Abstract
Objective:
To examine the cross-sectional association of functional performance with Alzheimer’s disease (AD) neuroimaging biomarkers in non-demented individuals (cognitively unimpaired (CU) and those with mild cognitive impairment (MCI)).
DESIGN:
Cross-sectional study
SETTING:
Olmsted County, Minnesota
PARTICIPANTS:
1,782 participants of the population-based Mayo Clinic Study of Aging (MCSA) (≥50 years of age; 1,578 CU; 204 with MCI) who underwent 11C-PiB PET.
MEASUREMENTS:
We defined abnormal (elevated) 11C-PiB-PET retention ratio as standardized uptake value ratio >1.42, abnormal (reduced) AD signature cortical thickness (N+; MRI measurement) as <2.67mm, and biomarker groups by the combination of abnormality for amyloid (A+/A-) and neurodegeneration (N+/N-). Functional performance was assessed by the Clinical Dementia Rating (CDR) sum of boxes (for functional domains) and the Functional Activities Questionnaire (FAQ).
RESULTS:
The study sample had a mean age (standard deviation (SD) of 71.3 (10.2) years; 53.4% were men and 28.3% were APOE ε4 allele carriers. Participants with a CDR-SOB (functional)> 0 (vs. 0) were almost four times more likely to be N+ (OR=3.92, 95%CI: 1.77, 8.67, adjusting for age, sex, education, global cognitive z-score and APOE ε4 allele status; p<.001) and those with FAQ score >0 were 1.5 times more likely to be A+ (OR=1.48, 95%CI: 1.04, 2.11, p=.031). Higher FAQ scores were associated with higher odds of A+N+ and A-N+ in CU participants.
CONCLUSIONS:
The findings in this study supplement limited available information that supports an association between functional performance and AD neuroimaging biomarkers earlier in the dementia pathophysiology. The associations should be validated in longitudinal studies.
Keywords: Amyloid, neurodegeneration, functional performance, CDR, FAQ
Research in the field of cognitive aging is now focusing on strategies for early detection of individuals with underlying Alzheimer’s disease (AD) pathology before clinical symptomatology is evident. Persons with preclinical disease could be candidates for early (secondary) preventive interventions to prevent or delay clinical symptoms. Although functional impairment is required for the definition of dementia, subtle changes are likely in the preclinical phase but have not been adequately assessed.1
It is vital to determine which changes in activities of daily living (ADL), particularly instrumental activities of daily living (i.e. requiring more complex skills; IADL) can be detected at an early stage and may be early markers of cognitive decline and herald changes in AD pathology. The Clinical Dementia Rating (CDR) scale is a global scale used to assess severity of dementia and simultaneously captures both functional and cognitive performance. Both the CDR score and the CDR sum of boxes (CDR-SOB) have been shown to be significantly associated with brain amyloid burden in AD patients. 2, 3
IADL impairment has been associated with greater amyloid burden in MCI patients4 and deteriorating ADLs in AD patients have been associated with greater pathologic burden (i.e., neuritic plaque and neurofibrillary tangle counts).5 Some IADL might start declining earlier in the disease course, even at the stage of mild cognitive impairment (MCI).1, 6 Higher IADL impairment (as assessed by the informant-based Functional Activities Questionnaire (FAQ))7 has been associated with higher global PiB retention in MCI but not in CU individuals.4 An association between lower IADL abilities and higher burden of brain amyloid has also been previously demonstrated in community dwelling older adults with good functional status, who reported memory complaints to their general practitioner.1 Studies also support an association between IADL impairment and AD neurodegenerative patterns in MRI studies8 and brain hypometabolism. 9
The objective of the current study was to examine the association of daily functioning (assessed by CDR and FAQ) with AD biomarkers in non-demented individuals (CU and with MCI).
METHODS
Study population.
The study design and protocol of the Mayo Clinic Study of Aging (MCSA) has been reported in detail previously.10, 11 In brief, the Rochester Epidemiology Project (REP) 12 resources were used to enumerate Olmsted County (MN) residents, aged 70–89 years (October 1, 2004). An age and sex-stratified random sample of Olmsted County residents was invited to participate in the MCSA.10 Ongoing recruitment has maintained the study sample. Recruitment of participants 50–69 years old was initiated in 2012. MRI was initiated in 2005 and 11C-PiB PET scans in 2008. As of March 2017, 1,782 MCSA participants had available data on 11C-PiB PET; of these N=1,763 had available CDR data and N=1,743 had available FAQ data.
Standard Protocol Approvals, Registrations, and Patient Consent.
The study was approved by the Institutional Review Boards of the Mayo Clinic and of Olmsted Medical Center. All participants provided written informed consent prior to participation in the study.
Clinical evaluation and diagnostic assessment.
