Summary
A 70-year-old female with exertional dyspnoea was found to have basal septal hypertrophy (BSH), or a ‘basal septal bulge’, with evidence of mild left ventricular outflow tract obstruction (LVOT) at rest on her initial echocardiogram. She was usually fit and well with no significant past medical history. She had no history of hypertension. She had never smoked. There was no family history of hypertrophic cardiomyopathy (HCM). A cardiac MRI did not demonstrate any typical features of HCM. ECG showed sinus tachycardia with a rate of 101 bpm but was otherwise unremarkable. She was referred for exercise echocardiography to assess for latent LVOT obstruction. Prior to commencing exercise, her LVOT gradient was re-assessed at rest. Her LVOT gradients were 30 mmHg at rest, 49 mmHg during Valsalva and 91 mmHg on standing. A diagnosis of significant latent LVOT obstruction was made and the patient was started on bisoprolol, a cardioselective beta-blocker. Bisoprolol was slowly uptitrated from 1.25 mg to 5 mg once daily, following which the patient reported a significant improvement in her symptoms with an improved exercise capacity. Follow-up echocardiography demonstrated a dramatic reduction in LVOT gradient, with a maximum of 11 mmHg assessed both with Valsalva and on standing. This case is a reminder that patients with a ‘common’ basal septal bulge can develop significant LVOT obstruction, the symptoms of which may respond to pharmacological therapy. Orthostatic assessment of LVOT gradient using echocardiography should be considered during standard LVOT obstruction provocation manoeuvres such as a Valsalva.
Learning points:
Differentiation between basal septal hypertrophy (BSH) and hypertrophic cardiomyopathy (HCM) may be challenging. Key factors favouring HCM include a positive family history of HCM or sudden cardiac death, septal thickness >15 mm/posterior wall thickness >11 mm, systolic anterior motion of the anterior mitral valve (SAM), late gadolinium enhancement on cardiac MRI, a causative genetic mutation associated with HCM and an abnormal ECG.
Significant LVOT obstruction may develop in patients with BSH and is potentially responsive to pharmacotherapy.
Standing reduces venous return, resulting in decreased LV volume. Compensatory mechanisms to maintain cardiac output involve sympathetic nervous system activation leading to increased LV contractility and subsequent increased LVOT gradient.
Significant LVOT obstruction may be unmasked by an orthostatic posture.
Orthostatic LVOT gradient assessment should be part of the routine echocardiographic assessment of all patients with an increased LVOT gradient at rest.
The post-prandial state has been associated with increased LVOT gradient due to splanchnic dilatation and the consequent increased cardiac output required to maintain blood pressure. Post-prandial status should therefore be considered when assessing LVOT gradient.
Keywords: basal septal hypertrophy, orthostatic LVOT assessment, latent LVOT obstruction, provocable gradient
Background
The 2015 European Society of Cardiology (ESC) guidelines recommend provocation testing to assess for latent LVOT obstruction in all patients with exertional symptoms who have either hypertrophic cardiomyopathy (HCM) or isolated basal septal hypertrophy (BSH) (1). LVOT obstruction is a major cause of symptoms in HCM and has been associated with a worse prognosis (1). BSH, often referred to as a ‘sigmoid septum’ or a ‘ventricular septal bulge’, is common in elderly patients, particularly those with hypertension. The differentiation between this very common finding and genetically inherited HCM can be difficult. Canepa et al. proposed that a family history of HCM or sudden cardiac death, presence of symptoms, septal thickness >15 mm/posterior wall thickness >11 mm, systolic anterior motion of the anterior mitral valve (SAM) and LVOT obstruction, late gadolinium enhancement on cardiac MRI, a genetic mutation associated with HCM and an abnormal ECG all make HCM more likely versus BSH (2). Importantly patients with BSH can have latent LVOT obstruction and there is evidence demonstrating improvement in symptoms with pharmacological treatment (3). Aortoseptal angulation has also been shown to be predictive of latent outflow tract obstruction, with smaller angles in patients with provocable LVOT obtruction (4).
In most echo departments, standard outpatient provocation testing involves measuring the LVOT gradient at rest and following a Valsalva manoeuvre. If no significant gradient is induced in a symptomatic patient, stress testing is usually implemented at this stage. Shah et al. demonstrated that 2/3rd (62.1%) of patients who had no previous documented LVOT obstruction (LVOT gradient ≤30 mmHg) developed LVOT obstruction during exercise (5). Approximately 20% of these patients went on to have invasive treatment with subsequent improvement in symptoms (5).
It is recognised that following a meal mesenteric vasodilatation occurs, resulting in reduced peripheral vascular resistance. As a compensatory mechanism, heart rate and stroke volume increase to maintain blood pressure (6). Using radionucleotide imaging, Kelbaek et al. demonstrated post-prandial increases in cardiac output of 62% due to increased heart rate and stroke volume (6). This post-prandial haemodynamic change is well recognised to increase LVOT gradient and exacerbate symptoms (7). Protocols used for the assessment of latent LVOT obstruction should therefore take into consideration post-prandial status.
