Abstract
Intracortical microelectrodes exhibit enormous potential for researching the nervous system, steering assistive devices and functional electrode stimulation systems for severely paralyzed individuals, and augmenting the brain with computing power. Unfortunately, intracortical microelectrodes often fail to consistently record signals over clinically useful periods. Biological mechanisms, such as the foreign body response to intracortical microelectrodes and self-perpetuating neuroinflammatory cascades, contribute to the inconsistencies and decline in recording performance. Unfortunately, few studies have directly correlated microelectrode performance with the neuroinflammatory response to the implanted devices. However, of those select studies that have, the role of the innate immune system remains among the most likely links capable of corroborating the results of different studies, across laboratories. Therefore, the overall goal of this review is to highlight the role of innate immunity signaling in the foreign body response to intracortical microelectrodes and hypothesize as to appropriate strategies that may become the most relevant in enabling brain-dwelling electrodes of any geometry, or location, for a range of clinical applications.
Keywords: intracortical microelectrodes, brain machine interfaces, neuroinflammatory response, neurodegeneration, biocompatibility, innate immunity
I. INTRODUCTION
A. Failure of Intracortical Microelectrodes
In order to become a clinical solution for many brain interfacing applications, intracortical microelectrodes should ideally operate consistently over time ranges of years to decades to prevent the repetition of highly invasive brain surgeries. Unfortunately, intracortical microelectrodes fail to consistently record neural signals over extended periods. Many labs studying various chronically implanted intracortical microelectrodes have demonstrated trends of the decreasing number of units detected,1–6 the number of channels detecting units,1,2,7–11 signal amplitudes,4,10,12 and decoder performance,4 as well as generally inconsistent recording performance1,7,13 and recording longevity,8,10,13 regardless of the animal model. Together, such characteristics are not ideal for the long-term performance of brain-computer and brain-machine interfaces.
Declining and inconsistent recording performance has been ascribed to many failure mechanisms, typically grouped into mechanical, material, or biological mechanisms.10 In their review, Barrese et al. defined mechanical failures as physical relocation or damage of the electrode array and hardware, material failures as breakdown of electrode array materials, and biological failures as a consequence of the foreign body response or implantation trauma following device insertion.10 Mechanical and materials related failure modes have recently been reviewed and will not be discussed here (see Prasad et al., Barrese et al., Jorfi et al., and Kozai et al. for examples7,9,10,14,15). Biological failures are typically associated with the interruption of electrical signals between cortical neurons and recording contacts. They will be further described and discussed in the sections that follow.
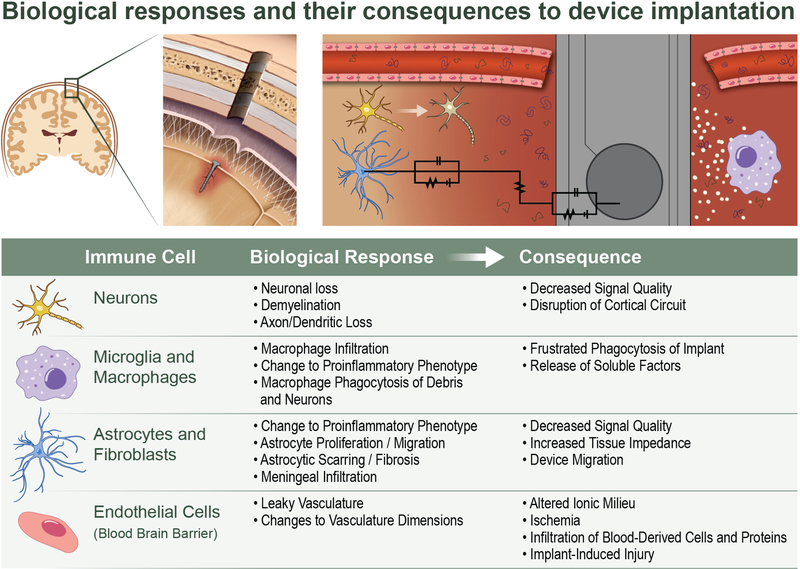
B. Foreign Body Response to Intracortical Microelectrodes
Intracortical microelectrodes fail, in part, due to biological mechanisms, many of which result from the foreign body response to the implant and subsequent chronic inflammatory cascades (for review, see Jorfi et al.14). As a microelectrode array is inserted in the brain, blood vessels are ruptured,16 releasing blood proteins into the brain parenchyma. Several components of blood, including proteins, can be neurotoxic.17 Furthermore, tissue is displaced and neurons and glia cells along the insertion path are damaged or killed.18 Blood proteins will adsorb to the surface of the electrode array and denature, upon which they are recognized by the brain’s major inflammatory cells, the microglia.19,20 Subsequently, microglia transition from a dormant ramified state to a pro-inflammatory amoeboid phenotype by retracting processes and upregulating lytic enzymes.18 In the activated state, microglia are able to release a variety of soluble pro-inflammatory and cytotoxic factors, such as nitric oxide, reactive nitrogen species, and reactive oxygen species.21–24 Evidence of oxidative gene expression and cellular oxidative damage have been detected at the electrode tissue interface.25 Factors released by pro-inflammatory microglia likely contribute to neuronal death and neurodegeneration (Fig. 1).21–24 Additionally, soluble factors released by microglia may damage the blood-brain barrier by disrupting tight adherens junctions connecting endothelial cells of the vasculature.26 Furthermore, oxidative factors may also contribute to material degradation (Fig. 1).27 Thus, consequences of electrode implantation and early inflammatory activation may contribute to the decline or unreliability of electrode performance.
FIG. 1:
Oxidative stress following neural probe implantation. The implantation of neural probes leads to the over-production of reactive oxygen species (ROS), which can consequently (1) perpetuate the foreign body response, (2) facilitate neuronal death, and (3) facilitate corrosion and delamination of the microelectrode surface. (Reprinted from Ereifej et al.25 under a Creative Commons Attribution International License, Copyright 2018: https://creativecom-mons.org/licenses/by/4.0/.)
Attracted by the release of soluble factors by nearby microglia, more microglia migrate from the parenchyma. Likewise, additional monocytes and other myeloid cells are recruited from circulation across the leaky blood-brain barrier.28,29 Blood proteins and necrotic cells from insertion damage, as well as the early inflammatory response and leaky blood-brain barrier promote pro-inflammatory activation in the newly recruited inflammatory cells.20,28,30 One pathway by which microglia and macrophages recognize such damage and enact pro-inflammatory responses is through Toll-like receptors (TLRs).31 Later sections of this paper will discuss TLRs in depth. Soluble factors from the total collection of inflammatory cells around the implant may cause further damage to neurons, recording materials (Fig. 1), and the blood-brain barrier.21–24 Repeated damage to cells and blood-vessels resulting in the recruitment and activation of more inflammatory cells is hypothesized to propagate self-perpetuated chronic inflammatory cascades indefinitely.20,28 The neuronal and material damage resulting from self-perpetuating inflammatory cascades may contribute to long-term biological failure mechanisms.20,28
As the microglial and macrophage response matures, within days to weeks, the fibroblast of the brain, astrocytes, begin their response to the implantation of the microelectrode. Astrocytes proliferate, become hypertrophic, and migrate to the implantation site, ultimately resulting in a dense encapsulation layer around the electrode.18 Astrocytic responses typically start wider and more diffuse, and then become denser and more compact over the course of several weeks.18 Astrocytic encapsulation isolates the inflammatory or damaged region around the electrode from the surrounding parenchyma. In addition to astrocytes, meningeal fibroblasts may migrate to the electrode-tissue interface and contribute to electrode encapsulation.6,10 Together, encapsulation of the electrode may interfere with diffusion of neuroactive substances,32 increase the electrical impedance between the electrode and neurons,13,33 increase the distance between neurons and electrodes,13 or in extreme cases promote extrusion of electrode arrays.10
Overall, chronic inflammation, neuronal loss, blood-brain barrier permeability, and encapsulation of microelectrode arrays may stem from the foreign body response to implanted intracortical microelectrodes and can be implicated in the recording performance of the implanted device.
C. Studies Linking Neuroinflammation to Electrode Failure
Despite all the efforts to overcome biological intracortical failure mechanisms (see Jorfi et al. or Hermann14,34), only a handful of studies have directly compared neuroinflammation to electrode performance. Interestingly, each study can be linked to the innate immunity, the body’s fast-acting response to pathogenic threats. Here we briefly discuss the studies directly comparing neuroinflammation to electrode performance and their links to innate immunity.
Rennaker et al. were the first to demonstrate a correlation between microelectrode recording performance and the inflammatory response.35 Specifically, in their study, Rennaker et al. investigated the effects of minocycline, chosen for its neuroprotective and neurorestorative effects,36 on intracortical microelectrode recording performance.35 Rats that were administered minocycline via water for two days before surgery through five days after surgery exhibited improved recording performance over controls after the first week of implantation, upon which the signal-to-noise ratio of controls steadily dropped.35 End-point histological analyses revealed that astrocytic encapsulation was decreased at one- and four-week time points after implantation in animals treated with minocycline.35 Improved recording performance was hypothesized to be caused by decreased inflammation, neuronal dieback, and microglia activation in addition to the observed decrease in astrocyte encapsulation. Minocycline was later shown to inhibit the pro-inflammatory phenotype of macrophages.37 Alternative explanations could factor in the antibiotic activity of minocycline. Perhaps a lower bacterial load activated less inflammation via innate immunity pathways. Regardless of the mechanism of action, minocycline is not suitable for long-term administration due to detrimental side effects including bone discoloration.38,39 The long-term clinical significance of minocycline induced bone discoloration remains unknown, yet is reported to be worrisome to orthopedic surgeons while further characterization is completed.40,41
In contrast to the previous study administering an anti-inflammatory compound, Harris42 examined the effects of a pro-inflammatory compound on intracortical microelectrode performance in an unpublished thesis study. At the end of a four-week study, rats administered a one-time surgical dose of the pro-inflammatory agent lipopolysaccharide (LPS) exhibited significantly lower neuronal density within the first 50 μm away from the implant.42 Additionally, administration of LPS significantly reduced firing rates in evoked neural recordings.42 Thus, inflammation is associated with poor neuronal survival and recording performance. Interestingly, the pro-inflammatory agent LPS is derived from the bacterial cell walls of gram-negative bacteria and predominantly recognized by the innate immunity receptors cluster of differentiation 14 (CD14) and Toll-like receptor 4 (TLR4) to induce robust inflammatory responses.43 The results of Harris42 indicate that activation of innate immunity can result in detrimental effects in recording performance and tissue integration.
Furthermore, Saxena et al. examined the effects of blood-brain barrier permeability on recording performance.28 In this study, extravasation of blood proteins and myeloid cells into the brain parenchyma around intracortical microelectrodes implanted in rats coincided with poor recording performance.28 The team hypothesized that chronic blood-brain barrier permeability following intracortical microelectrode implantation was part of a positive feedback loop with chronic inflammation, resulting in neurodegeneration,28 similar to the self-perpetuating neurodegenerative response described by Potter et al.20 One mechanism by which microglia and macrophages may recognize blood proteins and promote inflammation is through the innate immunity receptor Toll-like receptors.31,44 Thus, innate immunity may play a role in the positive feedback loop of blood-brain barrier permeability and chronic inflammation that leads to intracortical microelectrode failure.
Finally, Kozai et al. investigated the role of caspase-1 in the recording performance of intracortical microelectrodes.11 Caspase-1 was studied for its role in the activation of the pro-inflammatory cytokine IL-1β, as well as its role in neuronal death related to ischemia and chronic neurodegeneration.11 Knockout mice lacking caspase-1 implanted with intracortical microelectrodes exhibited significantly improved single unit yields over wild type controls out to ~ 150 days after implantation with similar yields extending to the end of the experiment around 180 days after implantation.45 Coincidentally, caspase-1 is involved in several innate immunity mechanisms, including inflammasome, RIG-like receptor, and TLR signaling.45 In this instance, attenuation of innate immunity improved chronic intracortical microelectrode performance. Thus, innate immunity appears to play a role in the failure of intracortical microelectrodes.
Overall, studies linking inflammation to intracortical microelectrode performance reveal several connections by which innate immunity may be involved in intracortical microelectrode performance.
II. DISCUSSION
A. Innate Immunity
As indicated above, innate immunity has ties to the foreign body response to intracortical microelectrodes. Thus, further understanding of the innate immunity may inform strategies to mitigate intracortical microelectrode failures.
