Abstract
Sphingolipids are diverse lipids with essential, and occasionally opposing, functions in the cell and therefore tight control over biosynthesis is vital. Mechanisms governing this regulation are not understood. Initial steps in sphingolipid biosynthesis take place on the cytosolic face of the endoplasmic reticulum (ER). Serine palmitoyltransferase (SPT) is an ER-resident enzyme catalyzing the first-committed step in sphingolipid biosynthesis. Not surprisingly, SPT activity is tightly regulated. ORMDLs are ER-resident proteins recently identified as regulators of SPT activity. ORMDL proteins interact directly with SPT but the nature of this interaction is unknown. ORMDL protein sequences contain hydrophobic regions, yet algorithm-based predictions of transmembrane segments are highly ambiguous, making topology of this key regulator unclear. Here we report use of substituted cysteine accessibility to analyze topology of mammalian ORMDLs. We constructed multiple mutant ORMDLs, each containing a single cysteine strategically placed along the protein length. Combined use of selective membrane permeabilization with an impermeant cysteine modification reagent allowed us to assign transmembrane and cytosolic segments of ORMDL. We confirmed that mammalian ORMDL proteins transit the membrane four times, with amino- and carboxy termini facing the cytosol along with a large cytosolic loop. This model will allow us to determine details of the ORMDL-SPT interaction and identify regions acting as the “lipid sensor” to detect changes in cellular sphingolipid levels. We also observe that SPT and ORMDL are substantially resistant to extraction from membranes with non-ionic detergent, indirectly suggesting that both proteins reside in a specialized subdomain of the ER.
Keywords: ORMDL, serine palmitoyltransferase, SPT, sphingolipid biosynthesis, SCAM™ analysis, detergent resistance, membrane insertion
1. Introduction
The sphingoid base, also known as the long chain base (LCB), was first reported by Thudichum in 1884 while analyzing brain tissue, and is now known to be the basic building block of all sphingolipids. Sphingolipids are essential components of cellular membranes as well as key signaling molecules with diverse and sometimes opposing roles in determining cell fate. Therefore, a clear understanding of the mechanisms controlling their metabolism is essential. The precursor for all sphingoid bases is 3-ketodihydrosphingosine which is produced by the enzyme, serine palmitoyltransferase (SPT) [1]. The activity of SPT is highly conserved across multiple species even though the overall profile of sphingolipids found in each species differs somewhat. Complexity and diversity in sphingolipid composition is brought about by variations in the chain length of the fatty acids utilized to acylate the sphingoid base in subsequent enzymatic steps, as well as the variety of head groups that can be attached to the C1 hydroxyl, such as acyl chains, sugars, phosphate, phosphoinositol, and phosphocholine [2]. The rapid conversion of intermediates, both biosynthetically and via degradative pathways, yields distinct pools of sphingolipids throughout the interior of the cell [1, 3].
Serine palmitoyltransferase activity has long been suspected as key to sphingolipid biosynthesis [4] and the molecular structure of the enzyme was first characterized in yeast [5]. SPT is an ER-resident, membrane-bound protein that requires multiple subunits in order to be catalytically active [1]. In yeast, these subunits include long chain base 1 (Lcb1), long chain base 2 (Lcb2), and Tsc3 proteins, with Lcb1 and Lcb2 making up the base complex required for SPT activity. Tsc3 is not required for basal activity of the SPT complex but it was shown to increase activity nearly 30-fold when present [6]. In mammals, the large subunits of SPT include SPTLC1-3, where SPTLC1 pairs with either SPTLC2 or SPTLC3 to form an active, heterodimeric complex. In addition to these large subunits, recent reports have identified small activating subunits in mammals, ssSPTa and ssSPTb [7]. These subunits are reported to confer acyl-CoA specificity and increase basal SPT activity nearly 100-fold.
As SPT catalyzes the first committed step for all sphingoid bases, regulation of this enzyme is key to flux through the sphingolipid metabolic pathway. It has long been known that the addition of exogenous sphingolipids, such as sphingosine [8] or short chain ceramides [9], to cells in culture has the ability to inhibit SPT activity thereby reducing the production of new sphingoid bases. Even though the original observation was made almost 30 years ago, the molecular mechanisms governing the regulation of SPT are still not known. Recent advances in the sphingolipid field have led to the discovery of a family of small, membrane-bound ER proteins, the Orms (in yeast and plants) and the ORMDLs (in mammals) that hold the key to SPT regulation. The first protein in this family, Orm1, was discovered in yeast and orthologues were discovered in the human genome and named ORM1-like or ORMDL[10]. It was subsequently found that both the yeast Orms [11, 12] and mammalian ORMDLs [9] mediate the inhibition of SPT in response to elevated levels of cellular sphingolipids. The Orm and ORMDL proteins form stable complexes with SPT to accomplish their regulatory function. How the Orm/ORMDL-SPT complex detects cellular sphingolipid levels and how changes in those levels result in altered SPT activity is unknown. The Orm/ORMDL proteins have recently been the subject of intense research efforts and are emerging as essential negative regulators of SPT activity [9, 11–17]
While it is becoming increasingly clear that ORMDL proteins play a key role in regulating SPT activity, and that they do so through direct physical contact with SPT, the nature of this protein-protein interaction has yet to be elucidated. To date, there is no crystal structure of the SPT enzyme to help guide this research. While there is ample evidence showing that the Orm/ORMDL proteins are localized to the ER [10, 13, 18, 19], the topology of these proteins has not been conclusively discerned. Prediction algorithms such as TopPred, TMPred, and HMMTOP, assign two, three or four possible transmembrane domains (TMDs) for the Orm/ORMDL proteins based on hydrophobicity. Topology of human ORMDL3 was probed using a fluorescence-based protease protection assay [20] and that of yeast Orm2 was probed using the insertion of glycosylation cassettes at key locations in the protein [16]. These studies revealed that both the N and C termini of Orm/ORMDL proteins are located on the cytosolic face of the ER. This orientation of the terminal regions of Orm/ORMDL proteins supports an even number of TMDs. A more precise qualification of the transmembrane topology of the ORMDLs would help to identify regions along the length of these proteins that are positioned to directly interact with SPT and possible domains, or discrete sequence elements, that could serve as a “lipid sensor” for detecting changes in the cellular levels of sphingolipids.
2. Materials and Methods
2.1. Materials –
HeLa cells were purchased from the American Type Tissue Collection (#CCL-2.2). Dulbecco’s minimal essential medium (DMEM), scintillation fluid (BetaMax) and HRP-conjugated secondary antibodies for mouse (#31430) and rabbit (#31460) were purchased from Thermo-Fisher (Waltham, MA). Fetal bovine serum was purchased from Gemini Bioproducts (West Sacramento, CA). Empty vector plasmid and the plasmid containing mouse ORMDL1 with carboxy-terminal FLAG and Myc epitopes were purchased from Origene Inc. (Rockville, MD). Q5™ Site-Directed Mutagenesis Kit (#E0552S) was from New England Biolabs (Ipswich, MA). All mutagenesis primers were designed in-house and purchased from Integrated DNA Technologies (San Diego, CA). A plasmid expressing the fusion protein of human SPTLC2/ssSPTa/SPTLC1 (hscSPT) was generated as previously described (Gable et al 2010 JBC). A plasmid expressing a fusion protein of human eGFP-Sec61β subunit was purchased from Addgene (plasmid #62008). Polyethylenimine (PEI) transfection reagent (#23966-1) was from Polysciences, Inc. (Warrington, PA). Polyclonal antibody for ORMDL (#ABN417) was from EMD Millipore (Temecula, CA). Monoclonal antibody for LCBI (#611304) was from BD Biosciences (San Jose, CA). Polyclonal antibody for Calnexin (#SPA-860) was from Enzo (Farmingdale, NY). Polyclonal antibody for GAPDH (#5143A-50) was from Imgenex (Ontario, Canada). Polyclonal antibody for GFP (#2555) was from Cell Signaling (Danvers, MA). Amersham ECL Prime Western Blot Detection Reagent (#RPN2322) was from GE Healthcare (Marlborough, MA). Maleimide-PEG 5000 (#63187), monoclonal antibody for FLAG-M2 (#F1804), essentially fatty acid-free bovine serum albumin (BSA) and myriocin were from Sigma-Aldrich Corp. (St. Louis, MO). Protease inhibitor cocktail (Complete, EDTA-free) was from Roche (Indianapolis, IN). 3H-serine was from American Radiochemical Corporation (St. Louis, MO) or Perkin-Elmer (Waltham, MA). PVDF was from BioRad (Hercules, CA). Unless otherwise indicated all other reagents were from commercial sources.
2.2. Cell Culture and Transfections –
HeLa cells were maintained in high glucose DMEM containing 10% fetal bovine serum, 2mM L-glutamine, 50IU/mL penicillin, 50μg/mL streptomycin and HEPES buffer then housed at 37°C with 5% CO2. Depending on the experiment outlined below, HeLa cells were plated in complete medium using either p100, 6-well, 12-well or 24-well tissue culture plates which were pre-treated with collagen for approximately 10 minutes prior to plating. The next day, media was changed to antibiotics-free DMEM and cells were transfected using PEI at a ratio of 6μg PEI to 1μg plasmid DNA, each diluted in OptiMEM. When co-transfecting two or more constructs in the same experiment, empty vector was used to maintain a constant PEI:DNA ratio for all wells. All transfections were done 24 hours prior to further experimentation.
2.3. Site-directed mutagenesis –
For each cysteine mutant construct that was generated, mutagenesis primers were designed using the specifications recommended in the Q5 Site-Directed Mutagenesis kit (New England Biosciences) and all mutagenesis steps were followed per manufacturer’s instructions. As mouse ORMDL1 contains one endogenous cysteine at residue 43, this cysteine was first mutated to a serine and the subsequent mORMDL1-C43S mutant was used as the template for the remaining constructs generated as well as being used as the no-cysteine, wild type ORMDL control as indicated in each figure. Mutagenesis results for each construct generated were verified via DNA sequencing (Eurofins USA, Louisville, KY) prior to use.
2.4. Modifying cysteine residues with maleimide-PEG in intact cells –
HeLa cells were plated in 12-well plates at 1×105 cells/well in complete DMEM. The next day, cells were transfected with 2μg/well of plasmid DNA using 12μg/well PEI transfection reagent and antibiotics-free DMEM. Twenty-four hours after transfection, media was removed and cells were washed with 1× PBS. Cells were then incubated with either BSA (0.1% final concentration) in complete medium or BSA complexed with C8-ceramide (0.1% BSA:10μM C8-ceramide final concentration) for one hour prior to permeabilization and labeling reactions. The plasma membrane was permeabilized using media containing digitonin (200μg/mL) for 3 minutes at 37°C. Digitonin was removed and cells were washed with 1×PBS. Maleimide-PEG was prepared as a 150mM stock solution in 20mM Tris (pH 7.5) then stored at −20°C until use. Working solutions of maleimide-PEG were prepared by diluting to 1.5mM using 1× PBS containing 0.1mM CaCl2and 1mM MgCl2 (PBSCM, pH 7.0). For some experiments, working solutions of maleimide-PEG were prepared by diluting to 1.5mM using PBSCM that was adjusted to pH 9.0. Cells were incubated in maleimide-PEG solutions (reagent alone, (+) 0.05% Digitonin, (+) 1% TX-100, (+) 10mM DTT, or (+) 0.5% Toluene) for 30 minutes on ice using a rocker. For sonication experiments, cells were lifted with trypsin, pelleted, then resuspended in modification reagent without detergent and sonicated in an ultrasonic water bath for 30 minutes at 4°C. At the end of each incubation, maleimide-PEG modification was stopped using a stop solution containing 10mM DTT in PBSCM with 2% BSA for 10 minutes on ice. At the end of the labeling time, cells were scraped into the modification reagent and transferred to 1.5 mL microfuge tubes containing appropriate volumes of protease inhibitor cocktail and TX-100 for making homogenates. Homogenization was carried out by passaging cells 20 times through a 26 gauge needle followed by a low-speed spin to clear unbroken cells. Samples were prepared for Western blot analysis by adding 5× Laemmli sample buffer, incubating at 60°C for 20 minutes then loading equal volumes of each condition on 12% SDS-PAGE gels.
2.5. Preparation of total cell lysates –
Cells targeted for protein quantitation (to normalize radiolabeled lipids) or Western Blot analysis, were harvested by trypsinization and washed with ice-cold PBS by centrifugation. Cell pellets were resuspended in lysis buffer [(25mM Tris, 250mM sucrose, pH 7.5), 2mM DTT, 0.1% TX-100] containing an EDTA-free protease inhibitor cocktail (Roche). Cells were kept on ice then broken by passage 20 times through a 26 gauge needle. Unbroken cells and debris were removed by centrifugation at 800 RPM for 10 minutes in a fixed angle rotor at 4°C (Eppendorf 5415R Centrifuge). Supernatants were transferred to clean microfuge tubes and subjected to Bradford assay for total protein quantitation [21].
