patients with CAD.
Keywords: coronary artery disease, obstructive sleep apnea, functional status, and continuous positive airway pressure
INTRODUCTION
Functional status, the ability to manage daily activities and meet basic needs, is an important measure of health.[1] Optimal functional status is vital to adequate disease self-management and survival in persons with chronic disease. Functional impairment can negatively impact adherence to therapies and lead to worse health outcomes.[2] In persons with coronary artery disease (CAD), decrements in functional status are only partially explained by the disease process and can occur even when the desired outcomes from therapies are obtained (e.g. absence of angina). Percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) improve survival after an initial event,[3] however, patients still have an increased risk for recurrence[4] and may not regain their pre-cardiac disease functional status.
OSA, characterized by repetitive apneas and hypopneas from upper airway collapse during sleep, is highly prevalent (up to 73%) in patients with CAD.[5,6] Growing evidence indicates that severe OSA is a risk factor for CAD and is associated with an increased risk for recurrence and poor functional recovery in revascularized patients with CAD.[7,8] The resultant sympathetic activation, sleep fragmentation, and intermittent hypoxia can lead to reduced daytime function and health-related quality of life (HRQoL), fatigue, and sleepiness.[5,9] Treatment of OSA using continuous positive airway pressure (CPAP) therapy may help to improve daytime symptoms. Large clinical trials[10,11] evaluated the effect of CPAP in patients with OSA and cardiac disease. While the results did not support benefit on cardiovascular outcomes,[10,11] CPAP therapy did result in significant improvements to daytime sleepiness[11] and HRQoL.[10,11]
Excessive daytime sleepiness (EDS) can negatively impact functional status (i.e. sleep-related functional impairment).[12] In patients with CAD and OSA, factors beyond OSA may contribute to EDS including cardiac function, surgery, depression, or medications.[13,14] The distinction between sleep-related functional impairment due to CAD versus impairment due to OSA in a CAD population with concomitant OSA has not been fully examined. The majority of studies that have explored the effect of OSA therapy on change in sleep-related functional impairment have been in non-cardiac populations.[15–18] A CAD population with comorbid OSA may yield discrete findings given that complaints of EDS as a result of OSA, are reported less frequently, suggesting factors other than OSA contribute to sleep-related functional impairment.[6] This study evaluated the impact of OSA on sleep-related functional impairment in patients from a CAD cohort and compared the effect of 1-year continuous positive airway pressure (CPAP) use on change in impairment between those with and those without excessive daytime sleepiness (EDS) and OSA. We hypothesized that sleep-related functional impairment is impacted by EDS independent of comorbid OSA in patients with CAD.
METHODS
The current study was a secondary analysis of baseline data from the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnea (RICCADSA) trial.[19] The RICCADSA trial was a randomized controlled trial designed to examine whether CPAP therapy in newly revascularized CAD patients with OSA reduced cardiovascular mortality or the need for an additional revascularization over three years. The trial complied with the Declaration of Helsinki and was approved by the local ethics committee. All persons gave their informed consent prior to their inclusion in the trial. Men and women with angiography-verified CAD who recently (within six months) underwent either PCI or CABG were recruited between 2005 and 2010 from Sweden and follow-up completed in May 2013.
The current baseline sample comprised of patients with either no OSA (apnea-hypopnea index [AHI] <5 per hour) or moderate to severe OSA (AHI ≥15 per hour), Figure 1. Case control matching was performed to control for age and CAD severity. For each OSA patient, one patient without OSA was randomly matched by age ± 2 years, left ventricular ejection fraction (LVEF) ± 5%, and baseline status of treated hypertension (HTN; no/yes). The final baseline sample (N = 210) consisted of 105 non-OSA patients matched to 105 OSA patients. Of those with OSA, 80 went on to use CPAP therapy for 1 year and were included in follow-up analyses.
Figure 1.