Innate immunity is often described as the body’s first line of defense against pathogenic threats.46 Innate immunity should be distinguished from the body’s other defense system, adaptive immunity, by several characteristics. Innate immunity activates much more quickly, responding within minutes or hours, as compared to several days.46,47 The quickness in responses is, in part, due to the widely distributed germline encoded effectors.47 In contrast, the adaptive immunity requires genetic recombination and clonal expansion of specific effectors.46,47 The disadvantage of germline encoded effectors is that innate immunity has less diversity and specificity of responses.47 However, the recognition of general patterns conserved among classes of pathogens allows the innate immunity to be versatile and have capable effector cells distributed throughout the body.46,47 In further contrast, the adaptive immunity features memory, meaning that adaptive responses to a previously encountered threat may be activated more quickly and with greater intensity.47 Innate immunity is not traditionally thought to feature memory; thus, innate immune responses enact with a relatively consistent activation time and intensity. However, there is growing evidence that innate immunity may feature some degree of memory.48 Innate and adaptive immunity are not completely independent, as the innate immunity often aids in the activation and regulation of adaptive immunity effectors.47
Innate immunity is comprised of physical barriers, chemical barriers, and cellular responses.47 Physical barriers include epithelial layers, mucosal tissues, and glandular tissues.47 Chemical barriers include acidic fluids, anti-microbial proteins, and anti-microbial peptides that reside near the physical barriers.47 Cellular innate immune responses are typically mediated by phagocytic cells, such as macrophages, neutrophils, dendritic cells, monocytes, and microglia, but can also involve natural killer cells, leukocytes, epithelial cells, and endothelial cells.47 Additionally, complement glycoproteins found in serum are included in innate immunity.47
In the event that pathogens bypass the physical and chemical barriers of the body, cellular effectors may address the threat. Cellular effectors of the innate immunity are generally activated via pattern-recognition receptors (PRRs), which recognize molecular patterns common to categories of pathogens referred to as pathogen associated molecular patterns (PAMPs). Some PRRs may also recognize molecular patterns on endogenous molecules released by the body called damage (or danger) associated molecular patterns (DAMPs).47 The family of PRRs employed by the innate immunity are TLRs,49 C-type lectin receptors,50 Retinoic acid-inducible gene-I-like receptors (RLRs),51,52 and Nod-like receptors (NLRs).53,54 The TLRs and CLRs are transmembrane proteins expressed across plasma membranes; however, TLRs may also be expressed on endosomes and lysosomes.47 In contrast, RLRs and NLRs are expressed in the cytosol.47 Both TLRs and NLRs have demonstrated recognition of DAMPs in addition to PAMPs.47 Activation of the various PRRs may result in pro-inflammatory activation, apoptosis, phagocytosis, coagulation cascades, opsonization, or complement activation.46
In the context of foreign body responses to implanted intracortical microelectrodes, TLRs may be the most relevant. Some TLRs are expressed on plasma membranes,55,56 so they may be able to respond to external threats directly. The TLRs also have the capability to recognize DAMPs and PAMPs,31,57 enabling the potential detection of tissue, cellular, and vascular damage associated with intracortical microelectrode implantation, in addition to pathogens introduced with the microelectrode. Thus, we will also discuss TLRs and their adaptor molecule, CD14.
1. Toll-Like Receptors and CD14
One major group of effectors in innate immunity is a class of PRRs called TLRs.58 The TLRs are transmembrane proteins that recognize molecular patterns associated with either pathogens or tissue damage and enact downstream inflammatory activation.58,59 The TLRs are expressed on peripheral immune cells, such as macrophages and dendritic cells, as well as several cells of the central nervous system, including microglia, astrocytes, and neurons.58,60,61
Toll-like receptors are named for their similarity to Toll,62,63 a protein responsible for directing dorsal-ventral patterning in Drosophila embryos64 and involved in innate immunity in adult Drosophila.65 The TLRs were later identified in humans based on their shared molecular structure and downstream signaling pathways.66
Toll-like receptors are type 1 transmembrane proteins that feature leucine-rich repeat (LRR) domains on the extracellular side and a Toll/IL-1 receptor (TIR) domain.58,62,66,67 The LRRs are sequence motifs with frequent interspersed instances of the amino acid leucine and are found on many proteins involved in protein-protein interactions such as signal transduction.58,68 The other major component of Toll-like receptors, the TIR domain, is a protein-protein interaction module found involved in the host responses of both plants and animals.58,69
Building off the common structural elements of LRR and TIR domains, the TLR family gives rise to at least 12 different functional members in mice and 10 different functional members in humans.49,51,58 The TLRs 1–9 are functional in both mice and humans, TLR10 is functional in humans but not mice,70 and TLRs 11–13 have been identified in mice but not humans.49 The members of the TLR family vary in membrane location and ligand specificity.58 Some TLRs, such as TLR 1, 2, 4–6, 8, and 11, are located on the cell membrane, with the LRR domain facing the extracellular environment and the TIR domain facing the cytosol.49,71,72 With an outward-facing ligand-binding domain, TLRs on the cell membrane monitor for external threats like bacteria. Other TLRs, such as TLR 3, 7, 8, and 9, are located on intracellular vesicles, with the LRR domains facing the interior of the vesicle and the TIR domain facing the cytosol.49,71,72 With a vesicle-facing ligand-binding domain, TLRs on vesicle membranes monitor for internal threats, such as viruses. Each TLR recognizes its own set of ligands, which may be pathogen associated molecular patterns (PAMPs) or damage/danger associated molecular patterns (DAMPs).
The PAMPs recognized by TLRs and other PRRs are molecular patterns shared among broad categories of pathogens, allowing rapid and versatile innate immune responses with a handful of widely available receptor types.58 The broad, fast-acting nature of TLRs contrasts against the highly specialized receptors and antibodies of the adaptive immunity, which require time-consuming clonal expansion to distribute effector cells to combat pathogens.58 The PAMPs are effective targets for immune recognition because they occur in pathogens but not host cells, allowing inflammatory effector cells to discriminate between self and non-self.58 Furthermore, PAMPs are typically molecular patterns involved in critical pathogenic survival mechanisms.58 Mutations in pathogens removing PAMPs are typically deadly, allowing TLR signaling to remain effective across many generations of evolution.58
Of note, PAMPs can be utilized in the recognition of bacterial, fungal, parasitic, and viral pathogens (for a detailed summary, see Akira et al.73). As opposed to PAMPs, DAMPs are patterns found in endogenous molecules released in the event of noninfectious tissue damage (Table 1) from Pineau and Lacroix,31 Tsan and Gao,57 and Beg.59 The DAMPs recognized by TLRs include heat shock proteins (HSP), extracellular matrix (ECM) components, components of necrotic cells, surfactant protein A, RNA, DNA, and fibrinogen.31,59 Heat shock proteins are molecules released when cells are exposed to various stresses.31,57,74,75 TLRs can recognize HSP22,76 HSP60,77–79 HSP70,80,81 and Gp96.82 Molecules may be cleaved from the ECM by proteolytic enzymes during the inflammatory response to traumatic injury,31 and TLRs can recognize heparan sulfate,83–85 hyaluronan-derived oligosaccharide,86,87 biglycan,88 and fibronectin extra domain A.89 Necrotic cells promote inflammation via TLR recognition of the released chromatin binding protein high mobility group box 1 (HMGB1)30,90–93 or unidentified ligands.94,95 Surfactant protein A is a component of pulmonary surfactant and may activate TLRs.96 Host RNA97–99 and DNA97,100 can activate TLRs, but the method of endosome localization is unclear.97 Fibrinogen escapes vasculature during inflammation and can bind TLRs to further propagate inflammatory mechanisms.44
TABLE 1:
Summary of major damage associated molecular patterns (DAMPs) recognized by Toll-like receptors (TLRs), based on data from Pineau and Lacroix,31 Tsan and Gao,57 and Beg59
| DAMP | TLR | Factors Produced | Refs. |
|---|---|---|---|
| Heat shock proteins (HSPs) | |||
| HSP60 | TLR2, TLR4 | TNF, NO | 77–79 |
| HSP70 | TLR2, TLR4 | TNF, IL-1b, IL-6, IL-12 | 80, 81 |
| Gp96 | TLR2, TLR4 | TNF, IL-12 | 82 |
| HSP22 | TLR4 | TNF, IL-6, IL-12 | 76 |
| Extracellular matrix components | |||
| Heparan sulfate | TLR4 | TNF | 83–85 |
| Hyaluronan-derived oligosaccharide | TLR2, TLR4 | TNF, MIP-1a, MIP-2, KC | 86, 87 |
| Biglycan | TLR2, TLR4 | TNF, MIP-2 | 88 |
| Fibronectin extra domain A | TLR4 | MMP-9 | 89 |
| Decorin | TLR2, TLR4 | PDCD4, IL-10 | 101 |
| Versican | TLR2, TLR6 | TNF-α, IL-6 | 102 |
| Necrotic cells | TLR2, TLR3 | TNF, IL-8, MIP-2, KC, MMP-3, iNOS | 94, 95 |
| RNA | TLR3, TLR7 | IL-12, IFN-α | 97, 98 |
| DNA (in the form of immune complexes) | TLR9 | IFN-α | 97, 100 |
| High mobility group box 1 protein (HMGB1) | TLR2, TLR4 | TNF, IL-1a,IL-1b, IL-6, IL-8, MIP-1a, MIP-1b, COX-2, iNOS | 30, 90–93 |
| Lung surfactant protein A | TLR4 | TNF-α, IL-10 | 96 |
| Fibrinogen | TLR4 | MIP-1α, MIP-1β, MIP-2, MCP-1 | 44 |
| S100A8 | TLR4 | TNF-α | 103 |
| S100A9 | TLR4 | IL-1β, TNF-α, IL-6, IL-8 and IL-10 | 104 |
| Fibrillar β-amyloid | TLR2, TLR4 | Superoxide radical | 105 |
| α-synuclein | TLR4 | TNF-α, IL-6, CXCL1, ROS | 106 |
Upon binding of a PAMP or DAMP to the sequence of LRR domains on a TLR, cytoplasmic signaling events occur (for a more detailed review, see Akira and Takeda107). Genes induced by TLR activation have been implicated in the production of pro-inflammatory cytokines, chemokines, major histocompatibility complex (MHC), co-stimulatory molecules, and antimicrobial peptides.58 Although cytokines and chemokines promote innate immunity and inflammatory responses, MHC and co-stimulatory molecules facilitate adaptive immune responses. Cytokines released in response to TLR activation include the interleukins (IL) IL-1α,90 IL-1β,90 IL-6,76,108 and IL-12,81,97 tumor necrosis factor (TNF, also listed as TNF-α),78,86–88,90,92,94,97,108 interferon-α (IFN- α),97 and the chemokines macrophage inflammatory protein-1α (MIP-1α),86,90,95 MIP-1β,90 MIP-2,86,88,95 MCP-1,109 and KC.86,95 Cytokines can promote further inflammatory activation and edema, and chemokines can promote cellular extravasation and trafficking.110
Other factors released after TLR activation include the free radical signaling molecule nitric oxide (NO)77,78 and an enzyme that produces NO, inducible nitric oxide synthetase (iNOS).92,94 Additionally, TLR activation may result in release of the matrix metalloprotease (MMP) 3 or MMP9,95 which are involved in tissue repair.89 Finally, TLR activation may release the pro-inflammatory enzyme cyclooxygenase 2 (COX-2),92 which activates arachidonic acid/prostaglandin inflammatory mechanisms.111 Overall, TLR signaling pathways utilize a broad range of downstream effectors to respond to pathogenic and endogenous threats, which can be a likely source of the self-perpetuating inflammatory response to microelectrodes described above.
Because of established roles of particular PRR in neurodegenerative disorders, we have taken particular interest in the potential role of TLR2, TLR4, and the co-receptor cluster of differentiation 14 (CD14) in the foreign body response to intracortical microelectrodes.
The cell-surface receptor TLR2 is expressed on endothelial cells and antigen-presenting cells, including macrophages and microglia.58,112,113 Rather than forming homodimers to activate downstream signaling pathways, TLR2 typically forms heterodimers with TLR1 or TLR6.58 The main function of TLR2 is the recognition of Gram-positive bacteria via peptidoglycan (disputed by Travassos et al.114) and lipoteichoic acid,115–119 but it also binds the PAMPs lipoproteins/lipopeptides,120 lipoarabinomannan,121 phenol-soluble modulin,122 glycoinositolphospholipids, 123 porins,124 atypical LPS,125,126 and zymosan.107,113 In addition to the aforementioned PAMPs, TLR2 recognizes the DAMPs HSP60,79 HSP70,80,81 GP96,82 hyaluronan-derived oligosaccharide,86 biglycan,88 necrotic cells,94,95 and HMGB1.91,93 The broad range of recognized ligands makes TLR2 an effective innate immunity activator throughout the body, but the important role of TLR2 in CNS inflammation and neurodegeneration will be discussed below.
