Abstract
Introduction:
Lead (Pb) crosses the placenta and can cause oxidative stress, reduced fetal growth and neurological problems. The principal source of oxidative stress in human cells is mitochondria. Therefore, disruption of normal mitochondrial function during pregnancy may represent a primary mechanism behind the adverse effects of lead. We sought to assess the association of Pb exposure during pregnancy with mitochondrial DNA (mtDNA) content, a sensitive marker of mitochondrial function, in cord blood.
Materials and methods:
This study comprised mother-infant pairs from the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study, a prospective birth-cohort that enrolled 1050 pregnant women from Mexico City who were receiving prenatal care between December 2007 to July 2011. Quantitative PCR was used to calculate relative MtDNA content (mitochondrial-to-nuclear DNA ratio (mtDNA/nDNA)) in cord blood. Lead concentrations in both maternal blood (2nd and 3rd trimester and at delivery day) and in cord blood were measured by ICP-MS. Multivariable regression models adjusting for multiple confounders were fitted with 410 mother-infant pairs for whom complete data for mtDNA content, lead levels, and covariates were available.
Results:
Maternal blood Pb measured in the second (mean 3.79 μg/dL, SD 2.63; β = 059, 95% CI 0.008, 0.111) and third trimester (mean 3.90 μg/dL; SD 2.84; β = 0.054, 95% CI 0.002, 0.107) during pregnancy and PB in cord blood (mean 3.50 μg/dL, SD 2.59; β = 0.050, 95% CI 0.004; 0.096) were associated with increased cord blood mtDNA content (mean 1.46, SD 0.44). In two-way interaction analyses, cord blood Pb marginally interacted with gestational age leading to an increase in mtDNA content for pre-term births (Benjamini-Hochberg False Discovery Rate correction; BH-FDR = 0.08).
Conclusion:
This study shows that lead exposure in pregnancy alters mtDNA content in cord blood; therefore, alteration of mtDNA content might be a mechanism underlying the toxicity of lead.
Keywords: mtDNA content, Lead exposure, Pregnancy, mitochondrial dysfunction and cord blood
1. Introduction
Efforts to decrease lead (Pb) exposure have resulted in lower blood Pb levels over time. The current recommended limit for blood Pb is 5 μg/dL [1]; however, the Environmental Protection Agency (EPA), the Centers for Disease Control and Prevention (CDC), and the American Academy of Pediatrics recognize there is no safe level [2, 3]. Pregnancy is one of the most vulnerable windows for exposure to pollution, and Pb is a well-known neurotoxic heavy metal that can cross the placenta [4]. Pb is associated with fetal growth restriction, preterm delivery, and premature rupture of membranes (PROM) [5–7]. Pb exposure during pregnancy is also associated with neurodevelopment issues in children, such as behavior and learning problems, hyperactivity, slowed growth, hearing problems, and anemia [3].
Among its various actions, Pb can reach cellular mitochondria and affect their function [8]. Pb is considered a potent oxidative stress inducer that in addition can also decreased mitochondrial antioxidant enzymes levels [9], leading to decrease energy production (ATP levels) and reduced Na(+)/K(+) ATPase activity [10, 11]. Mitochondria produce the majority of cellular energy, via oxidative phosphorylation, but also participate in regulating calcium stores, cell signaling, and apoptosis [12–15]. Since the main function of mitochondria is to provide energy to cells, disruption of mitochondria may affect a variety of organs and tissues, particularly those with the highest energetic demands, such as muscle, heart, liver, and brain [16]. Mitochondria have multiple copies of small circular mtDNA (~16.5kb), whose number is regulated through the processes of biogenesis (production of new mitochondria) and mitophagy (elimination of damaged mitochondria) [17, 18] to appropriately satisfy the metabolic and energy demands of tissues and cells [19]. MtDNA is especially sensitive to oxidative stress and is more prone to damage than nuclear DNA since compared to nuclear DNA, mtDNA lacks histone proteins and introns and has lower DNA repair activity, due to the lack of nuclear excision repair (NER) in mitochondria [8, 20]. In addition, it has been suggested that mitochondria compensate for mtDNA oxidative damage by increasing mtDNA content [21], hence mtDNA content has been suggested as a marker of mitochondrial response to damage. However, to our knowledge, no study has yet evaluated the effects of prenatal Pb exposure on cord blood mitochondrial DNA in humans. The goal of this study was to evaluate the association between prenatal Pb exposure at various gestational stages on mitochondrial DNA content—a marker of mitochondrial response to damage [21]—in cord blood from the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) cohort. Our hypothesis was that lead exposure during pregnancy will lead to increase mtDNA content, since increases in mtDNA content may represent a beneficial response to moderate stress induced by pregnancy Pb exposure [8, 22].
2. Methods
2.1. Study participants
The Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study is a prospective cohort comprised of pregnant women from Mexico City who received prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social– IMSS) between December 2007 and July 2011. Details of the cohort recruitment have been reported previously [23–25]. In brief, 1054 participants (< 20 weeks gestation; maternal age of ≥ 18 years and without medical history of heart or kidney disease) underwent clinical examinations at different hospitals from IMSS. Both premature rupture of membranes (PROM) and preeclampsia were medical diagnosis obtained from medical records. We collected venous umbilical cord blood from 540 of the 948 infants born into the study. Missing samples were mainly because births occurring late at night or in the very early morning hours or because mothers did not report the start of labor to the study workers. At the moment of the present study we only had 516 DNA samples and all of them were analyzed for mtDNA content. Due that maternal or cord blood were lack for some participants, Pb levels were not quantified for all of them in the time frames studied. Hence, the present study included up to 410 mother-infant pairs for whom there was complete information on Pb measurements, cord blood mitochondrial DNA content and covariates. At each visit (second trimester, third trimester and at delivery day), trained interviewers queried participants for detailed information on socio-demographic characteristics, medical history, medication, and lifestyle. Socioeconomic status (SES) was calculated based on an index created by the Mexican Association of Market and Public opinion Research Agencies using variables derived from a questionnaire. The institutional review boards at the Harvard T.H. Chan School of Public Health, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health approved the study. Women provided written informed consent.
