Abstract
Background
The long-term significance of radiological transmural response (TR) as a treatment goal at the first follow-up scan in small bowel Crohn’s disease (CD) has been previously shown. We examined the durability of a long-term strategy of treating to a target of radiological TR and the influence of baseline predictors on the maintenance of TR.
Methods
Small bowel CD patients between January 1, 2002, and December 31, 2014, were identified with serial computed tomography enterography (CTE)/magnetic resonance enterography (MRE) before and after initiation of therapy or on maintenance therapy. Overall TR (inflammatory lesions with/without strictures) w1as characterized by abdominal radiologists in up to 5 small bowel lesions per patient at each serial scan until last follow-up or small bowel resection, as response, partial response, or nonresponse. The rate of conversion between TR states and transition to surgery, including the effect of baseline patient/disease characteristics, was examined using a multistate model (mstate R-package).
Results
CD patients (n = 150, 705 CTE/MRE) with a median of 4 CTE/MRE during 4.6 years of follow-up, 49% with ileal-only distribution, had 260 examined bowel segments. Conversion from response to partial response/nonresponse was 37.4% per year of follow-up with no transitions seen directly from response to surgery. Current smoking status (hazard ratio [HR], 2.2; 95% confidence interval [CI], 1.1–4.3) and internal penetrating disease at baseline scan (HR, 2.2; 95% CI, 1.2–4.1) were associated with a 2-fold increased risk of transition from partial response/nonresponse to surgery.
Conclusions
Achievement and maintenance of radiological response is associated with avoidance of small bowel surgery. Continued follow-up with CTE/MRE is recommended to identify loss of response, especially in current smokers and patients with internal penetrating disease at baseline CTE/MRE.
Keywords: Crohn’s disease, small intestine/diagnostic imaging, x-ray computed tomography, magnetic resonance imaging, medication therapy management
INTRODUCTION
Crohn’s disease (CD) is a chronic immune-mediated condition of the gastrointestinal system characterized by a transmural inflammatory response that can lead to progressive damage with the development of strictures and/or fistulae that require surgical interventions and/or hospitalizations.1, 2 CD management driven by clinical symptoms is hampered by poor correlation between clinical symptoms and active small intestinal inflammation.3, 4 Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) are noninvasive cross-sectional imaging techniques that have been shown to be highly sensitive and specific in detecting inflammation in the small bowel.5–7 CTE and MRE can also provide a transmural pan-intestinal assessment of therapeutic response, and therefore often guide management decisions.5, 8–10
Radiological transmural response (TR) as a treatment goal at the first follow-up scan in small bowel CD has been shown to be associated with a reduced probability of CD-related surgeries, hospitalizations, and rescue corticosteroid usage.11 There is a paucity of data, however, regarding clinical outcomes of CD patients after the first follow-up imaging for TR. We examined the natural history of a long-term strategy of treating to a target of radiological TR, the durability of response, and the influence of baseline predictors on the maintenance of TR.
METHODS
We constructed a cohort of patients with established small bowel CD who had undergone serial CTE and/or MRE imaging at Mayo Clinic, Rochester, Minnesota, between January 1, 2002, and October 31, 2014. This was performed using a retrospective study design. These patients had either initiated medical therapy or were on stable maintenance therapy with at least 1 year of follow-up. In the study time period (2002–2014), no established protocol to perform a CTE/MRE as part of a treat-to-target approach was in place at our institution. Definitions for established CD and maintenance therapy were previously reported.11 The radiological response at the first follow-up CTE/MRE and association with long-term outcomes of rescue corticosteroids, hospitalizations, and surgeries were also reported in a prior publication.11
In this study, each inflammatory lesion (with/without associated stricturing or internal penetrating complication) was followed longitudinally over multiple scans beyond the first follow-up CTE/MRE until the last clinical follow-up or a small bowel resection occurred. Disease location and phenotype were defined based on the Montreal classification, whereas internal penetrating disease phenotype was defined as an abscess, inflammatory mass, and/or fistula, excluding patients with isolated perianal disease.
Scan Technique
Before CTE or MRE examinations, patients ingested approximately 1350cc of low-contrast barium solution (Volumen; Bracco Diagnostics, Princeton, NJ, USA), followed by 500cc of water over 60minutes before the scan.11 For CTE, iodinated contrast dye was given by intravenous injection using a weight-based protocol, typically at 4cc/s, followed 50 seconds later by scanning during the enteric phase (peak small bowel enhancement).10–13 The CTE were reconstructed with high spatial resolution (slice thickness≤ 3mm). MRE was performed with 1.5T magnets using an 8-channel phased array coil. Glucagon was administered intravenously to alleviate motion artifact from bowel peristalsis at the beginning of the MRE (0.5 mg) and before gadolinium enhancement (0.5mg). Precontrast sequences as part of the MRE included axial and coronal single-shot, fast-spin echo, and FIESTA (or TrueFISP) imaging. Postgadolinium contrast sequences included coronal 3D LAVA sequences and 2D SPGR and axial 3D LAVA images with fat saturation.
Image Analysis
Clinical information was extracted from the medical record by a gastroenterologist (P.D.) blinded to the radiological score. Radiologic scoring was performed by 5 of the co-authors with prior fellowship-level training in abdominal radiology (J.G.F., J.L.F., J.M.B., A.K., or S.P.S.), who were blinded to clinical data, using a standard reader’s manual to guide interpretation (Appendix 1). Images were evaluated using the clinical PACS system (GE Centricity, GE Healthcare). In each patient, radiologists were instructed to identify all small bowel segments with CT or MR evidence of inflammation, structuring, and fistulizing disease. The longest 5 inflamed small bowel segments were evaluated in each patient for characteristics including length (cm) and wall thickness (mm), hyperenhancement and comb sign, peri-enteric inflammation (mesenteric edema or inflammatory mass), and for characteristics of any associated stricture (including any proximal dilation).
Radiological Response
Individual lesions were compared with the characteristics of the same lesion on the CTE/MRE beyond the first follow-up scan. At the lesion level, inflammatory transmural response (TR) was first graded as improved, unchanged, or worsened lesion(s).11 Improvement required a decrease in enhancement or length of disease, without worsening of the other disease parameters of active inflammation (described above). Any increase in the score of any imaging parameter of active inflammation resulted in a “worsened” classification. Unchanged lesions were defined as those without worsening or improving inflammatory parameters.
