Abstract
For the past two decades, a great deal of research has been published concerning adiponectin (APN), an abundant protein responsible for regulating numerous biologic functions including antioxidative, antinitrative, anti-inflammatory, and cardioprotective effects. A review of APN and its two major receptors is timely because of new findings concerning the mechanisms by which APN signaling may be altered in pathologic processes such as diabetes and heart failure. In this review we elaborate on currently known information regarding the physiologic role of APN and the known mechanisms underlying pathologic APN resistance – namely, APN receptor downregulation and phosphorylation – and provide insight regarding the future directions of APN research including an assessment of the clinical applicability of preventing pathologic post-translational modification of the APN receptor.
Newly Identified Mechanisms of APN Resistance Hold Therapeutic Potential
APN is a protein hormone modulating numerous metabolic processes. In this review we briefly elaborate currently known information about APN and its receptors, discuss in detail the known mechanisms underlying pathologic APN resistance (namely, APN receptor downregulation and phosphorylation), and provide insight regarding the future of APN research stemming directly from cardiovascular APN resistance concepts. A review of APN and its two major receptors is timely because of new research findings concerning the mechanisms by which APN signaling may be deranged in diabetes and heart failure. Novel pathologic mechanisms identified involving post-translational modification of the APN receptor hold great therapeutic potential to restore APN’s cardioprotection in such disease processes.
APN, APN Receptors, and APN Cardiovascular Protection
A Brief Review of APN, Its Structure, and Epidemiology
First described in 1995, APN was first termed Acrp30, as it was an adipocyte complement-related protein of 30 kDa in size [1]. The protein received the names AdipoQ, apM1, and GBP28 from three different researchers who isolated it independently [2–4]. The human APN gene exists on chromosome 3q26, a region associated with type 2 diabetes mellitus and metabolic syndrome susceptibility [5].
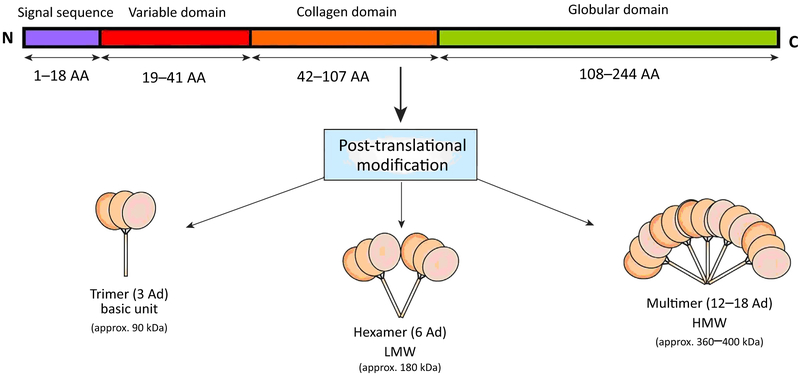
Modulating metabolic processes such as glucose regulation and fatty acid (FA) catabolism, APN has a primary sequence of 244 amino acids. It contains a signal sequence (which targets the protein for extracellular section and is cleaved from the mature peptide) and a non-conserved N-terminal domain, followed by 22 collagen repeats and a C-terminal globular domain (Figure 1). Full-length APN (fAPN) requires post-translational modification for biologic activity (e.g., hydroxylation, glycosylation). Leukocyte elastase (secreted by activated monocytes and neutrophils) cleaves full-length APN (fAPN) and generates globular APN (gAPN). The high molecular weight (HMW) isoform is the biologically active form in the liver. The ratio of HMW to total APN isoform quantity (the SA index) correlates to both insulin and APN sensitivity. Mutations of the APN gene (Arg112Cys and Ile164Thr) preventing trimer assemblage cause impaired cellular secretion and are clinically associated with hypoadiponectinemia, a state of low circulating APN concentration compared with baseline [6, 7]. Gly84Arg and Gly90Ser mutants can assemble into trimers and hexamers but are unable to form HMW multimers, resulting in clinical diabetes [7] and contributing more evidence to isoform specificity in insulin sensitization.
Figure 1. Structure of Adiponectin (APN) (Human).
Full-length APN (fAPN) requires post-translational modifications (e.g., hydroxylation, glycosylation) for its activity. APN molecules are secreted from adipocytes as trimers (~90 kDa; the basic unit), low molecular weight (LMW) hexamers (~180 kDa), and high molecular weight (HMW) isoforms (12–18-mer; >400 kDa). AA, amino acid (length); N, amino terminus; C, carboxy terminus.
At a circulatory concentration of 0.5–30 μg/ml, APN comprises approximately 0.01 % of all plasma proteins [1], thereby exceeding other adipokines 100-fold and most hormones threefold. Cnop and colleagues report APN concentrations to be significantly higher in women than men (7.4 ± 2.9 vs 5.4 ± 2.3 μg/ml, P < 0.000 1, 182 total subjects) [8]. Women in general have greater physiologic adipose tissue levels than men, but this gender difference in APN level has also been attributed to the inhibitory effect of testosterone on APN secretion by epidemiological puberty data [9, 10], reaffirmed in hormonal replacement therapy in male-to-female transgender studies [11]. Males harbor a predominance of the low molecular weight (LMW) APN isoform whereas females have a more balanced distribution of the LMW and HMW forms [12].
The origin of APN is primarily white adipose tissue but also osteoblasts, skeletal muscle, and cardiomyocytes. Cardiomyocyte-derived APN is biologically active and contributes to physiologic function [13]. Contrary to expectations given its major production source, APN levels are inversely correlated with body fat percentage in adults (in stark contrast with leptin levels, which are proportional to adipose mass). fAPN or gAPN decreases free FA release in the postprandial state. Systemic insulin sensitivity and plasma APN levels are positively correlated and APN augments post-prandial insulin’s suppression of hepatic glucose output [14]. In summary, APN exerts significantly positive metabolic effects on the body.