2.2. Maternal blood and cord blood lead levels
We measured Pb levels in maternal blood and cord blood samples using a previously described method [24]. Briefly, maternal blood (at second and third trimesters and at delivery) and venous umbilical cord blood were collected in trace metal-free tubes and stored at −20° C. Blood samples (1 mL) were thawed on ice, weighed, digested in 2 mL of ultra-pure concentrated HNO3 acid (1 mL) for 48 h, and diluted to 10 mL with deionized water after the addition of 0.5 mL of 30% hydrogen peroxide. Samples were handled in an ISO Class 5 laminar flow clean hood in an ISO Class 6 clean room and analyzed using an Agilent 8800 ICP Triple Quad (ICP-QQQ) instrument (Agilent technologies, Inc., Delaware, USA) in MS/MS mode with Lutetium as the internal standard. Laboratory recovery rates for quality control standards and spiked samples were 85 to 115% and precision (given as % relative standard deviation) was < 10% for samples with concentrations above the limit of quantitation. The limit of detection for Pb by this procedure was 0.065 μg/dL and limit of quantitation was 0.22 μg/dL.
2.3. Mitochondrial DNA (mtDNA) content
Total DNA extraction was done by two different DNA extraction methods, the first 260 samples were extracted using a QIAamp DNA Blood Kit (QIAGEN) while the rest of samples were extracted by conventional phenol–chloroform method after red cell lysis. Then, we measured venous cord blood relative mtDNA content through mtDNA/ nDNA, a biomarker representing the mitochondrial DNA copy number versus the nuclear DNA copy number using a previously described method [21].
Briefly, we adapted a multiplex quantitative real-time polymerase chain reaction method with minor modifications [26]. To measure mtDNA content, we used the mtDNA 12S ribosomal ribonucleic acid (RNA) TaqMan (Applied Biosystems, Waltham, MA) probe (6FAM-5′ TGCCAGCCACCGCG 3′-MGB). The sequences of primers used for amplification of mtDNA were mtF805 (5′CCACGGGAAACAGCAGTGATT3′) and mtR927 (5′CTATTGACTTGGGTTAATCGTGTGA3’). The quantity of mtDNA was corrected by simultaneous measurement of a single-copy nuclear Ribonuclease P gene (TaqMan RNase P Control Reagents Kit, Applied Biosystems). Real-time polymerase chain reaction assays were performed using the Bio-Rad CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA). A laboratory control reference DNA sample, which was a pool of 300 test samples (20 μL taken from each sample, final concentration 40 ng/μL), was used to construct standard curves with five-points (10 ng/μL, 3.33 ng/μL, 1.11 ng/μL, 0.37 ng/μL and 0.12 ng/μL.) to quantify mtDNA and nDNA copy numbers to standardize the mtDNA/nDNA obtained from all test samples, which were based on the linear relationship between the crossing point cycle values and the logarithm of the starting copy number. Therefore, a ratio value of 1 indicates that the mtDNA/nDNA of the test sample is equal to the mtDNA/nDNA in the reference DNA pool used in the assay. All samples were run in triplicate. The within-run and between-run coefficients of variation of this assay were 5% and 7%, respectively.
2.4. Statistical analysis
We fitted multivariate linear regression models to estimate the association of Pb levels in maternal blood at different gestational ages (second trimester, third trimester, and at delivery) and in cord blood with cord blood mtDNA content. Because DNA was extracted using two different methods, we conducted the following normalization procedure. We calculated the ratio of the geometric mean of the two batches and the inverse was applied to the batch that used the phenol-chloroform method because the QIAamp DNA Kit was shown to provide the more highly reproducible results [26]. Although values did not change after normalization, mtDNA content values were log-transformed (ln) [25]. Pb levels were log-transformed (ln) to approximate normality. Each model was adjusted for potential confounders selected a priori based on previous literature [21, 25, 27, 28]: mother’s age, body mass index (BMI) —we added 2 kg to traditional cut-off points to account for normal weight gain in the first trimester of pregnancy as previously reported [29]—socioeconomic status (SES), second-hand smoking was used as prenatal exposure to tobacco smoke, since the participants reported were not active smoker before or during pregnancy, gestational age, child’s sex, C-section, premature rupture of membranes (PROM), preeclampsia, and date of visit to control for possible long-term trends that affected Pb exposure. PM2.5 levels were included, since lead is a component of fine particles [30] and it has been reported that exposure to PM2.5 during pregnancy is associated with mtDNA content [25, 28]. In addition, we evaluated the relationship between the potential confounders and mtDNA content through t-tests and ANOVA. We also adjusted for platelet and leucocyte counts in venous cord blood because mtDNA content can vary by cell-type [31]. Q-Q plots of the residuals were used to test the assumptions of all linear models.
To evaluate the interaction between Pb exposure at different time frames and potential effect modifiers, including mother’s age, BMI, SES, second-hand smoking, gestational age, C-section, premature rupture of membranes and preeclampsia. We ran linear regression models that included two-way interaction terms between Pb levels and the effect modifiers of interest. For instance, to consider gestational age as an effect modifier of second trimester Pb levels, we fitted the term Yi = log_Pb2T + gestational age + log_PbM2T*gestational age + covariates. To account for the multiple testing in interaction analyses we calculated the Benjamini-Hochberg False Discovery Rate correction and a BH-FDR < 0.05 was considered as significant. All analyses were performed using SAS Studio 3.6 (SAS Institute, Cary NC) and R version 3.3.0 (Vienna, Austria).