At the patient level, inflammatory TR was classified as a responder if all lesions improved in the individual patient, nonresponder if any of the lesions worsened or a new lesion developed, and a partial responder if all lesions stayed the same without improvement/worsening or if some but not all lesions improved. Next, the stricture TR or change in degree of stenotic disease (including prestenotic dilation) of any small bowel strictures associated with inflammatory lesions was factored into a composite classification of TR as responders, partial responders, and nonresponders (Table 1). For example, a patient with 2 inflammatory lesions at the first follow-up (or second CTE/MRE), both of which improved at the third or subsequent CTE/MRE, would be classified as a “responder” at third or subsequent CTE/MRE. In the next step, we would account for associated stricture. If 1 of the lesions also had an associated stricture at first follow-up CTE/MRE (or second CTE/MRE), without prestenotic dilation (score of 1), that subsequently worsened at the third CTE/MRE to develop a prestenotic dilation (score 2 or 3), then the overall response would be downgraded from a “responder” inflammatory TR to a “partial response.”
Table 1:
Radiological Response Incorporating Inflammatory and Stricture Responsea
| Responder at Current Scan | Stricture Data at Current Scan | Partial Responder at Current Scan | Stricture Data at Current Scan | Nonresponder at Current Scan | Stricture Data at Current Scan | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||||||
| Stricture data at prior scan |
0 | R | R | P | P | Stricture data at prior scan |
0 | P | P | N | N | Stricture data at prior scan |
0 | N | N | N | N |
| 1 | R | R | P | P | 1 | P | P | N | N | 1 | N | N | N | N | |||
| 2 | R | R | P | P | 2 | P | P | P | N | 2 | N | N | N | N | |||
| 3 | R | R | R | P | 3 | P | P | P | P | 3 | N | N | N | N | |||
Abbreviations: N, nonresponse; P, partial response; R, response.
aThe first classification of response is based on inflammatory response. If a patient is a nonresponder by inflammatory classification, then regardless of stricture response (3→0), the patient would remain a nonresponder. If a patient is a partial responder by inflammatory classification and the associated stricture remains stable (same score) or improves, then patient stays a partial responder. If, however, a patient is a partial responder by inflammatory classification and the associated stricture worsens (higher score), then the patient would be reclassified as a nonresponder.
Statistical Analysis
Calculation of sample size was performed to investigate the association of baseline patient/disease characteristics with response status for an analysis comparing response vs partial/nonresponse.11 Assuming 50% prevalence, there was 80% power, for a 2-sided test of proportions in response (alpha = 0.05), to detect 0.21 vs 0.45 (odds ratio [OR], 3.0) with 124 patients.11 In this study, the start time for the study was the date of the first follow-up CTE/MRE. Patients were then followed until the time point when an individual patient underwent a small bowel resection for an inflammatory lesion alone or with an associated stricture or until the date of last known follow-up, if earlier than October 31, 2014.
Analyses were performed using a multistate model. This is a method of survival analysis that accounts for competing risks beyond the framework of the traditional Cox proportional hazards model. Multistate models (MSMs) have been utilized in cardiovascular clinical trials, liver cirrhosis, bone marrow transplant, breast cancer, cardiovascular revascularization outcomes, acute myeloid leukemia, psoriatic arthritis, and colon cancer.14–20 Analysis followed the methods found in Putter et al.,21 using the mstate package within the R statistical system.22
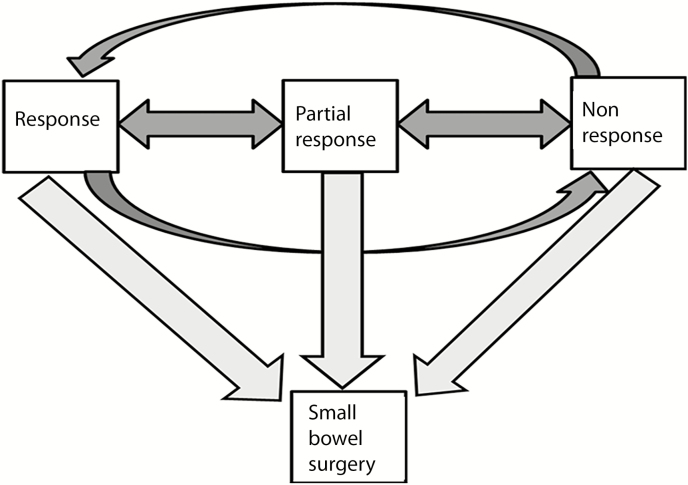
Multistate modeling is a continuous time process where subjects move among a finite number of states. Each subject’s initial state was defined to be the TR status at the first follow-up CTE/MRE. Subsequent states involve transitions from a response to a partial response or nonresponse state or vice versa (Fig. 1). Small bowel resection was defined to be a final or absorbing state from which a patient cannot transition back to any of the response states. The change in the states was evaluated using a time scale by the “clock reset” approach where the clock is reset to 0 each time the patient enters a new state. The transition rate between any 2 particular states assumes proportionality of hazards. The baseline covariates included in the model were sex, smoking (current vs former/never), penetrating disease at baseline scan, and history of perianal disease. An Aalen-Johansen estimate based on the fitted rates yielded further summaries such as the mean time in each state and the current proportion of individuals in each state.
FIGURE 1.
Four-state model of radiological transmural response identified on serial enterography in small bowel Crohn’s disease patients with 3 transient states (response, partial, or nonresponse) and an absorbent state (small bowel surgery).
ETHICAL CONSIDERATIONS
The study was approved by the Institutional Review Board of the Mayo Clinic at Rochester, Minnesota. We only included patients who had given authorization (at the original clinical encounter) to use their medical records for research purposes per the law of the State of Minnesota and HIPAA.
RESULTS
A total of 150 CD patients and 705 CTE/MREs were included, with a median follow-up (interquartile range [IQR]) of 4.6 (1.6–7) years after the first follow-up scan. The median age (IQR) at diagnosis was 23 (19–33) years, the median disease duration was 9 (3–21) years, and the female/male ratio was 1:1. Ileal distribution (n = 73, 49%) and nonstricturing/nonpenetrating phenotype (n = 73, 49%) were the most common locations or phenotypes. The distribution of baseline characteristics between responders vs partial/nonresponders is depicted in Table 2.