In contrast to TLR2, TLR4 communicates via homodimerization and recognizes Gram-negative bacteria.58,127–129 The cell-surface receptor TLR4 is expressed on macrophages, microglia, and several immune cells.58,66,130 In addition to bacterial recognition via LPS, TLR4 also recognizes several other PAMPs,73,107 including mannan,131 glucuronoxylomannan,73,132 viral fusion protein,133 and viral envelope proteins.134 Also, TLR4 is also involved in the recognition of several DAMPs,31,59 such as HSP60,77,78 HSP70,80,81 Gp96,82 HSP22,76 heparan sulfate,83–85 hyaluronan-derived oligosaccharide,86,87 biglycan,88 fibronectin extra domain A,89 HMGB1,91,93 and fibrinogen.44 Involvement of TLR4 signaling in CNS inflammation and neurodegeneration will also be discussed in below.
The co-receptor CD14, a 55 kD glycoprotein closely associated with TLR2 and TLR4, is predominantly known for its role in the recognition of LPS.135 Briefly, CD14 binds LPS monomers extracted from LPS aggregates by LPS binding protein (LBP)136,137 and the TLR4-MD2 complex subsequently binds LPS.135,138 Although the exact mechanism of LPS transfer from CD14 to TLR4-MD2 has not been elucidated, the presence of CD14 greatly improves the sensitivity of LPS recognition by effector cells.139 Macrophages and to a lesser extent microglia express CD14,140–142 either linked to a membrane by a glycophosphoinositol or released in a soluble form.58,135 In addition to LPS recognition, CD14 acts as a co-receptor to TLR2 in the recognition of the various PAMPs, such as virions143 and the cell wall components peptidoglycan (disputed by Travassos et al.114) and LTA.119,144 The co-receptor CD14 is thought to be an adaptor molecule to the TLRs for the binding of both PAMPs and DAMPs.144 For example, CD14 acts as a co-receptor to TLR2 and/or TLR4 in the recognition of HMGB1,145 β amyloid plaques,105 and HSP70.80,146 Furthermore, CD14 signals independently of TLR2 and TLR4, such as in the endosomal recognition of viral nucleic acids by TLR7 and TLR9,147 and the non-TLR mediated recognition of necrotic cells144 and apoptotic cells.148–150 Finally, CD14 facilitates the internalization of several molecules, such as phosphatidylinositol.151 The role of CD14 in CNS inflammation and neurodegeneration will also be discussed further in below.
2. TLR2, TLR4, and CD14 in Neurodegenerative Disorders
The innate immunity receptors TLR2, TLR4, and CD14 play a prominent role in several neurodegenerative disorders due to their expression in CNS tissue and ability to promote potent inflammatory mechanisms.152 The receptors TLR2 and TLR4 are expressed on microglia and astrocytes in both human and mice and on neurons in mice only.60,153 Additionally, TLR2 is expressed on oligodendrocytes.153 Although constitutive expression of CD14 in the brain is limited to perivascular, leptomeningeal, and choroid plexus macrophages, it can be upregulated in parenchymal microglia following injury.154,155 Subsequently, activation of TLR2, TLR4, and/or CD14 on microglia can induce neurodegeneration.156–158 In addition to parenchymal cells of the brain, TLR2 and TLR4 are expressed on cerebral endothelial cells159 and soluble CD14 may participate in the ligand recognition of endothelial cells lacking membrane-bound CD14.160 Injuries of the CNS may also recruit CD14 positive macrophages to enact and propagate inflammatory responses.154 The receptors TLR2 and TLR4 are commonly expressed in macrophages as well.
Neuroinflammatory mechanisms involving TLR2, TLR4, and CD14 activation have been demonstrated to contribute to neurodegeneration.156–158 Resident microglia or infiltrating macrophages may respond to TLR2, TLR4, and CD14 ligands linked to CNS disorders and injuries, such as α-synuclein,106,161 fibrillar β-amyloid,105 heparan sulfate proteoglycans,162 heat shock proteins,77,163,164 necrotic neurons,94,165 and whole and partial bacteria.156,166 Activation of TLRs on microglia can lead to microglial activation and the subsequent release of cytokines, chemokines, reactive oxygen species,106 and nitric oxide,156 as well as induce phagocytosis of both damaged and viable neurons.167 The release of reactive oxygen species by activated microglia can cause damage to neuronal membranes and dendrites.168 Additionally, activation of TLRs has been shown to interfere with remyelination.169 In summary, overreaction of microglia to TLR2, TLR4, and CD14 can cause neuronal damage in many ways.
Contrary to the major role of TLR2, TLR4, and CD14 signaling in neurodegeneration, TLR2, TLR4, and CD14 have also been linked to neuroprotection.170 Activation of TLR2, TLR4, and CD14 may protect neurons by reverting microglia to a pro-healing activation state,171 inducing cytokine release in a beneficial context,172 or to facilitate clearing of dangerous molecules from the CNS.173 Thus, well-regulated innate immune signaling is useful for maintaining neuronal health. These results contradict many reports of the current understanding of TLR2, TLR4, and CD14, but should be further explored for a more complete understanding of the role of innate immunity in microelectrode performance.
Activation of TLR2, TLR4, and CD14 has been implicated in the neurodegeneration caused by a variety of CNS diseases and injuries, including Parkinson’s disease (PD),174 dementia with Lewy bodies (DLB),174 multiple system atrophy (MSA),174 multiple sclerosis (MS),175 amyotrophic lateral sclerosis (ALS),176 Alzheimer’s disease,105,177 neuropathic pain,178 subarachnoid hemorrhage,179,180 traumatic brain injury,181 focal cerebral ischemia,182 spinal cord injury,183 and brain injury.184 Synucleinopathies (PD, DLB, and MSA), Alzheimer’s disease, and ALS will be discussed in further detail due to the involvement of multiple receptors of interest and heavy innate immunity involvement.
Alzheimer’s disease is a memory disorder involving plaques made of β amyloid in the parenchyma of the brain.185,186 Bystander damage caused by the chronic inflammatory mechanisms directed against β amyloid plaques is hypothesized to be a major source of neurodegeneration.187 The receptors TLR2, TLR4, and CD14 on microglia recognize fibrillar β amyloid and induce a pro-inflammatory state involving phagocytosis and ROS release.105 Inhibiting TLR2 and TLR4 by various means has resulted in decreased generation of pro-inflammatory factors and subsequent neurotoxicity.158,185,188–190 On the other hand, activation of TLR2, TLR4, and CD14 signaling has been shown to facilitate the clearance of β amyloid,191 and the removal of neurons damaged by Aβ.192 Thus, some degree of TLR activation while limiting neuronal damage is likely the optimal scenario for treating Alzheimer’s disease.
Recent studies have identified participation of TLR2 and TLR4 in synucleinopathies, such as Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy.106,161,173,174 Synucleinopathies involve misfolding and buildup of the protein α-synuclein, resulting in TLR-mediated neuroinflammation and neurodegeneration.106,193 Activation of TLR2 disrupts the clearance of α-synuclein via autophagy.161 Contrarily, activation of TLR4 promotes the clearance of α-synuclein via phagocytosis, while simultaneously promoting neurodegenerative inflammatory mechanisms.106,173 Thus, a proper balance of clearing harmful materials and limiting self-inflicted damage must be maintained to combat synucleinopathies. Alternatively, a TLR4 ligand similar to LPS, mono-phosphoryl lipid A, has been identified to selectively promote clearance and not destructive inflammatory cascades.174 Therefore, approaches more nuanced than simply shutting down the signaling of entire TLRs must be considered in the mitigation of TLR-mediated neuroinflammation and neurodegeneration.
Amyotrophic lateral sclerosis is a disorder involving death of motoneurons in the motor cortex and spinal cord resulting in muscle atrophy and paralysis.185,194,195 Although the initial cause of motoneuron damage is unclear, activated microglia with mutations in SOD1 have been shown to exacerbate neurodegeneration via the release of superoxide, nitrate, and nitrite.185,196 TLR2, TLR4, and CD14 have been shown to activate microglia by binding extracellular mutated SOD1.197 Furthermore, knocking out TLR4 resulted in increased survival and improved functional outcomes in a mouse model of ALS.198 Recently, natural and synthetic TLR4 antagonists have been shown to mitigate neurodegeneration and nitric oxide release (natural TLR4 antagonist only) by microglia, in vitro.176 Overall, persistent activation of microglia via TLR2, TLR4, and CD14 in ALS leads to further neurodegeneration, and inhibiting these pathways mitigates harmful outcomes.
In summary, TLR2, TLR4, and CD14 are expressed on tissues of the CNS, recognize PAMPS and DAMPS in the CNS, and promote inflammatory mechanisms involved in neurodegenerative disorders. Temporal and regulated inhibition of TLR2, TLR4, and CD14 holds the potential to mitigate neurodegenerative disorders, because clearance of the ligands that generate inflammation must be considered for proper healing to occur.
B. TLR2, TLR4, and CD14 in Foreign Body Response to Intracortical Microelectrodes
Although there is currently little direct evidence for TLR2, TLR4, and CD14 signaling in the foreign body response to intracortical microelectrodes, several connections exist that suggest their role in the recognition of damage to promote and perpetuate inflammation. Particularly, TLR2, TLR4, and CD14 are expressed in the cells at the electrode tissue interface;60,153–155 ligands recognized by TLR2, TLR4, and CD14 have been reported at the electrode tissue interface;89,199 and outcomes associated with the activation of TLR2, TLR4, and CD14 pathways have been reported by several groups.61,66,157,200–205 Together, the concurrent presence of receptors, ligands, and downstream effectors strengthens the likelihood for participation of TLR2, TLR4, and CD14 in the foreign body response to intracortical microelectrodes.
As discussed above, resident parenchymal microglia express TLR2 and TLR4 constitutively,60,153 and may express CD14 following injury.154,155 Additionally, infiltrating macrophages constitutively express TLR2, TLR4, and CD14. Following intracortical microelectrode implantation, both resident microglia and infiltrating macrophages accumulate at the electrode-tissue interface and revert to a pro-inflammatory activated state.29 Therefore, the receptors TLR2, TLR4, and CD14 may play a role in the pro-inflammatory activation of microglia and macrophages through the recognition of ligands.
Several of the ligands that activate TLR2, TLR4, and CD14 may be present at the electrode tissue interface. Starting with PAMPs, residual endotoxin (LPS) has been detected at levels above FDA guidelines for CNS devices on intracortical microelectrode probes even following standard sterilization protocols with ethylene oxide.199 Ravikumar et al. compared sterilization methods and found that probes with higher endotoxin levels exhibited higher microglial activation, astrocytic encapsulation, blood-brain barrier permeability, and neuronal dieback at acute time points.199 Since TLR4 and CD14 are involved in the main pathway of endotoxin recognition,58 their findings indicate that activation of TLR4 and CD14 may contribute to unfavorable inflammatory mechanisms at the electrode tissue interface. Immunohistochemical differences between sterilization methods were not observed 16 weeks after implantation, suggesting that endotoxin or LPS may not be a significant activating ligand for chronic inflammatory responses once the LPS introduced by the electrode has been cleared. Additionally, as mentioned above, Harris administered LPS systemically to rats at the time of intracortical microelectrode implantation and observed decreased neuronal survival paired with lower firing rates in evoked recordings compared to control rats,42 indicating that activation of TLR4 and CD14 may contribute to declining recording performance as well as the foreign body response to the electrode. Of note, Gram-positive bacterial cell wall components, recognized by TLR2, were not quantified in the sterilization study but may be present following imperfect sterilization methods.
In addition to PAMPs, several DAMPs may be present at the electrode tissue interface due to the damage caused by insertion trauma, micromotion, and chronic inflammatory mechanisms.199 Enhanced expression of fibronectin, a ligand to TLR4,89 has been detected in the reactive tissue around implanted intracortical microelectrodes,206 as well as cortical impact injures.207 HMGB1, recognized by TLR2,91,93 TLR4,91,93 and CD14,145 was observed to be upregulated around implanted intracortical microelectrodes208 as well as in the brain following ischemia.92,209,210 Furthermore, necrotic cells, recognized by TLR294,95 and CD14,144 have been observed around implanted intracortical microelectrodes.211 Other DAMPs have been observed in various brain injuries and illnesses. A ligand to TLR4, HSP70, has been observed in the brain following focal cerebral ischemia.212,213 In vitro studies of CNS cells also indicate evidence of DAMP release following nervous system injuries. The ligands HSP60 and HSP70, recognized by TLR2,79–81 TLR4,77,78,80,81 and CD14 (HSP60 only),80,146 were observed on the axons of cultured DRG neurons that were explanted from rats following sciatic nerve injury.163,164 Furthermore, HSP60 was observed being released from apoptotic and necrotic cells in mixed CNS culture (embryonic forebrain-derived cells of unspecified composition).77 Additionally, fibrinogen, a blood protein that activates TLR4,44 has been speculated to be present around implanted intracortical microelectrodes.14,21,214 The disruption of vasculature upon intracortical microelectrode implantation16 and chronic permeability of the blood brain barrier likely results in the presence of fibrinogen in the brain at both acute and chronic time points.28,215 Overall, a variety of DAMPs may be released in the brain following intracortical microelectrode implantation.
