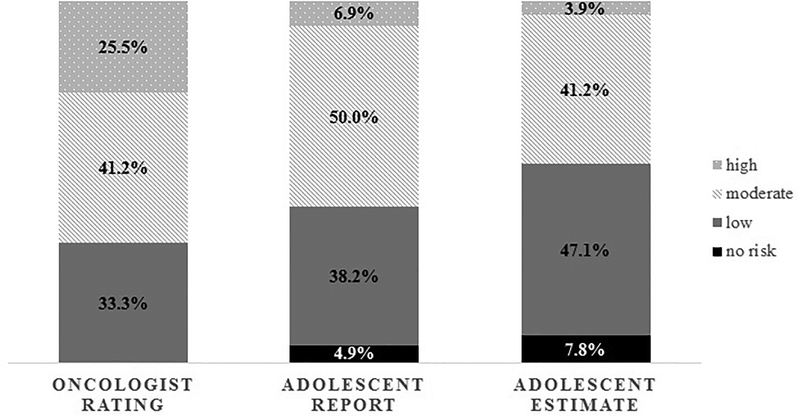Abstract
Objective:
To examine patient and parent understanding of infertility risk (relative to oncologists’ risk ratings) among adolescents newly diagnosed with cancer, and to identify background factors related to inaccurate reporting/estimating.
Methods:
Male patients (N=137; aged 13–21) and their parents completed self-report questionnaires. Those who reported a fertility-related conversation with their provider (N=102 adolescents, N=74 parents), reported their infertility risk (i.e., what oncologist had communicated) and all participants estimated risk (i.e., personal belief). Reports/estimates were compared to oncologists’ ratings to assess relative accuracy, and regression analyses assessed potentially related background factors.
Results:
Participants’ agreement of their risk reports with the oncologist was poor (kappa=.079/.122 for adolescents/parents), resulting in most adolescents (59.8%) and parents (58.7%) inaccurately reporting risk. Older adolescents were less likely to over-report risk (OR=0.69; 95%CI [0.49–0.97]) and parents of sons with the highest Tanner stage were less likely to under-report (OR=0.28; 95%CI [0.08–0.92]). Risk estimates were also in poor agreement with oncologists’ ratings among adolescents (kappa=.040) and parents (kappa=.088). Accordingly, incongruent estimates occurred in most adolescents (63.7%) and parents (62.2%), although all reported fertility-related conversations with their providers.
Conclusions:
Most adolescents and parents inaccurately reported infertility risk, and more poorly estimated risk. Research is needed to identify additional factors associated with accurate understanding of cancer-related infertility risk. Providers should be supported with user-friendly educational tools to promote awareness of infertility risk.
Keywords: adolescent, cancer, fertility, infertility risk, oncology, parents, provider communication, risk estimation
Background
With increasing survival rates among youth diagnosed with cancer, more attention has focused on physical late effects among long-term survivors. One commonly reported late effect is impaired fertility, especially among survivors treated with alkylating agents and pelvic/cranial radiation.(1) Therefore, fertility preservation prior to treatment initiation has been emphasized, but it remains underutilized in the pediatric setting.(2,3) This underutilization may be due to the attitudes, knowledge, and beliefs of families and providers, as well as due to time, institutional, and/or financial constraints.(3,4) However, if fertility preservation can be offered, utilization may largely be influenced by whether patients and parents accurately understand providers’ communication about infertility risk or personal beliefs, which could affect decision-making regarding preservation and treatment.
