Abstract
Background
Visceral obesity is associated with diabetogenic and atherogenic abnormalities, including insulin resistance and increased risk for cardiometabolic diseases and mortality. Rodent lipectomy studies have demonstrated a causal link between visceral fat (VF) and insulin resistance, yet human omentectomy studies have failed to replicate this metabolic benefit, perhaps owing to the inability to target the mesentery.
Objectives
We aimed to demonstrate that safe and effective removal of mesenteric fat (MF) can be achieved in obese insulin-resistant baboons using tissue liquefaction technology (TLT).
Setting
Southwest National Primate Research Center, San Antonio, Texas.
Methods
TLT has been developed to enable Mesenteric Visceral Lipectomy (MVL) to be safely performed without disturbing the integrity of surrounding nerves and vessels in the mesentary. Following an initial MVL optimization study (n=3), we then performed MVL (n=4) or Sham surgery (n=2) in a cohort of insulin-resistant baboons, and the metabolic phenotype was assessed via hyperinsulinemic-euglycemic clamps at baseline and 6 wks later.
Results
MVL led to a 75% improvement in glucose disposal at 6 wks follow-up (p=0.01). Moreover, despite removing only an average of 430 g of MF (~1% of total body mass), MVL led to a 14.4% reduction in total body weight (p=0.001). Thus, these data demonstrate that MF can be safely targeted for removal by TLT in a non-human primate, leading to substantial metabolic improvements, including reversal of insulin resistance and weight loss.
Conclusions
These data provide the first demonstration of successful adipose tissue removal from the mesentery in a mammal. Importantly, we have demonstrated that when MVL is performed in obese, insulin resistant baboons, insulin resistance is reversed and significant weight loss occurs. Therefore, trials performing MVL in humans with abdominal obesity and related metabolic sequelae should be explored as a potential clinical tool to ameliorate insulin resistance and treat type 2 diabetes.
Keywords: Mesenteric fat, tissue liquefaction technology, hypophagia, metabolic improvement, insulin-resistant baboons
Excess visceral fat (VF) has been strongly linked with development of insulin resistance and subsequent type 2 diabetes (T2D) in humans (1, 2), and rodent lipectomy studies have demonstrated that this relationship is causal (3–5). More than two-thirds of Americans are overweight or obese, and in ~90% of the 29 million individuals affected by T2D, onset appears secondary to being overweight or obese, with excess VF considered to be a key driver (6, 7). Despite current treatment advances, T2D often leads to numerous complications, co-morbidities, and early mortality (8, 9). Lifestyle changes (diet and exercise) are the first line of treatment for weight management and disease prevention (10, 11). However, the percentage of individuals who succeed in achieving long-term weight loss is low (12).
In an effort to identify an alternative treatment option, small and large volume liposuction has been explored, but because this only targets subcutaneous (SC) depots for removal, a metabolic benefit has not been consistently observed or maintained in most studies (13–15). Indeed, it is now appreciated that SC and VF are biologically distinct, with VF more pathogenic, due in part to its direct portal access and greater secretion of pro-inflammatory adipokines (6). Therefore, targeting the VF depots for removal, particularly the mesentery, where most VF is stored, should be explored as a potential surgical strategy to rapidly and significantly improve metabolic and overall health in humans. However, due to the dense innervation and vascularization within and around human mesenteric fat (MF), there are currently no feasible surgical methods (including classic liposuction) to safely perform this procedure.
As an alternative to removing adipose tissue deep in the viscera, human studies have targeted the greater omentum for surgical removal with mixed results on metabolic outcomes (16–21). The amount of omentum reportedly removed in these studies was ~0.5 kg. However, the average amount of total VF mass has been estimated to exceed 3kg in obesity, with levels as high as ~7–8kg detected in some individuals (22). Thus, it is not surprising that these studies did not observe a benefit given that only a fraction of total VF is removed by omentectomy in obese subjects. As rodent lipectomy studies demonstrating metabolic benefit surgically ablate >75% of total VF mass, targeting the mesentery, where most VF is harbored, may be required to resolve insulin resistance by lipectomy.
In order to overcome surgical barriers to targeting VF, we have utilized a novel surgical energy source, referred to as Tissue Liquefaction Technology (TLT), also referred to as Phaser™ Liquefaction Technology (23). TLT was initially invented for cataract extraction and became the AquaLase® Liquefaction Device, a modality on the Infiniti Vision System by Alcon, Inc (24). In 2013, Andrew Technologies (Haddonfield, NJ/Tustin, CA) launched HydraSolve®, which is FDA cleared for SC fat removal in aesthetic body contouring and autologous fat transfer (25). TLT delivers low levels of thermal and mechanical energy as a stream of warmed, low-pressurized, and pulsed saline, which causes susceptible non-connective tissues to undergo a phase transition from solid to liquid, while connective tissue-protected blood vessels and nerves are unharmed (25, 26). Because of the high safety margins and the clinically proven target-tissue specific liquefaction capability of both AquaLase® and HydraSolve® in surgical practice (27), we set out to determine if TLT could be safely adapted to perform Mesenteric Visceral Lipectomy (MVL) in non-human primates, which are close genetic relatives of humans, and harbor many similarities to human physiology, including regulation of glucose metabolism.