Finally, the downstream consequences of TLR2, TLR4, and CD14 activation may indicate a role in the foreign body response to implanted intracortical microelectrode. Activation of TLR2, TLR4, and CD14 on microglia and macrophages leads to the activation of NF-κB, which mediates pro-inflammatory genes responsible for the release of cytokines, chemokines, cyclooxygenase-2, and matrix metalloproteinase-9 (MMP-9).66,216,217 The factors generated following NF-κB activation may perpetuate chronic inflammatory mechanisms around the implanted device. Activated microglia and macrophages are present at the electrode tissue interface long after device implantation.29,215 Activation of TLRs has the capability to induce neuronal damage,61,157 and neuronal dieback is commonly observed along with the foreign body response to intracortical microelectrodes.21,215,218 Activation of TLRs by DAMP recognition may contribute to the loss of neurons around the implant. Additionally, the neurons killed around implanted intracortical microelectrodes may provide further stimulus for TLR activation via necrosis or the release of HSP60, HSP70, or HMGB1, thus self-perpetuating chronic inflammation. Furthermore, activation of TLRs may contribute to chronic blood-brain barrier permeability via the release of nitric oxide pro-inflammatory cytokines, NO, and reactive oxygen species (ROS), and MMP-9.26,66,200–205 Blood-brain barrier permeability is a persistent problem of the foreign body response to intracortical microelectrodes and has been associated with poor recording performance.28,215
Overall, the evidence presented here suggests that TLR2, TLR4, and CD14 play a role in the foreign body response to intracortical microelectrodes via the recognition of PAMPs and DAMPs by microglia and macrophages and subsequent pro-inflammatory, neurotoxic, and blood-brain barrier damaging actions (Fig. 2).
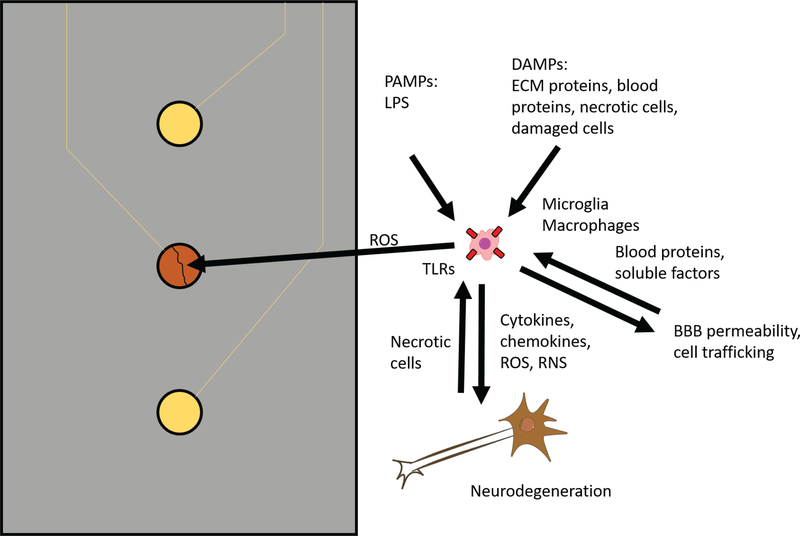
FIG. 2:
Potential role of TLR2, TLR4, and CD14 in the foreign body response to intracortical microelectrodes. Microglia and macrophages at the electrode tissue interface may recognize PAMPs and DAMPs at the electrode tissue interface via TLR2, TLR4, and CD14, resulting in the release of soluble factors such as cytokines, chemokines, reactive oxygen species, and reactive nitrogen species. The released soluble factors may damage neurons, blood vessels, and electrode materials, leading to poor intracortical microelectrode performance. Cells damaged and killed by the soluble inflammatory factors may release DAMPs that activate microglia and macrophages. Blood proteins released following damage to the blood brain barrier may also act as DAMPs. Inflammatory cells infiltrating across the blood-brain barrier permeability may become activated by DAMPs and contribute to the foreign body response. Thus, TLR2, TLR4, and CD14 signaling may contribute to the activation and perpetuation of chronic inflammatory mechanisms in the foreign body response to intracortical microelectrodes.
1. Current Strategies to Inhibit TLR2, TLR4, and CD14
Several strategies exist for inhibiting TLR2-, TLR4-, and CD14-mediated signaling, varying in target and molecular composition. The most direct strategies target the receptors themselves, however, inhibition strategies may target other components of the TLR2/TLR4/CD14 signal transduction pathways. A variety of peptides, antibodies, and RNA sequences may disrupt signaling at the various targets. In this section, several TLR2/TLR4/CD14 inhibition strategies under different stages of investigation will be reviewed (see Table 2).
TABLE 2:
Strategies for the inhibition of TLR2, TLR4, and CD14
| Inhibition Target Mechanism | Type of Therapeutic | Receptor Targeted | Therapeutic | Application(s) |
|---|---|---|---|---|
| Ligand binding | Small molecule | CD14/TLR4 | IAXO-101 | Sepsis,233 neuropathic pain,178 malaria234 |
| Ligand binding | Small molecule | CD14/TLR4 | IAXO-102 | Aneurysm235 |
| Ligand binding/ receptor dimerization | Small molecule | TLR2 | SSL3236 | N/A |
| Ligand binding | Antibody | CD14 | IC14 | LPS responsiveness,241 sepsis242,243 |
| Co-receptors (non-CD14) | Small molecule | TLR4 (via MD-2) | Eritoran | Sepsis250 |
| Co-receptors (non-CD14) | Small moleculee | TLR4 (via MD-2) | TAP2 | Inflammation251 |
| Co-receptors (non-CD14) | Small molecule | TLR4 (via MD-2) (natural) | Curcumin | Integration of intracortical microelectrodes208 |
| TLR4-MyD88 Complex formation | Small molecule | TLR4 | Wogonin | Inflammation in DRG neurons,255 traumatic brain injury216 |
| Intracellular TLR domain interactions | Small molecule | TLR4 | TAK-242/resatorvid | Sepsis,260 endotoxic shock,259 traumatic brain injury,261 cerebral ischemia,257 intracerebral hemorrhage,257 optic nerve crush262 |
| Receptor expression at cell surface | siRNA | TLR4 | N/A | Neuropathic pain220 |
| Receptor expression at cell surface | Small molecule | TLR4 | Oxymatrine | Traumatic brain injury181 |
| Receptor expression at cell surface | Small molecule | TLR4 | PACAP | Traumatic brain injury223 |
| Receptor expression at cell surface | Small molecule | TLR2 | Glycyrrhizin | Autoimmune thyroiditis221 |
| Receptor expression at cell surface | Small molecule | TLR4 | Melatonin | Subarachnoid hemorrhage,224 integration of intracortical microelectrode228 |
| Receptor expression at cell surface | Small molecule | TLR4 | Apigenin | Subarachnoid hemorrhage225 |
| Receptor expression at cell surface | Small molecule | TLR4 | Ursolic acid | Subarachnoid hemorrhage226 |
The first major option for inhibiting TLR2/TLR4/CD14 signaling we will discuss utilizes methods to alter the cell-surface expression of TLR2/TLR4/CD14, either by downregulation or internalization. Administration of rosmarinic acid,219 siRNA,220 oxymatrine,181 glycyrrhizin,221 argon,222 pituitary adenylate cyclase-activating polypeptide (PACAP),223 melatonin,224 apigenin,225 and ursolic acid,226 resulted in reduced TLR2, TLR4, and/ or CD14 expression; whereas, walnut extract was shown to promote receptor internalization.227 Of note, intrathecal siRNA that reduces TLR4 expression attenuated neuropathic pain in a rat model.220 Oxymatrine and PACAP conferred neuroprotection in a rat models of traumatic brain injury by inhibiting the expression and upregulation of TLR4.181,223 Glycyrrhizin inhibited TLR2-HMGB1 signaling, resulting in reduced lymphocyte trafficking in experimental autoimmune thyroiditis in mice.221 Melatonin, apigenin, and ursolic acid attenuated the effects of subarachnoid hemorrhage, including neurobehavioral deficits, blood-brain barrier permeability, and neuronal apoptosis.224–226 Even more promising, administration of melatonin has resulted in improved intracortical microelectrode recording and neuronal viability.228 Although melatonin was chosen for its ability to inhibit caspase activation, cytochrome c release, and oxidative stress, inhibition of TLR4 may have contributed to improved microelectrode integration and performance.224,228 Reducing the expression of TLR2, TLR4, and/or CD14 effectively attenuates the negative effects of overactive TLR signaling. Again, the success of cell-surface expression in CNS injuries and disorder demonstrate a potential application for integrating intracortical microelectrodes. Therefore, we utilized transgenic TLR2, TLR4, or CD14 knockout mice to investigate the effects of removal of each receptor in the integration and performance of intracortical microelectrodes. Studies with TLR2/TLR4 utilized dummy implants and investigated only the histological response.229 Endpoint histology at 2 and 16 weeks after implantation demonstrated that Tlr4−/− mice exhibited significantly lower BBB permeability at acute and chronic time points but also demonstrated significantly lower neuronal survival at the chronic time point. Additionally, inhibition of the TLR2 pathway had no significant histological effect compared to control animals. Together, our results indicate that complete genetic removal of TLR4 was detrimental to the integration of intracortical neural probes, while inhibition of TLR2 had no impact within the tests performed in this study. Implanting functional recording intracortical microelectrodes into Cd14−/− mice exhibited acute but not chronic improvements in intracortical microelectrode performance without significant differences in end point histology.230 However, when we used a mouse bone marrow chimera model to selectively knockout CD14 from either brain resident microglia or blood-derived macrophages, we were able to understand the most effective targets for future therapeutic options—brain-derived microglia or infiltrating blood-derived cells.231 Using single-unit recordings, we demonstrate that inhibiting CD14 from the blood-derived macrophages improves recording quality over the 16-week long study. We conclude that targeting CD14 in blood-derived cells should be part of the strategy to improve the performance of intracortical microelectrodes and that the daunting task of delivering therapeutics across the blood-brain barrier may not be needed to increase intracortical microelectrode performance. Knowing that both TLR4 and CD14 are important mediators of intracortical microelectrode integration and performance, other strategies to inhibit their activation must be explored.
Our next step was to consider inhibiting TLR2/TLR4/CD14 signaling via antagonism of the binding domain with a small molecule. The glycolipid IAXO-101 has demonstrated success as a CD14-TLR4 antagonist by competitively occupying CD14,232 resulting in mitigation of sepsis, neuropathic pain, and experimental malaria in mice.178,233,234 Therefore, we explored the use of IAXO-101 in an intracortical microelectrode model. Interestingly, mice receiving IAXO-101 exhibited significant improvements in recording performance over the entire 16-week duration without significant differences in endpoint histology.230 Combined with our chimera studies, we hypothesized that some degree of CD14 signaling may be necessary over chronic time ranges to facilitate wound healing mechanisms. We also remained unsure if IAXO-101 was simply not the most optimal method for small molecule inhibition and are exploring other options. For example, a similar compound IAXO-102 attenuated the development of an experimental form of aneurysm.235 The naturally derived antagonist to TLR2 staphylococcal superantigen-like protein 3 (SSL3) may inhibit TLR2 signaling by both blocking binding sites and disrupting heterodimerization.236 Because of the diversity of molecular structures recognized by the same TLR, the effectiveness of small molecule antagonists to interfere with ligand-receptor interactions may vary between ligand.237 Small molecule antagonists to the TLR2/TLR4/CD14 pathways have demonstrated varying degrees of success. The capability of small molecule antagonists to mitigate TLR2/TLR4/CD14 activity and attenuate detrimental inflammatory outcomes is promising for their utilization in intracortical microelectrode integration. When contemplating the effectiveness of a small molecule antagonist approach, it is important to consider delivery to the brain. Depending on the approach, delivery through the blood-brain barrier is complicated, despite the known “leakiness” associated with the neuroinflammatory response to intracortical microelectrodes.