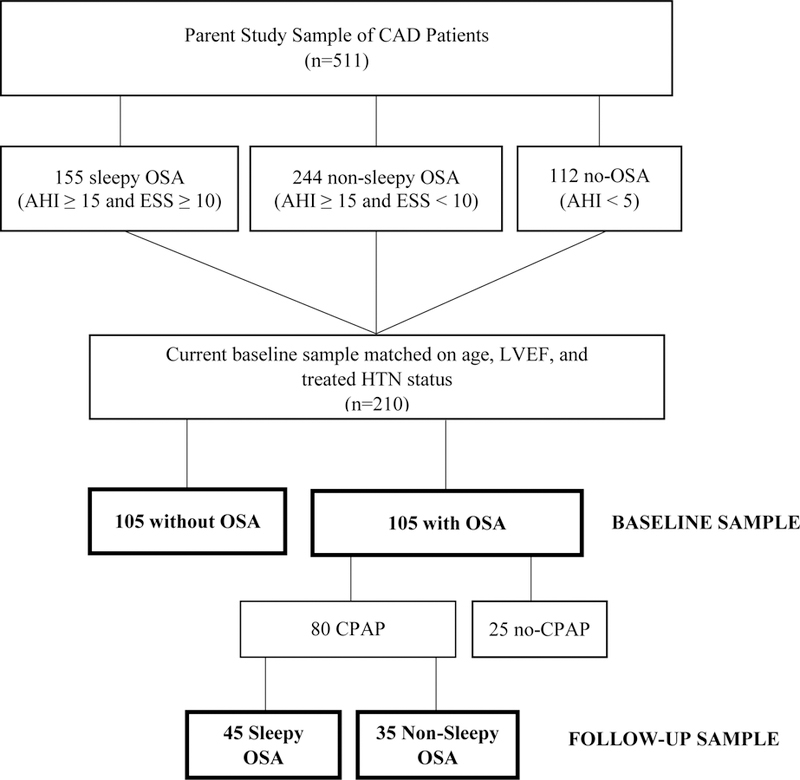
Flow diagram for the study sample. In bold, individuals who were included for baseline and follow-up analyses.
Abbreviations: CAD, coronary artery disease; ESS, Epworth sleepiness scale; LVEF, left ventricular ejection fraction; HTN, hypertension; OSA, obstructive sleep apnea.
Procedures
Patients were screened for EDS using the Epworth Sleepiness Scale (ESS), EDS defined as an ESS score ≥ 10. Sleep apnea was evaluated with in-home sleep testing using the EmblettaR PDS (Portable Digital System) device (Embla, Broomfield, CO, USA). Apneas were defined as the (≥ 90%) cessation of airflow. Hypopneas were defined as either a ≥50% reduction in chest-abdominal movement, a ≥50% decrease in the nasal airflow for ≥10 seconds, or a ≥30% reduction in chest-abdominal movement or nasal airflow accompanied by oxygen desaturation (≥4%). Patients were excluded if they had mild OSA (AHI 5–14) or predominantly central sleep apneas with Cheyne-Stokes respiration. Baseline data were collected in 511 patients meeting inclusion criteria. Patients with an OSA diagnosis during home sleep testing underwent an unattended overnight polysomnography in hospital at baseline. Those with nonsleepy OSA (ESS <10) were randomized to CPAP or no-treatment, and patients with sleepy OSA (ESS ≥10) received CPAP. Patients returned at 1 year for a post-evaluation.
Outcome Measures
Sleep-related functional impairment was assessed using the Functional Outcomes of Sleep Questionnaire (FOSQ).[20] The FOSQ is a validated measure[20] and includes 30-items categorized into five subscales (i.e., Activity Level, Vigilance, Intimacy and Sexual Relationships, General Productivity, and Social Outcomes). Item responses range from no difficulty (4) to extreme difficulty (1). The total score is the sum of the subscale scores. Total FOSQ scores range from 5 to 20 with lower scores indicate greater functional impairment. Impairment is indicated by a total FOSQ score of <17.9.[16] A change of 2.0 or more points in the FOSQ score is considered to indicate a clinically meaningful improvement in daily functioning.[16]
Daytime sleepiness was assessed by the ESS.[21] The ESS is a validated [22] subjective measure that assesses patients’ tendency to fall asleep in eight different situations. Responses are on a 0 to 3 Likert scale where 0 signifies “no chance of dozing” and 3 signifies a “high chance of dozing”; total scores range from 0 to 24.[21] Excessive daytime sleepiness (EDS) was indicated by a total score of ≥10.
Statistical Analyses
The sample distribution of demographic and clinical characteristics was examined using descriptive statistics. Continuous variables were reported as mean ± standard deviation; categorical variables were described as numbers and percentages. Comparisons between patients with and without OSA and between OSA patients with and without EDS who used CPAP therapy were done using Student’s independent t-test or Mann-Whitney U tests for continuous variables and Chi-square tests for categorical variables. Wilcoxon signed rank test was used to examine differences in FOSQ and ESS scores between baseline and 1-year follow-up within groups. Unadjusted linear regression modeling examined binary relationships between baseline FOSQ scores, demographics, and clinical characteristics selected a priori.
