Abstract
Background
The phosphatase actin regulator-1 (PHACTR-1) gene on chromosome 6 encodes an actin and protein phosphatase 1 (PP1) binding protein, Phactr-1, which is highly expressed in brain tissues. Phactr-1 expression is involved in physiological and pathological cerebral microvascular events. This study aimed to investigate the role of expression of Phactr-1 in a mouse brain capillary endothelial cell line, bEnd.3, by knockdown the PHACTR-1 gene.
Material/Methods
Three bEnd.3 cell groups were studied, CON (normal control cells), NC (control scramble transfected cells), and KD (cells with PHACTR-1 gene knockdown). The PHACTR-1 gene was knocked down using transfection with small hairpin RNA (shRNA). In the three cell groups cell proliferation, migration, and apoptosis were studied by MTT and colony formation assays, transwell and scratch assays, and flow cytometry. The related cell pathways of associated with Phactr-1 knockdown were studied by Western blot.
Results
Phactr-1 knockdown suppressed bEnd.3 cell proliferation and migration, promoted cell apoptosis, and downregulated the expressions of migration-associated proteins, including matrix metalloproteinase (MMP)-2 and MMP-9 and upregulated apoptosis-associated proteins, including Bax, Bcl-2, cleaved caspase-3, and caspase-3.
Conclusions
Phactr-1 was shown to have a role in the inhibition of endothelial cell proliferation and migration, promoted cell apoptosis, and regulated matrix metalloproteinases and apoptosis-associated proteins. These findings indicate that the expression of the Phactr-1 should be studied further in the cerebral microvasculature, both in vitro and in vivo, regarding its potential as a diagnostic and therapeutic target for cerebral microvascular disease.
MeSH Keywords: Acid Phosphatase, Actins, Apoptosis, Cell Migration Assays, Cell Proliferation, Endothelial Cells
Background
Angiogenesis is one of the most basic physiological processes occurring throughout life and leads to the formation of new blood vessels from existing endothelial cells, and promotes recovery from disease, including ischemic and hemorrhagic disease [1]. Angiogenesis is a complex process that requires a balance between pro-angiogenic and anti-angiogenic factors [2,3]. Changes in normal angiogenesis is associated with many disease processes, including tumor development, infarction, and hemorrhagic diseases [4].
The phosphatase actin regulator-1 (PHACTR-1) gene on chromosome 6 encodes the actin and protein phosphatase 1 (PP1) binding protein, Phactr-1, which is highly expressed in neurons and vascular endothelial cells [5]. Phactr-1 regulates the process of synaptic plasticity by modulating PP1 activity, which is critical for many neuronal functions [6]. In vascular endothelial cells, the expression of Phactr-1 regulates PP1 activity and F-actin expression during vascular remodeling, possibly through its actin-binding activity [7,8].
PP1 is a member of the phosphoprotein phosphatase superfamily of Ser/Thr-specific protein phosphatases [9]. Four PPI isozymes have been identified, including PP1α, PP1β, and splice variants PP1γ1 and PP1γ2. PP1 has key functions in a variety of cellular processes, including transcription and protein dephosphorylation, and also has neuroprotective functions during cerebral infarction and hemorrhagic traumatic brain injury as a major upstream regulator of cell apoptosis and cell survival pathways [10,11]. PP1 activity has a role in brain repair through modulation of JNK1/2, Erk1/2, and Bcl-related apoptotic and survival pathways [11]. PP1 also controls cell proliferation by dephosphorylation of downstream substrates and mediates ezrin/radixin/moesin dephosphorylation and apoptosis to protect primary mouse brain endothelial cells during aging, reducing breakdown of the blood-brain barrier and preventing neurocognitive impairment [12–14].
F-actin is biologically active actin that exists in various cells, including endothelial cells [15]. F-actin is involved in the maintenance of cell morphology and spatial structure, and also in like cell adhesion and cell transport [16]. The normal F-actin structure has been shown to be closely associated with cell migration in endothelial EA.hy926 cells in vitro [17]. The expression of Phactr-1 may be associated with the development of neurogenic and vascular disease.
Therefore, the aims of this study were to investigate the role of expression of Phactr-1 in a mouse brain capillary endothelial cell line, bEnd.3, by knockdown of the PHACTR-1 gene.
Material and Methods
Cell culture
Cells of the mouse brain vascular endothelial cell line, bEnd.3, were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 μg/mL penicillin at 37°C in an anaerobic chamber infused with a gas mixture consisting of 5% CO2 and 95% air. Six to eight cell passages were used for all experiments. Three bEnd.3 cell groups were studied, CON cells (normal control cells), NC cells (control scramble transfected cells), and KD cells (cells with PHACTR-1 gene knockdown).
