Abstract
Objective
Previous studies have shown sex-specific differences in all-cause and CHD mortality in type 2 diabetes. We performed a systematic review and meta-analysis to provide a global picture of the estimated influence of type 2 diabetes on the risk of all-cause and CHD mortality in women vs men.
Methods
We systematically searched PubMed, EMBASE and Web of Science for studies published from their starting dates to Aug 7, 2018. The sex-specific hazard ratios (HRs) and their pooled ratio (women vs men) of all-cause and CHD mortality associated with type 2 diabetes were obtained through an inverse variance-weighted random-effects meta-analysis. Subgroup analyses were used to explore the potential sources of heterogeneity.
Results
The 35 analyzed prospective cohort studies included 2 314 292 individuals, among whom 254 038 all-cause deaths occurred. The pooled women vs men ratio of the HRs for all-cause and CHD mortality were 1.17 (95% CI: 1.12–1.23, I2 = 81.6%) and 1.97 (95% CI: 1.49–2.61, I2 = 86.4%), respectively. The pooled estimate of the HR for all-cause mortality was approximately 1.30 in articles in which the duration of follow-up was longer than 10 years and 1.10 in articles in which the duration of follow-up was less than 10 years. The pooled HRs for all-cause mortality in patients with type 2 diabetes was 2.33 (95% CI: 2.02–2.69) in women and 1.91 (95% CI: 1.72–2.12) in men, compared with their healthy counterparts.
Conclusions
The effect of diabetes on all-cause and CHD mortality is approximately 17 and 97% greater, respectively, for women than for men.
Introduction
Diabetes is recognized as the world’s fastest growing chronic condition. Due to rapid increases in the prevalence of physical inactivity, overweight and obesity, the number of people with diabetes is projected to rise to 592 million by 2035 (1). In particular, type 2 diabetes (T2D) has attained the status of a global pandemic, with the total number of patients with T2D estimated at 425 million in 2015 (2).
Accumulating evidence documents T2D as an independent risk factor for all-cause mortality (3, 4, 5, 6). The risk of all-cause mortality in persons with T2D is approximately doubled (7). However, these conclusions are mainly based on the assumption that the risk of diabetes in women is the same as in men (8). However, evidence is accruing that the detrimental effects of diabetes are higher among women than among men (9). The sex-based difference in the risk of diabetes would not only result from patient management and treatment (10) but also from the diversity of biological factors (11). Epidemiological studies reported that women with T2D had a higher risk of mortality from cardiovascular diseases (10, 12) and cancer (13) than men.
However, the differences between men and women in the risk of all-cause and CHD mortality is still unclear. Moreover, none of the relevant meta-analyses summarized the differences in risk of all-cause and CHD mortality between men and women. Therefore, we performed a comprehensive meta-analysis to estimate reliably the effect of T2D on all-cause and CHD risk among women in comparison with men.
Methods
Search strategy
We systematically searched the PubMed (www.ncbi.nlm.nih.gov), EMBASE and Web of Science databases (from their starting dates to Aug 7, 2018) with the limitations object human and language English. A combined text word and medical subject heading (MeSH) search strategy was applied with the terms ‘mortality’, ‘death’, ‘Diabetes Mellitus, Type 2’, ‘Adult-Onset Diabetes’, ‘Non-Insulin-Dependent Diabetes’, ‘Gender’, ‘Sex’, ‘Cohort’, ‘Prospective’ and ‘Longitudinal’. We also scanned the reference lists of relevant reviews and meta-analyses to discern additional potentially relevant literature.
Inclusion and exclusion criteria
We included articles only when they had clearly reported hazard ratios (HRs) or equivalents for all-cause or CHD mortality in both genders (T2D patients vs healthy counterparts). We also included articles that did not report HRs for each gender directly, but from which we could calculate it. Studies in which the enrolled participants had stroke, coronary heart disease or other cardiovascular diseases (myocardial infarction, atherosclerosis etc.) were excluded. If more than two articles had been published about the same cohort, we enrolled the one with the longer follow-up period or a larger sample size. The search strategy and inclusion criteria were defined and agreed upon by all the authors. The quality of the included studies was evaluated by the NOS (Newcastle-Ottawa Scale) (14) (Supplementary data, see section on supplementary data given at the end of this article). Our meta-analysis was performed in accordance with the PRISMA statement (15) and registered at the International Prospective Register of Systematic Reviews (Prospero) (http://www.crd.york.ac.uk /PROSPERO, registration number: CRD42017074187).
Data extraction
For each study, we extracted the following variables: name of first author and study, baseline years of study, country of study, duration of follow-up, mean ages of participants, sample size, death count, adjusted variables, HRs and their 95% CIs in men and women and NOS score. Two authors (Guodong Xu and Dingyun You) independently extracted the data. If there was controversy, the discrepancy was resolved by an arbitrator (Liyuan Han).
Statistical analysis
We extracted gender-specific HRs and 95% CIs from each study (T2D patients vs healthy counterparts). Subsequently, gender-specific HRs and 95% CIs were used to estimate the pooled ratio of HR and the corresponding 95% CIs. Subgroup analyses were performed by year of study baseline (before 1980, 1980 to 1990 and after 1990); region (America, Europe, Asia, and Australia, Canada, New Zealand or Pacific); duration of study (<10, 10–14, >14 years); study quality (NOS score) (≥6 vs <6) (14) and adjusted status (unadjusted vs adjusted). Sensitivity analysis was conducted to ascertain the stability of the pooled results after removing one study at a time. The I 2 value was used to estimate heterogeneity. An I 2 value of 25, 50 and 75% represented a low, middle and high degree of heterogeneity, respectively (16). Meta-regression analyses were also performed to estimate the source of heterogeneity. We used funnel plots to estimate publication bias. Egger’s and Begg’s test were also applied to quantitatively estimate publication bias. Additionally, to explore the possible effect of publication bias, we employed trim-and-fill method (17) in our meta-analyses for more reliable estimates. All P values were two sided and P values less than 0.05 were considered as statistically significant. Software Stata 12.0 (StataCorp) was used to perform statistical analyses.
Results
Study characteristics
We systematically searched PubMed, EMBASE and Web of Science (from their starting dates to Aug 7, 2018). A total of 3907 articles were identified by assessment of titles and abstracts, and eight additional records were identified from the reference lists (Supplementary Fig. 1). After full-text assessment, 35 articles were finally included in our meta-analysis (18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52) for all-cause mortality, and 24 articles for CHD mortality (21, 25, 26, 29, 30, 40, 43, 47, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66). Table 1 shows the baseline characteristics of all 52 cohorts. A total of 2 314 292 T2D cases (46% women) were included, and 254 038 all-cause deaths (45% women) occurred. Among the 35 datasets included, 23 cohort studies were performed in Europe (19, 20, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 36, 37, 38, 39, 41, 42, 43, 45, 48, 49, 51), 5 in Australia, Canada, New Zealand or Pacific (22, 26, 35, 44, 46), 3 in America (18, 21, 22) and 6 in Asia (23, 26, 40, 47, 50, 52). All the included articles had NOS scores higher than four points, and 26 of them scored at least six points (18, 21, 23, 25, 26, 28, 29, 31, 32, 33, 35, 36, 38, 39, 40, 41, 43, 44, 46, 47, 48, 49, 50, 51, 52).