2.6. Preparation of total membranes –
Cells were harvested by trypsinization and washed with ice-cold PBS by centrifugation. Cell pellets were resuspended in hypotonic swelling buffer [10mM Tris, 15mM KCI, 1mM MgCl2, pH 7.5] then incubated on ice for 15 minutes. Volumes of swelling buffer differed depending on plate size. After swelling, buffer was adjusted to 250mM sucrose, 1mM EDTA and 1× protease inhibitor cocktail (Roche). Cells were broken with 20 strokes of a 7mL Dounce homogenizer (Kontes, Kimble/Chase, Rockwood, TN). Unbroken cells were removed by centrifugation at 600 RPM for 10 minutes in a swinging bucket centrifuge (Beckman Allegro25R). Supernatants were then centrifuged at 4°C for 20 minutes at 100,000 RPM in a TLA120.2 ultracentrifuge rotor (Beckman). Pellets were carefully resuspended with resuspension buffer [(25mM Tris, 250mM sucrose), pH 7.5] containing an EDTA-free protease inhibitor cocktail. After resuspension, membrane solutions were passed through a 26 gauge needle then frozen in aliquots using liquid nitrogen and stored at −80°C until use or prepared immediately for WB analysis by adding 5× Laemmli sample buffer and heating at 60°C for 20 minutes.
2.7. Analysis of degree of membrane association for serine palmitoyltransferase (SPT) and ORMDL proteins –
Total membranes were prepared from non-transfected HeLa cells as described in Section 2.6. Equal volumes of membrane were aliquoted into polycarbonate centrifuge tubes (Beckman #343776, 8×34mm) with three technical replicates prepared for each condition tested. Final concentrations for each treatment condition are as follows: no treatment = water only; high salt = [1M] NaCl; alkaline = [0.1M] Na2CO3; detergent treatments = 1% TX-100, 1% Digitonin or 1% SDS. All treatment solutions were prepared in distilled water containing 1× protease inhibitor cocktail. Prepared membrane samples were incubated at 4°C for 30 minutes with rotation except for SDS, which was incubated at room temperature for 30 minutes with rotation to avoid precipitation of detergent. Following the 30-minute incubation period with each treatment condition, samples were centrifuged at 4°C for 20 minutes at 100,000 rpm in a TLA120.1 ultracentrifuge rotor (Beckman). The supernatants were removed and prepared for Western blot analysis as described in Section 2.9. Pellets were resuspended in 5× Laemmli sample buffer to equivalent volumes of supernatants then passaged through a 26 gauge needle prior to heating samples for 20 minutes at 60°C. Equal volumes of prepared membrane and supernatant fractions were resolved on 12% SDS-PAGE gels. Under these conditions, proteins that remained in the pellet after treatment were considered to be resistant to extraction.
2.8. Assay of serine palmitoyltransferase (SPT) activity in intact cells –
Essentially as reported previously with slight modifications [9, 14]. HeLa cells were plated in 24-well plates at 7×104 cells/well in complete DMEM. The next day, cells were transfected with 1 μg/well of plasmid DNA using 6μg/well PEI transfection reagent. 24 hours after transfection, media was removed and cells were washed with 1× PBS. Serine-free media containing 10μCi/mL 3H-serine was used for radiolabeling cells at 200μL/well volume. Cells were labeled for 90 minutes after which labeling media was removed and cells were washed once with PBS. After washing, 200μL/well of 1× PBS was placed on cells. For lipids, cells were harvested directly on the plates by adding 400μL/well alkaline methanol (MeOH + 0.7g/100mL KOH). The cell/PBS/methanol mix was transferred to 2-mL screw cap tubes and total sphingolipids were extracted as follows: 100μl CHCI3 was added, followed by vortexing and brief centrifugation. Then 500μl CHCI3 was added to each tube followed by 300μl alkaline H2O (100μl 2N NH3OH) and 100μl 2N NH3OH to break the phases. Tubes were vigorously vortexed then centrifuged at 14,000 rpm for 1 minute in a microfuge (Beckman) to separate the phases. The upper, aqueous phase was aspirated. The lower, organic phase was washed twice with 1mL alkaline H2O by vortexing, centrifugation, and aspiration of the upper phase. After the final wash, 400μL of the organic phase was dried in mini scintillation vials (Perkin-Elmer, Waltham, MA) under N2, then 7mL of scintillation fluid was added (BetaMax ES, MP Biomedicals, Solon OH) and vials were counted for 5 minutes (Beckman-Coulter LS6500) to assess the 3H incorporated into total sphingolipids. CPM values were normalized to total protein for each condition.
2.9. SDS polyacrylamide electrophoresis and immunoblotting –
Lysates were prepared by adding Laemmli sample buffer then incubated at either 100°C for 5 minutes (whole cell lysates) or 60°C for 20 minutes (membranes and maleimide-PEG samples). Samples were then resolved using 1.5mm thick, 12% polyacrylamide gels and electrophoresed at 50mA. Proteins were transferred to PVDF at 250mA. Blots were blocked for 1 hour with 5% non-fat dry milk (w/v) in Tris-buffered saline/0.1% Tween20 (TBST) then incubated overnight at 4°C with primary antibodies in 5% non-fat dry milk (w/v) in TBST at 1:1000 dilutions. Membranes were then washed three times for 15 minutes at room temperature with TBST. HRP-coupled secondary antibodies were diluted 1:30,000 in TBST with 1% non-fat dry milk (w/v) in TBST and blots were incubated for either 1 hour at room temperature (FLAG, GAPDH) or overnight at 4°C (ORMDL, SPTLC1, Calnexin). Blots were washed three times for 15 minutes at room temperature with TBST followed by 5 minutes in dH2O then visualized with Amersham ECL-Prime detection reagent (GE Healthcare, Pittsburgh, PA) according to the manufacturer’s instructions using Hyblot CL Film (Denville Scientific, Meuchen, NJ). Densitometry was done using ImageJ software.
2.10. Immunofluorescence and confocal microscopy –
HeLa cells were plated @ 5×104 cells/well in 24-well plates on glass cover slips (Fisher, 12mm round) coated with fibronectin using complete DMEM. The next day, cells were transfected with either empty vector or plasmid DNA for ORMDL constructs using 0.5μ9 DNA plus 3μg PEI transfection reagent per well in antibiotics-free medium. After 24 hours, media was removed and cells were washed with 1×PBS. To fix cells, freshly prepared 4% paraformaldehyde was added to each well and incubated at room temperature for 10 minutes. After fixation, paraformaldehyde was removed and cells were washed twice with 0.1% TX-100 in PBS. After second wash, cells were covered completely with 0.1% TX-100 in PBS and incubated at room temperature for 10 minutes to permeabilize cells. Primary antibodies were diluted at 1:200 in 0.1% TX-100 containing 3% BSA. Cells were incubated on rocker with primary antibodies for 1 hour at room temperature then washed 3 times with 0.1% TX-100/3% BSA solution. Fluorescent secondary antibodies were diluted at 1:400 in 0.1% TX-100/3% BSA solution. Cells were then wrapped in foil and incubated on rocker with secondary antibodies for 1 hour at room temperature. Antibodies were removed and cells were washed 3 times with 0.1% TX-100/3% BSA solution. Cover slips were mounted on glass microscope slides using Dako Fluorescence mounting medium and allowed to dry overnight before viewing. Slides were viewed using a Zeiss LSM 700 confocal laser scanning microscope and images were acquired using a 63× objective and ZEN Software (V2.0).
3. Results and Discussion
3.1. Two topological models of mammalian ORMDL proteins are compatible with an even number of predicted transmembrane domains.
While there is sufficient evidence that ORMDL proteins interact directly with serine palmitoyltransferase (SPT) in order to inhibit the activity of this enzyme, the exact nature of this interaction is not known [11, 12, 16]. As an initial starting point, we used a number of online protein sequence analysis tools, each of which utilizes a unique combination of topology prediction algorithms, to explore the transmembrane topology of mammalian ORMDL proteins. Based on these prediction tools, human and mouse ORMDLs are predicted to have either two (TMHMM-2.0, CCTOP, PHILIUS), three (TMPred) or four transmembrane helices (MEMSAT3, HMMTOP). Examples of these topology predictions for all three human and mouse ORMDL proteins, as well as yeast Orm 1 and 2, are detailed in Table 1. These widely varying predictions make it difficult to assess which regions, or sequence elements, of ORMDL proteins might be orientated in a way that would allow direct interaction with serine palmitoyltransferase (SPT) subunits. Previously, topology of human ORMDL3 was probed using a fluorescence-based protease protection assay [20] and that of yeast Orm2 was probed using the insertion of glycosylation cassettes at key locations in the protein [16]. Both of these studies revealed that the N and C termini of Orm/ORMDL proteins are located on the cytosolic face of the ER. These data are compatible with an even number of transmembrane segments. Consequently we designed two working models with the N and C termini of ORMDL proteins on the cytosolic side of the ER membrane (Figure 1).
Table 1:
Predicted transmembrane domains (TMDs) for eukaryotic ORMDL proteins (TMDs are represented as amino acid positions with length of helices in parentheses)
| Protein | Accession # | Length | N-terminus | # of TMDs | TMD1 | TMD2 | TMD3 | TMD4 | Program |
|---|---|---|---|---|---|---|---|---|---|
| hORMDL1 | AAM43502.1 | 153 a.a. | lumenal | 3 | 23-50 (28) | 101-119 (19) | 121-137 (17) | N/A | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 21-43 (23) | 49-67 (19) | 100-115 (16) | 123-140 (18) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 2 | 21-43 (23) | 101-119 (19) | N/A | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 2 | 22-45 (24) | 101-117 (17) | N/A | N/A | PHILIUS4 |
| hORMDL2 | AAM43506.1 | 153 a.a. | lumenal | 3 | 23-51 (29) | 101-119 (19) | 121-140 (20) | N/A | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 21-43 (23) | 49-67 (19) | 100-115 (16) | 123-140 (18) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 1 | 21-43 (23) | N/A | N/A | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 2 | 22-45 (24) | 121-139 (19) | N/A | N/A | PHILIUS4 |
| hORMDL3 | AAM43507.1 | 153 a.a. | lumenal | 3 | 27-51 (25) | 100-119 (20) | 121-137 (17) | N/A | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 21-43 (23) | 49-67 (19) | 100-115 (16) | 123-140 (18) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 2 | 21-43 (23) | 95-117(23) | N/A | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 2 | 22-45 (24) | 122-139 (18) | N/A | N/A | PHILIUS4 |
| mORMDL1 | AAH23695.1 | 153 a.a. | lumenal | 3 | 23-50 (28) | 101-119 (19) | 121-137 (17) | N/A | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 21-43 (23) | 49-65 (17) | 100-115 (16) | 123-140 (18) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 2 | 21-40 (20) | 45-67 (23) | N/A | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 2 | 22-49 (28) | 101-117 (17) | N/A | N/A | PHILIUS4 |
| mORMDL2 | AAH02146.1 | 153 a.a. | lumenal | 3 | 23-50 (28) | 101-119 (19) | 121-140 (20) | N/A | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 21-43 (23) | 49-64 (16) | 100-115 (16) | 123-140 (18) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 2 | 21-43 (23) | 47-64 (18) | N/A | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 2 | 22-45 (24) | 121-139 (19) | N/A | N/A | PHILIUS4 |
| mORMDL3 | AAH46594.1 | 153 a.a. | lumenal | 3 | 27-51 (25) | 100-119 (20) | 121-137 (17) | N/A | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 21-43 (23) | 49-67 (19) | 100-115 (16) | 123-140 (18) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 2 | 21-43 (23) | 95-117(23) | N/A | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 2 | 22-45 (24) | 122-139 (18) | N/A | N/A | PHILIUS4 |
| Protein | Accession # | Length | N-terminus | # of TMDs | TMD1 | TMD2 | TMD3 | TMD4 | Program |
| yORM1 | CAA97026.1 | 222 a.a. | cytoplasmic | 4 | 84-107 (24) | 112-130 (19) | 163-181 (19) | 185-205 (21) | TMPred1 |
| “ | “ | “ | lumenal | 4 | 84-104 (21) | 111-129 (19) | 162-177 (16) | 186-205 (20) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 3 | 85-107 (23) | 120-142 (23) | 163-181 (19) | N/A | TMHMM3 |
| “ | “ | “ | cytoplasmic | 4 | 85-103 (19) | 112-130 (19) | 163-180 (18) | 185-204 (20) | PHILIUS4 |
| yORM2 | KZV09598.1 | 216 a.a. | cytoplasmic | 4 | 79-99 (21) | 106-124 (19) | 153-173 (21) | 177-199 (23) | TMPred1 |
| “ | “ | “ | cytoplasmic | 4 | 79-98 (20) | 105-123 (19) | 156-171 (16) | 178-198 (21) | MEMSAT32 |
| “ | “ | “ | cytoplasmic | 4 | 80-99 (20) | 114-136 (23) | 157-174 (18) | 179-198 (20) | TMHMM3 |
| “ | “ | “ | cytoplasmic | 4 | 80-99 (20) | 104-124 (21) | 152-172 (21) | 179-199 (21) | PHILIUS4 |
Abbreviations: h = human; m = mouse; y = yeast; TMD = transmembrane domain (predicted); a.a. = amino acids
References:
TMPred : K. Hofmann & W. Stoffel (1993) TMbase - A database of membrane spanning proteins segments Biol. Chem. Hoppe-Seyler 374,166
MEMSAT3 : Jones D.T. (2007) Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics. In press. The original method is described in the following reference: Jones, D.T., Taylor, W.R. and Thornton, J. M. (1994) Biochemistry. 33:3038-3049.
