Abstract
Background
Accumulating evidence suggests that plant-derived molecules may prove extremely beneficial in the development of chemotherapy for deadly cancer types. Multiple myeloma is a rare and incurable type of cancers. Very little research has been directed towards the development of chemotherapy for the management of multiple myeloma. Here, the anticancer effects of a plant-derived triterpenoid, Asiaticoside, were examined against the drug-resistant myeloma cell line KM3/BTZ.
Material/Methods
Cell viability was determined by CCK-8 assay and autophagy was checked by transmission electron microscopy. ROS levels were determined by flow cytometery. Cell migration and invasion were examined by Transwell assay. Protein expression was assessed by Western blotting.
Results
The results showed that Asiaticoside inhibits the growth of the KM3/BTZ cells and exhibited an IC50 of 12 μM. Further, it was observed that the anticancer effects of Asiaticoside are due to the induction of autophagy allied with upsurge of the expression of LC3-II. Moreover, the expression of the effector caspases in the KM3/BTZ cells was also altered. Asiaticoside also caused accretion of the ROS in the KM3/BTZ cells and inhibited their migratory and invasive properties via modulation of the STAT-3 signaling pathway.
Conclusions
Asiaticoside may prove useful in the management and treatment of the multiple myeloma and needs further investigation.
MeSH Keywords: Autophagy, Cell Migration Assays, Multiple Myeloma, Reactive Oxygen Species
Background
Multiple myeloma a rare but destructive type of cancer, characterized by unregulated division of clonal plasma cells [1]. The incidence of multiple myeloma is around 20 000 per year in the USA, and it still is considered an incurable disease. It causes hypercalcemia, kidney failure, and destruction of the bones [2]. The overall survival of multiple myeloma patients is just 7 years after diagnosis. The disease is often diagnosed at advanced stages and the chemotherapeutic agents used in treatment have a number of adverse effects [3]. Development of drug resistance in multiple myeloma cells also hinders the management of multiple myeloma [4]. Hence, there is a pressing need to find molecules that could halt the growth of plasma cells and at the same time exhibit minimal or no adverse effects. Plants are natural chemists with the capability to biosynthesize a wide array of chemical scaffolds [4]. Although these chemical scaffolds are synthesized by plants for their own defense, they have been used in their purest form for the alleviation of human aliments since early 19th century [5]. Tritepenoids are a large class of plant secondary metabolites that have been shown to exhibit tremendous pharmacological potential [6]. Asiaticoside is one such triterpenoid, generally extracted from the plant Centella asiatica [7]. Although the extracts of Centella asiatica have shown promising anticancer effects [8, 9], the anticancer effects of its principal component, Asiaticoside, have not been examined so far. Therefore, in this study, the anticancer effects of Asiaticoside were examined against the Bortezomib-resistant myeloma cell line KM3/BTZ. The results showed that Asiaticoside exerts considerable anti-proliferative effects on the KM3/BTZ cells, with an observed IC50 of 12 μM. The STAT3 signaling pathway is considered an important target for the management of several types of cancers as it is involved in the development and progression of several types of cancer [10]. Thus, Asiaticoside may prove essential in the development of chemotherapy for multiple myeloma.
Material and Methods
Chemicals and other reagents
Asiaticoside (purity >98%, determined by high-performance liquid chromatography), 3-(4, 5-dimethyl-2-thiazolyl) -2, 5-diphenyl-2H-tetrazolium bromide (MTT) were obtained from SigmaAldrich Chemical Co. (St. Louis, MO, USA). Annexin V-FITC and propidium iodide were purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Dulbecco’s modified Eagle’s medium (DMEM) and RPMI-1640 medium were purchased from HyClone (Logan, UT, USA). Fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Tianjin HaoYang Biological Manufacture Co., Ltd. (Tianjin, China). Horseradish peroxidase-labeled anti-mice and anti-rabbit secondary antibodies and all other antibodies were purchased from Cell Signaling Technology (MA, USA). Cell culture plasticware was purchased from BD Biosciences (San Jose, CA, USA).
