Abstract
Introduction:
Gastric cancer is one of the top causes of cancer-related death worldwide. How to eliminate gastric cancer is an urgent public-health issue.
Areas covered:
In this review, we present the up-to-date results of studies on gastric cancer prevention through the eradication of Helicobacter pylori and discuss the strategies and obstacles for the implementation of population-wide screening and treatment of this pathogen to prevent gastric cancer.
Expert commentary:
Gastric cancer is an inflammation-associated cancer with a multistep carcinogenesis. The process consists of H. pylori infection, ongoing inflammation, development of metaplastic epithelia and genetic instability eventuating in gastric cancer. H. pylori infection is critical to the development of the disease and studies have consistently shown that H. pylori eradication results in a reduction in (a) gastric mucosal inflammation, (b) progression of histologic damage, (c) risk of peptic ulcers and ulcer recurrence, and (d) risk of gastric cancer. Compared with the large number of clinical trials evaluating chemopreventive approaches, studies of population-wide screening and eradication of H. pylori have only recently begun and only in high-risk populations. To eliminate gastric cancer requires information on how to implement an effective program for screening and treatment of H. pylori taking into consideration the other health priorities in any specific population.
Keywords: H. pylori, atrophic gastritis, gastric cancer, screening, cancer prevention
1. Introduction
Gastric cancer remains one of the most common causes of cancer deaths worldwide. According to the GLOBOCAN 2018 database, 1,033,701 new cases of stomach cancer and 782,685 gastric cancer-related deaths occurred worldwide in 2015 with the highest burden in Asia [1]. The discovery that gastric cancer was related to chronic inflammation caused by infection with the bacterium, H. pylori [2], suggested that it might be possible to eradicate gastric cancer by eradicating H. pylori [3]. There is a long history of programs targeting different infectious diseases designed to reduce or eliminate major causes of mortality or morbidity. Until the mid-20th century infectious diseases were the major cause of death in humans and eradication and/or containment programs proved highly successful in markedly reducing deaths and disability from infectious diseases with examples such as polio, small pox, diphtheria, malaria, and typhoid [4]. Often success was achieved through vaccination such as with routine immunizations for polio [5] and smallpox [6] and through environmental changes such as provisions for improved sanitation, clean water and good diets. Studies with an oral recombinant H. pylori vaccine in H. pylori-naive children have been conducted and reported an efficacy of 60% in children aged younger than 10 years vs. 44.2% in children aged 10 years or older [7]. This success suggests that vaccination for H. pylori may eventually become feasible. However, challenge studies of other vaccine candidates in adults have proven ineffective suggesting development may continue to be slow [8].
2. Benefits of H. pylori eradication
H. pylori infection was almost universal among humans until the mid-20th century. In retrospect, it became apparent that programs to provide improved sanitation and fresh foods and vegetables also resulted in a marked reduction in acquisition of the infection among children [9]. The fall in the incidence of acquisition of H. pylori in many populations, especially in highly developed countries, also explains the marked reduction in the incidence of the H. pylori related diseases, peptic ulcer and gastric cancer occurring in the mid-20th century [9].
In 1994, the IARC designated H. pylori as a Class I carcinogen for gastric cancer based on the fact that H. pylori can result in the longstanding mucosal inflammation, which may trigger the carcinogenetic cascade by producing genotoxic molecules and result in both oxidative damage and nitrosation of DNA bases [10]. In 2014, the World Health Organization (i.e., the IARC) published a monograph regarding studies using elimination of H. pylori as a strategy to prevent gastric cancer [11]. In 2015, the Maastricht Consensus Report updated the evidence related to the complexity of gastric functions in both health and disease associated with H. pylori infection and its clinical management [12]. In 2015, and for the first time, the Kyoto Global Consensus Report on H. pylori gastritis declared that H. pylori gastritis should be described as an infectious disease irrespective of whether an individual had symptoms or complications such as peptic ulcer or gastric cancer [13]. They also stated that all individuals with H. pylori-infected should be offered eradication therapy, unless there are competing considerations. In 2018, the Houston Consensus Report provided statements with supporting literature to implement testing for H. pylori infection, advice for the clinical practice, and guidelines to be adopted by the health care systems in the United States [14].