Binary logistic regression analysis evaluated the impact of OSA on the likelihood of patients reporting sleep-related functional impairment. The model was adjusted for age, sex, BMI, and additional covariates identified from the unadjusted linear regression analyses and between group comparisons with a P value <0.10. EDS was categorized as no (ESS <10) and yes (ESS ≥10) and OSA was categorized as no OSA (AHI <5) or moderate-to-severe OSA (AHI ≥ 15). Only cases with complete data were used. Results are reported as odds ratio (OR) and 95% confidence interval (CI). A P value of <.05 was considered statistically significant. Analyses were performed using SPSS 24 Windows (IBM Corp., Armonk, NY). The normality of the dependent variable (sleep-related functional impairment) was violated, showing both skewness (−1.45) and kurtosis (1.56). The Kolmorgorov-Smirnov test was significant at p < .001. However, in reasonably large sample sizes (N ≥ 200), the risk from bias or ineffectiveness due violation of the normality assumption is adequately reduced [23].
RESULTS
Table 1 details baseline characteristics of the 105 patients with moderate-to-severe OSA and 105 patients without OSA. The sample was mostly men (87.6 %, n = 184); none of the women (n = 26) had OSA. Those with OSA were significantly more likely to be overweight, male, have EDS, higher oxygen desaturation index (ODI), higher C-reactive protein (CRP) values, and were less likely a smoker or have a baseline acute myocardial infarction (AMI) compared to those without OSA (all p-values <.05). The OSA group had significantly lower mean FOSQ total scores (18.11 ± 1.9 versus 18.50 ± 1.9; p = .023) and lower mean scores on the “general productivity”, “activity level”, and “vigilance” subscales compared to the no OSA group (all p-values <.05) (Table 2). There was no difference in the percentage of patients with sleep-related functional impairment between groups, 35% with OSA versus 27.3% without OSA (p = .288).
Table 1.
Comparison of characteristics of the baseline sample between those with and without obstructive sleep apnea.
| With OSA (n = 105) |
Without OSA (n = 105) |
P Value | |||
|---|---|---|---|---|---|
| Age, year | 63.03 ± 8.0 | 62.96 ± 8.1 | .946 | ||
| Sex, male (%) | 100% (105) | 75.2% (79) | <.001 | ||
| BMI (kg/m2) | 28.4 ± 3.8 | 25.5 ± 3.0 | <.001 | ||
| % >= 25 | 84.8% (89) | 56.2% (59) | <.001 | ||
| LVEF | 60 (55–65) | 60 (55–65) | .854 | ||
| CRP (mg/dL) | 3.5 ± 6.8 | 2.3 ± 3.3 | .013 | ||
| AHI | 29.8 ± 12.9 | 3.1 ± 1.3 | <.001 | ||
| Moderate (AHI 15–29) | 57% (60) | ||||
| Severe (AHI ≥ 30) | 42.9% (45) | ||||
| ODI | 17.8 ± 12.3 | 1.7 ± 1.3 | <.001 | ||
| Previous PCI/CABG | 21.9% (23) | 16.2% (17) | .292 | ||
| Treated HTN | 49.5% (52) | 49.5% (52) | 1.00 | ||
| Hx A-Fib | 14.3% (15) | 8.6% (9) | .193 | ||
| Hx Diabetes | 18.1% (19) | 13.3 (14) | .343 | ||
| AMI_baseline | 43.8% (46) | 58.1% (61) | .038 | ||
| Hx Stroke | 7.7 % (8) | 3.8% (4) | .234 | ||
| Current Smoker | 14.3% (15) | 25.7% (27) | .038 |
Abbreviations: A-fib, atrial fibrillation; AHI, apnea/hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CRP, C-reactive protein; HTN, hypertension; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention.
Data are presented as % (n) or mean ± SD.
Table 2.
Comparison of baseline FOSQ and ESS scores between patients with and without OSA.
| With OSA (n = 105) |
Without OSA (n = 105) |
P Value | ||||
|---|---|---|---|---|---|---|
| FOSQ (total) | 18.11 ± 1.9 | 18.50 ± 1.9 | .023* | |||
| FOSQ Subscales | ||||||
| General Productivity | 3.77 ± .29 | 3.82 ± .03 | .011* | |||
| Social Outcome | 3.87 ± .35 | 3.82 ± .41 | .411 | |||
| Activity Level | 3.50 ± .49 | 3.59 ± .50 | .007* | |||
| Vigilance | 3.57 ± .46 | 3.72 ± .38 | .009* | |||
| Intimacy | 3.43 ± .78 | 3.51 ± .08 | .450 | |||
| FOSQ Scores < 17.9 | 35.0% (36) | 27.3% (27) | .288 | |||
| ESS | 8.44 ± 4.4 | 5.63 ± 3.0 | <.001* | |||
| EDS (ESS ≥ 10) | 42.9% (45) | 6.7% (7) | <.001* |
Abbreviations: EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FOSQ, functional outcomes of sleep questionnaire.