Cell lines and cell culture conditions
The KM3/BTZ multiple myeloma cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in DMEM with l-glutamine supplemented with 10% fetal bovine serum (FBS) and 1.5% penicillin and streptomycin each. The MG-63 cells were grown in a highly humidified atmosphere with 5% CO2 at 37°C.
Cell counting kit-8 (CCK-8) assay
The multiple myeloma cell line KM3/BTZ cells in each group were inoculated in a 96-well plate, and subjected to treatment with Asiaticoside at various concentrations (0–100 μM) and the number of KM3/BTZ cells was measured at each concentration. The procedures were as follows: the culture medium was discarded and we add 100uLCCK-8 reagents (Beyotime Institute of Biotechnology (Shanghai, China) was added to a fresh medium. The 60-well plate was incubated in a carbon dioxide incubator for 2 h. The OD values were measured by a microplate reader at the wavelength of 450 nm. The cell proliferation rate (%) was calculated with (OD value of experimental well -OD value of control well)/OD value of control well ×100%.
Transmission electron microscopy (TEM)
For electron microscopy, the 0, 6, 12, and 24 μM Asiaticoside-treated cells were fixed in a solution of 4% glutaraldehyde 0.05 M sodium cacodylate, postfixed in 1.5% OsO4, and dehydrated in alcohol (70%). They were then prepared for flat embedding in Epon 812 and then observed using a Zeiss CEM 902 electron microscope.
Cell migration and invasion assay
The migration and invasion abilities of the 0, 6, 12, and 24 μM Asiaticoside-treated KM3/BTZ cells were examined by Transwell chamber assay. In brief, 1×104 KM3/BTZ cells were seeded in the upper chamber of the Transwell device (8-μm pore size polycarbonate filters). This was followed by the placement of the cells from the chambers into 24-well plates and subjected to incubation at 37°C for 48 h. However, for the invasion assay, the inserts were coated with extracellular matrix gel (50 μl) (ECM, Sigma, USA). Swabbing was performed to remove the non-migrated and non-invaded cells from the upper surface. However, the migrated and the invaded cells on the lower surface were subjected to fixation with 70% methanol for about 35 min, followed by staining with crystal violet (0.5%) for about 50 min, subjected to washing with PBS, and finally counted under a light microscope (5 fields).
Determination of the ROS levels
For determination of the endogenous ROS levels, the KM3/BTZ cells were treated with 0, 6, 12, and 24 μM concentrations of Asiaticoide or 12 μM of Asiaticoside for 0, 12, 24, and 48 h, and then the ROS levels in the KM3/BTZ cells were determined as described previously [10].
Western blotting
To determine the expression of the selected proteins in the Asiaticoside (0, 6, 12, and 24 μM)- treated KM3/BTZ cells, the cells were subjected to lysis with RIPA buffer and the protein content of each lysate was estimated by BCA assay. The 50-μg protein samples were then loaded on the SDS-PAGE. The gels were then transferred to nitrocellulose membranes and subjected to treatment with primary antibody [STAT-3 (cat. No. sc-293151), p-STAT-3 (cat. No. sc-56747) caspase 1 (cat. no. sc-622), caspase 2 (cat. no. sc-623), Actin (cat. no. sc-58673), and GAPDH (cat. no. sc-47724) obtained from Santa Cruz Biotechnology and light chain 3 (LC3)-I/II (cat. no. 12741), beclin-1 (cat. no. 3495) obtained from Cell Signaling Technology, dilution ratio 1: 1,000 for all antibodies)] at 4°C for 24 h. After this, the membranes were incubated with HRP-conjugated secondary antibody (cat. no. sc-2372) from Santa Cruz Biotechnology for 50 min at 25°C. Enhanced chemi-luminescence reagent was used to visualise the protein bands.