The risk of gastric cancer has long been known to be proportional to the extent and severity of gastric mucosal damage (i.e., atrophic gastritis) [15]. The discovery of H. pylori as the most common cause of progressive gastric damage suggested that elimination of the H. pylori, major cause of gastric cancer, would ultimately eliminate gastric cancer and that early intervention in those with the infection, before development of a high risk state, would likely also reduce gastric cancer incidence [16]. Recent studies have confirmed that eradication of H. pylori can reduce the risk of gastric cancer and, importantly, risk reduction extends even to those at highest risk (i.e., those with early gastric cancer after endoscopic submucosal dissection or endoscopic mucosal resection) [17,18]. This hypothesis has been confirmed by a meta-analysis that included 20,484 treated and 27,580 untreated individuals with a total follow-up of 340,255 person-years and demonstrated the significant reduction of gastric cancer risk by about 50% [15]. That magnitude of risk reduction was also supported by a recent placebo controlled trial in Korea [19]. However, in that synthetic analysis, most of included studies focused on patients who received endoscopic removal of an initial gastric cancer rather than on the general at-risk population; the latter may require a larger sample size and a longer follow-up period to yield a statistically significant result. Nonetheless, eradication of H. pylori infection has additional population benefits beyond the elimination of gastric cancer (Table 1) [12].
Table 1.
Benefits of H. pylori eradication on human health in addition to gastric cancer prevention
| Benefit of H. pylori eradication | Evidence level |
|---|---|
| Peptic ulcer disease | 1a |
| MALT lymphoma | 1a |
| Functional dyspepsia | 1a |
| Atrophic gastritis | 1a |
| Vitamin B12 deficiency | 3b |
| Iron deficiency anemia | 1a |
| Idiopathic thrombocytopenic purpura | 1b |
Adopted from the Maastricht IV/Florence Consensus Reports (Malfertheiner et al. Gut 2012;61:646–664 [12]), with permission.
3. Population strategies for gastric cancer prevention
A program for prevention of gastric cancer is more complicated that simply deciding to implement a strategy to achieve success based on the concept “no H. pylori, no gastric cancer”. Requirements for a successful program include the availability of accurate and affordable diagnostic tests to confirm the presence of H. pylori. Currently methods such as the 13carbon urea breath tests and stool antigen detection achieve high sensitivity and specificity results but are expensive, relatively time consuming (although not as time consuming as the culture identification), and require the subject’s cooperation [20]. In contrast, H. pylori serology is simple, inexpensive and widely available but with a lower specificity and sensitivity than the tests for active infection mentioned previously [21]. Prevention of the infection will also prevent development of H. pylori-associated diseases such as gastric cancer. However, the ability of eradication of an established infection to prevent gastric cancer depends in part on the risk of cancer at the time of H. pylori eradication. Thus, any eradication program must decide whether it offers only H. pylori eradication and no follow-up for those with a significant risk of gastric cancer or, alternately, the program could offer a variety of options depending risk stratification including surveillance for development of early gastric cancer (secondary prevention). This second option would require assessing the status and severity of atrophic gastritis at time of H. pylori eradication. This can be done non-invasively using testing for pepsinogen I and pepsinogen I/II ratios [22,23]. That approach can be further detailed such as by using the “ABC Method” [24,25], in which the combination of the serum anti-H. pylori antibody and the level of serum pepsinogens is used to stratify the gastric cancer risk according to the individual’s severity of atrophic gastritis. A systematic review that enrolled 20 studies indicated the pooled sensitivity and specificity of using this approach were 74.7% and 95.6%, respectively, to detect atrophic gastritis [26]. Another cohort study with 16 years of follow-up also demonstrated that those who had the positive pepsinogen tests at baseline were associated with an approximately 3.5 folds increasing risk of dying from gastric cancer [27].
An alternative approach would be to offer those potentially at risk (eg, over age 40 or 50) endoscopy to assess for atrophic gastritis and pre-malignant lesions [28]. Clearly, the choices entail markedly different commitments of resources and costs which can be very high especially when associated with the need for an extended period of surveillance.
Primary prevention means no H. pylori (i.e., preventing transmission-acquisition of the infection). Since the primary reservoir for infection is humans, H. pylori eradication also helps prevent transmission which occurs most often in children. For those with H. pylori infection, the goal is to eliminate the infection which stops the progression of damage and also prevents that individual from transmitting the infection to another host. As noted above, potentially the next step after eradication would be to consider risk stratification to identify those who need, or might benefit, from follow-up. That approach can likely only be considered and implemented in countries with considerable resources available to support such a program and where gastric cancer is currently a major problem such as Japan or Korea. For example, a rapidly developing country might design a program that started at age 18 and extended to age 25 with the goal of elimination of the infection in young individuals early in the disease while the risk of cancer was low and before they had children to ensure that their infections would not be transmitted to their children. Such a program could also include pre-marriage screening. Thus, over time the population of H. pylori free individuals would replace those with infection and eventually the country would by free of both H. pylori and gastric cancer. This is essentially what happened naturally in many western countries such as the United States that went from gastric cancer being the most common cancer to it becoming rare during the 20th century [9].
