Abstract
Traumatic brain injury and the long-term consequences of repeated concussions constitute mounting concerns in the United States, with 5.3 million individuals living with a traumatic brain injury-related disability. Attempts to understand mechanisms and possible therapeutic approaches to alleviate the consequences of repeat mild concussions or traumatic brain injury on cerebral vasculature depend on several aspects of the trauma, including: (1) the physical characteristics of trauma or insult that result in damage; (2) the time “window” after trauma in which neuropathological features develop; (3) methods to detect possible breakdown of the blood–brain barrier; and (4) understanding different consequences of a single concussion as compared with multiple concussions. We review the literature to summarize the current understanding of blood–brain barrier and endothelial cell changes post-neurotrauma in concussions and mild traumatic brain injury. Attention is focused on concussion and traumatic brain injury in humans, with a goal of pointing out the gaps in our knowledge and how studies of rodent model systems of concussion may help in filling these gaps. Specifically, we focus on disruptions that concussion causes to the blood–brain barrier and its multifaceted consequences. Importantly, the magnitude of post-concussion blood–brain barrier dysfunction may influence the time course and extent of neuronal recovery; hence, we include in this review comparisons of more severe traumatic brain injury to concussion where appropriate. Finally, we address the important, and still unresolved, issue of how best to detect possible breakdown in the blood–brain barrier following neurotrauma by exploring intravascular tracer injection in animal models to examine leakage into the brain parenchyma.
Keywords: Concussion, TBI, traumatic, blood–brain barrier, brain, injury
Introduction
Traumatic brain injury (TBI) and the long-term consequences of repeated concussions are a mounting concern in the United States, with 5.3 million individuals living with a TBI-related disability.1 TBI exists as a spectrum, ranging from mild to severe brain trauma, such as intracerebral hemorrhages (ICH) and penetrating missile wounds. However, on the mild end of the spectrum, concussions are far from trivial, as even mild concussions can result in prolonged deficits. The Centers for Disease Control (CDC) estimates that 1.4–3.8 million concussions occur annually in the United States alone.2,3 Efforts to develop therapeutic treatments to deal with the effects of concussions and TBI, as well as possible measures to prevent the secondary effects of head injury in military or sports-related situations, must be based on an understanding of what constitutes TBI, what conditions contribute to the severity of TBI, and what might be the treatment “window” for interventional therapeutics. Attempts to understand mechanisms and possible therapeutic approaches to alleviate the consequences of repeat mild concussions or TBI on cerebral vasculature have led researchers to focus on several aspects of the trauma, including: (1) the physical characteristics of trauma or insult that result in damage; (2) the time “window” after trauma in which neuropathological features develop; (3) methods to detect possible breakdown of the blood–brain barrier (BBB); and (4) understanding the possible different consequences of single as compared with multiple concussions.
The present paper briefly reviews literature on the milder TBI spectrum of concussions and mild TBI. Attention will first be focused on concussion and TBI in humans, with a goal to point out the gaps in our knowledge and how the solution to these gaps may be informed by results from studies in rodent models. Although the bulk of results demonstrate that laboratory rodents exhibit physiological and behavioral responses similar to neurotrauma to humans, it is crucial to bear in mind that lissencephalic rodent cortex, along with possible species differences in fundamental cell biology, may not allow experimental results in rodents to comprehensively model the complex changes in human cortical anatomy following injury.4
Specifically, we will focus on disruptions that concussion causes to the BBB and its multifaceted consequences. Secondary repercussions of post-concussion BBB dysfunction may manifest as neuronal loss, impaired consciousness, memory and motor impairment, cognitive decline and even increased dementia risk.5,6 Importantly, the magnitude of post-concussion BBB dysfunction may influence the time course and extent of neuronal recovery; hence, we include in this review comparisons of more severe TBI to concussion where appropriate.
Overview of the BBB
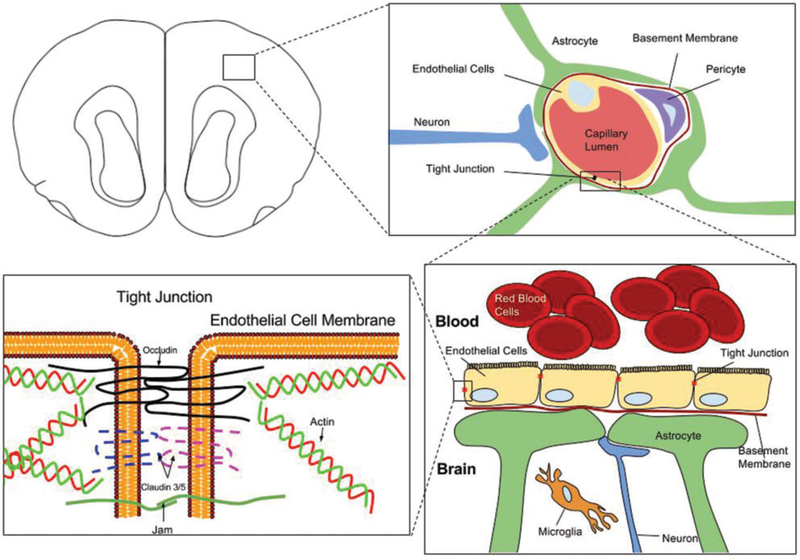
The BBB is a highly selective barrier, formed by specializations of endothelial cells (ECs), which separates circulating blood from neuroparenchymal extracellular fluid.7–11 In addition, astrocytes, microglia, and pericytes contribute to the formation and maintenance of the BBB, and also contribute to aspects of selectivity and specificity regarding the molecules able to traverse the BBB (Figure 1).12 ECs line all vascular structures, but those of the cerebral vasculature are characterized by different genetic profiles than those of other organs.13 Characteristics of ECs that contribute to the regulation of molecular movement between circulating blood and brain parenchyma include tight junctions between adjacent cells, specialized vesicles that contribute to the selectivity of transcellular movement of molecules, and active transport mechanisms. The tight junctions are composed of smaller subunits, often biochemical dimers,14 which are transmembrane proteins including occludins, claudins, and junctional associated proteins (shown in Figure 1). Moreover, glial and ECs communicate via paracrine signaling, contributing to the integrity of the BBB.
Figure 1.
Diagram depiction of the blood–brain barrier (BBB). In the upper left, a cross-sectional depiction of a mouse brain is shown. The upper right panel focuses on the intraparenchymal blood–brain barrier (BBB), demonstrating astrocyte foot-processes, in conjunction with pericytes, forming highly selective tight junctions between capillary endothelial cells. This restricts the movement of many microscopic and macromolecular compounds. The barrier is reinforced by a thick basement membrane. Disruption of the BBB results following concussion and traumatic brain injury (TBI). Microanatomical and functional changes in the cellular constituents of the BBB, such as the endothelial cells, following TBI, result in “leakiness” of the tight junctions and can allow toxic compounds to pass through, compromising neuronal integrity. The two lower panels focus on the molecular components of the tight junction, which include dimeric transmembrane proteins such as occludin, claudins, and junctional adhesion molecules (Jam).
Lipophilic15 and/or very small compounds including water and some gases can pass through the BBB with little resistance. Larger molecules, such as glucose or insulin, can be facilitated past the barrier by transporter proteins located in the endothelial layer of the brain’s blood vessel wall.16 BBB disruption can allow into the neuroparenchyma potentially toxic blood-borne factors (largely proteins), in addition to uncontrolled signaling fluxes from the cellular components of the BBB. These adverse effects will be explored in detail with regard to neurotrauma severity.
BBB studies in humans across the neurotrauma spectrum
The first challenge to understanding neurotrauma is the development of a reliable means of assessing neurotrauma severity. In this regard, standardized scales have proven to be advantageous. The Glasgow Coma Scale (GCS) is a standardized neurotrauma severity scale commonly used clinically in humans, which in conjunction with duration of post-traumatic amnesia (PTA), and loss of consciousness (LOC) duration can classify TBI severity.17–25 Although the GCS is a widely used index of TBI severity, there is widespread agreement that improved tools for the classification and subtyping of brain injuries in humans are needed; considerable efforts to develop such tools are underway.26,27 Other systems, including the Abbreviated Injury Scale (AIS), Trauma Score, and Abbreviated Trauma Score, have been developed to classify TBIs.28,29 The Mayo Classification System for Traumatic Brain Injury Severity has since been developed as a single TBI classification system that uses GCS, PTA, LOC duration, and the presence of skull fractures to accurately classify TBI severity.30 Although many of these approaches have focused on behavioral and clinical neurological assessments of the degree of trauma, additional studies have focused on measures of BBB breakdown.
