Abstract
Background
Chemoresistance is a main limitation in chemotherapy for therapeutic cancer. MicroRNA (miRNA) has been indicated in the progression and tumorigenesis of many types of cancer, but the effect of miR-34b-3p in bladder cancer (BCa) cells is still unknown.
Material/Methods
This research compared the multidrug-sensitive (5637) BCa cell line and the multidrug-resistant (EJ) BCa cell line. We found that CCND2 (G1/S-specific cyclin-D2) and P2RY1 (purinergic receptor P2Y1) were the targets of miR-34b-3p, as further validated by qRT-PCR (quantitative real-time polymerase chain reaction) and western blot analysis.
Results
Forced reversal of the levels of miR-34b-3p or CCND2/P2RY1 changed the chemoresistance profiles in both 5637 cells and EJ cells. Further experiments suggested that the CCND2 gene and the P2RY1 gene act in concert to negatively correlate with miR-34b-3p effect on BCa multidrug-chemoresistance.
Conclusions
These results not only reveal new players regulating BCa chemoresistance, but also provide clues for effective chemotherapy for BCa patients.
MeSH Keywords: Drug Resistance, MicroRNAs, Urinary Bladder Neoplasms
Background
MicroRNAs (miRNAs) are considered to be small non-coding RNA molecules, which play very important roles in multiple biological processes [1]. They have been found to be involved in disease generation, including cancer. The unusual expression of miRNAs in tumor cells has been explored in different fields of cancer pathology [2,3], including multidrug-chemoresistance [4], which is still a major obstacle to effective treatment of cancer patients [5]. Multidrug-resistant phenotypes differ greatly among cancer patients, and even in different tumor lesions [6]. Due to the complex regulatory network, our understanding of the mechanism of multidrug resistance in cancer is still very poor, despite research efforts in this area [7,8]. To date, the accumulating findings have shown that various miRNAs, such as miR-199a-5p [9], miR-193a-3p [5,10], miR-130a [11], and miR-140 [12] participate in the development of cancer chemoresistance.
As a well-researched miRNA group, the miR-34 family has been shown to be regulated by p53 transcription factor [13–15], which regulates different cellular events, such as cell apoptosis, cell cycle arrest, and cell aging [15,16]. All members of this group (which contains miR-34a and miR-34b/c) share homolog common sequences which resemble endogenous mRNA sequences [17]. Sequence analysis of the promoter regions of miR-34a, miR-34b, and miR-34c have shown that these regions harbor the canonical p53 binding site. Notably, miR-34b was reported to be a cancer suppressor in a series of malignant tumor studies, including breast, ovarian, colorectal, pancreatic, urothelial, renal cell, and soft tissue carcinomas [18].
Bladder cancer (BCa) is the most life-threatening urogenital cancer [19,20]. The mechanisms underlying BCa chemoresistance are not clear. In our previous studies, we found several miRNAs and their target genes involved in BCa chemoresistance [5,21–23]. In this study, we showed that miR-34b-3p can repress BCa multidrug-chemoresistance by regulating the CCND2 (G1/S-specific cyclin-D2) gene and the P2RY1 (purinergic receptor P2Y1) gene, 2 newly identified targets of miR-34b-3p. Our results give novel insights into BCa chemoresistance and might provide potential therapeutic targets for the effective therapy of BCa patients.
Material and Methods
Animal experiments were undertaken in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal research was approved by the biomedical ethics committee of Anhui Medical University. The animal study proposal was approved by the Institutional Animal Care and Use Committee (IACUC, No. 20170082) of Anhui Medical University. All of the mouse experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
Human BCa cell line studies and culture
The cell lines of 5637 (NO. HTB-9) [5] and EJ (by Marshall CJ established in 1977) [5,24] that were used in this study were bought from the Chinese Academy of Sciences Collection Committee of cultural collections. The cells were cultured in DMEM (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, PAN-Biotech), 100 U/mL of penicillin (Wisent Inc.), and 100 mg/mL of streptomycin (Wisent Inc.) in humidified air at 37°C with 5% CO2.
Transfection of BCa cells
All sequences for transfection were provided by Ribobio, China. The plasmids of CCND2 (EX-W0038-M98) and P2RY1 (EX-Q0127-M98-5) fused with the GFP tag were constructed by FulenGen, China. The transfection method was carried out in accordance with the product instructions. The following sequences were used in this study: si-CCND2 target sequence: CGGAGAAGCTGTGCATTTA; si-CCND2 sense sequence (5′-3′): CGGAGAAGCUGUGCAUUUA dTdT; si-CCND2 antisense sequence (3′-5′): dTdTGCCUCUUCGACACGUAAA; si-P2RY1 target sequence: CTGGGCTGTTACGGATTAA; si-P2RY1 sense sequence (5′-3′): CUGGGCUGUUACGGAUUAA dTdT; si-P2RY1 antisense sequence (3′-5′): dTdT GACCCGACAAUGCCUAAUU; miR-34b-3p antagomiR (5′-3′): AUGGCAGUGGAGUUAGUGAUUG; miR-34b-3p mimic sense (5′-3′): CAAUCACUAACUCCACUGCCAU; miR-34b-3p mimic antisense (5′-3′): GGCAGUGGAGUUAGUGAUUGUU.
Analysis of chemotherapeutic agents and multidrug-chemoresistance
Medical classification of the 5 drugs, paclitaxel, pirarubicin, epirubicin hydrochloride, adriamycin, and cisplatin have been described previously [5,25,26]. The Cell Counting Kit-8 (CCK8) method has also been described previously [27].
Cell apoptosis flow cytometry assay
Cell apoptosis was tested by staining with annexin V-FITC/propidium iodide. Cells were transfected with either miR-34b-3p mimic, miR-34b-3p antagomiR, si-CCND2, si-P2RY1, or their corresponding negative control (NC), then gathered and resuspended at a concentration of 5–10 x 106 cells/mL and then stained with 5 μL of annexin V-FITC (BD Biosciences) for 10 minutes and 5 μL propidium iodide for another 10 minutes, then subjected to cell apoptosis analysis. Apoptotic cells were assessed by gated propidium iodide and annexin V positive cells in the FACSCalibur apparatus. The experiments were conducted independently at least 3 times and showed representativeness.
Cell cycle flow cytometry assay
Cells were transfected with either miR-34b-3p mimic, miR-34b-3p antagomiR, si-CCND2, si-P2RY1, or their corresponding NC for 48 hours, then cells were gathered and resuspended at a concentration of 5–10×106 cells/mL, and fixed in 75% ice-cold ethanol for 24 hours at 4°C. The cells were stained with 150 μL propidium iodide (BD Biosciences) for 30 minutes at room temperature, at the same time avoiding light. Then the cells were subjected to cell cycle analysis with the FACSCalibur instrument (FACSVerse). The experiments were conducted independently at least 3 times and showed representativeness.
