Abstract
Background:
Previous studies have suggested that varenicline, an α4β2 nicotinic receptor partial agonist, and α7 nicotinic receptor full agonist, may be effective for the treatment of methamphetamine (MA) dependence due to dopaminergic effects, relief of glutamatergic and cognitive dysfunction, and activation of nicotinic cholinergic systems. This study aimed to determine if varenicline (1 mg BID) resulted in reduced methamphetamine use compared to placebo among treatment-seeking MA-dependent volunteers.
Methods:
Treatment-seeking MA-dependent volunteers were randomized to varenicline 1 mg twice daily (n = 27) or placebo (n = 25) and cognitive behavioral therapy for 9 weeks. The primary outcomes were the proportion of participants achieving end-of-treatment-abstinence (EOTA, MA-negative urine specimens during weeks 8 and 9) and the treatment effectiveness score (TES, number of MA-negative urine specimens) for varenicline versus placebo.
Results:
There was no significant difference in EOTA between varenicline (15%, 4/27) and placebo (20%, 5/25; p = 0.9). There was some suggestion that urinary confirmed medication compliance corresponded with EOTA in the varenicline condition, though it did not reach statistical significance, OR = 1.57 for a 100 ng/ml increase in urine varenicline, p = 0.10, 95% CI (0.99, 3.02). There was no significant difference in mean TES in the varenicline condition (8.6) compared to the placebo condition (8.1), and treatment condition was not a statistically significant predictor of TES, IRR = 1.01, p = 0.9, 95% CI (0.39, 2.70).
Conclusions:
The results of this study indicate that 1 mg varenicline BID was not an effective treatment for MA dependence among treatment-seeking MA-dependent volunteers.
Keywords: Methamphetamine dependence, Varenicline, Relapse
1. Introduction
Methamphetamine (MA) dependence is a significant source of deleterious consequences to individual and public health (Cruickshank and Dyer, 2009). Approximately 469,000 people aged 12 and older in the U.S. meet the DSM-IV criteria for MA dependence, and the economic burden of MA use in the U.S. is approximately $23.4 billion per year (Nicosia et al., 2009; Substance Abuse and Mental Health Services Administration, 2014). Available behavioral treatments, including cognitive behavioral therapy (CBT) and contingency management (CM), are only modestly effective (Lee and Rawson, 2008; Roll, 2007). Potential pharmacotherapies have been investigated in randomized, placebo-controlled trials for MA dependence, but results have failed to identify a medication with a robust effect in generalized populations of MA users (Anderson et al., 2015; Courtney and Ray, 2014; Heinzerling et al., 2014; Miles et al., 2013; Pérez-Mañá et al., 2013), instead only efficacious in subpopulations defined by baseline MA use (Elkashef et al., 2008; Ling et al., 2014; Shoptaw et al., 2008) or among men who have sex with men (Colfax et al., 2011).
Cholinergic mechanisms are important in the neurobiology of MA dependence (Hiranita et al., 2008; Williams and Adinoff, 2008). Varenicline is an α4β2 nicotinic receptor partial agonist and α7 nicotinic receptor full agonist that is approved for cigarette smoking cessation (Gonzales et al., 2006) and shows promise for treating alcohol dependence (de Bejczy et al., 2015; Litten et al., 2013; McKee et al., 2009). The rationale for varenicline as a treatment for MA dependence includes: (1) restoration of MA-related dopaminergic deficits via binding to α4β2 receptors in striatal DA neurons, (2) reductions in cigarette smoking and associated nicotine-mediated potentiation of MA effects, (3) activation of nicotinic cholinergic systems that mediate reductions in reinstatement of MA seeking, (4) relief of MA-related glutamatergic deficits via α7 nicotinic ACh receptor activation, and (5) reduction in MA-related cognitive dysfunction via the cognitive enhancing effects of cholinergic agonists.
While none of these putative mechanisms raise questions of safety of varenicline for methamphetamine dependence, the U.S. Food and Drug Administration (FDA) issued a “black box” warning regarding increased risks of neuropsychiatric and cardiovascular adverse effects with varenicline for cigarette smoking cessation (U.S. Food and Drug Administration, 2009). Our group found varenicline to be safe and without any psychiatric adverse events in a phase 1 safety study (n = 8) among MA-dependent cigarette smokers (Zorick et al., 2010). Another phase I trial (n = 17) by Verrico et al. (2014) showed that varenicline was safe and reduced subjective positive effects of MA compared to placebo.