3.1. Cost-effectiveness of an eradication program
On the global scale, populations vary in gastric cancer risk such that the costs and manpower needs must be taken into consideration to determine the relative benefits of any eradication program to that country in the face of other needs (Figure 1). One approach is to categorize populations according to the prevalence of H. pylori infection and gastric cancer risk. There is a tendency that the relative cost-effectiveness would be better when the population has a higher incidence of gastric cancer [29, 30]; for example, the incremental cost-effectiveness ratio (or the cost for a quality-adjusted life-year gain) may decline from about 25,000 USD in Western countries to only about 1,300 USD in China [31]. Populations with a high prevalence of H. pylori infection and high gastric cancer risk are most likely to find that a mass screening program would be close to cost-saving (i.e., more benefit at lower cost compared with no screening; see right lower quadrate of Figure 1). The cost-benefit ratio can be augmented when we take into consideration the benefit of H. pylori eradication on the prevention of peptic ulcer and its complications and when popualtion aging is a global phenomenon such that the use of aspirin and non-steroidal anti-inflammatory drug has increased the risk of peptic ulcer disease [32]. In such a country, the program could be actively initiated in an organized manner including definition of the target population, the choice of a reliable screening test, identification of an effective treatment based on the antibiotic susceptibility, and mechanisms to audit the process and results; examples can be found in Japan and in Taiwan (e.g., the Matsu Island) [33,34]. Similar to campaigns against liver and cervical cancers, mass vaccination should prove very worthwhile and further investigation should be a priority.
Figure 1.
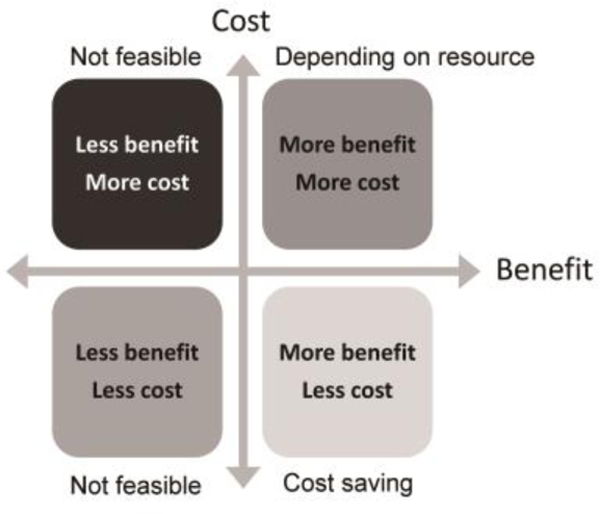
Relative cost-effectiveness of a preventive strategy, compared with no intervention at the grid origin
In a population with an intermediate risk for gastric cancer and a high prevalence of H. pylori, there will likely be a number of health-care priorities that compete for resources (such as cardiovascular diseases, diabetes, other top-ranked cancers, HIV, etc.). In general, the benefits obtained are associated with additional costs for the screening service (at the right upper quadrate in Figure 1) so applicability depends on whether the cost can be reduced to acceptable levels to justify the benefits (e.g., additional quality-adjusted life years). In such circumstance, a successful population screening/treatment program might target the high-risk subpopulations and integrate H. pylori screening within the established framework for health promotion (for example with colorectal cancer screening) [35]; by doing so, the additional costs for the manpower of healthcare delivery are minimized while the benefit from screening is augmented for both cancers. In this instance, the relative cost-effectiveness will be close to the right lower quadrate.
Populations with low gastric cancer burdens may have a high (example: India) or low prevalence rate of H. pylori infection (example: North American countries). In India, because atrophic gastritis and gastric cancer are infrequent outcomes of H. pylori infection, the additional costs and competing priorities may outweigh the benefits making a mass screening program impractical. Although the relative cost-effectiveness is still located at the right upper quadrate of Figure 1, it would be very close to the left-side quadrate. However, if one considers peptic ulcer and iron deficiency eradication instead of gastric cancer the calculations may change.