Table 2:
Baseline Characteristics of Patients With Small Bowel Crohn’s Disease Classified by Response at Second CT or MR Enterography
| Response (n = 55) | Partial/Nonresponse (n = 95) | |||
|---|---|---|---|---|
| Sex, No. (%) | Female | 27 (49.1) | 48 (50.5) | |
| Age at diagnosis, y | Median (IQR) | 24.0 (19.0–37.0) | 23.0 (19.0–32.0) | |
| Smoking, No. (%) | Current | 9 (16.4) | 12 (12.6) | |
| Never | 28 (50.9) | 56 (59.0) | ||
| Former | 18 (32.7) | 27 (28.4) | ||
| Disease duration, y | Median (IQR) | 9.0 (2.0–17.0) | 10.0 (4.0–22.0) | |
| Prior CD-related surgery, No. (%) | Yes | 33 (60.0) | 59 (62.1) | |
| Appendectomy, No. (%) | Yes | 18 (32.7) | 26 (27.4) | |
| BMI, kg/m2 | Median (IQR) | 25.0 (22.5–28.5) | 25.0 (22.1–28.7) | |
| Family history of IBD, No. (%) | Yes | 6 (10.9) | 19 (20.0) | |
| CRP, No. (%) | Missing | 14 | 24 | |
| High | 15 (27.3) | 33 (34.7) | ||
| Serum albumin, g/dL | Median (IQR) | 4.3 (3.9–4.5) | 4.0 (3.8–4.3) | |
| Montreal classification, No. (%) | Location | Ileal alone | 27 (49.1) | 46 (48.4) |
| Any upper gut disease | 5 (9.1) | 11 (11.6) | ||
| Any colonic disease | 23 (41.8) | 38 (40.0) | ||
| Stricturing phenotype | Nonstricturing | 35 (63.6) | 62 (65.3) | |
| B2 stricturing | 20 (36.4) | 33 (34.7) | ||
| Penetrating phenotype | Nonpenetrating | 48 (87.3) | 73 (76.8) | |
| B3 penetrating | 7 (12.7) | 22 (23.2) | ||
| Harvey Bradshaw Index | Median (IQR) | 6.0 (3.0–9.0) | 6.0 (3.0–9.0) | |
| Medication usage at second CTE/MRE, No. (%) | TNF-α inhibitor alone | 14 (25.5) | 16 (16.8) | |
| Thiopurine alone | 17 (30.9) | 37 (39.0) | ||
| Methotrexate alone | 2 (3.5) | 6 (6.3) | ||
| TNF-α inhibitor plus thiopurine | 16 (29.1) | 20 (21.1) | ||
| TNF-α inhibitor plus methotrexate | 3 (5.5) | 5 (5.3) | ||
| Budesonide alone or combination | 3 (5.5) | 9 (9.4) | ||
| Natalizumab | - | 2 (2.1) | ||
The median number of CTE/MREs was 4, and 142 patients (94.7%) underwent serial CTE/MREs after the first follow-up scan. A total of 260 lesions across 150 patients was present at the first follow-up CTE/MRE. The distribution of these lesions was: 209 inflammatory (80.4%), 135 with associated stricturing disease (51.9%), and 28 with penetrating disease (10.8%) (Table 3). Based on the first follow-up scan, 55 patients (37%) were responders, 39 were partial responders (26%), and 56 patients were nonresponders (37%), as previously reported.11 Based on the third CTE/MRE (Table 3), 44 patients (31%) were responders, 44 were partial responders (31%), and 54 patients were nonresponders (38%).
Table 3:
Radiological Response During Serial Enterography in Patients With Small Bowel Crohn’s Disease
| Scan No. | No. | Response Status | No. Lesionsa | Inflamm. Lesions, No. (%) | Stricturing Lesions, No. (%) | Penetrating Lesions, No. (%) | ||
|---|---|---|---|---|---|---|---|---|
| Response, No. (%) | Partial, No. (%) | Nonresponse, No. (%) | ||||||
| 2. | 150 | 55 (36.7) | 39 (26) | 56 (37.3) | 260 | 209 (80.4) | 135 (51.9) | 28 (10.8) |
| 3. | 142 | 44 (31) | 44 (31) | 54 (38) | 238 | 170 (71.4) | 119 (50) | 28 (11.8) |
| 4. | 108 | 36 (33.4) | 32 (29.6) | 40 (37.0) | 203 | 139 (68.5) | 103 (50.7) | 20 (9.9) |
| 5. | 73 | 16 (21.9) | 27 (37.0) | 30 (41.1) | 131 | 86 (35.7) | 67 (51.2) | 18 (13.7) |
| 6. | 42 | 9 (21.4) | 15 (35.7) | 18 (42.9) | 81 | 57 (70.4) | 36 (44.4) | 6 (7.4) |
| 7. | 20 | 4 (20) | 11 (55) | 5 (25) | 44 | 35 (79.6) | 21 (47.7) | 3 (6.8) |
| 8. | 8 | 1 (12.5) | 6 (75.0) | 1 (12.5) | 24 | 22 (91.7) | 12 (50.0) | 5 (20.8) |
| 9. | 5 | 1 (20.0) | - | 4 (80.0) | 16 | 14 (87.5) | 5 (31.3) | 8 (50) |
| 10. | 4 | 2 (50) | 2 (50) | - | 11 | 9 (81.8) | 2 (18.2) | 5 (45.5) |
| 11. | 2 | 1 (50) | 1 (50) | - | 7 | 7 (100) | - | 4 (57.1) |
| 12. | 1 | - | 1 (100) | - | 5 | 5 (100) | - | 3 (60) |
aIndividual lesion may have inflammatory, structuring, and penetrating phenotype.