Another mechanism of inhibiting TLR2/TLR4/CD14 signaling by blocking the ligand-receptor interactions utilizes blocking antibodies. Antibodies that bind a cellular receptor and prevent ligand binding have demonstrated success in various inflammatory disorders.238 Anti-CD14 antibodies may disrupt CD14 signaling by blocking the binding of LPS to CD14239,240 or through mechanisms independent of LPS binding.239 The commercially available monoclonal antibody IC14 recognizes human CD14 and saturates CD14 on monocytes and granulocytes.241 Intravenous administration of IC14 in humans reduced responsiveness to LPS, specifically attenuating the release of pro-inflammatory cytokines (TNF, IL-6, and IL-10), the activation of leukocytes and endothelial cells, the acute phase protein response, and various symptoms.241 The administration of IC14 has produced less promising results in severe cases of sepsis.242,243 One potential setback for the use of blocking antibodies is activation of the complement pathway by the Fc region of the antibody.244 Recent studies employing hybrid antibodies with inert Fc regions have been successful in attenuating CD14-mediated inflammatory responses without activating complement-related side effects in both in vivo porcine models and ex vivo human whole blood models of sepsis.244 Furthermore, pairing complement inhibition with hybrid anti-CD14 antibodies has demonstrated enhanced survival in a porcine model of polymicrobial sepsis.245 Finally, hybrid anti-CD14 antibodies with and without concurrent complement inhibition has outperformed the synthetic small molecule antagonist eritoran in mitigating monocyte activation in response to sepsis.246 Therapeutic antibodies have demonstrated success in mitigating TLR2/TLR4/CD14 mediated disorders despite potential complications. Thus, therapeutic antibodies may be applicable for integrating intracortical microelectrodes within the brain.
Alternative inhibition strategies to inhibit TLR2 and TLR4 focus on the non-CD14 co-receptors. The protein MD-2 (myeloid differentiation 2) is the other major cell-surface co-receptor involved in the recognition of LPS. Where CD14 is thought to facilitate recognition of both PAMPs and DAMPs, MD-2 is thought to facilitate response to PAMPs only.144 Limiting the types of ligands inhibited will likely result in different outcomes. Small molecule antagonists to TLR4-MD-2 may be derived from synthetic and natural sources, such as olive oil and curcumin.237 Synthetic antagonists to TLR4-MD-2 are typically similar in structure to the LPS component lipid A that is recognized by the TLR4 receptor complex.237 Eritoran is a synthetic small molecule antagonist derived from lipid A that binds MD-2 without promoting the dimerization of TLR4.237,247 Despite promising in vitro248 and animal model results,249 eritoran was unable to reduce mortality in clinical trials of severe sepsis.250 More recently, the TLR4/MD-2 antagonist peptide TAP2, hypothesized to bind the LPS-binding pocket of MD-2, was shown to attenuate LPS-induced inflammatory activation in vitro and in mice.251 However, clinical trials with TAP2 have not been reported. Becaust of the predominant interaction of MD-2 with PAMPS,144 the authors did not initially expect TLR4-MD-2 antagonists to be effective in mitigating chronic inflammation associated with the damage caused by intracortical microelectrodes. However, Potter et al. previously demonstrated that the natural TLR4-MD-2 antagonist curcumin improves neuronal survival and blood-brain barrier stability around intracortical microelectrodes implanted in rats.208 Their results may indicate a larger role of pathogens at the electrode tissue interface, involvement of MD-2 in DAMP recognition, or promiscuous activity of curcumin as an antioxidant, which was the author’s intended application. Regardless of the mechanism, this finding indicates the potential for TLR4-MD-2 inhibitors in the integration of intracortical microelectrodes.
Additional inhibition strategies to inhibit TLR2/TLR/CD14 signaling target downstream intracellular signal transduction pathways. Antagonists have been developed or identified to target the adaptor molecules MyD88 adaptor-like (Mal) and TRIF-related adaptor molecule (TRAM),252 the TLR2 TIR domain,253 JNK,166 ubiquitin chains,254 TLR4-MyD88 interactions,255 TLR1-TLR2 heterodimerization,256 and the intracellular domain of TLR4.257,258 Of note, administration of the flavonoid wogonin attenuated LPS-induced inflammation in dorsal root ganglion neurons255 and improved outcomes in a model of traumatic brain injury216 by interfering with TLR4MyD88 complex formation.255 Furthermore, cyclohexene-derived TAK-242/resatorvid inhibits TLR4 signaling by binding the intracellular domain of TLR4 and preventing interactions with downstream adapter molecules.257,259 In vivo administration of TAK-242 for the treatment of sepsis,260 endotoxic shock,259 traumatic brain injury,261 cerebral ischemia,257 intracerebral hemorrhage,257 and optic nerve crush262 resulted in positive outcomes, such as attenuated inflammation and neuroprotection. A phase III clinical trial (NCT00633477) investigated the utilization of TAK-242 to treat sepsis, but the trial was discontinued due to business concerns unrelated to safety.263 Strategies to inhibit TLR2/TLR4/CD14 signaling have demonstrated preliminary efficacy but have not demonstrated clinical success to this date. Nonetheless, many of the in vivo experiments have demonstrated efficacy in conditions related to the foreign body response to intracortical microelectrodes. Traumatic brain injury involves similar phenomena exhibited in the foreign body response to intracortical microelectrodes, such as blood-brain barrier permeability, neuroinflammation, and neuronal death.264 Additionally, ischemic conditions have been observed around intracortical microelectrodes.265 The efficacy of inhibiting intracellular signal transduction pathways downstream of TLR2/TLR4/CD14 in CNS applications is promising for the utilization in the integration of intracortical microelectrodes.
Overall, TLR2, TLR4, and CD14 may be attenuated at different points along their respective signaling pathways (Table 2). Many promising findings have been observed in vitro and in vivo, but strong clinical performance in humans has not been demonstrated. Human clinical trials mainly failed in severe cases of sepsis, which is likely not the best predictor of efficacy mitigating the foreign body response to intracortical microelectrodes. However, promising in vivo results in CNS injuries and diseases, coupled with our preliminary work in the role of TLR4/CD14 in microelectrode performance clearly indicate the potential success of additional strategies for integrating intracortical microelectrodes.
III. CONCLUSION: FUTURE PERSPECTIVES AND DIRECTIONS
Electrical signals recorded from the neurons of human patients by intracortical microelectrodes have been used to communicate with computers, control robotic limbs, and to control the patient’s own arm. The signal quality and the length of time that useful signals can be recorded are inconsistent. The widely held view of the community is that the inflammatory response of neural tissue that surrounds the microelectrodes, at least in part, compromises electrode reliability. Several studies have demonstrated the connection between neuroinflammation and microelectrode performance.
Inflammation is initiated when inflammatory cells recognize foreign biologics (i.e., damaged/infiltrating proteins and cells). Serum proteins and blood-derived cells invade the central nervous system following microelectrode implantation and aggravate the neuroinflammatory response. Cells and tissue are damaged from the trauma of microelectrode implantation. At the microelectrode surface, accumulation of pro-inflammatory molecules causes neuronal degeneration and increases the permeability of the blood-brain barrier, self-perpetuating the process. In rodents, the neuroinflammatory response to implanted microelectrodes in the motor cortex has been linked to decreased fine motor skills.266
Many labs, including ours, have worked to develop new microelectrode designs to reduce to neuroinflammatory response to intracortical microelectrodes. The extent to which neuroinflammation has been altered by electrode design varies between labs and designs. In the near term, the field must remember the effort that was taken to obtain FDA and CE approvals for clinical trials. The most direct route to translation is working with the devices further along in development. Ultimately, one electrode design will be unable to reach all the targets of the brain that may require interfacing. Therefore, simply making the intracortical microelectrode smaller, thinner, more flexible, mechanically dynamic, or inert may not be enough. We believe that the most likely route to enabling intracortical microelectrodes to interface with any region of the brain is to intimately understand the neuroinflammatory process, to allow for the most directed strategies for mitigation without interruption of the normal healthy response to disease or injury.
Here, we have outlined the rationale behind our hypothesis for the importance of the innate immune system in intracortical microelectrode performance. We have introduced our preliminary efforts and attempted to inspire others to also implement approaches that target the controlled immunosuppression of the localized and specific parts of the innate immune response to intracortical microelectrodes, as opposed to broader approaches that may lead to greater side effects. Much of the work reported here in understanding the role of specific innate immunity pathways was performed in mice. Although most basic neuroscience studies of the brain are also performed in mice, the majority of brain computer interface studies in rodents chose the rat over the mouse.267 We have previously reported that there is no difference in the inflammatory response to microelectrode in the mouse and rat models268 and thus chose to focus on the mouse as more transgenic models are available in mice than rats. The number and type of TLRs in mice varies to that found in humans; so as with all animal work, precaution should be taken in overanalyzing results from transgenic studies in mice when deciding pathways to clinical translation. Additionally, as chemists and biomaterialists, we appreciate the role of new materials and electrode designs in the overall picture. In the end, no one approach will work alone, indefinitely. Only after we understand the body’s temporally driven process for rejection of implanted devices can we better engineer the neural interface and our complete strategy for long-term integration.
ACKNOWLEDGMENTS
This work was supported in part by the Department of Biomedical Engineering and Case School of Engineering at Case Western Reserve University through laboratory start-up funds and the National Institute of Health, National Institute of Neurological Disorders and Stroke, (Grant No. 1R01NS082404-01A1). Additional support was provided by the Presidential Early Career Award for Scientists and Engineers (PECASE, JR. Capadona) and by Merit Review Award B1495-R from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service. None of the funding sources aided in collection, analysis, and interpretation of the data, in writing of the paper, or in the decision to submit the paper for publication. The authors have no conflict of interest related to this work to disclose. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
ABBREVIATIONS:
- ALS
amyotrophic lateral sclerosis
- CE
Conformité Européene
- COX-2
cyclooxygenase 2
- CD14
cluster of differentiation 14
- DAMPs
damage (or danger) associated molecular patterns
- DLB
dementia with Lewy bodies
- ECM
extracellular matrix
- FDA
Food and Drug Administration
- HSP
heat shock proteins
- HNMGB1
High Mobility Group Box 1
- iNOS
inducible nitric oxide synthetase
- IFN-α
interferon-α
- IL
interleukin
- LPS
lipopolysaccharide
- LRR
leucine-rich repeat
- MIP-1α
macrophage inflammatory protein-1α
- MHC
major histocompatibility complex
- MMP
matrix metalloprotease
- MS
multiple sclerosis
- MSA
multiple system atrophy
- NLRs
Nod-like receptors
- NO
nitrous oxide
- PD
Parkinson’s disease
- PAMPs
pathogen associated molecular patterns
- PRRs
pattern-recognition receptors
- PACAP
pituitary adenylate cyclase-activating polypeptide
- ROS
reactive oxygen species
- RLRs
retinoic acid-inducible gene-I-like receptors
- SSL3
staphylococcal superantigen-like protein 3
- TLRs
Toll-like receptors
- TNF or TNF-α
tumor necrosis factor
REFERENCES
- 1.Williams JC, Rennaker RL, Kipke DR. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res Brain Res Protoc. 1999;4(3):303–13. [DOI] [PubMed] [Google Scholar]
- 2.Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82(1):1–15. [DOI] [PubMed] [Google Scholar]
- 3.Freire MA, Morya E, Faber J, Santos JR, Guimaraes JS, Lemos NA, Sameshima K, Pereira A, Ribeiro S, Nicolelis MA. Comprehensive analysis of tissue preservation and recording quality from chronic multielectrode implants. PLoS One. 2011;6(11):e27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perge JA, Zhang S, Malik WQ, Homer ML, Cash S, Friehs G, Eskandar EN, Donoghue JP, Hochberg LR. Reliability of directional information in unsorted spikes and local field potentials recorded in human motor cortex. J Neural Eng. 2014;11(4):046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolta NF, Christensen MB, Crane PD, Skousen JL, Tresco PA. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials. 2015;53:753–62. [DOI] [PubMed] [Google Scholar]