METHODS
ANIMALS
For optimization studies performed in Phase I, a total of 3 male baboons with obesity (weight >37 Kg, % fat >21) were carefully selected (28). For the Phase II efficacy study, a total of 9 non-diabetic obese animals (mean age = 12.0 ± 1.2yrs; mean A1C 4.8 ± 0.4%) with the highest A1C levels (Table 1) were then enrolled into the study from the colony housed at the Southwest National Primate Research Center, Texas Biomedical Research Institute (San Antonio, TX). Baseline phenotypic assessments were then made and blood was sampled for clinical chemistries. Hyperinsulinemic-euglycemic clamps, considered the “gold standard” test in metabolic science for assessing insulin status, sensitivity or resistance, were performed using previously published methods (29). After obtaining the glucose rate of disappearance (Rd) as previously described (30), we further selected for 6 baboons with cut-off Rd values of 5.6 or < mg/kg/min, indicating insulin resistance (31). Animals were then subsequently assigned to either undergo MVL (n=4) or a control sham surgery (n=2). At 6 wks follow-up, all morphometric and metabolic study procedures were repeated. The protocol was approved by the Institutional Animal Care and Use Committees of the Texas Biomedical Research Institute. The study approval ID was IACUC 1435PC.
Table 1 Legend. Baseline metabolic characteristics of Phase II male baboons.
Nine non-diabetic obese, male baboons were subjected to a 2-hour hyperinsulinemic-euglycemic clamp, which is the gold-standard method to measure insulin-mediated glucose disposal. After obtaining the Rd, we further selected those baboons with cut-off Rd values of 5.6 or < mg/kg/min (n=6), indicating insulin resistance for further studies.
| Baboon ID | Body Weight (kg) | A1C (%) | Rd (mg/kg/min) |
|---|---|---|---|
| 28420 | 43.5 | 4.6 | 1.3 |
| 25358 | 36.7 | 5.7 | 3.5 |
| 27677 | 37.8 | 4.8 | 4.2 |
| 26103 | 45.4 | 5.1 | 4.3 |
| 19975 | 41.4 | 4.8 | 4.5 |
| 18344 | 38.4 | 4.8 | 5.6 |
| 26099 | 42.7 | 4.8 | 6.3 |
| 19424 | 44.7 | 4.1 | 7.8 |
| 19914 | 39.6 | 4.7 | 8.3 |
BODY WEIGHT AND COMPOSITION
Baboons were sedated and evaluated for morphometric measurements including body weight measured on an electronic scale (GSE 665, Texas Scales). Body composition was determined by dual-energy X-ray absorptiometry (DXA) using a Lunar Prodigy densitometer (GE Healthcare, Madison, WI) to determine fat and lean mass. Animals were first sedated and placed in the supine position on the DXA bed and extremities were positioned within the scanning region. Scans were approximately 5 min in duration and data were subsequently analyzed using encore2007 software (v.11.40.004; GE Healthcare).
DIETARY INTAKE AND PAIR-FEEDING STRATEGY
Before the surgical removal of MF, the quantity of food offered to each baboon daily was based on the estimated metabolizable energy requirements for adult captive baboons. Specifically, the animals were fed a commercial diet targeted to meet an expected energy requirement to sustain constant body weight (BW) of 40–51 kcal/kg BW (32). In order to account for unanticipated changes in food intake, we used a pair-feeding strategy in the two Sham control animals. This process entailed taking the average amount of food in grams eaten by two experimental baboons (who had received MVL surgery) and providing the same amount to two control sham-operated baboons housed under identical conditions the following day (33).
HYPERINSULINEMIC-EUGLYCEMIC CLAMPS
Insulin sensitivity was assessed with the hyperinsulinemic-euglycemic insulin clamp technique as previously described (29, 31). After an overnight fast (12 hrs), each baboon was sedated with ketamine hydrochloride (10 mg/kg i.m.) before arrival to the procedure room. Endotracheal intubation was performed using disposable cuffed tubes (6.5–8.0 mm diameter) under direct laryngoscopic visualization, and all animals were supported with 98–99.5% FiO2 by a pressure controlled ventilator adjusted, as necessary, to keep the oxygen saturation 95%. The maintenance of anesthesia consisted of an inhaled isofluorane (0.5–1.5%) and oxygen mix. Catheters were inserted into the femoral vein for insulin and glucose infusion and into the contralateral femoral artery for blood sampling. Fasting plasma glucose, free fatty acid (FFA), and insulin concentrations were measured at −10 and 0 min. At t=0 min, a primed continuous infusion of human regular insulin (Novolin; Novo Nordisk, Princeton, NJ) was started and continued at 60 mU/m2 body surface area per minute for 120 min. Body surface area (A) was estimated from the body mass (M) using the Meeh formula A = K.M2/3 as described for the baboon (34). During the clamp, plasma glucose was measured every 5 min, and a 20% glucose infusion was adjusted as necessary, to maintain a plasma glucose concentration of ~95mg/dl. Insulin sensitivity was calculated as the mean rate of insulin-stimulated glucose disposal (Rd) during the final 30 minutes of the clamp as hepatic glucose production is completely suppressed at the achieved level of hyperinsulinemia (31).