LVOT gradient is sensitive to preload. Bedside manoeuvres that affect preload will therefore change the outflow gradient. Squatting increases preload and therefore reduces the gradient through the outflow tract, whereas standing up reduces preload and has the opposite effect. The reduction in venous return caused by standing up also results in decreased LV volume. Compensatory mechanisms to maintain cardiac output involve sympathetic nervous system activation leading to increased LV contractility, which also contributes to increased LVOT gradient (8). A number of studies have demonstrated increased LVOT gradient in the standing versus the supine position (7, 8, 9).
Crucially, most patients will be in a fully upright position when symptoms are usually experienced. Orthostatic assessment of LVOT gradient is therefore more representative of ‘real-life’ haemodynamics and is commonly employed during stress testing to look for outflow obstruction.
Case presentation
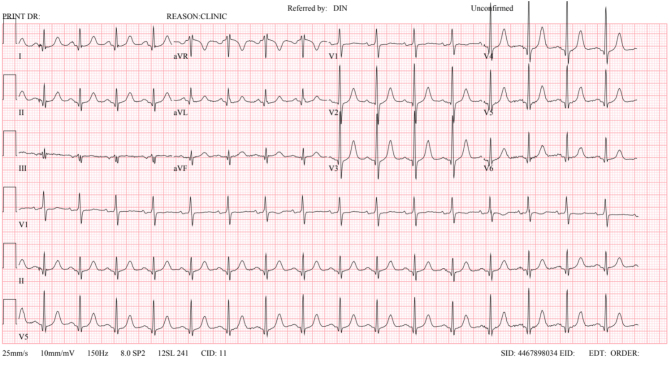
A 70-year-old female presented with an 18-month history of exertional breathlessness occurring on walking up inclines. She had no history of chest pain or syncope. She had no relevant past medical history. She specifically had no history of hypertension. She had never smoked. There was no family history of HCM or sudden unexplained death. Regular medications included only prophylactic antibiotics. Cardiac examination revealed a resting regular tachycardia with a heart rate of 101 bpm (Fig. 1). Resting blood pressure was 134/80 mmHg. She was comfortable at rest and was clinically euvolemic. Pulse character was normal. There was a loud ejection murmur heard throughout the precordium with normal first and second heart sounds. Examination was otherwise unremarkable.
Figure 1.
12 lead electrocardiogram at initial presentation.
Investigation
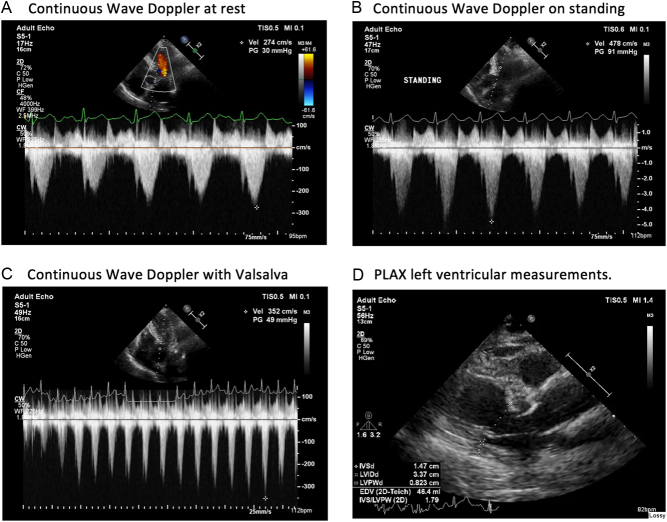
Her resting ECG showed a sinus tachycardia with a rate of 101 bpm and no other significant abnormality (Fig. 1). Initial echocardiogram demonstrated a hyperdynamic left ventricle with BSH measuring 15 mm (Fig. 2 and Videos 1, 2). There was a peak outflow gradient of 30 mmHg measured in the left lateral position at rest. This increased to 49 mmHg with a Valsalva. There was no significant SAM; however, there was mild mitral regurgitation. No other pathology was demonstrated.
Figure 2.
Continuous wave Doppler through the left ventricular outflow tract (A) at rest (B) on standing (C) with Valsalva and the (D) parasternal long axis view with measurements.
At initial presentation: parasternal long axis with colour. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-1.
Download Video 1 (1MB, mp4)
At initial presentation: parasternal long axis view. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-2.
Download Video 2 (1.3MB, mp4)
She had a cardiac MRI to investigate for any evidence of cardiomyopathy, specifically HCM. This reconfirmed isolated BSH and with a maximum thickness of 15 mm. There was no late gadolinium enhancement, and native T1 relaxation times were normal. Adenosine stress imaging was performed, which demonstrated no inducible ischaemia.