Previous studies indicate that a cancer diagnosis during childhood/adolescence can be stressful for the entire family.(5) Therefore, handling extensive medical information and anticipating future health problems may be outside the scope of what patients and parents can fully process at the time of diagnosis. Studies among families of survivors of childhood cancer indicate that parents felt unprepared for various late effects(6,7) and desired more information from providers.(8) Studies on awareness around infertility as a potential late effect are scarce, and to what extent parents and patients accurately understand risk is unclear. It was indicated that at diagnosis (i.e., when preservation decisions need to be made), future fertility may be a less relevant concern among patients and/or parents,(9–11) as treatment initiation/cure is prioritized. Nevertheless, many patients desire having children in the future(11) and rate it as an important life goal.(12) Over time, survivors may adopt an attitude of wait-and-see regarding their fertility,(9,13) although many also actively worry about infertility.(14–19) Importantly, research has shown survivors value both fertility-related information at diagnosis and the opportunity of choosing to engage in fertility preservation, regardless of their decision.(20) Concurrently, long-term survivors who did not have, or do not remember such conversations with their providers, report regret in hindsight(21) and increased worry, which may affect overall well-being.(22)
This study examined adolescents’ and parents’ infertility risk perceptions relative to risk reports of their attending oncologist at the time of diagnosis. Two different aspects were assessed: (1) adolescent- and parent-report of what their oncologist communicated about treatment-related infertility risk (i.e., risk reporting) and (2) adolescents’ and parents’ personal beliefs of what their (son’s) infertility risk is, irrespective of what they were told (i.e., risk estimates). It was further tested whether accurate understanding was associated with sociodemographic and medical factors. Therefore, this study may offer valuable targets of intervention for increasing understanding of infertility risk among families with adolescent sons newly diagnosed with cancer.
Methods
Procedure
Cancer patients treated at eight pediatric oncology centers in the US and Canada were eligible if they were: a) male, b) newly diagnosed with cancer for the first time, c) aged 13–21 years, d) at Tanner Stage ≥3, e) proficient in English or Spanish, f) cognitively able to complete questionnaires, and g) identified by their oncologist as being at increased risk for infertility due to impending gonadotoxic treatment. Newly admitted patients were screened for initial eligibility (criteria a-f). If these criteria were met, the patient’s attending oncologist was contacted to rate the patients’ infertility risk (none, low, moderate, or high). Those patients identified as being at increased risk for infertility (i.e., >none) were approached for study enrollment. Once a patient consented/assented, his parent(s) was also invited for participation. Study questionnaires were typically administered within 1–7 days post initiation of treatment, and thus close to when fertility-related discussions/decisions about fertility preservation occurred. All procedures followed the ethical standards of the US Federal Policy for the Protection of Human Subjects and were approved by the IRBs at all sites (SJCRH#Pro00001628). Data presented here are part of a larger study about sperm cryopreservation among adolescent cancer patients.(23)
Participants
A total of 193 adolescent males were eligible, of whom 156 (80.8%) provided written consent/assent for this study, and 137 returned questionnaires with complete data relevant here. Most participants (n=102; 74.5%) indicated that their provider had talked to them about fertility, and they were the focus of this study. These 102 males were ages 13–21 (M=16.6; SD=2.1), primarily White (68.6%), Christian (79.4%), at Tanner stage 5 (62.7%), and were diagnosed with leukemia/lymphoma (55.9%) or brain/solid tumors (44.1%; only 5 patients had a brain tumor; Table 1). Note that these patient demographics did not differ from males (n=35) who did not recall fertility-related conversations.
Table 1:
Description of the adolescent and parent sample
| Adolescents (N=102) |
Parents (N=74) |
|
|---|---|---|
| Racea | ||
| White | 70 (68.6%) | 54 (73.0%) |
| Other | 32 (31.4%) | 20 (27.0%) |
| Religious Orientationa | ||
| Christian | 81 (79.4%) | 67 (90.5%) |
| Other | 19 (18.6%) | 7 (9.5%) |
| Parent Marital Status | ||
| Married | 50 (67.6%) | |
| Other | 24 (32.4%) | |
| Parent Educationa | ||
| Less than Bachelor’s degree | 39 (52.7%) | |
| Bachelor’s degree or higher | 34 (45.9%) | |
| Patient Tanner Stagea | ||
| Stage 3–4 | 33 (32.4%) | 26 (35.1%) |
| Stage 5 | 64 (62.7%) | 45 (60.8%) |
| Patient Diagnosis | ||
| Leukemia/lymphoma | 57 (55.9%) | 37 (50.0%) |
| Solid/brain tumors | 45 (44.1%) | 37 (50.0%) |
| Children as Life Priority: | ||
| Endorsed | 35 (34.3%) | |
| Not endorse | 67 (65.7%) | |
| M (SD), range | M (SD), range | |
| Age | 16.6 (2.1), 13–21 | 44.6 (5.7), 32–57 |
| Anxiety (T-score) | 56.3 (14.0), 40–81 | 62.4 (12.1), 37–81 |
categories do not add to 100% due to missing data
The 102 adolescents had 86 parents who also participated. The majority of these parents (n=81, 94.1%) indicated that their provider had discussed their sons’ future fertility with them, and 74 provided complete data, including 44 mothers only, 12 fathers only, 17 cases where both parents completed the study, and one case where both parents jointly participated. If both parents participated (i.e., n=17), mother-report was used for categorical data and a parent aggregate for continuous data. This procedure has been used previously based on sensitivity analyses(23) and the fact that most participating parents were mothers. Parents were between the ages of 32–57 (M=44.6; SD=5.7), predominantly White (73.0%), Christian (90.5%), married (67.6%), and had less than a bachelor’s degree (52.7%; Table 1). Parents included in primary data analyses (n=74) did not differ from parents (n=12) who did not recall fertility-related conversations.