MVL AND SHAM SURGERY
Following an overnight fast (~12 hrs), each baboon was sedated as described above for the clamp procedure. The surgical field was prepared and an ~16–18 cm vertical midline open abdominal incision was made using aseptic technique, and the mesentery was located. Using a 3.0 mm sterile Miltex punch biopsy instrument, a round 3 mm diameter opening was made in the anterior mesenteric sheath. A 2.4 mm outer diameter, 15 cm long cannula (HydraSolve®, Andrew Technologies, Haddonfield, NJ/Tustin, CA) was inserted through the opening and into the mesenteric fat tissue. The MF was liquefied and simultaneously aspirated from the body. In 3 of the 7 baboons who underwent the MVL procedure in Phase 1 and 2, there was no active bleeding detected and no cautery devices were used. In 4 of the 7 baboons who had MVL, a minimal number (3 to 4) of tiny venous bleeders were encountered which were all quickly and easily rectified either by cauterization or the placing of a 3.0 Vicryl suture. Sham operations were identical in length and procedure as VML, but no MF was removed. Animals were then intensively monitored and evaluated in the post-operative period. All baboons demonstrated acceptable recoveries, regaining their normal behavior and bodily functions within 48 hrs after surgery (personal communication with Shannan Hall-Ursone, DVM).
STATISTICAL ANALYSIS
All values are presented as means ± SE. Parametric data were analyzed by paired-sample t-tests for VF removal and Sham groups, respectively. Square root transformation on the relative percentage change from baseline to post-op was performed when appropriate to ensure normality of distribution. Linear regression was used to determine relationships among metabolic and phenotypic responses to the MVL intervention. Statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL). A P≤0.05 was considered statistically significant.
RESULTS
OPTIMIZATION
For Phase I, we performed an initial safety trial in obese male baboons (n=3). For each procedure, approximately 125–150 grams of MF was surgically removed using TLT (Fig. 1A). In all surgeries, the majority of the operating time was spent starting and stopping the fat removal activity in an effort to determine the optimal parameters and settings for removing VF using the TLT system. As these studies were intended for optimization, the effect on MF removal efficiency was tested with numerous machine settings of the phaser™ stream temperature (PhST) and pounds per square inch (psi) pressure, as well as numerous vacuum power settings for fat aspiration. In addition, numerous cannulae were tested in this process, as well as various surgical techniques of handling the cannulas until achieving the ideal PhST, psi, cannulae and technique.
Figure 1 Legend. Surgical removal of MF by TLT.
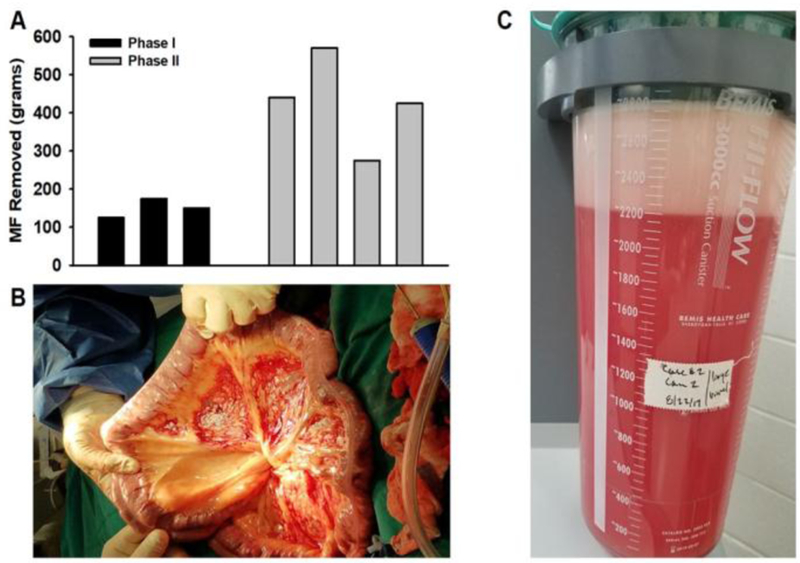
(A) Bar graph indicates amount of MF removed during Phase I optimization studies (black bars; n=3) and Phase II efficacy studies (gray bars; n=4). (B) Image taken during a Phase II MVL procedure showing that TLT can successfully remove adipose tissue from the mesentery without causing damage to the vascular supply or to the mesenteric sheath. (C) Lipoaspirate collected in a canister with ∼500 g of mesenteric visceral fat tissue removed (after complete gravity separation).