Lentiviral vector transfection with small hairpin RNAs (shRNAs)
The transfection induced knockdown of the PHACTR-1 gene in bEnd.3 cells with lentiviral vector-loaded PHACTR-1 small hairpin RNAs (shRNAs) designed by Shanghai Genechem Co., Ltd. (Shanghai, China). The sequences (PHACTR-1: 5′-ACTGGAACAGAGGAACATT-3′, Scramble sequence: 5′-TTCTCCGAACGTGTCACGT-3′) were used as the target sequence and scrambled control, respectively. The sequences were cloned into the pGV248 lentiviral vector. The recombinant lentiviral plasmid and two plasmid vectors, pHelper 1.0 and pHelper 2.0, were co-transfected into 293T cells. The medium was changed 8 h following transfection. The viral supernatants were collected and filtered at 48 h after transfection. For lentiviral (LV)-shRNA transfection, 5×103 bEnd.3 cells were cultured in 96-well plates for transfection after 24 h. Different media, including DMEM, DMEM + polybrene, enhanced transfection solution (Eni.S), and Eni.S with polybrene, and different multiplicities of infection (MOIs) were tested to determine the optimal conditions for cell transfection. After 12 h following transfection, the different media were replaced with DMEM and then cultured for between 48–72 h at 37˚C in 5% CO2. The transfection efficiency was evaluated by observing green fluorescent protein (GFP) expression using a CKX41-A32PH fluorescence microscope (Olympus Corp., Tokyo, Japan) and then further examined by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot. In this study, the cells studied included the three groups, CON, NC, and KD.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from bEnd.3 cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). After measurement of the RNA concentration using a NanoDrop 1000 spectrophotometer (ThermoFisher, Wilmington, DE, USA). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using a One-Step SYBR® PrimeScript™ PLUS RT-PCR Kit (Takara Bio Inc., Shiga, Japan). The ribosomal phosphoprotein large P0 (RPLP0) housekeeping gene was used. The primer sequences used to amplify the target genes were:
PHACTR-1, forward: 5′-GAGGCAAAGCAGAGAAGAGC-3′;
PHACTR-1, reverse: 5′-CATGATGTCTGACGGTTGGA-3′;
RPLP0, forward: 5′-CATTGCCCCATGTGAAGTC-3′;
RPLP0, reverse 5′-GCTCCCACTTTGTCTCCAGT-3′.
Relative mRNA expression levels were examined using the 7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Western blot
Total protein was extracted from bEnd.3 cells after cell lysis in lysis buffer. Following denaturation, aliquots containing equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% dried skimmed milk powder, the membranes were incubated overnight at 4°C with the following primary antibodies at 1: 1000 dilution: anti-Phactr-1 and anti-β-actin (Cat. No. ab229120, and ab8227), anti-MMP-2, anti-MMP-9, and anti-β-tubulin antibodies (Cat. No. ab92536, ab38898, ab15568), anti-Bax, anti-Bcl-2, and anti-GAPDH (Cat. No. ab32503, ab182858, and ab9485) (Abcam, Cambridge, UK), anti-cleaved caspase-3 and anti-caspase-3 (Cat. No. 9664 and 9665) (Cell Signaling Technology, Beverly, MA, USA).
The membranes were washed three times in 10 mM Tris-HCl buffer (pH 7.6) containing 150 mM NaCl and 0.05% Tween-20 for 10 min. The membranes were then incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (1: 5000 dilution) (Cat. No. ab6721) (Abcam, Cambridge, UK) for 1 h at room temperature, followed by incubation with enhanced chemiluminescence (ECL) reagent (Pierce, Rockford, IL, USA). The results were recorded using Quantity One image software (Bio-Rad, Hercules, CA, USA), and the relative intensity was evaluated by Gel-Pro Analyzer software (Media Cybernetics, Bethesda, MD, USA).
MTT colorimetric assay of bEnd.3 mouse brain capillary endothelial cells
Cells were seeded into 96-well plates at a density of 1,000 cells/100 μl per well, cultured for 24h, and transfected with LV- PHACTR-1-shRNA or LV-NC-shRNA. After 24, 48, 72, and 96 h of culture, 10 μl of MTT solution (5 mg/ml) (11465007001) (Sigma-Aldrich, St Louis, MO, USA) was added, and the cells were incubated at 37°C for 4 h. Then, 150 μl of dimethyl sulfoxide (DMSO) was added to terminate the reaction, and the plates were gently shaken for 10–15 min. The absorbance of the wells at 490 nm was measured in a Bio-Tek Elx800 absorbance microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The optical density (OD) using the OD490/fold method was used to evaluate the cell proliferation capacity, which was defined as the OD at 490 nm of each time point/OD at 490 nm at the first 24 h.
Colony formation assay of bEnd.3 mouse brain capillary endothelial cells
Colony formation assays were performed to assess cell growth. Cells (8×103) of CON, NC, and KD groups were seeded into the wells of 6-well plates and incubated at 37°C in 5% CO2 for 96 h. The cells were then fixed with 4% paraformaldehyde for 30 min and stained with Giemsa (A0909-0010) (Applichem GmbH, Darmstadt, Germany) for 15 min. The cells were washed twice with double-distilled H2O, and the colony numbers and cell numbers were quantified using a CKX41-A32PH light microscope (Olympus Corp., Tokyo, Japan).