3. Results
3.1. General characteristics of the cohort
We studied 410 mother-infant pairs from the PROGRESS cohort in Mexico. Mean age during pregnancy was 27 years (18 to 44 years) (Table 1). Participants had a mean BMI of 26.84 kg/m2 and 42% were overweight or obese. Twenty-nine percent were exposed to second-hand smoking and approximately 50% had low SES. Eleven percent of births were preterm (< 37 weeks) and almost 50% of the children were delivered through C-section. The mtDNA content observed in the whole population was 1.46 ± 0.44 with a range of 0.12 – 3.63. We observed a significant increase in mtDNA content associated with preterm birth, C-section, and premature rupture of membranes (p < 0.05) in bivariate analyses. Higher maternal BMI (> 32 kg/m2) during the second trimester was also associated with higher cord blood mitochondrial DNA content. The number of participants decreased over time through follow up; however, there were no significant differences between the characteristics of the participants at the different time points (Supplemental Table 1).
Table 1.
Characteristics of PROGRESS birth cohort participants
| n (%) | Mean ± SD (Range) | mtDNA content | |
|---|---|---|---|
| All | 410 | 1.46 ± 0.44 | |
| Child Sex | |||
| Male | 228 (56) | 1.46 ± 0.42 | |
| Female | 182 (44) | 1.45 ± 0.47 | |
| Gestational age | 38.34 ± 1.81 (29.00, 43.00) | ||
| Preterm <37 weeks | 45(11) | 1.81 ± 0.62* | |
| 38 weeks | 365 (89) | 1.41 ± 0.40 | |
| Birth weight (kg) | 3.06 ± 0.46 (0.70,4.15) | ||
| Maternal age (years) | 27.22 ± 5.37 (18.00, 44.00) | ||
| 18 - 23 | 111 (27) | 1.42 ± 0.48 | |
| 24 - 27 | 118 (29) | 1.46 ± 0.45 | |
| 28 - 31 | 82 (20) | 1.42 ± 0.34 | |
| 32 - 44 | 99 (24) | 1.52 ± 0.48 | |
| Body Mass Index (2T) | 26.84 ± 4.38 (17.42, 44.70) | ||
| < 27 kg/m2 | 239 (58) | 1.42 ± 0.44 | |
| ≥ 27 and < 32 kg/m2 | 120 (29) | 1.47 ± 0.43 | |
| ≥32 kg/m2 | 51 (13) | 1.59 ± 0.48* | |
| Body Mass Index (3T) | 355 | 29.49 ± 4.34 (19.88, 47.23) | |
| <27 kg/m2 | 107 (30) | 1.44 ± 0.45 | |
| ≥27 and < 32 kg/m2 | 151 (43) | 1.43 ± 0.41 | |
| ≥32 kg/m2 | 97 (27) | 1.50 ± 0.45 | |
| Smoking exposure | |||
| No household smoke exposure | 292 (71) | 1.45 ± 0.46 | |
| Household smoke exposure | 118 (29) | 1.47 ± 0.40 | |
| SES | |||
| Low | 201 (49) | 1.41 ± 0.43 | |
| Medium | 162 (40) | 1.49 ± 0.44 | |
| High | 47 (11) | 1.55 ± 0.53 | |
| Delivery method | |||
| Vaginal delivery | 209 (51) | 1.38 ± 0.38 | |
| C-section | 201 (49) | 1.53 ± 0.49* | |
| Complications | |||
| No Premature rupture of membranes | 366 (90) | 1.44 ± 0.44 | |
| Premature rupture of membranes | 44 (10) | 1.58 ± 0.48* | |
| No Preeclampsia | 391 (95) | 1.45 ± 0.45 | |
| Preeclampsia | 19 (5) | 1.51 ± 0.38 |
Significant differences p<0.05. P-value was obtained from T-test or ANOVA test.
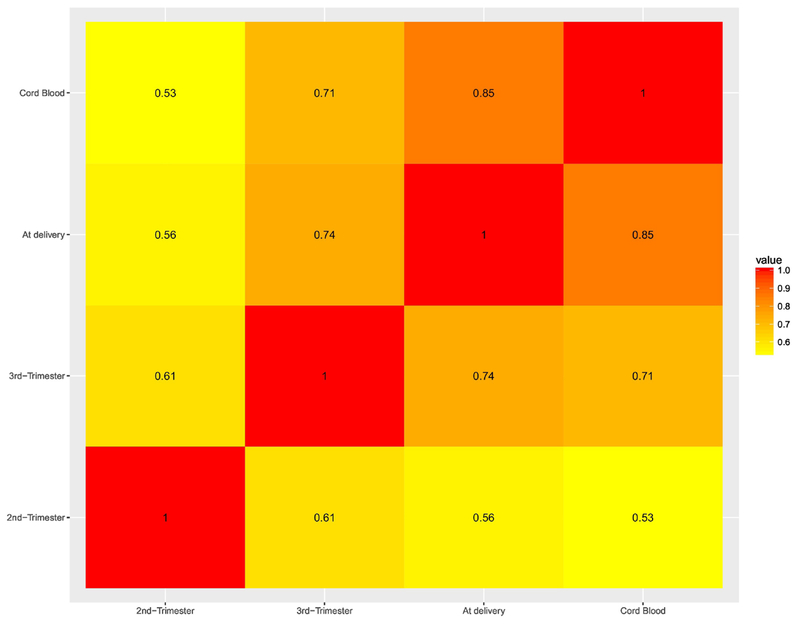
Pb exposure levels for the study period are summarized in Table 2. Second trimester Pb levels ranged from 0.75 μg/dL to 17.81 μg/dL with an average of 3.79 ± 2.63 μg/dL. Table 2 also reports the average Pb level in maternal blood samples from the third trimester and at delivery, as well as in cord blood. Maternal Pb blood levels at delivery were higher than at the 3rd (4.16 vs 3.90 μg/dL) and 2nd trimester (4.16 vs 3.79 μg/dL), while cord blood Pb levels were the lowest (3.50 μg/dL). Pb levels at the different time points were highly correlated (Figure 1 ).