TMHMM : Server V2.0 (http://www.cbs.dtu.dk/services/TMHMM/)
PHILIUS : Reynolds SM, Käll L, Riffle ME, Bilmes JA, Noble WS (2008) Transmembrane Topology and Signal Peptide Prediction Using Dynamic Bayesian Networks. PLoS Comput Biol 4(11): e1000213. doi:10.1371/journal.pcbi.1000213
Figure 1 – Two topological models of mammalian ORMDL proteins are compatible with an even number of predicted transmembrane domains.
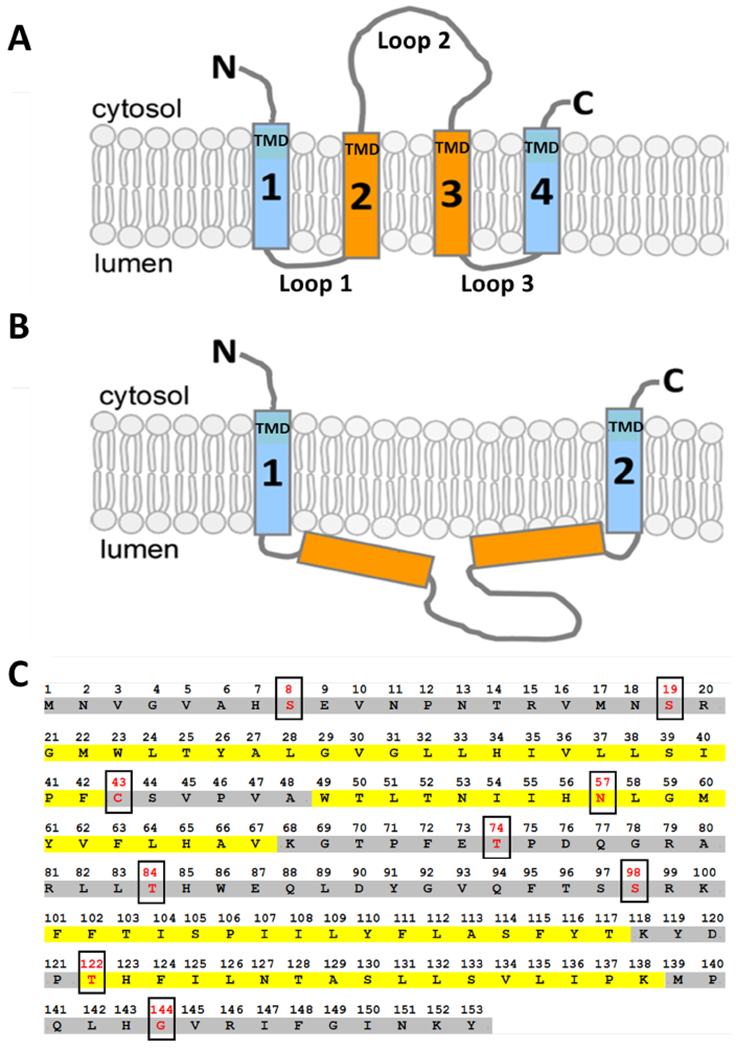
A) One possible model of the transmembrane topology for mammalian ORMDL proteins in the endoplasmic reticulum (ER) membrane that could result from four transmembrane domains with the N and C termini both located on the cytosolic side of the ER. B) An alternative topology for mammalian ORMDL proteins in the ER membrane that could result from two transmembrane domains with the N and C termini both located on the cytosolic side of the ER. C) Mouse ORMDL1 open reading frame produces a protein with 153 amino acids. Possible transmembrane domains as predicted based on hydrophobicity are highlighted in yellow. Residues highlighted in gray are not predicted to span the membrane based on hydrophobicity. Boxed amino acids (red type) represent the residues that were mutated to cysteines for SCAM™ analysis as reported in this study.
The topology model depicted in Fig. 1A shows ORMDL proteins with four transmembrane domains (TMDs), as predicted by HMMTOP and MEMSAT3. In Figure S1 (Supplemental Materials), we used ClustalW [22] to perform a protein sequence alignment of the ORMDL and Orm proteins listed in Table 1. The regions highlighted in yellow represent the predicted TMDs of each protein as reported by MEMSAT3 and agrees well with our proposed topology model in Fig. 1A which has four TMDs. The alternate model predicted by TMHMM-2.0 and PHILIUS, having only two transmembrane domains, is shown in Fig. 1B. For the ease of discussion, we have designated the less hydrophobic spans of amino acids found in the ORMDL sequence as “Loop” domains (Fig. 1A). If the topology model in Fig. 1A is demonstrated as being correct, then Loops 1 and 3 will be in the lumen of the ER and Loop 2 will be cytosolic. If, however, the model in Fig. 1B is shown to be favorable, then all of three Loop domains will be luminal. The amino acid sequence detailed in Fig. 1C is that of mouse ORMDL1 (NP_663492.3), which we used as the template for all of our single-cysteine protein constructs designed for this study. The putative transmembrane domains (TMDs) of mouse ORMDL1, as predicted by algorithms with slight modifications by us, are highlighted in yellow (Fig. 1C). The boxed amino acids (red type) in this figure are the residues that were mutated to cysteines for the purpose of our analysis by substituted cysteine accessibility methodology as applied to transmembrane domains (SCAM™) [23, 24]. This technique, as explained in more detail below, utilizes chemical modification of strategically placed cysteine residues to monitor protein transmembrane topology. In general, mammalian ORMDL proteins are well suited for SCAM™ analysis due to the low number of endogenous cysteine residues present. For example, mouse ORMDL1, which was used in this study, has only one cysteine residue at position 43 (Fig. 1C). In order to have a cysteine-free template for generating single cysteine proteins to test in this topology study, as well as a “pseudo WT” control lacking reactive cysteine residues, we mutated the cysteine at position 43 to a serine. We refer to this construct as “WT” or “pseudo WT” throughout this text.
3.2. ORMDL constructs with single cysteine substitutions are localized to the endoplasmic reticulum and maintain the ability to inhibit serine palmitoyltransferase activity.
Amino acid residues were chosen for site-directed mutagenesis to cysteines based on their predicted topology in our working models. In addition to verifying the localization of the N and C termini, we chose residues predicted to reside in the lumen of the ER (loops 1 and 3, Fig. 1A) as well as residues located within the longest non-hydrophobic region of ORMDL (loop 2, Fig. 1A). We also chose one residue located in the middle of the putative transmembrane domain 2 (TMD 2, Fig. 1A) to assess the efficacy of our modification reagent to react with intramembrane cysteines. Once the mutagenesis steps were complete, we verified the DNA and protein sequences for each single-cysteine construct to insure that the desired changes were made without any unwanted mutations elsewhere in the protein. We then subjected each construct to a series of quality control assays. There were several criteria we felt important for each single-cysteine protein to possess prior to using them for SCAM™ analysis: 1) the ability to produce recombinant protein when overexpressed in cells; 2) the ability to inhibit serine palmitoyltransferase in intact cells to the same degree as wild type; 3) localization of the constructs to the native ORMDL site in the endoplasmic reticulum as previously established. Once all of these criteria were met, the construct was added to the pool of single-cysteine mutants to be used for SCAM™ analysis.
The ORMDLs are well characterized as inhibitors of serine palmitoyltransferase [9, 11, 12, 16] and understanding this ORMDL-dependent regulation of SPTis one main focus of our research efforts. To confirm that the cysteine substitutions did not compromise this function, the ability of each single-cysteine construct to inhibit SPT activity was assessed using an in situ assay developed in our lab [9]. We previously reported that overexpression of human SPT, as a single polypeptide comprising the subunits SPTLC2, ssSPTa and SPTLC1 (scSPT), generated SPT activity that was responsive to inhibition by ectopic expression of ORMDLs [14]. Here, we have used a modification of our in situ assay that is a direct measure of de novo sphingolipid biosynthesis due to the nature of the alkaline extraction conditions used after labeling with tritiated serine [25]. As seen in Fig. 2A, each of the single-cysteine constructs used for this study was able to inhibit SPT activity to the same degree as wild type ORMDL when co-expressed with scSPT. While there are slight variations in the degree of SPT inhibition afforded by each construct tested, these differences were not statistically different from the level of inhibition seen with the wild-type ORMDL. Even though each of these single-cysteine constructs was made using the same plasmid DNA template, we sometimes noted variations in the expression levels of the overexpressed protein for each construct as shown in Fig. 2B (overexpressed or upper ORMDL bands). This could, in part, explain the differences in SPT inhibition as the activity assay is normalized to total protein not ORMDL expression level. Additionally, we have previously established a necessary stoichiometry between the ORMDL proteins and the SPT subunits [14] which might not be at the correct proportions for this type of overexpression assay. In contrast to the ORMDL constructs used, when we overexpressed human Sec61β subunit, which is also an integral membrane protein localized to the ER, there is no inhibition of SPT activity as compared to either WT ORMDL or the single cysteine mutant, S8C (Figure S2A, Supplemental Materials). This further validates the specificity of this assay as a measure of a functional ORMDL-SPT complex. In addition to inhibition of SPT activity, we also used fluorescent confocal microscopy (Fig. 2C, Supplemental Figure S3) to look at the cellular localization of our cysteine-substituted constructs to insure that the overexpressed protein was being localized to the ER membrane. HeLa cells were grown on glass coverslips then transfected with each single-cysteine ORMDL construct twenty-four hours prior to fixing and staining the cells. ORMDL constructs were visualized using their FLAG epitope tags (upper panels) and an antibody recognizing the KDEL sequence in ER proteins (lower panels) was used as an endogenous cellular marker for the ER. The immunofluorescence pattern documented for the ORMDL-FLAG constructs is not discernibly different from the pattern documented for the endogenous ER marker, KDEL, showing that our recombinant single-cysteine proteins are clearly localized to the ER, as expected. As seen in Supplemental Figure S3, there is good overlay with the ER marker, KDEL, and each of the ORMDL constructs tested. Shown in Fig. 2C and Fig. S3, are just four of the constructs used in this study, namely wild type, one N-terminal predicted (S8C), one C-terminal predicted (G144C) and one located in Loop 2 (T74C) as proposed in the model shown in Fig. 1A. The remaining constructs were submitted to the same immunofluorescent assay (results not shown).
Figure 2 – ORMDL constructs with single cysteine substitutions are localized to the endoplasmic reticulum and maintain the ability to inhibit serine palmitoyltransferase activity.
