Abstract
This study examined the state of the literature on the effectiveness of medication assisted treatment (MAT; methadone, buprenorphine, naltrexone) delivered in prisons and jails on community substance use treatment engagement, opioid use, recidivism, and health risk behaviors following release from incarceration. Randomized controlled trials (RCTs) and quasi-experimental studies published through December 2017 that examined induction to or maintenance on methadone (n=18 studies), buprenorphine (n=3 studies), or naltrexone (n=3 studies) in correctional settings were identified from PsycINFO and PubMed databases. There were a sufficient number of methadone RCTs to meta-analyze; there were too few buprenorphine or naltrexone studies. All quasi-experimental studies were systematically reviewed. Data from RCTs involving 807 inmates (treatment n = 407, control n = 400) showed that methadone provided during incarceration increased community treatment engagement (n=3 studies; OR = 8.69, 95% CI = 2.46; 30.75), reduced illicit opioid use (n=4 studies; OR = 0.22, 95% CI = 0.15; 0.32) and injection drug use (n=3 studies; OR = 0.26, 95% CI = 0.12; 0.56), but did not reduce recidivism (n=4 studies; OR = 0.93, 95% CI = 0.51; 1.68). Data from observational studies of methadone showed consistent findings. Individual review of buprenorphine and naltrexone studies showed these medications were either superior to methadone or to placebo, or were as effective as methadone in reducing illicit opioid use post-release. Results provide the first meta-analytic summary of MATs delivered in correctional settings and support the use of MATs, especially with regard to community substance use treatment engagement and opioid use; additional work is needed to understand the reduction of recidivism and other health risk behaviors.
Keywords: methadone, buprenorphine, naltrexone, medication assisted treatment, incarceration
1. Introduction
Heroin and other opioid use is associated with high rates of dependence (Lipari & Hughes, 2015), healthcare costs, and mortality, and is considered to be a significant public health problem in the United States (Birnbaum et al., 2011; Centers for Disease Control, 2016; Grant et al., 2016). Rates of opioid use in criminal justice populations are disproportionately high relative to the general population; the Survey of Inmates in State and Federal Correctional Facilities showed that 23% of state prisoners and 18% of federal prisoners report lifetime use of heroin and other opioids (Mumola & Karberg, 2006). Opioid use carries unique risks among justice-involved populations, as it is associated with elevated risk of death by overdose following release from incarceration due to loss of tolerance and increased availability of potent forms of synthetic opioids (e.g., fentanyl; Merrall et al., 2010; Binswanger et al., 2013). In addition, injection drug use (including opioids) increases risk for recidivism (Håkansson & Berglund, 2012). It is critically important that people with opioid use disorder in the criminal justice system receive effective treatment prior to release into the community.
1.1. Medication Assisted Treatments
Medication-assisted treatment (MAT), here referring to methadone, buprenorphine, and naltrexone, is a core component of opioid use treatment in the general population (Farré, Mas, Torrens, Moreno, & Camí, 2002). In 1991, the National Institutes of Health issued a consensus statement on the safety, efficacy, and utility of methadone (one form of MAT) for the treatment of opioid use disorders. Methadone maintenance is one of the most widely used form of MAT for opioid use disorders. Methadone is a full opioid agonist (administered orally) that effectively reduces opioid abstinence symptoms (e.g., withdrawal and cravings) in a dose-dependent manner (Marsch, 1998; Farré et al., 2002), reduces illicit opioid use (Mattick et al., 2009), and increases treatment retention (Fullerton et al., 2014). Buprenorphine, a partial agonist (administered sublingually, by implant, or injection for new formulations), is another widely used opioid substitution therapy that is effective in reducing illicit opioid use (Otiashvili et al., 2013; Mattick et al., 2014; Lee et al., 2017; Tanum et al., 2017) and reducing opioid cravings/withdrawal symptoms (Ling et al., 2010; Rosenthal et al., 2013), as well as increasing treatment retention (Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016; Weinstein et al., 2017). There is less research on naltrexone, an antagonist administered orally, injection, or by implant (though this administration method is not yet approved by the U.S. Food and Drug Administration); however, the available studies suggest it also increases treatment retention (Timko et al., 2016) and reduces illicit opioid use (Lee et al., 2017; Tanum et al., 2017).
1.2. Medication Assisted Treatment in Corrections
Despite the strong evidence base for MATs, they are rarely utilized in correctional settings for inmates with opioid use disorder or those who were treated with MATs in the community prior to incarceration (Rich et al., 2005; Nunn et al., 2009; Wakeman & Rich, 2015). The few correctional facilities utilizing MATs use methadone as opposed to other forms of MAT, and existing methadone programs for inmates only target special populations, such as pregnant women, or inmates with chronic pain (Fiscella, Moore, Engerman, & Meldrum, 2004). As a result, people with opioid use disorder entering the criminal justice system often undergo forced withdrawal from opioids or MAT upon incarceration, which involves aversive physiological symptoms (Aronowitz & Laurent, 2016), decreases the likelihood of re-engaging in MAT at a later point (Fu, Zaller, Yokell, Bazazi, & Rich, 2013; Maradiaga, Nahvi, Cunningham, Sanchez, & Fox, n.d.), and increases risk for fatal overdose on opioids post-release (Degenhardt et al., 2014). MAT induction and/or maintenance during incarceration may prevent relapse and overdose on opioids post-release, and reduce other consequences of opioid use (i.e., crime, health risk behaviors).
1.3. Current Study
Currently, there is no quantitative summary of research on the effectiveness of MAT (methadone, buprenorphine, naltrexone) in correctional settings with respect to post-release substance use treatment engagement, opioid use, criminal behavior/recidivism, and health risk behaviors. The aim of this study was to comprehensively review the state of the literature on MAT delivered in both prison and jail settings and determine whether sufficient data exists to permit meta-analysis. We hypothesized that studies would collectively show that inmates inducted to or maintained on any of the three forms of MAT while incarcerated would have: (1) increased engagement in community-based substance use treatment, (2) reduced opioid use, (3) reduced involvement in criminal activity and/or return to the criminal justice system, and (4) reduced engagement in health risk behaviors (e.g., injection drug use) post-incarceration.