Table 2.
Sample size and distribution of blood lead concentrations in pregnancy, delivery and in umbilical cord blood
| n (%) | Mean ± SD | 10pct | 25pct | 50pct | 75pct | 90pct | Range (μg/dL) | |
|---|---|---|---|---|---|---|---|---|
| Maternal blood lead | ||||||||
| 2nd trimester (μg/dL) | 410 | 3.79 ± 2.63 | 1.44 | 2.07 | 2.91 | 4.51 | 7.13 | 0.75, 17.81 |
| 3rd trimester (μg/dL) | 356 | 3.90 ± 2.84 | 1.45 | 2.11 | 3.10 | 4.73 | 7.21 | 0.77, 28.25 |
| At delivery (μg/dL) | 354 | 4.16 ± 2.85* | 1.61 | 2.28 | 3.35 | 5.28 | 7.64 | 0.77, 21.40 |
| Cord blood lead | ||||||||
| At delivery (μg/dL) | 346 | 3.50 ± 2.59 | 1.22 | 1.77 | 2.83 | 4.45 | 6.57 | 0.40, 18.56 |
Significant differences p<0.01, blood levels at 2nd trimester as a reference, P-value was obtained from ANOVA test.
Figure 1.
Heat map for Pearson pair-wise correlations of ln-transformed Pb levels within different time frames
3.2. Mitochondrial DNA content
We found in multivariate models that maternal Pb levels during the second (β = 0.059, 95% CI 0.008, 0.111) and third trimester (β = 0.054, 95% CI 0.002, 0.107) were significantly associated with higher mtDNA content. Cord blood Pb levels (β = 0.050, 95% CI 0.004; 0.096) were positively associated with mtDNA content, while maternal blood Pb levels at delivery were marginally associated (Table 3).
Table 3.
Association of blood ln-Lead levels at different time frames with cord blood mitochondrial DNA content, PROGRESS cohort study, 2007-2011
| Ln-Lead levels (n) | Estimate | P-value* | 95% Confidence Limits | ||
|---|---|---|---|---|---|
| Second trimester | (410) | 0.059 | 0.024 | 0.008 | 0.111 |
| Third trimester | (356) | 0.054 | 0.042 | 0.002 | 0.107 |
| At delivery | (354) | 0.050 | 0.075 | −0.005 | 0.104 |
| Cord blood | (346) | 0.050 | 0.035 | 0.004 | 0.096 |
P-values were obtained from linear regression models with log transformed measures of lead exposure.
Results were adjusted for sex, mother’s age, mother’s BMI, SES, smoke exposure, PM2.5 levels, gestational age, platelets and leucocytes in cord blood, C-section, PROM, preeclampsia and date of visit.
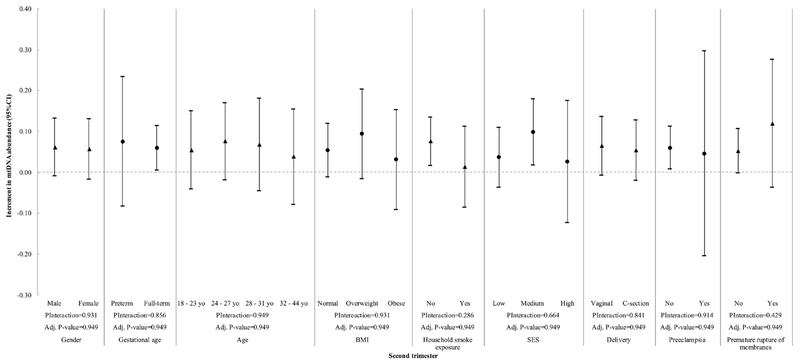
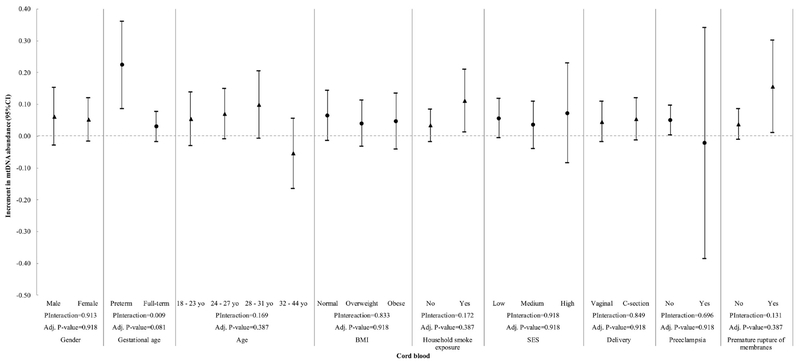
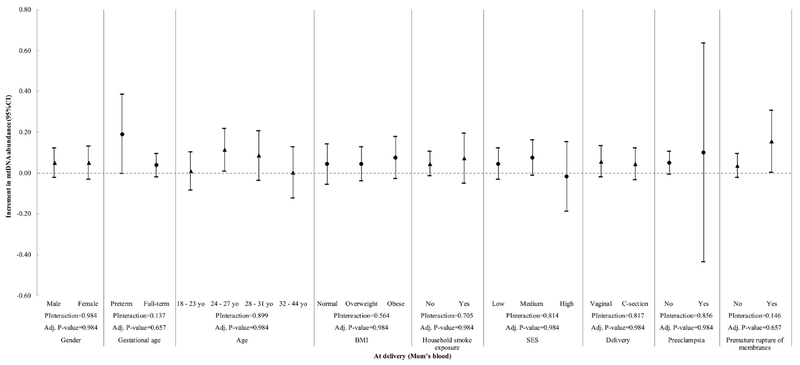
We then evaluated the interaction between the variables in Table 1 and Pb exposure at different gestational stages with mtDNA content (Figure 2 to 5). We observed a significant interaction between gestational age and Pb levels in cord blood (Pinteraction= 0.009) (Fig. 5). However, at a BH-FDR < 0.05, the interaction of Pb levels in cord blood and gestational age becomes marginal (adjusted P-value= 0.081). Pb levels in cord blood significantly increased mtDNA cord blood content in preterm children (β = 0.224, 95% CI 0.085; 0.362), but this association was not observed in full-term children (β = 0.030, 95% CI −0.018; 0.078, Fig. 5). Child sex, mother’s age, BMI, SES, second-hand smoking, C-section, premature rupture of membranes and preeclampsia did not significantly interact with Pb levels within any time frame.