In view of the mild left ventricular outflow tract (LVOT) gradient of 30 mmHg detected at rest and ongoing unexplained breathlessness, she was referred for stress echocardiography to assess for provocable LVOT obstruction during exercise.
The patient was instructed to eat a small meal, such as a sandwich, 1 h prior to the test. This is the recommendation made to all patients in order to standardise the impact of splanchnic dilatation. Pre-exercise LVOT gradient was measured at rest, with Valsalva and on standing. The resting gradient on continuous wave Doppler was 30 mmHg, on Valsalva this increased to 49 mmHg and on standing further increased to 91 mmHg (Fig. 2).
Treadmill stress was not deemed appropriate as significant obstruction had been demonstrated.
Treatment and outcome
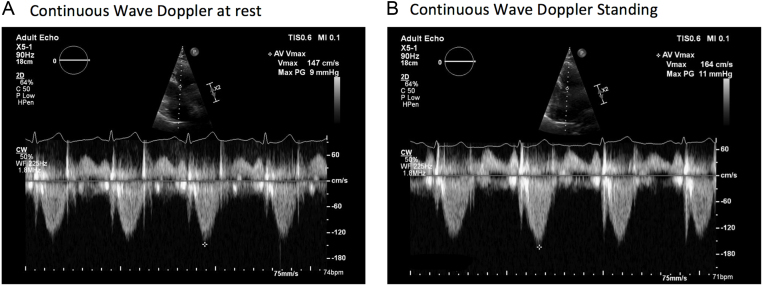
Given the demonstration of a significant outflow tract gradient on standing, the patient was commenced on 1.25 mg of bisoprolol. The dose was increased to 5 mg once daily, which resulted in an improvement in her symptoms. The echocardiogram was repeated, at which point the patient had a heart rate of 74 bpm. The ventricle no longer appeared hyperdynamic at rest and peak gradient through the LVOT with provocation manoeuvres fell to 11 mmHg (Fig. 3 and Videos 3, 4).
Figure 3.
Continuous wave Doppler through the left ventricular outflow tract following the introduction of bisoprolol 2.5 mg (A) at rest (B) on standing.
Following the introduction of 2.5 mg bisoprolol: parasternal long axis view. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-3.
Download Video 3 (1.4MB, mp4)
Following the introduction of 2.5 mg bisoprolol: parasternal long axis with colour. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-4.
Download Video 4 (691.5KB, mp4)
Discussion
Latent LVOT obstruction should be considered in all symptomatic patients with either HCM or BSH. In most echo departments, standard provocation testing in the outpatient setting involves measuring the LVOT gradient at rest and following a Valsalva manoeuvre. If no significant gradient is demonstrated in a symptomatic patient, stress testing is usually implemented at this stage.
It is possible, however, that a significant gradient could be induced simply by standing the patient up, as shown in this case. If provocable outflow obstruction is demonstrated on standing, there is no requirement for further stress testing at this stage.
Post-prandial status is crucial as the degree of splanchnic dilatation will have an impact on the LVOT gradient. A large meal will induce splanchnic dilatation with a resultant increase in LVOT gradient due to a compensatory increase in cardiac output (7). Starving patients prior to assessment of LVOT gradient will result in a lower inducible gradient and therefore the diagnosis of latent LVOT obstruction could be ‘missed’. Outflow tract gradient is also sensitive to preload. Bedside manoeuvres that affect preload will therefore change the outflow gradient. Squatting increases preload and therefore reduces the gradient through the outflow tract, whereas standing up reduces preload and has the opposite effect. Crucially, most patients will be in a fully upright position when symptoms are usually experienced. Orthostatic assessment of LVOT gradient is therefore more representative of ‘real-life’ haemodynamics and should be carried out during standard echocardiographic assessment. The ultimate goal should be to replicate the environment and haemodynamics during which a patient experiences symptoms.
A significant gradient is generally considered to be an LVOT gradient >50 mmHg (1). Lifestyle advice such as avoiding large meals and dehydration, which can exacerbate the LVOT gradient, should be given to all patients. First-line treatment is pharmacological and has been shown to be beneficial in LVOT obstruction caused by HCM and BSH (3). Bisoprolol is a cardioselective b-blocker, which is specific for beta-1 receptors found in the heart. It is negatively inotropic and therefore reduces contractility and heart rate. This has the effect of reducing the LVOT gradient and can improve symptoms in patients with hypertrophic obstructive cardiomyopathy. The maximum dose is 10 mg daily and patients should be uptitrated to the maximum tolerated dose.