Table 1.
Characteristics of included studies.
| Baseline years | Country | Follow-up duration (years) | Participants (n) | % women | Mean age (years) | Deaths (n) | % deaths in women | Ascertainment of diabetes | Variables used to standardize HR | Causes of death | NOS score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Framingham Heart Study (earlier) (19) | 1950–1975 | US | 25 | 399 | 52 | 56.7 | 109 | 45 | Self-reported, Measured | Age, sex | All-cause | 7 |
| Bedford Diabetes Study (19) | 1962–1971 | UK | 10 | 363 | 50 | NA | 108 | 56 | Measured | Age | All-cause | 5 |
| Reykjavik Diabetes Study (20) | 1967–1991 | Iceland | 17 | 477 | 44 | 55 | 213 | 31 | Measured | NA | All-cause | 5 |
| NHANES I (21) | 1971–1984 | US | 14 | 407 | 54 | 62.5 | 172 | 41 | NA | Age, smoking, SBP, TC, BMI | All-cause, CHD | 6 |
| WHO MSVDD (22) | 1975–1979 | US | 15 | 240 | 46 | NA | 51 | 35 | NA | Age | All-cause | 5 |
| GRIC (23) | 1975–1984 | India | 10 | 1266 | 58 | NA | 241 | 47 | Self-reported, Measured | Age | All-cause | 6 |
| Framingham Heart Study (later) (18) | 1975–2005 | US | 25 | 679 | NA | 59.5 | 159 | 31 | Self-reported, Measured | Age, sex | All-cause | 7 |
| WHO MSVDD (Switzerland) (24) | 1977–2006 | Switzerland | 30 | 308 | 44 | 48.6 | 214 | 43 | Self-reported | NA | All-cause | 5 |
| Swedish Annual Level-of-Living Survey (25) | 1979–1985 | Sweden | 7 | 776 | 46 | NA | 418 | 46 | Self-reported | Age | All-cause, CHD | 6 |
| Diabetes Melanesian Fijians Cohort Study (26) | 1980–1991 | Melanesian | 11 | 65 | 65 | NA | 25 | 48 | Measured | Age | All-cause, CHD | 6 |
| Diabetes Asian Indian Cohort Study (26) | 1980–1991 | India | 11 | 166 | 52 | NA | 21 | 34 | Measured | Age | All-cause, CHD | 6 |
| Denmark Diabetes Register (27) | 1981–1993 | Denmark | 13 | 228 | 60 | 68 | 75 | 48 | Measured | NA | All-cause | 5 |
| Kuopio Diabetes Register (28) | 1981–1995 | Finland | 15 | 133 | 47 | 55.7 | 59 | 47 | Measured | Age | All-cause | 6 |
| Finland Diabetes Study (29) | 1982–1997 | Finland | 17 | 962 | 48 | 42.7 | 399 | 48 | Self-reported, Measured | Age, education years, BMI, SBP, TC, and smoking | All-cause, CHD | 7 |
| Diabetes Finland Cohort Study (30) | 1982–2001 | Finland | 18 | 1059 | 45 | 58.1 | 768 | 45 | Measured | NA | All-cause, CHD | 5 |
| Poole Diabetes Registry (31) | 1983–1991 | UK | 8 | 917 | 48 | 60.8 | 295 | 47 | Self-reported, Measured | NA | All-cause | 6 |
| DISS (32) | 1983–1992 | Sweden | 10 | 661 | NA | NA | 14 | 21 | Self-reported, Measured | Age | All-cause | 6 |
| DISS (33) | 1983–1999 | Sweden | 9 | 1142 | NA | NA | 37 | 22 | Measured | Age, sex | All-cause | 7 |
| Verona Diabetes Study (34) | 1987–1991 | Italy | 5 | 7148 | 53 | NA | 1550 | 52 | Measured | NA | All-cause | 5 |
| Prospective Dubbo Study of Australian (35) | 1988–1993 | Australian | 5 | 207 | 49 | 69.9 | 61 | 39 | Self-reported, Measured | NA | All-cause | 6 |
| Diabetes New Zealand Cohort Study (36) | 1989–1999 | New Zealand | 10 | 447 | 53 | 62 | 187 | 54 | Self-reported, Measured | Age, sex | All-cause | 7 |
| FRESCO (37) | 1991–2005 | Spanish | 10 | 8627 | 47 | 60.9 | 781 | 44 | Self-reported | NA | All-cause | 5 |
| Diabetes Spain Cohort Study (38) | 1991–2006 | Spain | 9 | 469 | 54 | 60.4 | 80 | 40 | Measured | Age, HDL, smoking | All-cause | 6 |
| Norwegian Diabetes Register (39) | 1991–1999 | Italy | 9 | 29 656 | NA | NA | 6673 | 48 | Measured | Age, area of birth | All-cause | 6 |
| Takayama Diabetes Study (40) | 1992–1999 | Japan | 8 | 1217 | 65 | 60.1 | 176 | 36 | Measured | Age, smoking, BMI, physical activity, education years, hypertension, total energy intake, intake of vegetables, fat, and alcohol | All-cause, CHD | 7 |
| GPRD (41) | 1992–1999 | UK | 8 | 44 230 | 46 | 67 | 12 453 | 76 | Measured | Age, sex | All-cause | 7 |
| Record-linkage Databases (42) | 1993–2004 | UK | 12 | 10 532 | 48 | NA | 1863 | 47 | Measured | NA | All-cause | 5 |
| South Tees Diabetes Mortality Study (43) | 1994–1999 | UK | 6 | 4081 | 45 | NA | 1151 | 45 | Measured | Age, sex, calendar year | All-cause, CHD | 7 |
| CCDSS (44) | 1995–2008 | Canada | 10 | 15 152 | 19 | 59.9 | 3554 | 48 | Measured | Region of residence, socioeconomic status quintile, | All-cause | 6 |
| Diabetes Clinic of the San Giovanni Battista Hospital (45) | 1996–2000 | Italy | 5 | 2673 | NA | 70 | 428 | 46 | Self-reported, Measured | NA | All-cause | 4 |
| National Diabetes Services Scheme (1997–03) (46) | 1997–2003 | Australia | 7 | 10 60367 | 46 | 60 | 76 689 | 43 | Self-reported, Measured | Age, sex | All-cause | 7 |
| ET-CHD Registry (47) | 1997–2006 | China | 10 | 386 | 38 | 64.6 | 157 | 42 | Self-reported, Measured | Age, smoking status, HDL, TC, creatinine, stroke, cancer | All-cause, CHD | 6 |