Figure 2.
Interaction of maternal blood lead exposure (second trimester) and all covariates with mtDNA content in cord blood. P-Interaction values were calculated from multivariate models that include two-way interaction terms between Pb levels and the effect modifiers of interest plus covariates. Adjusted P-values were calculated using the Benjamini-Hochberg False Discovery Rate correction and a BH-FDR < 0.05 was considered as significant.
Figure 5.
Interaction of cord blood lead exposure and all covariates with mtDNA content in cord blood. P-Interaction values were calculated from multivariate models that include two-way interaction terms between Pb levels and the effect modifiers of interest plus covariates. Adjusted P-values were calculated using the Benjamini-Hochberg False Discovery Rate correction and a BH-FDR < 0.05 was considered as significant.
4. Discussion
In this study of participants from the PROGRESS cohort, we found that Pb levels at different time frames during pregnancy were associated with increased mtDNA content in cord blood. In particular, maternal Pb levels during the second and third trimester as well as Pb levels in cord blood were significantly associated with higher mtDNA content in cord blood. We also found that gestational age marginally modified the associations between cord blood mtDNA content and Pb levels in cord blood, as there was a significant increase in cord blood mtDNA content in children that were born preterm. It has been widely suggested that children, even if exposed to low doses of pollutants during in utero development may have increased risk of disabilities, diseases, and death in childhood or later in life [32]. This represents a substantial public health problem that burdens families and the health care system [32]. According to The Lancet Commission on pollution and health, the removal of Pb from gasoline dating from 1980 is an example of successful prevention that returned an estimated $200 billion to the economy every year in reduced morbidity, plus over $6 trillion thanks to the increased cognitive function and enhanced economic productivity of generations of children now exposed to lower levels of Pb [32]. In Mexico, since the phase-out of leaded gasoline, blood Pb levels in urban areas have decreased, but remain high in rural areas (5.36 μg/dL and 8.9 μg/dL, respectively) [33]. In our study, the mean levels of Pb were lower than those described above and were below the current reference level of 5 μg/dL from the Centers for Disease Control and Prevention [1]; however, some participants had higher levels; for instance, one participant had Pb levels of 28.25 μg/dL at the third trimester and 21.40 μg/dL at delivery. In urban areas such as Mexico City, where our population was recruited, lead-glazed ceramics are likely the primary exposure source [33].
We found that maternal blood Pb levels during the second and third trimester and cord blood Pb levels were associated with higher mtDNA content in cord blood. Pb is a well-known heavy metal that crosses the placenta and affects child cognitive function [4, 34]; Pb is also a mitochondrial toxicant that can accumulate in mitochondria by entry via calcium transporters due its molecular mimicry of calcium [8]. As has been reported mtDNA is more susceptible to oxidative stress (10 times) and mutations (5 times) than the nDNA [17]; however, also it has been suggested that mitochondria compensate for mtDNA oxidative damage by increasing mtDNA content under the challenge of toxics exposure [21]. -Hence, our results are consistent with studies that suggest an increase in mtDNA content under the challenge of exogenous or endogenous sources of oxidative stress [35–37]. We did find a significant association between maternal blood Pb levels during the second and third trimester with cord blood Pb levels and mtDNA content. We also observed a positive trend between lead in maternal blood at delivery time with mtDNA content, suggesting that the effect of lead by itself on mitochondria might be chronic (months), recent (weeks) and even acute (hours or days). Therefore, a constant monitoring of blood lead levels, especially among pregnant women, should be considered by governments and health care providers as a routine test in order to discard lead exposure and guaranty health to the population and indirectly improve familiar and country’s economy as the Lancet commission has documented.
Mitochondrial content is largely unexplored in environmental studies, especially for Pb exposure. Therefore, we included some variables as possible confounders and effect modifiers solely based on the literature on prenatal Pb exposure and child outcomes (i.e., sex, mother’s age, mother’s BMI, SES, smoke exposure, gestational age, platelets and leucocytes in cord blood, C-section, and premature rupture of membranes, preeclampsia, and date of visit). We found that C-section and premature rupture of membranes were related per se with a significant increase in mtDNA content. The proportion of complications during pregnancy, such as premature rupture of membranes (10%) and preeclampsia (5%), are similar to those found previously in Mexico [38, 39]. However, the proportion of C-section in our population (50%) was higher than the average global rate (18.6%) as well as the rate for high-income regions (27.2%) [40] and significantly higher than the World Health Organization recommended rate of 10–15% [41]. It was, albeit, very similar to the 2012 Mexican National Health and Nutrition Survey (ENSANUT) where 47% were either a planned or emergency cesarean sections [42].