Measures
Attending oncologists rated patients’ risk for infertility as low, moderate, or high (as part of initial study eligibility), referred to as oncologist rating. Adolescents and parents were asked to report their (son’s) infertility risk based on what they perceived their provider told them (i.e., none, low, moderate, high risk); referred to as adolescent/parent risk report (i.e., “What has the medical team told you about your [son’s] chances for developing fertility problems due to cancer treatment?”). Similarly, adolescents and parents were asked to estimate their (son’s) infertility risk, regardless of what they have been told; referred to as adolescent/parent risk estimate (i.e., “Regardless of what you’ve been told, what do you think your [son’s] chances are for developing fertility problems after cancer treatment?”). Additionally, patients and parents were queried as to whether they had fertility-related conversations with each other (i.e., “Have you talked with your [parent(s)/son] about [your/his] chances for fertility problems based on cancer treatment?” yes/no).
Adolescents were further asked to rank whether having children in the future would be a priority in their lives (coded as included in top 3 life priorities vs. not). All participants rated their level of anxiety during the previous week (i.e., when diagnosis and fertility-related discussions occurred) using the anxiety subscale of the Symptom Checklist 90-R.(24) This subscale contains 10 items, and participants’ responses were transformed into standardized age- and sex-specific T-scores (Table 1).
Statistical Analyses
Accuracy of infertility risk reports (i.e., agreement between adolescent-/parent-report and oncologist rating) was tested using Cohen’s Kappa (κ), as it adjusts for agreement by chance.(25) Based on the distribution of responses, adolescents and parents were grouped into under-, over-, or accurately reporting the patient’s risk for infertility relative to their oncologist. We further checked whether under-/over-/accurate reporting were clustered within families using Kappa. Coefficients <.20 were considered poor agreement, .20-.40 fair, .40-.60 moderate, and coefficients >.60 substantial.(26)
Factors potentially related to (in)accurate reporting were tested in two separate logistic regression analyses, using over- or under-reporting (relative to accurate) as dependent variables among adolescents and parents separately. Regressions utilized backward conditional selection of the following variables among adolescents: age (continuous), religion (Christian vs. other), Tanner stage (stage 5 vs. <5), diagnosis (leukemia/lymphoma vs. solid/brain tumor), recalling fertility-related conversations with parents (yes/no), anxiety (continuous), and having children as top 3 life priority (yes/no). Tested factors among parents included age, education (lower than bachelor’s degree vs. bachelor or higher), religion, recalling fertility-related conversations with son, anxiety, adolescent Tanner stage and age (as proxies for son’s development), and diagnosis.
The same analyses were conducted for infertility risk estimates. Thus, testing adolescents’ and parents’ estimates against the oncologists’ rating, testing whether estimates clustered within families, and running regression analyses as specified above.
Finally, agreement between risk reports and estimates was examined using kappa among adolescents and parents respectively.