| National Diabetes Registry (48) | 1998–2003 | UK | 5 | 736 | 45 | 64.2 | 147 | 52 | Self-reported, Measured | NA | All-cause | 6 |
| University Hospital Birmingham (49) | 2000–2007 | UK | 7 | 679 | 36 | NA | 100 | 42 | Measured | Age | All-cause | 6 |
| NHISNSC (50) | 2002–2004 | Korean | 3 | 29 807 | 48 | NA | 7103 | 44 | Self-reported, Measured | Age, sex | All-cause | 6 |
| National Diabetes Services Scheme (2004–10) (46) | 2003–2010 | Australia | 7 | 1 060 367 | 46 | NA | 134 393 | 44 | Self-reported, Measured | Age, sex | All-cause | 7 |
| GPRD (51) | 2004–2010 | UK | 7 | 21 789 | 40 | 55.1 | 2146 | 35 | Measured | Age, sex, and general practice | All-cause | 7 |
| DIAMOND Cohort Registry (52) | 2010–2012 | Korean | 2 | 1125 | 34 | 64.9 | 44 | 52 | Self-reported, Measured | NA | All-cause | 6 |
| Tecumseh Study (59) | 1959–1979 | US | 6 | 386 | 60 | NA | 230 | 55 | Measured | Age, BMI, smoking, use of hypertension medications | CHD | 6 |
| The Reykjavik Study (60) | 1961–1991 | Iceland | 9 | 547 | 31 | NA | 50 | 12 | Measured | Age, TC, SBP, ECG, education | CHD | 6 |
| Chicago Heart Association Detection Project (61) | 1967–1973 | US | 19 | 5729 | 46 | 51.4 | 183 | 41 | Self-reported, Measured | Age, education, smoking, alcohol intake, physical activity, BMI, hypertension, diabetes | CHD | 7 |
| NHANES I (62) | 1971–1992 | US | 20 | 462 | 62 | 59.2 | 127 | 54 | NA | Age, smoking, hypertension, TC, BMI | CHD | 5 |
| Framingham Heart Study (63) | 1971–1995 | US | 20 | 178 | 42 | NA | 35 | 46 | Measured | Age, hypertension, TC, BMI, smoking | CHD | 6 |
| The Rancho Bernardo Study (64) | 1972–1985 | US | 14 | 334 | 38 | 63.4 | 55 | 35 | Self-reported, Measured | Age | CHD | 7 |
| Hawaii-Los Angeles-Hiroshima Study (65) | 1976–1984 | Hawaii | 7 | 776 | 46 | 64.5 | 183 | 48 | Self-reported, Measured | Age | CHD | 6 |
| The Adventist Health Study (66) | 1977–1982 | US | 6 | 812 | 68 | NA | 33 | 61 | Self-reported | Age | CHD | 5 |
| Community-dwelling Elderly (53) | 1982–1988 | US | 11 | 166 | 52 | NA | 12 | 42 | Measured | Age, study year, smoking, TC, HDL, SBP, BMI | CHD | 6 |
| Finland Diabetes Study (54) | 1982–1990 | Finland | 20 | 113 | 62 | NA | 36 | 47 | NA | Age, sex | CHD | 7 |
| Finland National Hospital Discharge Register (55) | 1982–1994 | Finland | 12 | 14 786 | 52 | NA | 294 | 21 | Self-reported, Measured | Age, TC, TG, BMI, hypertension, smoking | CHD | 4 |
| Finnish Diabetes Study (56) | 1986–1988 | Finnish | 10 | 386 | 38 | NA | 105 | 50 | Measured | Age | CHD | 5 |
| NHANES III (57) | 1988–2006 | US | 12 | 133 | 56 | NA | 23 | 61 | Measured | Age, BMI, UA, TC, TG, hypertension, smoking | CHD | 7 |
| JACC Study (58) | 1988–2009 | Japan | 8 | 1217 | 35 | NA | 15 | 13 | Measured | Age, race, education, BMI, smoking, SBP, DBP, HDL, medication use, | CHD | 7 |
| GeneSTAR (57) | 1993–2005 | US | 7 | 292 | 48 | 46.5 | 17 | 41 | NA | Age | CHD | 6 |
| MESA (57) | 2000–2011 | US | 14 | 407 | 54 | 52.6 | 59 | 36 | NA | Age | CHD | 7 |
*Including UK, Switzerland, Poland, Germany, Croatia, China, Japan, Cuba, USA.
BMI, body mass index; CCDSS, Canadian Chronic Diseases: Surveillance System; DISS, Diabetes Incidence Study in Sweden; ET-CHD, Eastern Taiwan integrated health care delivery system of Coronary Heart Disease; FRESCO, Función de Riesgo ESpañola de acontecimientos Coronarios y Otros; GPRD, General Practice Research Database; GRIC, Gila River Indian Community; HDL, high density lipoprotein; HR, hazard ratio; JACC, The Japan Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; MSVDD, Multinational Study of Vascular Disease in Disease in Diabetes; NA, not available; NHANES, the National Health and Nutrition Examination Survey; NHISNSC, National Health Insurance ServiceNational Sample Cohort; TC, total cholesterol.
HRs for all-cause and CHD mortality between men and women
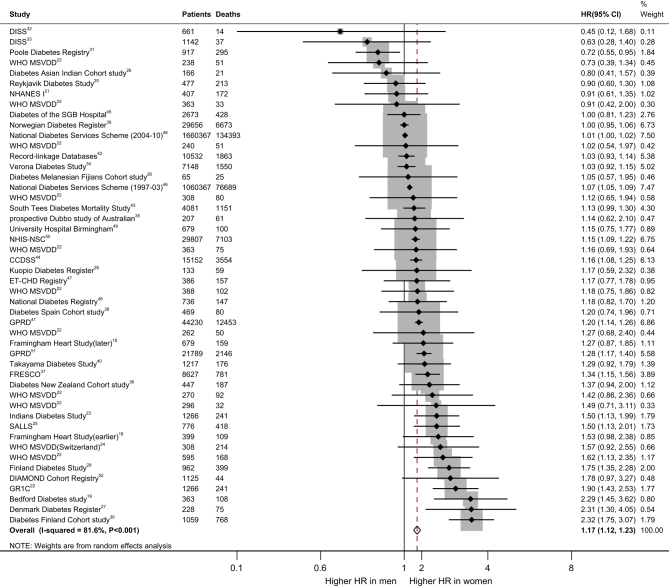
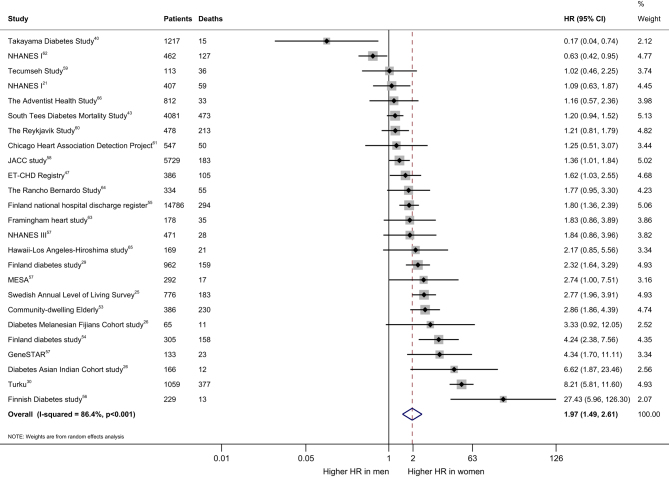
The effect of diabetes on all-cause mortality is 17% higher in women than men (HR 1.17. (95% CI: 1.12–1.23)) (Fig. 1). The pooled women vs men HR for CHD mortality was 1.97 (95% CI: 1.49–2.61) (Fig. 2). However, the I 2 value of 81.6 and 86.4%, respectively, implying the possibility of significant heterogeneity between studies. The pooled HR for all-cause mortality in patients with T2D was 2.33 (95% CI: 2.20–2.69) in women (Supplementary Fig. 3) and 1.91 (95% CI: 1.72–2.12) in men (Supplementary Fig. 4), when compared with their healthy counterparts. The pooled HR for CHD mortality in patients with T2D was 3.79 (95% CI: 3.01–4.78) in women (Supplementary Fig. 5) and 2.13 (95% CI: 1.86–2.44) in men (Supplementary Fig. 6), when compared with their healthy counterparts.