In the following sections, we review the current understanding of BBB and EC changes post-neurotrauma by injury “severity.” First, we will discuss BBB and EC changes post-concussion in humans; then we will examine common changes in mild, moderate, and severe TBI. An important and still unresolved issue is the question of how best to detect possible breakdown in the BBB following neurotrauma. This issue will be addressed more fully below in the section on animal models, where the general approach is to inject tracer molecules intravascularly and look for leakage into brain parenchyma. Studies in humans are constrained by procedural and ethical issues, but researchers have investigated both leakage of known brain parenchymal molecules into the circulating blood and the leakage of red blood cells or serum proteins into brain parenchyma.31
Concussion
Concussion and mild TBI (mTBI) are terms that historically have been used interchangeably, but technically are different entities with concussion constituting a subset of mTBI. Although the delineation between mTBI and concussion is blurry, we attempt to distinguish the two throughout. Concussion is broadly defined by the Quality Standards Subcommittee of the American Academy of Neurology (AAN) as transient neurological dysfunction due to biomechanical force that may or may not involve LOC; or “an event during which there has been the transmission of physical energy imparted to the head (and neck) by an acceleration.”32 Like in mTBI, LOC is usually present, but not required for a diagnosis of concussion. Additionally, a medical diagnosis of post-concussive syndrome (PCS), which refers to signs and symptoms frequently seen post mild head injury, can differentiate concussion from mTBI; however, the pathogenesis of PCS is not known.33
It is well known that concussive forces can have a multitude of neurological sequelae, including ionic fluxes (rodent),34 indiscriminate neuroparenchymal glutamate release (rodent),35 hyperglycolysis (rodent),36 lactate accumulation (rodent),37,38 and diffuse axonal injury (cat, human).39–41 Secondary consequences include intracellular hypercalcemia (rodent),42–44 mitochondrial dysfunction (rodent),45,46 impaired oxidative metabolism (cat),47 decreased glycolysis (rodent),48 diminished cerebral blood flow (rodent),49,50 axonal disturbances, neurotransmitter disarray, and apoptotic delay.51 Furthermore, the post-injury period increases the brain’s susceptibility to further damage. This suggests that even small concussive forces, which remain undetectable in humans by current techniques, may increase the risk for damage from subsequent impacts and thus have clinically relevant consequences, which are additive over time.
Marchi et al.52 used diffusion tensor imaging (DTI) and serum measurements of S100B (a glial-specific protein secreted by cells in the central nervous system (CNS) and used as a marker of BBB disruption) and S100B autoantibodies to evaluate whether low-force head impacts can disrupt the BBB even when they are not considered as classic neurotrauma.53 The study included 67 college football players. In a subset of the players (n=15), for whom pre- and post-game blood samples were available, the players with the most sub-concussive head impacts, based on self-report and review of corresponding game recordings, had detectable serum levels of S100B and elevated levels of auto-antibodies against S100B. Serum S100B antibodies predicted lasting changes in mean brain white matter diffusivity in a subset of players (n=10) who had pre-season, post-season, and six-month follow-up DTI scans. They also found that post-season S100B autoantibodies also correlated with impulse control and balance problems. Although the study sample is too small for conclusive findings, this research supports the hypothesis that repetitive head impacts—even of low force—may lead to BBB disruption. With recurrent concussions, the effects can be similar to a TBI, as will be discussed later.
In an alternative approach, some investigators have focused on cerebral microbleeds,54 which are small collections of red blood cells, or their iron-containing residues, that can be detected by magnetic resonance imaging. Such microbleeds have been demonstrated to result from a variety of cerebral traumas, including stroke, seizures, and head trauma.55 These microbleeds can occur following concussion and increase the risk for future ICH, which contributes to cognitive impairment and dementia.
Studies have found that after concussion, various neuropathological changes may persist for days or weeks in humans. For example, even in individuals with normal GCS scores, significant changes in cerebral glucose metabolism were found with the first month post-concussion.56 Specific interest is now turning to finding cerebrospinal fluid (CSF) and peripheral biomarkers that can be used to assay concussion, TBI, and BBB integrity—though none have been widely used clinically.57 One study examined individuals with PCS using electroencephalogram recordings and single photon emission computed tomography to examine the BBB. The results showed that abnormal rhythm generators were closely related to the anatomic location of the injury and corresponding BBB lesion. The authors suggested that focal cortical dysfunction, BBB disruption, and hypoperfusion might underlie the pathogenesis of PCS.33
Among proteins in circulating blood, in addition to S-100β discussed above, other potential biomarkers for concussion and mTBI include ubiquitin C-terminal hydroxylase L1 (UCH-L1), neuron-specific enolase (NSE), cleaved i protein (CTP), brain-derived neurotrophic factor (BDNF), creatine kinase brain isoenzyme (CKBB), glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP), but only UCH-L1, S-100β, NSE, and CTP have been studied in patients with mTBI.58–61 NSE, similar to S-100β, has a very variable sensitivity ranging from 40 to 89%, making it too insensitive to diagnose mTBI, and does not reliably provide prognostic information on mTBIs.62–65 Similarly, CTP has not been found to significantly differ between control subjects and those with mTBI.66,67 UCH-L1 has also been demonstrated to be elevated in TBI patients, with less severe TBI (GCS of 6–8) being associated with lower UCH-L1 levels in CSF.68–70 NSE, GFAP, BDNF, CKBB, and MBP all correlate with TBI severity but are also found in patients with polytrauma without TBI.71 With the exception of S100B, these other biomarkers have not been studied as well and do not appear to be promising for diagnostics.
Vascular changes post-concussion
Following direct impact to the brain, considerable variation in displacement characteristics between different structures, which can lead to intracranial shear, tensile, and compressive forces. Impact-induced shearing stresses result in axonal injury as well as primary vascular damage leading to the leakage of blood-borne proteins and extravasation of red blood cells into the brain parenchyma. In addition to these specific regions, isolated petechial hemorrhages were scattered throughout the brain and were sometimes located contralateral to injury—consistent with a coup-contracoup injury in mild head injuries.9,72 In addition to these clear damages to vascular elements, more subtle damage to the BBB can result from concussion. Although EC changes in humans post-concussion have not been well characterized, BBB damage likely causes significant changes to EC microstructure and function. The above studies, which focused on BBB changes post-concussion, suggest that neurological trauma of any magnitude has substantial clinical consequences. Next, the effects of mTBI on BBB and EC integrity will be examined.
mTBI
Concussion and mTBI share many similarities. The diagnostic criteria differentiating the two are sometimes elusive and non-delineative. The two terms are often used interchangeably, as concussion is normally considered the mildest arm of the TBI spectrum.73 A recent review of concussion and PCS helped distinguish PCS from mTBI.74 The model defines mTBI using the American Congress of Rehabilitation Medicine and the CDC criteria: Mechanical force to the head resulting in LOC for no more than 30min or amnesia and a GCS of 13–15. Concussion is defined using the AAN criteria of a trauma-induced alteration of mental state with or without LOC. This implies that concussion is relatively transient from a clinical perspective, with a shorter time course in comparison to mTBI, and no or very brief LOC; yet, both concussion and mTBI can lead to permanent alterations in brain function. Individuals with both mTBI and concussion may have clinically silent symptoms. PCS, as defined by Willer and Leddy, is the clinical persistence of concussive symptoms beyond the period where the individual would have been expected to recover, which is usually a three-week period, and subsequently the single incident would qualify as mTBI.75 Although this definition is clinically useful, it deemphasizes the potential longterm sequelae of concussion and mTBI.
The National Center for Injury Prevention and Control considers mTBI as a “silent epidemic” due to the subclinical symptomatology and lack of visible neurostructural changes normally seen in severe neurotrauma.76 Currently, no universal criteria of mTBI exist.77 Commonly accepted criteria include physiological disruption of cognitive function manifested by one or more of the following: alteration of mental state, LOC, memory loss, or focal neurological deficit.78,79
Humans demonstrate a particular vulnerability to mTBI in the frontal lobes. Interestingly, studies have shown that acute mTBI and chronic mTBI differ in fMRI-determined levels of anisotropy—with acute mTBI associated with elevated anisotropy values while chronic mTBI is correlated with depressed anisotropy.80
Studies of TBI and inflammatory responses
Currently, the utility of inflammatory biomarkers to assess the severity of neuropathology following mTBI remains elusive. However, recently some human studies have begun to explore the important relationship between inflammatory markers and mTBI. Importantly, Hergenroeder et al.81 found plasma levels of C-reactive protein (CRP), a sensitive but non-specific marker of systemic inflammation, to be a robust predictor of TBI pathological symptomatology. In fact, by Su et al. identified a significant association between continued elevated CRP levels and increased the incidence of PCS (OR=2.719; CI 95%: 1.609–4.594) in 213 patients with mTBI.82 Essentially, among this cohort of patients, persistent higher CRP levels forecasted clinically relevant cognitive impairment.