EFFICACY
For Phase II, we performed a safety and efficacy trial. We selected 9 non-diabetic obese animals [A1C levels <6.5% according to the American Diabetes Association (ADA) guidelines (35)] (mean age = 12.0 ± 1.2yrs) with the highest A1C levels to enroll into the study (Table 1). We next performed clamp studies to further identify animals with overt insulin resistance. The mean rate of whole-body glucose disappearance (Rd) was 5.0 ± 2.1 mg/kg/min, average body weight was 41.1 ± 3.1 Kg, and average A1C values were 4.8 ± 0.4%. Among those 9 baboons, 6 animals with overt insulin resistant were identified (mean Rd =3.8 ± 0.9 mg/kg/min) and selected for either MVL surgery (n=4) or as pair-fed Sham controls (n=2) (31)
For Phase II MVL studies, greater amounts of MF were targeted, with 75–80% of all visible fat in their mesentery removed in 3 animals, experimental baboon # 1 (EXP 1): ID19975, experimental baboon # 2 (EXP 2): ID18344, experimental baboon # 3 (EXP 3):ID27677) and ~50% of all visible MF removed in experimental baboon # 4 (EXP 4): ID28420 (Fig. 1A-C). Despite removing only an average of 430kg of adipose tissue, body weight decreased by an average of 6.6 Kg (14.4%) at 6 weeks follow up (Table 2) Similarly, total fat mass (p=0.001), percentage fat (p=0.001) and lean mass (p=0.02) all significantly decreased after MVL (Table 2). Interestingly, while MVL resulted in a significant amount of weight loss, it was noted that the extent of relative weight loss was dependent on baseline body mass, whereby larger pre-op body size positively predicted a greater weight loss response to MVL (R2=0.76; p=0.084; Figure 2) EXP 3 pre-op clamp weight 38 kg – total body weight (TBW) loss at post-op clamp 5.8 %; EXP 4 pre-op clamp weight 45 kg - TBW loss at post-op clamp 8.9 %; EXP 2 pre-op clamp weight 49 kg - TBW loss at post-op clamp 21.8 %; EXP 1 pre-op clamp weight 51 kg - TBW loss at post-op clamp 19.8 %.
Table 2 Legend. Metabolic data at baseline and at 6 weeks follow up after MVL surgery.
Values are means±SE. A total of 4 male insulin-resistant male baboons were assessed at baseline for various metabolic characteristics and at 6 wks following MVL.
| Measurement (n=4) | Baseline | Follow up | p value |
|---|---|---|---|
| Weight (kg) | 45.7±2.9 | 39.1±1.2 | 0.05 |
| Fat mass (kg) | 12.2±2.3 | 10.3±2.0 | 0.017 |
| Percent fat (%) | 27.8±4.5 | 25.9±4.3 | 0.026 |
| Lean mass (kg) | 30.6±.0.9 | 28.3±0.9 | 0.002 |
| Glucose (mg/dL) | 80.8±6.1 | 83.7±3.0 | 0.48 |
| Insulin (uIU/mL) | 36±11 | 20±6 | 0.248 |
| HOMA-IR | 4.0±1.2 | 2.4±0.8 | 0.22 |
| Rd (mg/kg/min) | 3.8±0.9 | 6.7±0.6 | 0.012 |
| Glucagon (pg/mL) | 22.6±8.1 | 18.3±2.3 | 0.59 |
| FFA (mEq/L) | 0.33±0.06 | 0.36±0.04 | 0.72 |
| Triglycerides (mg/dL) | 54.2±21.5 | 49.5±15.8 | 0.56 |
| Cholesterol (mg/dL) | 84.5±13.8 | 63.5±5.8 | 0.07 |
Figure 2 Legend. Baseline body mass predicts relative weight loss response to MVL.
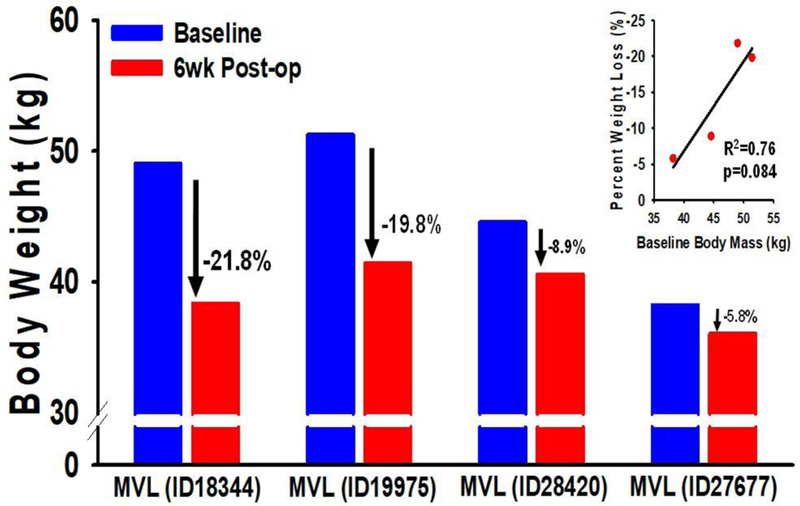
Baseline body mass of baboons assigned to MVL ranged from 38.2kg to 51.2kg. As a group, MVL resulted in a significant amount of weight loss (14.4%) at 6 wks follow-up (see Table 2). However, closer examination of this response revealed that greater baseline body mass tended to positively predicted a greater weight loss response (R2=0.76; p=0.084) with the largest intervened animals (ID19975 body wt 51.2kg; ID18344 body wt 49.0kg) demonstrating the greatest relative weight loss, while the smaller animals (ID28420 body wt 44.6kg; ID27677 body wt 38.2kg) lost much less weight.