Each participant was evaluated by a nurse or a study coordinator, a physician and underwent neuropsychological testing by a psychometrist. The nurse or study coordinator collected demographic information, asked questions about memory; and administered the CDR13 and the FAQ 14 to an informant. The physician’s evaluation included review of medical history, administration of the Short Test of Mental Status15 and a neurological examination. Nine neuropsychological tests, administered by a psychometrist, were used to assess cognitive performance in four domains: (i) memory [Logical Memory–II (delayed recall) and Visual Reproduction–II (delayed recall) from the Wechsler Memory Scale–Revised and the Auditory Verbal Learning Test];16, 17 (ii) executive function (Trail Making Test Part B, and Digit Symbol Substitution from Wechsler Adult Intelligence Scale–Revised);18, 19 (iii) language (Boston Naming Test and Category Fluency Test);20, 21 and (iv) visuospatial skills (Picture Completion and Block Design from the Wechsler Adult Intelligence Scale–Revised).19 The raw scores from each test were transformed into age-adjusted scores using independent normative data.22 Domain scores were obtained by averaging the adjusted scaled scores within each domain and then scaled to allow for comparisons across domains. For each domain, a score greater than one standard deviation (SD) below the age-specific mean was considered as possible cognitive impairment. At a weekly held conference, the nurse or study coordinator, the physician and a neuropsychologist discuss all the information for each subject and the final diagnosis (i.e., CU, MCI, dementia) is made by consensus decision. 10, 11 Individuals who performed in the normative range and did not meet criteria for MCI23 or dementia 24 were classified as CU.
Covariates.
For analytic assessment of performance on the various tests the raw scores for tests in each domain were z-scored, averaged and scaled to create domain-specific cognitive z-scores. A global z-score for overall performance was also created by averaging and scaling the four domain z scores. APOE ε4 genotype was determined from a blood draw at baseline assessment. Medical comorbidities were abstracted from participant medical records using the REP medical records-linkage system. The chronic disease burden was assessed from a weighted Charlson Comorbidity Index25 score based on electronic diagnosis codes (HICDA, ICD-9, ICD-10) using the REP resources.
11C-PiB PET acquisition.
Details are presented in previous reports.26,27, 28 A global PIB standardized uptake value ratio (SUVR) was calculated from weighted median pixel values in ROIs from both hemispheres for parietal, temporal, prefrontal, orbito-frontal, precuneus, anterior cingulate, and posterior cingulate regions and referenced to the cerebellar grey matter crus. An abnormal (elevated; A+) 11C-PiB-PET retention ratio was defined as SUVR >1.42.29 The first 11C-PiB-PET was used in current analysis.
MRI measures acquisition.
MRI was performed at 3 Tesla and cortical surface was parcellated using Freesurfer (v 5.3). A composite thickness measure representing an AD signature cortical thickness was computed to assess AD-related neurodegeneration based on individual cortical thickness for regions of interest (ROI) from entorhinal, inferior temporal, middle temporal and fusiform cortex from both hemispheres.30_ENREF_21 Abnormal (reduced) AD signature cortical thickness (N+) was defined as <2.67mm.29
Assessment of functional performance.
Functional performance was assessed by the CDR13 and the FAQ.14 The CDR scale measures impairment caused by cognitive loss and obtains information on an individual’s performance in six cognitive and functional domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care).2, 13 The global CDR score (0 [no dementia] and 0.5, 1, 2, 3 for questionable, mild, moderate, severe dementia) is derived from the ratings in each domain, which range from 0 (no impairment) to 3 (severe impairment). The CDR sum of boxes (SOB) is computed by summing the scores for each of the domain boxes (score ranges from 0 to 18). 2 We also computed the CDR-SOB only for the 3 functional domains,31 which we call in the present study “CDR-SOB (functional)”.
The FAQ is a 10-item questionnaire that assesses IADL (e.g., writing checks, assembling tax records, shopping alone for groceries, working on a hobby, turning off stove after use, traveling out of neighborhood, preparing a balanced diet, etc.). The informant rates the participant’s abilities (0=normal, 1= has difficulty, but does by self, 2= requires assistance, 3= dependent; 8= “Not applicable (e.g., never did)”, was not scored) resulting in a score range from 0 to 30; higher scores indicate greater impairment. The FAQ questionnaire was considered complete if 70% of the questions were answered. 1508 participants had completed all FAQ questions, 164 participants had one missing question, 59 participants had 2 missing and 12 participants had 3 missing questions. Thirty-nine participants had less than 70% completion and were excluded from analyses. In current analyses, functional performance was assessed by CDR-SOB (functional) and FAQ, over the past year.