2. Method
2.1. Search Strategy and Inclusion Criteria
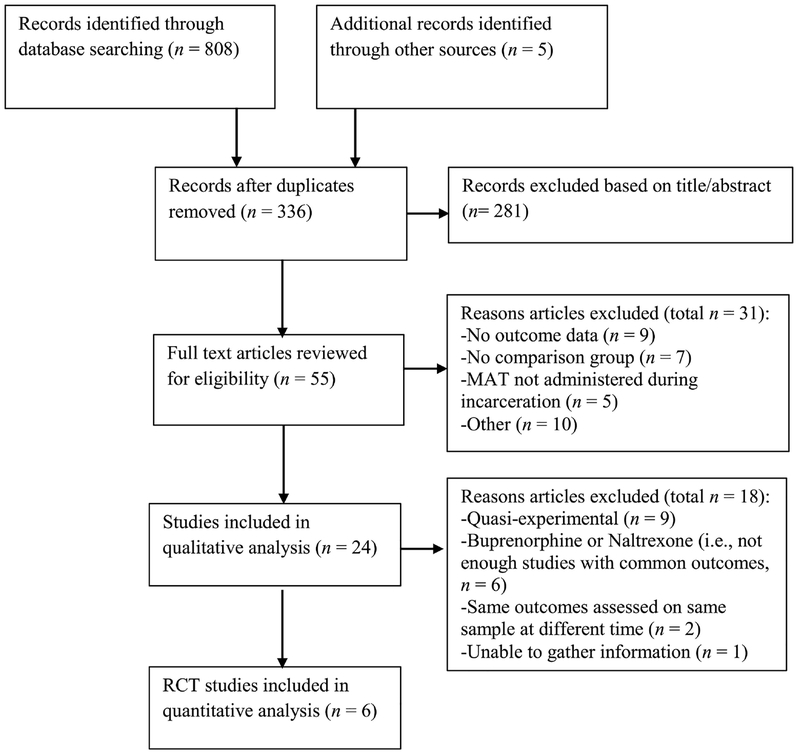
PubMed and PsycINFO databases were searched for combinations of the following terms: methadone, buprenorphine, naltrexone, medication assisted treatment, medication assisted therapy, opioid pharmacotherapy, incarceration, prison, jail, correctional facility, and offenders. Eligible studies were peer-reviewed, written in English, and published through December 2017. Preferred Reporting Items for Systematic reviews and Meta-Analyses (i.e., PRISMA) guidelines (Moher et al., 2009) were used to inform study search and selection (i.e., population, intervention, comparison, outcomes; PICO). The population of interest included people currently incarcerated in a prison or jail. Eligible interventions included any induction to or maintenance on methadone, buprenorphine, or naltrexone at any point during incarceration; medication assisted treatment solely used for detoxification during incarceration was not included. Eligible studies had a comparison group, which included randomized controlled trials or quasi-experimental studies with an administrative comparison group. Outcomes included substance use treatment engagement, opioid use, recidivism (i.e., re-arrest, re-incarceration), and any reported health risk behaviors. Study titles and abstracts were initially reviewed to select potentially eligible studies, and studies were further reviewed in full text to assess for all inclusion criteria (see Figure 1). We identified a total of 24 studies, including 11 quasi-experimental studies, 8 RCTs, and 5 follow-up studies of these RCTs, published between 1969 and 2017 (see Table 1–3).
Figure 1.
PRISMA Flow Diagram. This figure illustrates how studies were selected for inclusion in this meta-analysis and systematic review.
Table 1.
Studies of Methadone Maintenance Treatment among Incarcerated Populations
| Sample Size/Gender | Post-Release Study Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study Design | Setting | Treatment | Control | Intervention/dose | Substance Use Treatment Engagement | Opioid Use | Recidivism | Health Risk Behaviors | ||
| Dole et al. (1969) | Quasi-experimental | Jail | 12 | 16 | MMT prior to releasea; 35 mg on average | MMT less likely to use heroin daily 7–10 months post-release (clinic data, self-report) | MMT had lower reincarceration rates 7–10 months post-release (legal data) | ||||
| Magura et al. (1993) | Quasi-experimental | Jail | 195 (60% male) | 54 (61% male) | MMTa; 30–40 mg depending on prior MMT | MMT had higher substance use treatment engagement 6.5 months post-completion (self-report, clinic data) | No differences in heroin and cocaine use 6.5 months post-completion (hair test) | No differences in property offenses or past-week illegal income 6.5 months post-completion (self-report) | No differences in past-month drug injection 6.5 months post-completion | ||
| Dolan (1998) | Quasi-experimental | Prison | 48 (90% male) | 105 (67% male) | MMTa; target dose of 60 mg; 95 max dose on average | MMT less likely to inject heroin post-completion (self-report) | MMT less likely to share syringes, lower HIV risk behavior (self-report) | ||||
| Bellin et al. (1999) | Quasi-experimental | Jail | 1,423 (high dose; 76% male), 1,758 (low dose; 70% male), 6,898 (detox with methadone; 82% male) | 10,079 (89% male) | Methadone dosea; > 60mg; < 30mg; detoxified on methadone; no methadone | High dose MMT had lower risk of reincarceration compared to low dose MMT, higher risk of reincarceration compared to detox and no MMT (legal data) | |||||
| Dolan et al. (2003) * | RCT | Prison | 191+(100% male) | 191c (100% male) | MMTa; 61 mg dose on average | MMT had lower heroin use 4 months post-completion (hair test; self-report) ** | MMT had lower injection heroin use (self-report), and syringe sharing 4 months post-completion (self-report)** | ||||
| No differences in HIV and hepatitis C incidence (serology) | |||||||||||
| Dolan et al. (2005) * | Follow-up (4 years) | Prison | 191 (100% male) | 191c (100% male) | MMTa; length of MMT treatment while incarcerated; 69mg max dose on average ** | MMT episodes ≥ 8 months associated with reduced reincarceration rates 4 years after RCT ** (legal data) | MMT episodes ≥ 5 months associated with lower risk of hepatitis-C (serology) 4 years after RCT | ||||
| Heimer (2006) | Quasi-experimental | Prison | 20 (100% male) | 40 (100% male) | MMTa; target of 80–120 mg | MMT less likely to use heroin post-program completion (self-report, urine toxicology) | |||||
| Kinlock (2007) * | RCT | Prison | 71+(100% male) | 70d (100% male) | MMT + counselinga; target dose of 60mg | MMT+Counseling more likely to engage in community MMT compared to passive referral 1 month post-completion (clinic data)** | MMT+ Counseling less likely than passive referral to test positive or report heroin 1 month post-completion (urine toxicology, self-report)** | No significant differences in hospitalizations, mortality, overdose events (medical data) | |||
| Kinlock et al. (2008)* | Follow-up (3 months) | Prison | 68 (100% male) | 63d (100% male) | MMT + counselinga; target dose of 60mg | MMT+Counseling more likely to engage in community MMT compared to passive referral 3 month post-completion (clinic data) | MMT+ Counseling less likely to report using heroin than passive referral 3 months post-completion (self-report); no differences in opioid urine toxicology | MMT+ Counseling had lower rates of reincarceration and criminal activity compared to passive referral** (self-report) | |||
| Gordon et al. (2008) | Follow-up (6 months) | Prison | 70 (100% male) | 63d (100% male) | MMT + Counselinga; target dose of 60mg | MMT+Counseling spent more days in community MMT compared to passive referral 6 months post-completion (clinic data) | MMT+ Counseling had reduced opioid use compared to passive referral 6 months post-completion (self-report; urine toxicology) | MMT+ Counseling had reduced criminal activity but not recinarceration compared to passive referral 6 months post-completion (self-report) | |||
| McMillian (2008) | Quasi-experimental | Jail | 589 total | MMTb; 88 mg dose on average at release | No differences in time to rebooking post-incarceration (admin data) | ||||||
| Kinlock et al. (2009) | Follow-up (12 months) | Prison | 71+(100% male) | 70d (100% male) | MMT + counselinga; target dose of 60mg | MMT+Counseling spent more days in community MMT compared to passive referral 12 months post-completion (clinic data) | MMT+ Counseling had reduced opioid use (but not self-reported heroin use) compared to passive referral 12 months post-completion (urine toxicology) | No differences in criminal activity, arrests (self-report) | No differences in hospitalizations, mortality, overdose events (medical data) | ||