Statistical analyses
Data are shown as mean ±SD. Statistical analyses were done using the t test with GraphPad prism 7 software. Values of p<0.05 were taken as indicative of significant difference.
Results
Asiaticoside inhibits the growth of drug-resistant multiple myeloma cells
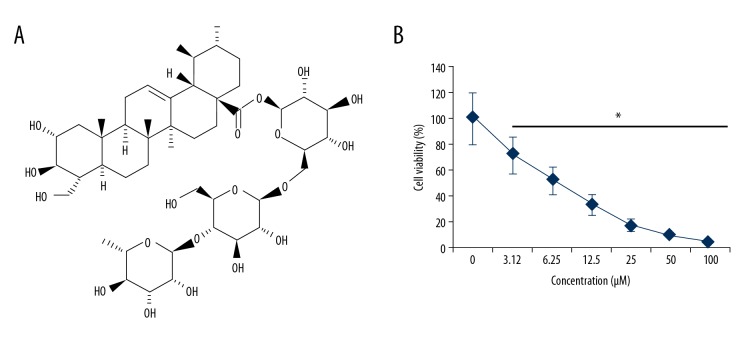
The effects of Asiaticoside (Figure 1A) on the proliferation of the drug-resistant multiple myeloma cells were examined on a panel KM3/BTZ and normal cell lines by CCK-8 assay. It was found that Asiaticoside exerts anti-proliferative effects on the Bortezomib-resistant KM3/BTZ cells. The IC50 of 12 μM was observed for Asiaticoside against the KM3/BTZ cell line. In addition, it was found that the anticancer effects of Asiaticoside on the drug-resistant multiple myeloma cells were concentration-dependent and the most effective was 24 μM (Figure 1B).
Figure 1.
(A) Structure of Asiaticoside. (B) Asiaticoside exhibits anti-proliferative effects on the KM3/BTZ cells, as depicted by CCK-8 assay. The experiments represent mean ±SD of 3 biological replicates (* p<0.05).
Asiaticoside induces autophagy in drug-resistant multiple myeloma cells
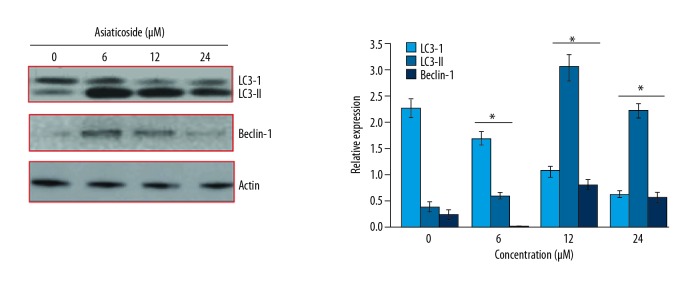
The effects of Asiaticoside on the KM3/BTZdrug resistant multiple myeloma cells were first evaluated by electron microscopy. It was found that Asiaticoside triggered the formation of autophagosomes in the KM3/BTZ drug-resistant multiple myeloma cells, indicative of autophagy (Figure 2). For the confirmation of autophagy, the expression of autophagy-associated proteins was examined and it was found that Asiaticoside caused an upsurge of Beclin-1 and LC3-I. However, no effects were observed on the expression of LC3-I (Figure 3). The effects of Asiaticoside were also examined on Caspase-1 and Caspase-2 expression. It was found that although the expression of Caspase 1 and 2 remained more or less unaltered, the expression of cleaved capase-1 increased and that of cleaved caspase-2 decreased concentration-dependently (Figure 4).
Figure 2.
Asiaticoside triggers autophagic cell death of the KM3/BTZ cells, as depicted by TEM. The experiments were performed in triplicate.
Figure 3.
Effect of Asiaticoside on the autophagy-associated protein expression, as depicted by Western blot analysis. The experiments represent mean ±SD of 3 biological replicates (* p<0.05).