Statistical analysis
The CDR-SOB (functional) was square root-transformed and the FAQ-total score was log-transformed (ln (x+1) of FAQ-total score), as they were substantially skewed. We examined cross-sectional associations of functional status measures with abnormal amyloid (A+) and neurodegeneration (N+) in non-demented individuals. The National Institute on Aging and Alzheimer’s Association (NIA-AA) preclinical AD stages were defined in CU participants by abnormal amyloid (A+/A-) and neurodegeneration (N+/N-): i.e., A-N-(stage 0), A+N-(stage 1), and A+N+ (stage 2+3).32–34 Suspected non-amyloid pathophysiology(SNAP) was defined as A-N+.34 For these cross-sectional analyses, we used binary (for binary outcomes; A-/A+ or N-/N+) or multinomial (for categorical outcomes; A/N biomarker combinations) logistic regression models (odds ratios [ORs], 95% confidence intervals [CIs]). For evaluation of possible confounding and/or effect modification, analyses were also performed stratifying by cognitive status (CU, MCI) as well as within the two cognitive status groups. We adjusted the binary logistic and multinomial models for age at PiB PET scan, sex, education, APOE ε4 allele status and global cognitive z-score. In addition, we run models including only participants with 100% complete FAQ questionnaires and estimates were very similar, thus we report analyses including all FAQ questionnaires with 70% of questions answered. Further adjustment for the Charlson Comorbidity Index (which takes into account disease severity and was developed to assess impact of disease burden on health outcomes)25, 35 did not change the estimates appreciably and is not included in the presented models. In stratified logistic regression models, we adjusted for age at PiB PET scan, sex, education and APOE ε4 allele status, using cognitive status (CU/MCI) as strata. Potential effect modification by age was examined using interaction terms in the models but interaction terms were not statistically significant. Associations were considered significant at a two-tailed p-value < .05. Analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, North Carolina) and Stata/SE statistical software version 15.1 (StataCorp LP, College Station, Texas).
RESULTS
Characteristics of participants
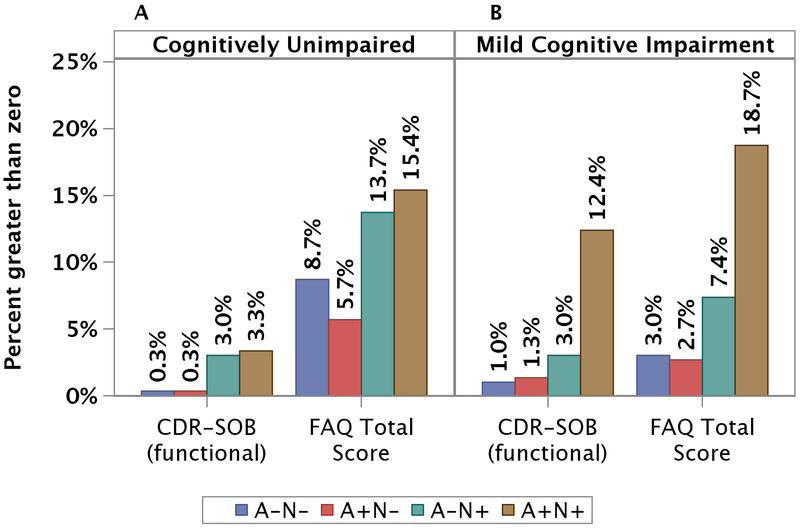
Table 1 presents characteristics of non-demented MCSA participants by amyloid (N=1,782) and neurodegeneration (N=1,749) status. Overall, mean age (SD) was 71.3 (10.2) years, 53.4% were men, and 28.3% were APOE ε4 allele carriers. Individuals with elevated amyloid (A+) or neurodegeneration (N+) were significantly older, had a lower mean global cognitive z-score and Short Test of Mental Status and were more likely to have CDR-SOB (functional)>0 and FAQ total >0 compared with A- or N- individuals respectively. There were 800 (45.7%) individuals with A-N-, 223 (12.8%) with A+N-, 379 (21.7%) with A-N+, and 347 (19.8%) with A+N+ biomarkers. A higher percentage of participants with CDR-SOB (functional) >0 or FAQ total score>0 were present in the A-N+ and A+N+ biomarker groups in CU (p<.001) and participants with MCI (p<.001) (Figure 1).
Table 1.
Characteristics of Participants by Amyloid and Neurodegeneration status.
| Characteristics | Elevated Amyloid(A+) | Neurodegeneration(N+) | ||
|---|---|---|---|---|
| No, N=1199 | Yes, N=583 | No, N=1023 | Yes, N=726 | |
| Age, mean (SD) | 68.4 (9.9)* | 77.3 (7.8) | 66.9 (9.05)* | 77.4 (8.1) |
| Sex, Male | 652 (54.4) | 299 (51.3) | 508 (49.7)* | 427 (58.8) |
| Education (years), mean (SD) | 14.8 (2.6)* | 14.4 (2.7) | 14.9 (2.5)* | 14.4 (2.9) |
| APOE ε24/ε34/ε44a | 244 (20.9)* | 248 (43.4) | 280 (28.20) | 204 (28.2) |
| Obesity (BMI≥30)b | 405 (34.0) | 182 (31.4) | 336 (33.04) | 234 (32.5) |
| Coronary artery diseasec | 271 (22.6)* | 216 (37.1) | 198 (19.35) * | 279 (38.5) |
| Congestive heart failurec | 42 (3.5)* | 45 (7.7) | 18 (1.76)* | 68 (9.4) |
| Strokec | 33 (2.8)* | 30 (5.2) | 22 (2.15) * | 38 (5.2) |
| Hypertensionc | 686 (57.2)* | 425 (73.0) | 533 (52.10) * | 556 (76.7) |
| Charlson Comorbidity Indexd | 2.53 (2.84)* | 3.65 (3.02) | 2.14 (2.40)* | 3.91 (3.24) |
| Global cognitive z- scoree | 1.11 (.95)* | .43 (1.04) | 1.20 (.89)* | .45 (1.05) |
| Short Test of Mental Status | 35.3 (2.6)* | 34.1 (2.9) | 35.6 (2.5)* | 34.2 (2.9) |