Figure 1.
Pooled women-to-men ratios of HRs for all-cause mortality, comparing people with type 2 diabetes vs those without the disorder. CCDSS, Canadian Chronic Diseases: Surveillance System; DISS, Diabetes Incidence Study in Sweden; ET-CHD, Eastern Taiwan integrated health care delivery system of Coronary Heart Disease; FRESCO, Función de Riesgo ESpañola de acontecimientos Coronarios y Otros; GPRD, General Practice Research Database; GR1C, Gila River Indian Community; HR, hazard ratio; MSVDD, Multinational Study of Vascular Disease in Disease in Diabetes; NHANES, the National Health and Nutrition Examination Survey; NHISNSC, National Health Insurance Service‑National Sample Cohort.
Figure 2.
Pooled women-to-men ratios of HRs for CHD mortality, comparing people with type 2 diabetes vs those without the disorder. ET-CHD, Eastern Taiwan integrated health care delivery system of Coronary Heart Disease; HR, hazard ratio; JACC, The Japan Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; NHANES, the National Health and Nutrition Examination Survey.
Subgroup analysis
We used subgroup analysis to explore the sources of heterogeneity. The year in which the study began did not explain the possible heterogeneity in our study (P = 0.072) (Fig. 3). Different quality scores between articles also did not account for the heterogeneity (P = 0.388). The presence or absence of adjustment for confounding factors in the articles also not the cause of heterogeneity (P = 0.729). The different regions and follow-up duration explained some of the heterogeneities (P = 0.035 and P = 0.014, respectively). However, the result of meta-regression indicated that the different regions had no effect on the pooled estimate of the HR (All P > 0.05), and study duration less than 10 years may be a source of heterogeneity (P = 0.035). The pooled estimate of the HR was 1.30 (95% CI: 1.12–1.52) in the articles with a follow-up duration longer than 15 years, compared with 1.33 (95% CI: 1.15–1.53) and 1.10 (95% CI: 1.05–1.15) in those with a follow-up duration between 10 and 15 years and less than 10 years, respectively.
Figure 3.
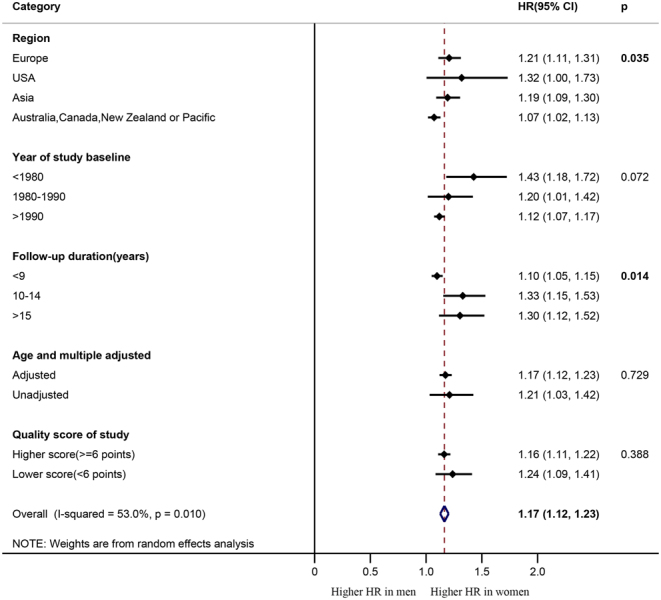
Forest plot of the subgroup analyses with pooled women-to-men ratios of HRs for all-cause mortality. HR, hazard ratio.
Sensitivity analysis and publication bias
Sensitivity analysis did not change the results of this meta-analysis. The Begg’s funnel plot was used to estimate the potential for publication bias (Supplementary Fig. 2). The result of Begg’s test (P = 0.001) indicated the possibility of publication bias in all-cause mortality. The trim-and-fill method was applied to test and adjust for publication bias, which conservatively hypothesized publication bias is the reason for funnel plot asymmetry. Some negative unpublished studies were used to produce a symmetrical funnel plot (Supplementary Fig. 2), which continued to reveal a statistically significant sex-specific association between T2D and all-cause mortality (HR: 1.08. (95% CI: 1.03–1.13)).
Discussion
In this meta-analysis of 35 prospective cohort studies, which included data for more than 2 314 292 individuals and 254 038 all-cause mortality events, T2D was demonstrated as one of the risk factor for all-cause and CHD mortality in both women and men. Diabetes-related mortality was higher in women than men, and the effect of diabetes on all-cause and CHD mortality was 17 and 97% higher in women than men, respectively.
Similarly, the effect of type 1 diabetes on mortality was 37% higher in women than men (67). In a recent collaborative meta-analysis, diabetes was associated with all-cause mortality, and the relative risks were 1.59 in men and 2.00 in women, respectively (68). It is not clear why the rate of diabetes-related death was higher in women than in men, but several mechanisms could help to explain. One hypothesis is that the excess risk of mortality in women is due to the combined effect of greater deterioration and more prolonged exposure to cardiovascular risk profile during their prediabetic period (12, 67, 69). Due to their poorer glycemic control, women with diabetes had an overall greater cumulative lifetime exposure to hyperglycemia (12). Several studies have suggested that women with diabetes had higher BMI (70, 71, 72) and were more insulin resistant (72) than their men counterparts. And they also had significantly higher blood pressure and lipid levels than in men (12).