Transwell migration assay of bEnd.3 mouse brain capillary endothelial cells
Cell migration was analyzed using a Transwell Migration Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, 2×105 bEnd.3 cells from the CON, NC, and KD groups were suspended in 100 μl of serum-free medium and seeded into the upper chambers of 24-well plates containing transwell polycarbonate membrane filters with an 8.0 μm pore size. The lower chambers were filled with 10% FBS-containing medium. Following cell culture for 24 h, the cells that had migrated into the membranes were fixed and stained with Giemsa solution, while the cells that had not migrated into the membranes were scraped with cotton tips. The migrated cells were observed and photographed under a CKX41-A32PH light microscope (Olympus Corp., Tokyo, Japan).
Wound healing assay of bEnd.3 mouse brain capillary endothelial cells
The bEnd.3 cells (2×105) of the CON, NC, and KD groups were seeded into the wells of 24-well plates and cultured overnight at 37°C under 5% CO2. A scratch was created using a sterile 200 μl pipette tip, followed by two washes with PBS. Images were captured at 0 and 24 h. Cell migration was observed and photographs were taken under a CKX41-A32PH light microscope (Olympus Corp., Tokyo, Japan). The results were quantified using Gel-Pro Analyzer software (Media Cybernetics, Bethesda, MD, USA).
Apoptosis assay of bEnd.3 mouse brain capillary endothelial cells using flow cytometry
Flow cytometry was performed to detect apoptosis of bEnd.3 cells. Following cell culture for 96 h, the cells in the CON, NC, and KD groups were washed with D-Hanks solution and digested with trypsinase. The digested cells were collected, washed twice with ice-cold PBS, suspended in 100 μl of binding buffer to 2×103 cells/μl with 5 μl of Annexin V and 5 μl of propidium iodide (PI), and incubated in the dark for 15 min at room temperature. A further 300 μl of binding buffer was added. All procedures were performed according to the manufacturer’s instructions of the E606336 apoptosis kit (Sangon Biotech, Shanghai, China). Flow cytometry was performed within 1 h using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) to measure the apoptosis rate as a percentage of the cell population.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 19 software (SPSS Inc., Chicago, IL, USA). Histograms were created using GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA). Data were presented as the mean ± standard deviation (SD) for at least five repeated individual experiments in each group. The statistical significance of differences in data between groups was determined by one-way analysis of variance (ANOVA) (three groups) or the Student’s t-test (two groups) for independent samples. P-values <0.05 and p<0.01 were considered to represent statistical significance.
Results
Transfection efficiency of bEnd.3 cells with LV- PHACTR-1-shRNA and the results of quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot
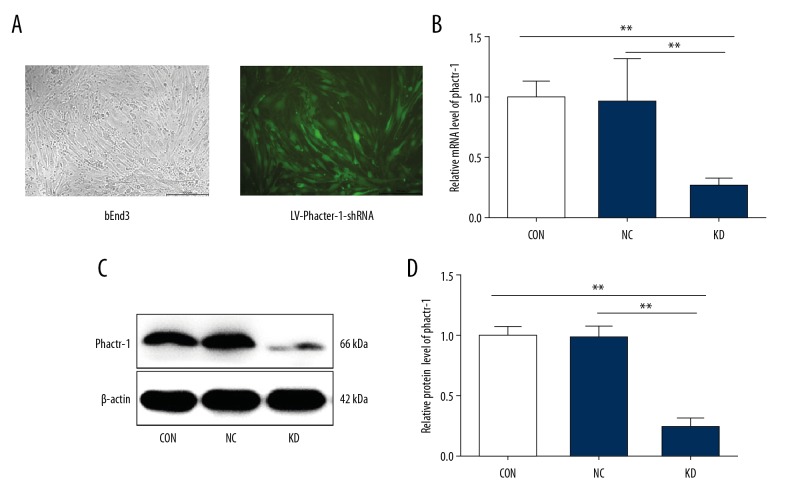
Three bEnd.3 cell groups were studied, CON (normal control cells) NC (control scramble transfected cells), and KD (cells with PHACTR-1 gene knockdown). A multiplicities of infection (MOI) of 10, cell culture in Dulbecco’s modified Eagle’s medium (DMEM), and a transfection time of 72 h were found to be most effective in bEnd.3 cells transfected by LV-PHACTR-1-shRNA, based on repeated experiments (Figure 1A). The knockdown efficiency of the PHACTR-1 gene at the mRNA level reached 75% by quantitative reverse transcription polymerase chain reaction (qRT-PCR) (p<0.01) (Figure 1B). Consistent with the qRT-PCR results, the expression of Phactr-1 in the KD group was significantly reduced compared with that in the other groups by Western blot analysis (p<0.01) (Figure 1C, 1D). These results demonstrated that highly efficient Phactr-1 knockdown was achieved after transfection.