| McKenzie et al. (2012)* | RCT | Prison/jail (combined) | 31+ (total sample = 71% male) | 30d | Pre-release MMTa; referral to community-MMT w/financial coverage; referral to community-MMT w/o financial coverage; 33 mg dose on average | MMT had greater, quicker community MMT engagement 1 month post-release (clinic data)** | MMT had reduced heroin and cocaine use compared to passive referral 6 months post-completion (self-report)** | No differences in arrest, re-incarceration 6 months post-completion (self-report, admin data)** | No differences in nonfatal overdose events (self-report) MMT had trend of lower rates of injection drug use compared to passive referral (self-report)** |
||
| Macswain et al. (2014) | Quasi-experimental | Federal prison | 642 (MMT-continued = 161, MMT-tenninated at release = 481; 100% male) | 214 (100% male) | MMTa; dose not reported | MMT-continued under community supervision for longest time compared to controls, no differences in return to custody 3 years post-release (admin data) | |||||
| Farrel-MacDonald et al. (2014) | Quasi-experimental | Federal prison | 92 (MMT-continued = 25, MMT-tenninated at release = 67; 100% female) | 45 (100% female) | MMTa; 89.1mg dose on average | MMT-continued had lower risk of return to custody than controls 2 years post-release (admin data) | |||||
| Rich et al. (2015)* | RCT | Prison/jail (combined) | 114+ (76% male) | 109e (79% male) | MMTb; 87.5mg on average | MMT had greater community MMT engagement and less time to engagement 1 month post-completion (clinic data)** | MMT had reduced opioid use 1 month post-completion (self-report)** | No differences in reincarceration rates (self-report) | MMT had reduced injection drug use (self-report)** No differences in unprotected sex, overdoses, hospital admissions (self-report) |
||
| Westerberger et al. (2016) | Quasi-experimental | Jail | 118 (total sample = 73.8% male) | 842 (no use = 385, alcohol detox = 220, opioid detox = 237) | MMTb; dose not reported | MMT had lower chance of being rebooked and longer time to rebooking compared to detox groups, no differences compared to no use group 1 year post-release (admin data) | |||||
| Moore et al. (in press) | Quasi-experimental | Prison/jail (combined) | 184 (100% male) | 198 (100% male) | MMTb; 68.3 mg dose on average | MMT had greater community MMT engagement 1 day, 1 month post-release (clinic data) | |||||
Study included in the meta-analysis.
Outcomes included in the meta-analysis.
Note. MMT = methadone maintenance treatment. Sample sizes listed reflect the analyzed sample (which may have been intent to treat [indicated by +]); in the case of multiple control groups, sample sizes reflect the control group that was analyzed in the meta-analysis (i.e., the condition involving the least amount of treatment). Gender not specified in Dole (1969). Dolan et al. (2003) reports that all interviews were conducted in custody (i.e., not upon release into the community); this study was included in analyses because of high rates of substance use reported in post-program follow up. Only total sample size is given for McMillian (2008); outcomes are analyzed as 727 “inmate intervals” (i.e., intervals of incarceration) from 589 inmates. Dolan (1998), Dolan (2003), and Heimer (2006) outcomes were post program completion, not post-release.
Study participants were inducted to methadone.
Study participants were continued on previously initiated methadone.
Control condition: Wait list for MMT.
Control condition: Treatment as usual during incarceration plus passive referral to community methadone program.
Control condition: Treatment as usual during incarceration plus referral and financial assistance for community methadone program.
Table 3.
Studies of Naltrexone Treatment among Incarcerated Populations
| Sample Size | Post-Release Study Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Study Design | Setting | Treatment | Contro l | Intervention | Substance Use Treatment Engagement | Opioid Use | Recidivism | Health Risk Behaviors |
| Lobmaier et al. (2010) | Quasi-experimental | Prison | 23+ (total sample = 93.2% male) | 21 | Naltrexone implantsa; 20 pellet naltrexone implants, methadone control group target dose of 80–130mg) | Naltrexone had higher drug treatment engagement 6 months post-release (self-report) | Naltrexone less likely to use heroin 6 months post-release (self-report) | Both groups had significantly less criminal activity (self-report), no differences between groups 6 months post-release (self-report) | |
| Lee et al. (2015) | RCT | Jail | 16+ (100% male) | 17 (100% male) | Extended release Naltrexone (XR-NTX) + Motivational Enhancementb; (380 mg injection of XR-NTX) | No differences in community treatment engagement (self-report) | XR-NTX less likely to relapse on opioids 1 and 2 months post-release (self-report, urinalysis) | No differences in reincarceration (admin data). XR-NTX who did 2 injections (n=8) had fewer reincarcerations compared to control & those who did 1 injection (n = 25) (admin data) | No differences in injection drug use, no mortality or overdose observed |
| Springer et al. (2017) | RCT | Jail and Prison | 66 (83% male) | 27 (78% male) | Extended release Naltrexone (XR-NTX)+ treatment as usualc (6 380 mg injections of XR-NTX starting week before release) | No differences in time to opioid use 6 months post-release, but those that got 3+ injections had longer time to opioid use compared to <2 injections | No differences in HIV treatment, XR-NTX more likely to improve & maintain viral suppression, less likely to decrease in viral suppression 6 months post-release | ||
Note. Sample sizes listed reflect the analyzed sample (which may have been intent to treat [indicated by +]).
Study participants inducted to naltrexone, controls inducted on methadone.
Study participants inducted to naltrexone, controls received treatment as usual (motivational enhancement + referral to community treatment).
Study participants inducted to naltrexone and access to treatment as usual (i.e., counseling, 12-step), controls received placebo + treatment as usual.
2.2. Data Analysis Approach
We determined that sufficient data existed to perform a meta-analysis of methadone delivered in prison and jail settings. To minimize bias from non-controlled study designs on treatment effects, only RCTs were included in the meta-analysis (Egger, Schneider, & Smith, 1998). There were too few buprenorphine (n=3) and naltrexone (n=3) RCTs with shared outcomes to meta-analyze. Outcomes needed to be present in at least 3 RCTs to meta-analyze. Follow up studies that examined the same outcomes as the primary study (i.e., on the same sample) were excluded from the meta-analysis. Quasi-experimental studies of all MATs were systematically reviewed and effect sizes are reported when available.
