Abstract
Antibodies that potently neutralize highly diverse HIV-1 variants offer great potential for therapy and prevention. Passive administration of HIV-specific neutralizing antibodies genetically modified to have a long serum half-life has now been shown to confer long-lasting protection from infection in the rhesus macaque model.
A number of features specific to primate lentiviral biology — such as very high genetic diversity, structural flexibility that allows escape from the host immune responses, and the ability to integrate into the host genome — have made it so far impossible to develop a safe and effective HIV-1 vaccine. The recent discovery of broadly neutralizing antibodies (bNAbs) that can be isolated from a subset of HIV-1-infected individuals has revitalized AIDS vaccine research. While those bNAbs are usually unable to control virus replication in the patient they were isolated from, they effectively neutralize a high percentage of global HIV isolates in vitro at very low concentrations by recognizing highly conserved epitopes in some key regions of the viral envelope. The antiviral effect of bNAbs was also confirmed in vivo in rhesus macaques in which both transmission and replication of chimeric simian–human immunodeficiency virus (SHIV) have been successfully inhibited by passive administration of such antibodies1. Since the elicitation of bNAbs by active immunization strategies (that is, conventional vaccines) has not yet been successful, passive immunization with ex-vivo-produced bNAbs to prevent HIV-1 infection presents a valuable alternative. In a recent issue of Nature Medicine, Gautam et al. describe two genetically modified bNAbs that each have an increased serum half-life, thereby significantly expanding the durability of protection by passive immunization, and explore the protective efficacy of these antibodies when used in combination through subcutaneous (s.c.) administration2. They demonstrate that these modified bNAbs confer longer protection from SHIV challenge in macaques than was previously possible, and provide initial evidence that s.c. administration may be as effective as intravenous (i.v.) infusion.
Previous work has established that two bNAbs, 3BNC117, which targets the CD4 receptor-binding site, and 10–1074, which is directed against a V3 loop site, protect rhesus macaques from repeated low-dose SHIV challenge1. However, the durability of protection from infection conferred by these antibodies was limited by relatively short median serum half-lives of 1.45 and 1.05 weeks for 3BNC117 and 10–1074, respectively1. To address this major obstacle to effective long-term protection, Gautam et al. introduced a two-amino-acid substitution (LS) that has been successfully used in a recent study to significantly increase the half-life of bNAb VRC01 (refs 1,3). Importantly, this LS modification does not reduce the in vitro neutralizing activity of 3BNC117-LS and 10–1074-LS antibodies. The effect of the LS modification on antibody serum half-life was first evaluated in rhesus macaques that received an i.v. injection of either 3BNC117-LS or 10–1074-LS — half-life was observed to increase 1.9-fold and 3.8-fold, respectively, as compared to the unmodified antibodies. To then determine whether this increase in bNAb serum half-life would translate into better protection from infection, these antibody-treated animals were challenged intrarectally with repeated low doses of virus, thus closely mimicking the natural mucosal exposure to HIV. Consistent with the extended bNAb serum half-life, Gautam et al. convincingly show that the modified 10–1074-LS conferred a 2.2-fold increase in the number of challenges that were needed to acquire an infection compared with the unmodified 10–1074 antibody, whereas the 3BNC117-LS conferred a more modest 1.3-fold increase.
Although 10–1074-LS and 3BNC117-LS successfully neutralize in vitro the vast majority of a representative panel of diverse HIV variants, passive immunization with either one of these bNAbs as single agents will probably fail to protect against resistant viruses. As such, passive administration of a combination of bNAbs with complementary neutralizing activity may be necessary to achieve a pan-HIV protective effect. For the global implementation of this strategy, it may also be essential to profile the HIV variants endemic to specific geographical regions to achieve the greatest breadth of neutralization as tailored by various possible combinations of bNAbs. In addition, to increase the clinical relevance of this approach to HIV prevention, routes of bNAb administration that are easier to implement than the i.v. route need to be explored. Gautam et al. thus tested the efficiency of s.c. injection using the combination of 3BNC117-LS and 10–1074-LS. When both 3BNC117-LS and 10–1074-LS antibodies were administered subcutaneously to rhesus macaques, they conferred protection for 15–24 challenges (that is, weeks) in 5 of the 6 animals, thus demonstrating the potential of this strategy for longer-term HIV/AIDS protection.