Data are presented as % (n) or mean ± SD.
Baseline Associations between Sleep-Related Functional Impairment and Covariates in Matched Sample (N=210)
Univariate analysis found two variables significantly associated with sleep-related functional impairment: higher CRP values (rho = .165) and daytime sleepiness (rho = .352). Comorbidities (atrial fibrillation, diabetes, stroke, AMI, previous PCI/CABG), ODI and LVEF were not associated with impairment (all p-values >0.05). The final regression model (Table 3) contained eight independent variables: sex, age, BMI, CRP, smoking status, and baseline AMI, OSA presence, and EDS. The full model was statistically significant, χ2 (9, N = 200) = 27.0, p = .001 and explained 17.8% (Nagelkerke R square) of the variance in sleep-related functional impairment. The model identified EDS and female sex as significant contributors. Those with EDS were 4.8 (95% CI 2.12–11.0, p < .001) times more likely to report sleep-related functional impairment, after controlling for all other factors in the model. Women were 3.8 (95% CI 1.37–10.6, p = .01) times more likely than men to report impairment.
Table 3.
Adjusted binary logistic regression analysisa examining predictors of impaired functional outcomes (FOSQ < 17.9) in 210 revascularized patients matched on CAD severity.
| OR | 95% CI | P Valueb | |
|---|---|---|---|
| Final Model | <.001* | ||
| BMI | 1.67 | .734–3.78 | .222 |
| Sex | 4.42 | 1.6–12.24 | .004* |
| Age | 1.24 | .611–2.50 | .556 |
| C-reactive protein | 1.04 | .976–1.11 | .227 |
| Current Smoker | 1.23 | .508–3.0 | .642 |
| AMI Baseline | 0.91 | .461–1.79 | .782 |
| OSA Severity | |||
| None (AHI <5) | .881 | ||
| Moderate (15–29) | 0.96 | .374–2.47 | .933 |
| Severe (≥ 30) | 1.20 | .446–3.22 | .720 |
| EDS | 4.68 | 2.06–10.60 | <.001* |
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index; EDS, Excessive Daytime Sleepiness; FOSQ, functional outcomes of sleep questionnaire; OSA, obstructive sleep apnea status.
Logistic regression analysis adjusted for BMI, body mass index, dichotomized as 0 = normal (BMI < 25) and 1 = overweight (BMI ≥ 25); sex, 0 = male and 1 = female; age, dichotomized as 0 = < 65 and 1 = ≥ 65; CRP, C-reactive protein, continuous; Current smoker dichotomized as 0 = no and 1 = yes; AMI, acute myocardial infarction, dichotomized as 0 = no and 1 = yes. OSA severity categorized as 0=no OSA, 1=moderate, and 2=severe; and EDS, Excessive Daytime Sleepiness, dichotomized as 0= no (ESS < 10) and 1= yes (ESS ≥ 10) were entered into block 2.
Final model after an enter stepwise approach, with entry and removal criteria of P < 0.05 and p < 0.10, respectively.
Comparison of FOSQ and ESS scores - baseline to 1 year follow-up
Among those patients with baseline OSA who went on to use CPAP for 1 year, 35 did not have EDS (nonsleepy OSA) and 43 had EDS (sleepy OSA). There was no significant difference in AHI between groups (p = .74). At baseline (Table 4), the sleepy OSA group had a mean FOSQ total score corresponding to impairment (17.2 ± 2.0) and was significantly lower (p <.05) versus the mean FOSQ total score of the nonsleepy OSA group (18.52 ± 1.6). At 1 year, the sleepy OSA group demonstrated significant improvements in the FOSQ total score (p = .002) from baseline which corresponded to a medium effect size [23] of 0.33. The median score went from being within the impaired range to being within the normal range (17.67 to 18.75) and at 1 year was comparable to the non-sleepy group. The sleepy OSA group also demonstrated significant improvements in the FOSQ subscales of “General productivity”, “Activity Level”, and “Vigilance”. The OSA sleepy group had significant reductions in ESS median scores (12.6 ± 2.5 to 9.4 ± 2.8, p <.001) which corresponded with a large effect size [23] of 0.53 and a significant reduction in the proportion with EDS (58%; n= 45 versus n=19). Overall, only the sleepy OSA group showed significant improvements in both the FOSQ and ESS scores after 1 year (Figure 2) when compared to the nonsleepy OSA and non-OSA group. Relative change in FOSQ total score from baseline to 1-year (Table 4) was significantly greater in the sleepy OSA group compared to the non-sleepy OSA group after 1 year of CPAP use (6.1% versus 0.9%, p = .004). Mean CPAP use (Table 4) did not differ between groups at 1 year (2.93 ± 2.93 versus 3.0 ± 3.09, p = .57). To explore if change in ESS has a significant effect on change in FOSQ (total) when controlling for CPAP adherence and other covariates, a post-hoc multiple linear hierarchical regression was completed. However, the fully adjusted model was found to not be significant (p > .05).