Building upon this, we conducted a randomized, double-blind Phase II clinical trial of varenicline (1 mg) versus placebo BID for MA dependence. We hypothesized that MA-dependent participants randomized to varenicline would be more likely to achieve end-of-treatment abstinence (EOTA), reduce MA use during active treatment, and delay time-to-relapse as compared to placebo. In addition, we hypothesized that varenicline would reduce cigarette smoking more than placebo among cigarette-smoking participants. We also explored whether varenicline compliance would be associated with treatment outcomes in the varenicline group and whether an inpatient detoxification period would be associated with better outcomes. Finally, we describe safety and tolerability data for varenicline among MA-dependent participants.
2. Methods
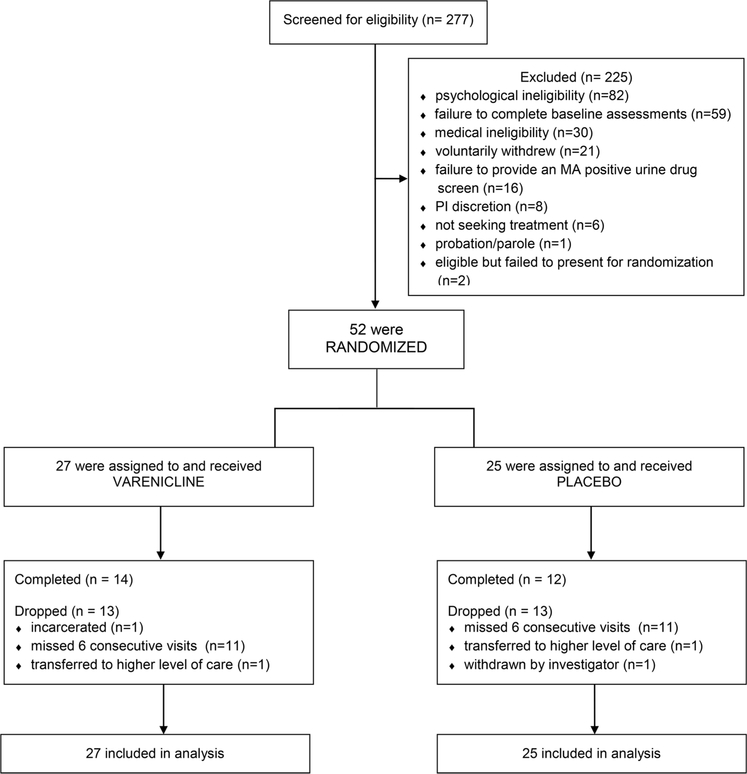
Prior to study initiation, ethical approval was obtained from the Institutional Review Boards at UCLA and LA Biomed and an independent data safety monitoring board and is registered with ClinicalTrials.gov, NCT01365819. A CONSORT study flowchart is presented in Fig. 1.
Fig. 1.
CONSORT Diagram showing flow of study participants.
2.1. Design
This randomized, double-blind, placebo-controlled Phase II clinical trial recruited participants from February 2012 through May 2015 and compared outcomes for varenicline and placebo conditions. Following randomization, participants underwent dose escalation to varenicline 1 mg/placebo BID over one week while completing thrice-weekly outpatient visits. On day 8 of the trial (steady state), participants were admitted to the Harbor-UCLA Clinical and Translational Research Center for 4-night inpatient detoxification and methamphetamine-abstinence initiation. Participants were discharged and returned to the UCLA outpatient clinic on a thrice-weekly basis to complete a nine-week medication phase. Participants then completed four additional weeks of medical and safety assessments; the full duration of the trial was 13 weeks. Due to funding constraints, the inpatient stay was discontinued approximately one-third of the way through the trial (n = 18 of 52 participants underwent inpatient stays), with subsequent participants visiting the outpatient clinic daily instead during week 2 to complete daily required assessments. Prior to study initiation, power calculations were based on 29 repeated measures of the binary outcome variable (MA-negative urine) for each subject (thrice weekly collected samples during study weeks 1, 3–9 and daily samples collected during week 2) with an average within-subject autocorrelation of 0.5 and a two-sided test with alpha = 0.05. The design provided adequate power to detect a medium effect size (Cohen’s f = 0.21) with a target enrollment of 90 participants. Due to lack of accrual, enrollment was halted at n =52.
Participants were reimbursed in gift cards, up to $595, for time spent completing study assessments and transportation to/from the clinic.
2.2. Screening and inclusion/exclusion criteria
In total, 277 participants opened informed consent; 225 screen failed, and 52 were randomized and received varenicline or placebo. Of the 52 randomized, 26 completed and 26 dropped (Fig. 1). Participants were recruited via websites, newspapers, radio, and referrals. Interested individuals called a toll-free number, completed telephone prescreening, were provided study information, and the opportunity to schedule a consent appointment.