Luciferase assay
The binding of miR-34b-3p to CCND2 and P2RY1 was tested with luciferase reporter assay, using methods described previously [28]. The 3′-untranslated region (3′-UTR) of CCND2 and P2RY1, including binding-domain of miR-34b-3p, was embedded into the pEZX-MT01-REPORT™ vector to construct pEZX-MT01-REPORT™-luc-CCND2 wild type (WT) and pEZX-MT01-REPORT™-luc-P2RY1 WT. The cells were inoculated into 96-well plates with the density of approximately 1×104 cells/well. Next, the transfection was carried out for the aforementioned cells with the mix of pEZX-MT01-REPORT™-luc-CCND2 WT or pEZX-MT01-REPORT™-luc-P2RY1 WT (50 ng), Renilla (5 ng), mimic, or NC nucleotides (5 pmol). After 24 hours of transfection, the cells were detected by Promega GloMax 20/20 photometer and double luciferase reporting analysis system (Promega). The relative luciferase activity of UTR constructs and pathway report constructs was analyzed as previously reported [5].
RNA analysis
According to the manufacturer’s instructions, TRIzol (Tiangen, China) was used to extract total RNA from cells [28]. The sequences for the primers and probes for the qRT-PCR (quantitative real-time polymerase chain reaction) experiments are as follows:
hP2RY1F: 5′-CTCATTGTGGTGGTGGCG-3′;
hP2RY1R: 5′-GTACTCGTCTGAGGTGGTGTCG-3′;
hP2RY1 probe: 5′-CY5-CATCCTCTTCTACTCAGGTACCGGGGTC-3′;
hCCND2F: 5′-TACACCGACAACTCCATCAAGC-3′;
hCCND2R: 5′-GCCAGGTTCCACTTCAACTTC-3′;
hCCND2 probe: 5′-CCACCAGTTCCCACTCCAGCAGC-3′;
hRBPJF: 5′-TTCACAGTCCGAGATGGCTACA-3′;
hRBPJR: 5′-TGCGGTCTGCTTATCAACTTTC-3′;
hRBPJ probe: 5′-CTTGTGTGCTCAGTTACTGGCATGGC-3′;
hTP53F: 5′-GACGGAGGTTGTGAGGCG-3′;
hTP53R: 5′-CTTCCACTCGGATAAGATGCTG-3′;
hTP53 probe: 5′-CCACCATGAGCGCTGCTCAGATAGC-3′;
hSMAD2F: 5′-CAGGCTCTCCAGCAGAACTATC-3′;
hSMAD2R: 5′-GTGAGTGAGGGCTGTGATGC-3′;
hSMAD2 probe: 5′-CTATCGAACACCAAAATGCAGGTTCTGAG-3′;
hNFKB1F: 5′-CAATGCCCTTTTCGACTACG-3′;
hNFKB1R: 5′-GGTGGATGATTGCTAAGTGTAAGA-3′;
hNFKB1 probe: 5′-ACGTGAAGATGCTGCTGGCCGTC-3′;
hFOSF: 5′-CTTACTCCAGGGCTGGCG-3′;
hFOSR: 5′-GCAGCCATCTTATTCCTTTCC-3′;
hFOS probe: 5′-CATTGGCAGGAGGGGCAAGGTG-3′;
hGLIF: 5′-AGCGTGAGCCTGAATCTGTG-3′;
hGLIR: 5′-GGATGTGCTCGCTGTTGATG-3′;
hGLI probe: 5′-CCAGGAATTTGACTCCCAAGAGCAGC-3′;
hNFATF: 5′-CAACACCAAAGTCCTGGAGATAC-3′;
hNFATR: 5′-CTCAATGTCGGCGTTTCTAAG-3′;
hNFAT probe: 5′-CATGAGGGCAACCATCGACTGTGC-3′;
hACTBF: 5′-GCCCATCTACGAGGGGTATG-3′;
hACTBR: 5′-GAGGTAGTCAGTCAGGTCCCG-3′;
hACTB probe: 5′-HEX-CCCCCATGCCATCCTGCGTC-3′.
The expression of miR-34b-3p was detected and quantified by Bulge-Loop™ miRNA qRT-PCR Primer Set (Ribobio) and quantified by PCR analysis based on SYBR Green. The Ct value of miRNA was quantified by 2−ΔΔCt method and then quantified as the Ct value of U6 RNA.
Western blot assay and antibodies
Cell lysate was incubated with 1× SDS buffer at 95°C for 10 minutes to facilitate the loading of samples for routine protein imprinting analysis [28]. Anti-CCND2 (10934-1-AP) and anti-P2RY1 (18273-1-AP) were purchased from San Ying, China. The target protein was then detected by anti-rabbit IgG peroxidase-binding antibody (SA00001-2). Enhanced chemiluminescence was used to detect the target band and Gel-Pro analyzer was used to quantitatively analyze the relative density of proteins on GAPDH band.
In vivo studies
A nude mouse xenograft model was established and analyzed according to the National Institutes of Health Guidelines for the Nursing and Use of Laboratory Animals. The analysis was carried out as previously reported [28]. The CCND2 and PYR1 protein expressions were detected by immunohistochemistry. The antigen was extracted by pretreatment dewaxing section and handled by the Super Sensitive Link-Labeled Detection System (Biogenex, Italy). The pictures were taken using a LEICA DM 4000B microscope. The animal research proposal was approved by IACUC of Anhui Medical University.
Nude mice were bought from Shanghai Slack Laboratory Animal Co., Ltd., and were sacrificed by euthanasia using CO2 inhalation. After the study, the animals were processed together by the IACUC.
Bioinformatics analysis
The key pathway genes served as querying genes to predict potential interactions in the GeneMANIA databases (http://genemania.org/). We used GeneMANI, which is a fast heuristic algorithm, to integrate functional associative networks containing all input genes. The shortest path among the genes was drawn from the network to simplify the model. The interactions reported were selected to gain the real function of each interaction in all query gene connection networks.
Statistical analysis
The data are presented as the means, and the error bars indicate the standard deviation (SD). The statistical analyses were performed using GraphPad Prism 5 and Excel. Statistical analysis among groups used the 2-tailed Student’s t-test, and one-way analysis of variance (one-way ANOVA). P<0.05 was considered statistically significant.