Importantly, even in low gastric cancer risk areas, there are often high-risk groups and ethnicities making targeted screening both practical and cost effective (examples: American Indians and Alaska Natives) [36,37]. Another group is immigrants from high-risk areas where active screening reduces their cancer incidence and also reduces the risks of reintroduction and transmission of H. pylori infections.
3.2. Implementation of a mass eradication program
As noted above, to largely eliminate the risk of death from gastric cancer, the targets of a gastric cancer prevention program should focus on early eradication of H. pylori (primary prevention), prevention of its transmission (primary prevention), and early detection of gastric cancer (secondary prevention) [38]. Treatment and monitoring programs must also take into account issues such as whether the prevalence of H. pylori and hence atrophic gastritis is falling locally. The problem of a residual risk of gastric cancer after H. pylori eradication is an important consideration. The program can be designed to eliminate or prevent gastric cancer or to accomplish both. If the primary purpose is eradication of gastric cancer, it is necessary to remove the all causes whereas cancer prevention may or may not include the complete elimination of all possible causes. The ideal program should give everyone the same opportunity and ability to participate. This requires correctly standardized diagnostic tests and treatments that are both cost-effective and widely accepted by the public [39].
Mass population-based H. pylori treatments generally require a new program that either integrates or replaces any current programs. Programs for the mass population should be easy to join and the benefits should not be limited to the relationship between physicians and patients. Therefore, whether the program is designed to be “good enough” and “cost-effective” will be an important consideration. At the same time, any mass population-based treatment program should address the following public health issues. Is this practice worthwhile? Who are possible candidates? Can such a program be implemented? Can it be done cost-effectively? Which prioritization should be the program in the nation’s resources?
Some issues of any mass population-based program should regularly be rethought. In principle, personal treatment is relevant to mass treatment. For example, after treatment of an individual patient, it is considered important to confirm the success of treatment. When one considers mass treatment, test of cure may be neither necessary nor cost effective and the focus may be on what is an acceptable cure rate. It is clear that clinicians should expect to relinquish control of the guidelines used in mass treatment programs. The development of the planning process should include determination about how to and whether it is even possible to define and invite the target populations. How can the quality of screening be ensured? Infected patients should receive standardized treatments. How can one assess adherence to treatment, the possible side effects of the treatment, and whether the desired cure rates are being achieved? Examples for such an information system and quality control can be found in some well-established programs for other cancers. For example, in the mass screening program for colorectal cancer in Taiwan, the screening results, including the coverage rate, positivity rate of screening tests, number of lower endoscopies performed, and endoscopic results, can be transmitted via a virtual private network to a central database and periodically generate standardized indicators for monitoring the screening quality [40]. Regional consensus for the management of H. pylori infection should be established in order to standardize the practice of primary care physicians [41].
Another important point is that mass population-based program will almost invariably treat patients who are actually non-infected (i.e., those with false positive tests). At the same time, some infected subjects will be screened as negative (false negative tests) and will not receive treatment. It is important to consider what are the acceptable proportions of patients who receive unnecessary therapy or who fail to receive treatment because of false negative screening results. For example, suppose there is a 50% infection rate among a population of 100,000. That is, 50,000 people are infected and another 50,000 are not infected. If a test with 90% sensitivity and specificity is applied, the result of screening will be 45,000 are positive and 5,000 are negative (i.e., false negative) among the 50,000 infected people. In the 50,000 who are uninfected, 45,000 people will be scored negative, and 5,000 as positive (false positive). So the target group for treatment will include 45,000 true positives plus 5,000 false positives and 5,000 false-negative people will miss the opportunity to be treated (Figure 2A). If the treatment regimen had a 100% cure rate the prevalence of infection after treatment would be 5% (5,000/100,000) among this population since 5,000 false-negative patients are not treated. Accordingly, if the cure rate of the regimen were only 70%, the prevalence of infection after treatment would be 18.5%. The proportion of people who really need to be treated (45,000) and wrongly treated (5,000 people who do not need treatment) is as high as nine to one (Figure 2B). Using the same screening method among population with a 10% infection rate would use nearly half of the treatment resources for false positive patients. In that program, use of a second test to confirm the active infection would probably be worthwhile.
Figure 2.