Inclusion criteria were: 1) at least 18 years of age, 2) met DSM-IV criteria for MA dependence verified by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (American Psychiatric Association, 2000; First et al., 2002), 3) had an MA-positive urine drug screen at any time during screening, 4) seeking treatment for MA problems, 5) willing and able to comply with study procedures, 6) willing and able to provide written informed consent and 7) if female, not pregnant or lactating and willing to use a medically reliable method of birth control during the trial. Exclusion criteria were: 1) a medical condition that, in the study physician’s judgment, might interfere with safe study participation, 2) a current or past history of cardiovascular disease, 3) systolic blood pressure > 160 or diastolic blood pressure > 100 at two or more screening visits, 4) a history of angioedema, 5) renal impairment, 6) a current neurological disorder (e.g., organic brain disease, dementia) or a medical history which would make study agent compliance difficult or which would compromise informed consent, 7) a current major psychiatric disorder (SCID-verified) not due to substance abuse (e.g., schizophrenia, bipolar disorder) 8) a history of attempted suicide in the past 10 years and/or active suicidal ideation in the past year, 9) current dependence on cocaine, opiates, alcohol, or benzodiazepines (SCID-verified), or 10) a history of sensitivity to varenicline or taking any medications that were contraindicated for use with varenicline or nicotine replacement therapy.
2.3. Randomization
Participants deemed eligible by the study physician, were randomized to varenicline or placebo utilizing an urn randomization procedure (Stout et al., 1994) that provided balance across conditions by gender, ethnicity, baseline frequency of MA use (≤ 18 versus > 18 of the past 30 days), cigarette smoking status (smoker versus non-smoker), and baseline cognitive function (score of ≥ 26 versus < 26) as assessed by the Montreal Cognitive Assessment (MOCA) tool to determine cognitive impairment (Nasreddine et al., 2005). The analysis is modified intent-to-treat in that two individuals were randomized but failed to present for randomization, did not receive study medication, did not contribute data and were considered part of n = 225 excluded participants (Fig. 1). A staff member not directly involved in the research maintained the randomization key and program off-site. Participants and study staff who had any participant contact were blind to treatment assignment.
2.4. Treatments
Varenicline 0.5 mg and 1.0 mg tablets were obtained from the manufacturer (Pfizer). Varenicline or matching placebo tablets were over-encapsulated in a #1 size capsule with 25 mg riboflavin (daily total). Varenicline dosing was titrated, starting at 0.5 mg daily for days 1–3, then 0.5 mg twice daily for days 4–7, and 1 mg twice daily from day 8 until completion of the medication phase. Urine specimens were collected every visit for qualitative measurement of riboflavin as a real-time measure of medication adherence (Herron et al., 2013), part of the comprehensive medication adherence counseling provided every visit. All study medications were prepared in blister packages by a compounding pharmacy, transported to the clinic and dispensed by a study clinician.
All study participants were offered a standard counseling program, consisting of one-hour weekly individual CBT sessions during the 9-week treatment phase. Concepts and materials included: (1) self-monitoring and relapse analysis, (2) identification of “triggers” and cravings, and strategies for coping with them, (3) teaching problem-solving skills, (4) education about MA and MA dependence, (5) HIV education and risk reduction, and (6) motivation/commitment to stopping drug use. This CBT program has demonstrated efficacy in reducing cocaine and alcohol use (Carroll et al., 2004) and has been adapted for use in MA medication studies.
2.5. Assessments and outcome measures
At baseline, participants completed a demographic interview, medical history, physical exam and timeline follow-back of substance use and cigarette smoking (past 30 days). Participants visited the clinic thrice weekly (daily during week 2) and provided a urine sample each visit, and any missing urine samples were considered MA-positive for primary outcome analyses. Participants also completed weekly timeline follow-back assessments of substance use (any use/none) and cigarette smoking (number of cigarettes smoked). Two urine samples to quantitatively measure varenicline adherence were collected during weeks 3 and 5; upon analysis at study completion, the two measurements were averaged.
Varenicline in urine was determined using liquid chromatography-electrospray ionization-tandem mass spectrometry with amphetamine-d5 as the internal standard. The urine was made basic (pH > 10) with ammonia hydroxide and then extracted with n-butyl chloride: acetonitrile (4:1). The organic layer was collected, acidified with HCl, evaporated and reconstituted with 0.1% formic acid. Varenicline and amphetamine-d5 were detected in the mass spectrometer using selected-reaction monitoring with respective transitions of m/z 212 to 169 and 141 to 93. Calibration used a duplicate set of blank human urine samples fortified with eight concentrations ranging from 1.0 to 1000 ng/mL. One set was run at the beginning of the batch, one at the end. Quality control samples were prepared at 3, 30 and 800 ng/mL, aliquoted and stored at − 20 °C until time of use with N ≥ 2 for each run.