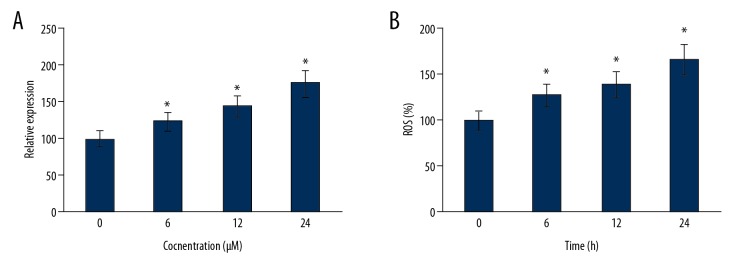
Figure 4.
Generation of ROS at different (A) concentrations of Asiaticoside and (B) time intervals, as determined by flow cytometry. The experiments represent mean ±SD of 3 biological replicates (* p<0.05).
Asiaticoside triggers the generation of the endogenous ROS
The effect of the Asiaticoide was also examined on the endogenous ROS levels of KM3/BTZ cells. The results showed that the levels of the endogenous ROS increased both concentration- and time-dependently in the KM3/BTZ cells upon treatment with Asiaticoside (Figure 5A, 5B).
Figure 5.

Effect of Asiaticoside on the effector caspase expression of the KM3/BTZ cells, as depicted by Western blot analysis. The experiments were performed in triplicate.
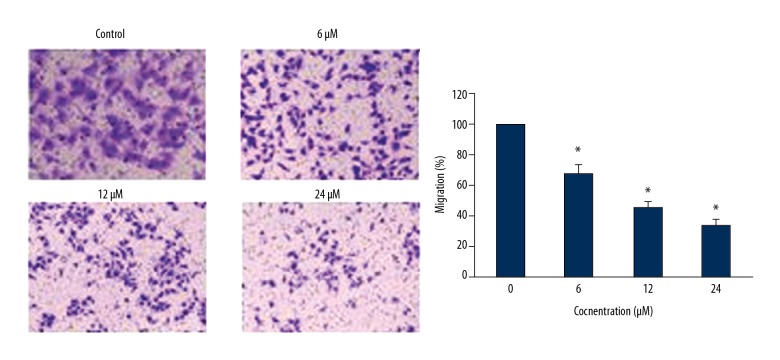
Asiaticoside inhibits the cell migration of KM3/BTZ cells
The effects of Asiaticoside on the migration and invasion of the KM3/BTZ cells were checked by Transwell chamber assays. It was found that Asiaticoside treatment inhibited the migration of the cancer cells in a dose-dependent fashion (Figure 6).
Figure 6.
Asiaticoside inhibits the migration of the KM3/BTZ cells, as depicted by Transwell assay. The experiments represent mean ±SD of 3 biological replicates (* p<0.05).
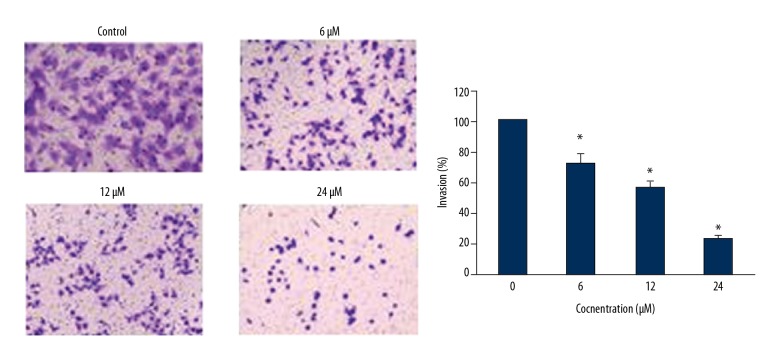
Asiaticoside inhibits the invasion of KM3/BTZ cells
The effects of Asiaticoside on the invasion of the KM3/BTZ cells were checked by Transwell chamber assays. It was found that Asiaticoside treatment suppressed the invasion of cancer cells in a dose-dependent manner (Figure 7).