Moreover, S100B is a calcium-binding protein that is notably detected in glial cells at physiologic concentrations. However, overproduction of S100B at levels >0.30μg/L of plasma at six months after mTBI was linked to neuroinflammation and injury.83,84 In particular, Zongo et al. examined CT scans and plasma S100-B for 1560 patients with mTBI and found that patients with positive CT scan had a higher S100B median relative to patients with negative CT scan results (0.46 vs. 0.22μg/L, p<0.001).85 Furthermore, prior studies have shown IL-6, a pro-inflammatory cytokine, to be an independent predictor of mTBI.86,87 Interestingly, the findings of a study that evaluated inflammatory markers in 16 pediatric patients demonstrated higher concentrations of IL-6 in patients with mTBI relative to controls without injury, (p<0.00).88 Finally, these investigations highlighted novel inflammatory markers that can, to some extent, uniquely classify mTBI in human patients, thereby translating theory into clinical practice.
While studies focusing on systemic inflammation after mTBI in humans are limited, the opposite trend is observed for studies on severe TBI. Specifically, Kossmann et al.89 examined IL-6 levels in the serum and CSF of 20 patients with severe mTBI and found maximum levels of IL-6 on day 1 and 2 post-trauma in both of these body fluids. Pointedly, peak IL-6 levels strongly correlated with severe BBB dysfunction (r=0.637, p=0.001) and CRP and α1-antitryptin reactant proteins (r=0.605, p=0.004; r=0.719, p=0.0002) in serum. Ultimately, these findings highlight the principal role of the CNS in initiating the acute-phase response in patients with severe TBI.
Likewise, Hergenroeder et al.90 assessed the relationship between IL-6 levels and intracranial pressure (ICP) in patients with severe TBI (GCS<8) and found a significant mean difference between healthy controls and severe TBI patients, with the latter having higher IL-6 levels and experiencing ICP ≥25mm Hg.90 To further refine this relationship, increases in ICP are dependent on the formation of brain edema; thus and not surprisingly, peak pressures were found three to five days post-injury. Ultimately, these studies all underline the importance of identifying prognostic inflammatory biomarkers in patients with TBIs in order to advance and support clinical management.
Studies of TBI in laboratory rodents
While a common goal is to understand mechanisms of TBI in humans and then develop therapeutic interventions, the combination of ethical and procedural challenges of doing mechanistic work in humans has led to efforts to develop neurological models in laboratory animals, primarily rodents, and test hypotheses in these models. Use of rodent models allows investigators to address the fundamental issues outlined above, including: (1) the physical characteristics of trauma or insult that result in damage; (2) methods to detect possible breakdown of the BBB; (3) the time “window” after trauma in which neuropathological features develop; and (4) understanding possible different consequences of single as compared with multiple impacts. The integrity of the BBB is of critical importance in determining the sequelae of cerebral impact; the use of laboratory rodents also has allowed a closer look at the organization of the BBB.
Rodent models of TBI
Exhaustive modeling of TBI has been well characterized in rodents. Both invasive and non-invasive models have been developed to mimic neurophysiological and pathological changes post-TBI. Several models with various parameters exist, such as the controlled cortical impact (CCI) model,91 the weight drop acceleration impact model,92–94 the blast model,95,96 and the fluid percussion model.97–99 Injuries produced in each model can range in severity based on varying levels of force applied to the skull or directly to the brain. Replication and reproducibility are consistent across laboratories, although a range of parameters has created a wide range of injury severity from mild to moderate to severe, relative to one another (Table 1).
Table 1.
Range of parameters used for TBI severity in rodent models.
| TBI severity | Mild | Moderate | Severe |
|---|---|---|---|
| CCI – mouse | 5.0 m/s, 2 mm depth, 3.5 mm tip100 | 6.0 m/s, 2 mm depth, 3.5 mm tip100 | 7.5 m/s, 2 mm depth, 3.5 mm tip100 |
| 5.25 m/s, 1.5 mm depth, 3.5 mm tip101 | 5.25 m/s, 2.0 mm depth, 3.5 mm tip101 | 5.25 m/s, 2.5 mm depth, 3.5 mm tip101 | |
| 6.0 m/s, 0.5 mm depth, 2.0 mm tip102 | 6.0 m/s, 1.0 mm depth, 2.0 mm tip102 | 6.0 m/s, 2.0 mm depth, 2.0 mm tip102 | |
| CCI – rat | 6.0 m/s, 1 mm depth, 5 mm tip103 | 6.0 m/s, 2 mm depth, 5 mm tip103 | 6.0 m/s, 3 mm depth, 5 mm tip103 |
| 6.0 m/s, 0.5 mm depth, 3.0 mm tip102 | 6.0 m/s, 1.0 mm depth, 3.0 mm tip102 | 6.0 m/s, 2.0 mm depth, 3.0 mm tip102 | |
| 3 m/s, 1 mm depth, 3.0 mm tip104 | 4 m/s, 2.5 mm depth, 5.0 mm tip104 |
For example, the CCI model utilizes commercially available devices that deliver impacts to either the skull or brain directly, at speeds ranging from 0 to 6.0m/s, depths of injury from 1 to 3.0mm, as well as different impactor tip diameters ranging from 1 to 5mm. Commercially available CCI devices have improved reproducibility of classic weight drop models that have variability due to imprecise impact location. When weights are dropped down a tube, lateral movement along the way down, and upon exiting the tube, can create an uneven, sided, and/or angled impact to the rodent’s skull. CCI devices have resolved this short-coming with much more controlled injury location setup. As a result, CCI devices have become quite popular in modeling repeated mild mTBI.105–107
No exact definition of mild, moderate, or severe has been widely accepted in regards to injury parameters, but rather, these terms are used relative to one another in a spectrum of disease progression and mimic the clinical neuropathological presentation of varying TBI severities. Most often, mTBI is characterized in rodent models as having minimal LOC as defined by righting time post injury, little to no BBB breakdown, diffuse axonal injury, neuronal and synaptic changes, as well as metabolic changes.
Methods of detection of vascular changes post-TBI
While studies of BBB changes in humans have relied largely on analyses of brain parenchymal proteins appearing in circulating blood, experimental studies in laboratory rodents typically have relied upon intravascular injections of tracer molecules, and searching for evidence of extravasation of those tracer molecules into brain parenchyma.
The detection of disruption of brain vasculature in general and the BBB in particular is reliant in part upon the ability to image the fine vascular elements in brain. The use of immunohistochemistry to detect endothelial related markers has commonly been used, particularly Von Wildebrand factor and CD-31 or PECAM-1, a protein found on the surface of a variety of circulating blood cells and also on the surface of endothelial cells.108 Recently, the use of intravascularly injected tomato lectin has been used to provide a detailed image of cerebral (and other) vascular structures (see Figure 2),109 and use of a zinc fixative before immunohistochemistry for CD-31 in thick vibratome sections has been shown to be helpful in visualizing brain vasculature.110
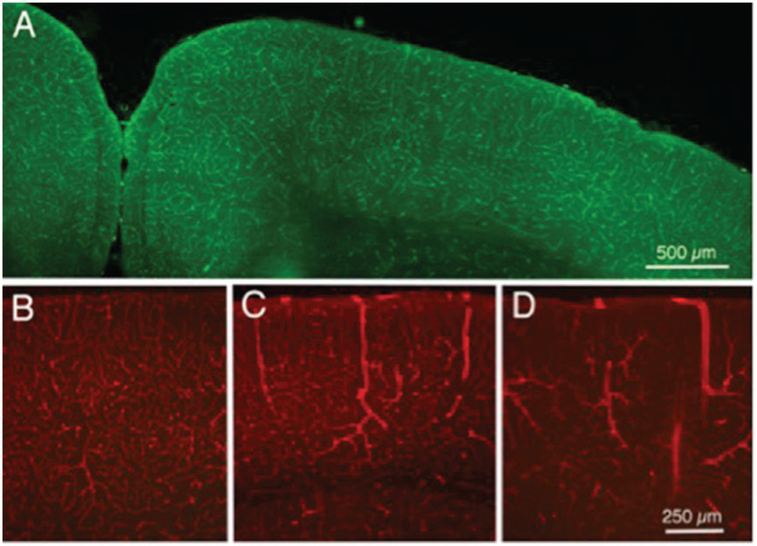
Figure 2.