Primary outcomes were end-of-treatment abstinence (EOTA) and treatment effectiveness score (TES). EOTA was defined as MA-negative urine samples during the final 2 weeks of treatment (weeks 8 and 9) and no more than one of the three possible urine samples missing per week. Participants with any urine sample during the final 2 weeks positive for MA or missing two or more specimens in either week were considered non-abstinent. TES was defined as the number of MA-negative urine samples provided during the 9 week treatment phase (range 0–29 specimens). Secondary outcomes included time-to-relapse among the subgroup of participants who achieved abstinence during the study (as monitored via urine samples provided, excluding missed visits) and a weekly number of cigarettes smoked in the tobacco smoker subgroup.
2.6. Statistical analyses
Baseline demographic and clinical characteristics were compared between conditions using t-tests for continuous variables and X2 tests for discrete variables. Three participants provided no smoking data during week 1, and their data were imputed as the average number of cigarettes smoked per week during the treatment phase (weeks 2–9).
The primary analyses compared medication conditions on primary outcomes of EOTA with logistic regression and TES with negative binomial regression [Poisson regression model indicated significant overdispersion, likelihood ratio X2 = 71 (1 df), p < 0.001]. Primary analyses were adjusted for age, gender, baseline MA use, and smoking status. Results are reported as adjusted odds ratios (OR) for EOTA and adjusted incidence rate ratios (IRR) for TES. For the two primary outcomes, a Bayes factor is reported (Beard et al., 2016; Dienes, 2014). Bayes factors represent the probability of a true difference between varenicline and placebo groups (the study hypothesis) given the data divided by the probability of no difference (null hypothesis). In this analysis, the treatment regression coefficients (the log OR/log IRR for varenicline vs. placebo) were assumed to be normally distributed with mean zero and SD = log 2 (corresponding to a 95% interval of 0.25–4 for plausible OR/IRR). Bayes factors were calculated using the R function Bf (Christie, 2011; Dienes, 2008).
Secondary analyses examined time-to-relapse in the subgroup of participants who achieved abstinence (provided two consecutive MA-negative urine specimens) and tobacco use in the smoker subgroup. Time-to-relapse was calculated as the number of days from Friday week 2 or if not abstinent during week 2, from the first point of two consecutive negative urines until the first positive urine. Relapse was analyzed with a right-censored Cox proportional hazards model, and multilevel models examined treatment condition and time effects on tobacco use. Varenicline compliance was tested as a predictor of clinical outcomes and relapse in the varenicline subgroup. Given the original expectation that varenicline would improve treatment outcomes after inpatient detoxification, post-hoc analyses examined main effects of inpatient detoxification and the inpatient by medication interaction on the primary outcomes. All analyses were conducted using R v.3.2.5 (R Core Team, 2016) with a two-tailed level of the significance of p < 0.05.
3. Results
3.1. Sample characteristics
Demographic and clinical characteristics for both treatment conditions are presented in Table 1. Results indicate no statistically significant differences between the treatment conditions at baseline.
Table 1:
Baseline demographic and clinical characteristics.
| Characteristic | Varenicline treatment n(%)/mean(sd) | Placebo | X2/t, p |
|---|---|---|---|
| Age in years (at consent) | 34.4(10) | 37.5(11) | 1.06, 0.29 |
| Male gender | 17(63%) | 16(64%) | 0.01, 0.94 |
| Number of days used methamphetamine in past 30 days | 20(8) | 19(10) | 0.53, 0.6 |
| Smoked at least one cigarette in past week at baseline | 14(52%) | 19(76%) | 3.26, 0.07 |
| Total number of cigarettes smoked in past 7 days at baseline | 38(55) | 64(87) | 1.29, 0.2 |
3.2. Medication condition effects on clinical outcomes
Primary analyses examined EOTA and TES, controlling for sex, age, baseline MA use, and smoking status. EOTA was achieved by 17% of the sample (n = 9), and rates of abstinence were comparable between treatment conditions; 15% of varenicline participants (4/27) vs. 20% of placebo participants (5/25), OR = 0.9, p = 0.9, 95% CI (0.17, 4.70). The Bayes factor for the hypothesis that varenicline impacted EOTA was 0.77, suggesting no difference between groups.