The conversion from response state to partial/nonresponse state was 37.4% (95% confidence interval [CI], 25.6%–54.8%) per year of follow-up. The reverse occurred at 12.2% (95% CI, 7.8%–19.2%) per year of follow-up. No transitions were seen from treatment response (of all lesions) to surgery, whereas a transition from partial/nonresponse to surgery was seen in 16.7% (95% CI, 12.9%–21.7%) per year of follow-up. Patients spent a mean (95% CI) of 2.7 (1.8–3.9) years in the response state during follow-up, before transitioning to a different state. Those who had a partial/nonresponse state spent a mean (95% CI) of 3.4 (2.7–4.4) years before transition.
During follow-up, patients could go through multiple transitions. Figure 2 depicts serial CTE images in a patient without initial response who achieved it slowly over time. Figure 3 shows serial CTE images in a patient where the inflammatory lesion improved over time but developed a stricture that did not improve over time. In Figure 4, the serial MRE images depict a patient who had improvement in both the inflammatory lesion and the associated stricture.
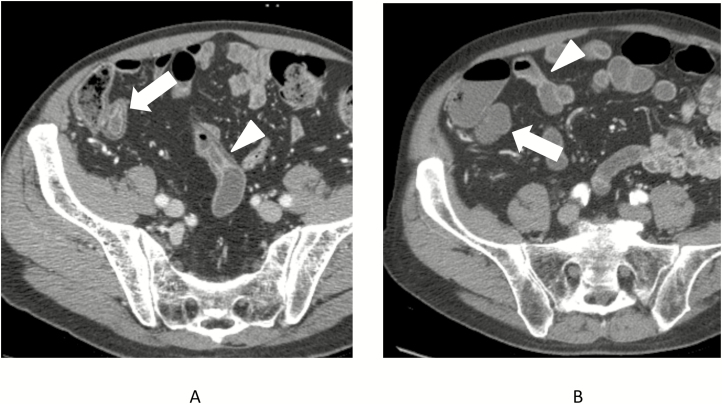
FIGURE 2.
Seventy-five-year-old male with CT enterography that demonstrated active inflammatory Crohn’s disease involving the terminal ileum (A, white arrow) and distal ileum over a distance of approximately 30 cm, with the proximal end of the inflamed segment also shown (A, arrowhead). The subsequent 2 CT enterography exams demonstrated no change, but subsequent CT enterography obtained 7 years after index scan show a normal-appearing terminal ileum (B, arrow) and equivocal enhancement and wall thickening (B, arrowhead) that involves only approximately 15 cm of the distal ileum in a discontinuous fashion.
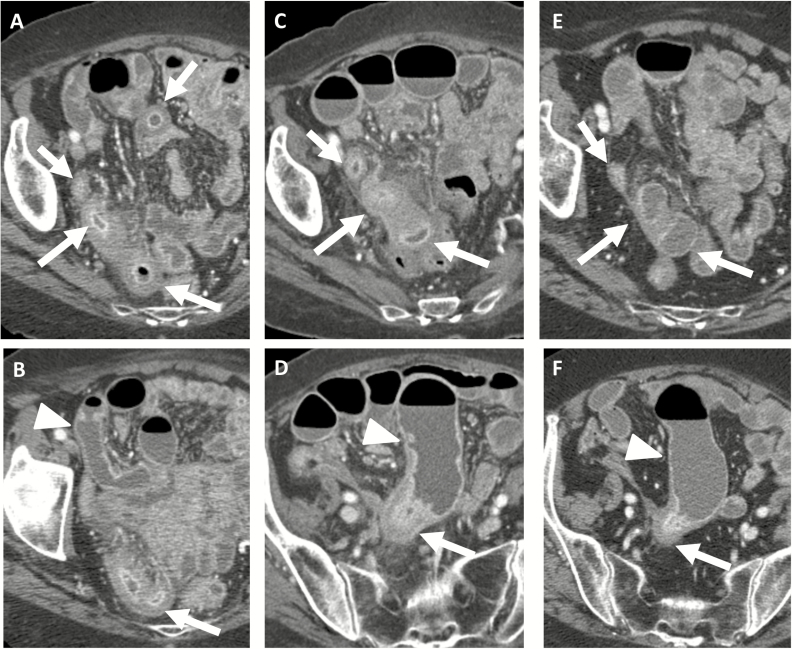
FIGURE 3.
Eighty-four-year-old female with CT enterography that demonstrated active inflammatory Crohn’s disease involving 25 cm of the distal ileum (A, white arrow) but without prestenotic dilation (B, arrowhead). Two years later, CT enterography demonstrates increasing ileal inflammation, as manifested by increasing wall thickness and peri-enteric stranding (C, arrows) with additional development of prestenotic dilation (D, arrowhead) indicating stricture development. Ten months later, repeat exam shows marked reduction in distal ileal inflammation (E, arrows) with persistent stricture (F, arrowhead).
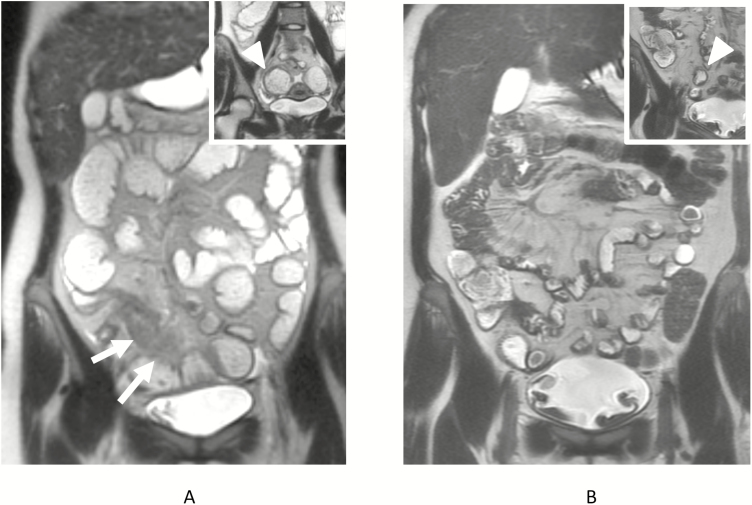
FIGURE 4.