We next determined the impact of MVL on insulin action by performing hyperinsulinemic-euglycemic clamp studies. Glucose disposal rates were 3.8 mg/kg/min at baseline, which was indicative of insulin resistance in these animals, but Rd significantly increased by 75% to 6.8 mg/kg/min at 6 wks after MVL (p=0.019; Table 2). Interestingly, the percentage improvement in insulin action after MVL was not correlated with the relative amount of weight loss in these baboons (R2=0.21; p=0.541). Meanwhile fasting insulin levels tended to decrease (p=0.248) without significant changes in glucose, leading to a numerical reduction in homeostatic model assessment for insulin resistance (HOMA-IR) from 4 to 2.4 (p=0.22). There was no significant difference in triglycerides or FFAs at follow up, but total cholesterol tended to be reduced (p=0.07; Table 2).
PAIR-FEEDING
In order to account for the sustained reduction in food intake following MVL on insulin sensitivity, we performed an experiment whereby sham-operated baboons (n=2) were pair-fed to MVL baboons (n=2). This strategy was designed to measure the precise contribution of the TLT procedure and MVL surgery to weight loss, hypophagia and metabolic improvement. Animals were carefully matched for weight EXP 4: [ID28420 (MVL), 44.6 kg vs. ID26103 (Sham), 46.7 kg; and EXP 3: ID27677 (MVL), 38.2 Kg vs. ID 25358 (Sham), 37.0kg,]. Mean caloric intake in both baboons assigned to MVL decreased from ~2250 calories/day to ~650 calories/day at 18 days follow up. After 6 weeks, calorie intake of animal EXP 4:ID28420 remained steady with an average daily intake of ~750 calories, while baboon EXP 3:ID27677 gradually increased his daily food intake after day 18 to ~1400 calories by day 42 (Fig. 3A-B). Both MVL baboons directly involved in pair-feeding, EXP 3: ID 27677 and EXP 4: ID 28420, lost an average of 7.3% of their total body weight at 6 wks post-op, while their pair-fed Sham control baboons, ID 25358 and ID 26103, lost 2.5% (Fig. 3C). Likewise, fat mass (−14%) (Fig. 3D), percentage fat (Fig. 3E) and waist circumference (−9.5%) (Fig. 3F) were all decreased following MVL, while these measures were unchanged in Sham animals. Metabolically, experimental baboons 3 and 4 who underwent MVL had markedly enhanced Rd,198% improvement, while pair-fed sham controls demonstrated a more modest 12.4% increase from baseline (Fig. 4A). MVL also tended to reduce insulin levels (Fig. 4C) and HOMA-IR (Fig. 4B), while increasing adiponectin (Fig. 4E) at 6 wks follow up. Meanwhile leptin levels (Fig. 4D) and triglyceride levels (not shown) were relatively unaffected.
Figure 3 Legend. Phenotypic characteristics of pair-fed animals assigned to Sham or MVL.
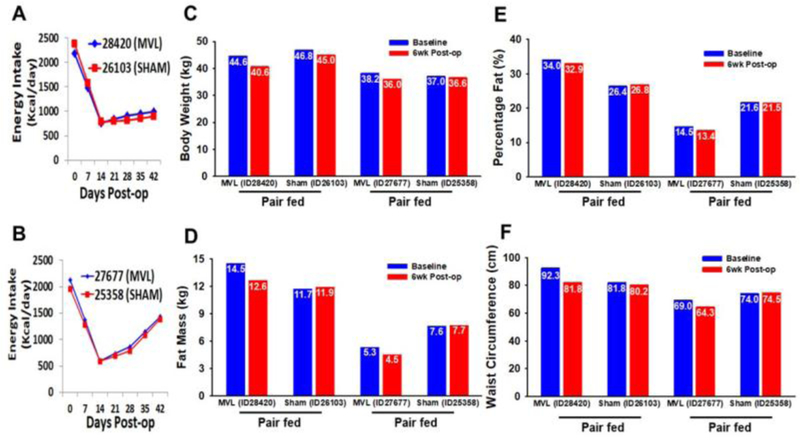
(A-B) To control for changes in food intake after MVL, Sham-operated animals (n=2) were pair-fed to animals assigned to MVL (n=2) over a 6wk period. (C-F) Body weight, fat mass, percentage fat, and waist circumference were all reduced in both animals 6 wks following MVL, but not in Sham controls.