| Cognitively unimpaired | 1,114 (92.9)* | 464 (79.6) | 960 (93.8)* | 591 (81.4) |
| Mild cognitive impairment | 85 (7.1) | 119 (20.4) | 63 (6.2) | 135 (18.6) |
| CDRf score >0 | 82 (6.9)* | 121 (20.9) | 57 (5.7)* | 143 (19.8) |
| CDR score, median (range) | 0 (1) | 0 (1) | 0 (.5) | 0 (1) |
| CDR-SOB>0 | 93 (7.9)* | 131 (22.6) | 63 (6.3)* | 157 (21.7) |
| CDR-SOB, median (range) | 0 (4.5) | 0 (6.5) | 0 (4.0) | 0 (6.5) |
| CDR (functional) >0 | 23 (1.9)* | 52 (9.0) | 9 (.9)* | 65 (9.0) |
| CDR (functional), median (range)g | 0 (3) | 0 (3.5) | 0 (1.5) | 0 (3.5) |
| FAQh score >0 | 101 (8.6)* | 129 (22.5) | 60 (6.0)* | 165 (23.1) |
| FAQ score, median (range) | 0 (19) | 0 (20) | 0 (11) | 0 (20) |
| N+i | 379 (32.2)* | 347 (60.9) | -- | -- |
| A+ | -- | -- | 223 (21.8)* | 347 (47.8) |
N (%) unless otherwise stated;
p<.05 (Kruskal Wallis or Chi-Square test).
Abbreviations: APOE - apolipoprotein E; BMI – body mass index; CU – cognitively unimpaired; A+ – Elevated amyloid (A+), is defined as 11C-Pittsburgh compound B standardized uptake value ratio > 1.42; N+ – Abnormal (reduced) AD signature cortical thickness (N+) was defined as <2.67mm; MCI – mild cognitive impairment; CDR SOB – Clinical dementia rating scale sum of boxes (0–18); CDR (functional): CDR-SOB only for the 3 functional domains; FAQ total: Functional activities questionnaire score (0-30, completed if 70% of questions answered).
41 missing;
12 missing;
1 missing;
Chronic disease burden was assessed from a weighted Charlson Comorbidity Index score; 24 missing in A+ comparisons; 16 missing in N+ comparison;
Cognitive z scores computed after scaling raw cognitive test scores (mean = 0, SD = 1) using data for cognitively normal subjects at baseline. Domain specific z scores are summed and scaled to obtain global z scores; 87 missing in A+ comparisons; 86 missing in N+ comparison;
19 missing;
Sum of boxes for functional domains (community affairs, home and hobbies and personal care);
39 missing;
33 missing.
Figure 1.
Percentage of participants (50+ years of age) with CDR-SOB (functional) and FAQ-total score higher than zero by neuroimaging biomarkers’ combinations in cognitively unimpaired (A) and participants with MCI (B). In cognitively unimpaired participants, there were overall 21 (out of 1532) participants with CDR-SOB (functional)>0 and 130 (out of 1518) with FAQ score>0. In MCI participants, there were overall 53 (out of 198) participants with CDR-SOB (functional)>0 and 95 (out of 192) with FAQ score>0.
Participants with PET studies (vs. non-participants) were more likely to be males (p=.001), were younger (p<.001), with higher education (p<.001), had a higher global cognitive z-score (<.001), less likely to have hypertension (p<.001), stroke (p=.001), congestive heart failure (p<.001) or coronary heart disease (p<.001), lower CDR-SOB (p=.040) and lower FAQ score (p<.001); there was no difference in frequency of APOE ε4 allele status carrier (p=0.23) (results not shown in tables).
Association between functional performance, amyloid and neurodegeneration status
Having CDR-SOB (functional)>0 was associated with a 2-fold increase in the odds of having A+ (OR=1.95, 95%CI: 1.04, 3.67, p=.038) and an almost 4-fold increase in the odds of having N+, in the total sample. Individuals with FAQ-total>0 had higher odds of elevated amyloid and neurodegeneration in the total sample (vs. FAQ=0). The associations between FAQ measures and A+ and N+ kept their significance when stratified models were employed.
Impairment (>0 vs. 0) in the “home and hobbies” CDR component was significantly associated with A+ (Supplementary Table S1). Both the “community” and “home and hobbies” components were significantly associated with N+. Participants with FAQ total score ≥4 (vs. <4) had significantly higher odds of neurodegeneration (Supplementary Table S2).
Association between functional performance, preclinical AD and SNAP
Individuals with an FAQ score> 0 (vs. 0) were over 3 times more likely to be A+N+ (vs. A-N-; OR=3.31 95% CI: 1.81, 6.04) and were 2.6 times more likely to have SNAP (vs. A-N-; OR=2.55, 95% CI: 1.45, 4.46). Estimates for CDR-SOB (functional) were increased as well but were very imprecise and inflated due to small numbers and are not included in the table; there were 1, 1, 9, 10 participants with CDR-SOB (functional) >0 in the A-N-, A+N-, A-N+ and A+N+ groups respectively.