Twenty-five-year-old female underwent MR enterography demonstrating marked inflammation in the 13 cm of neoterminal ileum (arrows, A) with stricture, as manifested by dilation of the proximal ileum to greater than 4 cm (arrowhead, inset A), and generalized proximal small bowel distension. Patient had 2 subsequent MR enterography exams, each showing decrease in length and severity of inflammation. A third subsequent MR exam performed 4 years later shows only 4 cm of neoterminal inflammation, with decrease in wall thickness as well (arrow, B), with minimal dilation of the proximal small bowel immediately proximal to the stricture (arrowhead, inset B) and normalization of jejunal and proximal ileal distension.
Summing up total time spent in the same state across multiple transitions, an average patient was in the “Response” state for 43.6% of their follow-up, with a mean time of 4.63 years in the response state and 5.98 years in the partial/nonresponse state. Among patients in the response state, after 1 year, 27.1% had converted to the partial/nonresponse state, and an additional 2.5% had converted to the surgery state (Table 4). After 2 years, 39.8% were in the partial/nonresponse state and 8.3% were in the surgery state.
Table 4:
Percentage of Patients at 1 and 2 Years From Entry Into Response and Partial/Nonresponse States
| Transition State | At 1 y | At 2 y | ||||||
|---|---|---|---|---|---|---|---|---|
| Response, % | Part/Non, % | Surgery, % | Response, % | Part/Non, % | Surgery, % | |||
| Overall | Response | 70.4 | 27.1 | 2.5 | 52.0 | 39.8 | 8.3 | |
| Partial/non | 8.8 | 76.5 | 14.6 | 13.0 | 61.0 | 26.0 | ||
| Sex | Female | Response | 66.6 | 30.6 | 2.7 | 47.7 | 43.7 | 8.6 |
| Partial/non | 10.5 | 75.9 | 13.5 | 15.0 | 60.9 | 24.1 | ||
| Male | Response | 75.8 | 22.0 | 2.2 | 58.9 | 33.8 | 7.3 | |
| Partial/non | 6.7 | 77.5 | 15.8 | 10.3 | 61.6 | 28.2 | ||
| Smoking status | Never/former | Response | 72.8 | 25.1 | 2.1 | 55.1 | 38.0 | 6.9 |
| Partial/non | 8.4 | 78.3 | 13.3 | 12.7 | 63.5 | 23.8 | ||
| Current | Response | 59.5 | 33.8 | 6.6 | 39.2 | 41.4 | 19.4 | |
| Partial/non | 11.0 | 63.0 | 26.1 | 13.4 | 43.3 | 43.2 | ||
| Internal penetrating disease | No | Response | 71.8 | 26.1 | 2.1 | 53.6 | 39.4 | 6.9 |
| Partial/non | 8.1 | 79.1 | 12.8 | 12.3 | 64.6 | 23.1 | ||
| Yes | Response | 60.2 | 33.4 | 6.4 | 41.0 | 40.4 | 18.6 | |
| Partial/non | 14.1 | 60.9 | 24.9 | 17.1 | 41.8 | 41.0 | ||
| History of perianal disease | No | Response | 70.8 | 26.9 | 2.3 | 52.4 | 40.0 | 7.6 |
| Partial/non | 8.4 | 77.9 | 13.6 | 12.6 | 63.0 | 24.4 | ||
| Yes | Response | 69.2 | 27.0 | 3.8 | 51.0 | 37.1 | 12.0 | |
| Partial/non | 11.3 | 68.2 | 20.4 | 15.5 | 49.6 | 34.8 | ||
Abbreviation: non, nonresponse.
Current smoking status influenced the transition from partial/nonresponse to surgery, which was 2-fold greater than that for never/former smokers (hazard ratio [HR], 2.2; 95% CI, 1.1–4.3; P = 0.02) (Table 5). Among current smokers in the response state, after 1 year, 33.8% had converted to the partial/nonresponse state and an additional 6.6% had converted to the surgery state (Table 5). After 2 years, 41.4% were in the partial/nonresponse state and 19.4% were in the surgery state. Current smoking status did not, however, have a significant effect on the rate of transition from response state to partial/nonresponse (HR, 1.7; 95% CI, 0.6–4.5; P = 0.28).
Table 5:
Hazard Ratios for Transition Between States by Baseline Covariate
| Covariate | Reference Category | Complete to Partial/Nonresponse, HR (95% CI) | Partial/Nonresponse to Complete, HR (95% CI) | Partial/Nonresponse to Surgery, HR (95% CI) |
|---|---|---|---|---|
| Male sex | Female sex | 0.7 (0.3–1.4) | 0.6 (0.2–1.5) | 1.2 (0.7–1.9) |
| Current smoking | Never/former | 1.7 (0.6–4.5) | 1.7 (0.4–6.4) | 2.2 (1.1–4.3) |
| Internal penetrating | Nonpenetrating | 1.6 (0.6–4.7) | 2.2 (0.7–7.6) | 2.2 (1.2–4.1) |
| Perianal disease | No perianal | 1.1 (0.4–2.8) | 1.5 (0.4–4.9) | 1.6 (0.8–3.1) |
Patients with internal penetrating disease at baseline scan also had a 2-fold greater transition from partial/nonresponse to surgery compared with those without internal penetrating disease (HR, 2.2; 95% CI, 1.2–4.1; P = 0.01). Internal penetrating disease at baseline did not have a significant effect on the rate of transition from response state to partial/nonresponse (HR, 1.6; 95% CI, 0.6–4.7; P = 0.36). Among patients in the response state and with internal penetrating disease at baseline scan, after 1 year, 33.4% had converted to the partial/nonresponse state and an additional 6.4% had converted to the surgery state (Table 5). After 2 years, 40.4% were in the partial/nonresponse state and 18.6% were in the surgery state.
Other clinical factors were not predictive in the multistate model. Male sex (compared with female) did not have a significant effect on the transition from response state to partial/nonresponse (HR, 0.7; 95% CI, 0.3–1.4; P = 0.29) or the transition from partial/nonresponse to surgery (HR, 1.2; 95% CI, 0.7–1.9; P = 0.6). A history of perianal disease also did not have a significant effect on the transition from response state to partial/nonresponse (HR, 1.1; 95% CI, 0.4–2.8; P = 0.85) or the transition from partial/nonresponse to surgery (HR, 1.6; 95% CI, 0.8–3.1; P = 0.2).