For the purposes of the meta-analysis, when studies utilized more than one control condition, we selected the control condition with the least treatment provided (i.e., passive referral); we used this comparison strategy because passive referral is the current standard of care for opioid use in correctional facilities in the U.S. When possible, we analyzed the intent to treat sample (i.e., the sample that was randomized at the start of the study). Most studies utilized dichotomous outcomes; continuous outcomes were converted to dichotomous outcomes (presence vs. absence) for consistency across studies in our analysis. When studies used multiple assessments of recidivism, all outcomes were reported in Tables 1–3; re-incarceration was selected for use in the meta-analysis. The presence of dichotomous outcomes (i.e., post-incarceration substance use treatment engagement, opioid use, re-incarceration, and engagement in health risk behaviors) was assessed by calculating pooled odds ratios (OR) with a 95% confidence interval (CI). ORs were pooled using the method recommended by DerSimian and Laird (1986). The level of significance to detect differences in outcomes between the treatment and control/comparison group was p < .05. The duration of follow up varied from 1 month to 6 months for most RCTs, though one RCT (Dolan et al., 2005) had a 4 year follow up. Because studies were heterogeneous (e.g., different dose of methadone, length of treatment, follow-up period), random effects models were used. The I2 statistic was used to assess heterogeneity of effect sizes (i.e., how inconsistent results were across studies).
2.3. Assessment of Bias
The Cochrane Risk of Bias (ROB) tool (Cochrane Handbook, 2008; Munder & Barthe, 2017) was used to rate bias of RCTs in five domains: selection (i.e., random sequence generation, allocation concealment), performance (i.e., blinding of participants and personnel), detection (i.e., blinding of outcome assessment), attrition (i.e., incomplete outcome data), and reporting (i.e., selective reporting of outcomes). Specific to the meta-analysis portion of the review, risk of reporting bias was evaluated using adjusted rank correlation test described by Begg and Mazumdar (1994).
3. Results
Characteristics of methadone studies are described in Table 1, buprenorphine studies in Table 2, and naltrexone studies in Table 3. A total of 8 RCTs (i.e., 4 original RCTs and 4 follow-up RCTs) examining methadone during incarceration were eligible for meta-analysis. Of the 4 original methadone RCTs, 2 took place in a prison setting and 2 took place in a combined prison/jail setting; 3 inducted inmates to methadone, and 1 maintained inmates on previously received methadone; 3 administered methadone doses >60mg and 1 administered <60mg.
Table 2.
Studies of Buprenorphine Treatment among Incarcerated Populations
| Sample Size/Gender | Post-Release Study Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Study Design | Setting | Treatment | Control | Intervention/dose | Substance Use Treatment Engagement | Opioid Use | Recidivism | Health Risk Behaviors |
| Magura et al. (2009) | RCT | Jail | 43 (100% male) | 38 (100% male) | Buprenorphine-naloxone sublingual tablets; 12mg average dose; methadone control group 30mg average dose)a | Buprenorphine group had increased community buprenorphine engagement 3 months post-release (self-report) | No differences in opioid use 3 months post-release (self-report) | No differences in re-arrest or re-incarceration 3 months post-release (self-report) | No overdoses or mortality observed |
| Gordon et al. (2014) | RCT | Prison | 104+ (during incarceration = 52, after incarceration = 52) (total sample = 70.1% male) | 107 | Buprenorphine/naloxone sublingual tabletsb; target dose of 8mg per day/16mg every other day | Buprenorphine-naloxone during incarceration group had greater community treatment engagement 1 month post-release compared to counseling only (clinic data) | |||
| Gordon et al. (2017) | 12 month follow up | Prison | 104+ (during incarceration = 52, after incarceration = 52) (total sample = 70.1% male) | 107 | Buprenorphine/naloxone sublingual tabletsb; target dose of 8mg per day/16mg every other day | Buprenorphine during incarceration group stayed in treatment longer than buprenorphine after incarceration (clinic data) | No differences in heroin use or opioid-positive urinalysis 1, 3, 6, or 12 months (self-report, urine toxicology) | No differences in criminal activity 1, 3, 6, or 12 months (self-report) | No differences in hospitalizatio ns |
Note. Sample sizes listed reflect the analyzed sample (which may have been intent to treat [indicated by +]).
Study participants inducted to buprenorphine/naloxone, control condition was inducted to methadone.
Study participants inducted to buprenorphine/naloxone and received counseling during incarceration plus passive referral to two types of community MAT (2 treatment conditions), control included counseling only during incarceration plus passive referral to two types of community MAT (2 control conditions).
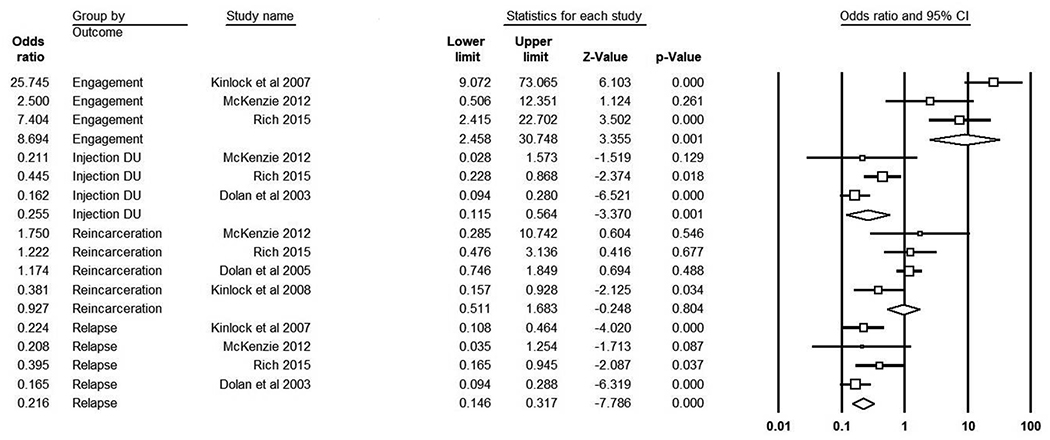
Of the 8 RCTs eligible for meta-analysis, 2 follow up RCTs were excluded because they analyzed the same outcomes for the same participants at a later point in time. Among the 6 remaining RCTs (4 original RCTs with 807 inmates; treatment n = 407, control n = 400; 2 follow-up RCTs), four outcomes were included in the meta-analysis: community substance use treatment engagement (n=3 studies), opioid use (n=4 studies), recidivism (n=4 studies), and injection drug use (n=3 studies). Other health risk behaviors were not present in enough studies and thus were not included in the meta-analysis, including hospitalization, infectious disease presence, overdose, syringe sharing, unprotected sex, and mortality. Results from the meta-analysis are depicted in Table 4.
Table 4.
Forest plot of randomized controlled trials evaluating methadone effectiveness grouped by post-release outcomes of community treatment engagement, injection drug use, re-incarceration, and relapse to opioid use.
|
Note. Random effects are presented. The overall effect is the last odds ratio listed in each outcome category.