Mean TES was 8.6 (10.1) in the varenicline condition and 8.1 (8.2) in the placebo condition. In adjusted analysis, treatment condition was not a statistically significant predictor of TES, IRR= 1.01, p = 0.9, 95% CI (0.39, 2.70). The Bayes factor for the hypothesis that varenicline impacted TES was 0.50, also suggesting no difference. Baseline MA use significantly predicted TES, as participants with greater pre-treatment MA use were less likely to provide MA-negative urine samples, IRR = 0.94 for one additional day of MA use in the month preceding enrollment, p = 0.01, 95% CI (0.88, 0.99). Varenicline treatment did not interact with baseline MA use to predict EOTA or TES, and age, sex, and smoking status did not have significant effects on abstinence or TES.
3.3. Time-To-Relapse
Forty-eight percent of varenicline participants (13/27) achieved abstinence during treatment compared to 56% of placebo (14/25). Of those achieving abstinence, 62% of varenicline participants relapsed (8/13) vs. 43% of placebo (6/14). Kaplan-Meier median time-to-relapse was 38 days in the varenicline group and not estimable in the placebo group, as overall “survival” did not drop below 50% during the treatment phase. In a Cox proportional hazards model, treatment condition did not predict relapse; Hazard Ratio (HR) = 1.42, p = 0.52, 95% CI (0.49, 4.10).
3.4. Medication compliance
Additional analyses examined whether urinary medication compliance associated with primary outcomes or time-to-relapse in the varenicline condition. There was some suggestion that compliance corresponded with EOTA, though it did not reach statistical significance, OR = 1.57 for a 100 ng/mL increase in urine varenicline, p = 0.10, 95% CI (0.99, 3.02). Varenicline compliance was not associated with TES; IRR = 1.14, p = 0.18, 95% CI (0.93, 1.41). Among varenicline participants who achieved abstinence, there were no statistically significant effects of compliance on time-to-relapse, though findings were in the expected direction; HR = 0.75 per 100 ng/mL increase in urine varenicline, p = 0.063, 95% CI (0.55, 1.02). Mean urine varenicline concentration was 791 ng/mL among participants who did not relapse as compared to 536 ng/mL among those who did.
3.5. Inpatient detoxification
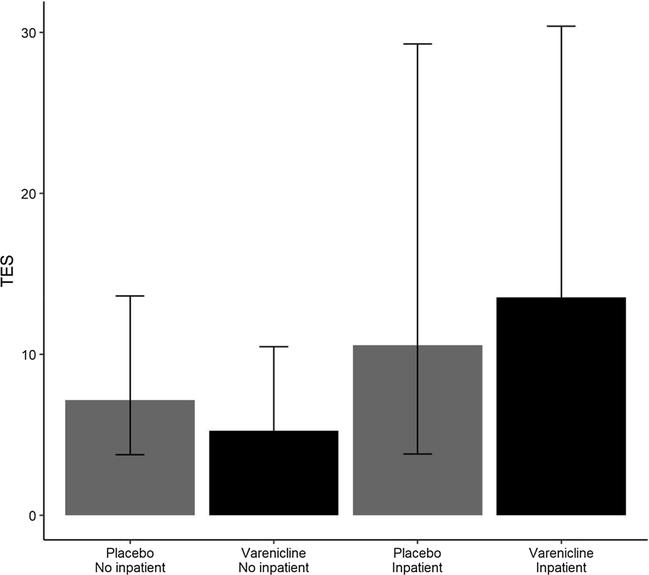
A final analysis examined whether inpatient detoxification corresponded with primary outcomes or moderated treatment effects. Eleven of 27 varenicline participants underwent inpatient detoxification (41%) vs. 7/25 placebo participants (28%). There was no main effect of inpatient detoxification on EOTA [OR = 0.93, p = 0.9, 95% CI (0.18, 4.09)], and the effect of detoxification on TES was positive, but did not reach statistical significance [IRR = 1.98, p = 0.1, 95% CI (0.91, 4.54)]. No inpatient placebo participants achieved EOTA, resulting in an undefined odds ratio in the strata of inpatient participants. Consequently, the interaction effect between treatment and inpatient detoxification on EOTA was not estimated. Additionally, inpatient detoxification did not modify the treatment-TES relationship (p value for interaction = 0.5). Fig. 2 presents model-fitted TES by treatment group and inpatient detoxification status.
Fig. 2.
Treatment effectiveness scores by treatment condition and inpatient detoxification status. Error bars indicate the 95% confidence interval of the model-fitted mean TES in each group.