Research indicates that men with diabetes were diagnosed earlier than women (73), as the early symptoms of diabetes in man may be more likely to be recognized by physicians (74). Men with diabetes may receive better therapeutic interventions and more comprehensive care (75, 76, 77). Alternatively, sex differences in the management and treatment of diabetes may play a crucial role in the disparity in mortality rates between men and women (74). In addition, men with diabetes are more likely to use aspirin (78), which was proven to decrease the risk of stroke and myocardial infarction (79, 80). Furthermore, it has been reported that more diabetic men than women received recommended care processes (62 vs 58%) (77). Notably, even under the same treatment regimen, women were less likely than men to achieve treatment targets for controlling mortality risk factors (81, 82). Moreover, it has been reported that women were less likely to achieve glycemic targets with insulin glargine and exhibited significantly less reductions in fasting blood glucose levels (83). Previous research also observed that women were more likely to experience hypoglycemia during insulin treatment (84, 85). Therefore, differences in treatment and management may explain a large component of the excess risk associated with diabetes in women. In addition, women have less stroke risk factors compared with men (86), so the effect of adding one risk factor (such as diabetes) on women may be more serious.
The other potential mechanisms for sex-specific differences in mortality may result from the differences in biological factors. A recent study (87) suggested that diabetic women had higher levels of endogenous testosterone, which could predict incident CHD risk (88, 89). Women with diabetes also had a greater change in insulin resistance than men (90). Mansfield and colleagues also found sex-based differences in the level of coagulation and fibrinolysis in individuals with diabetes (91, 92) and reported that factor VII and plasminogen activator inhibitor 1 activity levels were significantly higher in women than in men, contributing to the increased cardiovascular risk. Furthermore, higher levels of adiponectin were associated with all-cause mortality in people with T2D (92, 93), and diabetic women were found to have higher levels of adiponectin (92).
The large sample size is one of the strengths of this meta-analysis. We are also the first study to estimate reliably the effect of T2D on CHD mortality risk among women in comparison with men. Additionally, the included studies were limited to prospective cohort studies, which eliminated the possible recall and selection bias. The subgroup and sensitivity analysis was used to explore the possible heterogeneity and ensure the reliability of the results. The trim-and-fill method was applied to adjust the potential publication bias. For the quality control of this meta-analysis, we also registered it at Prospero and performed the study in accordance with the PRISMA statement.
However, there were several limitations in our meta-analysis. Firstly, the standard definition of diabetes and confounding variables adjusted varied across studies, which may have resulted in inconsistent estimation of mortality risks. Secondly, the follow-up duration of T2D was not directly reported in some studies. Moreover, in most studies, diabetic status was mainly based on self-report or past medical history; therefore, there was a higher probability of underestimation of the number of patients with T2D. Although we performed a range of sensitivity analyses, we were also unable to explain most of the heterogeneity among the studies for the outcome of all-cause mortality. In addition, some articles lacked specific data on patient’s age; therefore, we could not perform age-specific subgroup analysis.
Taken together, we found that the relative effect of diabetes on all-cause and CHD mortality was significantly greater in women than in their men counterparts. For future, we should avoid sexual prejudice in diabetes, take all necessary steps to diagnose early and control risk factors comprehensively to guarantee the most suitable treatments in women patients. Besides, it is necessary to perform further studies to determine the actual mechanisms that account for sex-based difference in diabetes-related mortality risk.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Funding
The study is supported by Natural Science Foundation of Zhejiang Province (LY17H260002), K C Wong Magna Fund in Ningbo University, China Postdoctoral Science Foundation funded project (156458), Jiangsu Postdoctoral Science Foundation funded project (1601121B), Natural Science Foundation of Ningbo (2016A610169), Public welfare technology and policy science (soft science) application research of Zhejiang Province (2017C35006), Ningbo Scientific Innovation Team for Environmental Hazardous Factor Control and Prevention (2016C51001), Project of Science and Technology Innovation for College Students in Zhejiang Province (2018R405092), Sanming Project of Medicine in Shenzhen (SZSM201803080).
Author contribution statement
L H, L L and D Y conceived the study, interpreted the data and drafted and critically revised the report. G X, J Y X and L W did the search, analyzed and interpreted the data and critically revised the report. J Z, X Z and L N Z critically revised the report. D D and F K participated in data collection, oversaw the data analysis and interpreted the data.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice 2014. 103 137–149. ( 10.1016/j.diabres.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016. 388 1545–1602. ( 10.1016/S0140-6736(16)31678-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Archives of Internal Medicine 2004. 164 1422–1426. ( 10.1001/archinte.164.13.1422) [DOI] [PubMed] [Google Scholar]
- 4.Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014. 57 1542–1551. ( 10.1007/s00125-014-3260-6) [DOI] [PubMed] [Google Scholar]
- 5.Chen HF, Lee SP, Li CY. Sex differences in the incidence of hemorrhagic and ischemic stroke among diabetics in Taiwan. Journal of Women’s Health 2009. 18 647–654. ( 10.1089/jwh.2008.0918) [DOI] [PubMed] [Google Scholar]
- 6.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess mortality among persons with Type 2 diabetes. New England Journal of Medicine 2015. 373 1720–1732. ( 10.1056/NEJMoa1504347) [DOI] [PubMed] [Google Scholar]
- 7.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Archives of Internal Medicine 2004. 164 1422–1426. ( 10.1001/archinte.164.13.1422) [DOI] [PubMed] [Google Scholar]
- 8.Taking sex into account in medicine. Lancet 2011. 378 1826 ( 10.1016/S0140-6736(11)61795-9) [DOI] [PubMed] [Google Scholar]
- 9.Peters SA, Huxley RR, Woodward M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3,980,359 individuals and 42,401 strokes. Stroke 2013. 44 2821–2828. ( 10.1161/STROKEAHA.113.002342) [DOI] [PubMed] [Google Scholar]