Preterm delivery and PROM have previously been associated with Pb exposure [5–7]. In a study of 262 mother–infant pairs from California (14–45 years), women with Pb higher than 10 μg/dL had increased risk for preterm birth (OR = 3.2, 95% CI 1.2–7.4) [5]. In a study of 348 women (16–35 years), a 1 unit increase in blood Pb levels during the first trimester was associated with increased risk of preterm birth (OR 1.41, 95% CI 1.08 to 1.84) [6]. In a study in 332 women (16–35 years) from Tehran, Iran, Pb levels during the first trimester of pregnancy were associated with increased risk of PROM [7]. In our study, PROM was responsible for 27% of the preterm births, which is similar to the 30% reported for the US [43] and Pb exposure was not associated with preterm birth and PROM, but we found Pb levels in cord blood interacted with preterm birth. While we saw that lead exposure, at different time frames (second and third trimester and in cord blood), by itself has an effect on cord blood mtDNA content, we only found a marginal interaction effect of cord blood lead and gestational age on mtDNA content; meaning that children preterm and exposed to Pb are more prone to have increased oxidative stress damage, since mtDNA content was also high and has been reported that mitochondria compensate oxidative damage by increasing mtDNA content [21]. These results suggest that lead exposure can also have an effect that would be potentialized from other factors, such as pregnancy complications, in particular preterm birth. MtDNA content has been suggested as a compensatory response for oxidative stress [44–47], which may contribute to diseases later in life, such as hypertension, cardiovascular disease, and diabetes. Therefore, while no data are currently available to determine whether Pb-induced mitochondrial toxicity affects children’s health, further studies are needed to prospectively evaluate clinical phenotypes, such as those mentioned above, particularly among children who were born preterm.
Our study has several strengths. We adjusted all analyses for relevant covariates. In addition to clinical and personal variables, we also adjusted for platelet and leucocyte counts, as changes in cell proportions in blood may affect blood mtDNA content. In addition, we included PM2.5 as a covariate in the models, since it has been shown that PM2.5 is associated with mtDNA content [25, 28], hence the associations observed were independently of the air pollution exposure. However, we also acknowledge some limitations. Some of the eligible participants were excluded from analysis because of missing mtDNA content or covariates data; however, there were no significant differences between the characteristics of the participants at the different time points, then selection bias attributable to informative missingness was unlikely. We measured mtDNA content only at one time point and therefore could not evaluate temporal variations in mtDNA content. It is possible we overestimated mtDNA content, since it has been reported that 90 - 97% of the mtDNA genome sequence can be found inserted in the nuclear genome as nuclear mitochondrial insertion sequence (NUMTs) [48, 49]. Therefore, it is likely we co-amplified NUMTs and mtDNA. However, because mtDNA copies greatly outnumber nDNA copies we anticipate the effect on our results to be minimal. Further, because our measure of mtDNAcn is relative, rather than absolute, the effect is likely mitigated. We cannot exclude false negative findings due to the relatively small sample size for the exploratory interaction analysis; however, our interaction findings were marginal using a stringent cutoff of 0.05 after accounting for multiple testing, limiting the chance of false-positive results. Our study is an observational study and, as such, cannot reveal particular mechanisms. The study population is Mexican and that may limit the generalizability of our findings. More studies involving other demographic groups and in different environments will be needed to confirm our findings.
In summary, our study showed that maternal second and third-trimester as well as cord blood Pb levels were associated with an increase of cord blood mtDNA content and that preterm delivery marginally interact with Pb to increase mtDNA content in cord blood. As the results of fetal programming can only be ascertained years after exposure, investigating the effects of prenatal risk factors requires longitudinal studies with accurate fetal assessments and prospective repeated child phenotyping. Therefore, future studies are warranted to identify health effects later in life to determine whether higher mtDNA content in cord blood predicts higher risk of Pb-related phenotypic alterations later in life, for instance cognitive, cardiovascular or bone health effects.
Supplementary Material
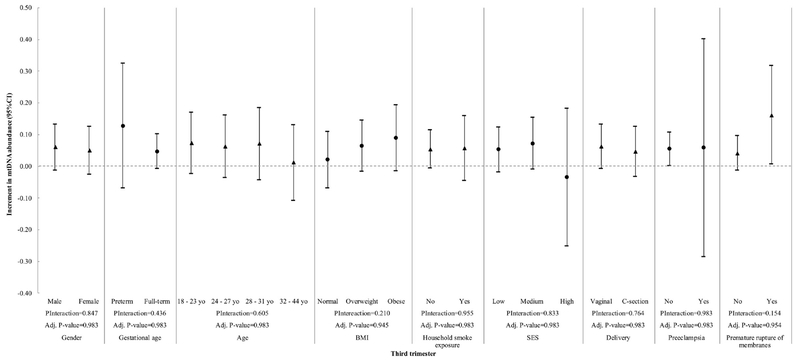
Figure 3.
Interaction of maternal blood lead exposure (third trimester) and all covariates with mtDNA content in cord blood. P-Interaction values were calculated from multivariate models that include two-way interaction terms between Pb levels and the effect modifiers of interest plus covariates. Adjusted P-values were calculated using the Benjamini-Hochberg False Discovery Rate correction and a BH-FDR < 0.05 was considered as significant.