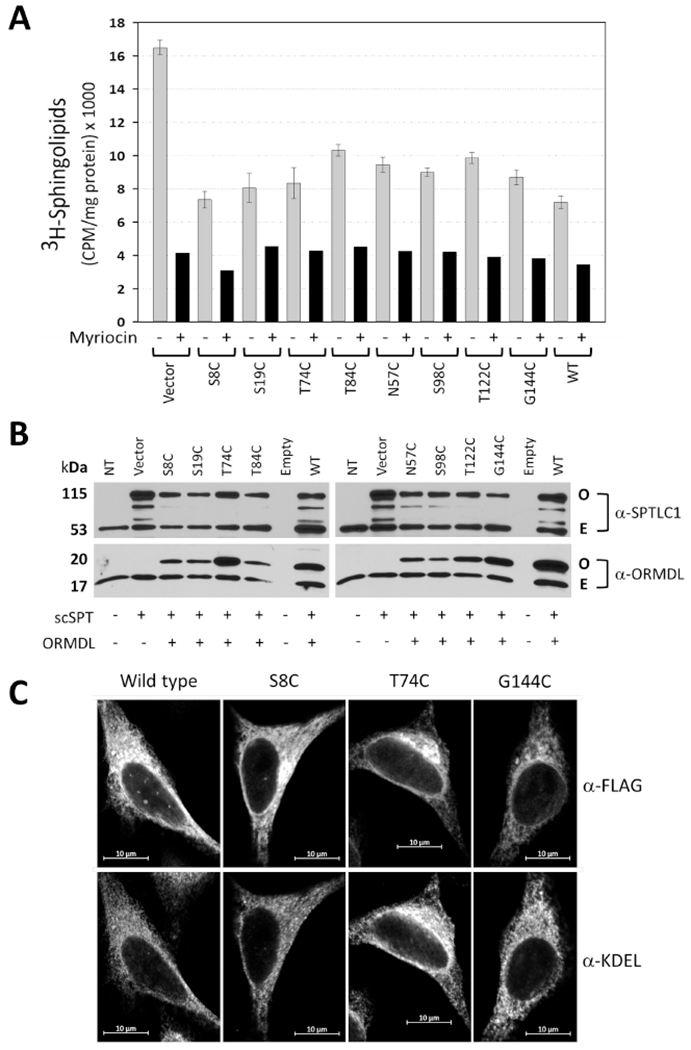
A) SPT activity assay using intact HeLa cells that were co-transfected with single chain-SPT fusion protein and either empty vector or cysteine mutant constructs as noted (numbers correspond to a specific amino acid residue that was mutated to cysteine). Cells were labeled with 3H-serine in the absence (gray bars) or presence (black bars) of the SPT-specific inhibitor myriocin after which total sphingolipids were harvested and the amount of 3H incorporated into sphingolipids was determined as described in Section 2.8 of Methods. Data shown are mean CPM/mg total protein ± SEM (n=5) and are representative of three independent experiments. Note that all constructs inhibited SPT activity to the same degree as WT. B) Western Blot analysis of protein samples generated from the transfected HeLa cells used in Figure 2A. scSPT: single-chain fusion construct of human SPTLC1, SPTCL2 and ssSPTa; O: overexpressed protein band; E: endogenous protein band. C) Fluorescent confocal microscopy of overexpressed mouse ORMDL1 constructs (top panels) labeled with anti-FLAG antibody in HeLa cells counter-stained with anti-KDEL antibody (bottom panels) as an endogenous endoplasmic reticulum (ER) marker. Wild type: mouse ORMDL1; S8C: serine 8 mORMDL1 mutant; T74C: threonine 74 mORMDL1 mutant; G144C: glycine 144 mORDLI mutant. Scale bars are 10μm.
3.3. Experimental strategy for transmembrane topology assessment of mammalian ORMDL, an ER-resident protein.
Scanning cysteine accessibility method (SCAM) has been used extensively to study the membrane topology of proteins residing in the plasma membrane [26–29]. However, there are only a limited number of studies using this method for proteins that reside in the ER membrane [24, 30]. The construction and analysis of the approach taken in this study is depicted diagrammatically in Figure 3 (note that the Western blots are cartoons for illustration, not actual data). Here, we employed low doses (0.02%) of the cholesterol-binding drug, digitonin, to selectively permeabilize the plasma membrane while keeping the ER membrane intact (Fig. 3A). At concentrations of 0.04% or less, the effects of digitonin are confined to the plasma membrane, which is cholesterol rich as compared to the ER membrane, whereas increasing the concentration to 0.1% or more results in leakage of ER luminal proteins [31–33]. We have successfully used this concentration for cell-based assays and achieve high levels of permeabilized cells without compromising function of the enzymes being studied [9, 34]. For each of the cartoon panels in Figure 3, permeabilized membranes are represented by dashed lines while intact membranes are represented by solid lines. We combined this digitonin-permeabilized cell technique with a membrane-impermeant sulfhydryl-reactive compound, maleimide-PEG, which is not able to cross membrane barriers in the absence of detergent [30, 31]. Maleimide readily and selectively reacts with the cysteinyl thiolate to form a mixed disulfide bond. Additionally, maleimides are virtually nonreactive until they encounter an available thiol group which makes them particularly well suited for SCAM™ analysis. Failure of our modification reagent, maleimide-PEG, to label the lumenal ER protein, PDI (protein disulfide isomerase), in the absence of detergent indicates that the ER membrane is still intact under the digitonin conditions used here to permeabilize the plasma membrane (Figure S4, Supplemental Materials).
Figure 3 – Experimental strategy for transmembrane topology assessment of mammalian ORMDL.
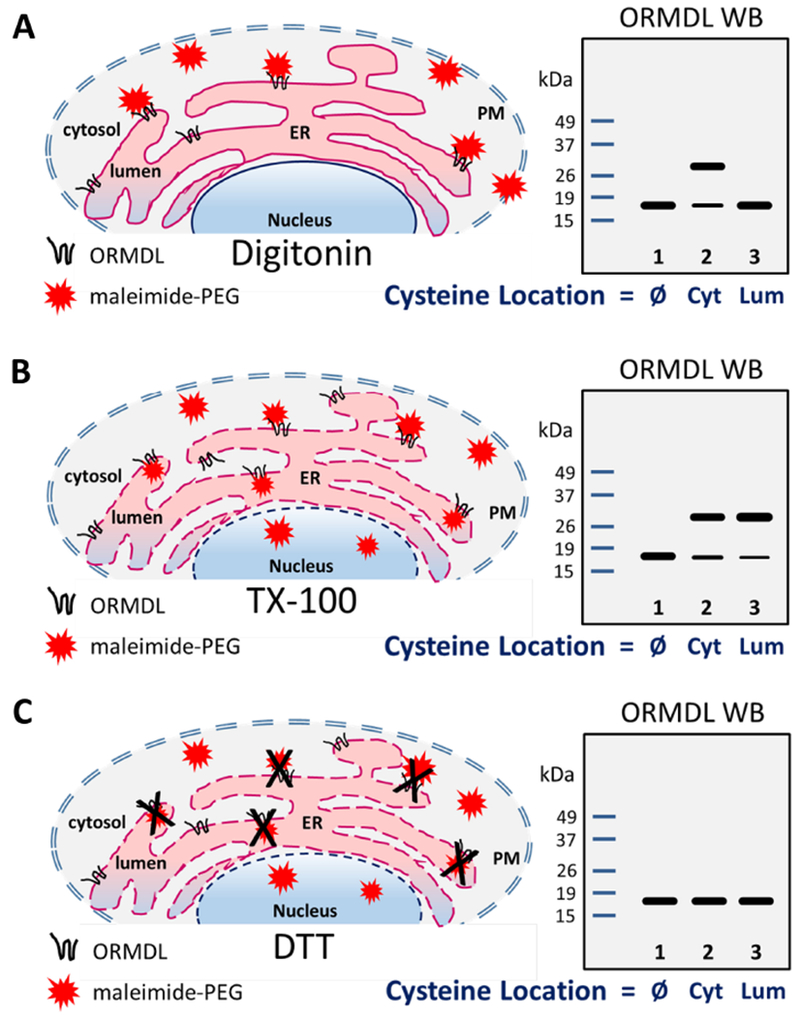
A) The cholesterol-binding drug, digitonin, is used to permeabilize the plasma membrane (PM) of adherent HeLa cells while leaving the internal membranes intact. For each of these cartoons, permeabilized membranes are represented by dashed lines while intact membranes are represented by solid lines. This allows the membrane impermeant maleimide-PEG compound to enter the cells where it can react with cysteine residues on the cytoplasmic side of the endoplasmic reticulum (ER) membrane. This PEG-modified version of ORMDL travels more slowly in SDS-PAGE gels and is depicted by the upper band in Lane 2 of the ORMDL Western blot cartoon (note that the blots are cartoons for illustration, not actual data). Under these conditions, cysteine residues located on the luminal side of the ER or within the membrane space, will not be labeled as in Lane 3. B) Addition of the detergent, TX-100, during the modification reaction will permeabilize all internal membranes and allow access of the maleimide-PEG compound to react with luminal cysteines as readily as it labels cytosolic cysteines. The only ORMDL constructs not labeled under these conditions would be the no-cysteine or wild-type control (i.e. Lane 1 in cartoons, Ø). C) Addition of 10m M DTT during the modification reaction will quench the reactive maleimide-PEG compound thereby rendering it incapable of labeling any cysteines. If the quenching is complete, the Western blot will show no modified ORMDL bands regardless of the location of the cysteine residues (all lanes). Ø: no cysteine residues; Cyt: cysteines located on cytosolic side of ER membrane; Lum: cysteines located on luminal side of ER membrane.
Maleimide-PEG (Mn 5,000), used in this study, is commonly employed to covalently attach polyethylene molecules (PEG) to proteins, after which the molecular weight of the modified protein will be larger and will run more slowly on SDS-PAGE gels. This is depicted in Figure 3A (lane 2, Cyt), where there are two bands drawn on our theoretical Western blot cartoon. The digitonin-permeabilized cells allowed the maleimide-PEG compound ready access to the intracellular space, thus labeling any cysteine residues on ORMDL on the cytosolic side (Cyt) of the ER membrane. Under our reaction conditions, maleimide-PEG is not able to cross the ER membrane unless a detergent is added to completely permeabilize internal cellular membranes (see Figure S4, lanes 2 and 3, Supplemental Materials). As depicted in Figure 3B, we used TritonX-100 to allow the maleimide-PEG reagent to reach the luminal side of the ER membrane. This resulted in labeling of luminal cysteine residues on ORMDL proteins to the same degree as cytosolic cysteines were labeled (Fig. 3B, lanes 2 and 3, upper bands). Maleimide-PEG is rendered non-reactive in the presence of a strong reducing agent, such as DTT or β-Mercaptoethanol. Here we used 10mM DTT during the labeling reaction to completely block the ability of maleimide-PEG to react with cysteinyl thiolates (Fig. 3C, no upper bands). The use of digitonin-permeabilized adherent cells combined with the membrane impermeant reagent, maleimide-PEG, in the presence or absence of TX-100, allowed us to assess the native topology of ORMDL protein residing in the ER membrane as determined by the labeling patterns for each single-cysteine ORMDL construct.
3.4. The N-terminus and C-terminus of mammalian ORMDL protein are readily labeled with maleimide-PEG with an intact ER membrane.
As mentioned earlier, we expected the N and C termini of ORMDL protein to be located on the cytosolic face of the ER membrane [16, 20]. To confirm this topology, we created the N-terminal predicted single-cysteine mouse ORMDL1-FLAG constructs, S8C and S19C, as well as the C-terminal predicted construct, G144C. These constructs were overexpressed in HeLa cells along with the no-cysteine “pseudo WT” control. We have previously shown that SPT activity is reduced in the presence of exogenous C8-ceramide in an ORMDL-dependent fashion [9, 14]. To assess the potential effect on topology of ORMDL protein in the presence of elevated ceramide levels, twenty-four hours after transfection the cells were treated with either BSAor BSA-C8 ceram ide for one hour prior to permeabilization and labeling with maleimide-PEG as detailed in Section 2.4 of Methods. The modification reaction was carried out in the presence and absence of TX-100 and DTT as indicated under each figure. As seen in Figure 4, the cysteine-less “pseudo WT” mORMDL1-FLAG protein failed to be modified by maleimide-PEG, indicated by the lack of a 32 kDa band in the Western blot panels for the WT control in both panels A and B. This confirms the cysteine-specificity of the labeling reaction under the conditions we employed. The S8C and S19C single-cysteine ORMDL proteins were readily labeled in the absence of TX-100 (Fig. 4A, lanes 1 and 4), indicating easy access of maleimide-PEG to these cysteine residues. Additionally, we noticed that the S19C single-cysteine protein was consistently modified to a lesser degree than the S8C construct resulting in reduced intensity of the upper band (modified ORMDL) and a more prominent lower band (unmodified ORMDL). This pattern suggests reduced accessibility of the maleimide-PEG reagent to the side chain of this particular cysteine residue location and is indicative of some type of steric hindrance. Inability of a modification reagent to access a reactive cysteinyl thiolate can result from several factors, including secondary protein structure, proximity to the membrane interface or possible intramembrane location (Fig. 4A, cartoon inset). This pattern of hindered accessibility is also seen with the S98C single-cysteine construct discussed in the next section. To distinguish between steric hindrance due to secondary protein structure vs. possible intramembrane location, we conducted modification reactions under alkaline conditions (Figure S5, Supplemental Materials). Increasing the reaction pH from 7.4 to 9.0 not only favors the modification reaction, it can help relax secondary protein structures to allow access to cysteinyl thiolates residing in previously inaccessible extramembrane environments [23, 24]. Integrity of the ER membrane is not compromised at pH 9.0, so residues which are truly embedded inside the membrane domain will not be modified under these conditions allowing for discrimination between intramembrane and sterically hindered residues. As seen in Figure S5, S19C and S98C constructs both showed improved labeling under alkaline conditions (compare intensity of upper bands at pH 7.4 vs pH 9.0). In contrast, the cytosolic construct, T74C, which is further away from the membrane interface (cartoon inset, Fig. 5), labeled equally well at either pH condition. This result suggests that residues S19 and S98 of ORMDL1 are located on cytosolic regions of the protein and that the reduced modification seen with cysteine substitutions at these locations are likely due to steric hindrance caused by proximity of the membrane interface. For the C-terminal predicted single-cysteine construct, G144C, there was also ample modification by maleimide-PEG in the absence of TX-100 (Fig. 4B, lanes 1 and 4) which was only slightly improved under alkaline conditions (Figure S5). All of the N- and C-terminal single-cysteine constructs tested displayed strong modification by maleimide-PEG in the presence of TX-100 (Panels A & B, lanes 2 and 5) and this modification was completely blocked by the addition of DTT (Panels A & B, lanes 3 and 6). Surprisingly, the pre-incubation with exogenous C8-ceramide did not appear to alter the topology of the single-cysteine ORMDL constructs tested (Panels A & B, compare lanes 1-3 with lanes 4-6). Overall, these results confirm our prediction that the N and C termini of mammalian ORMDL proteins are located on the cytosolic face of the ER membrane. This configuration also necessitates the existence of an even number of TMDs as predicted by TMHMM-2.0 and PHILIUS (two TMDs) or MEMSAT3 (four TMDs) which is reflected in our models presented in Figure 1 and highlighted in our sequence alignment (Figure S1).