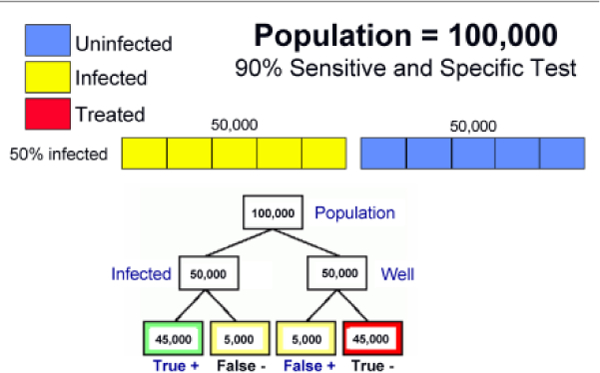
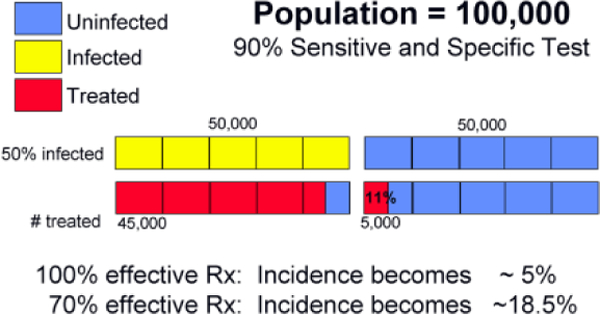
Population screening and treatment for H. pylori infection. (A) The results following the initial screening test in a high H. pylori prevalence population, and (B) the results in a low H. pylori prevalence population such as following one round of mass eradication therapy.
In terms of mass treatment programs, treating a population with 50% infection rate by a regimen of 90% success rate (that is, the treatment failure rate is 10%) results in 5% remaining infected after mass treatment. And so on. If the success rate of treatment is only 70%, then 15% are still infected. Regardless of the costs in terms of money, labor, or side effects, to achieve the best result every step from the recruitment of patients to diagnosis to treatment and treatment success must be carefully analyzed.
Evaluation of outcomes of any mass treatment program is necessary and the goals must be clearly defined. Data gathering and analysis must be performed in a timely manner and categorized into short-term end-points (e.g., surrogate outcome with premalignant gastric lesions), long-term end-points (e.g., gastric cancer incidence and mortality rate), cost-effectiveness analysis, and potential adverse effects [42]. There were many association studies that have suggested that the eradication of H. pylori may lead to the increase or decrease in some extra-gastric diseases (e.g., esophageal adenocarcinoma, childhood asthma and atopy, obesity, etc.); however, causal relationships have never been confirmed and most of these hypotheses have been falsified such that there is no solid evidence showing that H. pylori eradication could increase a personal or population risk [20]. Increase of the antibiotic resistance for H. pylori and other human bacteria, nonetheless, can be a potential concern, which may require the selection of an effective treatment to minimize the population exposure to unnecessary antibiotics. Routine re-testing after eradication treatment is also needed to identify those who failed because of antibiotic resistant strains and provide them with a tailored treatment according to antibiotic susceptibility.
4. Individually tailored approach
The natural history of H. pylori infection can be divided into periods. Figure 3 shows it divided into 4 periods starting with the acquisition and transmission phase. During this phase the infection is acquired and atrophic changes are rare. Between ages 20 and 40 the individuals often live with their children and may transmit the disease to them. During this period the infection increasing becomes chronic with acid secretion being reduced as the infection increasingly involves the corpus. The proportion with atrophic gastritis increases with age along with the risk of cancer becoming exponential. This third period of increasing atrophy (e.g., after 40 years old, the proportion with atrophy increase to the point that most patients have atrophy and the highest risk of gastric cancer. Based on the extent and severity of atrophic damage, the prevention strategists for each period should likely be different. For example in the population below 20 the strategist might focus on prevention of acquisition with vaccines and/or eradication of H. pylori in young adults possibly including pre-marital testing and treatment. The proportion with atrophy and the severity of atrophy increases with age. Treatment before development of a significant population cancer risk would likely preclude the need for surveillance. Older subjects likely contain a higher proportion with atrophy and an increased risk for gastric cancer. For this group risk-stratification using pepsinogen testing followed by endoscopy or simply endoscopy may be cost-effective. Where resources are available endoscopy has an advantage as pre-cancerous lesions can be identified and reliable risk-stratification done using a validated histologic method such as the OLGA/OLGIM staging systems [43].
Figure 3.