Fluorescence photomicrographs of sections of cerebral cortex from mice that received intravenous injections of labeled tomato lectin. (a) Normal control mouse, injected with labeled 488 nm lectin. (b) Another normal control mouse, injected with 594 (red) labeled lectin; most labeled vessels are likely capillaries, although arrows indicate larger vessels, likely venules. (c) Mouse injected with 594 lectin 24 h following a single TBI head impact of moderate strength; note the three dilated vessels in cerebral cortex. (d) Mouse injected with 594 lectin after five daily impacts of moderate strength; note the several dilated vessels in cortex (arrows), as well as the reduced numbers of normal appearing capillaries.
A common approach used intravascular injections of Evans blue to assess BBB leakage. Although Evans blue is a relatively small molecule, it binds rapidly and strongly to albumin, a ~68kDa protein that normally is prevented from extravasation into the brain by the BBB. Evans blue can be detected within brain parenchyma, either under bright field optics or fluorescent optics using a long wave length (deep red) filter, and continues to be a useful tool.111–115 Because Evans blue is tightly bound to albumin, other investigators have used intravascular injections of fluorescently labeled albumin.14
In addition to the use of Evans blue, investigators have also employed intravascular injections of horseradish peroxidase liposomes116–118—which also offers the advantage of being able to be detected electron microscopically15 and the extravasation of erythrocytes or endogenous plasma proteins from circulating blood into brain parenchyma.9,116,118
In an attempt to gain a better understanding of the cell biological bases of BBB breakdown, recent studies have employed dextrans.112 Dextrans are linear flexible carbohydrate molecules that are commercially available in varied lengths (molecular weights) ranging from 3kDa to 2000 kDa119 with varied fluorescent tags. Depending on their molecular size, the markers can be used to test both solute and ion permeability (low-molecular weight dextrans) and large molecule permeability (high-molecular weight dextrans). It should be noted that plasma proteins have been shown not to bind to FITC–dextran.112
Hoffman et al. evaluated assays from both EB and FITC–dextrans varying in molecular weight (4, 40, and 70kDa) in Wister rats following a 2-h middle cerebral artery (MCA) occlusion. Extravasation of EB and high-molecular FITC–dextrans (40 and 70kDa) in the infarcted region could be detected with all preparation methods use. However, there was no evidence of extravasation by the 4-kDa low-molecular weight dextran. Diffusion and washout of low-molecular weight FITC–dextran can be avoided when freshly frozen sections are used without a paraformaldehyde (PFA) or sucrose wash, thus minimizing false-negative results.119 Recent development of lysine fixable dextrans has reduced the problem of fixation and post-fixation diffusion of the dextrans.
Additionally, Nagaraja et al. conducted a study to evaluate if there is a limit on the size of a macromolecule before it leaks through the BBB. Following 3h of unilateral MCA occlusion and either 3 or 21h of reperfusion, 42 Wister rats were injected with both EB and fluorescent dextrans, ranging from 77 to 2000kDa. The study concluded that the 2000kDa dextran was too large to pass through the opening of the BBB after 3h of reperfusion in about 40% of the microvascular networks that were visualized.120
These studies give insight on the amount and extent of leakage that different dextrans could indicate, in regard to the disruption of particular components of the BBB. The studies indicate that selectivity in choosing a dextran size to assess BBB permeability as a result of neurotrauma is important. As described above, mTBI or concussion may result in decreased BBB permeability and requires the utilization of lower molecular weight dextrans to investigate BBB leakage. However, with more severe forms of TBI, dextrans of greater molecular weights can be used and will be easier to visualize BBB permeability. There is a need for further studies relating BBB permeability markers such as dextran with TBI visualization to test this hypothesis.
What component of the cerebral vasculature is “Leaky”?
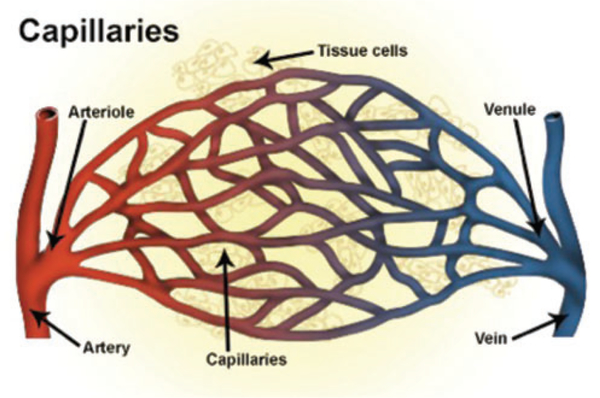
Cortical vasculature includes arterioles, capillaries, and venules (see Figure 3). While much attention to the components of the BBB has been focused on capillary endothelial cells, whether the leakage occurs exclusively or primarily from capillaries is not clear. Indeed, in an early study, Dietrich et al., studying effects of fluid percussion injury in rats, reported that intravascular injections of HRP-labeled small hemorrhages are associated with small venules.116 The involvement of venules was corroborated by Chodobski et al. who reported that extravasation of red blood cells occurs particularly from small venules.9 In lymphoid tissue, where movement of white blood cells between circulating blood and parenchyma is commonplace, movement of white cells occurs preferentially in post-capillary venules, through a specialized high endothelium. Despite the recent discovery of CNS lymphatics,121 such high endothelial linings of venules have not been reported for CNS vasculature. The identity of vascular components, when damaged, that allow movement of large molecules and leukocytes between brain and blood is an issue that awaits careful investigation.
Figure 3.
Diagram depiction of systemic blood vessels demonstrating blood flow from an artery to an arteriole, venule, and vein. The blood–brain barrier (BBB) is found at the level of the capillaries within the brain and is not present in normal circulation. Although most of the focus on BBB changes following TBI has focused on capillaries, a growing body of evidence is supporting the importance of small venules distal to the arterial and capillary circulation. No evidence has yet demonstrated leakiness at the level of the arterioles.122
The contribution from venules in breakdown of the BBB is supported by work in areas other than TBI. For example, Boroujerdi et al. studied a mouse model of multiple sclerosis (the EAE model) and found leakage of labeled fibrinogen from capillaries and venules at four to seven days following inoculation, while at 14 days after inoculation, leakage occurred only from venules.123 Further, Guerin et al. present evidence that in an experimental model of BBB breakdown, (from toxic poisoning of astrocytes) leakage of fluorescently labeled dextrans occurs from venules and some capillaries.124 No evidence currently is available suggesting leakage from arterioles.
Impact strength and cerebral vascular changes
Endothelial cell changes post-mTBI
mTBI can lead to increased BBB permeability via leakage of vasogenic factors and endothelial disruptors in human and rodent.110 Although Cornelius et al. pin-pointed primary and secondary damage resulting from any TBI injury, they do highlight BBB dysfunction and dysregulation following mTBI—defined by LOS <30 minutes, amnesia <24h, with transitory neuropsychiatric deficits and largely full recovery. These effects deplete energy and lead to alterations in calcium homeostasis.120 Excess cytoplasmic calcium is taken up by various calcium buffers of the cell, such as the mitochondria. Calcium intake by mitochondria affects the mitochondrial membrane potential and when its threshold is reached, the excess calcium is pumped back into cell cytoplasm. As cytosolic calcium levels rise, inflammatory cascades, apoptotic dependent and independent caspases and other protease pathways are activated in brain endothelial cells as well as in various other cell types of the brain, via studies in rodent brain.125
Although reactions such as vasogenic edema, EC activation, and calcium instability all work independently to initiate BBB breakdown at the level of EC tight junctions, they all synergistically weaken the BBB changes post-mTBI.126 This damage is dependent on time and severity of the head trauma. If the severity of the TBI is mild, there may not be this evolution of permanent BBB disruption, in contrast to moderate and severe TBI. Rat models of mTBI show that vascular breakdown is transient, lasting between four and six days.124 In this study, 1,3-Dinitrobenzene-induced glial degeneration resulted in BBB breakdown, which was investigated via fluorescent dextrans below 500,000mol wt in size. Disruption of the BBB was detected 18hfollowing dinitrobenzene dosage, and leakage continued for up to four days following lesion formation, but BBB integrity was re-established by day 6.
Timing of vascular changes post-TBI
The question of timing of onset of vascular, as well as parenchymal, changes following TBI are of great importance, but the temporal pattern may vary with species investigated as well as choice of method to detect changes. Most commonly used has been Evans blue, and Cernak et al. report that EB labeling of BBB breakdown peaked 20min after TBI for rat pups aged 7, 14, and 21 days.127 Adult animals showed a second peak at 24h post-TBI. Boyd et al. report that “stealth” liposomes enter the brain 0 to 8h after TBI.15 Shapira et al., using weight drop impact on ether anesthetized rats, reported extravasation of Evans blue as early as 15min after impact, and Evans blue labeling continued through four days post-impact.115 Glushakova et al. report that delayed increases in microvascular pathology after CCI-induced low- and high-magnitude TBI in rats is associated with prolonged BBB disruption.128 Tanno et al.118 studied fluid percussion injury in rats and intravascular HRP and report that the impact site was permeable to HRP for up to 72h after impact.