Figure 4 – The N-terminus and C-terminus of mammalian ORMDL protein are readily labeled with maleimide-PEG with an intact ER membrane.
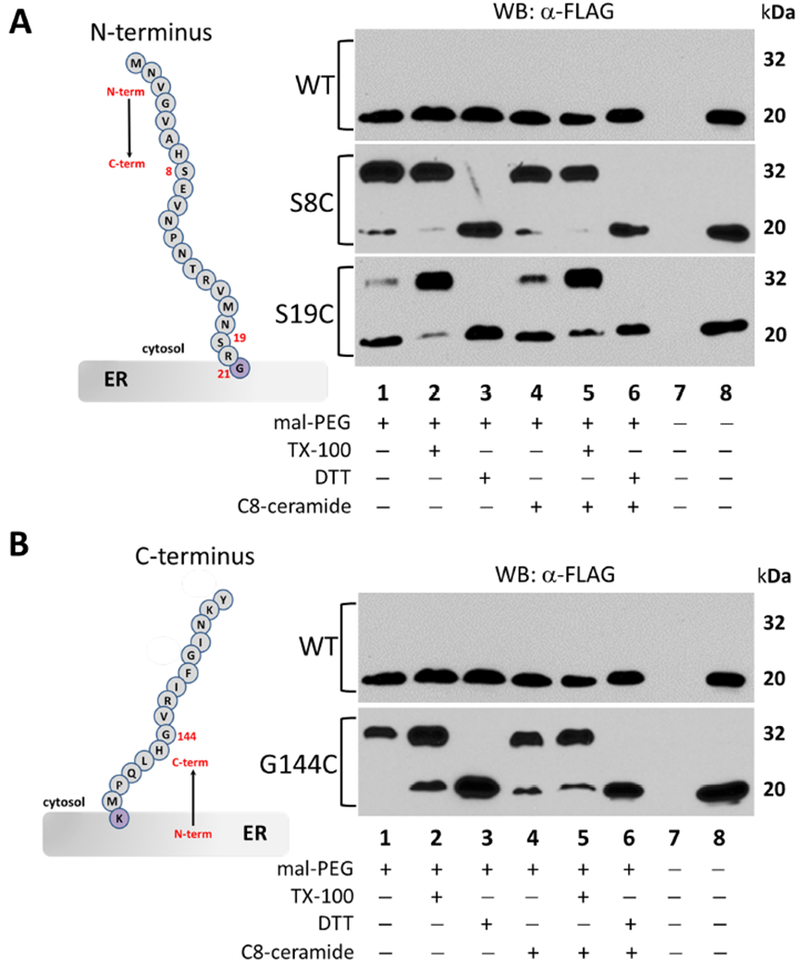
A) Western blot analysis of lysates from HeLa cells transfected with either the no-cysteine control mORMDL1-FLAG (WT) or the N-terminal (S8C, S19C) single-cysteine constructs of mORMDL1-FLAG then labeled with maleimide-PEG as outlined in Section 2.4 of Methods. Unmodified, FLAG-tagged ORMDL protein runs at approximately 20 kDa while the maleimide-PEG modified protein results in an additional band at approximately 32 kDa under these conditions. B) Western blot analysis of lysates from HeLa cells transfected with either the no-cysteine control mORMDL1-FLAG (WT) or the C-terminal (G144C) single-cysteine construct of mORMDL1-FLAG then labeled with maleimide-PEG exactly as outlined in Section 2.4 of Methods. Data in all panels are representative of multiple independent experiments and the lane conditions are as follows: 1) Maleimide-PEG reagent alone; 2) 1% TX-100 added; 3) 10mM DTT added; Lanes 4-6) same as lanes 1-3 after a 1-hour pre-incubation with 10μM C8-ceramide; 7) empty lane; 8) positive control for antibody staining.
Figure 5 – Cysteine substitutions located between residues 68 and 100 are readily labeled by maleimide-PEG in the absence of detergent.
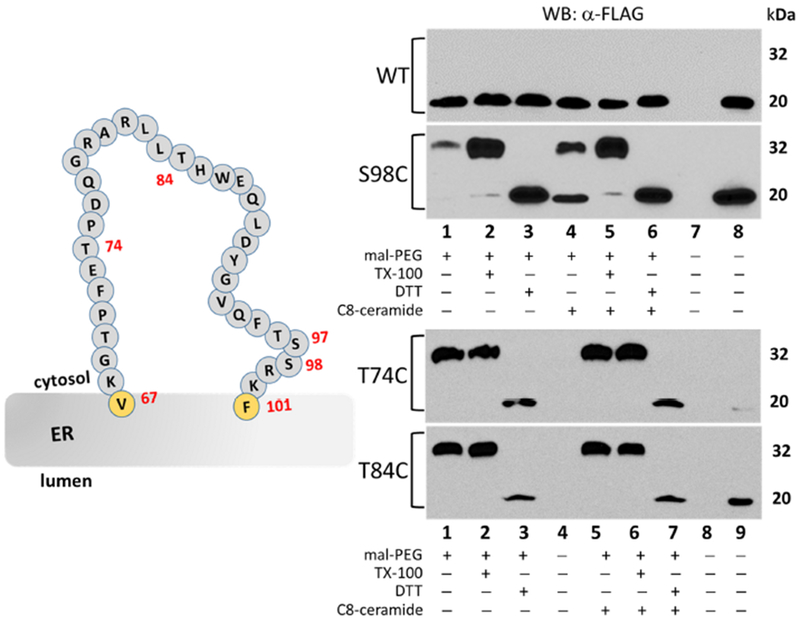
Western blot analysis of lysates from HeLa cells transfected with either the no-cysteine control ORMDL1-FLAG (WT) or the T74C, T84C and S98C single-cysteine constructs of mORMDL1-FLAG then labeled with maleimide-PEG as outlined in Section 2.4 of Methods. Unmodified, FLAG-tagged ORMDL protein runs at approximately 20 kDa while the maleimide-PEG modified protein results in an additional band at approximately 32 kDa under these conditions. Data in all panels are representative of multiple independent experiments and the lane conditions are as follows: upper panels 1) Maleimide-PEG reagent alone; 2) 1% TX-100 added; 3) 10mM DTT added; Lanes 4-6) same as lanes 1-3 after a 1-hour pre-incubation with 10μM C8-ceramide; 7) empty; 8) positive control for antibody staining; lower panels 1) Maleimide-PEG reagent alone; 2) 1% TX-100 added; 3) 10mM DTT added; 4) empty lane; Lanes 5-7) same as lanes 1-3 after a 1-hour pre-incubation with 10μM C8-ceramide; 8) empty lane; 9) positive control for antibody staining.
3.5. Cysteine substitutions located between 68 and 100 are readily labeled by maleimide-PEG in the absence of detergent.
After confirming the topology of the N and C termini of ORMDL, the next region of interest was the long span of relatively non-hydrophobic amino acids located between residues 68 to 100. As shown in Figure 1, this region (Loop 2) could either be forming a large cytosolic loop (Fig. 1A, four TMD model) or could be located entirely in the lumen of the ER (Fig. 1B, two TMD model). Although we focused primarily on selection of serine to cysteine mutations to keep the amino acid change as conservative as possible, the middle of this region of ORMDL1 protein lacked appropriate serines for substitution (Figure 5, cartoon inset). Therefore we decided to create three single-cysteine constructs to cover this span of amino acids, specifically threonines 74 and 84 (T74C and T84C) along with serine 98 (S98C). As before, these constructs were overexpressed in adherent HeLa cells along with the WT control mORMDL1-FLAG construct, then treated and labeled 24 hours after transfection. Similar to the modification profile seen with S19C (Fig. 4A), the S98C single-cysteine construct was weakly maleimide-PEG modified in the absence of TX-100, however labeling was enhanced in the presence of TX-100 (Figure 5, top panels, lanes 1 and 4 vs lanes 2 and 5). As stated earlier, we believe this pattern to be indicative of reduced accessibility of the maleimide-PEG reagent to this amino acid, possibly due to proximity to the membrane interface. As discussed above, we conducted additional modification reactions under alkaline conditions to confirm this observation so that we could designate this residue as being located either in the cytosol or lying within the intramembrane space. Figure S5 (Supplemental Materials) shows that S98C, as seen with S19C, was modified to a greater extent under alkaline conditions (compare intensity of upper bands for pH 7.4 to pH 9.0). In contrast, the single-cysteine constructs T74C and T84C, were modified equally well by maleimide-PEG irrespective of the absence or presence of TX-100 (Figure 5, bottom panels, lanes 1 and 5 vs lanes 2 and 6) or alkaline conditions (Figure S5, T74C). This pattern of modification indicates that these residue locations are indeed cytosolic in their topology, as well as being far enough removed from the membrane interface region of ORMDL as to afford easy access to the maleimide-PEG labeling compound regardless of the reaction conditions. Taken together, the modification profiles of the single-cysteine constructs tested thus far support the topology model depicted in Figure 1A. This model shows ORMDL firmly inserted in the ER membrane with four membrane spanning domains, as well as the N- and C-termini, along with a large loop between TMD2 and TMD3, facing the cytosol. This study is the first to report the presence of this cytosolic loop in ORMDL proteins.
3.6. Residues in regions between predicted transmembrane segments 1 and 2 and also between transmembrane segments 3 and 4 are either luminal or membrane embedded.
To further refine the emerging model of ORMDL, we selected additional residues that were predicted to be located either in the lumen of the ER or within the intramembrane space. If our proposed model is correct, these residues should not be accessible to maleimide-PEG modification when the ER membrane is intact (in the absence of detergent), yet readily accessible when the membrane is detergent solubilized. As stated above, mouse ORMDL1 protein has one endogenous cysteine residue at position 43. Based on our emerging topology model, this residue should be located in the luminal loop between TMD1 and TMD2 (Fig. 6A, cartoon inset; Fig. 1A, Loop 1). As shown in Figure 6A, when overexpressed in HeLa cells, the mouse ORMDL construct with the endogenous cysteine at residue 43 (C43) is only modified by maleimide-PEG in the presence of TX-100 (Fig. 6A, lanes 1 and 4 vs lanes 2 and 5). This modification profile also holds true for the single-cysteine substitution proteins, N57C (located within TMD2, Fig. 6A, lanes 1 and 4 vs lanes 2 and 5) and T122C (luminal loop between TMD3 and TMD4, Fig. 6B, lanes 1 and 5 vs lanes 2 and 6). These results confirm that cysteine residues at each of these locations are not accessible to maleimide-PEG unless the ER membrane is solubilized with TX-100, further strengthening the four-TMD model for ORMDL. The conditions used for the maleimide-PEG modification reactions thus far in this study do not distinguish between residues that are luminal versus residues that reside inside the membrane space of the ER. In an attempt to address this, we conducted experiments under two separate conditions that should have allowed access of the maleimide-PEG reagent to residues within an aqueous environment without allowing labeling to occur in the hydrophobic environment of the intramembrane space. Using alkaline conditions, cells overexpressing single-cysteine constructs were subjected to modification reactions in the presence of 0.5% Toluene or sonicated in an ultrasonic waterbath. Toluene has been shown to permeabilize membranes without completely solubilizing them as is the case with TX-100 [24]. In our hands, we did not see modification of our internal predicted loop constructs (C43 and T122C) in the presence of toluene (Figure S5). The presence of toluene did not alter the modification of our predicted cytosolic constructs (S19C, T74C, S98C, G144C) which implies that our system was functioning properly. As we did not check for PDI labeling under toluene conditions, we are not able to verify that the ER membrane was permeabilized during our reactions. In terms of sonication, only predicted cytosolic residues that were sufficiently far away from the membrane interface (S8C, T74C, G144C) showed labeling under these conditions (Figure S5). We did not expect the N57C construct to label under either of these conditions as our model predicts the amino acid to be located within the intramembrane space. Taken together, we cannot firmly distinguish amino acids C43 or T122 as being located within luminal loops existing between TMDs or within the intramembrane space. We can, however, designate them as being non-cytosolic in location.