Figure 1.
Transfection efficiency of bEnd.3 mouse brain capillary endothelial cells. (A) The transfection effects of bEnd.3 cells with LV-PHACTR-1-shRNA were observed using fluorescent microscopy. Magnification ×100. (B) Bar graphs showed the quantification of PHACTR-1 gene mRNA expression in CON, NC, and KD cell groups. (C) Protein expression of Phactr-1 detected by Western blot in the different cell groups. β-actin was used as the housekeeping protein. (D) Bar graphs show the quantification of Phactr-1 protein expression in CON, NC, and KD cell groups. Data are presented as the mean ±SD. ** P<0.01. shRNA – small hairpin RNA; CON – normal control cells; NC – control scramble transfected cells; KD – cells with PHACTR-1 gene knockdown.
Phactr-1 knockdown reduced bEnd.3 mouse brain capillary endothelial cell proliferation
The MTT and colony formation assays assessed cell proliferation. During the 96-hour observation period, the MTT assay showed that the OD490/fold in the KD group was significantly lower compared with the CON and NC groups from 72 h (p<0.01), indicating that the bEnd.3 cell proliferation decreased when Phactr-1 was suppressed. In the first 48 h, cell proliferation showed no significant difference between the three cell groups (p>0.05) (Figure 2).
Figure 2.
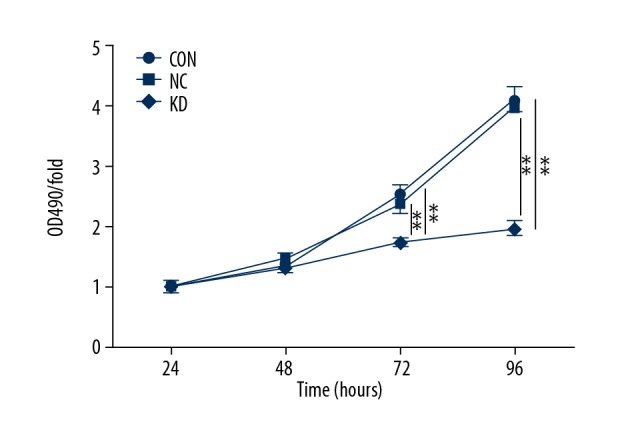
Proliferation of bEnd.3 mouse brain capillary endothelial cells by MTT assays in the CON, NC, and KD cell groups. Optical density (OD) 490. Data are presented as the mean ±SD. ** P<0.01. CON – normal control cells; NC – control scramble transfected cells; KD – cells with PHACTR-1 gene knockdown.
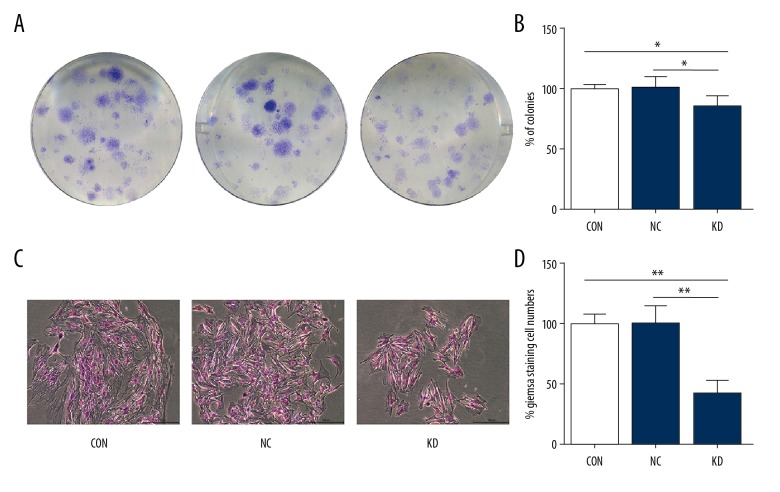
The results of the colony formation assays were observed at 96 h after shRNA transfection. The KD group had a significantly reduced number of colonies compared with the CON and NC groups (p<0.05) (Figure 3A, 3B). Also, the numbers of Giemsa-stained cells for each colony in the KD group were significantly less when compared with those in the CON and NC groups (p<0.01) (Figure 3C, 3D). Therefore, the MTT and colony formation assays showed that PHACTR-1 gene silencing in bEnd.3 cells significantly reduced cell proliferation.
Figure 3.
Proliferation of bEnd.3 mouse brain capillary endothelial cells by colony formation assays. (A) The results of the colony formation assays were observed using Giemsa staining in CON, NC, and KD cell groups. (B) Bar graphs showed the relative clone numbers of the CON, NC, and KD cell groups. (C) Cell numbers of each clone were detected by Giemsa staining in the CON, NC, and KD cell groups. Magnification ×100. (D) Bar graphs showed relative cell numbers determined by Giemsa staining of each clone in the CON, NC, and KD cell groups. Data were presented as the mean ±SD. * P<0.05, ** P<0.01. CON – normal control cells; NC – control scramble transfected cells; KD – cells with PHACTR-1 gene knockdown.