Table 4.
Comparison of FOSQ and ESS scores at baseline to 1 year in patients with nonsleepy and sleepy OSA who used CPAP.
| Baseline | 1 year | Mean Difference (Baseline – 1 year) | P Value | |
|---|---|---|---|---|
| Non-Sleepy CPAP | ||||
| N | 35 | 35 | ||
| FOSQ (total) | 18.52 ± 1.6 | 18.58 ± 1.8 | −.10 (−0.69, 0.49)† | |
| Median | 19.0 | 19.1 | .873 | |
| Interquartile Range | 18.0–19.9 | 18.2–19.9 | ||
| FOSQ Subscales | ||||
| General Productivity | 3.81 ± .21 | 3.82 ± .33 | .169 | |
| Social Outcome | 3.86 ± .45 | 3.82 ± .48 | .785 | |
| Activity Level | 3.58 ± .45 | 3.63 ± .41 | .859 | |
| Vigilance | 3.74 ± .35 | 3.79 ± .37 | .361 | |
| Intimacy | 3.65 ± .55 | 3.50 ± .75 | .090 | |
| Sleepiness-Related | 22.9% (8) | 20.6% (7) | 1.00 | |
| Functional Impairment | ||||
| (FOSQ < 18) | ||||
| ESS | 5.4 ± 2.5 | 5.5 ± 3.2 | −.03 (−1.1, 1.05) | |
| Median | 6.0 | 5.0 | .860 | |
| Interquartile Range | 4.0–8.0 | 3.0–8.0 | ||
| CPAP Adherencea | N/A | 2.93 ± 2.93 | ||
| Median | 3.19 | |||
| Interquartile Range | (0–5.8) | |||
| Sleepy CPAP | ||||
| N | 45 | 45 | ||
| FOSQ (total) | 17.2 ± 2.0 | 18.15 ± 1.7 | −.89 (−1.5, −0.32)† | |
| Median | 17.67 | 18.75 | .002* | |
| Interquartile Range | 16.2–18.86 | 17.11–19.46 | ||
| FOSQ Subscales | ||||
| General Productivity | 3.66 ± .33 | 3.80 ± .21 | .016* | |
| Social Outcome | 3.81 ± .38 | 3.88 ± .30 | .160 | |
| Activity Level | 3.30 ± .54 | 3.53 ± .41 | .003* | |
| Vigilance | 3.31 ± .43 | 3.59 ± .30 | <.001* | |
| Intimacy | 3.12 ± .88 | 3.31 ± .92 | .101 | |
| Sleepiness-Related | 55.8% (24) | 40% (16) | .092 | |
| Functional Impairment | ||||
| (FOSQ < 18) | ||||
| ESS | 12.6 ± 2.5 | 9.4 ± 2.8 | 3.23 (2.2, 4.2) | |
| Median | 12.0 | 9.0 | <.001* | |
| Interquartile Range | 11.0–14.0 | 7.0–12.0 | ||
| EDS (ESS ≥ 10) | 100% (45) | 42% (19) | ||
| CPAP Adherencea | N/A | 3.0 ± 3.09 | ||
| Median | 3.09 | |||
| Interquartile Range | (0–5.11) |
Abbreviations: CPAP, continuous positive airway pressure; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FOSQ, functional outcomes of sleep questionnaire.
Data are presented as % (n), mean ± SD, or median (IQR).
Data available for n = 35 nonsleepy and n = 44 sleepy patients.
Relative difference (1 year – Baseline / Baseline x 100) in FOSQ (total) between groups was found to be significantly greater in the sleepy OSA group compared to the non-sleepy OSA group after 1 year of CPAP use (6.1% versus 0.9%, respectively).
Figure 2.