3.1. Risk of Bias
Of the RCTs and follow-up RCTs included in the meta-analysis, all had low risk of bias in random sequence generation (i.e., utilized appropriate methods for random assignment). Half of the studies had low risk and the other half had unclear risk regarding allocation concealment. None of the studies were blinded to treatment condition and all involved cross over during the study, resulting in high risk of bias. All studies had low risk of bias with regard to blinding of outcome assessments; outcomes were assessed biochemically, via self-report, and via administrative/clinic databases in all studies, which are all considered low risk (Munder & Barthe, 2017. Kinlock et al. (2007), Kinlock et al., (2008), and Dolan et al. (2003) were rated as having low risk of bias around incomplete data, as they used intent to treat analyses and had similar rates/reasons for attrition across conditions. McKenzie et al. (2012) and Rich et al. (2015) were rated as having high risk of bias around incomplete data due to unequal attrition and reasons for attrition across conditions. Dolan et al. (2005) lacked sufficient information in this domain. All studies were rated as having low risk of bias around selective reporting of outcomes.
3.2. Community-based Substance Use Treatment Engagement
3.2.1. Methadone.
Three original RCTs (see Table 4) examined the impact of methadone on post-incarceration substance use treatment engagement (combined treatment n = 216). Inmates who received methadone during incarceration were more likely to engage in treatment post-release than controls (pooled OR = 8.96, 95% CI = 2.46−−30.75, p<.001, Q = 6.31, p = 0.043, I2 = 68.32). There was no evidence of bias, τ = −.67, p = 0.15. Two observational studies examined the effect of methadone on substance use treatment engagement post-release and found similar results. The first study (Magura et al., 1993; treatment n = 195, control n = 54) found that participants inducted to 30–40mg of methadone during incarceration were more likely to engage in community-based substance use treatment 6.5 months post-release compared to those who received methadone for detox purposes (27% vs. 9% respectively). The second study (Moore et al., in press; treatment n =184, control n = 198) found that participants continued on an average of 68mg of methadone during incarceration were more likely to re-engage in MAT 1 month post-release (41% vs. 10%).
3.2.2. Buprenorphine.
Two original studies (RCTs) examined the effect of induction to buprenorphine during incarceration on substance use treatment engagement post-incarceration. The first study (Magura et al., 2009; treatment n = 43, control n = 38) found that participants inducted to buprenorphine-naloxone sublingual tablets during incarceration were more likely to report to a community-based post-release treatment (48% vs. 14%) and engage in MAT 3 months post-release (48% vs. 23% respectively) compared to a control group receiving 30 mg of methadone. Similarly, a second study (Gordon et al., 2014; treatment n = 104, control n = 107) found that participants inducted to a combination of buprenorphine-naloxone during incarceration were more likely to engage in substance use treatment post-incarceration compared to a counseling only control group (47.5% vs. 33.7%, OR = 1.5, 95% CI = 1.1, 2.1).
3.2.3. Naltrexone.
Two original studies (one quasi-experimental, one RCT) examined the effect of induction to naltrexone during incarceration on substance use treatment engagement post-incarceration. The first study (Lobmaier et al., 2010; treatment n = 23, control n = 21) found that participants given naltrexone implants during incarceration were more likely to engage in substance use treatment 6 months post-incarceration compared to a control group receiving 80 to 130 mg of methadone (69.6% vs. 23.8% respectively). A second study (Lee et al., 2015; treatment n = 16, control n = 17) found that participants who received a naltrexone extended release injection prior to release were no more likely than those in the control group (motivational enhancement and referrals) to engage in non-study substance use treatment 1 month post-release (19% vs. 12% respectively). However, it is important to note that 75% of participants in the naltrexone group remained engaged in study treatment (i.e., received a second naltrexone injection) around 4 weeks post-release, indicating high levels of post-release engagement.
3.3. Post-release Opioid Use
3.3.1. Methadone.
Four original RCTs (see Table 4) examined relapse to illicit opioid use (combined treatment n = 407) following release. Inmates who received methadone while incarcerated were less likely to use opioids following release compared to controls (pooled OR = 0.22, 95% CI = 0.15–0.32, p<.001, Q = 2.76, p = 0.43, I2 = 0.00). There was no evidence of bias, τ = 0.17, p = 0.37. Four observational studies examined the effect of methadone during incarceration on post-release opioid use and showed similar results. Dole et al. (1969) (treatment n = 12, control n = 16) found that participants inducted to 35 mg of methadone prior to release were less likely to self-report using heroin daily 7–10 months post-release (0% vs. 100%). Similarly, Dolan (1998) (treatment n = 48, control n = 105) found that participants inducted to an average of 60mg of methadone during incarceration were less likely to use heroin post-release compared to those who received treatment as usual (15% vs. 38% respectively), and Heimer (2006) (treatment n = 20, control n = 40) found that participants inducted to an average of 80–120mg of methadone during incarceration were less likely to report heroin use post-program completion compared to a random general population sample (5.6% vs. 37.5% respectively), results which were supported by urine toxicology. On the other hand, Magura et al. (1993) found no differences in hair analysis of heroin and cocaine use 6 months post-release between participants who inducted to methadone compared to those who received methadone for detoxification (76% vs. 85% respectively).
3.3.2. Buprenorphine.
Magura and colleagues (2009) found no differences in opioid use 3 months post-release between participants inducted to buprenorphine-naloxone sublingual tablets during incarceration and a methadone control group (53% vs. 66% respectively). Similarly, a follow up to an RCT (Gordon et al., 2017; treatment n = 104, control n = 107) found no differences in opioid use at 1 (23.5% vs. 24.6%), 3 (28.8% vs. 33.8%), 6 (31.4% vs. 41.4%), and 12 (29.5% vs. 33.3%) months post-release between participants inducted to buprenorphinenaloxone during incarceration and a counseling only control group.
3.3.3. Naltrexone.
Lobmaier and colleagues (2010) found that there were no differences in self-reported heroin use between participants given naltrexone implants during incarceration and those on methadone (15.6% vs. 20.2% respectively), however, survival analyses indicated that those given naltrexone took longer to relapse post-release (p = .012). Two original RCTs examined the impact of naltrexone on opioid use post-release. Lee and colleagues (2015) found that participants who received an extended release naltrexone injection prior to release were less likely to use opioids 1 and 2 months post-release compared to the control group (38% vs. 88%, OR = .08, 95% CI = 0.01–0.48). Springer et al., 2017; treatment n=66, control n=27) examined the effect of extended-release naltrexone injections initiated 1 week prior to release and continued for 6 months post-release on opioid use and found no differences between treatment and placebo groups 6 months post-release, but did find that participants who received 3 or more injections had a longer time to opioid use (136 days) compared to those who received 2 or fewer (53 days).