Results
MiR-34b-3p repressed the multidrug-chemoresistance of BCa
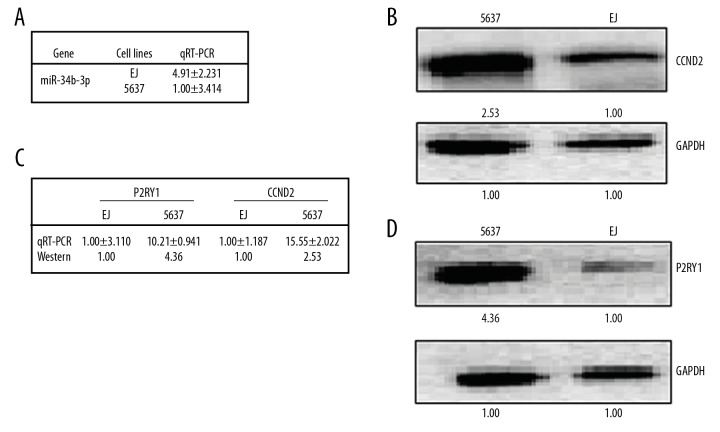
As shown previously, the cell lines of 5637 cells and EJ cells are multidrug-chemosensitive and multidrug-resistant BCa cell lines, respectively [5]. Thus, we used these 2 cell lines to study the potential mechanism of multidrug resistance in BCa cells. First, miR-34b-3p was selected as one of our targets, as it was previously reported to be regarded as a tumor suppressor [29]. At the same time, the expression of miR-34b-3p was 4.91-fold higher in EJ cells than in 5637 cells by qRT-PCR analysis (Figure 1A).
Figure 1.
Different expression patterns of miR-34b-3p/CCND2/P2RY1 in BCa cells 5637 and EJ. The relative miR-34b-3p levels (fold) in 5637 cells and EJ cells, as measured by real-time PCR analyses, are shown in A. The relative level (fold) of the CCND2 and P2RY1 genes in 5637 cells and EJ cells, as measured by western analyses, are shown in B–D, respectively. mRNA analyses are shown in table C. CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1; BCa – bladder cancer; PCR – polymerase chain reaction.
MiRNA always inhibits a target gene’s expression, then regulates downstream pathways. Therefore, based on the databases of TargetScan (http://www.targetscan.org/) and microRNA.org (http://www.microrna.org/microrna/getMirnaForm.do), we predicted the target genes of miR-34b-3p. Then we compared the expression patterns of common predictive RNA between 5637 cells and EJ cells by qRT-PCR and western blot analysis. The results showed that dozens of genes showed differential expression patterns in the 2 cell lines. Among them, CCND2 and P2RY1 genes were 2 of the most significantly differentially expression genes, and were negatively correlated with miR-34b-3p. The expression levels of CCND2 and P2RY1 were higher in 5637 cells than in EJ cells at the mRNA level (qRT-PCR analysis: 15.55: 1 for CCND2 and 10.21: 1 for P2RY1) and at the protein level (western blot analysis: 2.53: 1 for CCND2 and 4.36: 1 for P2RY1) (Figure 1B–1D). The lower expression of CCND2 and P2RY1 in multidrug-chemoresistant EJ cells suggests that CCND2 and P2RY1 are negative regulators of BCa multidrug-chemoresistance.
MiR-34b-3p targeted the CCND2 gene and P2RY1 gene in BCa cells
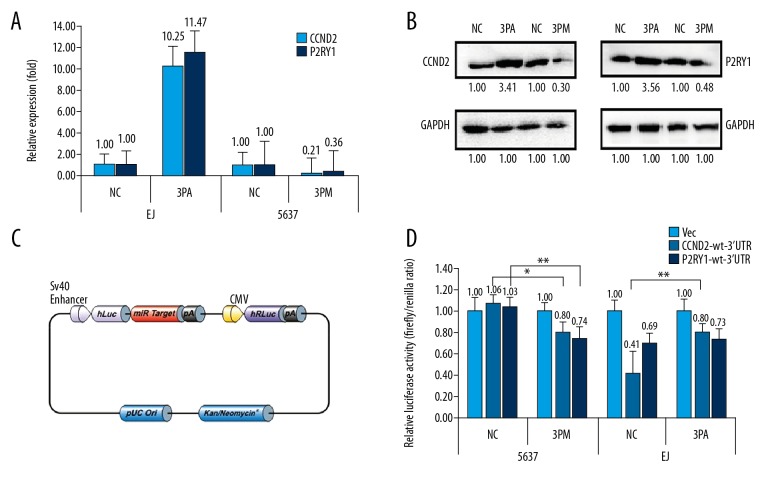
The miR-34b-3p level was significantly higher in EJ cells than in 5637 cells. In addition, we identified that CCND2 and P2RY1 negatively correlate with the level of miR-34b-3p in BCa cells. To check whether CCND2 and P2RY1 were the authentic targets of miR-34b-3p, we detected the levels of CCND2 and P2RY1 in the miR-34b-3p mimic-transfected 5637 cells or miR-34b-3p antagomiR-transfected EJ cells versus the NC (scramble sequence control)-transfected cells. MiR-34b-3p mimic transfection decreased the CCND2 mRNA to 21% and decreased the P2RY1 mRNA to 36% (Figure 2A). Accordingly, the protein levels of CCND2 and P2RY1 were decreased to 30% and 48%, respectively (Figure 2B), compared with those of the control cells. On the contrary, miR-34b-3p antagomiR transfection increased the mRNA levels of CCND2 and P2RY1 by 10.25-fold and 11.47-fold, respectively, (Figure 2A) and the protein levels by 3.41-fold and 3.56-fold, respectively, in EJ cells (Figure 2B).
Figure 2.
CCND2 and P2RY1 are direct targets of miR-34b-3p in BCa cells. Levels of CCND2 and P2RY1 mRNA (A) and protein (B) in miR-34b-3p mimic (3PM)-transfected 5637 cells and the miR-34b-3p antagomiR (3PA)-transfected EJ cells versus negative control (NC) cells, as determined by real-time PCR or western blot analyses. Vector information of CS-HmiT021075-MT01 used in the untranslated region (UTR) luciferase activities (C). Relative luciferase activities (fold) of the reporter with the wild-type (WT) CCND2-UTR/P2RY1-UTR or without the UTR (Vec) were determined in the BCa cells transfected with the 3PM (in 5637), 3PA (in EJ) or Mock (Vec) (D) sequences. The Renilla luciferase activity of a co-transfected control plasmid was used as a control for the transfection efficiency. Representative results from 3 independent experiments are shown. * P value <0.05, ** P value <0.01 by Student’s t-test. CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1; BCa – bladder cancer; PCR – polymerase chain reaction.