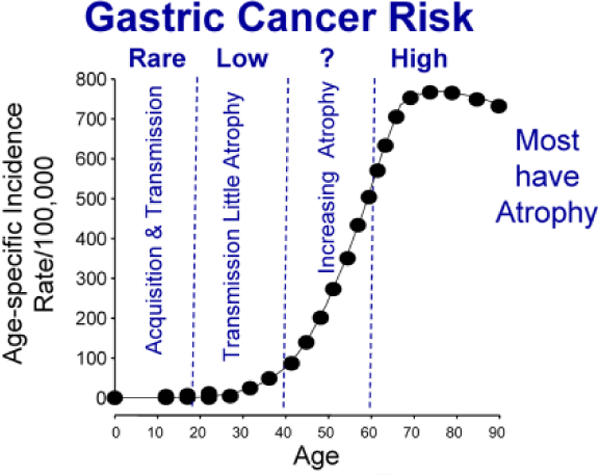
The age specific risk of cancer in a population with a high risk of gastric cancer such as Japan shown as risk/year/100,000 population. This is superimposed on the population divided into different age categories based on the progression of their H. pylori infection in which the risk of cancer increases as the proportion with atrophic gastritis increases with age.
The goal of treatment should be to halt the process in the low or moderate risk groups. There will still be a high risk group after therapy that is identified on the basis of the extent and severity of atrophic damage. This group is the one where surveillance may be indicated. Consensus meetings must be held to develop the treatment guidelines. Clinicians will play a relatively minor role. Increasingly important considerations relate to the natural history of the infection in that population. What is cost-effective? What are all the costs? What is “effective”? Trade-off’s will always be necessary.
5. Conclusions and perspectives
Even if these strategies are accompanied by incontrovertible evidence to support the effectiveness, not every proposed strategy can be realized at the population level whereas cost-benefit becomes critical determinants. The greatest impediment is costs, which reduce competitiveness with other health priorities. The key to success is careful and detailed planning. We know what we want to do is to eradicate H. pylori and prevent gastric cancer. Though there are many options but even with the most ideal, gastric cancer will be with us for decades. As Sir Authur Hurst speaking about gastric cancer said “. The ideal form of prophylaxis for gastric cancer would be not merely to recognize and treat the common precancerous gastric conditions, chronic gastritis and chronic gastric ulcer, but to prevent their development” [44]. That goal can be accomplished.
6. Expert Commentary:
Although the importance of H. pylori as a cause of human disease was recognized decades ago, gastric cancer, remains the sixth most prevalent cancer worldwide and accounts for the second most common cancer-related deaths. A number of consensus meetings have strongly recommended screening and treatment for H. pylori infection be adopted as a public health priority to eliminate the burden of gastric cancer; however, mass eradication programs are rare to absent in most high-risk populations, indicating a barrier between treatment of individuals and implementation of population-based programs. Given the strong evidence of benefits derived from H. pylori eradication relating to peptic ulcer, gastric cancer, iron deficiency anemia, etc., it is clear that research effects should be allocated to identifying the most cost-effective ways to integrate H. pylori eradication into the healthcare priorities especially in high-risk populations, to raising population awareness of this common but potentially deadly disease, establishing efficient delivery systems of screening and timely referral after a positive test, and implementation of standardized and validated treatment regimens. In the meanwhile, it should be recognized that the uppermost goal of a gastric cancer prevention program is to reduce the cancer related deaths while taking into consideration how to reduce the number of new incident cases and how to prolong cancer survival upon diagnosis. A dual approach has gradually come of age that integrates H. pylori eradication (reducing cancer risk in the future) with the targeted endoscopic surveillance (improving cancer survival through early detection) based on the information based on results at the individual level; these tailored approaches may include serological biomarkers, the gastric histology, and the genomic markers.
7. Five year view
The understanding that gastric cancer results from H. pylori-induced chronic inflammation suggested that eradication of H. pylori would also eliminate gastric cancer as well as the other H. pylori-related diseases. This realization is also forcing current gastric cancer prevention programs emphasizing early detection to also consider gastric cancer prevention via eradication of the causative agent. For low or moderate risk groups H. pylori eradication alone is likely the most cost effective strategy whereas high risk groups identified on the basis of the extent and severity of atrophic damage are likely to benefit from continuing surveillance. We believe that in the next 5 years many regions and countries will consider or implement H. pylori eradication programs that will vary from mass population-based H. pylori eradication in high risk countries to limited programs in low risk countries that are tailored to specific high risk groups. In high risk countries, the new programs will either integrate or more likely replace current programs. Planning will need to address (a) who are the candidates? (b) can the program be implemented cost-effectively? and (c) how to prioritize it in relation to other health care programs and in relation to use of the nation’s resources? A number of pilot programs are already underway. In high H. pylori prevalence countries with low gastric cancer burdens, preventive or curative vaccination would likely be the only cost effective alternative such that developed countries should support vaccine development.