If non-hydropyridine beta-blockers are not tolerated or contraindicated, non-hydropiridine calcium channel blockers, such as verapamil, can be started at 40 mg TDS and uptitrated to 480 mg SR once daily if required (1). ESC HCM guidelines recommend caution when using verapamil in patients with an LVOT gradient >100 mmHg due to the risk of pulmonary oedema (1).
In patients with resistant symptoms, disopyramide can be introduced in addition to either beta-blockers or calcium channel blockers. Disopyramide is a class IA anti-arrhythmic drug and works by blocking sodium channels. It reduces LVOT gradient due to its negative inotropic effect. It should be used with caution in patients with atrial arrhythmia due to the risk of enhanced AV conduction and therefore increased ventricular rates (1). It is usually started at a dose of 100 mg BD and can be increased to 400–600 mg/day (1). Its use is generally limited by anti-cholinergic side effects such as a dry mouth, urinary retention and dry eyes. ESC guidelines state that the QTc interval should be monitored during dose uptitration and the dose should be reduced if the QTc exceeds 480 ms (1).
Patients with HCM who have persistent moderate-severe symptoms and LVOT obstruction despite optimal pharmacotherapy should be referred for consideration of invasive therapy, such as myectomy or alcohol septal ablation (1). The use of invasive therapies in patients with LVOT obstruction due to BSH is not well documented.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
Author contribution statement
The case report was written by Dr Hannah Sinclair and Paul Russhard and overseen by Dr Chris Steadman and Dr Chris Critoph. Dr Chris Critoph is also the local specialist consultant in inherited cardiac conditions. The patient is under the care of Dr Jehangir Din, who gave permission, along with the patient, for this case report to be written.
References
- 1.Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, et al 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). European Heart Journal 2014. 35 2733–2779. ( 10.1093/eurheartj/ehu284) [DOI] [PubMed] [Google Scholar]
- 2.Canepa M, Pozios I, Vianello PF, Ameri P, Brunelli C, Ferrucci L, Abraham TP. Distinguishing ventricular septal bulge versus hypertrophic cardiomyopathy in the elderly. Heart 2016. 102 1087–1094. ( 10.1136/heartjnl-2015-308764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranasinghe I, Yeoh T, Yiannikas J. Negative ionotropic agents for the treatment of left ventricular outflow tract obstruction due to sigmoid septum and concentric left ventricular hypertrophy. Heart, Lung and Circulation 2011. 20 579–86. ( 10.1016/j.hlc.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 4.Critoph CH, Pantazis A, Tome Esteban MT, Salazar-Mendiguchía J, Pagourelias ED, Moon JC, Elliott PM. The influence of aortoseptal angulation on provocable left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Open Heart 2014. 1 1–8. ( 10.1136/openhrt-2014-000176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah JS, Esteban T, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, et al Prevalence of exercise induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart 2008. 94 1288–1294. ( 10.1136/hrt.2007.126003) [DOI] [PubMed] [Google Scholar]
- 6.Kelbaek H, Munck O, Christensen NJ, Godtfredsen J. Central haemodynamic changes after a meal. British Heart Journal 1989. 61 506–509. ( 10.1136/hrt.61.6.506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feiner E, Arabadjian M, Winson G, Kim B, Chaudhry F, Sherrid MV. Post-prandial upright exercise echocardiography in hypertrophic cardiomyopathy. Journal of the American College of Cardiology 2013. 61 2487–2488. ( 10.1016/j.jacc.2013.02.079) [DOI] [PubMed] [Google Scholar]
- 8.Joshi S, Patel UK, Yao SS, Castenada V, Isambert A, Winson G, Chaudhry FA, Sherrid MV. Standing and exercise doppler echocardiography in obstructive hypertrophic cardiomyopathy: the range of gradients with upright activity. Journal of the American Society of Echocardiography 2011. 24 75–82. ( 10.1016/j.echo.2010.10.006) [DOI] [PubMed] [Google Scholar]
- 9.Miranda R, Cotrim C, Cardim N, Almeida S, Lopes L, Loureiro MJ, Simões O, Cordeiro P, Fazendas P, João I, et al Evaluation of left ventricular outflow tract gradient during treadmill exercise and in recovery period in orthostatic position, in patients with hypertrophic cardiomyopathy. Cardiovascular Ultrasound 2008. 6 19 ( 10.1186/1476-7120-6-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
At initial presentation: parasternal long axis with colour. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-1.
Download Video 1 (1MB, mp4)
At initial presentation: parasternal long axis view. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-2.
Download Video 2 (1.3MB, mp4)
Following the introduction of 2.5 mg bisoprolol: parasternal long axis view. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-3.
Download Video 3 (1.4MB, mp4)
Following the introduction of 2.5 mg bisoprolol: parasternal long axis with colour. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0072/video-4.
Download Video 4 (691.5KB, mp4)



 This work is licensed under a
This work is licensed under a