Results
Infertility Risk Reporting
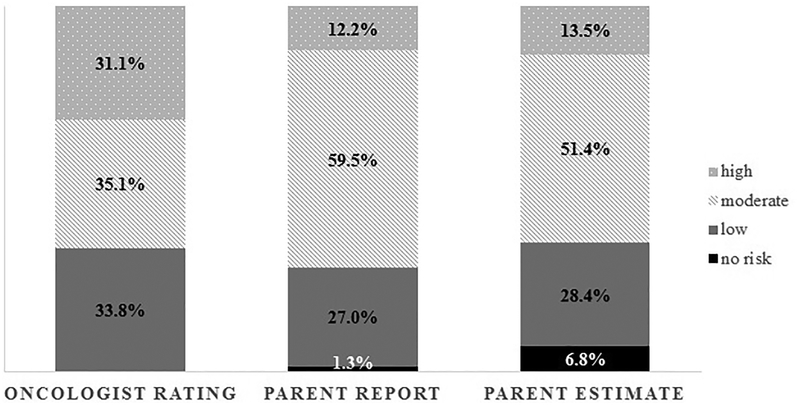
Oncologists rated many adolescents (n=42/102; 41.2%) at moderate risk for future infertility. Similarly, half of adolescents (n=51/102; 50.0%) and most parents (n=44/74; 59.7%) reported their oncologist had categorized their risk as moderate (Figure 1&2). Nevertheless, agreement between adolescent and oncologist risk report was poor (Kappa (κ)=.079, p=.235), and most adolescents (n=61; 59.8%) reported their risk inaccurately (i.e., 43.1% under-reported, 16.7% over-reported; Table 2). Parents also had poor agreement with the oncologists (κ=.122, p=.104), with the majority (n=43; 58.1%) reporting risk inaccurately (i.e., 35.1% under-reported, 23.0% over-reported; Table 2). Agreement within families was moderate (κ=.578, p<.001), with 73.0% of adolescent-parent dyads (n=54/74) falling into the same category of under-/over-/accurate reporting. Given these findings, we further tested whether accurate reporting was related to sperm banking attempts. It was indicated that, irrespective of whether adolescents (or parents) under-/over-/or accurately reported risk, they were equally likely to attempt sperm banking (Fisher’s exact test=0.185/ χ2=3.322, p=.190 [adolescents], Fisher’s exact test=0.555/ χ2=1.370, p=.504 [parents]; note that although Fisher’s exact test accounts for small cell frequencies, the smallest cell included only n=3). For more details regarding sperm banking outcomes, please see a previously published manuscript.(23)
Figure 1:
Infertility risk reporting and estimates among 102 adolescents and the corresponding oncologist rating
Figure 2:
Infertility risk reporting and estimates among 74 parents and the corresponding oncologist rating
Table 2:
Accuracy of risk reporting and risk estimates among adolescents and parents (relative to attending oncologist, as depicted in Figure 1&2)
| Adolescents | Parents | |
|---|---|---|
| Agreement with oncologist | N=102 | N=74 |
| Reporting: | ||
| Under-reporting risk | 44 (43.1%) | 26 (35.1%) |
| Accurately reporting risk | 41 (40.2%) | 31 (41.9%) |
| Over-reporting risk | 17 (16.7%) | 17 (23.0%) |
| Estimate: | ||
| Underestimating risk | 52 (51.0%) | 29 (39.2%) |
| Congruent risk estimation | 37 (36.3%) | 28 (37.8%) |
| Overestimating risk | 13 (12.7%) | 17 (23.0%) |
analyses among mothers and fathers separately showed similarly poor agreement (κ=.107-.295); 56.6% of mothers and 46.7% of fathers inaccurately reported risk, while 61.0% and 60.0% incongruently estimated risk respectively; agreement between mothers and fathers was not tested as only 17 mother-father dyads participated
Logistic regression analyses revealed that among adolescents, only age was associated with inaccurate risk reporting. Older adolescents were less likely to over-report infertility risk relative to younger adolescents (OR=0.69, 95%CI [0.49–0.97], p=.031). Among parents, those with a son at Tanner stage 5 were less likely to under-report risk relative to those with a son at lower Tanner stages (OR=0.28, 95%CI [0.08–0.92], p=.036). All other tested factors were unrelated to risk reporting.