BBB disruption normalizes within one week in several injury models, including CCI and weight drop models, yet recent studies show BBB permeability up to 30 days after ischemic insult.129 Collectively, these observations suggest that BBB integrity represents complex and dynamic sequelae meriting attention during acute and delayed stages post-severe TBI.
Comparison of single vs. multiple impacts
The emerging literature from studies of TBI in humans is indicating that multiple incidents of trauma produce markedly greater brain trauma than do single incidents. As with other aspects of TBI, the variation of sites and strengths of multiple head traumas has led investigators to search for mechanisms using animal models.
A study of functional consequences using a lateral fluid percussion model has demonstrated that rats sustaining three impacts display deficits in long-term potentiation (a model of learning), while a single impact results in non-detectable deficit.130 A series of contributions from Petraglia et al.131–134 have described the pathological and behavioral effects of repetitive TBI in a closed skull model of TBI in mice and demonstrated the clearly greater effects of multiple impacts on a variety of behavioral measures.
While much attention has been focused on the cellular (both neural and glial) response to TBI, as well as its behavioral consequences, less attention has been directed toward understanding possible effects of TBI on cerebral vasculature. Our laboratory has begun a series of preliminary experiments, studying the effects of varied impact parameters of TBI on cerebral cortical vascular elements, as well as the possible “leakiness” of those cortical blood vessels. These studies required a combination of techniques, including: (1) intravascular injections of fluorescently labeled lectin109 as a general label for the endothelium of cerebral cortical blood vessels, combined with (2) intravascular injections of tracers, including Evans blue, labeled albumin, or dextrans of varied molecular weights to study the possible movement of molecules of varied sizes from circulating blood into brain parenchyma. Injections of lectins and tracers were made at varied times, ranging from just prior to TBI injury to one month post injury. The pilot study consisted of three groups, (1) a repeat-hit group, (2) a single-hit group, and (3) a sham group. With IACUC approval, a 1cm region on the top of the head was shaved in isofluorane-anesthetized mice. Mice then received unilateral injuries over the right hemisphere either once, or five consecutive injuries over five days using a TBI-0310 Impactor (Precision Systems and Instrumentation, Lexington, KY). Parameters were set at 4m/s for impact velocity, a 50-ms dwell time, and a 1mm penetration depth to produce a moderate strength injury consistent with concussion-like behavioral symptoms in the mice.
Preliminary experiments were undertaken to determine if TBI impact had discernable effect on morphological features of cortical vasculature elements, and for these studies intravascular injections of lectins were used. Figure 2(a) shows green lectin labeling of cortical vascular elements from a normal control mouse, revealing the dense network of small vessels in the cortical tissue. Figure 2(b) is a slightly higher magnification view, in this case of red-labeled cortical vascular elements, again from a normal control mouse. Most labeled vessels appear to be small capillaries, although some slightly larger vessels (likely to be venules) also can be seen. Figure 2(b) presents an image from cortex of a mouse 24h after receiving a single TBI impact of moderate impact strength. Note the relatively normal appearing vasculature, with the exception of three apparently dilated vessels (possibly venules) as indicated by the arrows. Figure 2(c) presents a comparable photomicrograph of the cortex of a mouse after five daily TBI impacts of moderate strength. Note the marked increase in the number of apparently dilated vessels and the apparent reduction of the normal cortical small vessel network. Current work in our laboratory is directed at developing quantitative techniques so that reliable assessments can be made of the effects on cortical vasculature of a varied of parameters of TBI, including impact strength, number of impacts, and time after impact before assessment.
While these impacts appear to result in dilation of some cortical vessels, the more important question is likely to be whether the integrity of the blood vessel wall, and thus the BBB, is affected by the impacts. Figure 4 presents preliminary results from a case in which a mouse received a single TBI impact. In this case, three views of a single section of cerebral cortex are seen under different fluorescence optics. Figure 4(a) is the section under fluorescein optics to show the green lectin-labeled vasculature. Note the cortical network of vessels, and the one dilated vessel (arrow) near the center. Figure 4(b) shows the section under rhodamine optics, which reveals both the 594-labeled 10Kd dextran as well as the Evans blue (fluorescing red). It is clear that some dextran and/or Evans blue was extravasated from the circulating blood; the punctate appearance of the red labeling suggests that much of the dextran and/or Evans blue has been taken up by local neurons or glial cells. Figure 4(c) presents an image of the same section, as seen under UV optics to reveal the 4’,6-diamidino-2-phenylindole (DAPI)-labeled nuclei of neurons and glial cells. The DAPI does not provide a particularly sensitive indicator of the integrity of cortical parenchyma, but no evidence of gross damage is visible.
Figure 4.
Fluorescence photomicrographs of a single section of cerebral cortex from a mouse that received an intravenous injection of a combination of Evans blue and 594 labeled 10 Kd dextran, followed 5 min later by a single TBI impact, followed by a intracardiac injection of 488 labeled lectin. (a) Lectin labeling showing abnormal cortical vascular elements including a widely dilated vessel in the center (arrow). (b) Dextran labeling showing evidence on extravasation, and probable cellular uptake, of 10 Kd dextran in the regions surrounding the dilated vessel in (a). (c) DAPI labeling of cell nuclei in the cortex, showing no gross loss of neuropil; DAPI was applied as part of the mounting medium when cover-slipping the section. Calibration bar in C = 250 μm for all images.
Work in our laboratory now is directed toward using different molecular weights of dextrans and different times between TBI and impact in an attempt to understand what aspects of the BBB may be affected by the TBI impacts.
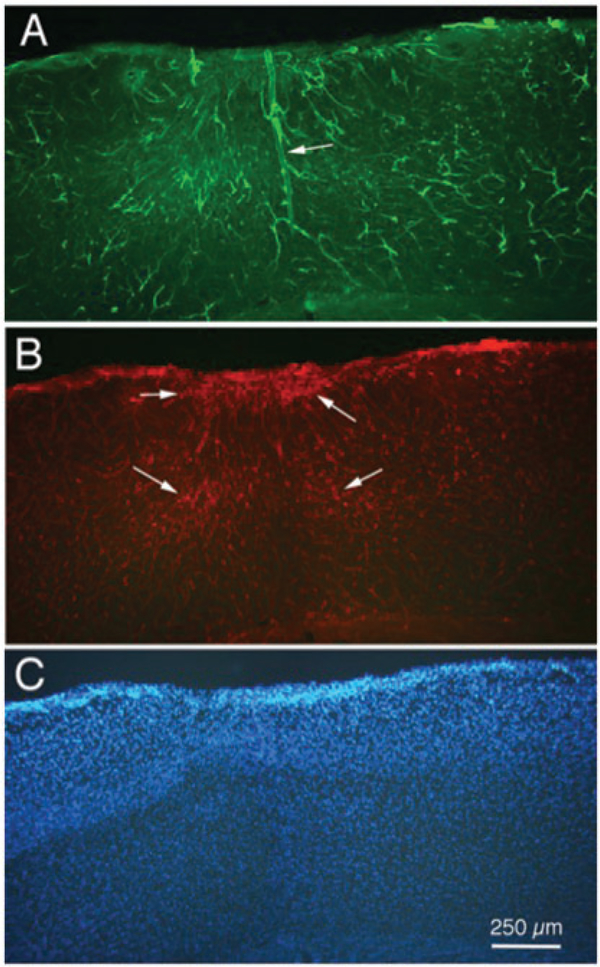
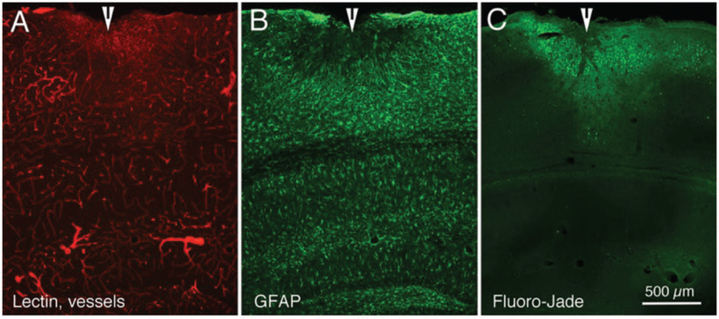
It is becoming clear that multiple TBI impacts have greater effects on a several measures of cortical integrity. For example, Figure 5 presents preliminary results from a mouse that was injected with lectin and euthanized seven days following a series of five hits of moderate strength. Figure 5(a) shows red lectin-labeled cerebral cortical (and hippocampal) vessels, with several clearly dilated blood vessels. GFAP labeling of astrocytes normally is very scant in cerebral cortex, but the image in Figure 5(b) shows evidence of proliferated astrocytes surrounding the impact site in cortex. Figure 5(c) shows Fluoro-Jade labeling in an adjacent section; Fluoro-Jade can be used as an indicator of degenerating neuronal tissue.135,136
Figure 5.