Figure 7.
Asiaticoside inhibits the invasion of the KM3/BTZ cells as depicted by Transwell assay. The experiments represent mean ±SD of 3 biological replicates (* p<0.05).
Asiaticoside inhibits the STAT-3 signaling pathway
The STAT3 signaling pathway is considered an important target for the management of several types of cancer, as it is involved in their development and progression. The effects of Asiaticoside were also examined on the STAT-3 signaling pathway of KM3/BTZ drug-resistant multiple myeloma cells. It was found that Asiaticoside caused a concentration-dependent decline in the expression of p-STAT3, but had no apparent effect on the expression of total STAT-3 in KM3/BTZ cells (Figure 8).
Figure 8.
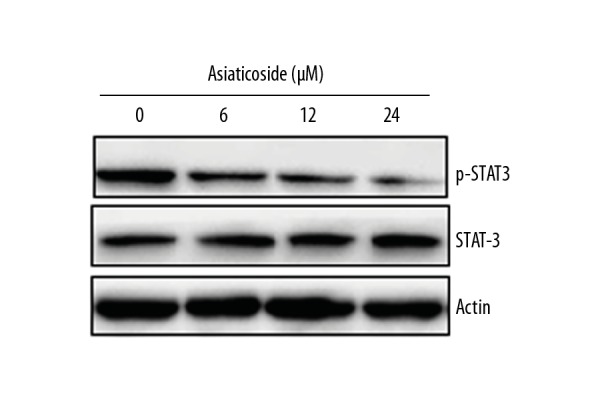
Effect of Asiaticoside on the phosphorylation of STAT-3 in the KM3/BTZ cells, as depicted by western blotting. The experiments were performed in triplicate.
Discussion
Multiple myeloma a rare but still incurable type of cancer [11]. It causes relapsing high calcium levels due to the destruction of bones, ultimately leading to renal failure [12]. In multiple myeloma, the plasma cells divide uncontrollably, leading to development of this devastating disease. Plant-derived anticancer agents have attained remarkable attention in the recent past due to their minimal toxic effects. Thus, more and more plant-derived natural products are being evaluated against cancer cells for their anticancer activity [13]. Herein, the anticancer effects of Asiaticoside were examined against multiple myeloma KM3/BTZ cell line cells. It was found that Asiaticoside caused a considerable decline in the viability of the multiple myeloma cells. In a previous study, the extract of Centella asiatica, which is the rich source of Asiaticoside, has also been reported to inhibit the growth of cancer cells [8,9]. Autophagy is a vital process that triggers death of the harmful cells and survival of the normal cells [14]. In this study, the investigation of the mechanism of action of Asiaticoside revealed that Asiaticoside prompts autophagy of the KM3/BTZ multiple myeloma cells. This was also associated with changes in the expression of autophagy as protein expression, especially LC3-II. Moreover Capsase-1 has been reported to promote autophagy of cancer cells [15], and in this study it was found that Asiaticoside increased the expression of Caspase-1. Moreover, ROS generation has been implicated in the onset of autophagy in cancer cells [10]. Herein, we observed that Asiaticoside triggered increased levels of ROS, both concentration- and time-dependently. Cell migration and invasion are important determinants of cancer cell metastasis [16] and it was found that Asiaticoside inhibited migration and invasion of KM3/BTZ cells concentration-dependently. It has been found that the STAT-3 pathway is activated in many cancer types, and this promotes cancer cell proliferation [10]. In this study, we found that Asiaticoside inhibited the expression of p-STAT-3 in KM3/BTZ cells concentration-dependently, indicating the anticancer potential of Asiaticoside.