3.4. Recidivism
3.4.1. Methadone.
Four original RCTs (see Table 4) examined recidivism outcomes (combined treatment n = 404). There was no significant effect of methadone received during incarceration on post-release recidivism rates (pooled OR = 0.93, 95% CI = 0.51–1.68, p = .810, Q = 5.53, p = 0.137, I2 = 45.79). There was no evidence of bias, τ = 0.00, p = 0.50. Five observational studies examined the effect of methadone on recidivism outcomes and results were mixed. Dole et al. (1969) found that participants inducted to methadone prior to release had lower reincarceration rates 7–10 months post-release (25% vs. 94%), and Macswain et al. (2014) (treatment n = 642, control n = 214) found no differences in return to custody rates but found that participants continued on methadone during incarceration and post-release were under community supervision the longest (358 days) compared to participants who terminated methadone at release (274 days) and those who never received methadone during incarceration (325 days). Magura et al. (1993) found no differences in property offenses (66% vs. 49%) or recent illegal income (67% vs. 65%) 6.5 months post-release between participants who did and did not receive MMT during incarceration. Bellin et al. (1999) (treatment n = 1,423, control n = 10,079) found that participants inducted to >60mg of methadone had a longer time to reincarceration compared to those inducted to <30mg (253 days vs. 187 days), however they had less time to reincarceration than participants who did not receive methadone during incarceration (634 days) or who received it for detoxification purposes during incarceration (337 days). Similarly, Westerberger et al. (2016) found that participants who were continued on previously received methadone during incarceration had a lower chance of being rebooked compared to participants who received MAT for detoxification (53% vs. 72% respectively), but there were no differences in rebooking compared to participants in the general population who did not receive methadone during incarceration (53% vs. 50%).
3.4.2. Buprenorphine.
With regard to buprenorphine, Magura and colleagues (2009) found no differences in reincarceration 3 months post-release between participants inducted to buprenorphine-naloxone sublingual tablets during incarceration and the methadone control group (40% vs. 50% respectively). Similarly, Gordon and colleagues (2017) found no differences in criminal activity at 1, 3, 6, and 12 months post-release between participants inducted to buprenorphine-naloxone during incarceration and the counseling only control group.
3.4.3. Naltrexone.
Lobmaier and colleagues (2010) found no differences in criminal activity 6 months post-incarceration between participants inducted to naltrexone implants and those given a high dose of methadone (21.7% vs. 23.8% respectively). Similarly, Lee and colleagues (2015) found no differences in reincarceration 1 month post-release between participants who were inducted to a naltrexone extended release injection prior to release and the treatment as usual control group (31% vs. 41% respectively), although participants who completed two injections (n=8) had significantly fewer reincarcerations compared to the control group and those who only had one injection (n = 25, 13% vs. 44% respectively).
3.5. Health Risk Behaviors
3.5.1. Methadone.
Three original studies (see Table 4) examined the effect of methadone on injection drug use post-incarceration (combined treatment n = 336). Methadone received during incarceration reduced the odds of injection drug use post-release (pooled OR = 0.26, 95% CI = 0.12–0.56, p=.001, Q = 5.27, p = 0.072, I2 = 62.03). There was no evidence of bias, τ = 0.00, p = 0.50. Across other methadone RCTs, there were no observed differences between participants who received methadone during incarceration and those who did not on most other health risk behaviors including hospitalization, overdose, sexual risk, or mortality outcomes (Kinlock et al., 2007; 2009; McKenzie et al., 2012; Rich et al. 2015). Two observational methadone studies examined health risk behaviors. Magura et al. (1993) found no differences in daily injection drug use 6.5 months post-release between participants who were and were not inducted to methadone during incarceration (44% vs. 48% respectively), but Dolan (1998) found that participants inducted to methadone were less likely to share syringes (21% vs. 39%) and had lower self-reported HIV risk behavior (3% vs. 6%) compared to participants not inducted to methadone during incarceration.
3.5.2. Buprenorphine and naltrexone.
One study examining injection drug use as an outcome (Lee et al., 2015) found no significant differences at 1 month post-release between participants who were inducted to extended release naltrexone and the control group (25% vs. 6% respectively). Springer et al. (2017) found that participants who received naltrexone injections prior to release were more likely to achieve (30% vs. 19%) and maintain (30% vs. 27%) viral suppression, and less likely to lose viral suppression (8% vs. 33%) compared to those who received a placebo. The only other health risk behavior examined in buprenorphine and naltrexone studies was hospitalization rate (Gordon et al., 2017), and there was no evidence of differences in hospitalization rate between participants who were inducted to buprenorphinenaloxone during incarceration and the control group.
4. Discussion
This study built upon previous systematic reviews (Sharma et al., 2016) to comprehensively review the literature on MAT delivered in correctional settings. We determined there was sufficient data to meta-analyze RCTs of methadone maintenance treatment, but not other forms of MAT (i.e., buprenorphine, naltrexone). This study is the first to quantify the effect of methadone in correctional settings on a variety of post-release behaviors. Our results showed strong support for the use of methadone as well as other forms of MAT for currently incarcerated populations, with almost all studies showing that MAT provided during incarceration increased community-based substance use treatment engagement, and in the case of methadone, decreased illicit opioid use and injection drug use post-release.
There were strong effects of methadone received during incarceration on post-incarceration substance use treatment engagement. Pooled effects from the meta-analysis suggest that inmates who received methadone during incarceration were more than 8 times as likely to engage in community-based substance use treatment compared to those who did not receive methadone during incarceration, and there was consistent support for engagement in treatment across observational studies. There was also strong support for the effectiveness of methadone in reducing illicit opioid and injection drug use following release from incarceration, with rates reduced by 78% and 74% respectively. Reductions in both behaviors have important implications; decreased illicit opioid use serves to decrease the heightened overdose risk that prisoners have just after release from incarceration (Merrall et al., 2010; Degenhardt et al., 2014), and reductions in injection drug use may serve to reduce risk for infectious disease transmission (Bruneau, Roy, Arruda, Zang, & Jutras-Aswad, 2012) and crime (Håkansson & Berglund, 2012). Buprenorphine and naltrexone were either superior to methadone or to placebo, or were as effective as methadone in reducing illicit opioid use post-release.
Recidivism did not consistently decrease among people receiving methadone, buprenorphine, or naltrexone during incarceration. This lack of effect may be related to differences in the follow-up periods studied, which ranged from 6 months to 4 years post-release for recidivism outcomes. For instance, studies showed significantly less self-reported post-release criminal activity among participants receiving methadone in prison up to 2 months post-incarceration; however, this difference did not consistently persist at 6-months and 12-months post-incarceration (Gordon, Kinlock, Schwartz, & O’Grady, 2008; Kinlock, Gordon, Schwartz, Fitzgerald, & O ‘grady, n.d.). In addition, differences in the operationalization and measurement of recidivism across studies (i.e., criminal behavior vs. re-arrest/reincarceration, self-reported vs. official records) may influence the ability to detect effects. Alternatively, the lack of effect may reflect the fact that people who receive MAT during incarceration but do not continue MAT post-release may not experience any reductions in criminal behavior or arrest, relative to controls. Studies that consider recidivism among those engaged in community treatment post-release demonstrate significant reductions in criminal behavior (Magura et al., 1993; Moore et al., in press). The provision of MAT during incarceration increases post-release community treatment engagement, and recidivism is lowered among those who remain engaged in community-based MAT.
While methadone delivered during incarceration did significantly reduce injection drug use post-release, there was no evidence that methadone, buprenorphine, or naltrexone reduced other health risk behaviors, partly due to methodological quality of the studies examined. Specifically, inconsistent assessment of health risk behaviors paired with low prevalence of behaviors such as hospitalizations, overdoses, and mortalities likely decreased power to detect such differences. The use of behavioral treatments in adjunct to MAT is supported, especially for certain types of high-risk patients (Carroll & Weiss, 2017), and the delivery of evidence-based care alongside MAT for opioid use may serve to reduce other health-compromising behaviors.