3.6. Cigarette smoking
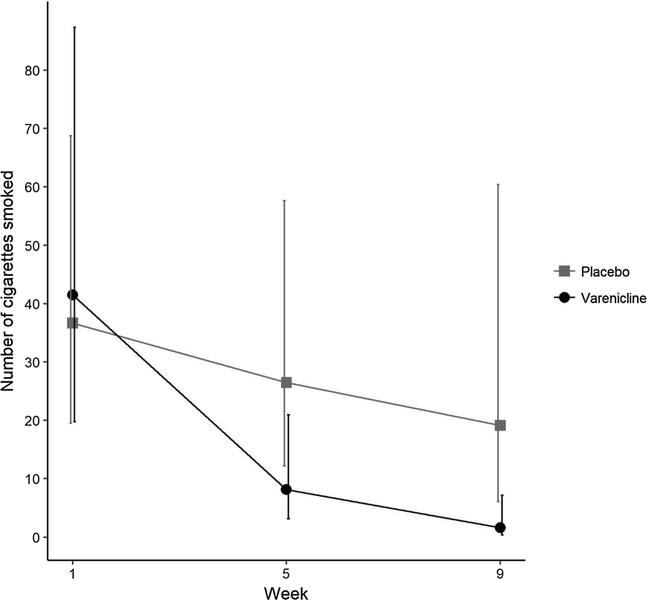
A mixed negative binomial regression model was used to examine the weekly number of cigarettes smoked among participants who smoked any cigarettes during the treatment phase (N = 35, n = 15 varenicline, n = 20 placebo). Greater reductions in cigarette smoking were observed in the varenicline group, as evidenced by a significant, negative time by varenicline treatment interaction (b = −0.56, se = 0.21, p = 0.007). Pre-specified comparisons of fitted values showed no difference between treatment groups at baseline (p = 0.83), a non-significant difference of about 14 cigarettes at 5 weeks (p = 0.056) and a statistically significant difference of about 18 cigarettes at 9 weeks (p = 0.01, Fig. 3).
Fig. 3.
Model fitted number of cigarettes smoked at weeks 5 and 9. Error bars indicate the 95% confidence interval of the model-fitted number of cigarettes smoked in each group at each time point.
3.7. Adverse events
Generally, varenicline was well tolerated, with 92% (23/25) of placebo participants reporting at least one adverse event (AE) compared to 92.6% (25/27) of varenicline participants [t(50) = −1.502, p = 0.139] (Supplemental material). The frequency of AEs reported was greater in the varenicline group compared to placebo, with AEs rated mild to moderate in severity. One AE, flushing, reached statistical significance with 16% of placebo participants reporting the event compared to no reports in varenicline group (p = 0.047). Despite concerns that varenicline use may lead to suicidal ideation or adverse cardiac events, no relationship between varenicline treatment and these adverse events was observed in this study. Two participants in each group reported any cardiac events (all were mild and resolved without sequelae). Only one placebo participant reported suicidal ideation and one participant in the varenicline group reported severe anger; medication was discontinued, and both were referred for treatment and subsequently dropped due to missing six consecutive visits. The most common AE was vivid dreams, reported by 44% of the varenicline group and 20% of the placebo group (p = 0.08) and headache, reported by 37% of the varenicline group and 28% of the placebo group (p = 0.56). Other common AEs included insomnia, nausea and dry mouth.
4. Discussion
In this randomized, double-blind, placebo-controlled trial among MA-dependent participants, there were no statistically significant differences between varenicline and placebo on the primary outcomes: EOTA and TES. Additionally, varenicline compliance was not associated with treatment outcomes among participants in the varenicline group. Varenicline treatment did not impact time-to-relapse among participants who achieved abstinence during the treatment phase, and a 4-day inpatient detoxification period did not predict treatment outcomes nor modify medication condition-treatment outcome associations. There was some evidence that varenicline compliance lowered the risk of relapse in the varenicline group, although this relationship did not reach statistical significance. Varenicline demonstrated a positive effect on cigarette smoking; analyses showed a significant decline in the number of cigarettes smoked during the treatment period in the varenicline group compared to placebo. Thus, levels of the medication were sufficient to reduce ad-libitum cigarette smoking but were not sufficient to reduce methamphetamine use.
Addiction researchers have promoted the use of Bayes factors to enhance the interpretation of trial results, both positive and negative (Dienes, 2014; West, 2016). Although Bayes factors are continuous measures of evidence, conventionally, Bayes factors > 3 are interpreted as evidence that the study hypothesis is likely to be true, while factors < 1/3 are taken as evidence in favor of the null hypothesis. Bayes factors in the range of 1/3–3 are inconclusive. We computed Bayes factors for our primary hypothesis tests, both of which failed to reach statistical significance. While neither Bayes factor crossed the conventional cutoff of 1/3 indicating “conclusive” support for the null hypothesis, both indicated that the null hypotheses of no difference between treatment groups were more likely than the study hypotheses. For EOTA, our data suggest the null hypothesis was about 30% more likely (1/0.77); for TES, the null hypothesis was twice as likely (1/0.5). In sum, our study suggests that varenicline is not an effective treatment for MA dependence at the dose tested.