DISCUSSION
In this study, we examined the natural history of radiological TR in small bowel CD over an extended period of follow-up with serial monitoring of response using CTE/MRE. The study demonstrated that no patient who stayed in a state of response progressed to small bowel surgery. However, 37.4% of the patients experienced transition from a state of response to partial/nonresponse per year of follow-up. Additionally, current smoking and the presence of penetrating disease at baseline scan were associated with a 2-fold increase in a loss-of-response transition from a state of partial/nonresponse to small bowel surgery. The findings indicate the need for ongoing follow-up monitoring after patients have achieved a TR status of response, especially in current smokers and those with internal penetrating disease detected by CT or MR enterography.
The findings of this study build upon previous publications.11 Our prior study demonstrated that achievement of complete response (renamed in this study as response) decreased the risk of subsequent surgery by more than two-thirds (HR, 0.3; 95% CI, 0.2–0.6). In this study, we demonstrated that there was no direct transition from a state of response to small bowel surgery, unless the patient lost response and transitioned to a state of partial/nonresponse. The current study strongly suggests that maintaining radiological response is a powerful treatment target to avoid small bowel surgery on long-term follow-up. It also demonstrates the importance of continued monitoring of radiological TR with serial enterography to allow appropriate changes medical therapy to recapture response.
In our study, current smoking resulted in a 2-fold increase in a loss-of-response transition from partial/nonresponse state to surgery. These results are consistent with findings from a prospective cohort of 43 patients with symptomatic small bowel CD who commenced immunomodulator or biologic therapy and had assessment of mucosal healing (MH) at week 52 using capsule endoscopy.23 There was a trend toward less MH in those who smoked (OR, 2.5; P < 0.31). In this study, patients with internal penetrating disease at baseline scan also had a 2-fold greater transition from partial/nonresponse to surgery compared with those without internal penetrating disease. Our results are consistent with a prior study of 112 CD patients where the presence of intra-abdominal fistulas on baseline magnetic resonance imaging (MRI) was independently predictive (OR, 10.6; 95% CI, 2–46, P = 0.002) of an increased risk of abdominal resection surgery.24 Finally, among 67 patients with CD who were followed in a treat-to-target of MH, female sex was associated with a 2-fold increase in MH.25 This finding was not replicated in our study.
The significance of the study findings is the context of increasing recognition of radiological response as a treatment target. Mucosal healing noted on ileocolonoscopy has been proposed as a treatment target in CD patients associated with better outcomes (steroid-free remission, hospitalizations, or major abdominal surgeries) than those in CD patients who do not achieve MH.26–29 More recently, deep remission (Crohn’s disease activity index [CDAI] < 150, absence of corticosteroids, and MH) as a composite target in the adalimumab 52-week EXTEND maintenance trial (after induction) has been associated with significantly fewer adalimumab treatment adjustments, hospitalizations, CD-related surgeries, less activity impairment, and a better quality of life/physical function.30 However, the EMBARK study demonstrated that a combined scoring of disease activity using ileocolonoscopy (SES-CD) and CTE correlated much better with biomarkers of inflammation (fecal calprotectin, IL-22, and serum matrix metalloproteinase-9) than ileocolonoscopy alone.31 Additionally, a recent multicenter study (n = 214 CD patients) by the Grupo de Estudos de Doença Inflamatόria Intestinal compared outcomes with a target of transmural healing on MRE compared with MH on ileocolonoscopy.32 At 12 months, transmural healing, compared with MH, was associated with lower rates of therapy escalation (15.2% vs 36.5%, P = 0.03) and surgery (0% vs 11.5%, P = 0.047), and longer times to therapy escalation and surgery (P = 0.046 and P = 0.045, respectively). Moreover, multiple studies have now shown that small bowel inflammation is present in approximately 50% of patients with normal or nonspecific appearance to the ileal mucosa at ileocolonoscopy.22
Additionally, in a multicenter survey study (n = 477), patients have also expressed a preference for serial disease activity assessment in CD using MRE over repetitive colonoscopy.33 However, the cost-effectiveness of such a strategy of treating to a target of radiological response may be a concern. A decision analysis model that explored treatment strategies for the management of moderate to severe CD with infliximab showed that MH as an end point was a cost-effective strategy as compared with a strategy based on clinical symptoms.34 In population-based cohorts, however, up to one-third of patients with CD have evidence of bowel damage with stricturing or penetrating complications at diagnosis, findings that are underdiagnosed without the aid of cross-sectional imaging.2 Recent studies have also demonstrated that CD patients with worsening bowel damage on subsequent scans, measured by the Lemann Index, have worse outcomes, including an increased risk of surgery.35 There is precedence for radiological techniques being more cost-effective than endoscopic techniques in other gastrointestinal diseases. For example, imaging modalities have been shown to be more cost-effective than endoscopic methodologies in the evaluation of patients with suspected common bile duct stones.36 Similarly, a strategy of abdominal CT plus endoscopic ultrasound has been found to be the most cost-effective staging strategy for nonmetastatic proximal rectal cancer.37 Recent studies have also demonstrated bowel ultrasound to be equally sensitive for detecting active small bowel disease similar to CTE/MRE.38 This modality is less expensive compared with CTE/MRE, potentially performed at the bedside, and could be the cross-sectional imaging modality of choice at centers with the appropriate expertise.
Limitations of this study include the retrospective study design, which limited our ability to assess a specific medication or therapeutic plan. This study was designed to assess the changes in response over the period of follow-up, regardless of the medication used. In addition, this study was performed at a tertiary referral institution, potentially limiting the generalizability of the study findings to small bowel CD patients in community practice. No established clinical protocol was in place to perform a CTE/MRE as part of a treat-to-target approach during the study period (2002–2014). Hence, patients with more severe or active CD maybe over-represented in the data set due to clinicians ordering a CTE/MRE more often in such patients compared with those with milder disease. Additionally, the intervals between scans were also variable. However, we adjusted for both variable follow-up and variable intervals between scans using the advanced survival analysis technique of MSM. Finally, assessment of response was undertaken interchangeably with both CTE and/or MRE. However, a prior study comparing MRE and CTE has demonstrated similar sensitivities for detecting active small bowel CD.6, 39
In summary, the natural history of radiological TR with medical therapy varies dramatically in CD patients. Achievement and maintenance of radiological TR is associated with avoidance of small bowel surgery. Continued follow-up with CTE/MRE is recommended to identify loss of response, especially in patients with a history of current smoking or internal penetrating disease at baseline CTE/MRE. Further research is required to validate the findings of this study in a prospective cohort.