4.1. Limitations
Though this study presents the first meta-analytic review of MAT in correctional settings, there were a small number of RCTs and there was heterogeneity in study characteristics, such as the duration of follow-up and method of substance use measurement (e.g., urine toxicology, self-report). Though heterogeneity statistics are provided, they must be interpreted cautiously given the small number of studies. Only one study in the meta-analysis examined continuation of methadone as opposed to induction, which may have been substantively different. There were too few studies of buprenorphine and naltrexone to conduct meta-analyses, and the studies examining these forms of MAT compared their superiority or equivalency to methadone versus a placebo-controlled condition. Many studies did not include key outcomes such as sexual risk behaviors, medical treatment utilization while incarcerated, and healthcare utilization post-release; thus, we were unable to address the effects of MAT on these outcomes. Finally, due to the small number of RCTs in the meta-analysis, we were unable to test for moderation by methadone dose, continuation vs. induction, or type of correctional facility (i.e., jail vs. prison, which differ in length and environment). Higher methadone doses (i.e., ≥ 60 mg per day [blocking dose] vs. < 60 mg/day [Fullerton et al., 2014]) may produce better outcomes. These are all directions for future research. Additionally, more RCTs of methadone are needed in both jails and prisons that test different dosages of methadone to identify factors that impact post-release treatment retention and opioid use. In addition, there is a need for RCTs examining continuation of methadone during incarceration. Future research should also focus on understanding how to target criminogenic risk and health risk behaviors within the context of MAT delivered to prison and jail populations.
5. Conclusions
This meta-analysis and systematic review shows strong support for the utility of MAT in increasing community-based substance-use treatment engagement post-incarceration in prison and jail, and strong support for the use of methadone in reducing illicit opioid use and injection drug use post-incarceration. Additional evaluations of naltrexone and buprenorphine are needed. To date, there is equivocal support for the use of MATs for reducing recidivism post-release, as re-engagement in community-based MAT after incarceration is often not considered in the prediction of recidivism. However, studies that account for post-release re-engagement in MAT support positive outcomes in these areas. More RCTs are needed to permit meta-analysis on a larger number of studies, particularly studies examining methadone continuation, buprenorphine, and naltrexone delivered during incarceration.
Highlights.
Medication assisted treatment (MAT) is effective for opioid use but rarely used during incarceration.
This meta-analysis and systematic review examined the effectiveness of MAT in correctional settings.
Methadone increases treatment entry and reduces opioid use and injection drug use post-release.
Buprenorphine and naltrexone are as effective as methadone in increasing treatment entry.
MATs did not decrease recidivism, but studies did not account for post-release engagement in MATs.
Acknowledgments
Funding source: National Institute on Drug Abuse (T32DA019426–12; KEM), State of Connecticut Department of Mental Health and Addiction Services. The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The work described in this article does not express the views of the State of Connecticut or NIDA. The views and opinions expressed are those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews D. a., & Bonta J (2010). Rehabilitating criminal justice policy and practice. Psychology, Public Policy, and Law, 16(1), 39–55. 10.1037/a0018362 [DOI] [Google Scholar]
- Anne Fullerton C, Meelee Kim M, Cindy Parks Thomas M, Russell Lyman D, Montejano LB, Richard Dougherty CH, … Delphin-Rittmon ME (2014). Assessing the Evidence Base Series Medication-Assisted Treatment With Methadone: Assessing the Evidence. Psychiatric Services, 65, 146–157. 10.1176/appi.ps.201300235 [DOI] [PubMed] [Google Scholar]
- Aronowitz SV, & Laurent J (2016). Screaming Behind a Door: The Experiences of Individuals Incarcerated Without Medication-Assisted Treatment. Journal of Correctional Health Care, 22(2), 98–108. 10.1177/1078345816634079 [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50, 1088–1101. [PubMed] [Google Scholar]
- Bellin E, Wesson J, Tomasino V, Nolan J, Glick AJ, Oquendo S (1999). High dose methadone reduces criminal recidivism in opiate addicts. Addiction Research, 7(1), 19–29. [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, & Stern MF (2013). Mortality after prison release: Opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine, 159, 592–600. 10.7326/0003-4819-159-9-201311050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, & Roland CL (2011). Societal Costs of Prescription Opioid Abuse, Dependence, and Misuse in the United States. Pain Medicine, 12(4), 657–667. 10.1111/j.1526-4637.2011.01075.x [DOI] [PubMed] [Google Scholar]
- Bruneau J, Roy É, Arruda N, Zang G, & Jutras-Aswad D (2012). The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction, 107(7), 1318–1327. 10.1111/j.1360-0443.2012.03803.x [DOI] [PubMed] [Google Scholar]
- Carroll KM, & Weiss RD (2017). The role of behavioral interventions in buprenorphine maintenance treatment: A review. American Journal of Psychiatry, 174(8), 738–747. 10.1176/appi.ajp.2016.16070792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. (2016). Increases in drug and opioid-involved overdose deaths--United States, 2010–2015. Retreived from https://www.cdc.gov/mmwr/volumes/65/wr/mm655051e1.htm [DOI] [PubMed]
- Higgins JPT, & Green S (2008). Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. The Cochrane Collaboration. [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor R, et al. (2007). Methadone and buprenorphine for the management of opioid dependence: A systematic review and economic evaluation. NIHR Health Technology Assessment programme: Executive Summaries. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Larney S, Kimber J, Gisev N, Farrell M, Dobbins T, … Burns L (2014). The impact of opioid substitution therapy on mortality post-release from prison: Retrospective data linkage study. Addiction, 109(8), 1306–1317. 10.1111/add.12536 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, & Laird N (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Egger M, Schneider M, & Smith GD (1998). Spurious precision? Meta-analysis of observational studies. BMJ, 316, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré M, Mas A, Torrens M, Moreno V, & Camí J (2002). Retention rate and illicit opioid use during methadone maintenance interventions: A meta-analysis. Drug and Alcohol Dependence, 65(3), 283–290. 10.1016/S0376-8716(01)00171-5 [DOI] [PubMed] [Google Scholar]