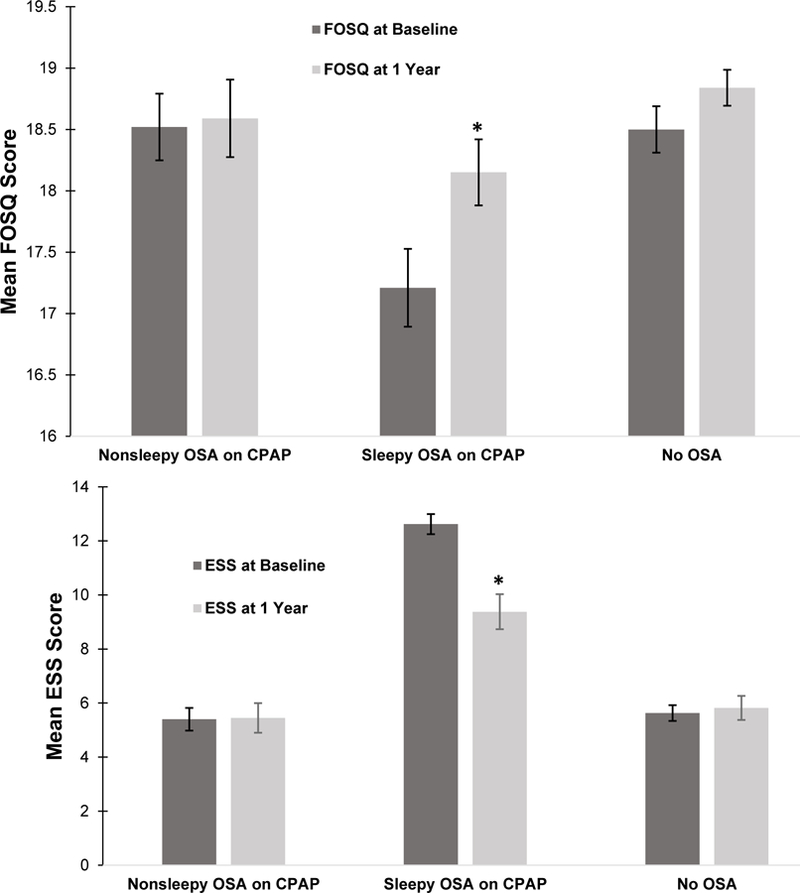
The figures depict group mean functional outcomes of sleep questionnaire (FOSQ) total scores and Epworth Sleepiness Scale (ESS) scores at baseline and 1 year for those who used CPAP (nonsleepy OSA and sleepy OSA) and those who did not use CPAP (no OSA). Only the sleepy OSA group demonstrated significant improvements in both FOSQ and ESS scores from baseline to 1-year follow-up.
DISCUSSION
In this study, we assessed the impact of OSA on sleep-related functional impairment in a CAD cohort matched for CAD severity. In addition, we investigated the effect of long-term CPAP use on change in functional impairment from baseline to 1 year follow-up. We found that following revascularization, CAD patients frequently report sleep-related functional impairment regardless of the presence of comorbid OSA. Secondly, moderate-to-severe OSA was not related to baseline sleep-related functional impairment in this CAD cohort. Finally, treatment of OSA with CPAP therapy in patients with moderate-to-severe sleepy OSA significantly improved FOSQ scores despite suboptimal adherence (approximately 3 hours per night).
In support of our hypothesis, moderate-to-severe OSA was not associated with sleep-related functional impairment in the full CAD cohort. The lack of a cross-sectional association between the FOSQ and AHI is consistent with prior studies in young and middle-aged adults without cardiac disease.[24–27] The FOSQ assesses impairment in function due to daytime sleepiness as a result of impaired sleep; the cause of sleepiness cannot be determined from this measure. In cardiac populations, OSA may not be the dominant cause of sleepiness and impaired functioning may not be entirely sleep related but rather associated with recovery of cardiac functioning following intervention, recovery from the intervention itself, or medications. Even with an improved LVEF, difficulty in performing daily activities could be perceived by patients as due to post-surgical deconditioning, fatigue[14], or depression[13]. Beta-adrenergic blocking agents, 86% use in the current sample, can also exacerbate vulnerability to daytime fatigue, somnolence, falls, and functional decline.[28] These non-sleep related factors, unavailable for the current study, may have contributed to the low variance in FOSQ scores that was explained by the full regression model.
The FOSQ, specifically developed to measure sleep-related impairment to function, correlated with EDS. Prior work emphasizes the contribution of daytime sleepiness to daily functioning [12] especially in those persons with OSA [5]. Daytime sleepiness was significantly associated with AHI in our study. In the current study, only 35% of the OSA patients reported sleep-related functional impairment and of those, less than half reported EDS. FOSQ scores did improve in the sleepy OSA group with CPAP use, but this improvement was not considered clinically meaningful. ESS significantly improved with CPAP use in this same group. These results suggest that the improvement in daytime sleepiness may have contributed to improvement in FOSQ scores, not OSA, which further corroborates our hypothesis.
Female sex was significantly associated with an increased risk for reporting impairment in functional outcomes. Evidence suggests a gender effect in the expression of impaired sleep including sleep-related functional impairment among women and men with suspected sleep disordered breathing.[29] However, the present study was underpowered to fully examine sex differences and highlights the need for a sufficiently larger sample of women.