A Brief Review of Known APN Receptors
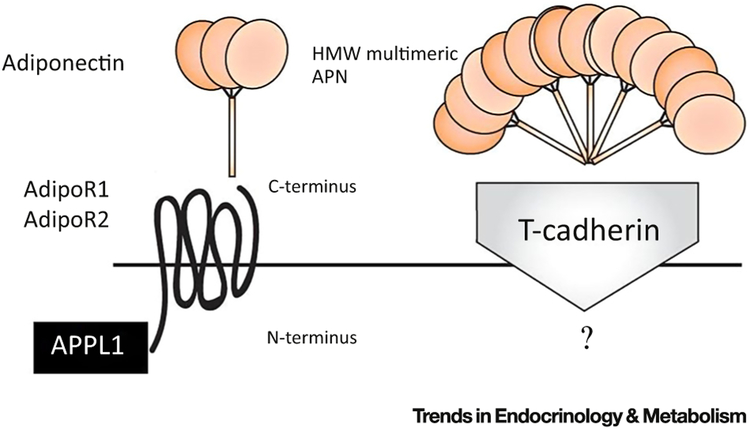
APN regulates cellular function via the binding and activation of its specific receptors APN receptor 1 (AdipoR1) and APN receptor 2 (AdipoR2), each encoded by their respectively named genes. The APN receptors belong to a family of membrane receptors structurally predicted to contain seven transmembrane domains but are topologically distinct from G protein-coupled receptor (GPCR) proteins. APN binds to the C-terminal extracellular domain of the APN receptor, whereas the N-terminal cytoplasmic domain interacts with an adaptor protein, APPL1 (Figure 2). AdipoR1 is abundantly expressed in skeletal muscle and endothelial cells and AdipoR2 is predominantly expressed in the liver [15]. Both AdipoR1 and AdipoR2 are constitutively expressed in adult cardiomyocytes and have different affinities for various APN isoforms [15, 16].
Figure 2. Adiponectin (APN) and the APN Receptor.
APN interacts with the extracellular C terminus region of the APN receptor, which spans the membrane in seven domains with its intracellular N terminus interacting with the adapter protein APPL1. T-cadherin is postulated to be a receptor for multimeric high molecular weight (HMW) APN isoforms with as-yet-unknown biologic functions. Adi-poR1/R2, APN receptor 1/2.
In addition to the two APN receptors, T-cadherin is a tethering molecule for the hexameric and HMW APN forms. T-cadherin binds APN in C2C12 myoblasts but not in hepatocytes [17]. However, as T-cadherin lacks an intracellular domain, the specific biologic function of any APN–T-cadherin interaction remains unknown. Calreticulin (CRT), a multifunctional chaperone protein that binds calcium ions, in concert with its adaptor protein CD91, has been identified by Walsh’s group [18, 19] to be yet another receptor for APN, responsible for endothelial anti-inflammatory and vasculoprotective effects. Walsh’s group has acknowledged that CRT–CD91 does not constitute a classical receptor pathway, but, in addition to its overwhelming serum abundance, APN is not a typical ligand, with its unique property of higher-order oligomerization [20] (Figure 2).
Relationship between Hypoadiponectinemia and Cardiac Injury
Numerous epidemiological studies have revealed the correlation of reduced APN levels with increased cardiovascular disease risk [21–23], a relationship that persists even after adjustment for diabetes, dyslipidemia, hypertension, smoking, and body mass index (BMI) [24, 25]. Increased plasma APN concentrations are associated with a lower risk of myocardial infarction (MI) (colloquially, a heart attack) in men [26, 27]. Ethnic disparities in APN levels might explain the predilection in African-Americans and those of South Asian descent for increased type 2 diabetes and coronary artery disease [28, 29]. Persistently low plasma APN concentration after acute MI is predictive of future adverse cardiac events [30, 31]. Clinical observations have demonstrated that post-MI plasma APN levels correlate positively with myocardial salvage index and ejection fraction recovery [32, 33]. Thus, hypoadiponectinemia is recognized as a risk factor for cardiovascular disease.
Experimental studies have generated direct evidence supporting the link between hypoadiponectinemia and cardiomyocyte injury. In vitro studies have demonstrated that APN promotes cell survival and inhibits cell death, suggesting that APN may have direct cardioprotective effects in addition to its vasculoprotective properties, which indirectly shield cardiomyocytes from ischemia–reperfusion (IR) injury. APN knock-out (APNKO) mice manifest exacerbated myocardial IR (MIR) injury compared with control mice, as evidenced by increased myocardial apoptosis and infarct size and decreased cardiac function [34, 35]. Compared with controls, MIR injury is increased in heterozygous APNKO mice (+/−), in which circulating APN levels are reduced by approximately half, with significantly reduced severity compared with homozygous knockout animals [35]. This finding suggests that APN levels are not only inversely correlated with the risk of development of ischemic heart disease (as indicated by available human epidemiological data), but also inversely related to the severity of a MIR insult. Provision of fAPN or gAPN in a dose-dependent manner ameliorates exacerbated MIR injury in APNKO mice [35–37]. A more recent study demonstrated that administration of AdipoRon, an orally active APN receptor activator, effectively attenuated post-ischemic cardiac injury, supporting the notion that treatment of cardiovascular complications caused by obesity-related disorders (such as type 2 diabetes) is possible with APN or APN analogs [37].
APN Resistance in Cardiovascular Disease
Although reduced APN circulation level is a recognized risk factor for diabetic cardiovascular complications, emerging experimental and clinical evidence demonstrates that, besides hypoadiponectinemia, reduced biologic response to APN (APN resistance) develops in metabolically active organs including adipocytes, skeletal muscle, liver, the vasculature, and the heart, contributing to diabetic cardiovascular injury.