Figure 4 Legend. Metabolic characteristics of pair-fed animals assigned to Sham or MVL.
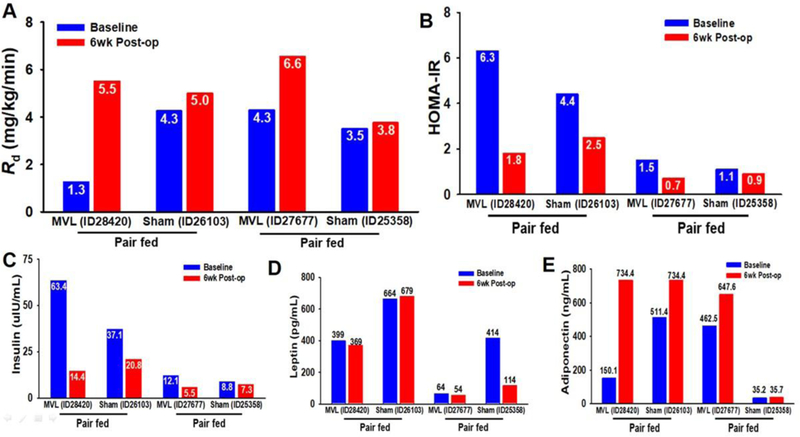
(A) Insulin action in male, insulin-resistant baboons was assessed at baseline and at 6 wks follow-up by hyperinsulinemic-euglycemic clamps, which demonstrated markedly enhanced Rd (198% improvement) following MVL, while pair-fed controls only demonstrated an increase of 12.4% from baseline. (B-C) Circulating insulin levels in pair-fed MVL vs Sham obese insulin-resistant baboons. MVL tended to reduce insulin levels and HOMA-IR in previously insulin-resistant animals. (D) Circulating leptin levels, which is predominantly produced by SC fat, were unaffected by MVL at follow-up. (E) Circulating adiponectin levels, which is a favorable marker of metabolic health, were increased at 6 wks follow up in both animals assigned to the MVL procedure.
DISCUSSION
Here, we have demonstrated that safe and effective removal of MF can be achieved in obese insulin-resistant baboons using TLT. We were able to remove up to 75–80% of all visible adipose tissue in the mesentery without complications, which we estimate to be ~40% of total VF stores, based upon prior estimates of abdominal fat mass in baboons (36). To our knowledge, this is the first report of significant dipose tissue removal from the mesentery in any mammalian species. Importantly, the MVL procedure reversed insulin resistance in all 4 experimental baboons and resulted in an average 14.4% reduction in body mass (~6.6kg) at 6 wks follow up, despite removing only ~430g of fat tissue during the surgery.
It is well known that obesity increases the risk of numerous diseases and all-cause mortality (37, 38). Given that a significant proportion of fat accrual in obesity occurs in SC fat depots, investigations have examined the possibility that cardiometabolic risk factors could be improved by removing large quantities of SC adipose tissue using classic liposuction. However, these studies largely failed to observe improvements in metabolic outcomes (39, 40), including a study by Klein et al. (13) which removed >10kg of SC abdominal fat in obese subjects, but did not observe any improvement in insulin action, inflammatory markers, or cardiovascular risk factors. Likewise, studies in rodents have observed no benefit to surgically removing SC fat on insulin sensitivity (4), while insulin-resistant models engineered to expand their SC fat pads had improved metabolic profiles (41), suggesting that SC fat may be beneficial. Instead, the vast majority of population-based and observational studies accounting for body fat distribution have concluded that central obesity is the strongest risk factor for insulin resistance, T2D (42), cancer (43) and mortality (37, 38), and rodent lipectomy studies have demonstrated that VF is directly linked to these outcomes (3–5).
In an effort to translate these observations from rodent studies, investigators have attempted to determine the effect of visceral lipectomy in insulin-resistant humans. However, unlike rodents, where VF pads can be completely ablated, only the greater omentum can be safely excised in humans, as removal of adipose tissue from the mesentery and peri-renal areas has until now, not been feasible. As a result, these studies were largely ineffective at providing metabolic benefit, leading some to conclude that VF may not be important to the pathology of human insulin resistance. However, we have demonstrated here that insulin sensitivity can be dramatically improved in a non-human primate model by surgically removing large quantities of MF.