- Fiscella K, Moore A, Engerman J, & Meldrum S (2004). Jail Management of Arrestees/Inmates Enrolled in Community Methadone Maintenance Programs. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 81(4), 645–654. 10.1093/jurban/jth147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JJB, Zaller ND, Yokell MAB, Bazazi AR, & Rich JD (2013). Forced withdrawal from methadone maintenance therapy in criminal justice settings: A critical treatment barrier in the United States. Journal of Substance Abuse Treatment, 44, 502–505. 10.1016/j.jsat.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O’Grady KE, & Vocci FJ (2014). A randomized controlled trial of prison-initiated buprenorphine: Prison outcomes and community treatment entry. Drug and Alcohol Dependence, 142(2014), 33–40. 10.1016/j.drugalcdep.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, & O’Grady KE (2008). A randomized clinical trial of methadone maintenance for prisoners: Findings at 6 months post-release. Addiction, 103(8), 1333–1342. 10.1111/j.1360-0443.2008.002238.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE, Fitzgerald TT, & Vocci FJ (2017). A randomized clinical trial of buprenorphine for prisoners: Findings at 12-months post-release. Drug and Alcohol Dependence, 172, 34–42. 10.1016/j.drugalcdep.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, … Hasin DS (2015). Epidemiology of DSM-5 Drug Use Disorder. JAMA Psychiatry, 20852, 1 10.1001/jamapsychiatry.2015.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson A, & Berglund M (2012). Risk factors for criminal recidivism - a prospective follow-up study in prisoners with substance abuse. BMC Psychiatry, 12, 111 10.1186/1471-244X-12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, Catania H, Newman RG, Zambrano J, Brunet A, & Ortiz AM (2006). Methadone maintenance in prison: Evaluation of a pilot program in Puerto Rico. Drug and Alcohol Dependence, 83, 122–129. [DOI] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, & O ‘grady KE (n.d.) A randomized clinical trial of methadone maintenance for prisoners: Results at 12 months postrelease. Journal of Substance Abuse Treatment, 37, 277–285. 10.1016/j.jsat.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Mcdonald R, Grossman E, Mcneely J, Laska E, Rotrosen J, & Gourevitch MN (2015). Opioid treatment at release from jail using extended-release naltrexone: A pilot proof-of-concept randomized effectiveness trial. Addiction, 110(6), 1008–1014. 10.1111/add.12894 [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, … Rotrosen J (2017). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. The Lancet, 6736(17), 1–10. 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, Bailey GL, … Beebe KL (2010). Buprenorphine Implants for Treatment of Opioid Dependence. Jama, 304(14), 1576 10.1001/jama.2010.1427 [DOI] [PubMed] [Google Scholar]
- Lipari RN, Hughes A (2015). The NSDUH Report: Trends in Heroin Use in the United States: 2002 to 2013. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 1–11. [PubMed] [Google Scholar]
- Lobmaier PP, Kunøe N, Gossop M, Katevoll T, & Waal H (2010). Naltrexone implants compared to methadone: Outcomes six months after prison release. European Addiction Research, 16(3), 139–145. 10.1159/000313336 [DOI] [PubMed] [Google Scholar]
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, & Rosenblum A (2009). Buprenorphine and methadone maintenance in jail and post-release: A randomized clinical trial. Drug and Alcohol Dependence, 99, 222–230. 10.1016/j.drugalcdep.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradiaga JA, Nahvi S, Cunningham CO, Sanchez J, & Fox AD (n.d.). " I Kicked the Hard Way. I Got Incarcerated. " Withdrawal from Methadone During Incarceration and Subsequent Aversion to Medication Assisted Treatments 10.1016/j.jsat.2015.11.004 [DOI] [PMC free article] [PubMed]
- Marsch L (1998). The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior, and criminality: A meta-analysis. Addiction, 93(4), 515–532. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M (2009). Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Library, (3), 1–32. 10.1002/14651858.CD002209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2014). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence (Review) Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. The Cochrane Library, 2(2), 1–84. 10.1002/14651858.CD002207.pub4.Copyright [DOI] [Google Scholar]
- McMillan GP, Lapham S, & Lackey M (2008). The effect of a jail methadone maintenance therapy (MMT) program on inmate recidivism. Addiction, 103, 2017–2023. doi: 10.1111/j.1360-0443.2008.02361.x [DOI] [PubMed] [Google Scholar]
- Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, … Bird SM (2010). Meta-analysis of drug-related deaths soon after release from prison. Addiction, 105(9), 1545–1554. 10.1111/j.1360-0443.2010.02990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6, e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Kelly E., Oberleitner L, Smith KMZ, Maurer K, McKee S (n.d.). Feasibility and effectiveness of continuing methadone maintenance treatment during incarceration compared to forced withdrawal. Journal of Addiction Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumola CJ, Karberg JC (2006). Drug use and dependence, state and federal prisoners, 2004. US Department of Justice, Office of Justice Programs, Bureau of Justice Statistics. Retrieved from https://www.bjs.gov/content/pub/pdf/dudsfp04.pdf [Google Scholar]
- Munder T, & Barth J (2017). Cochrane’s risk of bias tool in the context of psychotherapy outcome research. Psychotherapy Research, doi: 10.1080/10503307.2017.1411628 [DOI] [PubMed] [Google Scholar]
- Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, & Rich JD (2009). Methadone and buprenorphine prescribing and referral practices in US prison systems: Results from a Nationwide Survey. Drug and Alcohol Dependence, 105(1–2), 83–88. 10.1016/j.drugalcdep.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otiashvili D, Piralishvili G, Sikharulidze Z, Kamkamidze G, Poole S, & Woody GE (2013). Methadone and buprenorphine-naloxone are effective in reducing illicit buprenorphine and other opioid use, and reducing HIV risk behavior-Outcomes of a randomized trial. Drug and Alcohol Dependence, 133(2), 376–382. 10.1016/j.drugalcdep.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JD, Boutwell AE, Shield DC, Key RG, McKenzie M, Clarke JG, & Friedmann PD (2005). Attitudes and practices regarding the use of methadone in US State and federal prisons. Journal of Urban Health-Bulletin of the New York Academy of Medicine, 82(3), 411–419. 10.1093/jurban/jti072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RN, Ling W, Casadonte P, Vocci F, Bailey GL, Kampman K, … Beebe KL (2013). Buprenorphine implants for treatment of opioid dependence: Randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction, 108(12), 2141–2149. 10.1111/add.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, … Hall SM (2000). Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA: The Journal of the American Medical Association, 283(10), 1303–1310. 10.1097/00132586-200104000-00058 [DOI] [PubMed] [Google Scholar]
- Sharma A, O’Grady KE, Kelly SM, Gryczynski J, Mitchell SG, Schwartz RP (2016). Pharmacotherapy for opioid dependence in jails and prisons: Research review update and future directions. Substance Abuse and Rehabilitation, 7, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanum L, Solli KK, Latif Z-H, Benth JŠ, Opheim A, Sharma-Haase K, … Kunøe N (2017). The Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence. JAMA Psychiatry, 74(12), 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases, 35(1), 22–35. 10.1080/10550887.2016.1100960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, & Rich JD (n.d.). Addiction treatment within u.s. correctional facilities: bridging the gap between current practice and evidence-based care. 10.1080/10550887.2015.1059217 [DOI] [PubMed]
- Weinstein ZM, Kim HW, Cheng DM, Quinn E, Hui D, Labelle CT, … Samet JH (2017). Long-term retention in Office Based Opioid Treatment with buprenorphine. Journal of Substance Abuse Treatment, 74, 65–70. 10.1016/j.jsat.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberger VS, McCrady BS, Owens M, Guerin P (2016). Community-based methadone maintenance in a large detention center is associated with decreases in inmate recidivism. Journal of Substance Abuse Treatment, 70, 1–6. 10.1016/j.jsat.2016.07.007 [DOI] [PubMed] [Google Scholar]