Phactr-1 knockdown reduced of bEnd.3 mouse brain capillary endothelial cell migration
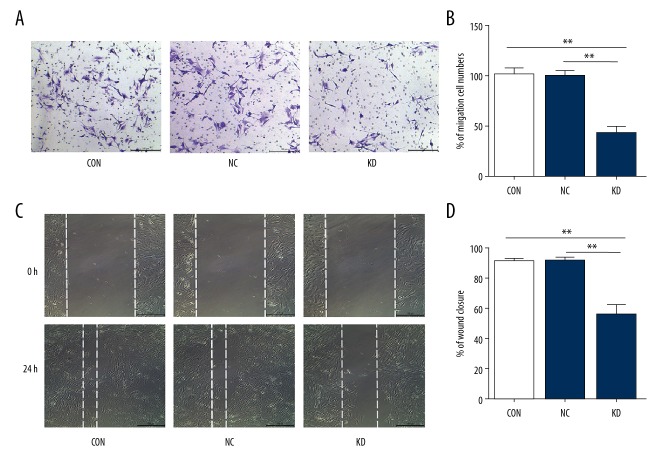
Transwell and wound healing assays were performed to investigate cell migration. Phactr-1 knockdown resulted in a significantly reduced number of migratory cells compared with the CON and NC groups in the transwell assay (p<0.01) (Figure 4A, 4B). Wound healing assays were performed at 24 h after scratch creation. Consistent with the results for the transwell assays, the migration rates were significantly lower in the KD group compared with the CON and NC groups (p<0.01) (Figure 4C, 4D). These results showed that Phactr-1 knockdown reduced endothelial cell migration.
Figure 4.
Cell migration of bEnd.3 mouse brain capillary endothelial cells. (A) Cell migration assays were performed in bEnd.3 cells of the CON, NC, and KD groups by a transwell assay. Magnification ×100. (B) Bar graphs show the relative migration cell numbers of the CON, NC, and KD cell groups. (C) Wound healing assay performed for bEnd.3 cells of the CON, NC, and KD groups. Magnification ×100. (D) Bar graphs showed the relative wound closure of the CON, NC, and KD groups. Data are presented as the mean ±SD. ** P<0.01. CON – normal control cells; NC – control scramble transfected cells; KD – cells with PHACTR-1 gene knockdown.
Phactr-1 knockdown promoted bEnd.3 mouse brain capillary endothelial cell apoptosis
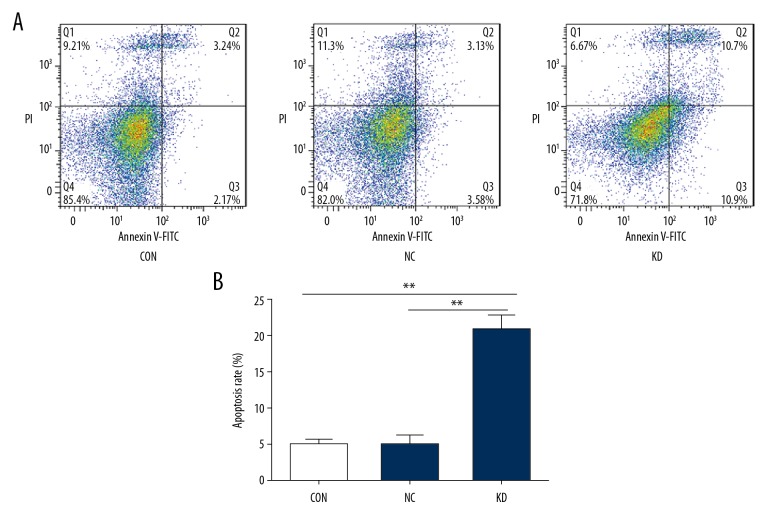
Flow cytometry was performed to determine the apoptosis rates of bEnd.3 cells before and after Phactr-1 suppression. The apoptosis rates in the CON, NC, and KD groups were 4.85±0.63%, 4.87±1.28%, and 20.67±1.99%, respectively. The results showed that the apoptosis rate differed significantly between the KD group and the other two groups (p<0.01) (Figure 5A, 5B). These findings indicated that Phactr-1 expression had a role in bEnd.3 cell apoptosis.
Figure 5.
Apoptosis of bEnd.3 mouse brain capillary endothelial cells. (A) Flow cytometry assays determined cell apoptosis of the CON, NC, and KD cell groups. (B) Bar graphs showed the apoptosis rate of the CON, NC, and KD cell groups. Data are presented as the mean ±SD. ** P<0.01. CON – normal control cells; NC – control scramble transfected cells; KD – cells with PHACTR-1 gene knockdown.