Key issues.
H. pylori infection is the underlying cause of the majority of cases of gastric cancer.
The Kyoto Global Consensus Report defined H. pylori-associated gastritis as an infectious disease. Currently H. pylori eradication is the only practical method of eliminating gastric cancer and other H. pylori-related diseases.
H. pylori eradication after development of atrophic changes will reduce but not completely prevent development of gastric cancer
In regions with a high gastric cancer burden the addition of cancer risk stratification and continued surveillance to identify and treat early gastric cancer may be cost effective.
The actual design and implementation of mass H. pylori eradication programs must consider the population cancer risk and the program’s value in relation to other health care priorities.
Acknowledgments
Financial support:
The study was funded by the Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan (kmtth-103–020). Dr. Lee is supported by the Population Health Research Center (PHRC), College of Public Health, National Taiwan University from the Ministry of Science and Technology (MOST 107–3017-F-002–003) and Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (NTU-107L9003). Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH.
Footnotes
Conflict of interests:
JY Wu and YC Lee do not have conflict of interests. Dr. Graham is a consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. September 12. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 3.Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to pylori. Int J Cancer. 2015;136:487–490. [DOI] [PubMed] [Google Scholar]
- 4.World Health Assembly (2012) World Health Assembly Resolution: Completion of polio eradication programmatic emergency for global public health.
- 5.Callaway E Public health: Polio’s moving target. Nature. 2013;496:290–292. [DOI] [PubMed] [Google Scholar]
- 6.Fenner F, Henderson DA, Arita I, et al. Smallpox and Its Eradication. World Health Organization; Geneva, Switzerland: 1988. [Google Scholar]
- 7.Zeng M, Mao XH, Li JX et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1457–1464. ** The first controlled clinical trial of oral vaccination to prevent H. pylori infection in H. pylori in children was done in China and was positive suggesting that vaccination may be a viable approach to preventing H. pylori and ultimately gastric cancer. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Selgard M, Wex T, et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: a randomised, placebo-controlled phase 1/2 study. Lancet Gastroenterol Hepatol. 2018;S2468–1253:30125–30130. [DOI] [PubMed] [Google Scholar]
- 9.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–5204.** Review of the history of H. pylori and it disease emphasizing the importance of environmental changes resulting in change of the pattern of gastritis leading to change in the manifestations of H. pylori infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rugge M, Genta RM, Di Mario F, et al. Gastric cancer as preventable disease. Clin Gastroenterol Hepatol. 2017;15:1833–1843. [DOI] [PubMed] [Google Scholar]
- 11.IARC Helicobacter pylori Working Group (2014). Helicobacter pylori eradication as a strategy for preventing gastric cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). Available from: http://www.gastro-health-now.org/wp/wp-content/uploads/2014/09/WHO-IARC-Report-2014.pdf .** Compilation of clinical trials of H. pylori eradication setting the stage for consideration of H. pylori eradication as a strategy for the gastric cancer prevention.