- 10.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014. 383 1973–1980. ( 10.1016/S0140-6736(14)60040-4) [DOI] [PubMed] [Google Scholar]
- 11.Peters SA, Huxley RR, Sattar N, Woodward M. Sex differences in the excess risk of cardiovascular diseases associated with Type 2 diabetes: potential explanations and clinical implications. Current Cardiovascular Risk Reports 2015. 9 36 ( 10.1007/s12170-015-0462-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006. 332 73–78. ( 10.1136/bmj.38678.389583.7C) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008. 300 2754–2764. ( 10.1001/jama.2008.824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology 2010. 25 603–605. ( 10.1007/s10654-010-9491-z) [DOI] [PubMed] [Google Scholar]
- 15.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLOS ONE 2013. 8 e83138 ( 10.1371/journal.pone.0083138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002. 21 1539–1558. ( 10.1002/sim.1186) [DOI] [PubMed] [Google Scholar]
- 17.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000. 56 455–463. ( 10.1111/j.0006-341X.2000.00455.x) [DOI] [PubMed] [Google Scholar]
- 18.Preis S, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Sr, Savage P, Levy D, Fox C. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009. 119 1728–1735. ( 10.1161/CIRCULATIONAHA.108.82917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarrett RJ, Mccartney P, Keen H. The Bedford survey: ten year mortality rates in newly diagnosed diabetics, borderline diabetics and normoglycaemic controls and risk indices for coronary heart disease in borderline diabetics. Diabetologia 1982. 22 79–84. ( 10.1007/BF00254833) [DOI] [PubMed] [Google Scholar]
- 20.Vilbergsson S, Sigurdsson G, Sigvaldason H, Sigfusson N. Coronary heart disease mortality amongst non-insulin-dependent diabetic subjects in Iceland: the independent effect of diabetes. The Reykjavik Study 17-year follow up. Journal of Internal Medicine 1998. 244 309–316. ( 10.1046/j.1365-2796.1998.00368.x) [DOI] [PubMed] [Google Scholar]
- 21.Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997. 315 722–729. ( 10.1136/bmj.315.7110.722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001. 44 (Supplement 2) S14–S21. ( 10.1007/PL00002934) [DOI] [PubMed] [Google Scholar]
- 23.Sievers ML, Nelson RG, Knowler WC, Bennett PH. Impact of NIDDM on mortality and causes of death in pima Indians. Diabetes Care 1992. 15 1541–1549. ( 10.2337/diacare.15.11.1541) [DOI] [PubMed] [Google Scholar]
- 24.Allemann S, Saner C, Zwahlen M, Christ ER, Diem P, Stettler C. Long-term cardiovascular and non-cardiovascular mortality in women and men with type 1 and type 2 diabetes mellitus: a 30-year follow-up in Switzerland. Swiss Medical Weekly 2009. 139 576–583. (https://doi.org/smw-12785) [DOI] [PubMed] [Google Scholar]
- 25.Nilsson PM, Johansson SE, Sundquist J. Low educational status is a risk factor for mortality among diabetic people. Diabetic Medicine 1998. 15 213–219. () [DOI] [PubMed] [Google Scholar]
- 26.Collins VR, Dowse GK, Ram P, Cabealawa S, Zimmet PZ. Non-insulin-dependent diabetes and 11-year mortality in Asian Indian and Melanesian Fijians. Diabetic Medicine 1996. 13 125–132. () [DOI] [PubMed] [Google Scholar]
- 27.Damsgaard EM, Frøland A, Mogensen CE. Over-mortality as related to age and gender in patients with established non-insulin-dependent diabetes mellitus. Journal of Diabetes and Its Complications 1997. 11 77–82. ( 10.1016/S1056-8727(97)00095-0) [DOI] [PubMed] [Google Scholar]
- 28.Niskanen L, Turpeinen A, Penttilä I, Uusitupa MI. Hyperglycemia and compositional lipoprotein abnormalities as predictors of cardiovascular mortality in type 2 diabetes: a 15-year follow-up from the time of diagnosis. Diabetes Care 1998. 21 1861–1869. ( 10.2337/diacare.21.11.1861) [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Jousilahti P, Qiao Q, Katoh S, Tuomilehto J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia 2005. 48 856–861. ( 10.1007/s00125-005-1730-6) [DOI] [PubMed] [Google Scholar]
- 30.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a ‘coronary heart disease equivalent’: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 2005. 28 2901–2907. ( 10.2337/diacare.28.12.2901) [DOI] [PubMed] [Google Scholar]
- 31.Gatling W, Tufail S, Mullee MA, Westacott TA, Hill RD. Mortality rates in diabetic patients from a community-based population compared to local age/sex matched controls. Diabetic Medicine 1997. 14 316–320. () [DOI] [PubMed] [Google Scholar]
- 32.Wibell L, Nyström L, Ostman J, Arnqvist H, Blohmé G, Lithner F, Littorin B, Sundkvist GIncreased mortality in diabetes during the first 10 years of the disease. A population-based Study (DISS) in Swedish adults 15-34 years old at diagnosis. Journal of Internal Medicine 2001. 249 263–270. ( 10.1111/j.1365-2796.2001.00802.x) [DOI] [PubMed] [Google Scholar]
- 33.Waernbaum I, Blohmé G, Ostman J, Sundkvist G, Eriksson J, Arnqvist H, Bolinder J, Nyström L. Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 2006. 49 653–659. ( 10.1007/s00125-005-0135-x) [DOI] [PubMed] [Google Scholar]
- 34.de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care 1999. 22 756–761. ( 10.2337/diacare.22.5.756) [DOI] [PubMed] [Google Scholar]
- 35.Simons LA, McCallum J, Friedlander Y, Simons J. Diabetes, mortality and coronary heart disease in the prospective Dubbo study of Australian elderly. Australian and New Zealand Journal of Medicine 1996. 26 66–74. ( 10.1111/j.1445-5994.1996.tb02909.x) [DOI] [PubMed] [Google Scholar]
- 36.Florkowski CM, Scott RS, Coope PA, Moir CL. Predictors of mortality from type 2 diabetes mellitus in Canterbury, New Zealand; a ten-year cohort study. Diabetes Research and Clinical Practice 2001. 53 113–120. ( 10.1016/S0168-8227(01)00246-7) [DOI] [PubMed] [Google Scholar]
- 37.Baenadíez JM, Peñafiel J, Subirana I, Ramos R, Elosua R, Marínibañez A, Guembe MJ, Rigo F, Tormodíaz MJ, Morenoiribas C. Risk of cause-specific death in individuals With diabetes: A competing risks analysis. Diabetes Care 2016. 39 1987–1995. ( 10.2337/dc16-0614) [DOI] [PubMed] [Google Scholar]
- 38.Mata-Cases M, Prado-Lacueva CD, Salido-Valencia V, Fernández-Bertolín E, Casermeiro-Cortés J, García-Durán M, Jabalera-López S, Fernández-Sanmartín MI. Incidence of complications and mortality in a type 2 diabetes patient cohort study followed up from diagnosis in a primary healthcare centre. International Journal of Clinical Practice 2011. 65 299–307. ( 10.1111/j.1742-1241.2010.02503.x) [DOI] [PubMed] [Google Scholar]
- 39.Gnavi R, Petrelli A, Demaria M, Spadea T, Carta Q, Costa G. Mortality and educational level among diabetic and non-diabetic population in the Turin Longitudinal Study: a 9-year follow-up. International Journal of Epidemiology 2004. 33 864–871. ( 10.1093/ije/dyh089) [DOI] [PubMed] [Google Scholar]