To further confirm whether CCND2 and P2RY1 were targets of miR-34b-3p, we cloned the downstream of either the WT CCND2 gene or P2RY1 gene into the pEZX-MT01-REPORT™ vector (GeneCopoeia™) to construct pEZX-MT01-REPORT™-CCND2 UTR WT and pEZX-MT01-REPORT™-P2RY1 UTR WT (Figure 2C). To determine the function of miR-34b-3p in different BCa cells, the constructs pEZX-MT01-REPORT™-CCND2 UTR WT or pEZX-MT01-REPORT™-P2RY1 UTR WT and pEZX-MT01-REPORT™ control were transfected into 5637cells and EJ cells, respectively. The pEZX-MT01-CCND2 UTR WT gave the relative luciferase activities of 1.06 and 0.41 in 5637 cells and EJ cells, respectively (Figure 2D). The transfection of miR-34b-3p mimic into 5637 cells reduced the luciferase activity to approximately 0.80 (Figure 2D). At the same time, the transfection of miR-34b-3p antagomiR into EJ cells raised the luciferase activity from 0.41 to 0.80 of the pEZX-MT01-CCND2 UTR WT construct (Figure 2D). Similar to CCND2, the transfection of miR-34b-3p mimic also decreased the luciferase activity of P2RY1 in 5637 cells, whereas the transfection of miR-34b-3p antagomiR increased the luciferase activity of P2RY1 in EJ cells (Figure 2D). In short, CCND2 and P2RY1 were the targets of miR-34b-3p and promote miR-34b-3p effect on BCa multidrug-chemoresistance.
CCND2 and P2RY1 negatively correlated with the miR-34b-3p effect on BCa drug resistance
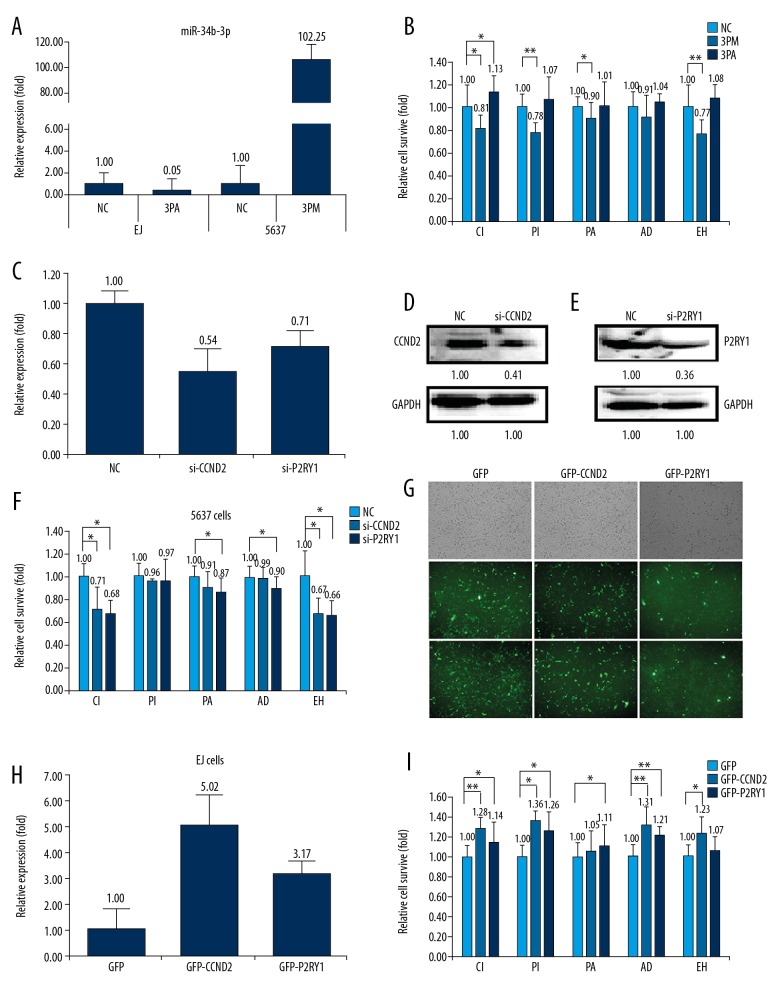
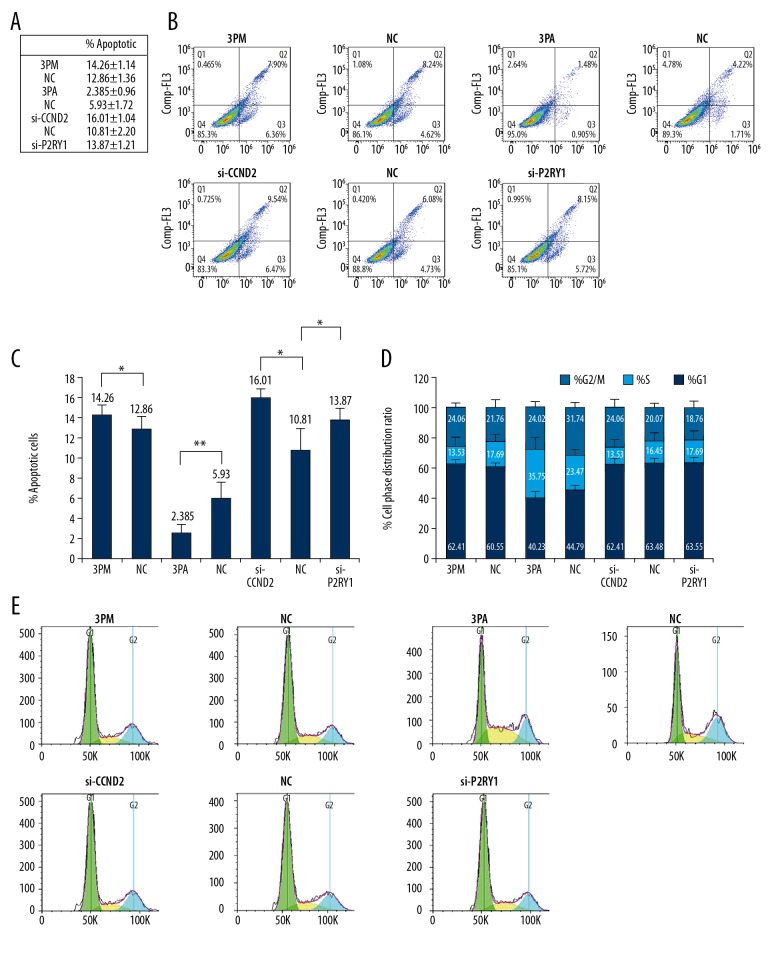
We further wanted to understand the roles of miR-34b-3p, as well as its target genes CCND2 and P2RY1 on BCa multidrug-chemoresistance. First, we transfected miR-34b-3p mimic into 5637 cells or miR-34b-3p antagomiR into EJ cells. Transfection of miR-34b-3p antagomiR into EJ cells decreased its expression to 0.05%, whereas transfection of miR-34b-3p mimic into 5637 cells dramatically increased its level to 102.25-fold (Figure 3A). Accordingly, the cell apoptosis rate, as indicated by IC50 values, was measured to check the effect of miR-34b-3p on BCa multidrug-chemoresistance. The results showed that transfection of miR-34b-3p mimic somewhat decreased the cell survival rate against the 5 drugs paclitaxel, pirarubicin, epirubicin hydrochloride, adriamycin, and cisplatin, indicating reduced chemoresistance (Figure 3B). By contrast, the transfection of miR-34b-3p antagomiR increased the cell survival rate to varying degrees (Figure 3B). Next, we detected the roles of CCND2 and P2RY1 in BCa multidrug-chemoresistance by transfection of siRNAs into 5637 cells. The transfection of si-CCND2 or si-P2RY1 significantly decreased both the mRNA (0.54: 1.00 for CCND2, 0.71: 1.00 for P2RY1) and protein levels (0.41: 1.00 for CCND2, 0.36: 1.00 for P2RY1) (Figure 3C–3E). In agreement with the lower levels of CCND2 and P2RY1, the transfection of either si-CCND2 or si-P2RY1 decreased the cell survival rate to varying degrees (Figure 3F). Thereafter, we increased the levels of CCND2 and P2RY1 by the overexpression of GFP-CCND2 or GFP-P2RY1 in EJ cells. The results showed that overexpression of GFP-CCND2 or GFP-P2RY1 increased the levels of CCND2 and P2RY1 by 5.02-fold and 3.17-fold, respectively (Figure 3G, 3H). In consensus with the elevated levels of CCND2 and P2RY1, the cell survival rate was slightly increased for the 5 drugs (Figure 3I). The results showed that CCND2 and P2RY1 negatively correlated with the miR-34b-3p effect on BCa drug resistance. According to negative effects on multidrug resistance, siRNA-mediated CCND2 and P2RY1 repression, and then