Supplementary Material
ACKNOWLEDGMENTS
Author contributions: Dr. Deepak was involved in the conception and design, collection, analysis, and interpretation of the data, drafting of the first draft of the article, and final approval of the article. Guarantor of the article: David H. Bruining, MD. Dr. Fletcher was involved in conception and design, collection, analysis, and interpretation of the data, initial drafting and critical revision of the article for important intellectual content, and final approval of the article. Dr. Fidler was involved in conception and design, collection and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Dr. Sheedy was involved in collection and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Dr. Kolbe was involved in collection and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Mr. Harmsen was involved in analysis and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Dr. Therneau was involved in analysis and interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Dr. Loftus was involved in interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Dr. Hansel was involved in interpretation of the data, critical revision of the article for important intellectual content, and final approval of the article. Ms. Becker was involved in collection of the data, critical revision of the article for important intellectual content, and final approval of the article. Dr. Bruining was involved in conception and design, study supervision, analysis and interpretation of the data, initial drafting of the article, critical revision of the article for important intellectual content, and final approval of the article.
APPENDIX: OVERVIEW
1 Scoring to be performed per patient and per lesion, of not more than 5 of the longest lesions identified on CTE or MRE.
2. Lesion to be identified on index CTE.
3. Skip lesion defined as 2 areas of active Crohn’s disease that must be separated by circumferential normal intestine.
4. Please note if surgery was performed in the interim in case a previously involved segment has disappeared/improved.
5. Location of segment involved (free text or selected): Series and image No.
6. Length of segment in centimeters.
7. Mural thickness in millimeters.
- 8. Enhancement defined in comparison with nearby small bowel, renal cortex, and vascular enhancement, as below:
- ◦ 0 = none (same as nearby, noncontracted normal small bowel segments);
- ◦ 1 = equivocal;
- ◦ 2 = mild (>nearby, noncontracted normal small bowel segments and less than renal cortex);
- ◦ 3 = moderate (similar to renal cortex);
- ◦ 4 = severe (greater than renal cortex and similar to intravascular enhancement in nearby vascular structures).
- 9. Stricture (obstruction) defined as a narrowed diameter in a distended bowel loop (that persists on multiple images when MRI is the modality):
- ◦ 0 = none;
- ◦ 1 = yes without upstream dilation;
- ◦ 2 = upstream dilation <4 cm;
- ◦ 3 = upstream dilation >4 cm.
10. Overall radiologist impression whether disease improved, unchanged, or worsened.
Sample Data Collection Sheet for All 5 Lesions per Scan for the Radiologist
MRN_____________Case number_________________
Patient last name _______________
Date of study ___________________
Type of study (circle answer): 1 = CTE 2 = MRE
| Lesion | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Location | |||||
| 1 = distal ileum | |||||
| 2 = proximal ileum | |||||
| 3 = jejunum | |||||
| Slice (representative) | |||||
| Length (record in cm) | |||||
| Mural thickness small bowel | |||||
| Enhancement | |||||
| 0 = none | |||||
| 1 = equivocal | |||||
| 2 = mild | |||||
| 3 = mod (renal cortex) | |||||
| 4 = severe (vessel) | |||||
| Comb sign | |||||
| 0 = none | |||||
| 1 = equivocal | |||||
| 2 = mild | |||||
| 3 = moderate | |||||
| 4 = severe | |||||
| Perienteric | |||||
| 1 = mesenteric edema | |||||
| 2 = phlegmon (inflammatory mass) | |||||
| 3 = abscess | |||||
| Type of fistula | |||||
| 0 = none | |||||
| 1 = enteroenteric | |||||
| 2 = enterovesical | |||||
| 3 = enterocutaneous | |||||
| Stricture (obstruction) | |||||
| 0 = none | |||||
| 1 = yes without upstream dilation | |||||
| 2 = upstream dilation ≤ 4 cm | |||||
| 3 = upstream dilation > 4 cm | |||||
| Radiologist impression | |||||
| 1 = improved | |||||
| 2 = unchanged | |||||
| 3 = worsened |
Mesenteric venous thromoses (circle): 0 = no; 1 = portal/SMV; 2 = peripheral (a or c). Perianal fistula: 0 = no; 1 = yes. If 5 lesions on index, new inflammatory lesions appearing on f/u scans? 0 = no; 1 = yes.
Abbreviations: a = acute; c = chronic.
Conflicts of interest: Dr. Parakkal Deepak: consultant for Pfizer; research support from Takeda. Dr. Fletcher: none. Dr. Fidler: none. Dr. Barlow: none. Dr. Sheedy: none. Dr. Kolbe: none. Scott Harmsen: none. Dr. Terry Therneau: none. Dr. Loftus: consultant for Janssen, Takeda, UCB, AbbVie, Amgen, Pfizer, Salix, Eli Lilly, Mesoblast, CVS Caremark; research support from Janssen, Takeda, UCB, AbbVie, Genentech, Pfizer, Amgen, Robarts Clinical Trials, Gilead, Receptos, Celgene, MedImmune, Seres. Dr. Hansel: none. Brenda Becker: none. Dr. Bruining: none.