Figure 6 – Residues in loops between predicted transmembrane segments 1 and 2 (C43) and also between transmembrane segments 3 and 4 (T122) are not readily labeled by maleimide-PEG in the absence of detergent.
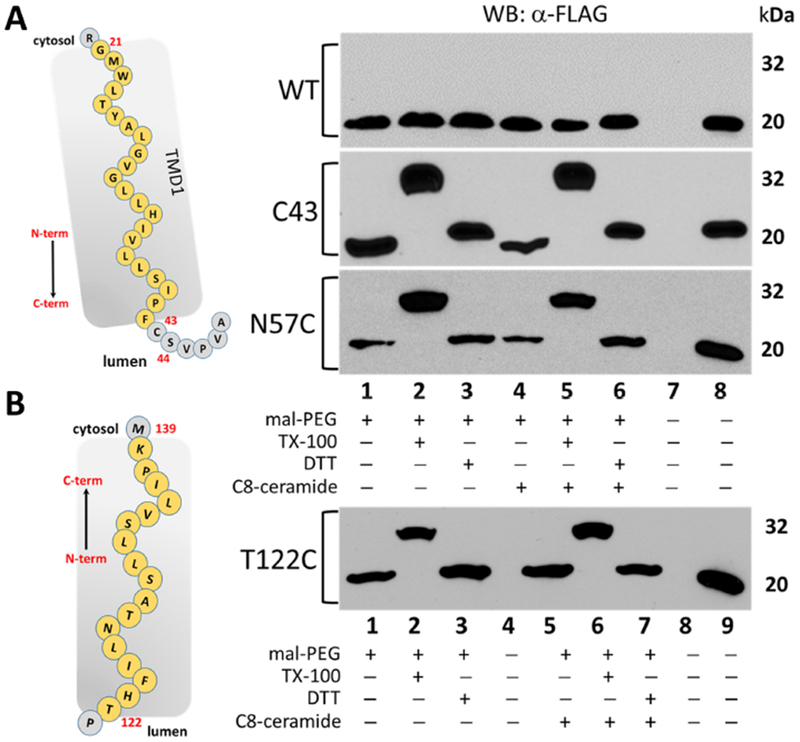
Western blot analysis of lysates from HeLa cells transfected with either the no-cysteine ORMDL1-FLAG (WT) or single-cysteine constructs of mORMDL1-FLAG predicted to be luminal or in the intramembrane space (C43, N57C, T122C) then labeled with maleimide-PEG as outlined in Section 2.4 of Methods. Unmodified, FLAG-tagged ORMDL protein runs at approximately 20 kDa while the maleimide-PEG modified protein results in an additional band at approximately 32 kDa under these conditions. Data in all panels are representative of multiple independent experiments and the lane conditions are as follows: upper panels 1) Maleimide-PEG reagent alone; 2) 1% TX-100 added; 3) 10mM DTT added; Lanes 4-6) same as lanes 1-3 after a 1-hour pre-incubation with 10μM C8-ceramide; 7) empty; 8) positive control for antibody staining; lower panel 1) Maleimide-PEG reagent alone; 2) 1% TX-100 added; 3) 10mM DTT added; 4) empty lane; Lanes 5-7) sameas lanes 1-3 after a 1-hour pre-incubation with 10μM C8-ceramide; 8) empty lane; 9) positive control for antibody staining.
3.7. Ceramide levels do not alter ORMDL topology.
The ORMDL proteins are essential for the homeostatic regulation of SPT in response to elevated sphingolipid levels [9]. We routinely use incubation of cells with a short-chain ceramide, in this case the sphingosine backbone N-acylated to an 8 carbon fatty acid (C8-ceramide), to trigger ORMDL-dependent inhibition of SPT activity [9, 14]. The precise molecular mechanisms governing this response to exogenously added sphingoid bases are not known. To test whether sphingolipid inhibition of SPT by ORMDL involves a possible change in the membrane topology of ORMDL protein, we included this pre-incubation step in our SCAM™ analysis for each single-cysteine construct tested. As detailed in Section 2.4 of Methods, prior to digitonin permeabilization and maleimide-PEG labeling, cells were pre-incubated with either BSA or BSA complexed with C8-ceramide under conditions previously shown to trigger ORMDL-dependent inhibition of SPT (Figures 4–6, lanes indicated as (+) C8-ceramide). None of the modification profiles observed in this study appear to be different in the presence of C8-ceramide when compared to profiles in the absence of exogenous sphingolipid. This result indicates there are no profound changes in the membrane topology of mammalian ORMDL protein under conditions known to trigger the ORMDL-dependent inhibition of SPT activity.
3.8. Based on the results of SCAM™ analysis, mammalian ORMDL protein contains four transmembrane domains with both the N and C termini on the cytosolic side of the ER.
Collectively, the modification patterns established here for each of our single-cysteine protein constructs, support the detailed model presented in Figure 7. Specifically, when residues S8, T74, T84 and G144, were replaced with cysteine residues, they were readily modified by the membrane-impermeant maleimide-PEG compound used under conditions where the ER membrane remains intact (i.e. absence of TX-100). The modification of cysteinyl thiolates at these predicted cytosolic positions was not enhanced or hindered under alkaline conditions or in the presence of toluene. Additionally, cysteines at these locations were the only constructs modified during sonication, which implies that these residues exist in an aqueous environment. In contrast, the positions represented by C43, N57 and T122 were not accessible to the maleimide-PEG compound under these same conditions. Only when the ER membrane was solubilized by the addition of TritonX-100 during the modification reaction, was the maleimide-PEG reagent able to modify cysteine residues placed at these predicted internal locations. Additionally, we consistently observed changes in the labeling pattern when cysteines were substituted for residues in close proximity to the membrane interface based on the predicted transmembrane segments, namely S19 or S98. Cysteines placed at these locations revealed a weaker modification profile at pH 7.4 that was improved under alkaline conditions, which have been shown to enhance modification reactions using maleimide compounds as well as relaxing secondary protein structures. This result supports the proximity of these ORMDL residues to the interfacial region of the ER membrane as depicted in our model. These same residue locations, however, were labeled to the same degree as all other single-cysteine constructs in the presence of TX-100 indicating that these cysteine molecules are equally reactive. In our final model presented in Figure 7, the amino acids that are part of the predicted transmembrane segments of mammalian ORMDL are shown in yellow. All other residues are shown in gray. The amino acids used for cysteine substitutions are numbered in red. This proposed membrane topology for mammalian ORMDL protein is based on the labeling work done in this study combined with the predictions made by online algorithms using unique combinations of prediction methods (Table 1). Additionally, when constructing our model, we considered the placement of arginines and lysines, which often define the boundary of transmembrane segments. Exact boundaries of TMDs can be difficult to assess and would require further experimentation beyond the scope of this study.
Figure 7 – Based on the results of SCAM™ analysis, mammalian ORMDL protein contains four transmembrane domains with both the N and C termini on the cytosolic side of the ER.
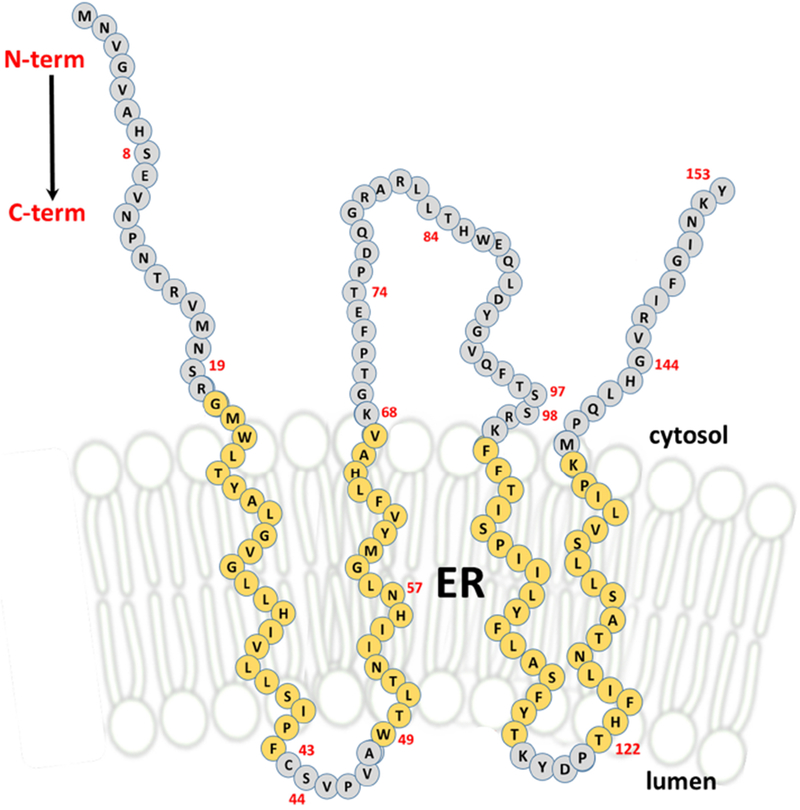
Each circle in this drawing depicts a single amino acid of the 153 residues of mouse ORMDL1 protein. The red numbers are the locations (based on N-terminal methionine being #1) of each residue that was used as a substitution site for a cysteine during our SCAM™ analysis. Amino acids colored yellow are predicted to reside within the ER membrane and constitute the four transmembrane domains (TMDs) of mammalian ORMDL protein. Amino acids colored gray are predicted to be outside of the ER membrane, located on either the luminal or cytosolic sides of this membrane boundary.
3.9. Serine palmitoyltransferase and ORMDL proteins are tightly associated with the ER membrane and are resistant to non-ionic detergent extraction.
The experimental hallmark of an integral membrane protein is resistance to extraction from membranes with high salt or alkaline washes [35, 36]. Although it is widely, and reasonably, assumed that the ORMDLs and the major SPT subunits are membrane-embedded based on their predicted transmembrane segments, we felt it was important to confirm this experimentally for the endogenous proteins in their native environment. To our knowledge, these experiments have not been reported. To this end, non-transfected HeLa cells were harvested and total membranes were isolated as described in Section 2.6 of Methods. Equal aliquots of membranes were then subjected to treatment with either high salt or alkaline sodium carbonate as described in Section 2.7 of Methods. Resulting supernatant and pellet fractions following high-speed centrifugation were subjected to SDS-PAGE then immunoblotted for ORMDL, SPTLC1, Calnexin or GAPDH to ascertain protein levels in each fraction. As shown in Figure 8A (quantitated in Figure 8B), ORMDL protein is resistant to high salt (NaCl) and alkaline (Na2CO3) treatments as evidenced by the lack of a protein band in the post-100k rpm supernatant (S) with all of the protein remaining in the pelleted fraction (P). This was similar to the profiles seen for both major subunit 1 of serine palmitoyltransferase (SPTLC1) and calnexin, two ER-resident proteins known to contain transmembrane domains. Resistance of membrane proteins to extraction with non-ionic detergents, although controversial as a measure of residence in distinct membrane microdomains, is indicative of distinct interactions of membrane proteins with the lipid environment in which they are imbedded. We tested the ability of the non-ionic detergents Triton X-100 and digitonin to extract the ORMDLs and subunit 1 of SPT (SPTLC1). Surprisingly to us, ORMDL and SPTLC1 proteins both exhibited resistance to extraction with these detergents, as evidenced by substantial amounts of protein remaining in the pellet (P) after treatment. These proteins were, however, completely extracted into the supernatant fraction with SDS, as expected. In contrast to the results for ORMDL and SPT, another membrane protein of the ER, calnexin, was completely extracted from membranes by non-ionic detergents as evidenced by lack of calnexin protein in the pellet fraction for both TX-100 and digitonin treatments. These results reveal a tight association with the membrane environment for both ORMDL and the large subunit of SPT, SPTLC1, possibly indicating a discrete subdomain of the ER that is enriched in sphingolipids.