Infertility Risk Estimation
Among all participants (who reportedly had fertility-related conversations with their provider, but irrespective of what they have been told), a substantial portion of adolescents (n=48, 47.1%) estimated their infertility risk as low, while most parents (n=38, 51.4%) estimated moderate risk (Figure 1&2). Accordingly, agreement between oncologists’ rating and adolescents’ estimate was poor (κ=.040, p=.532), and most adolescents (n=65; 63.7%) incongruently estimated risk (i.e., 51.0 % underestimated, 12.7% overestimated; Table 2). Similarly, agreement between parents’ risk estimates and the oncologists’ rating was poor (κ=.088, p=.230), as most parents (n=46; 62.2%) underestimated (39.2%) or overestimated risk (23.0%; Table 2). Estimates clustered only moderately within families (κ=.400, p<.001), with 62.2% of adolescent-parent dyads falling into the same estimate categories. Congruent risk estimates were unrelated to sperm banking attempts, but again some of the cells were small (Fisher’s exact test=0.431/ χ2=1.702, p=.427 [adolescents]; Fisher’s exact test=0.944/ χ2=0.200, p=.905 [parents]).
Logistic regressions analyses yielded no significant results, but two trends were identified. Among adolescents, those who listed having future children as a priority were less likely to overestimate infertility risk (OR=0.21, 95%CI [0.04–1.12], p=.068). Among parents, those that had older sons were less likely to overestimate relative to those with younger sons (OR=0.68, 95%CI [0.45–1.02], p=.065). All other included factors were unrelated to risk estimates.
Note that adolescents who reportedly did not have fertility-related conversations with providers (n=35; see Methods) were not able to report (what their oncologist had conveyed), but estimated their infertility risk. Their estimates were in poor agreement with oncologists (κ=.174, p=.111; 54.3% incongruent: 42.9% underestimated; 11.4% overestimated), which was just as poor as the estimates of the 102 adolescents presented above (χ2=0.988, p=.610). Likewise, parents from the larger study who did not recall conversations with their providers (n=21), poorly estimated risk (κ=.032, p=.813; 61.9% incongruent: 47.6% underestimated; 14.3% overestimated), which was comparable to the 74 parents presented above (χ2=0.870, p=.647).
Risk Report versus Estimates
Adolescents’ risk report and estimates were rather strongly correlated (ρ=.701; p<.001). The majority of adolescents (n=77; 75.5%) endorsed the same category when reporting and estimating potential infertility risk. However, 19.6% (n=20) estimated their risk lower than they had reported (based on provider communication), while 4.9% (n=5) estimated their risk higher than they initially reported. Similarly, parents’ risk reports and estimates were moderately to strongly related (ρ=.659, p<.001). Most parents (n=55; 74.3%) reported and estimated the same risk categories, while 16.2% (n=12) estimated lower and 9.5% (n=7) higher risk than they had reported.
Conclusions
This study examined infertility risk reporting and estimates among families of adolescent males newly diagnosed with cancer within days of receiving fertility-related information, rather than retrospectively assessing risk understanding months or years after diagnosis. Accurate risk reporting and congruent estimates were rare, and few factors associated with inaccuracies were identified.
Numerous adolescents and parents under-reported and underestimated risk, which could result in distress following treatment if survivors discover they are infertile. It may also cause regret or resentment in hindsight if, for example, risk had been underestimated and families opted against fertility preservation. In contrast, ~17% of adolescents and 23% of parents over-reported and/or overestimated risk, which can also significantly affect survivors’ well-being (e.g., unnecessary worry/concern, or unplanned pregnancies if incorrectly assuming to be infertile). Thus, there should be a general focus on accurate understanding among families of youth with cancer to prevent both inflating and downplaying potential fertility problems. Importantly, if family members are not in agreement about risk, it could also hinder communication within families about fertility-related decisions/preservation.