DISCUSSION
Our findings suggest that higher FAQ scores were cross-sectionally associated with abnormal (elevated) amyloid and neurodegeneration in non-demented MCSA participants, and with A+N+ (NIA-AA preclinical stages 2+3) and A-N+ (SNAP) in CU participants. CDR-SOB (functional) was associated with neurodegeneration in the total sample, in CU and in individuals with MCI. Impairment in “community” and “home and hobbies” CDR components were significantly associated with neurodegeneration. The findings in this study supplement limited available information that supports an association between functional performance and AD neuroimaging biomarkers earlier in the dementia pathophysiology when clinical symptomatology might not be evident. Current study findings are in line with previous studies suggesting that lower ADL and IADL abilities are associated with increased amyloid1−4and also studies showing an association between IADL impairment, brain hypometabolism 9, 36, 37 and MRI biomarkers of neurodegeneration.8, 38, 39
Cognitive impairment is a determinant of functional decline.1 Due to the method of the CDR assessment (involving informant information for functional status changes compared to previous performance), investigators have supported that its score is less influenced by factors that usually affect cognitive tests, such as age, education, depression, or practice effects.40 Other researchers4 however prefer to adjust analyses for global cognitive impairment to minimize spurious associations (i.e., associations potentially confounded by cognitive status. In MCSA, CDR is considered during the cognitive assessment of the participants by the nurse or study coordinator, as one of several assessments available for the evaluator; we chose to pursue analyses that also adjusted for global cognitive performance or took into account (in stratified analysis) the consensus diagnosis of CU/MCI. Findings from both analyses are consistent that FAQ measures are associated with AD neuroimaging biomarkers, especially neurodegeneration, in the total sample. CDR-SOB (functional) was not significantly associated with A+ when stratified analysis was used; however, CDR-SOB (functional) also included ADL related to personal care, which we expect to be impaired later in the AD trajectory.
Only a small minority of non-demented participants in the present study had lower than perfect functional performance (4.25% had CDR-SOB (functional)>0; 13.2% had FAQ>0). In general, we would expect that impairment in function follows impairment in cognition,41–43 with functional deficits not apparent until later in the AD process. AD, however, is a continuum with variable clinical course and subtle impairment at the earliest stages. Subtle functional difficulties could be associated with AD biomarkers before cognitive impairment1 and baseline MRI imaging biomarkers could predict worsening of IADL overtime across the AD spectrum8 (CU, MCI, mild AD individuals).
In the current study, the statistically significant association between FAQ measures and neurodegeneration was consistent throughout all analyses; this was not consistently the case for analyses related to abnormal amyloid, which might be important considering that biomarkers of neurodegeneration rather than of amyloid accumulation are directly related to cognitive symptoms.43
We found that higher FAQ total score and FAQ total score>0 were associated with A+N+ and A-N+ biomarker groups in CU. Findings are very important as persons with A-N+ could have a greater rate of cognitive decline than A-N- individuals and individuals with both elevated amyloid and neurodegeneration could have accelerated cognitive decline compared to individuals who are A-N-.33 Current study is in agreement with a previous report1 in community-dwelling older adults with good functional status who reported spontaneous memory complaints; although not all previous research4 is in agreement. We need to explore further whether subtle functional limitations in non-demented individuals could be part of the earliest manifestations of increased amyloid burden and/or neurodegeneration. CU individuals with lower functional performance who are A+N+ may be good candidates for surveillance and for preventive interventions when these become available.
In the present study, “community” and “home and hobbies” subscale impairment were significantly associated with neurodegeneration in non-demented individuals. Investigators have reported that individuals with amnestic MCI who progress to AD had a higher baseline CDR orientation component score,40 and individuals with MCI with impaired IADL in the CDR scale had more widespread gray matter thinning in frontal and parietal lobe areas, poorer cognitive performance and higher 2-year disease progression rate compared with MCI individuals with similar CDR global score but not IADL impairment.44 However, there is a paucity of studies on the association of CDR domains with amyloid positivity and neurodegeneration and additional research is needed to supplement our findings.
Most participants had an FAQ score of 0, and we could not dichotomize the FAQ score at very high values. However, FAQ score ≥4 (vs. <4) or FAQ ≥5 (vs. <5) had significant associations with neurodegeneration (but not with amyloid) and although findings might be of uncertain clinical significance, the general concordance of current results with previous studies showing that the FAQ total score can effectively discriminate between CU and patients with dementia with cut-off ranging from ≥5 to ≥8 and also discriminate between MCI and dementia with an optimal cut-off of 5/6 45 underscore the need to develop measures of very early functional changes (as suggested by the NIA-AA workgroup),32 in persons at the earliest or pre-symptomatic stages of AD.46
The study has certain limitations. The cross-sectional associations that we observed cannot be construed to assume that elevated amyloidosis was the cause of functional impairment. As the study is cross-sectional, we cannot assess causality. In addition, we lack information on Tau PET imaging and we cannot also exclude that other unmeasured factors confound estimations. Findings help generate research hypotheses for future studies. During the MCSA evaluation, CDR was considered by the nurse or study coordinator (one of the three independent evaluators) during the participant’s cognitive assessment. We tried to remedy this limitation by including the global cognitive score in the analysis models or using stratified analysis, but residual bias might remain.