A preclinical rodent study showed no difference in MA self-administration or reinstatement following abstinence between varenicline and saline (Pittenger et al., 2016). However, rats receiving a low dose of varenicline plus a low “reinstatement trigger” dose of MA relapsed at greater rates than rats receiving a high dose of varenicline and the same trigger. Somewhat in parallel, our findings pointed toward the possibility that compliance could be associated with lower risk of relapse among varenicline participants who achieved abstinence during the trial, though this relationship was not statistically significant.
Our study also indicated a positive and clinically significant effect of varenicline on cigarette smoking. Among participants in the varenicline group, the model-fitted number of cigarettes smoked during the final week of treatment was nearly zero. Notably, our trial did not provide any treatment for smoking cessation. As the prevalence of cigarette smoking is high among MA-dependent individuals, the public health impact of a simultaneous pharmacotherapy is similarly high and underscores significance of using varenicline for reducing smoking during quit attempts for methamphetamine dependence.
The stringent exclusion criteria in this study may limit the generalizability of the results. Concerns regarding rare cardiac and neuropsychiatric adverse events with varenicline used for smoking cessation led the FDA to issue warnings regarding safety of varenicline (U.S. Food and Drug Administration, 2009) and, as a result, participants with any history of cardiac disease or suicide attempt or serious suicidal ideation were excluded from the trial, making accrual challenging. This small trial in a highly-selected group of MA users cannot rule out rare cardiac or psychiatric adverse events although recent meta-analyses support the safety of varenicline in general populations including those with psychiatric illness (Cahill et al., 2016; Chelladurai and Singh, 2014; Thomas et al., 2018). Interestingly, in December 2016 the FDA approved updates to the CHANTIX® (varenicline) labeling, including removal of the boxed warning regarding serious neuropsychiatric events (U.S. Food and Drug Administration, 2016). Another limitation was our inability to provide inpatient treatment to all participants. Many participants in our trial failed to achieve even an initial period of abstinence, limiting our ability to examine varenicline’s impact on relapse to a small subset of participants. Finally, rates of loss to follow-up were high in this study, as in other trials involving MA-dependent individuals. We attempted to mitigate this loss by utilizing appropriate analytic strategies for missing data, e.g., modified intent-to-treat analysis, models stratified on follow-up time, and mixed-effect models. However, loss to follow-up likely reduced the study power, and there is the potential for bias whenever loss to follow-up is non-negligible.
5. Conclusion
In conclusion, there was not a significant difference between varenicline and placebo on the primary outcomes, EOTA and TES. Bayes factors for these hypotheses indicated that the null hypotheses of no difference were more likely than the study hypotheses; however, neither factor met the conventional threshold for persuasive evidence. On the contrary, our trial found that varenicline treatment resulted in reductions in cigarette smoking in the absence of any cessation treatment. In addition, varenicline compliance was protective against MA relapse among participants who were able to achieve abstinence. Thus, combinations of varenicline with other interventions aimed at sustaining MA abstinence and reducing or preventing relapses, such as voucher-based reinforcement of MA-negative urine samples or varenicline-bupropion combination treatment may warrant further study.
Supplementary Material
Acknowledgements
We would like to acknowledge the UCLA Vine Street Clinic Staff and the Harbor-UCLA Clinical Translational Science Institute and members of the Harbor-UCLA Department of Psychiatry for their support in conducting this research.
Role of funding source
This work was supported by the National Institute on Drug Abuse (R01DA030577; S. Shoptaw).
Footnotes
Conflict of interest
No conflicts declared.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2018.04.023.
References
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Text Revision, Retrieved from. fourth ed. American Psychiatric Association, Washington D.C: (Accessed May 21, 2018). https://dsm.psychiatryonline.org/doi/pdf/10.1176/appi.books.9780890420249.dsm-iv-tr. [Google Scholar]
- Anderson AL, Li SH, Markova D, Holmes TH, Chiang N, Kahn R, Campbell J, Dickerson DL, Galloway GP, Haning W, Roache JD, Stock C, Elkashef AM, 2015. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 150, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E, Dienes Z, Muirhead C, West R, 2016. Using Bayes Factors for testing hypotheses about intervention effectiveness in addictions research. Addiction 111, 2230–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T, 2016. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ, 2004. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch. Gen. Psychiatry 61, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelladurai Y, Singh S, 2014. Varenicline and cardiovascular adverse events: a perspective review. Ther. Adv. Drug Saf 5, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J, 2011. R function “Bf”. Retrieved from. (Accessed 30 June 2016). http://www.lifesci.sussex.ac.uk/home/Zoltan_Dienes/inference/bayesFactorCalc2.R.