Figure 8 – Serine palmitoyltransferase and ORMDL proteins are tightly associated with the ER membrane and are resistant to non-ionic detergent extraction.
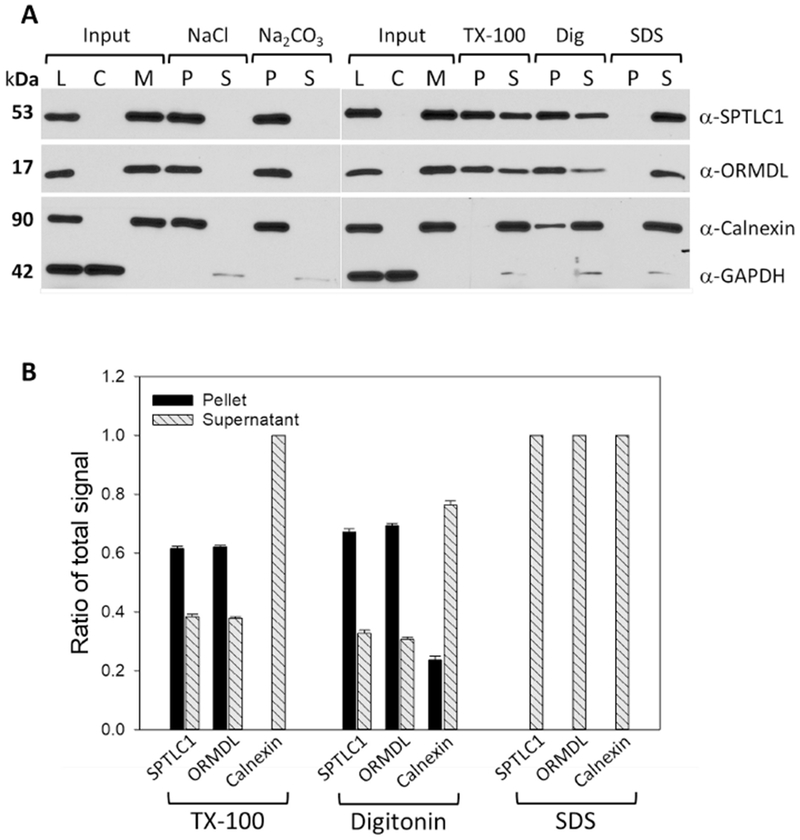
A) Western blot analysis of samples generated after treatment of isolated total membrane fractions from untransfected HeLa cells with various conditions as detailed in Section 2.7 of Methods. TX-100 and digitonin treatments were performed at 4°C with rotation and final detergent concentrations of 1% (v/v). SDS treatments were performed at RT to avoid precipitation with rotation and final detergent concentration of 1% (v/v). Results shown are representative blots from one of several independent experiments. L = whole cell lysate; C = 100K rpm supernatant fraction (cytosol); M = 100K rpm total membrane fraction used for input in all treatment groups; P = pellet after treatment; S = supernatant after treatment. B) Densitometry of Western blots resulting from experiments outlined in 8A above. Values are the ratio of the total signal (pellet and supernatant) measured in each fraction. Blots from independent experiments were quantitated and mean values are shown ± SD (n=3).
4. Conclusions
Here we offer a detailed model of the transmembrane topology of mammalian ORMDL proteins. This model is based on the results of our SCAM™ analysis combined with predicted transmembrane segments as assigned by sophisticated algorithms and the placement of arginine and lysine residues in keeping with their frequent assignment at the boundaries of membrane spanning segments. This model can now be used to target specific regions of ORMDL that may play key roles in either interacting directly with SPT or acting as a putative “lipid sensor” to monitor the levels of sphingolipids in the ER. ORMDLs have been well established to be negative regulators of SPT activity but it is unknown how this regulation is achieved, especially in mammals where the ORMDL proteins lack the phosphorylation sites found in yeast Orm proteins. In yeast, multiple pathways have now been linked to phosphorylation of the Orm proteins on distinct residues in the N-terminal region [11, 12, 37–40]. When Orm 1/2 are phosphorylated in yeast, they are no longer able to inhibit SPT and de novo synthesis of sphingolipids is elevated. Mammalian ORMDL proteins lack this level of regulation, making it even more important to have a precise model of membrane topology in order to identify putative regions that might lie in close proximity to active sites on SPT or other proteins known to be complexed with SPT [12]. Additionally, we are interested in determining how ORMDL proteins are able to “sense” the lipid levels in the cell. Our current working hypothesis for regulation of SPT activity in mammals shows that when cellular lipid levels are high, SPT activity is inhibited in an ORMDL-dependent manner. If the level of sphingolipids drops below a certain threshold, ORMDL proteins release their inhibition on SPT allowing de novo synthesis to resume. Many questions surrounding this working model still exist and the current study detailing the transmembrane topology of mammalian ORMDL protein presented here lays the groundwork to addressing some of those questions.
Supplementary Material
Figure S1 – Protein sequence alignment for mammalian Orm/ORMDL proteins. ClustalW (Network Protein Sequence Analysis, Combet C., Blanchet C., Geourjon C. and Deleage G., TIBS 2000 March Vol. 25 [291]: 147-150) was used to create a protein sequence alignment for the following proteins human ORMDL1 (AAM43502.1), human ORMDL2 (AAM43506.1), human ORMDL3 (AAM43507.1), mouse ORMDL1 (AAH23695.1), mouse ORMDL2 (AAH02146.1), mouse ORMDL3 (AAH46594.1), yeast Orm1 (CAA97026.1), and yeast Orm2 (KZV09598.1). Residues highlighted in yellow represent the putative transmembrane domains (TMDs) as predicted by MEMSAT3 (Detailed in Table 1). The amino acids labeled in red text for mouse ORMDL1 represent the putative TMDs as depicted in our model (Figure 7) which is based on results of our SCAM™ analysis combined with topology predictions.
Figure S2 – Overexpressed human Sec61 beta subunit does not inhibit SPT activity in vivo and is readily modified by maleimide-PEG in the absence of detergent. A) SPT activity assay using intact HeLa cells that were co-transfected with plasmid DNA constructs for single chain-SPT fusion protein and either empty vector, human Sec61 beta subunit or cysteine mutant constructs as noted (numbers correspond to a specific amino acid residue that was mutated to cysteine). Cells were labeled with 3H-serine in the absence (gray bars) or presence (black bars) of the SPT-specific inhibitor myriocin after which total sphingolipids were harvested and the amount of 3H incorporated into sphingolipids was determined as described in Section 2.8 of Methods. Data shown are mean CPM per well ± SEM (n=4) and are representative of two independent experiments. Note that both ORMDL constructs inhibited SPT activity to the same degree whereas human Sec61 beta subunit did not inhibit SPT activity. B) Protein sequence for human Sec61 beta subunit (UniProt #P60468). The residues highlighted in yellow represent the single membrane spanning region for this protein which anchors it in the ER membrane. There is one cysteine residue (red text) in the cytosolic domain which is available for modification by maleimide-PEG. C) Western Blot analysis of protein samples generated from transfected HeLa cells subjected to modification reactions using maleimide-PEG under the conditions noted for each lane in the panels. WT ORMDL has no cysteines and therefore did not exhibit a higher MW band (lanes 1-2). Human Sec61 beta was readily modified in the absence and presence of detergent (lanes 4-5) which confirms cytosolic placement of the single cysteine.
Figure S3 – ORMDL constructs with single cysteine substitutions are localized to the endoplasmic reticulum. Fluorescent confocal microscopy of overexpressed mouse ORMDL1 constructs (left panels, green) labeled with anti-FLAG antibody in HeLa cells counter-stained with anti-KDEL antibody (middle panels, red) as an endogenous endoplasmic reticulum (ER) marker. Third row of panels shows the merged images (FLAG + KDEL, yellow) and the last row of panels is for the transmitted light images (PMT). Wild type: mouse ORMDL1; S8C: serine 8 mORMDL1 mutant; T74C: threonine 74 mORMDL1 mutant; G144C: glycine 144 mORDLI mutant. Scale bars are 10μm.
Figure S4 – Lack of modification of the luminal protein, PDI (protein disulfide isomerase), in the absence of detergent suggests that the ER membrane is still intact. Western blot analysis of samples from HeLa cells transfected with “pseudo WT” ORMDL then subjected to maleimide-PEG modification reactions as detailed in Section 2.4 of Methods. Blots were probed with polyclonal anti-PDI antibody. In Lanes 1-4, HeLa cells were treated with 200μg/mL digitonin to permeabilize the plasma membrane prior to the modification reactions with maleimide-PEG as detailed in Methods and discussed in Section 3.3. Lane 2 represents modification reactions that were performed on digitonin permeabilized cells in the presence of 50μg/mL digitonin during modification to permeabilize the ER membrane without completely solubilizing all the cellular membranes. Lane 4 represents reactions that were carried out in the presence of 10mM DTT which blocks reactivity of maleimide-PEG and allows us to identify any non-specific modification bands that appear on Western blots.
Figure S5 – Western blot analysis of maleimide-PEG modification of single cysteine ORMDL constructs under various conditions. Western blot analysis of lysates from HeLa cells transfected with either the no-cysteine ORMDL1-FLAG (WT) or single-cysteine constructs of mORMDL1-FLAG predicted to be either luminal or located within the transmembrane space (C43, N57C, T122C), located close to the ER membrane interface (S19C, S98C), or cytosolic (T74C, G144C). Twenty-four hours after transfection, cells were labeled with maleimide-PEG as outlined in Section 2.4 of Methods using the conditions shown under each lane in the figure. Conditions used include: pH 7.4 (regular reaction condition used for Figures 4–6); pH 9.0 (alkaline conditions); Sonicate (ultrasonic waterbath in the absence of detergent); TX-100 (1%, v/v); Toluene (0.5%, v/v); DTT (10mM). Unmodified, FLAG-tagged ORMDL protein runs at approximately 20 kDa while the maleimide-PEG modified protein results in an additional band at approximately 32 kDa under these conditions. Data in all panels are representative of multiple independent experiments.
Highlights.
Sphingolipid synthesis is initiated by the activity of serine palmitoyltransferase (SPT)
SPT activity is negatively regulated by ORMDL proteins to control sphingolipid levels
Membrane topology of ORMDL proteins was explored using SCAMTM analysis
Mammalian ORMDLs transit the ER membrane 4 times with N and C termini facing the cytosol
SPT and ORMDL are resistant to detergent extraction from their native ER membrane
Acknowledgements –
The work presented here was supported by National Institute of Health Grant RO1HL131340. We would like to acknowledge the intellectual and editing contributions of Dr. Muthukumar Kannan in the preparation of this manuscript. The microscopy work presented here was done at the Virginia Commonwealth University Department of Anatomy & Neurobiology Microscopy Facility, supported, in part, by funding from NIH-NINDS Center Core Grant 5-P30-NS047463 and, in part, by funding from the NIH-NCI Cancer Center Support Grant P30-CA016059.