The study has also important strengths. Informants were not aware of the participant’s objective cognitive performance scores or amyloid and neurodegeneration status, thus avoiding recall bias. Functional performance was assessed by a study partner avoiding limitations due to participant’s cognitive status or factors that affect cognitive tests (e.g., education, depression, etc.).
Our observations add valuable information to limited available data that support an association between functional performance, amyloid deposition and neurodegeneration earlier in the AD pathophysiology when clinical symptomatology might not be evident. However, longitudinal studies are needed to replicate current findings. Functional status assessment is simple, inexpensive and readily available in clinical settings and additional research is needed on whether and in what form it could be part of a battery of tests to predict progression of cognitive decline or useful for participant selection for AD prevention trials.
Supplementary Material
Association between CDR functional domains with elevated amyloid (A+) and neurodegeneration (N+) in individuals 50 years old and older.
Association between FAQ score and elevated amyloid (A+) and neurodegeneration (N+) in participants 50 years old and older.
Table 2.
Association between CDR SOB (functional) and FAQ measures with elevated amyloid (A+) and neurodegeneration (N+) in individuals 50 years old and older.
| Elevated amyloid (A+)a | Neurodegeneration (N+)a | |||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Nondemented individualsb | ||||
| CDR SOB (functional)c, d | 2.07 (1.02, 4.20) | .043 | 4.93 (1.95, 12.49) | .001 |
| >0 vs. 0e | 1.95 (1.04, 3.67) | .038 | 3.92 (1.77, 8.67) | .001 |
| FAQ scored | 1.40 (1.07, 1.83) | .015 | 1.86 (1.36, 2.54) | <.001 |
| >0 vs. 0e | 1.48 (1.04, 2.11) | .031 | 2.14 (1.46, 3.12) | <.001 |
| Stratified modelf | ||||
| CDR-SOB (functional)c, d | 1.70 (.88, 3.29) | .113 | 6.53 (2.56, 16.71) | <.001 |
| >0 vs. 0e | 1.77 (.96, 3.25) | .066 | 5.03 (2.27, 11.12)i | <.001 |
| FAQ scored | 1.30 (1.00, 1.68) | .047 | 2.06 (1.51, 2.80) | <.001 |
| >0 vs. 0e | 1.43 (1.01, 2.02) | .045 | 2.33 (1.61, 3.37) | <.001 |
| Cognitively unimpairedg,j | ||||
| CDR-SOB (functional) c, d | 1.48 (.47, 4.70) | .507 | ---i | |
| >0 vs. 0e | 1.70 (.64, 4.55) | .289 | ---i | |
| FAQ scored | 1.36 (.96, 1.93) | .081 | 2.20 (1.46, 3.30) | <.001 |
| >0 vs. 0e | 1.48 (.99, 2.23) | .059 | 2.22 (1.45, 3.42) | <.001 |
| Participants with MCIg,j | ||||
| CDR-SOB (functional) c, d | 1.86 (.81, 4.28) | .144 | 3.16 (1.06, 9.41) | .038 |
| >0 vs. 0e | 1.82 (.82, 4.05) | .144 | 2.79 (1.06, 7.34) | .037 |
| FAQ scored | 1.26 (.85, 1.88) | .248 | 1.79 (1.11, 2.89) | .017 |
| >0 vs. 0e | 1.36 (.68, 2.72) | .388 | 2.62 (1.23, 5.54) | .012 |
Abbreviations: A – amyloid; CDR-SOB (functional) – Clinical dementia rating scale sum of boxes for the 3 functional domains; FAQ - Functional activities questionnaire score; OR (95%CI) – odds ratio (95% Confidence interval); MCI – mild cognitive impairment.
A+, is defined as 11C-Pittsburgh compound B SUVR > 1.42 and A- if otherwise; N+, Abnormal (reduced) AD signature cortical thickness (N+) was defined as <2.67mm and N- if otherwise.
Adjusted for age, sex, education, global cognitive z-score and APOE e4 allele. For analysis related to A+, data were available for 532 A+ and 1,105 A- participants overall for analyses related to CDR (functional) and 562 A+/1,141 A- for analyses related to FAQ measures. For analysis related to N+, data were available for 674 N+ and 939 N- participants overall for analyses related to CDR (functional) and 667 N+ and 927 N- for analyses related to FAQ measures.
Sum of boxes for functional domains (community affairs, home and hobbies and personal care).
CDR-SOB (functional) was square root-transformed and FAQ score was log-transformed (ln(x+1) of FAQ score).
Comparison of >0 vs. 0 (reference) of the CDR-SOB (functional) or FAQ measurement.
Adjusted for age, sex, education and APOE e4 allele status, stratifying by cognitive status (CU/MCI).
Adjusted for age, sex, education and APOE e4 allele status
For CU: Global cognitive z- score (SD): 1.08 (.88) N=1,514; STMS: 35.5 (2.1); 12 missing.