Fluorescence photomicrographs of three adjacent sections from mouse cerebral cortex demonstrating the effects of a series of five impacts (moderate strength), seven days after the last impact. (a) Cortical vasculature, as indicated by 594 (red) labeled lectin. (b) Astrocyte proliferation, as indicated by 488 (green) labeling for GFAP immunohistochemistry. (c) Neuronal degeneration, as indicated by Fluoro-Jade labeling. Arrowheads indicate site of cortical damage following the TBIs. Calibration bar in C = 500 μm for all images.
Conclusion
The consequences of TBI and repeated concussions are a growing concern in the United States. Despite increasing evidence linking head injury to a variety of cognitive alterations including dementia, the mechanisms and therapeutic approaches to alleviating the consequences of repeat concussions or TBI on cerebral vasculature have yet to be fully understood. It is clear that a variety of BBB microstructural and subsequent functional changes occur in a graded manner following head injury. As researchers work toward developing interventional therapeutics to alleviate the deleterious effect of TBI, it will be important that further studies are undertaken using tools that allow quantitative assessments of changes in both the morphological features of the vasculature, as well as the functional integrity of the BBB. Such studies will allow a better understanding of injury severity on the time “window” in which neuropathological features develop, and the effects of repeat vs. single injuries on the BBB and vascular endothelial cells.
Acknowledgments
Funding
This work was supported by a Seed Gift to Brian Cummings, Stem Cell Research Center, UC Irvine, and a grant from the Department of Defense, W81XWH-15-1-0435, to Brian Cummings and Cathy Cahill.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Roozenbeek B, Maas AI and Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nature Rev Neurol 2013; 9: 231–236. [DOI] [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald MM, et al. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths (2002–2006). Atlanta, GA: Centers for Disease Control and Prevention. National Center for Injury Prevention and Control, 2010. [Google Scholar]
- 3.Laker SR. Epidemiology of concussion and mild traumatic brain injury. PM&R 2011; 3: S354–S358. [DOI] [PubMed] [Google Scholar]
- 4.Cernak I Animal models of head trauma. NeuroRx 2005; 2: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005; 57: 128–134. [DOI] [PubMed] [Google Scholar]
- 6.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005; 57: 719–726. [DOI] [PubMed] [Google Scholar]
- 7.Ballabh P, Braun A and Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16: 1–3. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt B and Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 2009; 31: 497–511. [DOI] [PubMed] [Google Scholar]
- 9.Chodobski A, Zink BJ and Szmydynger-Chodobska J.Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2011; 2: 492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obermeier B, Daneman R and Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nature Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014; 82: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broux B, Gowing E and Prat A. Glial regulation of the blood-brain barrier in health and disease. Semin Immunopathol 2015; 37: 577–590. [DOI] [PubMed] [Google Scholar]
- 13.Daneman R, Zhou L, Agalliu D, et al. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PloS One 2010; 5: e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krueger M, Härtig W, Reichenbach A, et al. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One 2013; 8: e56419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd BJ, Galle A, Daglas M, et al. Traumatic brain injury opens blood–brain barrier to stealth liposomes via an enhanced permeability and retention (EPR)-like effect. J Drug Target 2015; 23: 847–853. [DOI] [PubMed] [Google Scholar]
- 16.Palmer AM. The role of the blood–CNS barrier in CNS disorders and their treatment. Neurobiol Dis 2010; 37: 3–12. [DOI] [PubMed] [Google Scholar]
- 17.Klonoff PS, Snow WG and Costa LD. Quality of life inpatients 2 to 4 years after closed head injury. Neurosurgery 1986; 19: 735–743. [DOI] [PubMed] [Google Scholar]
- 18.Levin HS. Prediction of recovery from traumatic brain injury. J Neurotrauma 1995; 12: 913–922. [DOI] [PubMed] [Google Scholar]
- 19.Wilson B, Vizor A and Bryant T. Predicting severity of cognitive impairment after severe head injury. Brain Injury 1991; 5: 189–197. [DOI] [PubMed] [Google Scholar]
- 20.Brown AW, Leibson CL, Malec JF, et al. Long-term survival after traumatic brain injury: a population-based analysis. NeuroRehabilitation 2004; 19: 37–43. [PubMed] [Google Scholar]
- 21.Carlsson CA, von Essen C and Löfgren J. Factors affecting the clinical course of patients with severe head injuries: part 1: influence of biological factors part 2: significance of posttraumatic coma. J Neurosurg 1968; 29: 242–251. [DOI] [PubMed] [Google Scholar]
- 22.Whyte J, Cifu D, Dikmen S, et al. Prediction of functional outcomes after traumatic brain injury: a comparison of 2 measures of duration of unconsciousness. Arch Phys Med Rehabil 2001; 82: 1355–1359. [DOI] [PubMed] [Google Scholar]
- 23.Brooks DN and McKinlay W. Personality and behavioural change after severe blunt head injury – a relative’s view. J Neurol Neurosurg Psychiatry 1983; 46: 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikmen S, Machamer J, Temkin N, et al. Neuropsychological recovery in patients with moderate to severe head injury: 2 year follow-up. J Clin Exp Neuropsychol 1990; 12: 507–519. [DOI] [PubMed] [Google Scholar]
- 25.Sherer M, Sander AM, Nick TG, et al. Early cognitive status and productivity outcome after traumatic brain injury: findings from the TBI model systems. Arch Phys Med Rehabil 2002; 83: 183–192. [DOI] [PubMed] [Google Scholar]
- 26.Rowley G and Fielding K. Reliability and accuracy of the Glasgow Coma scale with experienced and inexperienced users. Lancet 1991; 337: 535–538. [DOI] [PubMed] [Google Scholar]
- 27.Gabbe BJ, Cameron PA and Finch CF. The status of the Glasgow coma scale. Emerg Med 2003; 15: 353–360. [DOI] [PubMed] [Google Scholar]
- 28.Levin HS, Gary HE Jr, Eisenberg HM, et al. Neurobehavioral outcome 1 year after severe head injury: experience of the Traumatic Coma Data Bank. J Neurosurg 1990; 73: 699–6709. [DOI] [PubMed] [Google Scholar]
- 29.Williams DH, Levin HS and Eisenberg HM. Mild head injury classification. Neurosurgery 1990; 27: 422–428. [DOI] [PubMed] [Google Scholar]
- 30.Malec JF, Brown AW, Leibson CL, et al. The Mayo classification system for traumatic brain injury severity. J Neurotrauma 2007; 24: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 31.Zetterberg Henrik and Blennow Kaj. Fluid biomarkers for mild traumatic brain injury and related conditions. Nature Rev Neurol 2016; 9: 201–210. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JP and Rosenberg JH. Practice parameters: the management of concussion in sports: reports of the quality standards committee. Neurology 1997; 48: 581–585. [DOI] [PubMed] [Google Scholar]
- 33.Korn A, Golan H, Melamed I, et al. Focal cortical dysfunction and blood–brain barrier disruption in patients with postconcussion syndrome. J Clin Neurophysiol 2005; 22: 1–9. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi H, Manaka S and Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. J Neurosurg 1981; 55: 708–717. [DOI] [PubMed] [Google Scholar]
- 35.Katayama Y, Becker DP, Tamura T, et al. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg 1990; 73: 889–900. [DOI] [PubMed] [Google Scholar]
- 36.Yoshino A, Hovda DA, Kawamata T, et al. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper-and subsequent hypometabolic state. Brain Res 1991; 561: 106–119. [DOI] [PubMed] [Google Scholar]
- 37.Corbett RJ, Laptook AR, Nunnally RL, et al. Intracellular pH, lactate, and energy metabolism in neonatal brain during partial ischemia measured in vivo by 31P and 1H nuclear magnetic resonance spectroscopy. J Neurochem 1988; 51: 1501–1509. [DOI] [PubMed] [Google Scholar]
- 38.Richards TL, Keniry MA, Weinstein PR, et al. Measurement of lactate accumulation by in vivo proton NMR spectroscopy during global cerebral ischemia in rats. Magn Reson Med 1987; 5: 353–357. [DOI] [PubMed] [Google Scholar]
- 39.Pettus EH, Christman CW, Giebel ML, et al. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma 1994; 11: 507–522. [DOI] [PubMed] [Google Scholar]