Abbreviations
- SPT
serine palmitoyltransferase
- ORMDL
ORM1-like
- ER
endoplasmic reticulum
- PM
plasma membrane
- PEG
polyethylene glycol
- mPEG
maleimide-PEG
- SCAM™
substituted cysteine accessibility method as applied to transmembrane domains
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest Conflicts – none
References
- [1].Hanada K, Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism, Biochimica et biophysica acta, 1632 (2003) 16–30. [DOI] [PubMed] [Google Scholar]
- [2].Merrill AH Jr., Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics, Chemical reviews, 111 (2011) 6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harrison PJ, Dunn TM, Campopiano DJ, Sphingolipid biosynthesis in man and microbes, Natural product reports, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Merrill AH Jr., Nixon DW, Williams RD, Activities of serine palmitoyltransferase (3-ketosphinganine synthase) in microsomes from different rat tissues, J. Lipid Res, 26 (1985) 617–622. [PubMed] [Google Scholar]
- [5].Hanada K, Hara T, Fukasawa M, Yamaji A, Umeda M, Nishijima M, Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin. Genetic and biochemical evidence for complex formation of the LCB1 protein with the LCB2 protein for serine palmitoyltransferase, J. Biol. Chem, 273 (1998) 33787–33794. [DOI] [PubMed] [Google Scholar]
- [6].Gable K, Slife H, Bacikova D, Monaghan E, Dunn TM, Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity, J Biol Chem, 275 (2000) 7597–7603. [DOI] [PubMed] [Google Scholar]
- [7].Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, Eichler F, Brown RH Jr., Harmon JM, Dunn TM, Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities, Proc. Natl. Acad. Sci. U. S. A, 106 (2009) 8186–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mandon EC, van Echten G, Birk R, Schmidt RR, Sandhoff K, Sphingolipid biosynthesis in cultured neurons. Down-regulation of serine palmitoyltransferase by sphingoid bases, European journal of biochemistry, 198 (1991) 667–674. [DOI] [PubMed] [Google Scholar]
- [9].Siow DL, Wattenberg BW, Mammalian ORMDL Proteins Mediate the Feedback Response in Ceramide Biosynthesis, J. Biol. Chem, 287 (2012) 40198–40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hjelmqvist L, Tuson M, Marfany G, Herrero E, Balcells S, Gonzalez-Duarte R, ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins, Genome Biol, 3 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS, Orm family proteins mediate sphingolipid homeostasis, Nature, 463 (2010) 1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Han S, Lone MA, Schneiter R, Chang A, Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control, Proc. Natl. Acad. Sci. U. S. A, 107 (2010) 5851–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li J, Yin J, Rong C, Li KE, Wu JX, Huang LQ, Zeng HY, Sahu SK, Yao N, Orosomucoid Proteins Interact with the Small Subunit of Serine Palmitoyltransferase and Contribute to Sphingolipid Homeostasis and Stress Responses in Arabidopsis, The Plant cell, 28 (2016) 3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Siow D, Sunkara M, Dunn TM, Morris AJ, Wattenberg B, ORMDL/serine palmitoyltransferase stoichiometry determines effects of ORMDL3 expression on sphingolipid biosynthesis, J Lipid Res, 56 (2015) 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Siow D, Sunkara M, Morris A, Wattenberg B, Regulation of de novo sphingolipid biosynthesis by the ORMDL proteins and sphingosine kinase-1, Adv Biol Regul, 57 (2015) 42–54. [DOI] [PubMed] [Google Scholar]
- [16].Kimberlin AN, Han G, Luttgeharm KD, Chen M, Cahoon RE, Stone JM, Markham JE, Dunn TM, Cahoon EB, ORM Expression Alters Sphingolipid Homeostasis and Differentially Affects Ceramide Synthase Activity, Plant physiology, 172 (2016) 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chueasiri C, Chunthong K, Pitnjam K, Chakhonkaen S, Sangarwut N, Sangsawang K, Suksangpanomrung M, Michaelson LV, Napier JA, Muangprom A, Rice ORMDL controls sphingolipid homeostasis affecting fertility resulting from abnormal pollen development, PloS one, 9 (2014) e106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Araki W, Takahashi-Sasaki N, Chui DH, Saito S, Takeda K, Shirotani K, Takahashi K, Murayama KS, Kametani F, Shiraishi H, Komano H, Tabira T, A family of membrane proteins associated with presenilin expression and gamma-secretase function, Faseb j, 22 (2008) 819–827. [DOI] [PubMed] [Google Scholar]
- [19].Wang S, Robinet P, Smith JD, Gulshan K, ORMDL orosomucoid-like proteins are degraded by free-cholesterol-loading-induced autophagy, Proceedings of the National Academy of Sciences of the United States of America, 112 (2015) 3728–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R, The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress, Hum. Mol. Genet, 19 (2010) 111–121. [DOI] [PubMed] [Google Scholar]
- [21].Bradford MM, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal Biochem, 72 (1976) 248–254. [DOI] [PubMed] [Google Scholar]
- [22].Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG, Clustal W and Clustal X version 2.0, Bioinformatics, 23 (2007) 2947–2948. [DOI] [PubMed] [Google Scholar]
- [23].Bogdanov M, Mapping of Membrane Protein Topology by Substituted Cysteine Accessibility Method (SCAM), Methods in molecular biology (Clifton, N.J.), 1615 (2017) 105–128. [DOI] [PubMed] [Google Scholar]
- [24].Bogdanov M, Zhang W, Xie J, Dowhan W, Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAM(TM)): application to lipid-specific membrane protein topogenesis, Methods (San Diego, Calif.), 36 (2005) 148–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rutti MF, Richard S, Penno A, von EA, Hornemann T, An improved method to determine serine palmitoyltransferase activity, J. Lipid Res, 50 (2009) 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Akabas MH, Stauffer DA, Xu M, Karlin A, Acetylcholine receptor channel structure probed in cysteine-substitution mutants, Science, 258 (1992) 307–310. [DOI] [PubMed] [Google Scholar]
- [27].Loo TW, Clarke DM, P-glycoprotein. Associations between domains and between domains and molecular chaperones, J Biol Chem, 270 (1995) 21839–21844. [DOI] [PubMed] [Google Scholar]
- [28].Tang XB, Fujinaga J, Kopito R, Casey JR, Topology of the region surrounding Glu681 of human AE1 protein, the erythrocyte anion exchanger, J Biol Chem, 273 (1998) 22545–22553. [DOI] [PubMed] [Google Scholar]
- [29].Zhu Q, Lee DW, Casey JR, Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1, J Biol Chem, 278 (2003) 3112–3120. [DOI] [PubMed] [Google Scholar]
- [30].Zhu Q, Casey JR, Topology of transmembrane proteins by scanning cysteine accessibility mutagenesis methodology, Methods (San Diego, Calif.), 41 (2007) 439–450. [DOI] [PubMed] [Google Scholar]
- [31].Le Gall S, Neuhof A, Rapoport T, The endoplasmic reticulum membrane is permeable to small molecules, Mol Biol Cell, 15 (2004) 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lange Y, Disposition of intracellular cholesterol in human fibroblasts, J Lipid Res, 32 (1991) 329–339. [PubMed] [Google Scholar]
- [33].Ridsdale A, Denis M, Gougeon PY, Ngsee JK, Presley JF, Zha X, Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins, Mol Biol Cell, 17 (2006) 1593–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Siow DL, Wattenberg BW, An assay system for measuring the acute production of sphingosine 1-phosphate in intact monolayers, Analytical Biochemistry, 371 (2007) 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fujiki Y, Hubbard AL, Fowler S, Lazarow PB, Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum, J Cell Biol, 93 (1982) 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hack E, Lin C, Yang H, Horner HT, T-URF 13 Protein from Mitochondria of Texas Male-Sterile Maize (Zea mays L.) : Its Purification and Submitochondrial Localization, and Immunogold Labeling in Anther Tapetum during Microsporogenesis, Plant physiology, 95 (1991) 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gururaj C, Federman RS, Chang A, Orm proteins integrate multiple signals to maintain sphingolipid homeostasis, J. Biol. Chem, 288 (2013) 20453–20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu M, Huang C, Polu SR, Schneiter R, Chang A, Regulation of sphingolipid synthesis through Orm1 and Orm2 in yeast, J. Cell Sci, 125 (2012) 2428–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun Y, Miao Y, Yamane Y, Zhang C, Shokat KM, Takematsu H, Kozutsumi Y, Drubin DG, Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways, Mol. Biol. Cell, 23 (2012) 2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shimobayashi M, Oppliger W, Moes S, Jeno P, Hall MN, TORC1-regulated protein kinase Npr1 phosphorylates Orm to stimulate complex sphingolipid synthesis, Mol. Biol. Cell, 24 (2013) 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 – Protein sequence alignment for mammalian Orm/ORMDL proteins. ClustalW (Network Protein Sequence Analysis, Combet C., Blanchet C., Geourjon C. and Deleage G., TIBS 2000 March Vol. 25 [291]: 147-150) was used to create a protein sequence alignment for the following proteins human ORMDL1 (AAM43502.1), human ORMDL2 (AAM43506.1), human ORMDL3 (AAM43507.1), mouse ORMDL1 (AAH23695.1), mouse ORMDL2 (AAH02146.1), mouse ORMDL3 (AAH46594.1), yeast Orm1 (CAA97026.1), and yeast Orm2 (KZV09598.1). Residues highlighted in yellow represent the putative transmembrane domains (TMDs) as predicted by MEMSAT3 (Detailed in Table 1). The amino acids labeled in red text for mouse ORMDL1 represent the putative TMDs as depicted in our model (Figure 7) which is based on results of our SCAM™ analysis combined with topology predictions.
Figure S2 – Overexpressed human Sec61 beta subunit does not inhibit SPT activity in vivo and is readily modified by maleimide-PEG in the absence of detergent. A) SPT activity assay using intact HeLa cells that were co-transfected with plasmid DNA constructs for single chain-SPT fusion protein and either empty vector, human Sec61 beta subunit or cysteine mutant constructs as noted (numbers correspond to a specific amino acid residue that was mutated to cysteine). Cells were labeled with 3H-serine in the absence (gray bars) or presence (black bars) of the SPT-specific inhibitor myriocin after which total sphingolipids were harvested and the amount of 3H incorporated into sphingolipids was determined as described in Section 2.8 of Methods. Data shown are mean CPM per well ± SEM (n=4) and are representative of two independent experiments. Note that both ORMDL constructs inhibited SPT activity to the same degree whereas human Sec61 beta subunit did not inhibit SPT activity. B) Protein sequence for human Sec61 beta subunit (UniProt #P60468). The residues highlighted in yellow represent the single membrane spanning region for this protein which anchors it in the ER membrane. There is one cysteine residue (red text) in the cytosolic domain which is available for modification by maleimide-PEG. C) Western Blot analysis of protein samples generated from transfected HeLa cells subjected to modification reactions using maleimide-PEG under the conditions noted for each lane in the panels. WT ORMDL has no cysteines and therefore did not exhibit a higher MW band (lanes 1-2). Human Sec61 beta was readily modified in the absence and presence of detergent (lanes 4-5) which confirms cytosolic placement of the single cysteine.
Figure S3 – ORMDL constructs with single cysteine substitutions are localized to the endoplasmic reticulum. Fluorescent confocal microscopy of overexpressed mouse ORMDL1 constructs (left panels, green) labeled with anti-FLAG antibody in HeLa cells counter-stained with anti-KDEL antibody (middle panels, red) as an endogenous endoplasmic reticulum (ER) marker. Third row of panels shows the merged images (FLAG + KDEL, yellow) and the last row of panels is for the transmitted light images (PMT). Wild type: mouse ORMDL1; S8C: serine 8 mORMDL1 mutant; T74C: threonine 74 mORMDL1 mutant; G144C: glycine 144 mORDLI mutant. Scale bars are 10μm.
Figure S4 – Lack of modification of the luminal protein, PDI (protein disulfide isomerase), in the absence of detergent suggests that the ER membrane is still intact. Western blot analysis of samples from HeLa cells transfected with “pseudo WT” ORMDL then subjected to maleimide-PEG modification reactions as detailed in Section 2.4 of Methods. Blots were probed with polyclonal anti-PDI antibody. In Lanes 1-4, HeLa cells were treated with 200μg/mL digitonin to permeabilize the plasma membrane prior to the modification reactions with maleimide-PEG as detailed in Methods and discussed in Section 3.3. Lane 2 represents modification reactions that were performed on digitonin permeabilized cells in the presence of 50μg/mL digitonin during modification to permeabilize the ER membrane without completely solubilizing all the cellular membranes. Lane 4 represents reactions that were carried out in the presence of 10mM DTT which blocks reactivity of maleimide-PEG and allows us to identify any non-specific modification bands that appear on Western blots.
Figure S5 – Western blot analysis of maleimide-PEG modification of single cysteine ORMDL constructs under various conditions. Western blot analysis of lysates from HeLa cells transfected with either the no-cysteine ORMDL1-FLAG (WT) or single-cysteine constructs of mORMDL1-FLAG predicted to be either luminal or located within the transmembrane space (C43, N57C, T122C), located close to the ER membrane interface (S19C, S98C), or cytosolic (T74C, G144C). Twenty-four hours after transfection, cells were labeled with maleimide-PEG as outlined in Section 2.4 of Methods using the conditions shown under each lane in the figure. Conditions used include: pH 7.4 (regular reaction condition used for Figures 4–6); pH 9.0 (alkaline conditions); Sonicate (ultrasonic waterbath in the absence of detergent); TX-100 (1%, v/v); Toluene (0.5%, v/v); DTT (10mM). Unmodified, FLAG-tagged ORMDL protein runs at approximately 20 kDa while the maleimide-PEG modified protein results in an additional band at approximately 32 kDa under these conditions. Data in all panels are representative of multiple independent experiments.