19 of 21 with CDR-SOB (functional) > 0 have abnormal thickness; thus, estimations are inflated and imprecise: OR=23.69 95%CI (2.95, 190.06) for CDR-SOB (functional) as continuous measure and OR=11.39 95% CI (2.41, 53.80) for CDR-SOB (functional) >0 vs. 0.
For MCI: Global cognitive z- score (SD): −.70 (.81) N=181; STMS: 30.5 (3.1); 4 missing.
Table 3.
Association between CDR and FAQ measures with NIA-AA preclinical AD stages and SNAP in cognitively unimpaired 50 years old and older participants.
| Stage 1 (A+N−) a | Stages 2+3 (A+N+) | SNAP (A-N+) | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) b | p | OR (95%CI) | p | OR (95%CI) | p | |
| FAQ scorec | 1.79 (.92, 3.46) | .086 | 3.16 (1.78, 5.59) | <.001 | 2.60 (1.50, 4.49) | <.001 |
| >0 vs. 0d | 1.86 (.94, 3.67) | .075 | 3.31 (1.81, 6.04) | <.001 | 2.55 (1.45, 4.46) | .001 |
Abbreviations: A – amyloid; N – neurodegeneration; OR (95%CI) – odds ratio (95% Confidence interval); FAQ: Functional activities questionnaire score (0-30, completed if 70% of questions answered).
Imaging biomarker groups defined by the combination of abnormality for amyloid (A+/A−) and neurodegeneration (N+/N−); A+, is defined as 11C-Pittsburgh compound B SUVR > 1.42; N+ is defined as AD signature cortical thickness< 2.67 mm.
Multinomial logistic regression adjusted for age, sex, education and APOE e4 allele; each row represents results from 1 model, using A−N− as the reference biomarker group. Data were available for 1,489 (711 A−N−, 193 A+N−, 333 A−N+, 252 A+N+) participants overall for analyses related to FAQ measures. There were 1, 1, 9, 10 participants with CDR-SOB (functional)>0 in the A−N−, A+N−, A−N+ and A+N+ groups respectively; thus, estimates for Clinical dementia rating scale sum of boxes functional (sum of boxes for functional domains) were increased but very imprecise and not included in table.
FAQ score was log-transformed (ln(x+1) of FAQ score).
Comparison of >0 vs. 0 (reference) of the FAQ score.
IMPACT STATEMENT.
We certify that this work is novel. Alzheimer’s disease (AD) clinical signs and symptoms occur several years after amyloid deposition in the brain, offering a lag time of several years when persons with preclinical Alzheimer’s disease (AD) could be candidates for early (secondary) preventive interventions to prevent or delay clinical symptoms. Although functional impairment is required for the definition of dementia, subtle changes are likely in the preclinical phase but have not been adequately assessed. The findings in this study supplement limited available information that supports an association between functional impairment, amyloid deposition (i.e., one of the hallmarks of Alzheimer’s disease) and neurodegeneration earlier in the dementia pathophysiology.
ACKNOWLEDGMENTS
The study was supported by the NIH (U01 AG006786, P50 AG016574, R01 AG011378, R01 AG041851, R01 NS097495), F. Hoffman-La Roche, the GHR Foundation, the Elsie and Marvin Dekelboum Family Foundation, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Alice Weiner Postdoctoral Research Fellowship in Alzheimer’s Disease Research, Mayo Foundation for Medical Education and Research, the Liston Award, the Schuler Foundation, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Footnotes
Author Disclosures
Maria Vassilaki – Receives research funding from NIH, Roche and Biogen.
Jeremiah Aakre – Reports no disclosures.
Walter Kremers – Receives research funding from Department of Defense, NIH, Astra Zeneca, Biogen and Roche.
Michelle Mielke – Consults for Eli Lilly and Lysosomal Therapeutics, Inc.; receives unrestricted research grants from Biogen, Lundbeck, and Roche, and research funding from the NIH/NIA and Department of Defense.
Yonas Geda – Receives funding from NIH and Roche, and serves on Lundbeck Advisory Board
Mary Machulda – Receives NIH funding.
David Knopman –Serves on a Data Safety Monitoring Board for the DIAN study; is an investigator in clinical trials sponsored by Lilly Pharmaceuticals, Biogen and the Alzheimer’s Treatment and Research Institute at USC; and receives research support from the NIH.
Prashanthi Vemuri – Receives NIH Funding.
Preciosa M. Coloma - Full-time employee of, and own shares at F. Hoffmann-La Roche Ltd.
Barbara Schauble- Full-time employee of, and owns shares at F. Hoffmann-La Roche Ltd.
Val Lowe – Serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences, Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI)
Clifford Jack –Receives research support from the NIH/NIA, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation.
Ronald Petersen – Consultant for Roche, Inc., Biogen, Inc., Merck, Inc., Eli Lilly and Company, and Genentech, Inc.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the National Institute of Health
Rosebud Roberts – Receives research funding from the NIH/NIA, Biogen, and Roche.
Sponsor’s Role: The sponsor had no role in methods, subject recruitment, data collections, and analysis. Dr. Coloma’s and Dr. Schauble’s contributions to the manuscript are listed in “Author contributions”.