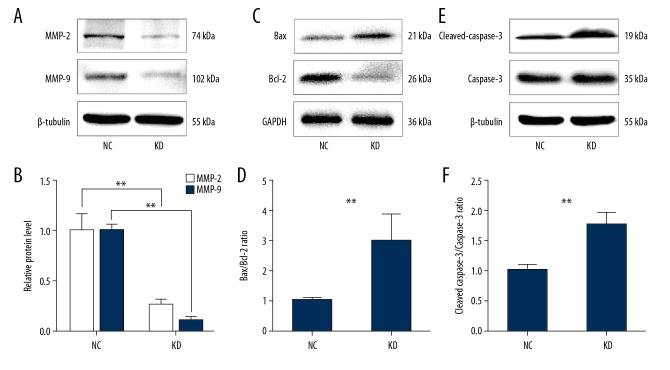
Phactr-1 knockdown suppressed the expression of migration-associated proteins and promoted the expression of apoptosis-associated proteins in bEnd.3 mouse brain capillary endothelial cells
To investigate the regulatory mechanisms of Phactr-1, the expression of migration-associated proteins were studied, including the matrix metalloproteinases (MMPs), MMP-2 and MMP-9, and apoptosis-associated proteins were studied, including Bax, Bcl-2, caspase-3, and cleaved-caspase-3 by Western blot. The results showed that MMP-2 and MMP-9 were significantly downregulated in the KD group compared with the NC group (p<0.01) (Figure 6A, 6B), while the Bax/Bcl-2 and cleaved caspase-3/caspase-3 ratios were significantly increased in the KD group compared with the NC group (p<0.01) (Figure 6C–6F). These findings indicated that Phactr-1 knockdown inhibited cell proliferation and migration and induced cell apoptosis in bEnd.3 cells and that the expression of Phactr-1 might have a role in the regulation of MMP-associated factors and the apoptosis pathway.
Figure 6.
The expression of MMP-2, MMP-9, Bax, Bcl-2, cleaved caspase-3, and caspase-3 in the bEnd.3 mouse brain capillary endothelial cells. (A, C, E) Western blot s for protein expressions of MMP-2, MMP-9, Bax, Bcl-2, cleaved caspase-3, and caspase-3 in the NC and KD cell groups. β-tubulin and GAPDH were used as the housekeeping proteins. (B, D, F) Bar graphs showed the quantification of MMP-2, MMP-9 protein expression and the Bax/Bcl-2 ratio and cleaved caspase-3/caspase-3 ratio in the NC and KD cell groups. Data are presented as the mean ±SD. ** P<0.01. NC – control scramble transfected cells; KD – cells with PHACTR-1 gene knockdown; MMP – matrix metalloproteinase.
Discussion
The phosphatase actin regulator-1 (PHACTR-1) gene on chromosome 6 encodes an actin and protein phosphatase 1 (PP1) binding protein and is a member of the newly characterized mammalian scapinin family [5,18]. Four isozymes, Phactr-1 to Phactr-4, encoded as transcript variants, have been identified in the scapinin family. Phactr-1 protein is selectively expressed in brain tissues, and high expression levels have been detected in the hippocampus, cortex, and striatum, with enrichment of the protein at neural synapses [5]. Expression of Phactr-2 protein has been shown to be limited to specific neurons, including those of the olfactory bulb, hippocampal nucleus, piriform cortex, CA3 area of the hippocampus, and Purkinje cell layer in the cerebellum [19]. Low expression levels of Phactr-3 protein have been shown to be present in the heart as well as in leukemia, melanoma, and lung cancer cells, and has also been shown to be enriched in normal human brain tissues [18,20]. High expression levels of Phactr-4 was found in proliferating neural stem/progenitors of adult brain tissues, and Phactr-4 mRNA has been shown to be highly expressed in the subventricular zone, subependymal zone, and dentate gyrus, which are areas of the brain that are enriched with neural stem cells and neuronal progenitor cells [19].
In the present study, the PHACTR-1 gene was knocked down to investigate its role in bEnd.3 mouse brain capillary endothelial cells. The findings of this study showed that bEnd.3 cells with Phactr-1 knockdown showed increased cell apoptosis, and decreased cell proliferation and migration. To our knowledge, this was the first study to show that the expression of Phactr-1 in bEnd.3 cells had an impact on endothelial cell function. Angiogenesis is known to be strongly dependent on apoptosis, proliferation, and migration of endothelial cells [21]. In previous studies, vascular endothelial growth factor (VEGF)-A165-induced PHACTR-1 gene binding to neuropilin-1 and vascular endothelial growth factor receptor 1 (VEGF-R1) was closely related to DR4/DR5/Fas/FADD/caspase-8/caspase-3 cell signaling, while Phactr-1 knockdown reduced the activity of protein phosphatase 1 (PP1) and abolished actin polymerization, resulting in impairment of cell dynamics [8,21].