Diabetes, Obesity, Metabolic Syndrome, and APN Resistance
APN metabolically mimics insulin, promoting glucose uptake and inhibiting hepatic glucose production [38–40]. Diets high in fats impair the insulin-signaling cascade by incremental skeletal muscle lipid accumulation [41, 42]. Animals subjected to high-fat diets manifest APN resistance, demonstrating blunted FA oxidation in response to APN administration, in addition to decreased maximal insulin-stimulated glucose transport [43, 44]. Mullen’s later studies investigating high-fat-fed rats reveal temporal relevance and importance for APN resistance; the loss of APN’s stimulatory effect on FA oxidation preceded the increase in plasmalemmal FA transporters. Through the resultant accumulation of intramuscular diacylglycerol (DAG) and ceramide, insulin signaling was consequently blunted, and maximal insulin-stimulated glucose transport in skeletal muscle impairment occurred after the onset of APN resistance [44]. When studying obese patients manifesting no significant insulin resistance, Bruce and colleagues discovered their subjects had deficient serum APN levels. Furthermore, blunted AMP-activated protein kinase (AMPK) activation in response to exogenous gAPN in obese muscle further suggested the development of APN resistance in obesity [45]. Taking these findings together, APN system derangement is likely to play a central role in obesity or high-fat-diet-induced insulin resistance.
Vascular System and APN Resistance
APN exerts anti-inflammatory and antiatherogenic (fatty plaque deposition) properties by stimulating endothelial nitric oxide (NO) production. NO is a soluble gas produced by the endothelium that maintains vascular homeostasis (including regulating vasodilation and local cell growth) and normal endothelial function. Obesity and hyperlipidemia alter the vascular response to APN by multiple mechanisms. Plasma APN level in animals fed high-fat diets peaked by 8 weeks and declined rapidly thereafter. Regardless of circulatory APN levels, phosphorylated AMPK and endothelial nitric oxide synthase (eNOS) in vascular tissue remained significantly reduced at all observed time points. Phosphorylation of AMPK (an important cellular protein regulating energy homeostasis) and eNOS (responsible for NO production) leads to their activation and activity. Recombinant full-length APN (rAPN)-induced AMPK/eNOS phosphorylation and vasodilatation were significantly reduced in 16-week obese/hyperlipidemic aortic segments [46, 47].
IR Injury and APN Resistance
Many basic science studies have demonstrated that APN reduces MIR injury [48, 49]. In the diabetic state (in which endogenous APN levels are decreased), APN has reduced cardioprotective effects. High-fat-diet-induced obesity/diabetic mice and age-matched control mice on a normal diet (ND) were subjected to MIR and were treated with gAPN before reperfusion. Compared with ND mice, high-fat-diet mice endured greater MIR injury. A nearly threefold-greater gAPN dose was required to achieve significant cardioprotection comparable with that observed in ND mice treated with a standard APN dose. High-fat-diet-induced diabetes diminished both the AMPK-dependently and AMPK-independently mediated cardioprotective effects of gAPN, suggesting APN resistance in the diabetic heart [50].
Heart Failure and APN Resistance
Hypoadiponectinemia during diabetes is correlated with increased acute MI risk [51, 52] as well as worse cardiac functional recovery after reperfusion (MIR) [53–55]. However, the role of APN in heart failure is controversial. Given the known beneficial effects of APN, increased APN levels are observed clinically during worsening heart failure – the opposite of expectations. Clinical observations demonstrate that increased plasma APN levels are associated with poor cardiac function, symptomatic clinical status, and high mortality in this patient population [56–58]. Moreno et al. employed a Mendelian randomization technique to evaluate this counterintuitive data and demonstrated that the paradoxical association between high plasma APN level and increased cardiovascular congestive heart failure (CHF) mortality is based on a cause–effect relationship, suggesting an unexpected deleterious role for APN’s action on metabolism and atherosclerotic processes [59–61]. CHF failure is a complex disease, often a consequence of MI, when the heart is unable to effectively pump blood to meet physiologic demands. However, several recent experimental studies demonstrate that APN deficiency significantly exacerbates the progression of heart failure [59, 60]. Clinical studies demonstrate that the biologic response to APN is significantly blunted in liver and skeletal tissues from advanced heart failure patients [62, 63]. Moreover, recent clinical studies demonstrate that exercise training enhances APN sensitivity in the skeletal muscle of HF patients [64] and reduces circulating APN levels. Together these results suggest that augmented APN production in advanced heart failure patients is likely to be a compensatory mechanism due to the development of ‘APN resistance’ similar to hyperinsulinemia during insulin resistance. To obtain direct supporting evidence, APNKO, AdipoR1KO, and AdipoR2KO mice were subjected to MI. Both the severity of the post-MI heart failure and the animals’ response to APN treatment were determined. Post-MI heart failure was significantly exacerbated in AdipoR1KO mice to an extent comparable with that observed in APNKO mice. More significantly, the cardioprotective effect of APN was abolished in AdipoR1KO mice. By contrast, the severity of post-MI remodeling and cardiac dysfunction was only modestly enhanced in AdipoR2KO mice, with the cardioprotective effect of APN preserved (Y. Wang, unpublished). These results are consistent with the fact that AdipoR1 is the primary APN receptor isoform in skeletal/cardiac muscles, whereas AdipoR2 is predominantly expressed in the liver. Moreover, a recent study demonstrated that AdipoR1 is expressed independently on the cell surface whereas AdipoR2 must form heterodimers with AdipoR1 to be expressed on the cell surface, because the nonconserved AdipoR2 N-terminal region prevents its cell surface expression [65].
The Molecular Mechanisms of APN Resistance
We have delineated multiple pieces of evidence demonstrating the blunted biologic effect of APN against disease processes such as IR injury and heart failure in the diabetic condition. Next we discuss the etiology of this loss of response and dissect the known underlying mechanisms responsible.