- 40.Oba S, Nagata C, Nakamura K, Takatsuka N, Shimizu H. Self-reported diabetes mellitus and risk of mortality from all causes, cardiovascular disease, and cancer in Takayama: a population-based prospective cohort study in Japan. Journal of Epidemiology 2008. 18 197–203. ( 10.2188/jea.JE2008004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulnier HE, Seaman HE, Raleigh VS, Soedamahmuthu SS, Colhoun HM, Lawrenson RA. Mortality in people with type 2 diabetes in the UK. Diabetic Medicine 2006. 23 516–521. ( 10.1111/j.1464-5491.2006.01838.x) [DOI] [PubMed] [Google Scholar]
- 42.Barnett KN, Ogston SA, McMurdo ME, Morris AD, Evans JM. A 12-year follow-up study of all-cause and cardiovascular mortality among 10,532 people newly diagnosed with Type 2 diabetes in Tayside, Scotland. Diabetic Medicine 2010. 27 1124–1129. ( 10.1111/j.1464-5491.2010.03075.x) [DOI] [PubMed] [Google Scholar]
- 43.Roper NA, Bilous R, Kelly W, Unwin N, Connolly V. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 2001. 322 1389–1393. ( 10.1136/bmj.322.7299.1389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roche MM, Wang PP. Sex differences in all-cause and cardiovascular mortality, hospitalization for individuals with and without diabetes, and patients with diabetes diagnosed early and late. Diabetes Care 2013. 36 2582–2590. ( 10.2337/dc12-1272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bo S, Ciccone G, Rosato R, Gancia R, Grassi G, Merletti F, Pagano GF. Renal damage in patients with Type 2 diabetes: a strong predictor of mortality. Diabetic Medicine 2005. 22 258–265. ( 10.1111/j.1464-5491.2004.01394.x) [DOI] [PubMed] [Google Scholar]
- 46.Harding JL, Shaw JE, Peeters A, Guiver T, Davidson S, Magliano DJ. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997–2010. Diabetes Care 2014. 37 2579–2586. ( 10.2337/dc14-0096) [DOI] [PubMed] [Google Scholar]
- 47.Lin GM, Li YH, Lin CL, Wang JH, Han CL. Gender differences in the impact of diabetes on mortality in patients with established coronary artery disease: A report from the Eastern Taiwan integrated health care delivery system of Coronary Heart Disease (ET-CHD) registry, 1997–2006. Journal of Cardiology 2013. 61 393–398. ( 10.1016/j.jjcc.2013.02.007) [DOI] [PubMed] [Google Scholar]
- 48.Guzder RN, Gatling W, Mullee MA, Byrne CD. Early mortality from the time of diagnosis of Type 2 diabetes: a 5-year prospective cohort study with a local age- and sex-matched comparison cohort. Diabetic Medicine 2007. 24 1164–1167. ( 10.1111/j.1464-5491.2007.02223.x) [DOI] [PubMed] [Google Scholar]
- 49.Schoepf D, Potluri R, Uppal H, Natalwala A, Narendran P, Heun R. Type-2 diabetes mellitus in schizophrenia: increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. European Psychiatry 2012. 27 33–42. ( 10.1016/j.eurpsy.2011.02.009) [DOI] [PubMed] [Google Scholar]
- 50.Yu MK, Kim YJ, Park JY, Lee WJ, Chang HJ. Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovascular Diabetology 2016. 15 131 ( 10.1186/s12933-016-0451-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor KS, Heneghan CJ, Farmer AJ, Fuller AM, Adler AI, Aronson JK, Stevens RJ. All-cause and cardiovascular mortality in middle-aged people With Type 2 diabetes compared With people Without diabetes in a Large U.K. Primary Care Database. Diabetes Care 2013. 36 2366–2371. ( 10.2337/dc12-1513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Won KB, Hur SH, Cho YK, Yoon HJ, Nam CW, Kim KB, Bae JH, Choi DJ, Ahn YK, Park JS. et al Comparison of 2-year mortality according to obesity in stabilized patients with type 2 diabetes mellitus after acute myocardial infarction: results from the DIAMOND prospective cohort registry. Cardiovascular Diabetology 2015. 14 141 ( 10.1186/s12933-015-0305-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seeman T, Mendes de Leon C, Berkman L, Ostfeld A. Risk factors for coronary heart disease among older men and women: a prospective study of community-dwelling elderly. American Journal of Epidemiology 1993. 138 1037–1049. ( 10.1093/oxfordjournals.aje.a116822) [DOI] [PubMed] [Google Scholar]
- 54.Laakso M, Rönnemaa T, Lehto S, Puukka P, Kallio V, Pyörälä K. Does NIDDM increase the risk for coronary heart disease similarly in both low- and high-risk populations? Diabetologia 1995. 38 487–493. ( 10.1007/BF00410288) [DOI] [PubMed] [Google Scholar]
- 55.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999. 99 1165–1172. ( 10.1161/01.CIR.99.9.1165) [DOI] [PubMed] [Google Scholar]
- 56.Kuusisto J, Mykkänen L, Pyörälä K, Laakso M. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes 1994. 43 960–967. ( 10.2337/diab.43.8.960) [DOI] [PubMed] [Google Scholar]
- 57.Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, Becker D, Vaidya D. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care 2014. 37 830–838. ( 10.2337/dc13-1755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsunaga M, Yatsuya H, Iso H, Yamashita K, Li Y, Yamagishi K, Tanabe N, Wada Y, Wang C, Ota A. et al Similarities and differences between coronary heart disease and stroke in the associations with cardiovascular risk factors: the Japan Collaborative Cohort Study. Atherosclerosis 2017. 261 124–130. ( 10.1016/j.atherosclerosis.2017.03.003) [DOI] [PubMed] [Google Scholar]
- 59.Butter WJ, Jr, Ostrander LD, Carman WJ, Lamphiear DE. Mortality from coronary heart disease in the Tecumseh study long-term effect of diabetes mellitus, glucose tolerance and other risk factors. American Journal of Epidemiology 1985. 121 541–547. ( 10.1093/oxfordjournals.aje.a114031) [DOI] [PubMed] [Google Scholar]
- 60.Vilbergsson S, Sigurdsson G, Sigvaldason H, Sigfusson N. Coronary heart disease mortality amongst non-insulin-dependent diabetic subjects in Iceland: the independent effect of diabetes. The Reykjavik Study 17-year follow up. Journal of Internal Medicine 1998. 244 309–316. ( 10.1046/j.1365-2796.1998.00368.x) [DOI] [PubMed] [Google Scholar]
- 61.Pan W, Cedres LB, Liu K, Dyer A, Schoenberger JA, Shekelle RB, Stamler R, Smith D, Collette P, Stamler J. Relationship of clinical diabetes and asymptomatic hyperglycemia to risk of coronary heart disease mortality in men and women. American Journal of Epidemiology 1986. 123 504–516. ( 10.1093/oxfordjournals.aje.a114266) [DOI] [PubMed] [Google Scholar]
- 62.Natarajan S, Liao Y, Sinha D, Cao G, Mcgee DL, Lipsitz SR. Sex differences in the effect of diabetes duration on coronary heart disease mortality. Archives of Internal Medicine 2005. 165 430–435. ( 10.1001/archinte.165.4.430) [DOI] [PubMed] [Google Scholar]
- 63.Natarajan S, Liao Y, Cao G, Lipsitz SR, Mcgee DL. Sex differences in risk for coronary heart disease mortality associated With diabetes and established coronary heart disease. Archives of Internal Medicine 2003. 163 1735–1740. ( 10.1001/archinte.163.14.1735) [DOI] [PubMed] [Google Scholar]