- 40.Johnson VE, Stewart W and Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013; 246: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang-Schomer MD, Johnson VE, Baas PW, et al. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol 2012; 233: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortez SC, McIntosh TK and Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res 1989; 482: 271–282. [DOI] [PubMed] [Google Scholar]
- 43.Fineman I, Hovda DA, Smith M, et al. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45 Ca autoradiographic study. Brain Res 1993; 624: 94–102. [DOI] [PubMed] [Google Scholar]
- 44.Osteen CL, Moore AH, Prins ML, et al. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma 2001; 18: 141–162. [DOI] [PubMed] [Google Scholar]
- 45.Mata M, Staple J and Fink DJ. Changes in intra-axonal calcium distribution following nerve crush. J Neurobiol 1986; 17: 449–467. [DOI] [PubMed] [Google Scholar]
- 46.Maxwell WL, McCreath BJ, Graham DI, et al. Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. J Neurocytol 1995; 24: 925–942. [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal M, LaManna J, Yamada S, et al. Oxidative metabolism, extracellular potassium and sustained potential shifts in cat spinal cord in situ. Brain Res 1979; 162: 113–127. [DOI] [PubMed] [Google Scholar]
- 48.Ackermann RF and Lear JL. Glycolysis-induced discordance between glucose metabolic rates measured with radiolabeled fluorodeoxyglucose and glucose. J Cereb Blood Flow Metab 1989; 9: 774–785. [DOI] [PubMed] [Google Scholar]
- 49.Yuan XQ, Prough DS, Smith TL, et al. The effects of traumatic brain injury on regional cerebral blood flow in rats. J Neurotrauma 1988; 5: 289–301. [DOI] [PubMed] [Google Scholar]
- 50.Yamakami I and McIntosh TK. Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J Cereb Blood Flow Metab 1989; 9: 117–124. [DOI] [PubMed] [Google Scholar]
- 51.Giza CC and Hovda DA. The neurometabolic cascade of concussion. J Athletic Train 2001; 36: 228. [PMC free article] [PubMed] [Google Scholar]
- 52.Marchi N, Bazarian JJ, Puvenna V, et al. Consequences of repeated blood-brain barrier disruption in football players. PloS One 2013; 8: e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blyth BJ, Farahvar A, He H, et al. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood–brain barrier function after traumatic brain injury. J Neurotrauma 2011; 28: 2453–2462. [DOI] [PubMed] [Google Scholar]
- 54.Offenbacher H, Fazekas F, Schmidt R, et al. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. Am J Neuroradiol 1996; 17: 573–578. [PMC free article] [PubMed] [Google Scholar]
- 55.Charidimou A and Werring DJ. Cerebral microbleeds: detection, mechanisms and clinical challenges. Future Neurol 2011; 6: 587–611. [Google Scholar]
- 56.Bergsneider M, Hovda DA, Lee SM, et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma 2000; 17: 389–401. [DOI] [PubMed] [Google Scholar]
- 57.Zetterberg H, Smith DH and Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nature Rev Neurol 2013; 9: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bazarian JJ, Blyth B and Cimpello L. Bench to bedside: evidence for brain injury after concussion – looking beyond the computed tomography scan. Acad Emerg Med 2006; 13: 199–214. [DOI] [PubMed] [Google Scholar]
- 59.Begaz T, Kyriacou DN, Segal J, et al. Serum biochemical markers for post-concussion syndrome in patients with mild traumatic brain injury. J Neurotrauma 2006; 23: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 60.Diaz-Arrastia R, Wang KK, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma 2014; 31: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang KK, Ottens AK, Liu MC, et al. Proteomic identification of biomarkers of traumatic brain injury. Expert Rev Proteom 2005; 2: 603–614. [DOI] [PubMed] [Google Scholar]
- 62.Stålnacke BM, Björnstig U, Karlsson K, et al. One-year follow-up of patients with mild traumatic brain injury: post-concussion symptoms, disabilities and life satisfaction at follow-up in relation to serum levels of S-100B and neuron-specific enolase in acute phase. J Rehabil Med 2005; 37: 300–305. [DOI] [PubMed] [Google Scholar]
- 63.Zemlan FP, Jauch EC, Mulchahey JJ, et al. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res 2002; 947: 131–139. [DOI] [PubMed] [Google Scholar]
- 64.Bulut M, Koksal O, Dogan S, et al. Tau protein as a serum marker of brain damage in mild traumatic brain injury: preliminary results. Adv Therapy 2006; 23: 12–22. [DOI] [PubMed] [Google Scholar]
- 65.Ma M, Lindsell CJ, Rosenberry CM, et al. Serum cleaved tau does not predict postconcussion syndrome after mild traumatic brain injury. Am J Emerg Med 2008; 26: 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Povlishock JT, Hayes RL, Michel ME, et al. Workshop on animal models of traumatic brain injury. J Neurotrauma 1994; 11: 723–732. [DOI] [PubMed] [Google Scholar]
- 67.Ashley MJ (ed.) Traumatic brain injury: rehabilitation, treatment, and case management. Boca Raton, FL, USA: CRC Press, 2016. [Google Scholar]
- 68.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med 2010; 38: 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu MC, Akinyi L, Scharf D, et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur J Neurosci 2010; 31: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mondello S, Akinyi L, Buki A, et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 2012; 70: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papa L, Ramia MM, Kelly JM, et al. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma 2013; 30: 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Head J Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8: 86–87. [Google Scholar]
- 73.Grandizio C, Lawson B, King M, et al. Development of a fitness-for-duty assessment battery for recovering dismounted warriors. ARMY Aeromedical Research Lab Fort Rucker AL Warfighter Health Div; 2014. April 7 http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA601358. [Google Scholar]
- 74.Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: review and meta-analysis. NeuroImage Clin 2014; 4: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hardman JM and Manoukian A. Pathology of head trauma. Neuroimaging Clin North Am 2002; 12: 175–187. [DOI] [PubMed] [Google Scholar]
- 76.Van der Naalt J, van Zomeren A, Sluiter W, et al. One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complaints and return to work. J Neurol Neurosurg Psychiatry 1999; 66: 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teasdale G and Jennett B. Assessment of coma and impaired consciousness. Lancet 1974; 304: 81–84. [DOI] [PubMed] [Google Scholar]
- 78.Levin HS. Outcomes from mild head injury In: Narayan RK, Wilberger JE and Povlishock JT (eds) Neurotrauma. New York, NY: McGraw-Hill, 1996. [Google Scholar]
- 79.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the US and the public health response: 1995–2009. J Saf Res 2012; 43: 299–307. [DOI] [PubMed] [Google Scholar]
- 80.Khan F, Baguley IJ and Cameron ID. 4: rehabilitation after traumatic brain injury. Med J Australia 2003; 178: 290–297. [DOI] [PubMed] [Google Scholar]
- 81.Hergenroeder G, Redell JB, Moore AN, et al. Identification of serum biomarkers in brain-injured adults: potential for predicting elevated intracranial pressure. J Neurotrauma 2008; 25: 79–93. [DOI] [PubMed] [Google Scholar]
- 82.Su SH, Xu W, Li M, et al. Elevated C-reactive protein levels may be a predictor of persistent unfavourable symptoms in patients with mild traumatic brain injury: a preliminary study. Brain Behav Immunity 2014; 38: 111–117. [DOI] [PubMed] [Google Scholar]
- 83.Michetti F, Corvino V, Geloso MC, et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem 2012; 120: 644–659. [DOI] [PubMed] [Google Scholar]
- 84.Van Eldik LJ and Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restorative Neurol Neurosci 2003; 21: 97–108. [PubMed] [Google Scholar]
- 85.Zongo D, Ribéreau-Gayon R, Masson F, et al. S100-B protein as a screening tool for the early assessment of minor head injury. Ann Emerg Med 2012; 59: 209–218. [DOI] [PubMed] [Google Scholar]
- 86.Yang SH, Gustafson J, Gangidine M, et al. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J Surg Res 2013; 184: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang SH, Gangidine M, Pritts TA, et al. Interleukin 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock (Augusta, Ga.) 2013; 40: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berger RP, Ta’Asan S, Rand A, et al. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr Res 2009; 65: 97–102. [DOI] [PubMed] [Google Scholar]
- 89.Kossmann T, Hans V, Imhof HG, et al. Interleukin-6 released in human cerebrospinal fluid following traumatic brain injury may trigger nerve growth factor production in astrocytes. Brain Res 1996; 713: 143–152. [DOI] [PubMed] [Google Scholar]