APN Receptor Downregulation
Both AdipoR1 and AdipoR2 expression in skeletal muscle and adipose tissue correlate with insulin level [66, 67]. Receptor expression is reduced in diabetic mouse models. Additionally, downregulation of APN receptor expression is one of the mechanisms by which obesity/hyperlipidemia alters the vascular response to APN [68]. After 16 weeks of high-fat diet, vascular AdipoR1 and AdipoR2 expression was significantly reduced. Decreased vascular AdipoR1/R2 expression and reduced circulating APN levels contributed to vascular APN resistance at varying stages of obesity [68].
Whereas circulating APN levels increase in proportion with the severity of heart failure, AdipoR1 levels decrease in patients with reduced left ventricular ejection fraction [69, 70]. In an interesting study investigating specifically APN and APN receptors, CHF patients exhibited elevated plasma APN levels but very low myocardial APN expression. Furthermore, an miRNA was identified (mir-150) that specifically repressed AdipoR2 expression in this patient population [71]. Accumulating reports demonstrate that APN resistance is related to decreased APN receptor expression, reduced receptor sensitivity, and dysfunctional downstream signaling. Table 1 tabulates investigations concerning the etiology of APN resistance pertaining specifically to alterations of APN receptor content.
Table 1.
Investigations of the Etiology of APN Resistance Pertaining to APN Receptor Change
Basic science studies have demonstrated that expression levels of AdipoR1 and AdipoR2 are unchanged 14 days after MI but significantly reduce 4 weeks post-MI and thereafter, suggesting that receptor alteration may be responsible for cardiac APN resistance at a late stage (such as in chronic heart failure).
APN Receptor Phosphorylation
It bears repeating that the expression levels of AdipoR1 and AdipoR2 are significantly reduced 4–8 weeks post-MI. Therefore, receptor downregulation is a plausible explanation for APN resistance in disease processes of a chronic nature (e.g., diabetes/metabolic syndrome [72], CHF). APN receptor downregulation has been identified in disease processes outside the cardiovascular system, including allergic/immunologic [73] and renal disease [74]. However, as noted above, there is significant evidence from both basic science and clinical investigations that APN resistance occurs in pathologic instances at times much earlier than the onset of receptor downregulation, indicating that other responsible mechanisms exist.
As described above, the APN receptors belong to a new family of membrane receptors (the PAQRs) predicted to contain seven transmembrane domains, but are structurally and topologically distinct from GPCRs. APN binds the C-terminal extracellular domain of APN receptors whereas the N-terminal cytoplasmic domain interacts with multiple intracellular molecules such as APPL1, APPL2, and neutral ceramidase (nCDase), transferring APN signaling. It is well recognized that β-adrenoceptors (β-ARs), a type of GPCR, are altered in the failing heart. β-ARs are desensitized in a process known as phosphorylation, contributing to the progression of heart failure [67]. In a recent study, Wang et al. provided the first direct evidence that AdipoR1 is phosporylatively modified in the failing heart, contributing to post-MI cardiac remodeling and heart failure development [75]. While AdipoR1 expression remained unchanged within 7 days of ischemic injury, AdipoR1 phosphorylation at serine and threonine residues was detectable as early as 1 day post-MI, reaching statistical significance 3 days post-MI. Peak cardiac AdipoR1 phosphorylation occurred 7–14 days post-MI, a time period notably coinciding with the period of significant APN resistance onset after ischemia, the critical transitional period when adaptation to pathologic remodeling occurs. The phosphorylation of these residues was highly associated with GRK2 overexpression in the remote regions of hearts subjected to permanent ischemia. More importantly, GRK2 knockout blocked AdipoR1 phosphorylation in the failing heart, whereas GRK2 overexpression potentiated AdipoR1 phosphorylation. These results indicate that GRK2 is the primary kinase phosphorylating AdipoR1 in the failing heart. Mass spectrophotometric analysis identifed that Ser7, Thr24, and Thr53 are sensitive to/selective for GRK2 phosphorylation (Table 2). Importantly, similar to functional impairment of β-ARs by phosphorylation [76], AdipoR1 phosphorylation at serine/threonine residues results in receptor desensitization, blocking the antioxidative, antinitrative, anti-inflammatory, and cardioprotective function ofAPN. Collectively, strong evidence exist supporting GRK2-mediated AdipoR1 phosphorylation with resultant impaired APN signaling as a significant risk factor contributing to cardiometabolic dysfunction, the inflammatory response, and heart failure progression, a process exacerbated in the diabetic condition.
Table 2.