increased the apoptotic cell rate from 10.81% to 16.01% for CCND2, and from 10.81% to 13.87% for P2RY1, indicating an decreased cell survival rate (Figure 4A–4C). A similar effect was also found in the miR-34b-3p mimic-transfected 5637 cells (Figure 4A–4C). By contrast, the transfection of miR-34b-3p antagomiR has a reverse effect, significantly decreasing the apoptotic cells from 5.93% to 2.39%. Taken together, the data revealed that miR-34b-3p and its target genes CCND2 and P2RY1 contribute to BCa multidrug-chemoresistance. FACS analysis of the cell cycle in 5637 cells showed that the percentage of G2/M cells significantly increased after miR-34b-3p mimic transfection or CCND2 and P2RY1 knockdown (Figure 4D, 4E). By contrast, the transfection of miR-34b-3p antagomiR in EJ cells has a reverse effect (Figure 4D, 4E).
Figure 3.
Effects of forced reversal of the miR-34b-3p or CCND2 and P2RY1 levels on the drug resistance of 5637 and EJ cells. Relative level of miR-34b-3p in the mimic (3PM)-transfected 5637 cells and miR-34b-3p antagomiR (3PA)-transfected EJ cells versus the negative control (NC) cells, as determined by real-time PCR (A). Cell death triggered by an IC50 dose of drug in 5637 and EJ cells and cells transfected with 3PM or 3PA versus the NC assayed 72 hours. after treatment with the IC50 dose of drugs (B). Relative levels of CCND2 and P2RY1 as determined by real-time PCR in siRNA-transfected 5637 cells versus NC-transfected cells (C). CCND2 and P2RY1 protein levels (western blot analysis) in siRNA-transfected versus NC-transfected 5637 cells (D, E). Cell survival of 5637 cells transfected with siRNA relative to that of NC-transfected 5637 cells 72 hours. after treatment with the IC50 dose of drugs (F). Expression of CCND2 and P2RY1 in overexpression constructs transfected into EJ cells and representative areas of EJ cells transfected with the GFP-CCND2/GFP-P2RY1 ectopic expression construct were shown, and GFP was used as a negative control (G). CCND2 and P2RY1 mRNA relative levels by real-time PCR in GFP-tagged overexpression construct-transfected versus NC-transfected EJ cells (H). Cell survival of EJ cells transfected with relative to the NC-transfected corresponding cells assayed 72 hours. Post-treatment with the IC50 dose of drugs (I). * P value <0.05, ** P value <0.01 by Student’s t-test. CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1; PCR – polymerase chain reaction.
Figure 4.
Effects of the forced reversal of the miR-34b-3p, CCND2 and P2RY1 levels on apoptosis (A–C) and the cell cycle (D, E) in 5637 and EJ cells, with a graph of the analyzed data and plots of the original FACS data. * P value <0.05 by Student’s t-test. CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1.
MiR-34b-3p repressed the tumor xenografts growth and paclitaxel chemoresistance
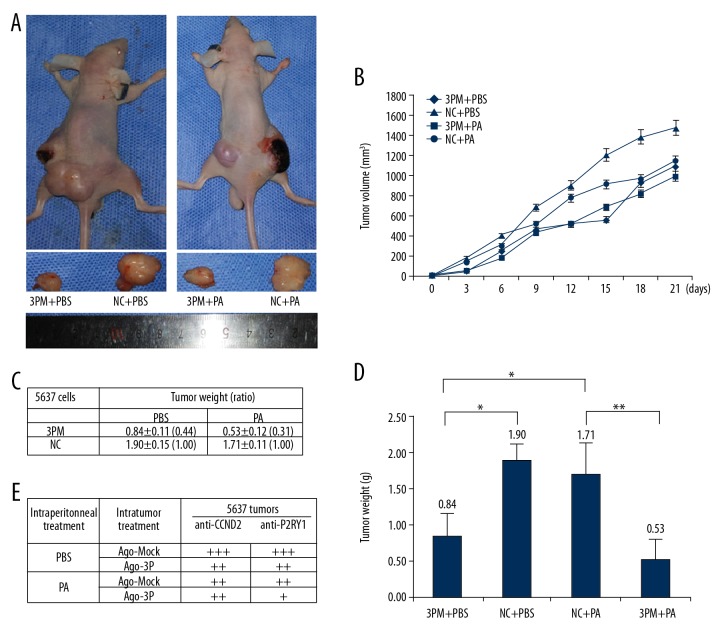
In vivo experiments were performed by the intratumoral injection of miR-34b-3p agomiR, Mock or PBS into 5637-derived tumors in nude mice. Transfection of miR-34b-3p agomiR into 5637-derived tumors decreased the tumor mass (Figure 5A, 5B). These results suggested that miR-34b-3p inhibits tumor growth in vivo. In addition, the tumor weight for the miR-34b-3p agomiR/paclitaxel in 5637 mice was lighter than that for the miR-34b-3p Mock counterpart, and the contrary was true for the paclitaxel treated group of 5637 cells with or without miR-34b-3p agomiR transfection (Figure 5C, 5D). These results showed that miR-34b-3p inhibits BCa drug resistance capability on paclitaxel.