This present study also investigated the changes in matrix metalloproteinase (MMP), MMP-2 and MMP-9 expression levels. Low levels of MMP-2 and MMP-9 have previously been reported to inhibit the migration of endothelial cells [22]. In the present study, the migration ability of bEnd.3 cells were reduced after knockdown of expression of Phactr-1 influenced the expression of MMP-2 and MMP-9. This study also we compared apoptosis-related protein ratios, Bax/Bcl-2 and cleaved caspase-3/caspase-3 [23], between the NC group and KD group. The high Bax/Bcl-2 and cleaved caspase-3/caspase-3 ratios in the KD group were likely to be the major reasons for the suppression of cell proliferation and promotion of cell apoptosis. These findings are consistent with previously published studies on primary human umbilical vein endothelial cells (HUVECs) [21,24]. These effects may lead to the suppression of endothelial cell proliferation and migration and promotion of cell apoptosis following Phactr-1 knockdown. Therefore, Phactr-1 should be studied further in both in vitro and in vivo studies to determine its role as a potential therapeutic target for vascular disease.
Debette and colleagues performed a genome-wide association study on 1,393 cases of cervical artery dissection cases and 14,416 control subjects [25]. These investigators examined six single nucleotide polymorphism (SNP) markers at the five most significantly associated loci in the genotype panel (P<1×10−5 in the genome-wide association study) and found that the rs9349379[G] allele (PHACTR-1) provided the most substantial evidence of an association in the genome-wide association data, and also showed a significant association with cervical artery dissection in the follow-up samples [25]. Also, the PHACTR-1 gene was previously shown to be associated with a lower risk of migraine and an increased risk of myocardial infarction [26–29]. However, the common pathological processes involved remain unclear. The key SNP variant, rs9349379, is intronic to the PHACTR-1 gene and was a risk locus for cervical artery dissection, migraine, and coronary artery disease [30]. The results of these previous studies suggest that different disease processes might be related to the gene polymorphisms of PHACTR-1 [31–34]. Therefore, the PHACTR-1 gene might not only have a role in angiogenesis of the cerebral microvasculature in vitro but might also have positive roles in vascular-associated events in vivo, such as cervical artery dissection, migraine, and myocardial infarction.
A previous study on breast cancer showed that the expression of transforming growth factor-β (TGF-β) could silence the expression of miR-584, resulting in enhanced Phactr-1 expression, and also resulted in actin re-arrangement and breast cancer cell migration [35]. These findings suggest that Phactr-1 expression may impact tumor behavior, and indicates that future studies on the role of this gene should include its effects in neoplasia.
Conclusions
The findings of this study showed that knockdown of Phactr-1 suppressed cell proliferation and migration and promoted cell apoptosis of bEnd.3 mouse brain capillary endothelial cells in vitro. Knockdown of the PHACTR-1 gene and its effects on microvascular endothelial cells might be associated with the expression of matrix metalloproteinases (MMPs), including MMP-2, MMP-9, and apoptosis-related pathways, including Bax/Bcl-2 and cleaved caspase-3/caspase-3. Although the precise mechanisms associated with Phactr-1 expression in endothelial cells remain to be elucidated, these preliminary in vitro findings suggest that Phactr-1 might have a regulatory role in the microvasculature, and possibly in brain-related vascular disease.
Footnotes
Source of support: National Nature and Science Foundation of China (No 81501048, 81671207, and 81471245)
Conflict of interest
None.
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–74. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–36. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Allen PB, Greenfield AT, Svenningsson P, et al. Phactrs 1–4: A family of protein phosphatase 1 and actin regulatory proteins. Proc Natl Acad Sci USA. 2004;101:7187–92. doi: 10.1073/pnas.0401673101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 7.Sagara J, Arata T, Taniguchi S. Scapinin, the protein phosphatase 1 binding protein, enhances cell spreading and motility by interacting with the actin cytoskeleton. PLoS One. 2009;4:e4247. doi: 10.1371/journal.pone.0004247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allain B, Jarray R, Borriello L, et al. Neuropilin-1 regulates a new VEGF-induced gene, PHACTR-1, which controls tubulogenesis and modulates lamellipodial dynamics in human endothelial cells. Cell Signal. 2012;24:214–23. doi: 10.1016/j.cellsig.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Verheyen T, Görnemann J, Verbinnen I, et al. Genome-wide promoter binding profiling of protein phosphatase-1 and its major nuclear targeting subunits. Nucleic Acids Res. 2015;43:5771–84. doi: 10.1093/nar/gkv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi KD, Chung J, Pang P, et al. Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci. 2005;25:7191–98. doi: 10.1523/JNEUROSCI.1328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedou GF, Koshibu K, Farinelli M, et al. Protein phosphatase 1-dependent bidirectional synaptic plasticity controls ischemic recovery in the adult brain. J Neurosci. 