Bioinformatics Analysis of Phosphorylation Sites in AdipoR1 and AdipoR2
| AdipoR1 | |||
|---|---|---|---|
| Position | Code | Kinase | Peptide |
| 2 | S | AGC/GRK | ******MSSHKGSAG |
| 2 | S | AGC/GRK/BARK | ******MSSHKGSAG |
| 2 | S | AGC/GRK/BARK/GRK-2 | ******MSSHKGSAG |
| 3 | S | AGC/GRK | *****MSSHKGSAGA |
| 3 | S | AGC/GRK/BARK/GRK-2 | *****MSSHKGSAGA |
| 3 | S | AGC/GRK/GRK/GRK-1 | *****MSSHKGSAGA |
| 3 | S | AGC/GRK/GRK/GRK-5 | *****MSSHKGSAGA |
| 7 | S | AGC/GRK | *MSSHKGSAGAQGNG |
| 7 | S | AGC/GRK/GRK/GRK-5 | *MSSHKGSAGAQGNG |
| 17 | S | AGC/GRK | AQGNGAPSGNREADT |
| 43 | S | AGC | EKGKRAASSPAKAEE |
| 43 | S | AGC/PKA | EKGKRAASSPAKAEE |
| 43 | S | AGC/GRK/BARK/GRK-3 | EKGKRAASSPAKAEE |
| 43 | S | AGC/GRK/GRK/GRK-1 | EKGKRAASSPAKAEE |
| 197 | S | AGC/GRK/GRK/GRK-4 | FHTVYCHSEKVSRTF |
| 201 | S | AGC/GRK/GRK/GRK-1 | YCHSEKVSRTFSKLD |
| 205 | S | AGC/PKA | EKVSRTFSKLDYSGI |
| 205 | S | AGC/GRK/GRK/GRK-5 | EKVSRTFSKLDYSGI |
| 240 | S | AGC/GRK/GRK/GRK-5 | QPRLIYLSIVCVLGI |
| 260 | T | AGC/GRK/GRK/GRK-5 | AQWDRFATPKHRQTR |
| 266 | T | AGC/PKA | ATPKHRQTRAGVFLG |
| 277 | S | AGC/GRK/GRK/GRK-1 | VFLGLGLSGVVPTMH |
| 370 | T | AGC/GRK | YGLEGGCTDDSLL** |
| 370 | T | AGC/GRK/BARK | YGLEGGCTDDSLL** |
| 370 | T | AGC/GRK/GRK | YGLEGGCTDDSLL** |
| 370 | T | AGC/GRK/BARK/GRK-2 | YGLEGGCTDDSLL** |
| 373 | S | AGC | EGGCTDDSLL***** |
| 373 | S | AGC/GRK | EGGCTDDSLL***** |
| 373 | S | AGC/GRK/BARK | EGGCTDDSLL***** |
| 373 | S | AGC/GRK/GRK | EGGCTDDSLL***** |
| 373 | S | AGC/GRK/BARK/GRK-2 | EGGCTDDSLL***** |
| 373 | S | AGC/GRK/GRK/GRK-1 | EGGCTDDSLL***** |
| AdipoR2 | |||
| Position | Code | Kinase | Peptide |
| 30 | T | AGC/GRK/GRK/GRK-4 | KGHQLDDTRGSNNDN |
| 33 | S | AGC/GRK/BARK | QLDDTRGSNNDNYQG |
| 33 | S | AGC/GRK/BARK/GRK-2 | QLDDTRGSNNDNYQG |
| 45 | S | AGC/GRK | YQGDLEPSLETPVCS |
| 45 | S | AGC/GRK/GRK | YQGDLEPSLETPVCS |
| 45 | S | AGC/GRK/GRK/GRK-4 | YQGDLEPSLETPVCS |
| 69 | S | AGC/GRK | PECHDDNSQEDEGFM |
| 69 | S | AGC/GRK/BARK | PECHDDNSQEDEGFM |
| 173 | S | AGC/GRK/GRK | YMFRPNISFVAPLQE |
| 173 | S | AGC/GRK/GRK/GRK-1 | YMFRPNISFVAPLQE |
| 208 | S | AGC/GRK/GRK/GRK-4 | FHTVYCHSEGVSRLF |
| 216 | S | AGC/GRK/GRK/GRK-5 | EGVSRLFSKLDYSGI |
| 381 | T | AGC/GRK/BARK | FMIGGGCTFFDAL** |
| 381 | T | AGC/GRK/GRK | FMIGGGCTFFDAL** |
| 381 | T | AGC/GRK/BARK/GRK-2 | FMIGGGCTFFDAL** |
| 381 | T | AGC/GRK/GRK/GRK-1 | FMIGGGCTFFDAL** |
Bioinformatics analysis provides information concerning the amino acid residues susceptible to phosphorylation in the APN receptors (AdipoR1 and AdipoR2). Both the activated kinase and kinase substrate peptide sequences are presented. S, serine; T, threonine;
omitted amino acid.
Concluding Remarks and Future Directions
It is clear that APN is an adipocyte-derived hormone with diverse biologic functions in multiple organ systems including the vascular tissue and heart. However, many studies addressing unanswered critical questions are ongoing in this field. These questions include (but are not limited to): why multiple APN isoforms exist in the circulation; why circulating gAPN concentrations are extremely low but have a biologic potency markedly exceeding that of other isoforms; whether it is possible that HMW APN functions as a ‘storage isoform’ in the circulation that is locally reduced/cleaved to produce gAPN for biologic function; which enzymes reduce and cleave HMWAPN; whether there is an isoform-specific cardiovascular regulatory function; and whether there is isoform/receptor-selective intracellular signaling regulation of APN’s cardiovascular functions (see Outstanding Questions).
Outstanding Questions.
Does APN resistance occur in every organ system? If so, are the underlying mechanisms similar? Comprehension of any existing divergent mechanisms may open exciting opportunities for therapeutic intervention in various systemic disorders.
What are the resultant specific biologic signaling sequelae of differently phosphorylated residues within the APN receptor protein? How is each of these signaling pathways regulated? Testing the resultant signaling of various phosphorylated APN receptor forms may lead to unknown science and answers to questions we do not yet know to ask.
How can we pharmacologically dephosphorylate the APN receptor to maintain its normal state during pathologic periods? Given the potential promising clinical applicability, exploitation of this pathologic post-translational modification of the APN receptor protein may yield significant clinical benefit for many patients in need.
Moreover, it is generally accepted that, similar to insulin resistance, APN resistance develops in obesity, diabetes, and heart failure. However, whether the same or different molecular mechanisms are involved in the development of APN resistance in different cell types under different pathologic conditions requires clarification. Additionally, basic experimental studies and clinical observations demonstrate that APN receptors are downregulated in pathological conditions, impairing APN signaling. However, the underlying molecular mechanisms responsible for such receptor downregulation remain unknown. Similarly, whether interventions restoring APN receptor expression may protect against diabetic cardiovascular complications are currently hot topics of investigation, due to the significant inherent translational potential.