Figure 5.
Effect of miR-34b-3p on the in vivo growth and paclitaxel drug resistance of 5637-derived xenografts in nude mice. (A) Image of representative mice with tumors on day 45. (B) Tumor volume of every step from intratumoral injection of the miR-34b-3p. (C, D) The mean ±SD of the tumor weight of the tumor for the same treatment was calculated, plotted (* P value <0.05), and summarized. (E) The protein levels of CCND2 and P2RY1 in each group were determined by immunostaining and are summarized in the table (magnification: 200×). * P value <0.05, ** P value <0.01 by Student’s t-test. SD – standard deviation; CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1.
Further investigation of the role of miR-34b-3p in paclitaxel resistance arose from the immunohistological analysis of CCND2 and P2RY1 in the tumor sections of the paclitaxel-treated versus PBS-treated mice (Figure 5E). Intratumoral injection of miR-34b-3p agomiR into 5637 cells decreased CCND2 and P2RY1 expression. The results again showed that miR-34b-3p had a meaningful negative effect on the growth of BCa cell-derived tumor xenografts in nude mice, and also had an obvious negative effect on the chemoresistance.
MiR-34b-3p regulated BCa multidrug resistance related chemoresistance signal transduction pathway
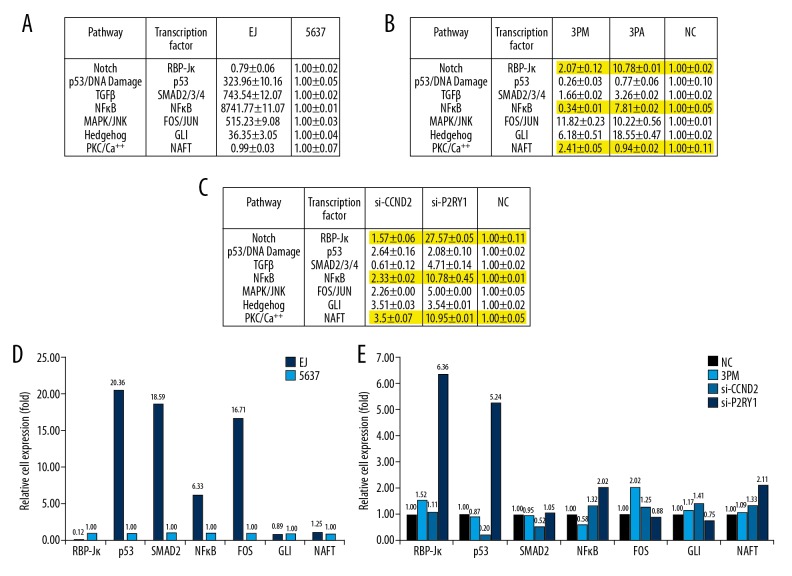
To further elucidate the molecular mechanism that governs BCa multidrug-chemoresistance, we determined the activities of the following 7 signaling pathways in 5637 cells versus EJ cells. The results showed that the activities of p53/DNA damage, TGFβ, NF-κB, MAPK/ERK, and Hedgehog were significantly upregulated in EJ cells compared with those in 5637 cells, whereas those of Notch and PKC/Ca++ were slightly lower in EJ cells than in 5637 cells (Figure 6A). Further transfection of miR-34b-3p mimic into 5637 cells showed that only 3 pathways: Notch, NF-κB, and PKC/Ca++ showed reverse effects compared with the transfection of miR-34b-3p antagomiR into EJ cells (Figure 6B–6E). Next, we downregulated the levels of CCND2 and P2RY1 by transfection of either si-CCND2 or si-CCND2 into 5637 cells. Only 2 pathways, Notch and PKC/Ca++, were upregulated, correlating well with the transfection of miR-34b-3p mimic into 5637 cells (Figure 6B–6E). The results strongly suggest that Notch and PKC/Ca++ pathways might be involved in miR-34b-3p-mediated BCa chemoresistance. Further studies are needed to elucidate the fine regulatory networks of BCa chemoresistance.
Figure 6.
Effects of the forced reversal of the miR-34b-3p, CCND2, and P2RY1 levels on the activity of the signaling pathways in EJ cells versus 5637 cells. (A) Relative activities of the 7 indicated pathways in EJ cells versus 5637 cells. (B) Relative pathway activities in the miR-34b-3p mimic (3PM)- or miR-34b-3p antagomiR (3PA)- versus the NC-transfected 5637 cells and EJ cells. (C) Relative pathway activities in the si-CCND2- or si-P2RY1- versus the NC-transfected 5637 cells. (D) The relative expression ratio of the 7 transcription factors in EJ cells and 5637 cells. (E) The relative expression ratio of the 7 transcription factors in the 3PM- or si-CCND2- or si-P2RY1- versus the NC-transfected 5637 cells (NC was normalized). CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1; PCR – polymerase chain reaction; NC, negative control.
Discussion
As well-studied miRNAs, the miR-34 family is thought to be a tumor suppressor [30]. MiR-34b has different expression modes and tissue-specific functions in many cancers [31]. For example, miR-34b expression is abnormal in many types of leukemias, such as acute myeloid leukemia [32], chronic lymphocytic leukemia [33], and acute promyelocytic leukemia [34]. Additionally, miR-34b-3p is downregulated in small cell lung cancer and significantly inhibits cancer cell aggressiveness [29]. Despite extensive studies of miR-34b-3p in various cancers, the involvement of miR-34b-3p and cancer chemoresistance remains unknown. In this study, we showed that miR-34b-3p was involved in the multidrug-chemoresistance of BCa. MiR-34b-3p was upregulated in multidrug-chemoresistant (EJ) BCa cells compared with multidrug-chemosensitive (5637) BCa cells (Figure 1).
By comparing the expression patterns of common predictive RNA between 5637 cells and EJ cells by qRT-PCR and western blot analysis, we found a group of genes that was differentially expressed, including the CCND2 gene and the P2RY1 gene, which negatively correlate with chemoresistance. Both the role and mechanism of the CCND2 gene and the P2RY1 gene in the context of BCa chemoresistance were systematically investigated in cultured cells and tumor xenografts in nude mice.