It is of particular concern that risk reporting was in poor agreement with oncologists, while risk estimates were even more discrepant. Although most participants reported and estimated the same risk categories, up to 20% of adolescents estimated a lower risk than they had initially reported based on provider communication. Additionally, it is of greater concern that the 102 adolescents and 74 parents who recalled fertility-related conversations with their oncologist were just as likely to incongruently estimate risk as those who did not report such conversations. Several adolescents and parents also reported ‘no’ risk (although all were at increased risk per oncologists’ ratings), which doubled among adolescents and increased 5-fold among parents when estimating risk (Figure 1&2). All of these findings indicate that there are barriers to receiving accurate information, potentially including disbelief/distrust in providers’ risk assessment, difficulties processing information at the distressing time of diagnosis, a focus on other aspects of diagnosis/treatment, and/or a need for families to remain hopeful while undergoing treatment. Research among adults has, for example, shown that trust in primary care physicians increases over time,(27) and as adolescents in this study were newly diagnosed, such basis of trust may not have been developed yet. At the same time, pediatric providers’ communication and attitudes are vital. For example, providers’ concerns about families’ reactions(28) and their own comfort surrounding fertility-related issues(3) influence their communication about infertility risk and fertility preservation with families. Therefore, providers should receive training and have specific guidelines about effective communication of such delicate topics. They should further be supported by multidisciplinary teams and supplementary educational materials (e.g., pamphlets, online resources), and have the opportunity for follow-up visits to repeatedly discuss infertility risk across the cancer continuum (see also Clinical Implications below). Additionally, it should be highlighted that anticipating future fertility in and of itself is difficult for providers, as cancer treatment is complex and various factors (including unexpected intensification of treatment plans) affect fertility outcomes long-term. However, creating awareness among families and emphasizing that it is impossible to predict future fertility with absolute certainty may help families to understand that fertility preservation is generally recommended and that they should consider fertility testing as part of survivorship care in the future.
Given the high frequency of inaccurate understanding, additional analyses were performed and indicated that accurate risk reports/estimates were not significantly related to sperm banking attempts, but replications in larger studies are needed. Nevertheless, this finding challenges a common view that accurate understanding of infertility risk may lead to greater initiation of fertility preservation. Previous research has found that provider referrals and recommendations can significantly increase participation in fertility preservation.(3,23) However, these previous and current findings combined beg the question as to whether families may opt for a procedure they do not regard as necessary or do not fully understand. While undergoing fertility preservation may not be a disadvantage for patients even if they do not desire biological children in the future, accurate understanding of risk may be important when survivors grow older to fully grasp the potential consequences of their cancer treatment more generally. Furthermore, this null finding aligns with previous research which found that families worry about potential infertility irrespective of treatment-related risk.(29) This study also highlighted that most parents (70%) were dissatisfied with the amount of fertility-related information they received. Thus, it remains important to determine factors that influence families’ understanding and decision-making in the fertility-preservation process.
In this study, few factors related to inaccurate reporting among families were identified, perhaps partly due to small subgroups (e.g., over- vs. accurate reporting). Nevertheless, older adolescents more accurately reported risk (i.e., less likely to over-report). This accuracy may be due to their ability to better comprehend fertility-related information, while also holding greater awareness/forethought of sexuality, fertility, and/or family planning, relative to young adolescents. Similarly, parents who had sons with a high Tanner stage were less likely to under-report risk (OR=0.28). Thus, general adolescent maturation may be a relevant factor for parents to accurately realize/consider their sons’ future fertility. Importantly, none of the adolescents in the larger study sample were under Tanner Stage 3, a developmental level appropriate for risk-related conversations/offering fertility preservation.(30,31) Nevertheless, many additional factors likely exist, given that families’ risk estimates aligned even less with the oncologist’s rating than reporting (and given that participants who recalled fertility-related conversations did not estimate risk better than participants who did not report such conversations). Factors related to estimates could not be identified, but a trend indicated that a priority of having future children may compel adolescents to be more attentive to infertility risk. However, more research is needed to examine what factors are related to discrepancies of families’ understanding of risk relative to providers.