Finally, most recent studies have demonstrated that AdipoR1 is phosphorylated in the failing heart. This receptor phosphorylation results in loss of responsiveness to APN. However, many unanswered questions warrant further investigation. Although Ser7, Thr24, and Thr53 are sensitive to/selective for GRK2 phosphorylation, it remains to be determined which residue’s phosphorylation is responsible for receptor desensitization, and whether one, two, or all three residues must be phosphorylated to maximally block APN signaling remains unknown. This has important implications for the design of a clinically applicable therapeutic. Any effective therapeutic agent must prevent the phosphorylation of residues within the receptor protein leading to its desensitization. Whether the agent must block one, two, or all three amino acid residues from alteration must be determined through basic science studies with clear clinical relevancy. Obtaining a clear answer to these questions is scientifically important, enhancing our understanding of APN receptor biology, but may also help us develop specific interventions that selectively enhance APN metabolic and/or anti-inflammatory signaling under non-diabetic and/or diabetic conditions.
It is well known that AdipoR1 phosphorylation inhibits APN signaling, albeit via unknown molecular mechanisms. Multiple possibilities exist. First, AdipoR1 is internalized in a ligand-independent fashion [77]. AdipoR1 phosphorylation may increase its internalization, thereby blocking its function, similar to β-AR. Second, multiple AdipoR1 intracellular binding proteins, including APPL1, nCDase, Cav3, activated protein kinase C1 (RACK1), and protein kinase CK2 β subunit (CK2β) have been identified. The interactions between these proteins and AdipoR1 are respectively required in APN-mediated metabolic regulation, anti-inflammation, and cellular protection [78–80]. The binding of APPL2 to AdipoR1, by contrast, inhibits APN signaling. AdipoR1 phosphorylation may plausibly alter one or more of these interactions in a manner blocking APN function. Third, APN exerts an antioxidative function in a cAMP-initiated, PKA-dependent fashion [81]. As PAQR5, 7, and 8 interact with G proteins [82], it is possible that G proteins may influence AdipoR1 signaling. Similar to β-AR, phosphorylation of AdipoR1 may alter its affinity, recruiting β-arrestin [83] and thereby blocking AdipoR1 function. As the ultimate goal of experimental investigation is to advance our understanding of human disease and enhance human health, it is of great importance to determine whether pharmacologic or genetic interventions capable of blocking AdipoR1 phosphorylation will reestablish the cardiometabolic regulatory, anti-inflammatory, and cardioprotective effects of APN in the failing heart, particularly in diabetic conditions.
In the past decade, our comprehension of APN biology and signaling in the cardiovascular system has seen great advances. Knowledge gaps remain and represent opportunities for novel work seeking improved therapeutics against the cardiovascular complications of diabetes.
Trends.
Adiponectin (APN) resistance is multifactorial, with origins from metabolic dysfunction leading to derangement of intracellular signaling.
Recent evidence demonstrates the integral role that APN receptor 1 (AdipoR1) plays in APN resistance. Pathologic modification of AdipoR1 disrupts its downstream signaling function, contributing to ineffectual increased APN expression and, ultimately, APN resistance in the heart failure and diabetic disease states.
Inhibition of APN receptor phosphorylation may hold promise as a novel therapeutic target against the development of heart failure.
Acknowledgments
This work is supported by the following grants: NIHHL-096686, HL-123404, American Diabetes Association 1-15-BS-122 (X.L.M.), and American Diabetes Association 1-14-BS-228 (Y.W.).
Abbreviation list
- AMPK
AMP-activated protein kinase
- APN
adiponectin
- APPL1
Adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1
- CHF
congestive heart failure, or heart failure (HF)
- eNOS
endothelial nitric oxide synthase
- FA
fatty acid
- fAPN
full length adiponectin
- gAPN
globular domain of adiponectin
- HMW
high molecular weight isoform (of adiponectin)
- Hypoadiponectinemia
state of decreased adiponectin concentration compared to baseline
- KO
knock-out, genetic deletion
- LMW
low molecular weight isoform (of adiponectin)
- MI
myocardial infarction
- MI/R injury
myocardial infarction/reperfusion injury
- NO
nitric oxide
References
- 1.Scherer PE et al. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 2.Hu E et al. (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem 271, 10697–10703 [DOI] [PubMed] [Google Scholar]
- 3.Maeda K et al. (2012) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). Biochem. Biophys. Res. Commun. 425, 556–559 [DOI] [PubMed] [Google Scholar]
- 4.Nakano Y et al. (1996) Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem 120, 803–812 [DOI] [PubMed] [Google Scholar]
- 5.Al Khaldi R et al. (2015) Associations of TERC single nucleotide polymorphisms with human leukocyte telomere length and the risk of type 2 diabetes mellitus. PLoS One 10, e0145721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M et al. (2015) Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes. J. Biol. Chem 290, 10143–10148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waki H et al. (2003) Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem 278, 40352–40363 [DOI] [PubMed] [Google Scholar]
- 8.Cnop M et al. (2003) Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46, 459–469 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Jaramillo P (2016) Ssa 04–3 leptin/adiponectin in cardi-ometabolic disease. J. Hypertens 34 (Suppl. 1), e7 [Google Scholar]
- 10.Martos-Moreno GA (2006) Normative data for adiponectin, resistin, interleukin 6, and leptin/receptor ratio in a healthy Spanish pediatric population: relationship with sex steroids. Eur. J. Endocrinol 155, 429–434 [DOI] [PubMed] [Google Scholar]
- 11.Frederiksen L et al. (2012) Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur. J. Endocrinol 166, 469–476 [DOI] [PubMed] [Google Scholar]
- 12.Chang CS et al. (2016) Role of adiponectin gene variants, adipokines and hydrometry-based percent body fat in metabolically healthy and abnormal obesity. Obes. Res. Clin. Pract. Published online May 25, 2016 10.1016/j.orcp.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y et al. (2010) Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia–reperfusion injury. Am. J. Physiol. Endocrinol. Metab 298, E663–E670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy A et al. (2015) Postprandial adiponectin and gelatinase response to a high-fat versus an isoenergetic low-fat meal in lean, healthy men. Nutrition 31, 863–870 [DOI] [PubMed] [Google Scholar]