This pre-clinical trial was primarily designed as a “proof-of-concept” to demonstrate that MF can be safely and effectively targeted for removal (Phase I) with demonstrable metabolic improvement following the procedure (Phase II) in a species closely modeling human anatomical and physiological characteristics. While benchmarks for success were met or exceeded for these primary endpoints, important limitations and unanswered questions remain that will need to be addressed in future trials. First, we observed a remarkable, yet unexpected decrease in food consumption following MVL, along with dramatic reductions in body weight and adiposity, and these latter observations could not be completely accounted for by the reduction in food intake per se. Thus, future studies will need to carefully account for changes in energy balance following this procedure in order to more clearly define probable mechanism(s) mediating the insulin-sensitizing effects of MVL. Second, while we generally aimed to remove >50% of all visible MF, a careful titration of fat removal should be conducted to determine the therapeutic range and dose response of this intervention. Third, given the relatively short follow-up period and small sample size utilized here, longer duration trials in larger cohorts of animals will be needed to definitively determine efficacy, safety and sustainability of this procedure over the long-term. Along these lines, as MVL removes a major storage site for excess nutrients, to what extent, if any, this procedure may lead to complications involving ectopic fat storage in metabolically-relevant tissues should also be evaluated. Forth, while we demonstrate efficacy of MVL in an insulin resistant cohort, whether this intervention can prove as efficacious with more prolonged exposure and/or disease severity, including T2D, is unknown, nor is it clear if sex differences may play a role in this response. Collectively, addressing these important issues will be critical to inform on the optimal surgical ‘dose’, therapeutic window and patient population(s) expected to benefit from MVL in clinical trials.
CONCLUSION
We have demonstrated here that significant quantities of mesenteric visceral fat can be safely and effectively surgically excised, using Tissue Liquefaction Technology, in adult male baboons, thereby providing the first important proof-of-concept that such a surgical intervention may be feasible in humans. Importantly, this procedure produced marked metabolic benefits in previously insulin resistant male, obese baboons, reversing insulin resistance and promoting significant weight loss. This suggests that meaningful clinical benefits may be uniquely achieved with Mesenteric Visceral Lipectomy (MVL) which have otherwise failed to be observed using previous lipectomy approaches, including omentectomy and classic liposuction. Clinical trials designed to explore both the feasibility and efficacy of MVL as a strategy to treat insulin resistance and T2D in abdominally-obese humans are warranted and should be pursued.
Acknowledgments
SOURCE OF FUNDING: supported by NIH Grant R43DK112428
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith U Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest 2015;125(5):1790–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Souza LR, Kogan E, Berger H, Alves JG, Lebovic G, Retnakaran R, et al. Abdominal adiposity and insulin resistance in early pregnancy. J Obstet Gynaecol Can 2014;36(11):969–75. [DOI] [PubMed] [Google Scholar]
- 3.Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell 2008;7(3):438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 2002;51(10):2951–8. [DOI] [PubMed] [Google Scholar]
- 5.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 1999;48(1):94–8. [DOI] [PubMed] [Google Scholar]
- 6.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta 2009;1790(10):1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell 2013;152(4):673–84. [DOI] [PubMed] [Google Scholar]
- 8.Nseir W, Artul S, Nasrallah N, Mograbi J, Mahamid M. Hospitalization and 1-year all-cause mortality in type 2 diabetic patients with chronic kidney disease at Stages 1 and 2: Effect of mild anemia. J Diabetes 2015. [DOI] [PubMed]
- 9.Munoz-Rivas N, Mendez-Bailon M, Hernandez-Barrera V, de Miguel-Yanes JM, Jimenez-Garcia R, Esteban-Hernandez J, et al. Time Trends in Ischemic Stroke among Type 2 Diabetic and Non-Diabetic Patients: Analysis of the Spanish National Hospital Discharge Data (2003–2012). PLoS One 2015;10(12):e0145535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, et al. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes 2010;59(3):627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16(6):1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Corral P, Bryan DR, Garvey WT, Gower BA, Hunter GR. Dietary adherence during weight loss predicts weight regain. Obesity (Silver Spring) 2011;19(6):1177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350(25):2549–57. [DOI] [PubMed] [Google Scholar]
- 14.Robles-Cervantes JA, Martinez-Abundis E, Gonzalez-Ortiz M, Cardenas-Camarena L, Hernandez-Salazar E, Olvera-Ozuna R. Behavior of insulin sensitivity and its relation to leptin and tumor necrosis factor-alpha in obese women undergoing liposuction: 6-month follow-up. Obes Surg 2007;17(9):1242–7. [DOI] [PubMed] [Google Scholar]