Figure 4.
Interaction of maternal blood lead exposure (mother’s blood at delivery) and all covariates with mtDNA content in cord blood. P-Interaction values were calculated from multivariate models that include two-way interaction terms between Pb levels and the effect modifiers of interest plus covariates. Adjusted P-values were calculated using the Benjamini-Hochberg False Discovery Rate correction and a BH-FDR < 0.05 was considered as significant.
Highlights:
Third trimester maternal blood Pb is associated with greater mtDNA content
Preterm birth, C-section and premature rupture of membranes altered mtDNA content
Gestational age marginally influences Pb effects on mtDNA content
Acknowledgements:
This work was supported by: R01ES013744; R01 ES021357; P30 ES023515; P30ES09089; and R01 ES014930; ABC – hospital and National Institute of Public Health (INSP), Mexico. MSG was financially supported by the Fundación México en Harvard, A.C. and Consejo Nacional de Ciencia y Tecnologia (CONACYT, Mexico).
Abbreviations:
- Pb
lead
- mtDNA
mitochondrial DNA
- ROS
reactive oxygen species
- BMI
body mass index
- SES
socioeconomic status
- RT-PCR
real time polymerase chain reaction
- PROM
premature rupture of membranes
- PROGRESS
Programing Research in Obesity, Growth, Environment and Social Stressors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.CDC: President’s Task Force on Environmental Risks and Safety Risks to Children. In.; 2016.
- 2.Council On Environmental H: Prevention of Childhood Lead Toxicity. Pediatrics 2016,138(1). [DOI] [PubMed] [Google Scholar]
- 3.Zartarian V, Xue J, Tornero-Velez R, Brown J: Children’s Lead Exposure: A Multimedia Modeling Analysis to Guide Public Health Decision-Making. Environ Health Perspect 2017,125(9):097009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen KA: Is Prenatal Lead Exposure a Concern in Infancy? What Is the Evidence? Adv Neonatal Care 2015,15(6):416–420. [DOI] [PubMed] [Google Scholar]
- 5.Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V: Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J Perinatol 2006, 26(3):154–162. [DOI] [PubMed] [Google Scholar]
- 6.Vigeh M, Yokoyama K, Seyedaghamiri Z, Shinohara A, Matsukawa T, Chiba M, Yunesian M: Blood lead at currently acceptable levels may cause preterm labour. Occup Environ Med 2011, 68(3):231–234. [DOI] [PubMed] [Google Scholar]
- 7.Vigeh M, Yokoyama K, Shinohara A, Afshinrokh M, Yunesian M: Early pregnancy blood lead levels and the risk of premature rupture of the membranes. Reprod Toxicol 2010, 30(3):477–480. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS: Mitochondria as a target of environmental toxicants. Toxicol Sci 2013,134(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottipolu RR, Davuljigari CB: Perinatal exposure to lead: reduction in alterations of brain mitochondrial antioxidant system with calcium supplement. Biol Trace Elem Res 2014,162(l-3):270–277. [DOI] [PubMed] [Google Scholar]
- 10.Baranowska-Bosiacka I, Gutowska I, Marchetti C, Rutkowska M, Marchlewicz M, Kolasa A, Prokopowicz A, Wiernicki I, Piotrowska K, Baskiewicz M et al. : Altered energy status of primary cerebellar granule neuronal cultures from rats exposed to lead in the pre- and neonatal period. Toxicology 2011, 280(1-2):24–32. [DOI] [PubMed] [Google Scholar]
- 11.Caito SW, Aschner M: Mitochondrial Redox Dysfunction and Environmental Exposures. Antioxid Redox Signal 2015, 23(6):578–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchen MR: Mitochondria and calcium: from cell signalling to cell death. J Physiol 2000, 529 Pt 1:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tait SW, Green DR: Mitochondria and cell signalling. Cell Sci 2012,125(Pt 4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradelli LA, Beneteau M, Ricci JE: Mitochondrial control of caspase-dependent and -independent cell death. Cell Mol Life Sci 2010, 67(10):1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stotland A, Gottlieb RA: Mitochondrial quality control: Easy come, easy go. Biochim BiophysActa 2015,1853(10 Pt B):2802–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magner M, Kolarova H, Honzik T, Svandova I, Zeman J: Clinical manifestation of mitochondrial diseases. Dev Period Med 2015,19(4):441–449. [PubMed] [Google Scholar]
- 17.Byun HM, Baccarelli AA: Environmental exposure and mitochondrial epigenetics: study design and analytical challenges. Hum Genet 2014,133(3):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carelli V, Maresca A, Caporali L, Trifunov S, Zanna C, Rugolo M: Mitochondria: Biogenesis and mitophagy balance in segregation and clonal expansion of mitochondrial DNA mutations. Int J Biochem Cell Biol 2015, 63:21–24. [DOI] [PubMed] [Google Scholar]
- 19.Mishra P, Carelli V, Manfredi G, Chan DC: Proteolytic cleavage of Opal stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 2014, 19(4):630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazak L, Reyes A, Holt IJ: Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol 2012, 13(10):659–671. [DOI] [PubMed] [Google Scholar]