- 12.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. * Global consensus statements for the management of H. pylori-related disease, diagnosis, and treatment. [DOI] [PubMed] [Google Scholar]
- 13.Sugano K, Tack J, Kuipers EJ, el al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. ** A position paper focus on the manifestation and outcome of H. pylori related gastritis. The first to formally declare H. pylori gastritis as an infectious disease that should be treated and cured. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Serag HB, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124. ** The first synthetic analysis to confirm the association between H. pylori eradication on the subsequent gastric cancer incidence in populations with different gastric cancer risk. [DOI] [PubMed] [Google Scholar]
- 16.Graham DY. Roadmap for elimination of gastric cancer in Korea. Korean J Intern Med. 2015;30:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–397. ** The first prospective randomized study to confirm that H. pylori eradication reduced subsequent gastric cancer incidence after endoscopic removal of an initial gastric cancer. [DOI] [PubMed] [Google Scholar]
- 18.Yoon SB, Park JM, Lim CH, et al. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014;19:243–248. * Meta-analysis to confirm the association between H. pylori eradication and reduction in subsequent gastric cancer incidence after endoscopic resection of gastric tumors. [DOI] [PubMed] [Google Scholar]
- 19.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–1095. * The most definitive study, hopefully the last placebo controlled trial, needed to prove that H. pylori eradication reduces the risk of gastric cancer even in the most high risk group (ie, prevention metachronous gastric cancer after endoscopic removal of an initial gastric cancer). [DOI] [PubMed] [Google Scholar]
- 20.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731. * Recent consensus regarding diagnostic testing for H. pylori and its related diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YC, Tseng PH, Liou JM, et al. Performance of a one-step fecal sample-based test for diagnosis of Helicobacter pylori infection in primary care and mass screening settings. J Formos Med Assoc. 2014;113:899–907 [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi S, Kato M, Katsuyama T, et al. Design and planned analyses of an ongoing randomized trial assessing the preventive effect of Helicobacter pylori eradication on occurrence of new gastric carcinomas after endoscopic resection. Helicobacter. 2006;11:147–151. [DOI] [PubMed] [Google Scholar]
- 23.Kitahara F, Kobayashi K, Sato T, et al. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134:1445–1457. [DOI] [PubMed] [Google Scholar]
- 25.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagari RM, Rabitti S, Greenwood DC, et al. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther. 2017;46:657–667. [DOI] [PubMed] [Google Scholar]
- 27.Chiang TH, Chiu SY, Chen SL, et al. Serum pepsinogen as a predictor for gastric cancer death: A 16-year community-based cohort study. J Clin Gastroenterol. 2018. January 23. doi: 10.1097/MCG.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 28.Dore MP, Cipolli A, Ruggiu MW, et al. Helicobacter pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions: A retrospective study. Medicine (Baltimore). 2018;97(4):e9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lansdorp-Vogelaar I, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol. 2013;27:933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YC, Lin JT, Wu HM, et al. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:875–885. [DOI] [PubMed] [Google Scholar]
- 31.Yeh JM, Kuntz KM, Ezzati M, et al. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer 2009;124:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford AC, Delaney BC, Forman D, et al. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833–55. [DOI] [PubMed] [Google Scholar]
- 33.Sugano K Strategies for prevention of gastric cancer: Progress from mass eradication trials. Dig Dis. 2016;34:500–504. [DOI] [PubMed] [Google Scholar]
- 34.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–682. ** The first mass eradication program of H. pylori for gastric cancer prevention in a high-risk community cohort to show the benefit on the incidences of peptic ulcer and gastric cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Screening for stomach diseases and colorectal neoplasms with the fecal testing. The study protocol is available at: https://clinicaltrials.gov/ct2/show/NCT01741363
- 36.Saumoy M, Schneider Y, Shen N, et al. Cost effectiveness of gastric cancer screening according to race and ethnicity. Gastroenterology. 2018;S0016–5085:34546–34553. * A cost-effectiveness analysis to evaluate the screening for high-risk subpopulations in a country with low gastric cancer burden (United States). [DOI] [PubMed] [Google Scholar]
- 37.Lee YC, Asaka M, Graham DY. Editorial: Helicobacter pylori and gastric cancer in Alaskan Natives: Time to stop studying H. pylori and to eradicate it. Helicobacter. 2018;23:e12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasechnikov V, Chukov S, Fedorov E, et al. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leja M, You W, Camargo MC, et al. Implementation of gastric cancer screening - the global experience. Best Pract Res Clin Gastroenterol. 2014;28:1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YC, Hsu CY, Chen SL, et al. Effects of screening and universal healthcare on long-term colorectal cancer mortality. Int J Epidemiol. 2018. September 3. doi: 10.1093/ije/dyy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheu BS, Wu MS, Chiu CT, et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22. doi: 10.1111/hel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YC, Chiang TH, Liou JM, el al. Mass eradication of Helicobacter pylori to prevent gastric cancer: Theoretical and practical considerations. Gut Liver. 2016;10:12–26. ** A review article to extensively discuss the scientific rationale, policy implementation, and outcome evaluation by using the mass eradication of H. pylori following the principle of a population-based, organized screening program. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut. 2018:0:1–7. doi: 10.1136/gutjnl-2017-314600. [DOI] [PubMed] [Google Scholar]
- 44.Hurst AF. Schorstein lecture on the precursors of carcinoma of the stomach. Lancet. 1929;214:1023–1028. [Google Scholar]