2008;28:154–62. doi: 10.1523/JNEUROSCI.4109-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Luo W, Howe PH. Dab2 stabilizes Axin and attenuates Wnt/beta-catenin signaling by preventing protein phosphatase 1 (PP1)-Axin interactions. Oncogene. 2009;28:2999–3007. doi: 10.1038/onc.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CY, Lv X, Li T, et al. PP1 cooperates with ASPP2 to dephosphorylate and activate TAZ. J Biol Chem. 2011;286:5558–66. doi: 10.1074/jbc.M110.194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park MH, Lee JY, Park KH, et al. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron. 2018;100(3):579–92. doi: 10.1016/j.neuron.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Heemskerk N, Schimmel L, Oort C, et al. F-actin-rich contractile endothelial pores prevent vascular leakage during leukocyte diapedesis through local RhoA signaling. Nat Commun. 2016;7:10493. doi: 10.1038/ncomms10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang DH, Lee JW, Lee J, et al. Dynamic rearrangement of F-Actin is required to maintain the antitumor effect of trichostatin A. PLos One. 2014;9:e97352. doi: 10.1371/journal.pone.0097352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagat M, Grzanka D, Izdebska M, et al. Nornicotine impairs endothelial cell-cell adherens junction complexes in EA.hy926 cell line via structural reorganization of F-actin. Folia Histochem Cytobiol. 2013;51:179–92. doi: 10.5603/FHC.2013.0026. [DOI] [PubMed] [Google Scholar]
- 18.Sagara J, Higuchi T, Hattori Y, et al. Scapinin, a putative protein phosphatase-1 regulatory subunit associated with the nuclear nonchromatin structure. J Biol Chem. 2003;278:45611–19. doi: 10.1074/jbc.M305227200. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Choi SY, Moon Y, et al. Different expression patterns of Phactr family members in normal and injured mouse brain. Neurosci. 2012;221:37–46. doi: 10.1016/j.neuroscience.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 20.Bankovic J, Stojsic J, Jovanovic D, et al. Identification of genes associated with non-small-cell lung cancer promotion and progression. Lung Cancer. 2010;67:151–59. doi: 10.1016/j.lungcan.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Jarray R, Allain B, Borriello L, et al. Depletion of the novel protein PHACTR-1 from human endothelial cells abolishes tube formation and induces cell death receptor apoptosis. Biochimie. 2011;93:668–75. doi: 10.1016/j.biochi.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Gao X, Zhang T, Zeng X, et al. Effect of silencing lncRNATUG1 on rapamycin-induced inhibition of endothelial cell proliferation and migration. Exp Ther Med. 2018;16:1891–99. doi: 10.3892/etm.2018.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Gan D, Fan C, et al. The Secretion from neural stem cells pretreated with lycopene protects against tert-butyl hydroperoxide-induced neuron oxidative damage. Oxid Med Cell Longev. 2018;2018:5490218. doi: 10.1155/2018/5490218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarray R, Pavoni S, Borriello L, et al. Disruption of phactr-1 pathway triggers pro-inflammatory and pro-atherogenic factors: New insights in atherosclerosis development. Biochimie. 2015;118:151–61. doi: 10.1016/j.biochi.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Debette S, Kamatani Y, Metso TM, et al. Common variation in PHACTR-1 is associated with susceptibility to cervical artery dissection. Nat Genet. 2015;47:78–83. doi: 10.1038/ng.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freilinger T, Anttila V, De Vries B, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2013;44:777–82. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anttila V, Winsvold BS, Gormley P, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–17. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deloukas P, Kanoni S, Willenborg C, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiando SR, Tucker NR, Castrovega LJ, et al. PHACTR-1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLoS Genet. 2016;12:e1006367. doi: 10.1371/journal.pgen.1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gormley P, Winsvold BS, Nyholt DR, et al. Migraine genetics: From genome-wide association studies to translational insights. Genome Med. 2016;8:86. doi: 10.1186/s13073-016-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szpakowicz A, Kiliszek M, Pepinski W, et al. The rs12526453 polymorphism in an intron of the PHACTR-1 gene and its association with 5-year mortality of patients with myocardial infarction. PLoS One. 2015;10:e0129820. doi: 10.1371/journal.pone.0129820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matoo S, Fallah MS, Daneshpour MS, et al. Increased risk of CHD in the presence of rs7865618 (A allele): Tehran lipid and glucose study. Arch Iran Med. 2017;20:153–57. [PubMed] [Google Scholar]
- 34.Rodríguezpérez JM, Blachmanbraun R, Pomerantz A, et al. Possible role of intronic polymorphisms in the PHACTR-1 gene on the development of cardiovascular disease. Med Hypotheses. 2016;97:64–70. doi: 10.1016/j.mehy.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Fils-Aimé N, Dai M, Guo J, et al. MicroRNA-584 and the protein phosphatase and actin regulator 1 (PHACTR-1), a new signaling route through which transforming growth factor-β Mediates the migration and actin dynamics of breast cancer cells. J Biol Chem. 2013;288:11807–23. doi: 10.1074/jbc.M112.430934. [DOI] [PMC free article] [PubMed] [Google Scholar]