- Colfax GN, Santos G-M, Das M, Santos DM, Matheson T, Gasper J, Shoptaw S, Vittinghoff E, 2011. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch. Gen. Psychiatry 68, 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA, 2014. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 143, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR, 2009. A review of the clinical pharmacology of methamphetamine. Addiction 104, 1085–1099. [DOI] [PubMed] [Google Scholar]
- de Bejczy A, Lof E, Walther L, Guterstam J, Hammarberg A, Asanovska G, Franck J, Isaksson A, Soderpalm B, 2015. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin. Exp. Res 39, 2189–2199. [DOI] [PubMed] [Google Scholar]
- Dienes Z, 2008. Understanding Psychology as a Science: An Introduction to Scientific and Statistical Inference. Palgrave MacMillan, Hampshire, England. [Google Scholar]
- Dienes Z, 2014. Using Bayes to get the most out of non-significant results. Front. Psychol 5, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D, 2008. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology 33, 1162–1170. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/ P) Biometrics Research. New York State Psychiatric Institute, New York. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, 2006. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296, 47–55. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Hall TM, Yi Y, Wu YN, Shoptaw SJ, 2014. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction 109, 1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron AJ, Mariani JJ, Pavlicova M, Parrinello CM, Bold KW, Levin FR, Nunes EV, Sullivan MA, Raby WN, Bisaga A, 2013. Assessment of riboflavin as a tracer substance: comparison of a qualitative to a quantitative method of riboflavin measurement. Drug Alcohol Depend. 128, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Yamamoto T, 2008. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology 55, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Lee NK, Rawson RA, 2008. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 27, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Chang L, Hillhouse M, Ang A, Striebel J, Jenkins J, Hernandez J, Olaer M, Mooney L, Reed S, Fukaya E, Kogachi S, Alicata D, Holmes N, Esagoff A, 2014. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction 109, 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R, 2013. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J. Addict. Med 7, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E, 2009. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol. Psychiatry 66, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SW, Sheridan J, Russell B, Kydd R, Wheeler A, Walters C, Gamble G, Hardley P, Jensen M, Kuoppasalmi K, Tuomola P, Fohr J, Kuikanmaki O, Vorma H, Salokangas R, Mikkonen A, Kallio M, Kauhanen J, Kiviniemi V, Tiihonen J, 2013. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction 108, 1279–1286. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J, 2009. The Economic Cost of Methamphetamine Use in the United States, 2005. Retrieved at. RAND Drug Policy Research Center (Accessed May 21, 2018). https://www.rand.org/pubs/monographs/MG829.html.
- Pérez-Mañá C, Castells X, Torrens M, Capellà D, Farre M, 2013. Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst. Rev CD009695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Barrett ST, Chou S, Bevins RA, 2016. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in female rats. Behav. Brain Res 300, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. R: A Language and Environment for Statistical Computing. Retrieved from. (Accessed May 21, 2018). https://www.r-project.org/.
- Roll JM, 2007. Contingency management: an evidence-based component of methamphetamine use disorder treatments. Addiction 102, 114–120. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W, 2008. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 96, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK, 1994. Ensuring balanced distribution of prognostic factors in treatment outcome research. J. Stud. Alcohol (Suppl. s12), 70–75. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Retrieved from. (Accessed June 29, 2016). http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf.
- Thomas KH, Martin RM, Knipe DW, Higgins JP, Gunnell D, 2018. 2015. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2009. Information for Healthcare Professionals: Varenicline (Marketed as Chantix) and Bupropion (Marketed as Zyban, Wellbutrin, and Generics). Retrieved from. (Accessed July 1, 2009). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm.
- U.S. Food and Drug Administration, 2016. FDA Drug Safety Communication: FDA Revises Description of Mental Health Side Effects of the Stop-Smoking Medicines Chantix (Varenicline) and Zyban (Bupropion) to Reflect Clinical Trial Findings. Retrieved from. (Accessed December 16, 2016). https://www.fda.gov/Drugs/DrugSafety/ucm532221.htm.
- Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, Bennett RS, Newton TF, De La Garza R 2nd, 2014. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int. J. Neuropsychopharmacol 17, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, 2016. Using Bayesian analysis for hypothesis testing in addiction science. Addiction 111, 3–4. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B, 2008. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology 33, 1779–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Sevak RJ, Miotto K, Shoptaw S, Swanson AN, Clement C, De La Garza R 2nd, Newton TF, London ED, 2010. Pilot safety evaluation of varenicline for the treatment of methamphetamine dependence. J. Exp. Pharmacol 2, 13–18. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.