REFERENCES
- 1.Lilamand M, Cesari M, del Campo N, et al. Brain Amyloid Deposition Is Associated With Lower Instrumental Activities of Daily Living Abilities in Older Adults. Results From the MAPT Study. J Gerontol a-Biol. 2016;71: 391–397. [DOI] [PubMed] [Google Scholar]
- 2.Grimmer T, Henriksen G, Wester HJ, et al. Clinical severity of Alzheimer’s disease is associated with PIB uptake in PET. Neurobiol Aging. 2009;30: 1902–1909. [DOI] [PubMed] [Google Scholar]
- 3.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68: 1718–1725. [DOI] [PubMed] [Google Scholar]
- 4.Marshall GA, Olson LE, Frey MT, et al. Instrumental Activities of Daily Living Impairment Is Associated with Increased Amyloid Burden. Dement Geriatr Cogn Disord. 2011;31: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall GA, Fairbanks LA, Tekin S, et al. Neuropathologic correlates of activities of daily living in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20: 56–59. [DOI] [PubMed] [Google Scholar]
- 6.Perneczky R, Pohl C, Sorg C, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21: 158–162. [DOI] [PubMed] [Google Scholar]
- 7. Pfeffer RI, Kurosaki TT, Harrah CH Jr., et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37: 323–329. [DOI] [PubMed] [Google Scholar]
- 8.Marshall GA, Lorius N, Locascio JJ, et al. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;41: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32: 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men The Mayo Clinic Study of Aging. Neurology. 2010;75: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. Am J Epidemiol. 2011;173: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer RI, Kurosaki TT, Harrah CH Jr., et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37: 323–329. [DOI] [PubMed] [Google Scholar]
- 15.Kokmen E, Smith GE, Petersen RC, et al. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48: 725–728. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D Manual for the Wechsler Memory Scale - Revised Psychological Corp. NewYork,NY, 1987. [Google Scholar]
- 17.Rey A L’examen Clinique en Psychologie. Paris,France: Presses Universitaires de France, 1964. [Google Scholar]
- 18.Reitan R Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8: 271–276. [Google Scholar]
- 19.Wechsler D Wechsler Adult Intelligence Scale–Revised Psychological Corp. San Antonio, TX, 1981. [Google Scholar]
- 20.Kaplan EGH WS. The Boston Naming Test. 2nd ed. ed. Boston, MA: Lea & Fabiger, 1978. [Google Scholar]
- 21.Strauss ESE SO. A Compendium of Neuropsychological Tests. New York, NY: Oxford University Press, 2006. [Google Scholar]
- 22.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s older americans normative studies: WAIS-R norms for ages 56 to 97. Clinical Neuropsychologist. 1992;6: 1–30. [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256: 183–194. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Washington, DC, 1994. [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45: 613–619. [DOI] [PubMed] [Google Scholar]
- 26.Lowe VJ, Kemp BJ, Jack CR Jr., et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR Jr., Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knopman DS, Jack CR Jr., Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78: 1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR Jr., Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack CR Jr., Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar frequencies of amyloid and neurodegeneration biomarker groups by age among cognitively non-impaired individuals. Brain. 2015;138: 3747–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tractenberg RE, Weiner MF, Cummings JL, et al. Independence of changes in behavior from cognition and function in community-dwelling persons with Alzheimer’s disease: a factor analytic approach. J Neuropsychiatry Clin Neurosci. 2005;17: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84: 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr., Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts RO, Cha RH, Mielke MM, et al. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84: 1854–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy K, Pepin LC, Philiossaint M, et al. Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer’s disease spectrum. J Alzheimers Dis. 2014;42: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salmon E, Lespagnard S, Marique P, et al. Cerebral metabolic correlates of four dementia scales in Alzheimer’s disease. J Neurol. 2005;252: 283–290. [DOI] [PubMed] [Google Scholar]
- 38.Cahn-Weiner DA, Farias ST, Julian L, et al. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007;13: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer’s disease. J Alzheimers Dis. 2010;19: 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, Byun MS, Sohn BK, et al. Clinical Dementia Rating Orientation Score as an Excellent Predictor of the Progression to Alzheimer’s Disease in Mild Cognitive Impairment. Psychiat Invest. 2017;14: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahodne LB, Manly JJ, MacKay-Brandt A, et al. Cognitive declines precede and predict functional declines in aging and Alzheimer’s disease. PLoS One. 2013;8: e73645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu-Seifert H, Siemers E, Price K, et al. Cognitive Impairment Precedes and Predicts Functional Impairment in Mild Alzheimer’s Disease. J Alzheimers Dis. 2015;47: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology. 2010;9: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang YL, Bondi MW, McEvoy LK, et al. Global clinical dementia rating of 0.5 in MCI masks variability related to level of function. Neurology. 2011;76: 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng E, Becker BW, Woo E, et al. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fieo R, Zahodne L, Tang MX, et al. The historical progression from ADL scrutiny to IADL to advanced ADL: Assessing functional status in the earliest stages of dementia. J Gerontol A Biol Sci Med Sci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between CDR functional domains with elevated amyloid (A+) and neurodegeneration (N+) in individuals 50 years old and older.
Association between FAQ score and elevated amyloid (A+) and neurodegeneration (N+) in participants 50 years old and older.