- 64.Barrettconnor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991. 265 627–631. ( 10.1001/jama.265.5.627) [DOI] [PubMed] [Google Scholar]
- 65.Imazu M, Sumii K, Yamamoto H, Toyofuku M, Tadehara F, Okubo M, Yamakido M, Kohno N, Onaka AT. Influence of type 2 diabetes mellitus on cardiovascular disease mortality: findings from the Hawaii–Los Angeles–Hiroshima study. Diabetes Research and Clinical Practice 2002. 57 61–69. ( 10.1016/S0168-8227(02)00016-5) [DOI] [PubMed] [Google Scholar]
- 66.Fraser GE, Strahan TM, Sabaté J, Beeson WL, Kissinger D. Effects of traditional coronary risk factors on rates of incident coronary events in a low-risk population. The Adventist Health Study. Circulation 1992. 86 406–413. ( 10.1161/01.CIR.86.2.406) [DOI] [PubMed] [Google Scholar]
- 67.Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes and Endocrinology 2015. 3 198–206. ( 10.1016/S2213-8587(14)70248-7) [DOI] [PubMed] [Google Scholar]
- 68.Prospective Studies C & Asia Pacific Cohort Studies C. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes and Endocrinology 2018. 6 538–546. ( 10.1016/S2213-8587(18)30079-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014. 57 1542–1551. ( 10.1007/s00125-014-3260-6) [DOI] [PubMed] [Google Scholar]
- 70.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S. et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 2011. 54 3003–3006. ( 10.1007/s00125-011-2313-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paul S, Thomas G, Majeed A, Khunti K, Klein K. Women develop type 2 diabetes at a higher body mass index than men. Diabetologia 2012. 55 1556–1557. ( 10.1007/s00125-012-2496-2) [DOI] [PubMed] [Google Scholar]
- 72.Kwon SK. Women are diagnosed with type 2 diabetes at higher body mass indices and older ages than men: Korea national health and nutrition examination survey 2007–2010. Diabetes and Metabolism Journal 2014. 38 74–80. ( 10.4093/dmj.2014.38.1.74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S. et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 2011. 54 3003–3006. ( 10.1007/s00125-011-2313-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014. 57 1542–1551. ( 10.1007/s00125-014-3260-6) [DOI] [PubMed] [Google Scholar]
- 75.Krämer HU, Raum E, Rüter G, Schöttker B, Rothenbacher D, Rosemann T, Szecsenyi J, Brenner H. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: results from the DIANA study. Cardiovascular Diabetology 2012. 11 88 ( 10.1186/1475-2840-11-88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brännström J, Hamberg K, Molander L, Lövheim H, Gustafson Y. Gender disparities in the pharmacological treatment of cardiovascular disease and diabetes mellitus in the very old: an epidemiological, cross-sectional survey. Drugs and Aging 2011. 28 993–1005. ( 10.2165/11594730-000000000-00000) [DOI] [PubMed] [Google Scholar]
- 77.Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, Becker D, Vaidya D. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care 2014. 37 830–838. ( 10.2337/dc13-1755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cull CA, Neil HAW, Holman RR. Changing aspirin use in patients with Type 2 diabetes in the UKPDS. Diabetic Medicine 2004. 21 1368–1371. ( 10.1111/j.1464-5491.2004.01328.x) [DOI] [PubMed] [Google Scholar]
- 79.Antithrombotic Trialists’ (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P. et al Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009. 373 1849–1860. ( 10.1016/S0140-6736(09)60503-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Berardis G, Sacco M, Strippoli GF, Pellegrini F, Graziano G, Tognoni G, Nicolucci A. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ 2009. 339 b4531 ( 10.1136/bmj.b4531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Franzini L, Ardigò D, Cavalot F, Miccoli R, Rivellese AA, Trovati M, Zavaroni I, Vaccaro O. Women show worse control of type 2 diabetes and cardiovascular disease risk factors than men: results from the MIND.IT Study Group of the Italian Society of Diabetology. Nutrition, Metabolism, and Cardiovascular Diseases 2013. 23 235–241. ( 10.1016/j.numecd.2011.12.003) [DOI] [PubMed] [Google Scholar]
- 82.Winston GJ, Barr RG, Carrasquillo O, Bertoni AG, Shea S. Sex and racial/ethnic differences in cardiovascular disease risk factor treatment and control among individuals with diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009. 32 1467–1469. ( 10.2337/dbib9-0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kautzky-Willer A, Kosi L, Lin J, Mihaljevic R. Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient-level pooled data of six randomized controlled trials. Diabetes, Obesity and Metabolism 2015. 17 533–540. ( 10.1111/dom.12449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sämann A, Lehmann T, Heller T, Müller N, Hartmann P, Wolf GB, Müller UA. A retrospective study on the incidence and risk factors of severe hypoglycemia in primary care. Family Practice 2013. 30 290–293. ( 10.1093/fampra/cms071) [DOI] [PubMed] [Google Scholar]
- 85.Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G. et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010. 340 b5444 ( 10.1136/bmj.b5444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA. et al Sex and race differences in the association of incident ischemic stroke With risk factors. JAMA Neurology 2018. 10 E1–E8. ( 10.1001/jamaneurol.2018.3862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006. 295 1288–1299. ( 10.1001/jama.295.11.1288) [DOI] [PubMed] [Google Scholar]
- 88.Barrett-Connor E. The Rancho Bernardo Study 40 years studying why women have less heart disease than men and how diabetes modifies women’s usual cardiac protection. Global Heart 2013. 8 95–104. ( 10.1016/j.gheart.2012.12.002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, Gapstur SM, Golden SH. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. Journal of Clinical Endocrinology and Metabolism 2009. 94 4127–4135. ( 10.1210/jc.2009-0910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wannamethee SG, Papacosta O, Lawlor DA, Whincup PH, Lowe GD, Ebrahim S, Sattar N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012. 55 80–87. ( 10.1007/s00125-011-2284-4) [DOI] [PubMed] [Google Scholar]
- 91.Mansfield MW, Heywood DM, Grant PJ. Sex differences in coagulation and fibrinolysis in white subjects with non-insulin-dependent diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology 1996. 16 160–164. ( 10.1161/01.ATV.16.1.160) [DOI] [PubMed] [Google Scholar]
- 92.Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M. Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care 2007. 30 354–359. ( 10.2337/dbib6-1772) [DOI] [PubMed] [Google Scholar]
- 93.Singer JR, Palmas W, Teresi J, Weinstock R, Shea S, Luchsinger JA. Adiponectin and all-cause mortality in elderly people with type 2 diabetes. Diabetes Care 2012. 35 1858–1863. ( 10.2337/dc11-2215) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a