Patient-reported health status, a construct that includes functional status,[1] is becoming increasingly important to evaluate the benefits of CPAP therapy. Similar to our longitudinal results, the FOSQ has been shown to be responsive to CPAP therapy in sleepy OSA patients.[15,18] We found that while 3 hours of CPAP use per night significantly improved FOSQ scores after 1 year in patients with sleepy OSA, it was not adequate to change the proportion with impairment or to exceed the 2.0-point increase that is considered to be a clinically meaningful improvement. Weaver et al. found a linear dose-response relationship between increased CPAP use and normalized functioning with 7.5 hours of CPAP use needed to achieve normalization.[16] Antic et al.[18] failed to identify a linear dose-response relationship with many patients not achieving normal functioning after 3 months of CPAP therapy despite CPAP use of ≥4 hours. Baseline FOSQ scores in the Antic[18] and Weaver[16] studies were considerably lower than the baseline FOSQ scores in our study, which could account for the nonsignificant change in proportion with impairment and the lack of a clinically meaningful improvement.
Study Limitations
Strengths of our study include the ability to evaluate the unique association of OSA with sleep-related functional impairment in a modest sample of adults with CAD and OSA. Limitations include that, it is unknown whether patients had co-existing sleep disorders and/or depression that contributed to their self-reported sleep-related functional impairment. Secondly, although a validated instrument, the FOSQ may have lower levels of discrimination in persons with cardiac disease who may also have competing causes of sleepiness.
Conclusions
These findings suggest that following revascularization in patients with CAD, (a) perceived sleep-related functional impairment may be more reflective of persistent EDS independent of OSA, (b) exploring for OSA and pursuing treatment is worthwhile in persons with CAD and comorbid sleepy OSA, and (c) although the low CPAP use as seen in the current study may not be adequate to reduce cardiovascular risk, this level of adherence was helpful to improve patient-centered outcomes. Because OSA and EDS appear to be independently associated with patient outcomes, both are important to guide appropriate therapy in patients with CAD.
Acknowledgements:
We gratefully acknowledge the Swedish Research Council, (521-2011-537, and 521-2013-3439); the Swedish Heart-Lung Foundation, (20080592, 20090708, 20100664, and 20110469); the “Agreement concerning research and education of doctors” of Vastra Gotalandsregionen, (ALFGBG-11538, and ALFGBG-150801); Research fund at Skaraborg Hospital; Skaraborg Research and Development Council; Heart Foundation of Kärnsjukhuset, ResMed Foundation and ResMed Ltd for the research grants that supported the parent RICCADSA study.
Funding: The National Institute of Health Heart Lung and Blood Institute provided financial support in the form of an institutional grant, Translational Sleep Medicine (HL82610) at the University of Pittsburgh School of Medicine. The sponsor had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. However, Thunström received consultant fees from ResMed and Pfizer outside the submitted work. Strollo received institutional grants from Philips-Respironics, ResMed, Inspire Medical Systems, National Football League PinMed, and advisory fees from ResMed, Emmi Solutions, Jazz Pharmaceuticals, Itamar Medical, Inspire Medical Systems, Seperation Design Group, all outside the submitted work. Peker received institutional grants from ResMed for the main RICCADSA trial, consultant fees from BresoTec, and lecture fees from ResMed and Philips-Respironics all outside the submitted work.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: For this type of study, formal consent is not required.