Study Limitations
Although this study offers novel insights into families’ reports of anticipated infertility risk, certain limitations have to be considered. Potentially relevant factors for (in)accurate understanding may have been overlooked due to limited sample size, while other factors not included in this study need to be considered (e.g., trust, family-provider relationship, provider communication, families’ health literacy, personality). Given the focus of the larger project on adolescent sperm banking, the presented analyses excluded female patients and conclusions for the adolescent cancer population as a whole cannot be drawn. Nevertheless, assessment of families’ reports and estimates occurred in ‘real-time’ rather than retrospectively assessing understanding years after diagnosis, offering valid insights into families’ understanding of infertility as a potential late effect at cancer treatment initiation. In this light, our findings raise concern that if understanding is already poor at diagnosis, it may continue to decline over time. However, the study setting did not allow to control and assess oncologists’ conversations/communicated risk with families. Concerns about providers’ ambiguous communication regarding potential infertility have been voiced by survivors previously,(32) but more research is needed.
Clinical Implications
Improving physician-family communication is warranted. For example, facilitating and supporting providers with appropriate training, specific guidelines, and additional resources (e.g., educational pamphlets, online resources, fertility specialists available for referral, etc.) could be helpful. Currently, guidelines recommend fertility preservation and timely conversations with families/patients with cancer,(30) but little guidance is provided regarding the specific content and delivery of such conversations, especially for youth with cancer.(33) Meanwhile, one easily implementable communication technique may be the “teach-back method”(34) (e.g., could you summarize the information I just reviewed with you?), which would allow for provider confirmation of families’ understanding and give providers the opportunity to clarify potential misunderstandings/misperceptions immediately. Tailored communication about potential late effects has indeed been found to increase awareness among survivors.(35) Additionally, providing families with written documentation regarding their infertility risk could be beneficial, both for recall among patients/parents, as well as documentation in the medical charts to ensure that interdisciplinary team members (e.g., nurses, psychologists, and/or other staff) could be utilized to reinforce physicians’ messages and help facilitate the fertility preservation process, if desired.
More clinical and research attention is needed to examine factors relevant to the understanding of fertility-related late effects and how it relates to fertility preservation decision-making among youth with cancer and their families.
Acknowledgement
This study was supported by the National Institute of Child Health and Human Development (HD061296, Klosky), National Cancer Institute (CA021765, Roberts), and support provided to St. Jude by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest
The authors declare to have no existing conflict of interest.
References
- (1).Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014. 10;15(11):1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Salih SM, Elsarrag SZ, Prange E, Contreras K, Osman RG, Eikoff JC, et al. Evidence to incorporate inclusive reproductive health measures in guidelines for childhood and adolescent cancer survivors. J Pediatr Adolesc Gynecol 2015;28(2):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Klosky JL, Anderson LE, Russell KM, Huang L, Zhang H, Schover LR, et al. Provider influences on sperm banking outcomes among adolescent males newly diagnosed with cancer. J Adol Health 2017;60(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Köhler TS, Kondapalli LA, Shah A, Chan S, Woodruff TK, Brannigan RE. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet 2011;28(3):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Long KA, Marsland AL. Family adjustment to childhood cancer: A systematic review. Clin Child Fam Psychol Rev 2011;14(1):57–88. [DOI] [PubMed] [Google Scholar]
- (6).Greenzang KA, Cronin AM, Mack JW. Parental preparedness for late effects and long-term quality of life in survivors of childhood cancer. Cancer 2016;122(16):2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kaye E, Mack JW. Parent perceptions of the quality of information received about a child’s cancer. Peds Blood Cancer 2013;60(11):1896–1901. [DOI] [PubMed] [Google Scholar]