- 6.Cody PA, Eles JR, Lagenaur CF, Kozai TD, Cui XT. Unique electrophysiological and impedance signatures between encapsulation types: An analysis of biological Utah array failure and benefit of a biomimetic coating in a rat model. Biomaterials. 2018;161:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad A, Xue Q-S, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9(5):056015. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, McCreery DB, Bullara LA, Agnew WF. Evaluation of the stability of intracortical microelectrode arrays. IEEE Trans Neural Syst Rehabil Eng. 2006;14(1):91–100. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A, Xue Q-S, Dieme R, Sankar V, Mayrand R, Nishida T, Streit WJ, Sanchez JC. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front Neurosci. 2014;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6):066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozai TD, Li X, Bodily LM, Caparosa EM, Zenonos GA, Carlisle DL, Friedlander RM, Cui XT. Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials. 2014;35(36):9620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chestek CA, Gilja V, Nuyujukian P, Foster JD, Fan JM, Kaufman MT, Churchland MM, Rivera-Alvidrez Z, Cunningham JP, Ryu SI, Shenoy KV. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J Neural Eng. 2011;8(4):045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, McCreery DB, Carter RR, Bullara LA, Yuen TG, Agnew WF. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Rehabil Eng. 1999;7(3):315–26. [DOI] [PubMed] [Google Scholar]
- 14.Jorfi M, Skousen JL, Weder C, Capadona JR. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2015;12(1):011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozai TDY, Catt K, Li X, Gugel ZV, Olafsson VT, Vazquez AL, Cui XT. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials. 2015;37:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006;3(3):196–207. [DOI] [PubMed] [Google Scholar]
- 17.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. [DOI] [PubMed] [Google Scholar]
- 18.Polikov V, Tresco P, Reichert W. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter KA, Buck AC, Self WK, Callanan ME, Sunil S, Capadona JR. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials. 2013;34:7001–15. [DOI] [PubMed] [Google Scholar]
- 21.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–26. [DOI] [PubMed] [Google Scholar]
- 22.Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. [DOI] [PubMed] [Google Scholar]
- 23.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clinica chimica acta; international journal of clinical chemistry. 2010;411(21–22):1570–9. [DOI] [PubMed] [Google Scholar]
- 24.Quagliarello VJ, Wispelwey B, Long WJ Jr, Scheld WM. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Invest. 1991;87(4):1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ereifej ES, Rial GM, Hermann JK, Smith CS, Meade SM, Rayyan JM, Chen K, Feng H, Capadona JR. Implantation of neural probes in the brain elicits oxidative stress. Front Bioeng Biotechnol. 2018;6(9):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett C, Samikkannu M, Mohammed F, Dietrich WD, Rajguru SM, Prasad A. Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials. 2018;164:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takmakov P, Ruda K, Phillips KS, Isayeva IS, Krauthamer V, Welle CG. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species. J Neural Eng. 2015;12(2):026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB, Bellamkonda RV. The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials. 2013;34(20):4703–13. [DOI] [PubMed] [Google Scholar]
- 29.Ravikumar M, Sunil S, Black J, Barkauskas D, Haung AY, Miller RH, Selkirk SM, Capadona JR. The roles of blood-derived macrophages and resident microglia in the neuroinflammatory response to implanted intracortical microelectrodes. Biomaterials. 2014;S0142–9612(35):8049–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5. [DOI] [PubMed] [Google Scholar]
- 31.Pineau I, Lacroix S. Endogenous signals initiating inflammation in the injured nervous system. Glia. 2009;57(4):351–61. [DOI] [PubMed] [Google Scholar]
- 32.Roitbak T, Sykova E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28(1):40–8. [DOI] [PubMed] [Google Scholar]
- 33.Schultz RL, Willey TJ. The ultrastructure of the sheath around chronically implanted electrodes in brain. J Neurocytol. 1976;5(6):621–42. [DOI] [PubMed] [Google Scholar]
- 34.Hermann JK. The role of innate immunity in the response to intracortical microelectrodes [dissertation]: Cleveland, OH, Case Western Reserve University; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rennaker RL, Miller J, Tang H, Wilson DA. Minocycline increases quality and longevity of chronic neural recordings. J Neural Eng. 2007;4(2):L1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shultz RB, Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. 2017;12(5):702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4(3):e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsendorf F Minocycline in acne vulgaris: benefits and risks. Am J Clin Dermatol. 2010;11(5):327–41. [DOI] [PubMed] [Google Scholar]
- 39.Reed DN, Gregg FO, Corpe RS. Minocycline-induced black bone disease encountered during total knee arthroplasty. Orthopedics. 2012;35(5):e737–9. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Takakubo Y, Kobayashi S, Asano T, Sasaki A, Sasaki K, Ohki H, Tamaki Y, Takagi M. Minocycline-induced periarticular black bones in inflamed joints which underwent arthroplastic reconstruction. Clin Orthop Surg. 2012;4:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashukem MT, Levy JC, Formaini N. Minocycline induced black bone disease: an incidental finding during total shoulder arthroplasty. Curr Orthop Pract. 2016;27(6):698–701. [Google Scholar]
- 42.Harris JP. The glia-neuronal response to cortical electrodes: interactions with substrate stiffness and electrophysiology [dissertation]. Cleveland (OH): Case Western Reserve University; 2012. [Google Scholar]
- 43.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microb Infect. 2002;4:903–14. [DOI] [PubMed] [Google Scholar]
- 44.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J Immunol. 2001;167(5):2887–94. [DOI] [PubMed] [Google Scholar]
- 45.Kozai TDY, Li X, Bodily LM, Caparosa EM, Zenonos GA, Carlisle DL, Friedlander RM, Cui XT. Effects of caspase-1 knockout on chronic neural recording quality and longevity: Insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials. 2014;35(36):9620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medzhitov R, Janeway CJ. Innate immunity. N Engl J Med. 2000;343(5):338–44. [DOI] [PubMed] [Google Scholar]
- 47.Owen J, Punt J, Stranford S, Jones P. Kuby immunology, 7th ed. New York: WH Freeman; 2013;27(692): 109–130. [Google Scholar]
- 48.Netea MG, Latz E, Mills KHG, O’Neill LAJ. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol. 2015;16:675–9. [DOI] [PubMed] [Google Scholar]
- 49.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84. [DOI] [PubMed] [Google Scholar]
- 50.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawai T, Akira S. Toll‐like receptor and RIG‐1‐like receptor signaling. Ann N Y Acad Sci. 2008;1143(1):1–20. [DOI] [PubMed] [Google Scholar]
- 52.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG‐I‐like receptors. Immunol Rev. 2009;227(1):54–65. [DOI] [PubMed] [Google Scholar]
- 53.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27: 229–65. [DOI] [PubMed] [Google Scholar]
- 54.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21(1):335–76. [DOI] [PubMed] [Google Scholar]
- 56.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. [DOI] [PubMed] [Google Scholar]
- 57.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76(3):514–9. [DOI] [PubMed] [Google Scholar]
- 58.Medzhitov R Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–45. [DOI] [PubMed] [Google Scholar]
- 59.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23(11):509–12. [DOI] [PubMed] [Google Scholar]
- 60.Kong Y, Le Y. Toll-like receptors in inflammation of the central nervous system. Int Immunopharmacol. 2011;11(10):1407–14. [DOI] [PubMed] [Google Scholar]
- 61.Lehnardt S Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll‐like receptor‐mediated neuronal injury. Glia. 2010;58(3):253–63. [DOI] [PubMed] [Google Scholar]
- 62.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95(2):588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiang C, Beachy PA. Expression of a novel Toll-like gene spans the parasegment boundary and contributes to hedgehog function in the adult eye of Drosophila. Mech Dev. 1994;47(3):225–39. [DOI] [PubMed] [Google Scholar]
- 64.Morisato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76(4):677–88. [DOI] [PubMed] [Google Scholar]
- 65.Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–83. [DOI] [PubMed] [Google Scholar]
- 66.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640): 394–7. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52(2):269–79. [DOI] [PubMed] [Google Scholar]
- 68.Kobe B, Deisenhofer J. Proteins with leucine-rich repeats. Curr Opin Struct Biol. 1995;5(3):409–16. [DOI] [PubMed] [Google Scholar]
- 69.Aravind L, Dixit VM, Koonin EV. Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science. 2001;291(5507):1279–84. [DOI] [PubMed] [Google Scholar]
- 70.Oosting M, Cheng S-C, Bolscher JM, Vestering-Stenger R, Plantinga TS, Verschueren IC, Arts P, Garritsen A, van Eenennaam H, Sturm P. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci U S A. 2014;111(42):E4478–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Celhar T, Magalhaes R, Fairhurst A-M. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 2012;53(1–3):58–77. [DOI] [PubMed] [Google Scholar]
- 73.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. [DOI] [PubMed] [Google Scholar]
- 74.Lindquist S The heat-shock response. Annu Rev Biochem. 1986;55(1):1151–91. [DOI] [PubMed] [Google Scholar]
- 75.Tsan M-F, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1(4):274–9. [PubMed] [Google Scholar]
- 76.Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176(11):7021–7. [DOI] [PubMed] [Google Scholar]
- 77.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28(10): 2320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164(2):558–61. [DOI] [PubMed] [Google Scholar]
- 79.Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17(11):1567–9. [DOI] [PubMed] [Google Scholar]
- 80.Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70 role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–34. [DOI] [PubMed] [Google Scholar]
- 81.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277(17):15107–12. [DOI] [PubMed] [Google Scholar]
- 82.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee H-G, Wagner H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277(23):20847–53. [DOI] [PubMed] [Google Scholar]
- 83.Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. FASEB J. 2005;19(7):872–4. [DOI] [PubMed] [Google Scholar]
- 84.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168(10):5233–9. [DOI] [PubMed] [Google Scholar]
- 85.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172(1):20–4. [DOI] [PubMed] [Google Scholar]
- 86.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–9. [DOI] [PubMed] [Google Scholar]
- 87.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schaefer L, Babelova A, Kiss E, Hausser H-J, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–33. [DOI] [PubMed] [Google Scholar]
- 90.Andersson U, Wang H, Palmblad K, Aveberger A-C, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279(9):7370–7. [DOI] [PubMed] [Google Scholar]
- 92.Kim J-B, Choi JS, Yu Y-M, Nam K, Piao C-S, Kim S-W, Lee M-H, Han P-L, Park J-s, Lee J-K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26(24):6413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim J-Y, Strassheim D, Sohn J-W, Yamada S, Maruyama I, Banerjee A. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–C24. [DOI] [PubMed] [Google Scholar]
- 94.Lee H, Jo E-K, Choi S-Y, Oh SB, Park K, Kim JS, Lee SJ. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochem Biophys Res Commun. 2006;350(3):742–7. [DOI] [PubMed] [Google Scholar]
- 95.Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the nf-kb/toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–35. [DOI] [PubMed] [Google Scholar]
- 96.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol. 2002;168(12):5989–92. [DOI] [PubMed] [Google Scholar]
- 97.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202(8):1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279(13):12542–50. [DOI] [PubMed] [Google Scholar]
- 99.Ni H, Capodici J, Cannon G, Communi D, Boeynaems J-M, Karikó K, Weissman D. Extracellular mRNA induces dendritic cell activation by stimulating tumor necrosis factor-α secretion and signaling through a nucleotide receptor. J Biol Chem. 2002;277(15):12689–96. [DOI] [PubMed] [Google Scholar]
- 100.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–7. [DOI] [PubMed] [Google Scholar]
- 101.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4(199):75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim S, Takahashi H, Lin W-W, Descargues P, Grivennikov S, Kim Y, Luo J-L, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–9. [DOI] [PubMed] [Google Scholar]
- 104.Riva M, Källberg E, Björk P, Hancz D, Vogl T, Roth J, Ivars F, Leanderson T. Induction of nuclear factor‐κB responses by the S100A9 protein is Toll‐like receptor‐4‐dependent. Immunology. 2012;137(2):172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and Toll-like receptors 2 and 4 are required for fibrillar a-stimulated microglial activation. J Neurosci. 2009;29(38):11982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll‐like receptor 4 is required for α‐synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. [DOI] [PubMed] [Google Scholar]
- 108.Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundbäck P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. PAIN®. 2014;155(9):1802–13. [DOI] [PubMed] [Google Scholar]
- 109.Gaudreault E, Fiola S, Olivier M, Gosselin J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol. 2007;81(15):8016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hanisch U-K. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–55. [DOI] [PubMed] [Google Scholar]
- 111.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–73. [PubMed] [Google Scholar]
- 112.Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from Gram‐negative, not Gram‐positive, bacteria cause a profound induction of the geneencoding Toll‐like receptor 2 in the CNS. J Neurochem. 2001;79(3):648–57. [DOI] [PubMed] [Google Scholar]