REFERENCES
- 1.Rumsfeld JS, Alexander KP, Goff DC, et al. (2013) Cardiovascular health: The importance of measuring patient-reported health status: A scientific statement from the American Heart Association. Circulation 127(22):2233–2249. 10.1161/CIR.0b013e3182949a2e [DOI] [PubMed] [Google Scholar]
- 2.Kaul P, Naylor CD, Armstrong PW, Mark DB, Theroux P, Dagenais GR (2009) Assessment of activity status and survival according to the Canadian Cardiovascular Society angina classification. Can J Cardiol 25(7):e225–e231. 10.1016/S0828-282X(09)70506-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff G, Dimitroulis D, Andreotti F, et al. (2017) Survival Benefits of Invasive Versus Conservative Strategies in Heart Failure in Patients With Reduced Ejection Fraction and Coronary Artery Disease. Circ Hear Fail 10(1):e003255 10.1161/CIRCHEARTFAILURE.116.003255 [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Benjamin EJ, Bonow RO, et al. (2011) AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 124(22):2458–2473. 10.1161/CIR.0b013e318235eb4d [DOI] [PubMed] [Google Scholar]
- 5.Kapur VK, Auckley DH, Chowdhuri S, et al. (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 13(3):479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glantz H, Thunstroöm E, Herlitz J, et al. (2013) Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc 10(4):350–356. 10.1513/AnnalsATS.201211-106OC [DOI] [PubMed] [Google Scholar]
- 7.Zhang J-J, Gao X-F, Ge Z, et al. (2016) Obstructive sleep apnea affects the clinical outcomes of patients undergoing percutaneous coronary intervention. Patient Prefer Adherence 10:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C-H, Sethi R, Li R, et al. (2016) Obstructive sleep spnea and cardiovascular events after percutaneous coronary intervention. Circulation 133(21):2008–2017. 10.1161/CIRCULATIONAHA.115.019392 [DOI] [PubMed] [Google Scholar]
- 9.Jilek C, Krenn M, Sebah D, et al. (2011) Prognostic impact of sleep disordered breathing and its treatment in heart failure: An observational study. Eur J Heart Fail 13(1):68–75. 10.1093/eurjhf/hfq183 [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Whellan DJ, Fiuzat M, et al. (2017) Cardiovascular outcomes with minute ventilation–targeted adaptive servo-ventilation therapy in heart failure: The CAT-HF trial. J Am Coll Cardiol 69(12):1577–1587. 10.1016/j.jacc.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 11.McEvoy RD, Antic NA, Heeley E, et al. (2016) CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 375(10):919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 12.Gooneratne NS, Weaver TE, Cater JR, et al. (2003) Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc 51(5):642–649. 10.1034/j.1600-0579.2003.00208.x [DOI] [PubMed] [Google Scholar]
- 13.Gu G, Zhou Y, Zhang Y, Cui W. (2016) Increased prevalence of anxiety and depression symptoms in patients with coronary artery disease before and after percutaneous coronary intervention treatment. BMC Psychiatry 16(1):1–9. 10.1186/s12888-016-0972-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnason SA, Zimmerman LM, Brey B, Catlin S, Nieveen JL. (2006) Patterns of recovery following percutaneous coronary intervention: A pilot study. Appl Nurs Res 19(1):31–37. 10.1016/j.apnr.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Mancini C, Maislin G, et al. (2012) Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: Results of the CPAP apnea trial north american program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med 186(7):677–683. 10.1164/rccm.201202-0200OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver TE, Maislin G, Dinges DF, et al. (2007) Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 30(6):711–719. 10.1016/S8756-3452(08)70707-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. (2017) Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med 13(11):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antic NA, Catcheside P, Buchan C, et al. (2011) The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 34(1):111–119. 10.1016/j.yneu.2011.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peker Y, Glantz H, Thunström E, Kallryd A, Herlitz J, Ejdebäck J. (2009) Rationale and design of the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnoea--RICCADSA trial. Scand Cardiovasc J 43(1):24–31. 10.1080/14017430802276106 [DOI] [PubMed] [Google Scholar]
- 20.Weaver TE, Laizner AM, Evans LK, et al. (1997) An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 20(10):835–843. http://www.ncbi.nlm.nih.gov/pubmed/9415942. [PubMed] [Google Scholar]
- 21.Johns MW. (1991) A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14(6):540–545. 10.1016/j.sleep.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. (1992) Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 15(4):376–381. 10.1093/sleep/15.4.376 [DOI] [PubMed] [Google Scholar]
- 23.Pallant J In: SPSS survival manual, a step by step guide to data analysis using SPSS for windows Third Edit. Sydney; McGraw Hill; 2007: pp 179–200. [Google Scholar]
- 24.Cohen J A power primer. (1992) Quant Methods Psychol 112(1):155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 25.Weaver EM, Woodson BT, Steward DL. (2005) Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol - Head Neck Surg 132(2):255–262. 10.1016/j.otohns.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Khan A, Harrison SL, Kezirian EJ, et al. (2013) Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in Osteoporotic Fractures in Men (MrOS) Sleep Study. J Clin Sleep Med 9(3):191–198. 10.5664/jcsm.2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kezirian EJ, Harrison SL, Ancoli-Israel S, et al. (2009) Behavioral correlates of sleep-disordered breathing in older men. Sleep 32(2):253–261. http://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=105447435&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billings ME, Rosen CL, Auckley D, et al. (2014) Psychometric performance and responsiveness of the Functional Outcomes of Sleep Questionnaire and Sleep Apnea Quality of Life Index in a randomized trial: The HomePAP study. Sleep 37(12):2017–2024. 10.5665/sleep.4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosendorff C, Lackland DT, Allison M, et al. (2015) Treatment of Hypertension in Patients with Coronary Artery Disease: A Scientific Statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension 65: 10.1161/HYP.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 30.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. (2007) Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: A clinical population study using the Sleep Disorders Questionnaire. Sleep 30(3):312–319. [DOI] [PubMed] [Google Scholar]