While the current study is of great interest, several concerns remain in terms of broad applicability and clinical implementation of this concept for global HIV prevention (Fig. 1). First, to evaluate passive immunization strategies for use in regions such as sub-Saharan Africa, where non-clade B virus variants are endemic, the results of the current experiments will have to be confirmed and expanded in experiments in which the treated macaques are challenged with SHIVs bearing envelopes from clade A and clade C HIV-1 variants. Second, while the LS mutation results in a prolonged protection as compared to the ‘wild-type’ version of these bNAbs, the number of weeks of full protection that would be sufficient to warrant a large-scale implementation of the passive administration strategy remains unclear, especially considering deployment in resource-limited settings, and in comparison with competing or complementary approaches (that is, long-acting antiretroviral drugs used for prevention). Third, this study revealed that, at least in the rhesus macaque model system, passive administration of bNAbs is followed by an anti-antibody immune response that reduces antibody serum half-life and has the potential to severely limit the efficacy of further administration of the same antibodies1. Future studies will need to determine whether this anti-antibody response can be mitigated by means such as simianization, Fc engineering or chemical modification, in order to facilitate bNAb animal studies and clinical trials. Initial clinical trials have demonstrated that bNAbs 3BNC117 and 10–1074 are safe and well-tolerated in humans and, notably, an anti-antibody response has not been reported as a major issue4–6.
Fig. 1 |. Goals for the effective implementation of a passive bNAb immunization.
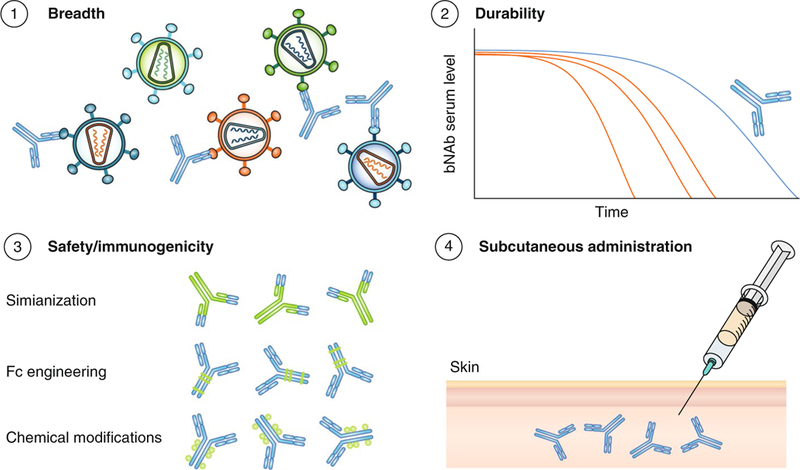
To successfully employ this prophylaxis strategy at the global level, it has to provide long-term protection from infection with diverse HIV variants following easy and safe s.c. administration and without inducing an anti-antibody immune response, for which antibody engineering might prove useful.
Passive immunization through administration of bNAbs is far superior, in terms of efficacy and breadth of protection, to any HIV-1 vaccine strategy that has been evaluated in vivo as of yet. However, large-scale, low-cost production of bNAbs of sufficiently long half-life is needed to effectively implement this strategy as prophylaxis at the global level, and in particular in those low-income countries with limited healthcare infrastructure that have been most severely affected by the AIDS pandemic. Gautam et al. have provided a much-needed step forward in this direction by demonstrating, in the SHIV macaque model, the efficacy of genetically modified bNAbs with longer serum half-life, and have successfully tested the s.c. route for delivery. ❐
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Gautam R et al. Nature 533, 105–109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautam R et al. Nat. Med 10.1038/s41591-018-0001-2 (2018). [DOI] [Google Scholar]
- 3.Ko SY et al. Nature 514, 642–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledgerwood JE et al. Clin. Exp. Immunol 182, 289–301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caskey M et al. Nature 522, 487–491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caskey M et al. Nat. Med 23, 185–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