- 15.Yan J et al. (2014) Adiponectin-impaired adipocyte differentiation negatively regulates fat deposition in chicken. J. Anim. Physiol. Anim. Nutr. (Berl.) 98, 530–537 [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi T et al. (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogalion of adiponectin binding and melabolic actions. Nat. Med 13, 332–339 [DOI] [PubMed] [Google Scholar]
- 17.Nicolas A et al. (2017) T-cadherin gene variants are associated with type 2 diabetes and the fatty liver index in the French population. Diabetes Metab 43, 33–39 [DOI] [PubMed] [Google Scholar]
- 18.Liu SH et al. (2015) Honokiol confers immunogenicity by dictating calreticulin exposure, activating ER stress and inhibiting epithelial-to-mesenchymal transition. Mol. Oncol 9, 834–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takemura Y et al. (2007) Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J. Clin. Invest 117, 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi K et al. (2009) Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol. Cell. Biol 29, 3487–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell S and Britton A (2015) The role of alcohol consumption in regulating circulating levels of adiponectin: a prospective cohort study. J. Clln. Endocrinol. Metab 100, 2763–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ntzouvani A et al. (2016) Reduced circulating adiponectin levels are associated with the metabolic syndrome independently of obesity, lipid indices and serum insulin levels: a cross-sectional study. Lipids Health Dis 15, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaghootkar H et al. (2013) Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes 62, 3589–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto N et al. (2006) Association of hypoadiponectinemia in men with early onset of coronary heart disease and multiple coronary artery stenoses. Metabolism 55, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 25.Kojima S et al. (2003) The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart 89, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittencourt C et al. (2014) Association of classical risk factors and coronary artery disease in type 2 diabetic patients submitted to coronary angiography. Diabetol. Metab. Syndr 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung CY et al. (2014) Adiponectin gene variants and the risk of coronary heart disease: a 16-year longitudinal study. Eur. J. Endocrinol 171, 107–115 [DOI] [PubMed] [Google Scholar]
- 28.Takemoto F et al. (2009) Plasma adiponectin: a predictor of coronary heart disease in hemodialysis patients – a Japanese prospective eight-year study. Nephron. Clin. Pract 111, c12–c20 [DOI] [PubMed] [Google Scholar]
- 29.Ohman-Hanson RA (2016) Ethnic and sex differences in adiponectin: from childhood to adulthood. J. Clin. Endocrinol. Metab 101, 4808–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Shafey EM and Shalan M (2014) Plasma adiponectin levels for prediction of cardiovascular risk among hemodialysis patients. Ther. Apher. Dial 18, 185–192 [DOI] [PubMed] [Google Scholar]
- 31.Reinstadler SJ (2014) Relation of plasma adiponectin levels and aortic stiffness after acute ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 3, 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajer GR et al. (2007) Low plasma levels of adiponectin are associated with low risk for future cardiovascular events in patients with clinical evident vascular disease. Am. Heart J 154, 750.e1–750.e7. [DOI] [PubMed] [Google Scholar]
- 33.Buturak A et al. (2016) Elective percutaneous coronary intervention leads to significant changes in serum resistin, leptin, and adiponectin levels regardless of periprocedural myocardial injury: an observational study. Anatoi. J. Cardiol 16, 940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruyama S et al. (2011) Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J. Biol. Chem 286, 32790–32800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao L et al. (2007) Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 115, 1408–1416 [DOI] [PubMed] [Google Scholar]
- 36.Zhang C et al. (2013) Recombinant adiponectin ameliorates liver ischemia reperfusion injury via activating the AMPK/eNOS pathway. PLoS One 8, e66382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y et al. (2013) Adiponectin inhibits oxidative/nitrative stress during myocardial ischemia and reperfusion via PKA signaling. Am. J. Physiol. Endocrinol. Metab 305, E1436–E1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migrenne S et al. (2009) Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am. J. Physiol. Regul. integr. Comp. Physiol 296, R929–R935 [DOI] [PubMed] [Google Scholar]
- 39.Tishinsky JM et al. (2012) Insulin-sensitizing properties of adiponectin. Biochimie 94, 2131–2136 [DOI] [PubMed] [Google Scholar]
- 40.Kuryszko J et al. (2016) Secretory function of adipose tissue. Pol. J. Vet Sci 19, 441–446 [DOI] [PubMed] [Google Scholar]
- 41.Matczuk J et al. (2016) Insulin resistance and obesity affect lipid profile in the salivary glands. J. Diabetes Res 2016, 8163474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Putti R et al. (2015) Skeletal muscle mitochondrial bioenergetics and morphology in high fat diet induced obesity and insulin resistance: focus on dietary fat source. Front. Physiol 6, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catalano KJ et al. (2010) Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 59, 1416–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullen KL et al. (2009) Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am. J. Physiol. Regul. integr. Comp. Physiol 296, R243–R251 [DOI] [PubMed] [Google Scholar]
- 45.Bruce CR et al. (2005) The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes 54, 3154–3160 [DOI] [PubMed] [Google Scholar]
- 46.Li R et al. (2010) Reduced vascular responsiveness to adiponectin in hyperlipidemic rats – mechanisms and significance. J. Mol. Cell. Cardiol 49, 508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L et al. (2015) Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J. Physiol 593, 4067–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo J et al. (2013) Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca2+-ATPase activity and inhibiting endoplasmic reticulum stress. J. Cardiovasc. Pharmacol 62, 143–153 [DOI] [PubMed] [Google Scholar]