- 113.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755): 811–15. [DOI] [PubMed] [Google Scholar]
- 114.Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, Boneca IG. Toll‐like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004;5(10):1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1-and TLR2-deficient mice. Nat Med. 2002;8(8):878–84. [DOI] [PubMed] [Google Scholar]
- 116.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97(25):13766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13(7):933–40. [DOI] [PubMed] [Google Scholar]
- 118.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169(1):10–4. [DOI] [PubMed] [Google Scholar]
- 119.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan-and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274(25):17406–9. [DOI] [PubMed] [Google Scholar]
- 120.Aliprantis AO, Yang R-B, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285(5428):736–9. [DOI] [PubMed] [Google Scholar]
- 121.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163(7):3920–7. [PubMed] [Google Scholar]
- 122.Hajjar AM, O’Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166(1):15–9. [DOI] [PubMed] [Google Scholar]
- 123.Coelho PS, Klein A, Talvani A, Coutinho SF, Takeuchi O, Akira S, Silva JS, Canizzaro H, Gazzinelli RT, Teixeira MM. Glycosylphosphatidylinositol‐anchored mucin‐like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP‐1 production by IFN‐γ‐primed‐macrophages. J Leukoc Biol. 2002;71(5):837–44. [PubMed] [Google Scholar]
- 124.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168(4):1533–7. [DOI] [PubMed] [Google Scholar]
- 125.Werts C, Tapping RI, Mathison JC, Chuang T-H, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2(4):346–52. [DOI] [PubMed] [Google Scholar]
- 126.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69(3):1477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8. [DOI] [PubMed] [Google Scholar]
- 128.Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med. 1999;189(4):615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting Edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 130.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22(7):2478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, Ohno N, Tamura H, Shibata Ki, Akashi S. Saccharomyces cerevisiae‐and Candida albicans‐derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14‐and Toll-like receptor 4‐dependent manner. Microbiol Immunol. 2002;46(7):503–12. [DOI] [PubMed] [Google Scholar]
- 132.Shoham S, Huang C, Chen J-M, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol. 2001;166(7):4620–6. [DOI] [PubMed] [Google Scholar]
- 133.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;5:398–401. [DOI] [PubMed] [Google Scholar]
- 134.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101(5):1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–3. [DOI] [PubMed] [Google Scholar]
- 136.Yu B, Wright SD. Catalytic properties of lipopolysaccharide (LPS) binding protein transfer of LPS to soluble CD14. J Biol Chem. 1996;271(8):4100–5. [DOI] [PubMed] [Google Scholar]
- 137.Thomas CJ, Kapoor M, Sharma S, Bausinger H, Zyilan U, Lipsker D, Hanau D, Surolia A. Evidence of a trimolecular complex involving LPS, LPS binding protein and soluble CD14 as an effector of LPS response. FEBS Lett. 2002;531(2):184–8. [DOI] [PubMed] [Google Scholar]
- 138.Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microb Infect. 2004;6(15):1361–7. [DOI] [PubMed] [Google Scholar]
- 139.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyer SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–14. [DOI] [PubMed] [Google Scholar]
- 140.Franklin W, Mason D, Pulford K, Falini B, Bliss E, Gatter K, Stein H, Clarke L, McGee J. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986;54(3):322–35. [PubMed] [Google Scholar]
- 141.Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol (Berl). 2002;103(6):541–9. [DOI] [PubMed] [Google Scholar]
- 142.Ziegler-Heitbrock HWL, Ulevitch RJ. CD14: Cell surface receptor and differentiation marker. Immunol Today. 1993;14(3):121–5. [DOI] [PubMed] [Google Scholar]
- 143.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77(8):4588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chun KH, Seong SY. CD14 but not MD2 transmit signals from DAMP. Int Immunopharmacol. 2010;10(1):98–106. [DOI] [PubMed] [Google Scholar]
- 145.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of High Mobility Group Box 1 (HMGB1) through Toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–42. [DOI] [PubMed] [Google Scholar]
- 147.Baumann CL, Aspalter IM, Sharif O, Pichlmair A, Bluml S, Grebien F, Bruckner M, Pasierbek P, Aumayr K, Planyavsky M, Bennett KL, Colinge J, Knapp S, Superti-Furga G. CD14 is a coreceptor of Toll-like receptors 7 and 9. J Exp Med. 2010;207(12):2689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392(6675):505–9. [DOI] [PubMed] [Google Scholar]
- 149.Devitt A, Marshall LJ. The innate immune system and the clearance of apoptotic cells. J Leukoc Biol. 2011;90(3):447–57. [DOI] [PubMed] [Google Scholar]
- 150.Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr Opin Immunol. 2000;12(1):27–34. [DOI] [PubMed] [Google Scholar]
- 151.Wang P-y, Munford RS. CD14-dependent internalization and metabolism of extracellular phosphatidylinositol by monocytes. J Biol Chem. 1999;274(33):23235–41. [DOI] [PubMed] [Google Scholar]
- 152.Arroyo DS, Soria JA, Gaviglio EA, Rodriguez-Galan MC, Iribarren P. Toll-like receptors are key players in neurodegeneration. Int Immunopharmacol. 2011;11(10):1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bsibsi M, Ravid R, Gveric D, Van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61(11):1013–21. [DOI] [PubMed] [Google Scholar]
- 154.Beschorner R, Schluesener HJ, Gözalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002;126(1–2):107–15. [DOI] [PubMed] [Google Scholar]
- 155.Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor κ B activity in the brain during endotoxemia. J Neurosci. 2000;20(9):3456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177(1):583–92. [DOI] [PubMed] [Google Scholar]
- 157.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18(1):203–5. [DOI] [PubMed] [Google Scholar]
- 159.Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Hasko J, Krizbai IA. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int. 2010;57(5):556–64. [DOI] [PubMed] [Google Scholar]
- 160.Andersson J, Nagy S, Bjourk L, Abrams J, Holm S, Andersson U. Bacterial toxin‐induced cytokine production studied at the single‐cell level. Immunol Rev. 1992;127(1):69–96. [DOI] [PubMed] [Google Scholar]
- 161.Kim C, Rockenstein E, Spencer B, Kim H-K, Adame A, Trejo M, Stafa K, Lee H-J, Lee S-J, Masliah E. Antagonizing neuronal toll-like receptor 2 prevents synucleinopathy by activating autophagy. Cell Rep. 2015;13(4):771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.O’Callaghan P, Li J-P, Lannfelt L, Lindahl U, Zhang X. Microglial heparan sulfate proteoglycans facilitate the cluster-of-differentiation 14 (CD14)/toll-like receptor 4 (TLR4)-dependent inflammatory response. J Biol Chem. 2015;290(24):14904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Willis D, Li KW, Zheng J-Q, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, Van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25(4):778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178(6):965–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pais TF, Figueiredo C, Peixoto R, Braz MH, Chatterjee S. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation. 2008;5:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leow-Dyke S, Allen C, Denes A, Nilsson O, Maysami S, Bowie AG, Rothwell NJ, Pinteaux E. Neuronal Toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J Neuroinflammation. 2012;9(1):230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front Pharmacol. 2012;3:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Milatovic D, Zaja-Milatovic S, Montine KS, Shie F-S, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. J Neuroinflammation. 2004;1(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sloane J, Batt C, Ma Y, Harris Z, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107(25):11555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hanisch U-K, Johnson TV, Kipnis J. Toll-like receptors: roles in neuroprotection? Trends Neurosci. 2008;31(4):176–82. [DOI] [PubMed] [Google Scholar]
- 171.Hayakawa K, Okazaki R, Morioka K, Nakamura K, Tanaka S, Ogata T. Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J Neurosci Res. 2014;92(12):1647–58. [DOI] [PubMed] [Google Scholar]
- 172.Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29(5):1319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179(2):954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy. Mol Neurodegener. 2017;12(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hossain MJ, Tanasescu R, Gran B. Innate immune regulation of autoimmunity in multiple sclerosis: Focus on the role of Toll-like receptor 2. J Neuroimmunol. 2017;304:11–20. [DOI] [PubMed] [Google Scholar]
- 176.De Paola M, Sestito SE, Mariani A, Memo C, Fanelli R, Freschi M, Bendotti C, Calabrese V, Peri F. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res. 2016;103:180–7. [DOI] [PubMed] [Google Scholar]
- 177.Reed-Geaghan EG, Reed QW, Cramer PE, Landreth GE. Deletion of CD14 attenuates Alzheimer’s disease pathology by influencing the brain’s inflammatory milieu. J Neurosci. 2010;30(46):15369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B. Glial TLR4 receptor as new target to treat neuropathic pain: Efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia. 2008;56(12):1312–9. [DOI] [PubMed] [Google Scholar]
- 179.Kawakita F, Fujimoto M, Liu L, Nakano F, Nakatsuka Y, Suzuki H. Effects of Toll-like receptor 4 antagonists against cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2016:1–10. [DOI] [PubMed] [Google Scholar]
- 180.Hanafy KA. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation. 2013;10(1):868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dong X-Q, Yu W-H, Hu Y-Y, Zhang Z-Y, Huang M. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflamm Res. 2011;60(6):533–9. [DOI] [PubMed] [Google Scholar]
- 182.Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171(1):258–67. [DOI] [PubMed] [Google Scholar]
- 183.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll‐like receptor (TLR)‐2 and TLR‐4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. 2007;102(1):37–50. [DOI] [PubMed] [Google Scholar]
- 184.Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26(49):12826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trotta T, Porro C, Calvello R, Panaro MA. Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol. 2014;268(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 186.Landreth GE, Reed-Geaghan EG. Toll-like receptors in Alzheimer’s disease. 2009;336:137–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schüffer W. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20(6):947–56. [DOI] [PubMed] [Google Scholar]
- 189.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-β peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol. 2008;181(10):7254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Udan ML, Ajit D, Crouse NR, Nichols MR. Toll‐like receptors 2 and 4 mediate Aβ (1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104(2):524–33. [DOI] [PubMed] [Google Scholar]
- 191.Tahara K, Kim H-D, Jin J-J, Maxwell JA, Li L, Fukuchi K-i. Role of Toll-like receptor signalling in Aβ uptake and clearance. Brain. 2006;129(11):3006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bate C, Veerhuis R, Eikelenboom P, Williams A. Microglia kill amyloid-β1–42 damaged neurons by a CD14-dependent process. Neuroreport. 2004;15(9):1427–30. [DOI] [PubMed] [Google Scholar]
- 193.Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of α-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121(6):675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Casula M, Iyer AM, Spliet WG, Anink JJ, Steentjes K, Sta M, Troost D, Aronica E. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience. 2011;179:233–43. [DOI] [PubMed] [Google Scholar]
- 195.Zhang R, Hadlock KG, Do H, Yu S, Honrada R, Champion S, Forshew D, Madison C, Katz J, Miller RG. Gene expression profiling in peripheral blood mononuclear cells from patients with sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2011;230(1):114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU. 1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103(43):16021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, Appel SH. Extracellular mutant SOD1 induces microglial‐mediated motoneuron injury. Glia. 2010;58(2):231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee JY, Lee JD, Phipps S, Noakes PG, Woodruff TM. Absence of toll-like receptor 4 (TLR4) extends survival in the hSOD1 G93A mouse model of amyotrophic lateral sclerosis. J Neuroinflammation. 2015;12(1):90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ravikumar M, Hageman DJ, Tomaszewski WH, Chandra GM, Skousen JL, Capadona JR. The effect of residual endotoxin contamination on the neuroinflammatory response to sterilized intracortical microelectrodes. J Mater Chem B. 2014;2:2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD. Peroxynitrite mediates neurotoxicity of amyloid β-peptide1–42-and lipopolysaccharide-activated microglia. J Neurosci. 2002;22(9):3484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115(12):1599–608. [DOI] [PubMed] [Google Scholar]
- 202.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190(1):101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Thiel VE, Audus KL. Nitric oxide and blood–brain barrier integrity. Antioxid Redox Signal. 2001;3(2):273–8. [DOI] [PubMed] [Google Scholar]
- 204.Merrill JE, Murphy SP. Inflammatory events at the blood brain barrier: regulation of adhesion molecules, cytokines, and chemokines by reactive nitrogen and oxygen species. Brain Behav Immun. 1997;11(4):245–63. [DOI] [PubMed] [Google Scholar]