- 15.Ybarra J, Blanco-Vaca F, Fernandez S, Castellvi A, Bonet R, Palomer X, et al. The effects of liposuction removal of subcutaneous abdominal fat on lipid metabolism are independent of insulin sensitivity in normal-overweight individuals. Obes Surg 2008;18(4):408–14. [DOI] [PubMed] [Google Scholar]
- 16.Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord 2002;26(2):193–9. [DOI] [PubMed] [Google Scholar]
- 17.Csendes A, Maluenda F, Burgos AM. A prospective randomized study comparing patients with morbid obesity submitted to laparotomic gastric bypass with or without omentectomy. Obes Surg 2009;19(4):490–4. [DOI] [PubMed] [Google Scholar]
- 18.Dillard TH, Purnell JQ, Smith MD, Raum W, Hong D, Laut J, et al. Omentectomy added to Roux-en-Y gastric bypass surgery: a randomized, controlled trial. Surg Obes Relat Dis 2013;9(2):269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010;139(2):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera MF, Pantoja JP, Velazquez-Fernandez D, Cabiedes J, Aguilar-Salinas C, Garcia-Garcia E, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care 2010;33(7):1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, et al. Visceral fat resection in humans: effect on insulin sensitivity, beta-cell function, adipokines, and inflammatory markers. Obesity (Silver Spring) 2013;21(3):E182–9. [DOI] [PubMed] [Google Scholar]
- 22.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes 2016;6(7):e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly MKPA. Advanced and Future Phaco Technology. In: BA H, editor. Essentials of Cataract Surgery Thorofare, NJ: SLACK Incorporated; 2007. p. 293. [Google Scholar]
- 24.Barsam A, Chandra A, Bunce C, Whitefield LA. Prospective randomized controlled trial to compare the effect on the macula of AquaLase liquefaction and ultrasound phacoemulsification cataract surgery. J Cataract Refract Surg 2008;34(6):991–5. [DOI] [PubMed] [Google Scholar]
- 25.Davis K, Rasko Y, Oni G, Bills J, Geissler P, Kenkel JM. Comparison of adipocyte viability and fat graft survival in an animal model using a new tissue liquefaction liposuction device vs standard Coleman method for harvesting. Aesthet Surg J 2013;33(8):1175–85. [DOI] [PubMed] [Google Scholar]
- 26.Mackool RJ, Brint SF. AquaLase: a new technology for cataract extraction. Curr Opin Ophthalmol 2004;15(1):40–3. [DOI] [PubMed] [Google Scholar]
- 27.Borab ZM, Godek CP. Tissue Liquefaction Liposuction for Body Contouring and Autologous Fat Transfer: A Retrospective Review Over 3 Years. Eplasty 2016;16:e36. [PMC free article] [PubMed] [Google Scholar]
- 28.Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, et al. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res 2003;11(1):75–80. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 30.Allsop JR, Wolfe RR, Burke JF. The realiability of rates of glucose appearance in vivo calculated from constant tracer infusions. The Biochemical journal 1978;172(3):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chavez AO, Lopez-Alvarenga JC, Tejero ME, Triplitt C, Bastarrachea RA, Sriwijitkamol A, et al. Physiological and molecular determinants of insulin action in the baboon. Diabetes 2008;57(4):899–908. [DOI] [PubMed] [Google Scholar]
- 32.Bastarrachea RA, Veron SM, Vaidyanathan V, Garcia-Forey M, Voruganti VS, Higgins PB, et al. Protocol for the measurement of fatty acid and glycerol turnover in vivo in baboons. J Lipid Res 2011;52(6):1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidyanathan V, Bastarrachea RA, Higgins PB, Voruganti VS, Kamath S, DiPatrizio NV, et al. Selective cannabinoid-1 receptor blockade benefits fatty acid and triglyceride metabolism significantly in weight-stable nonhuman primates. American journal of physiology Endocrinology and metabolism 2012;303(5):E624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van As A, Lombard F. Body surface area of the Chacma baboon (Papio ursinus). Growth 1981;45(4):322–8. [PubMed] [Google Scholar]
- 35.Standards of medical care in diabetes−−2010. Diabetes Care 2010;33 Suppl 1:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni T, Slaughter G, Ego-Osuala C, Kochunov P, Bastarrachea RA, Mattern V, et al. Hyperglycemic Challenge and Distribution of Adipose Tissue in Obese Baboons. Int J Diabetol Vasc Dis Res 2014;2(1). [PMC free article] [PubMed] [Google Scholar]
- 37.Rissanen A, Heliovaara M, Knekt P, Aromaa A, Reunanen A, Maatela J. Weight and mortality in Finnish men. J Clin Epidemiol 1989;42(8):781–9. [DOI] [PubMed] [Google Scholar]
- 38.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. Jama 1999;282(16):1530–8. [DOI] [PubMed] [Google Scholar]
- 39.Enzi G, Cagnoni G, Baritussio A, De Biasi F, Favaretto L, Inelmen EM, et al. Effects of fat mass reduction by dieting and by lipectomy on carbohydrate metabolism in obese patients. Acta Diabetol Lat 1979;16(2):147–56. [DOI] [PubMed] [Google Scholar]
- 40.Lambert EV, Hudson DA, Bloch CE, Koeslag JH. Metabolic response to localized surgical fat removal in nonobese women. Aesthetic Plast Surg 1991;15(2):105–10. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117(9):2621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 2004;159(12):1150–9. [DOI] [PubMed] [Google Scholar]
- 43.Yamaji T, Iwasaki M, Sasazuki S, Kurahashi N, Mutoh M, Yamamoto S, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol 2009;170(12):1502–11. [DOI] [PubMed] [Google Scholar]