Supported by: This publication was made possible by Centers for Translational Science Awards Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
REFERENCES
- 1. Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991;325:928–37. [DOI] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. . The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. . Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 4. Cellier C, Sahmoud T, Froguel E, et al. . Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. a prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siddiki HA, Fidler JL, Fletcher JG, et al. . Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. Am J Roentgenol. 2009;193:113–21. [DOI] [PubMed] [Google Scholar]
- 6. Lee SS, Kim AY, Yang SK, et al. . Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–61. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher JG, Fidler JL, Bruining DH, et al. . New concepts in intestinal imaging for inflammatory bowel diseases. Gastroenterology. 2011;140:1795–806. [DOI] [PubMed] [Google Scholar]
- 8. Deepak P, Fletcher JG, Fidler JL, et al. . Computed tomography and magnetic resonance enterography in Crohn’s disease: assessment of radiologic criteria and endpoints for clinical practice and trials. Inflamm Bowel Dis. 2016;22:2280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solem CA, Loftus EV Jr, Fletcher JG, et al. . Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255–66. [DOI] [PubMed] [Google Scholar]
- 10. Siddiki H, Fletcher JG, Hara AK, et al. . Validation of a lower radiation computed tomography enterography imaging protocol to detect Crohn’s disease in the small bowel. Inflamm Bowel Dis. 2011;17:778–86. [DOI] [PubMed] [Google Scholar]
- 11. Deepak P, Fletcher JG, Fidler JL, et al. . Radiological response is associated with better long-term outcomes and is a potential treatment target in patients with small bowel Crohn’s disease. Am J Gastroenterol. 2016;111:997–1006. [DOI] [PubMed] [Google Scholar]
- 12. Fletcher JG, Fidler JL, Huprich JE, et al. . Small-bowel imaging with CT and MR: overview of techniques and indications. Appl Radiol. 2012;41:18–24. [Google Scholar]
- 13. Schindera ST, Nelson RC, DeLong DM, et al. . Multi-detector row CT of the small bowel: peak enhancement temporal window–initial experience. Radiology. 2007;243:438–44. [DOI] [PubMed] [Google Scholar]
- 14. Keiding N, Klein JP, Horowitz MM. Multi-state models and outcome prediction in bone marrow transplantation. Stat Med. 2001;20:1871–85. [DOI] [PubMed] [Google Scholar]
- 15. Andersen PK, Esbjerg S, Sorensen TI. Multi-state models for bleeding episodes and mortality in liver cirrhosis. Stat Med. 2000;19:587–99. [DOI] [PubMed] [Google Scholar]
- 16. Putter H, van der Hage J, de Bock GH, et al. . Estimation and prediction in a multi-state model for breast cancer. Biom J. 2006;48:366–80. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Li Q, Rogatko A, et al. . Analysis of the bypass angioplasty revascularization investigation trial using a multistate model of clinical outcomes. Am J Cardiol. 2015;115:1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eefting M, de Wreede LC, Halkes CJ, et al. . Multi-state analysis illustrates treatment success after stem cell transplantation for acute myeloid leukemia followed by donor lymphocyte infusion. Haematologica. 2016;101:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen B, Yi GY, Cook RJ. Analysis of interval-censored disease progression data via multi-state models under a nonignorable inspection process. Stat Med. 2010;29:1175–89. [DOI] [PubMed] [Google Scholar]
- 20. Conlon AS, Taylor JM, Sargent DJ. Multi-state models for colon cancer recurrence and death with a cured fraction. Stat Med. 2014;33:1750–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. [DOI] [PubMed] [Google Scholar]
- 22. Mansuri I, Fletcher JG, Bruining DH, et al. . Endoscopic skipping of the terminal ileum in pediatric Crohn disease. Am J Roentgenol. 2017:W1–w9. [DOI] [PubMed] [Google Scholar]
- 23. Hall B, Holleran G, Chin JL, et al. . a prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601–9. [DOI] [PubMed] [Google Scholar]
- 24. Jauregui-Amezaga A, Rimola J, Ordás I, et al. . Value of endoscopy and mri for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut. 2015;64:1397–402. [DOI] [PubMed] [Google Scholar]
- 25. Bouguen G, Levesque BG, Pola S, et al. . Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:978–85. [DOI] [PubMed] [Google Scholar]
- 26. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 27. Baert F, Moortgat L, Van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–8; quiz e10. [DOI] [PubMed] [Google Scholar]
- 28. Schnitzler F, Fidder H, Ferrante M, et al. . Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 29. Rutgeerts P, Diamond RH, Bala M, et al. . Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433–42; quiz 464. [DOI] [PubMed] [Google Scholar]
- 30. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. . Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414–22.e5. [DOI] [PubMed] [Google Scholar]
- 31. Faubion WA Jr, Fletcher JG, O’Byrne S, et al. . Emerging biomARKers in inflammatory bowel disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013;108:1891–900. [DOI] [PubMed] [Google Scholar]
- 32. Fernandes SR, Rodrigues RV, Bernardo S, et al. . Transmural healing is associated with improved long-term outcomes of patients with Crohn’s disease. Inflamm Bowel Dis. 2017;23:1403–9. [DOI] [PubMed] [Google Scholar]
- 33. Buisson A, Gonzalez F, Poullenot F, et al. . Patients’ point of view regarding acceptability and usefulness of inflammatory bowel diseases monitoring tools: results from a Nationwide Multicenter Study (the ACCEPT Study). Gastroenterology. 2016;150(Supplement 1):S–S984. [Google Scholar]
- 34. Ananthakrishnan AN, Korzenik JR, Hur C. Can mucosal healing be a cost-effective endpoint for biologic therapy in Crohn’s disease? a decision analysis. Inflamm Bowel Dis. 2013;19:37–44. [DOI] [PubMed] [Google Scholar]
- 35. Bhagya Rao B, Koutroubakis IE, Ramos Rivers C, et al. . Delineation of Crohn’s disease trajectories using change in Lémann Index: a natural history study. J Clin Gastroenterol. 2016;50:476–82. [DOI] [PubMed] [Google Scholar]
- 36. Morris S, Gurusamy KS, Sheringham J, et al. . Cost-effectiveness analysis of endoscopic ultrasound versus magnetic resonance cholangiopancreatography in patients with suspected common bile duct stones. PLoS One. 2015;10:e0121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harewood GC, Wiersema MJ. Cost-effectiveness of endoscopic ultrasonography in the evaluation of proximal rectal cancer. Am J Gastroenterol. 2002;97:874–82. [DOI] [PubMed] [Google Scholar]
- 38. Greenup AJ, Bressler B, Rosenfeld G. Medical imaging in small bowel Crohn’s disease-computer tomography enterography, magnetic resonance enterography, and ultrasound: “which one is the best for what?” Inflamm Bowel Dis. 2016;22:1246–61. [DOI] [PubMed] [Google Scholar]
- 39. Samuel S, Bruining DH, Loftus EV Jr, et al. . Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012;10:1253–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.