- 205.Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. [DOI] [PubMed] [Google Scholar]
- 206.Kim YT, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25(12):2229–37. [DOI] [PubMed] [Google Scholar]
- 207.Tate CC, Tate MC, LaPlaca MC. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J Neurotrauma. 2007;24(1):226–30. [DOI] [PubMed] [Google Scholar]
- 208.Potter KA, Jorfi M, Householder KT, Foster EJ, Weder C, Capadona JR. Curcumin-releasing mechanically-adaptive intracortical implants improve the proximal neuronal density and blood-brain barrier stability. Acta Biomater. 2014;10(5):2209–22. [DOI] [PubMed] [Google Scholar]
- 209.Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, Yang H, Na S-Y, Akhisaroglu M, Fleming T. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Faraco G, Fossati S, Bianchi M, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103(2):590–603. [DOI] [PubMed] [Google Scholar]
- 211.Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156(1):33–49. [DOI] [PubMed] [Google Scholar]
- 212.Kinouchi H, Sharp FR, Hill MP, Koistinaho J, Sagar SM, Chan PH. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993;13(1):105–15. [DOI] [PubMed] [Google Scholar]
- 213.Kinouchi H, Sharp FR, Koistinaho J, Hicks K, Kamii H, Chan PH. Induction of heat shock hsp70 mRNA and HSP70 kDa protein in neurons in the ‘penumbra’ following focal cerebral ischemia in the rat. Brain Res. 1993;619(1–2):334–8. [DOI] [PubMed] [Google Scholar]
- 214.Skousen J, Merriam S, Srivannavit O, Perlin G, Wise K, Tresco P. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog Brain Res. 2011;194C:167–80. [DOI] [PubMed] [Google Scholar]
- 215.Potter KA, Buck AC, Self WK, Capadona JR. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J Neural Eng. 2012;9(4):046020. [DOI] [PubMed] [Google Scholar]
- 216.Chen C-C, Hung T-H, Wang Y-H, Lin C-W, Wang P-Y, Lee C-Y, Chen S-F. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-κB signaling after experimental traumatic brain injury. PLoS One. 2012;7(1):e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82(7):434–48. [DOI] [PubMed] [Google Scholar]
- 218.McConnell GC, Rees HD, Levey AI, Gutekunst CA, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6(5):056003. [DOI] [PubMed] [Google Scholar]
- 219.Wei Y, Chen J, Hu Y, Lu W, Zhang X, Wang R, Chu K. Rosmarinic acid mitigates lipopolysaccharide-induced neuroinflammatory responses through the inhibition of TLR4 and CD14 expression and NF-κB and NLRP3 inflammasome activation. Inflammation. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 220.Wu F-x, Bian J-j, Miao X-r, Huang S-d, Xu X-w, Gong D-j, Sun Y-m, Lu Z-j, Yu W-f. Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int J Med Sci. 2010;7(5):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li C, Peng S, Liu X, Han C, Wang X, Jin T, Liu S, Wang W, Xie X, He X. Glycyrrhizin, a direct HMGB1 antagonist, ameliorates inflammatory infiltration in a model of autoimmune thyroiditis via inhibition of TLR2-HMGB1 signaling. Thyroid. 2017;27(5):722–31. [DOI] [PubMed] [Google Scholar]
- 222.Ulbrich F, Kaufmann K, Roesslein M, Wellner F, Auwärter V, Kempf J, Loop T, Buerkle H, Goebel U. Argon mediates anti-apoptotic signaling and neuroprotection via inhibition of toll-like receptor 2 and 4. PLoS One. 2015;10(12):e0143887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mao SS, Hua R, Zhao XP, Qin X, Sun ZQ, Zhang Y, Wu YQ, Jia MX, Cao JL, Zhang YM. Exogenous administration of PACAP alleviates traumatic brain injury in rats through a mechanism involving the TLR4/MyD88/NF-small ka, CyrillicB pathway. J Neurotrauma. 2012;29(10):1941–59. [DOI] [PubMed] [Google Scholar]
- 224.Wang Z, Wu L, You W, Ji C, Chen G. Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4‐mediated inflammatory pathway. J Pineal Res. 2013;55(4):399–408. [DOI] [PubMed] [Google Scholar]
- 225.Zhang T, Su J, Guo B, Wang K, Li X, Liang G. Apigenin protects blood–brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int Immunopharmacol. 2015;28(1):79–87. [DOI] [PubMed] [Google Scholar]
- 226.Zhang T, Su J, Guo B, Zhu T, Wang K, Li X. Ursolic acid alleviates early brain injury after experimental subarachnoid hemorrhage by suppressing TLR4-mediated inflammatory pathway. Int Immunopharmacol. 2014;23(2):585–91. [DOI] [PubMed] [Google Scholar]
- 227.Willis LM, Bielinski DF, Fisher DR, Matthan NR, Joseph JA. Walnut extract inhibits LPS-induced activation of BV-2 microglia via internalization of TLR4: possible involvement of phospholipase D2. Inflammation. 2010;33(5):325–33. [DOI] [PubMed] [Google Scholar]
- 228.Golabchi A, Wu B, Li X, Carlisle DL, Kozai TD, Friedlander RM, Cui XT. Melatonin improves quality and longevity of chronic neural recording. Biomaterials. 2018;180:225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hermann JK, Lin S, Soffer A, Wong C, Srivastava V, Chang J, Sunil S, Sudhakar S, Tomaswzeski W, Protasiewicz G, Selkirk S, Miller R, Capadona JR. The role of Toll-like receptor 2 and 4 innate immunity pathways in intracortical microelectrode-induced neuroinflammation. Front Bioeng Biotechnol. 2018;6:113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hermann JK, Ravikumar M, Shoffstall AJ, Ereifej ES, Kovach KM, Chang J, Soffer A, Wong C, Srivastava V, Smith P, Protasiewicz G, Jiang J, Selkirk SM, Miller RH, Sidik S, Ziats NP, Taylor DM, Capadona JR. Inhibition of the cluster of differentiation 14 innate immunity pathway with IAXO-101 improves chronic microelectrode performance. J Neural Eng. 2018;15(2):025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bedell HW, Hermann JK, Ravikumar M, Lin S, Rein A, Li X, Molinich E, Smith PD, Selkirk SM, Miller RH, Sidik S, Taylor DM, Capadona JR. Targeting CD14 on blood derived cells improves intracortical microelectrode performance. Biomaterials. 2018;163:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Piazza M, Yu L, Teghanemt A, Gioannini T, Weiss J, Peri F. Evidence of a specific interaction between new synthetic antisepsis agents and CD14. Biochemistry. 2009;48(51):12337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Piazza M, Rossini C, Della Fiorentina S, Pozzi C, Comelli F, Bettoni I, Fusi P, Costa B, Peri F. Glycolipids and benzylammonium lipids as novel antisepsis agents: synthesis and biological characterization. J Med Chem. 2009;52(4):1209–13. [DOI] [PubMed] [Google Scholar]
- 234.Barboza R, Lima FA, Reis AS, Murillo OJ, Peixoto EPM, Bandeira CL, Fotoran WL, Sardinha LR, Wunderlich G, Bevilacqua E. TLR4-mediated placental pathology and pregnancy outcome in experimental malaria. Sci Rep. 2017;7(1):8623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Huggins C, Pearce S, Peri F, Neumann F, Cockerill G, Pirianov G. A novel small molecule TLR4 antagonist (IAXO-102) negatively regulates non-hematopoietic toll like receptor 4 signalling and inhibits aortic aneurysms development. Atherosclerosis. 2015;242(2):563–70. [DOI] [PubMed] [Google Scholar]
- 236.Koymans KJ, Feitsma LJ, Brondijk THC, Aerts PC, Lukkien E, Lössl P, van Kessel KP, de Haas CJ, van Strijp JA, Huizinga EG. Structural basis for inhibition of TLR2 by staphylococcal superantigen-like protein 3 (SSL3). Proc Natl Acad Sci U S A. 2015;112(35):11018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Peri F, Calabrese V. Toll-like receptor 4 (TLR4) modulation by synthetic and natural compounds: an update: miniperspective. J Med Chem. 2013;57(9):3612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10(5):301–16. [DOI] [PubMed] [Google Scholar]
- 239.Leturcq DJ, Moriarty AM, Talbott G, Winn RK, Martin TR, Ulevitch RJ. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 1996;98(7):1533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schimke J, Mathison J, Morgiewicz J, Ulevitch RJ. Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc Natl Acad Sci U S A. 1998;95:13875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Verbon A, Dekkers PE, ten Hove T, Hack CE, Pribble JP, Turner T, Souza S, Axtelle T, Hoek FJ, van Deventer SJ. IC14, an anti-CD14 antibody, inhibits endotoxin-mediated symptoms and inflammatory responses in humans. J Immunol. 2001;166(5):3599–605. [DOI] [PubMed] [Google Scholar]
- 242.Axtelle T, Pribble J. An overview of clinical studies in healthy subjects and patients with severe sepsis with IC14, a CD14-specific chimeric monoclonal antibody. J Endotoxin Res. 2003;9(6):385–9. [DOI] [PubMed] [Google Scholar]
- 243.Reinhart K, Glück T, Ligtenberg J, Tschaikowsky K, Bruining A, Bakker J, Opal S, Moldawer LL, Axtelle T, Turner T. CD14 receptor occupancy in severe sepsis: results of a phase I clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14). Crit Care Med. 2004;32(5):1100–8. [DOI] [PubMed] [Google Scholar]
- 244.Lau C, Gunnarsen KS, Høydahl LS, Andersen JT, Berntzen G, Pharo A, Lindstad JK, Ludviksen JK, Brekke O-L, Barratt-Due A. Chimeric anti-CD14 IGG2/4 Hybrid antibodies for therapeutic intervention in pig and human models of inflammation. J Immunol. 2013;191(9):4769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Skjeflo EW, Sagatun C, Dybwik K, Aam S, Urving SH, Nunn MA, Fure H, Lau C, Brekke O-L, Huber-Lang M. Combined inhibition of complement and CD14 improved outcome in porcine polymicrobial sepsis. Crit Care. 2015;19(1):415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gustavsen A, Nymo S, Landsem A, Christiansen D, Ryan L, Husebye H, Lau C, Pischke SE, Lambris JD, Espevik T. Combined inhibition of complement and CD14 attenuates bacteria-induced inflammation in human whole blood more efficiently than antagonizing the Toll-like receptor 4–MD2 complex. J Infect Dis. 2016;214(1):140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim HM, Park BS, Kim J-I, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130(5):906–17. [DOI] [PubMed] [Google Scholar]
- 248.Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8(6):483–8. [DOI] [PubMed] [Google Scholar]
- 249.Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304(3):1093–102. [DOI] [PubMed] [Google Scholar]
- 250.Opal SM, Laterre P-F, Francois B, LaRosa SP, Angus DC, Mira J-P, Wittebole X, Dugernier T, Perrotin D, Tidswell M. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154–62. [DOI] [PubMed] [Google Scholar]
- 251.Park S, Shin H-J, Shah M, Cho H-Y, Anwar MA, Achek A, Kwon H-K, Lee B, Yoo TH, Choi S. TLR4/MD2 specific peptides stalled in vivo LPS-induced immune exacerbation. Biomaterials. 2017;126:49–60. [DOI] [PubMed] [Google Scholar]
- 252.Lysakova-Devine T, Keogh B, Harrington B, Nagpal K, Halle A, Golenbock DT, Monie T, Bowie AG. Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule. J Immunol. 2010;185(7):4261–71. [DOI] [PubMed] [Google Scholar]
- 253.Mistry P, Laird MH, Schwarz RS, Greene S, Dyson T, Snyder GA, Xiao TS, Chauhan J, Fletcher S, Toshchakov VY. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci U S A. 2015;112(17):5455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.McGuire VA, Diez TR-Z, Emmerich CH, Strickson S, Ritorto MS, Sutavani RV, Weiβ A, Houslay KF, Knebel A, Meakin PJ. Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci Rep. 2016;6:31159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen S, Xiong J, Zhan Y, Liu W, Wang X. Wogonin inhibits LPS-induced inflammatory responses in rat dorsal root ganglion neurons via inhibiting TLR4–MyD88–TAK1-mediated NF-κB and MAPK signaling pathway. Cell Mol Neurobiol. 2015;35(4):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhong Z, Liu L-J, Dong Z-Q, Lu L, Wang M, Leung C-H, Ma D-L, Wang Y. Structure-based discovery of an immunomodulatory inhibitor of TLR1–TLR2 heterodimerization from a natural product-like database. Chem Commun. 2015;51(56):11178–81. [DOI] [PubMed] [Google Scholar]
- 257.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79(1):34–41. [DOI] [PubMed] [Google Scholar]
- 258.Takashima K, Matsunaga N, Yoshimatsu M, Hazeki K, Kaisho T, Uekata M, Hazeki O, Akira S, Iizawa Y, Ii M. Analysis of binding site for the novel small‐molecule TLR4 signal transduction inhibitor TAK‐242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol. 2009;157(7):1250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571(2–3):231–9. [DOI] [PubMed] [Google Scholar]
- 260.Fenhammar J, Rundgren M, Hultenby K, Forestier J, Taavo M, Kenne E, Weitzberg E, Eriksson S, Ozenci V, Wernerson A. Renal effects of treatment with a TLR4 inhibitor in conscious septic sheep. Crit Care. 2014;18(5):488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang D, Li H, Li T, Zhou M, Hao S, Yan H, Yu Z, Li W, Li K, Hang C. TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: implication in the treatment of human brain injury. Neurochem Int. 2014;75:11–8. [DOI] [PubMed] [Google Scholar]
- 262.Nakano Y, Shimazawa M, Ojino K, Izawa H, Takeuchi H, Inoue Y, Tsuruma K, Hara H. Toll-like receptor 4 inhibitor protects against retinal ganglion cell damage induced by optic nerve crush in mice. J Pharmacol Sci. 2017;133(3):176–83. [DOI] [PubMed] [Google Scholar]
- 263.Hennessy EJ, Parker AE, O’Neill LAJ. Targeting Toll-like receptors: emerging theraputics? Nat Rev Drug Discov. 2010;9:293–307. [DOI] [PubMed] [Google Scholar]
- 264.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kozai TDY, Vazquez AL, Weaver CL, Kim S-G, Cui XT. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J Neural Eng. 2012;9(6):066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Goss-Varley M, Dona KR, McMahon JA, Shoffstall AJ, Ereifej ES, Lindner SC, Capadona JR. Microelectrode implantation in motor cortex causes fine motor deficit: Implications on potential considerations to brain computer interfacing and human augmentation. Sci Rep. 2017;7:15254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jorfi M, Skousen JL, Weder C, Capadona JR. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2015;12(1):011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Potter-Baker KA, Ravikumar M, Burke AA, Meador WD, Householder KT, Buck AC, Sunil S, Stewart WG, Anna JP, Tomaszewski WH, Capadona JR. A comparison of neuroinflammation to implanted microelectrodes in rat and mouse models. Biomaterials. 2014;34:5637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]