Conclusions
Asiaticoside inhibits the proliferation of drug-resistant multiple myeloma cells by triggering autophagy and generation of ROS. It can also inhibit the migration and invasion of multiple myeloma cells via inhibition of the STAT-3 pathway. Hence, Asiaticoside may prove beneficial in the management of multiple myeloma and deserves further investigation.
Footnotes
Source of support: This study is funded by Shenyang Science and Technology project (F15-199-1-52)
Conflict of interest
None.
References
- 1.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 2.Pineli M, Pinho W, Amigo C, et al. Multiple myeloma: Epidemiology and burden of disease analysis in Latin America. Value in Health. 2017;20(9):A872. [Google Scholar]
- 3.Acquavella J, Garabrant D, Marsh G, et al. Glyphosate epidemiology expert panel review: A weight of evidence systematic review of the relationship between glyphosate exposure and non-Hodgkin’s lymphoma or multiple myeloma. Crit Rev Toxicol. 2016;46(Supp 1):28–43. doi: 10.1080/10408444.2016.1214681. [DOI] [PubMed] [Google Scholar]
- 4.Marinac CR, Birmann BM, Lee IM, et al. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: A prospective analysis in three large cohorts. Br J Cancer. 2018;118(7):1013–19. doi: 10.1038/s41416-018-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler MS. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25(3):475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 6.Li YX, Kim SK. Handbook of anticancer drugs from marine origin. Springer; Cham: 2015. Triterpenoids as anticancer drugs from marine sponges; pp. 15–27. [Google Scholar]
- 7.He L, Hong G, Zhou L, et al. Asiaticoside, a component of Centella asiatica attenuates RANKL-induced osteoclastogenesis via NFATc1 and NF-κB signaling pathways. J Cell Physiol. 2018 doi: 10.1002/jcp.27195. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Hamid IS, Widjaja NM, Damayanti R. Anticancer activity of Centella asiatica Leaves extract in benzo (a) pyrene-induced mice. Int J Pharmacog Phytochem Res. 2016;8(1):80–84. [Google Scholar]
- 9.Kaushik P, Kaushik PP. A comprehensive review on medicinal plants with anticancer activity. Global Journal of Pharmaceutical Education and Research. 2018;3(1–2) [Google Scholar]
- 10.Banerjee K, Resat H. Constitutive activation of STAT 3 in breast cancer cells: A review. Int J Cancer. 2016;138(11):2570–78. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang K, Wang W, Jin X, et al. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep. 2015;33(6):2711–18. doi: 10.3892/or.2015.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birmann BM, Andreotti G, De Roos AJ, et al. Young adult and usual adult body mass index and multiple myeloma risk: A pooled analysis in the International Multiple Myeloma Consortium (IMMC) Cancer Epidemiol Preven Biomarkers. 2017;2:5–9. doi: 10.1158/1055-9965.EPI-16-0762-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang TW, Weaver AL, Brewer JD, et al. Risk of melanoma in patients with multiple myeloma: a surveillance, epidemiology, and end results population-based study. J Am Acad Dermatol. 2018;78(3):621–23. doi: 10.1016/j.jaad.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Xie S, Zhou J. Harnessing plant biodiversity for the discovery of novel anticancer drugs targeting microtubules. Front Plant Sci. 2017;8:720. doi: 10.3389/fpls.2017.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenific CM, Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015;25(1):37–45. doi: 10.1016/j.tcb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabir MS, Ritchie ND, Li D, et al. Caspase-1 cleavage of the TLR adaptor TRIF inhibits autophagy and β-interferon production during Pseudomonas aeruginosa infection. Cell Host Microbe. 2014;15(2):214–27. doi: 10.1016/j.chom.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Yabasin IB, Sanches JG, Ibrahim MM, et al. Cisatracurium retards cell migration and invasion upon upregulation of p53 and inhibits the aggressiveness of colorectal cancer. Front Physiol. 2018;9:941. doi: 10.3389/fphys.2018.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]