- 21.Zhong J, Cayir A, Trevisi L, Sanchez-Guerra M, Lin X, Peng C, Bind MA, Prada D, Laue H, Brennan KJ et al. : Traffic-Related Air Pollution, Blood Pressure, and Adaptive Response of Mitochondrial Abundance. Circulation 2016, 133(4):378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer JN, Hartman JH, Mello DF: Mitochondrial Toxicity. Toxicol Sci 2018, 162(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burris HH, Braun JM, Byun HM, Tarantini L, Mercado A, Wright RJ, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM: Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 2013, 5(3):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodosthenous RS, Burris HH, Svensson K, Amarasiriwardena CJ, Cantoral A, Schnaas L, Mercado-Garcia A, Coull BA, Wright RO, Tellez-Rojo MM et al. : Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environ Int 2017, 99:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosa MJ, Just AC, Guerra MS, Kloog I, Hsu HL, Brennan KJ, Garcia AM, Coull B, Wright RJ, Tellez Rojo MM et al. : Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ Int 2017, 98:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreu AL, Martinez R, Marti R, Garcia-Arumi E: Quantification of mitochondrial DNA copy number: pre-analytical factors. Mitochondrion 2009, 9(4):242–246. [DOI] [PubMed] [Google Scholar]
- 27.Vriens A, Plusquin M, Baeyens W, Bruckers L, Den Hond E, Loots I, Nelen V, Schoeters G, Janssen BG, Nawrot TS: Cord blood leptin and insulin levels in association with mitochondrial DNA content. J Transl Med 2018, 16(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W et al. : Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect 2012, 120(9):1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burris HH, Baccarelli AA, Byun HM, Cantoral A, Just AC, Pantic I, Solano-Gonzalez M, Svensson K, Tamayo y Ortiz M, Zhao Y et al. : Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal BMI, gestational age, and birth weight. Epigenetics 2015, 10(10):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow JC, Watson JG, Edgerton SA, Vega E: Chemical composition of PM2.5 and PM10 in Mexico City during winter 1997. Sci Total Environ 2002, 287(3):177–201. [DOI] [PubMed] [Google Scholar]
- 31.Hurtado-Roca Y, Ledesma M, Gonzalez-Lazaro M, Moreno-Loshuertos R, Fernandez-Silva P, Enriquez JA, Laclaustra M: Adjusting MtDNA Quantification in Whole Blood for Peripheral Blood Platelet and Leukocyte Counts. PLoS One 2016, 11(10):e0163770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI et al. : The Lancet Commission on pollution and health. The Lancet. [DOI] [PubMed] [Google Scholar]
- 33.Caravanos J, Dowling R, Tellez-Rojo MM, Cantoral A, Kobrosly R, Estrada D, Orjuela M, Gualtero S, Ericson B, Rivera A et al. : Blood lead levels in Mexico and pediatric burden of disease implications. Ann Glob Health 2014, 80(4):269–277. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Hu H, Wright R, Sanchez BN, Schnaas L, Bellinger DC, Park SK, Martinez S, Hernandez-Avila M, Tellez-Rojo MM et al. : Prenatal Lead Exposure Modifies the Impact of Maternal Self-Esteem on Children’s Inattention Behavior. J Pediatr 2015, 167(2):435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas RR, Khan SM, Portell FR, Smigrodzki RM, Bennett JP Jr.: Recombinant human mitochondrial transcription factor A stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion 2011, 11(1):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HC, Wei YH: Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 2005, 37(4):822–834. [DOI] [PubMed] [Google Scholar]
- 37.Malik AN, Czajka A: Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13(5):481–492. [DOI] [PubMed] [Google Scholar]
- 38.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ: Preeclampsia. Lancet 2016, 387(10022):999–1011. [DOI] [PubMed] [Google Scholar]
- 39.Wallace ME, Grantz KL, Liu D, Zhu Y, Kim SS, Mendola P: Exposure to Ambient Air Pollution and Premature Rupture of Membranes. Am J Epidemiol 2016, 183(12):1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR: The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990-2014. PLoS One 2016, 11(2):e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appropriate technology for birth. Lancet 1985, 2(8452):436–437. [PubMed] [Google Scholar]
- 42.Heredia-Pi I, Servan-Mori EE, Wirtz VJ, Avila-Burgos L, Lozano R: Obstetric care and method of delivery in Mexico: results from the 2012 National Health and Nutrition Survey. PLoS One 2014, 9(8):e104166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simhan HN, Canavan TP: Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. BJOG 2005, 112 Suppl 1:32–37. [DOI] [PubMed] [Google Scholar]
- 44.Carugno M, Pesatori AC, Dioni L, Hoxha M, Bollati V, Albetti B, Byun HM, Bonzini M, Fustinoni S, Cocco P et al. : Increased mitochondrial DNA copy number in occupations associated with low-dose benzene exposure. Environ Health Perspect 2012, 120(2):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou L, Zhu ZZ, Zhang X, Nordio F, Bonzini M, Schwartz J, Hoxha M, Dioni L, Marinelli B, Pegoraro V et al. : Airborne particulate matter and mitochondrial damage: a cross-sectional study. Environ Health 2010, 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen M, Zhang L, Bonner MR, Liu CS, Li G, Vermeulen R, Dosemeci M, Yin S, Lan Q: Association between mitochondrial DNA copy number, blood cell counts, and occupational benzene exposure. Environ Mol Mutagen 2008, 49(6):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou L, Zhang X, Dioni L, Barretta F, Dou C, Zheng Y, Hoxha M, Bertazzi PA, Schwartz J, Wu S et al. : Inhalable particulate matter and mitochondrial DNA copy number in highly exposed individuals in Beijing, China: a repeated-measure study. Part Fibre Toxicol 2013, 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dayama G, Emery SB, Kidd JM, Mills RE: The genomic landscape of polymorphic human nuclear mitochondrial insertions. Nucleic Acids Res 2014, 42(20):12640–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simone D, Calabrese FM, Lang M, Gasparre G, Attimonelli M: The reference human nuclear mitochondrial sequences compilation validated and implemented on the UCSC genome browser. BMC Genomics 2011, 12:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.