- 90.Hergenroeder GW, Moore AN, McCoy JP, et al. SerumIL-6: a candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J Neuroinflamm 2010; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hannay HJ, Feldman Z, Phan P, et al. Validation of a controlled cortical impact model of head injury in mice. J Neurotrauma 1999; 16: 1103–1114. [DOI] [PubMed] [Google Scholar]
- 92.Marmarou A, Foda MA, Brink WV, et al. A new model of diffuse brain injury in rats: part I: pathophysiology and biomechanics. J Neurosurg 1994; 80: 291–300. [DOI] [PubMed] [Google Scholar]
- 93.Abd-Elfattah Foda MA and Marmarou A. A newmodel of diffuse brain injury in rats: part II: morphological characterization. J Neurosurg 1994; 80: 301–313. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Zhang L, Kallakuri S, et al. Quantitative relationship between axonal injury and mechanical response in a rodent head impact acceleration model. J Neurotrauma 2011; 28: 1767–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cernak I, Wang Z, Jiang J, et al. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma Acute Care Surg 2001; 50: 695–706. [DOI] [PubMed] [Google Scholar]
- 96.Bailey ZS, Hubbard WB and Vandevord PJ. Cellular mechanisms and behavioral outcomes in blast-induced neurotrauma: comparing experimental setups. Meth Mol Biol Inj Models CNS 2016; 1462: 119–38. [DOI] [PubMed] [Google Scholar]
- 97.Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg 1987; 67: 110–119. [DOI] [PubMed] [Google Scholar]
- 98.Carbonell WS, Maris DO, McCALL TO, et al. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma 1998; 15: 217–229. [DOI] [PubMed] [Google Scholar]
- 99.Hylin MJ, Orsi SA, Zhao J, et al. Behavioral and histopathological alterations resulting from mild fluid percussion injury. J Neurotrauma 2013; 30: 702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao Z, Loane DJ, Murray MG, et al. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma 2012; 29: 2475–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Washington PM, Forcelli PA, Wilkins T, et al. The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J Neurotrauma 2012; 29: 2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu S, Kaneko Y, Bae E, et al. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res 2009; 1287: 157–163. [DOI] [PubMed] [Google Scholar]
- 103.Dixon CE, Clifton GL, Lighthall JW, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Meth 1991; 39: 253–262. [DOI] [PubMed] [Google Scholar]
- 104.Baki SG, Kao HY, Kelemen E, et al. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res 2009; 1280: 98–106. [DOI] [PubMed] [Google Scholar]
- 105.Mouzon B, Chaytow H, Crynen G, et al. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma 2012; 29: 2761–2773. [DOI] [PubMed] [Google Scholar]
- 106.Petraglia AL, Plog BA, Dayawansa S, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma 2014; 31: 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Namjoshi DR, Cheng WH, McInnes KA, et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Mol Neurodegen 2014; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosenblum WI, Nelson GH, Wormley B, et al. Role of platelet-endothelial cell adhesion molecule (PECAM) in platelet adhesion/aggregation over injured but not denuded endothelium in vivo and ex vivo. Stroke 1996; 27: 709–711. [DOI] [PubMed] [Google Scholar]
- 109.Robertson RT, Levine ST, Haynes SM, et al. Use of labeled tomato lectin for imaging vasculature structures. Histochem Cell Biol 2015; 143: 225–234. [DOI] [PubMed] [Google Scholar]
- 110.Nikolajsen GN, Jensen MS and West MJ. A zinc fixative for 3D visualization of cerebral capillaries and pericytes. J Neurosci Meth 2016; 257: 1–6. [DOI] [PubMed] [Google Scholar]
- 111.Lucke-Wold BP, Logsdon AF, Smith KE, et al. Bryostatin-1 restores blood brain barrier integrity following blast-induced traumatic brain injury. Mol Neurobiol 2015; 52: 1119–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoffmann A, Bredno J, Wendland M, et al. High and Low molecular weight fluorescein Isothiocyanate (FITC)–dextrans to assess blood-brain barrier disruption: technical considerations. Transl Stroke Res 2011; 2: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McIntosh TK, Vink R, Noble L, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 1989; 28: 233–244. [DOI] [PubMed] [Google Scholar]
- 114.Ren Z, Iliff JJ, Yang L, et al. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab 2013; 33: 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shapira Y, Setton D, Artru AA, et al. Blood-brain barrier permeability, cerebral edema, and neurologic function after closed head injury in rats. Anesth Analg 1993; 77: 141–148. [DOI] [PubMed] [Google Scholar]
- 116.Dietrich WD, Alonso O and Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J Neurotrauma 1994; 11: 289–301. [DOI] [PubMed] [Google Scholar]
- 117.Schmidt RH and Grady MS. Loss of forebrain cholinergic neurons following fluid-percussion injury: implications for cognitive impairment in closed head injury. J Neurosurg 1995; 83: 496–502. [DOI] [PubMed] [Google Scholar]
- 118.Tanno H, Nockels RP, Pitts LH, et al. Breakdown of the blood–brain barrier after fluid percussive brain injury in the rat. Part 1: distribution and time course of protein extravasation. J Neurotrauma 1992; 9: 21–32. [DOI] [PubMed] [Google Scholar]
- 119.Juhler M, Barry DI, Offner H, et al. Blood-brain and blood-spinal cord barrier permeability during the course of experimental allergic encephalomyelitis in the rat. Brain Res 1984; 302: 347–355. [DOI] [PubMed] [Google Scholar]
- 120.Nagaraja TN, Keenan KA, Fenstermacher JD, et al. Acute leakage patterns of fluorescent plasma flow markers after transient focal cerebral ischemia suggest large openings in blood-brain barrier. Microcirculation 2008; 15: 1–4. [DOI] [PubMed] [Google Scholar]
- 121.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.SEER Training: Classification & Structure of Blood Vessels. Training.seer.cancer.gov. http://training.seer.cancer.gov/anatomy/cardiovascular/blood/classification.html (accessed 14 July 2016).
- 123.Boroujerdi A, Welser-Alves JV and Milner R. Extensive vascular remodeling in the spinal cord of pre-symptomatic experimental autoimmune encephalomyelitis mice; increased vessel expression of fibronectin and the α5β1 integrin. Exp Neurol 2013; 250: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guerin CJ, Nolan CC, Mavroudis G, et al. The dynamics of blood–brain barrier breakdown in an experimental model of glial cell degeneration. Neuroscience 2001; 103: 873–883. [DOI] [PubMed] [Google Scholar]
- 125.Cornelius C, Crupi R, Calabrese V, et al. Traumatic brain injury: oxidative stress and neuroprotection. Antioxidants Redox Signal 2013; 19: 836–853. [DOI] [PubMed] [Google Scholar]
- 126.Alluri H, Wiggins-Dohlvik K, Davis ML, et al. Blood–brain barrier dysfunction following traumatic brain injury. Metabolic brain Dis 2015; 30: 1093–10104. [DOI] [PubMed] [Google Scholar]
- 127.Cernak I, Chang T, Ahmed FA, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci 2010; 32: 442–453. [DOI] [PubMed] [Google Scholar]
- 128.Glushakova OY, Johnson D and Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood–brain barrier disruption, and progressive white matter damage. J Neurotrauma 2014; 31: 1180–1193. [DOI] [PubMed] [Google Scholar]
- 129.Balabanov R, Goldman H, Murphy S, et al. Endothelial cell activation following moderate traumatic brain injury. Neurol Res 2013; 23: 175–182. [DOI] [PubMed] [Google Scholar]
- 130.Aungst SL, Kabadi SV, Thompson SM, et al. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab 2014; 34: 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Petraglia AL, Plog BA, Dayawansa S, et al. The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int 2014; 5: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Petraglia AL, Dashnaw ML, Turner RC, et al. Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery 2014; 75: S34–S49. [DOI] [PubMed] [Google Scholar]
- 133.Turner RC, Lucke-Wold BP, Logsdon AF, et al. Modeling chronic traumatic encephalopathy: the way forward for future discovery. Front Neurol 2015; 6: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Turner RC, Lucke-Wold BP, Logsdon AF, et al. The quest to model chronic traumatic encephalopathy: a multiple model and injury paradigm experience. Front Neurol 2015; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmued LC, Stowers CC, Scallet AC, et al. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 2005; 1035: 24–31. [DOI] [PubMed] [Google Scholar]
- 136.Schmued LC and Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 2000; 874: 123–130. [DOI] [PubMed] [Google Scholar]