The CCND2 gene belongs to a highly conserved cyclin family, and its members have significant periodicity of protein abundances throughout the cell cycle [35,36]. Cyclin is a regulator of cyclin-dependent kinase (CDK) kinase. Different cyclins exhibit different expression and degradation patterns, which help to coordinate the time of each mitotic event. Cyclin forms a complex with CDK4 or CDK6, which acts as a regulatory subunit of the complex. Its activity is necessary for the G1/S transition of the cell cycle [35,36]. Our study results showed that CCND2 regulates the expression of different genes involved in many physiological functions. Another target gene, P2RY1, encodes a G-protein-coupled receptor in which adenine di-phosphate (ADP) is the physiological agonist [37,38]. P2RY1 couples to phospholipase C, which triggers Ca2+ release from the intracellular reservoir, leading to reversible platelet aggregation [39,40]. The P2RY1 polymorphism (C893T, rs1065776) was reported to be associate with an increase in aspirin resistance in Caucasian male patients and was found in patients with coronary artery disease on aspirin [41]. These results suggested that P2RY1 might also joined in the control of various physiological functions.
The transfection of either si-CCND2 or si-P2RY1 decreased the cell survival rate to varying degrees (Figure 3F). Thereafter, the overexpression of GFP-CCND2 or GFP-P2RY1 slightly increased the cell survival rate for the 5 drugs (Figure 3I). The results are closely related to the negative regulation of CCND2 and P2RY1 in the multidrug-chemoresistance of BCa cells. According to negative effects on multidrug resistance, siRNA-mediated CCND2 and P2RY1 repression, and then increased the apoptotic cell rate, indicating an decreased cell survival rate (Figure 4A). A similar effect was also found in the miR-34b-3p mimic-transfected 5637 cells (Figure 4A). By contrast, the transfection of miR-34b-3p antagomiR had a reverse effect, significantly decreasing the apoptotic cells. Taken together, the data revealed that miR-34b-3p and its target genes CCND2 and P2RY1 contribute to BCa multidrug-chemoresistance.
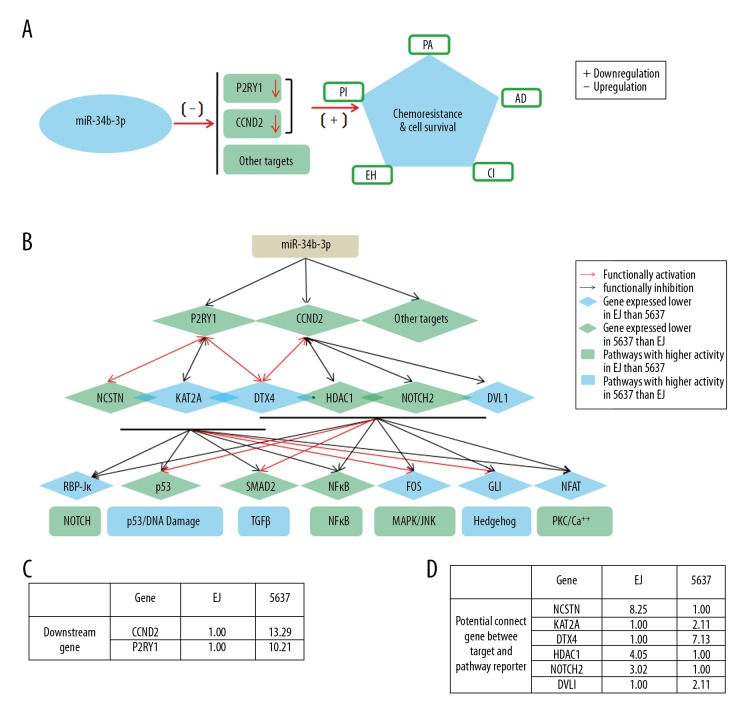
As summarized in Figure 7, we analyzed the gene networks that might dictate BCa chemoresistance. The results revealed a potential complicated regulatory network, and several potential genes were found that linked the target genes CCND2/P2RY1 and signaling pathways. Moreover, CCND2 and P2RY1 have a cross-talk effect that might act in concert to better coordinate the downstream pathways. However, the fine mechanism of the involvement of CCND2 and P2RY1 in cancer chemoresistance remains elusive. Here, we provide evidence of the relationship of CCND2 and P2RY1 with BCa chemoresistance. Moreover, these 2 genes are regulated by miR-34b-3p. Further studies are needed to elucidate the mechanism for this complicated regulation network.
Figure 7.
The working model for the role of miR-34b-3p in the regulation of the chemoresistance of BCa cells by repression of CCND2 and P2RY1. (A) The expression of CCND2 and P2RY1 genes under the negative regulation of miR-34b-3p is positively correlated with the chemoresistance to the 5 indicated chemotherapeutics. (B) Network model: the lines signify the potential interactions between genes obtained from GeneMANIA (http://genemania.org/). The green diamond nodes indicate genes expressed at a lower level in 5637 cells than in EJ cells; the green rectangle nodes indicate the pathways with higher activity in EJ cells than in 5637 cells. The blue diamond nodes indicate genes expressed at a lower level in EJ cells than in 5637 cells; the blue rectangle nodes indicate the pathways with higher activity in 5637 cells than in EJ cells. Seven pathways (transcription factor): Notch (RBP-Jκ), p53/DNA damage (p53), TGFβ (SMAD2/3/4), NFκB (NFκB), MAPK/JNK (FOS/JUN), Hedgehog (GLI), and PKC/Ca++ (NFAT). (C) The relative expression levels of the downstream genes CCND2 and P2RY1 are summarized in the table. (D) The relative expression levels of the potential connecting genes (NCSTN, KAT2A, DTX4, HDAC1, NOTCH2, and DVL1) between the targets and pathway reporter are summarized. BCa – bladder cancer; CCND2 – G1/S-specific cyclin-D2; P2RY1 – purinergic receptor P2Y1.
Conclusions
This study confirmed that CCND2 and P2RY1 are 2 target genes of miR-34b-3p, which have a negative regulatory effect on multidrug resistance of BCa. We conclude that the expression of miR-34b-3p in BCa cells might be a marker of chemotherapy resistance of BCa in patients. New therapies targeting miR-34b-3p and its target genes CCND2 and P2RY1 might provide strategies for overcoming BCa chemoresistance.
Abbreviations
- miR
microRNA
- BCa
bladder cancer
- UTR
untranslated region
- WT
wild-type vector
- PI
pirarubicin
- PA
paclitaxel
- AD
adriamycin
- EH
epirubicin hydrochloride
- CI
cisplatin
- CCND2
G1/S-specific cyclin-D2
- P2RY1
purinergic receptor P2Y1
Footnotes
Source of support: This work was supported by the Innovation Program of Science and Technology Bureau of Hefei (81572350 granted to CZL), and the Youth Technical Backbone Fund of West Branch of the First Affiliated Hospital of USTC (granted to YAT)
Conflict of interests
None.