- 49.Nduhirabandi F et al. (2014) Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J. Pineal Res 57, 317–332 [DOI] [PubMed] [Google Scholar]
- 50.Yi W et al. (2011) Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antioxid. Redox Signal 15, 1779–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata R et al. (2007) Potential of adiponectin as a cardioprotective agent. Future Cardiol 3, 647–656 [DOI] [PubMed] [Google Scholar]
- 52.Ouchi N et al. (2006) Cardioprotection by adiponectin. Trends Cardiovasc. Med 16, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koentges C et al. (2015) Myocardial mitochondrial dysfunction in mice lacking adiponectin receptor 1. Basic Res. Cardiol 110, 37. [DOI] [PubMed] [Google Scholar]
- 54.Durrani S et al. (2015) Relationship of adiponectin level with lipid profile in type-2 diabetic men with coronary heart disease. J. Ayub Med. Coll. Abbottabad 27, 32–35 [PubMed] [Google Scholar]
- 55.Antonopoulos AS (2015) Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64, 2207–2219 [DOI] [PubMed] [Google Scholar]
- 56.Persson J et al. (2012) High plasma adiponectin concentration is associated with all-cause mortality in patients with carotid atherosclerosis. Atheroscerosis 225, 491–496 [DOI] [PubMed] [Google Scholar]
- 57.Aso Y et al. (2012) Elevation of serum high molecular weight adiponectin in patients with type 2 diabetes and orthostatic hypotension: association with arterial stiffness and hypercoagulability. Diabet. Med 29, 80–87 [DOI] [PubMed] [Google Scholar]
- 58.Pischon T et al. (2011) Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atheroscerosis 219, 322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortega Moreno L et al. (2016) Evidence of a causal relationship between high serum adiponectin levels and increased cardiovascular mortality rate in patients with type 2 diabetes. Cardiovasc. Diabetol 15, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortega Moreno L et al. (2016) The combined effect of adiponectin and resistin on all-cause mortality in patients with type 2 diabetes: evidence of synergism with abdominal adiposity. Atheroscerosis 250, 23–29 [DOI] [PubMed] [Google Scholar]
- 61.Laoutaris ID et al. (2010) High plasma adiponectin is related to low functional capacity in patients with chronic heart failure. Int. J. Cardiol 144, 230–231 [DOI] [PubMed] [Google Scholar]
- 62.Sente T et al. (2016) Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J. Cachexia Sarcopenia Muscle 7, 261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sente T et al. (2016) Primary skeletal muscle myoblasts from chronic heart failure patients exhibit loss of anti-inflammatory and proliferative activity. BMC Cardiovasc. Disord 16, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sente T et al. (2016) The evolving role of adiponectin as an additive biomarker in HFrEF. Heart Fail. Rev 21, 753–769 [DOI] [PubMed] [Google Scholar]
- 65.Keshvari S et al. (2013) Characterisation of the adiponectin receptors: the non-conserved N-terminal region of AdipoR2 prevents its expression at the cell-surface. Biochem. Biophys. Res. Commun 432, 28–33 [DOI] [PubMed] [Google Scholar]
- 66.Wu YY et al. (2016) Effect of berberine on the ratio of high-molecular weight adiponectin to total adiponectin and adiponectin receptors expressions in high-fat diet fed rats. Chin. J. integr. Med. Published online November 17, 2016 10.1007/s11655-016-2518-x [DOI] [PubMed] [Google Scholar]
- 67.Kim JC (2016) The effect of exercise training combined with PPARγ agonist on skeletal muscle glucose uptake and insulin sensitivity in induced diabetic obese Zucker rats. J. Exerc. Nutrition Biochem 20, 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Landrier JF et al. (2016) Reduced adiponectin expression after high-fat diet is associated with selective up-regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue. FASEB J 31, 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Francisco C et al. (2016) Can adiponectin help us to target diastolic dysfunction? Cardiovasc. Drugs Ther 30, 635–644 [DOI] [PubMed] [Google Scholar]
- 70.Loncar G et al. (2013) Association of adiponectin with peripheral muscle status in elderly patients with heart failure. Eur. J. Intern. Med 24, 818–823 [DOI] [PubMed] [Google Scholar]
- 71.Kreth S et al. (2014) MicroRNA-150 inhibits expression of adiponectin receptor 2 and is a potential therapeutic target in patients with chronic heart failure. J. Heart Lung Transplant 33, 252–260 [DOI] [PubMed] [Google Scholar]
- 72.Fang X et al. (2005) Hyperglycemia- and hyperinsulinemia-induced alteration of adiponectin receptor expression and adiponectin effects in L6 myoblasts. J. Mol. Endocrinol 35, 465–476 [DOI] [PubMed] [Google Scholar]
- 73.Kim HK et al. (2011) The expression of adiponectin receptor is altered in mild and moderate/severe persistent allergic nasal mucosa. Am. J. Rhinol. Allergy 25, 318–322 [DOI] [PubMed] [Google Scholar]
- 74.Martinez Cantarin MP et al. (2014) Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrol. Dial. Transplant 29, 2268–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y et al. (2015) G-protein-coupled receptor kinase 2-mediated desensitization of adiponectin receptor 1 in failing heart. Circulation 131, 1392–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y et al. (2008) Norepinephrine- and epinephrine-induced distinct β2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J. Biol. Chem 283, 1799–1807 [DOI] [PubMed] [Google Scholar]
- 77.Ding Q et al. (2009) Endocytosis of adiponectin receptor 1 through a clathrin- and Rab5-dependent pathway. Cell Res 19, 317–327 [DOI] [PubMed] [Google Scholar]
- 78.Wang Y et al. (2014) Adiponectin inhibits tumor necrosis factor-alpha-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ. Res 114, 792–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y et al. (2012) Essential role of caveolin-3 in adiponectin signalsome formation and adiponectin cardioprotection. Arterioscler. Thromb. Vasc. Biol 32, 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao X et al. (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol 8, 516–523 [DOI] [PubMed] [Google Scholar]
- 81.Ouchi N et al. (2000) Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102, 1296–1301 [DOI] [PubMed] [Google Scholar]
- 82.Tang YT et al. (2005) PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol 61, 372–380 [DOI] [PubMed] [Google Scholar]
- 83.Gesty-Palmer D et al. (2006) Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem 281, 10856–10864 [DOI] [PubMed] [Google Scholar]