References
- 1.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–26. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–38. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 3.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen KE, Weiss GJ. Resistance may not be futile: MicroRNA biomarkers for chemoresistance and potential therapeutics. Mol Cancer Ther. 2010;9:3126–36. doi: 10.1158/1535-7163.MCT-10-0397. [DOI] [PubMed] [Google Scholar]
- 5.Lv L, Deng H, Li Y, et al. The DNA methylation-regulated miR-193a-3p dictates the multi-chemoresistance of bladder cancer via repression of SRSF2/PLAU/HIC2 expression. Cell Death Dis. 2014;5:e1402. doi: 10.1038/cddis.2014.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin JJ, Briz O, Monte MJ, et al. Genetic variants in genes involved in mechanisms of chemoresistance to anticancer drugs. Curr Cancer Drug Targets. 2012;12:402–38. doi: 10.2174/156800912800190875. [DOI] [PubMed] [Google Scholar]
- 8.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–80. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Su SF, Chang YW, Andreu-Vieyra C, et al. MiR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene. 2013;32:4694–701. doi: 10.1038/onc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Li N, Wang H, et al. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep. 2012;28:592–600. doi: 10.3892/or.2012.1823. [DOI] [PubMed] [Google Scholar]
- 12.Song B, Wang Y, Xi Y, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–74. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corney DC, Flesken-Nikitin A, Godwin AK, et al. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–38. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 15.He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–34. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–18. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Vogt M, Munding J, Gruner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Archiv. 2011;458:313–22. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 19.Chavan S, Bray F, Lortet-Tieulent J, et al. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Chang JS, Lara PN, Jr, Pan CX. Progress in personalizing chemotherapy for bladder cancer. Adv Urol. 2012;2012:364919. doi: 10.1155/2012/364919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng H, Lv L, Li Y, et al. The miR-193a-3p regulated PSEN1 gene suppresses the multi-chemoresistance of bladder cancer. Biochim Biophys Acta. 2015;1852:520–28. doi: 10.1016/j.bbadis.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Deng H, Lv L, et al. The miR-193a-3p-regulated ING5 gene activates the DNA damage response pathway and inhibits multi-chemoresistance in bladder cancer. Oncotarget. 2015;6:10195–206. doi: 10.18632/oncotarget.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv L, Li Y, Deng H, et al. MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. Cancer Lett. 2015;357:105–13. doi: 10.1016/j.canlet.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Redwood SM, Liu BC, Weiss RE, et al. Abrogation of the invasion of human bladder tumor cells by using protease inhibitor(s) Cancer. 1992;69:1212–19. doi: 10.1002/cncr.2820690524. [DOI] [PubMed] [Google Scholar]
- 25.Heiser LM, Sadanandam A, Kuo WL, et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci USA. 2012;109:2724–29. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrisano V, Bartolini M, Gotti R, et al. Determination of inhibitors’ potency (IC50) by a direct high-performance liquid chromatographic method on an immobilised acetylcholinesterase column. J Chromatogr B Biomed Sci Appl. 2001;753:375–83. doi: 10.1016/s0378-4347(00)00571-5. [DOI] [PubMed] [Google Scholar]
- 27.Tarazona S, Garcia-Alcalde F, Dopazo J, et al. Differential expression in RNA-seq: A matter of depth. Genome Res. 2011;21:2213–23. doi: 10.1101/gr.124321.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu Y, Zhao F, Wang H, et al. MiR-34a-5p promotes the multi-drug resistance of osteosarcoma by targeting the CD117 gene. Oncotarget. 2016;7(19):28420–34. doi: 10.18632/oncotarget.8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno K, Mataki H, Arai T, et al. The microRNA expression signature of small cell lung cancer: Tumor suppressors of miR-27a-5p and miR-34b-3p and their targeted oncogenes. J Hum Genet. 2017;62:671–78. doi: 10.1038/jhg.2017.27. [DOI] [PubMed] [Google Scholar]
- 30.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–99. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Li X, Ge X, et al. MiR-34b-3 and miR-449a inhibit malignant progression of nasopharyngeal carcinoma by targeting lactate dehydrogenase A. Oncotarget. 2016;7:54838–51. doi: 10.18632/oncotarget.10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pigazzi M, Manara E, Baron E, Basso G. miR-34b targets cyclic AMP-responsive element binding protein in acute myeloid leukemia. Cancer Res. 2009;69:2471–78. doi: 10.1158/0008-5472.CAN-08-3404. [DOI] [PubMed] [Google Scholar]
- 33.Deneberg S, Kanduri M, Ali D, et al. MicroRNA-34b/c on chromosome 11q23 is aberrantly methylated in chronic lymphocytic leukemia. Epigenetics. 2014;9:910–17. doi: 10.4161/epi.28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng HY, Wan TS, So CC, Chim CS. Epigenetic inactivation of DAPK1, p14ARF, mir-34a and -34b/c in acute promyelocytic leukemia. J Clin Pathol. 2014;67:626–31. doi: 10.1136/jclinpath-2014-202276. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Zhu Y, Fan G, Hu S. MicroRNA-2682-3p inhibits osteosarcoma cell proliferation by targeting CCND2, MMP8 and Myd88. Oncol Lett. 2018;16:3359–64. doi: 10.3892/ol.2018.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu W, Liu Q, Pan J, Sui Z. MiR-373-3p enhances the chemosensitivity of gemcitabine through cell cycle pathway by targeting CCND2 in pancreatic carcinoma cells. Biomed Pharmacother. 2018;105:887–98. doi: 10.1016/j.biopha.2018.05.091. [DOI] [PubMed] [Google Scholar]
- 37.Volonte C, D’Ambrosi N. Membrane compartments and purinergic signalling: The purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009;276:318–29. doi: 10.1111/j.1742-4658.2008.06793.x. [DOI] [PubMed] [Google Scholar]
- 38.Smyth SS, Woulfe DS, Weitz JI, et al. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009;29:449–57. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- 39.Gremmel T, Yanachkov IB, Yanachkova MI, et al. Synergistic inhibition of both P2Y1 and P2Y12 adenosine diphosphate receptors as novel approach to rapidly attenuate platelet-mediated thrombosis. Arterioscler Thromb Vasc Biol. 2016;36:501–9. doi: 10.1161/ATVBAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanachkov IB, Chang H, Yanachkova MI, et al. New highly active antiplatelet agents with dual specificity for platelet P2Y1 and P2Y12 adenosine diphosphate receptors. Eur J Med Chem. 2016;107:204–18. doi: 10.1016/j.ejmech.2015.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timur AA, Murugesan G, Zhang L, et al. P2RY1 and P2RY12 polymorphisms and on-aspirin platelet reactivity in patients with coronary artery disease. Int J Lab Hematol. 2012;34:473–83. doi: 10.1111/j.1751-553X.2012.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]