- (8).Vetsch J, Rueegg CS, Gianinazzi ME, Bergsträsser E, von der Weid, Nicolas X, Michel G. Information needs in parents of long-term childhood cancer survivors. Peds Blood Cancer 2015;62(5):859–866. [DOI] [PubMed] [Google Scholar]
- (9).Nieman CL, Kinahan KE, Yount SE, Rosenbloom SK, Yost KJ, Hahn EA, et al. Fertility preservation and adolescent cancer patients: lessons from adult survivors of childhood cancer and their parents In: Woodruff TK, Snyder KA, editor. Oncofertility: Fertility Preservation for Cancer Survivors New York: Springer; 2007. p. 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Edge B, Holmes D, Makin G. Sperm banking in adolescent cancer patients. Arch Dis Child 2006. February;91(2):149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Quinn GP, Knapp C, Murphy D, Sawczyn K, Sender L. Congruence of reproductive concerns among adolescents with cancer and parents: pilot testing an adapted instrument. Pediatrics 2012. April;129(4):e930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Klosky JL, Simmons JL, Russell KM, Foster RH, Sabbatini GM, Canavera KE, et al. Fertility as a priority among at-risk adolescent males newly diagnosed with cancer and their parents. Support Care Cancer 2015;23(2):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Somerfield MR, Curbow B, Wingard JR, Baker F, Fogarty LA. Coping with the physical and psychosocial sequelae of bone marrow transplantation among long-term survivors. J Behav Med 1996;19(2):163–184. [DOI] [PubMed] [Google Scholar]
- (14).Zebrack BJ, Casillas J, Nohr L, Adams H, Zeltzer LK. Fertility issues for young adult survivors of childhood cancer. Psycho-Oncology 2004. 10;13(10):689–699. [DOI] [PubMed] [Google Scholar]
- (15).Frederick NN, Recklitis CJ, Blackmon JE, Bober S. Sexual Dysfunction in Young Adult Survivors of Childhood Cance. Peds Blood & Cancer 2016;63(9):1622–1628. [DOI] [PubMed] [Google Scholar]
- (16).Thompson AL, Long KA, Marsland AL. Impact of childhood cancer on emerging adult survivors’ romantic relationships: A qualitative account. J Sex Med 2012;10(S1):65–73. [DOI] [PubMed] [Google Scholar]
- (17).Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: a qualitative investigation. Psycho-oncology 2003;12(2):141–152. [DOI] [PubMed] [Google Scholar]
- (18).Crawshaw M, Sloper P. ‘Swimming against the tide’–the influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care 2010;19(5):610–620. [DOI] [PubMed] [Google Scholar]
- (19).Langeveld NE, Grootenhuis MA, Voute PA, de Haan RJ, van den Bos C. Quality of life, self-esteem and worries in young adult survivors of childhood cancer. Psycho-Oncology 2004. December;13(12):867–881. [DOI] [PubMed] [Google Scholar]
- (20).Crawshaw MA, Glaser AW, Hale JP, Sloper P. Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. Eur J Cancer Care 2009. July;18(4):381–390. [DOI] [PubMed] [Google Scholar]
- (21).Stein DM, Victorson DE, Choy JT, Waimey KE, Pearman TP, Smith K, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J AYA Onc 2014;3(2):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hohmann C, Borgmann-Staudt A, Rendtorff R, Reinmuth S, Holzhausen S, Willich SN, et al. Patient counselling on the risk of infertility and its impact on childhood cancer survivors: results from a national survey. J Psychosoc Oncol 2011;29(3):274–285. [DOI] [PubMed] [Google Scholar]
- (23).Klosky JL, Wang F, Russell KM, Zhang H, Flynn JS, Huang L, et al. Prevalence and predictors of sperm banking in adolescents newly diagnosed with cancer: Examination of adolescent, parent, and provider factors influencing fertility preservation outcomes. J Clin Oncol 2017;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the SCL-90: a study in construct validation. J Clin Psychol 1977;33(4):981–989. [Google Scholar]
- (25).Landis JR, Koch GG. A review of statistical methods in the analysis of data arising from observer reliability studies (Part I). Statistica Neerlandica 1975;29(3):101–123. [Google Scholar]
- (26).Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- (27).Thom DH, Ribisl KM, Stewart AL, Luke DA. Further validation and reliability testing of the Trust in Physician Scale. Med Care 1999;37(5):510–517. [DOI] [PubMed] [Google Scholar]
- (28).Armuand GM, Nilsson J, Rodriguez-Wallberg KA, Malmros J, Arvidson J, Lampic C, et al. Physicians’ self-reported practice behaviour regarding fertility-related discussions in paediatric oncology in Sweden. Psycho-Oncology 2017;[accepted]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Oosterhuis BE, Goodwin T, Kiernan M, Hudson MM, Dahl GV. Concerns about infertility risks among pediatric oncology patients and their parents. Peds Blood & Cancer 2008;50(1):85–89. [DOI] [PubMed] [Google Scholar]
- (30).Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31(19):2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol 2010;28(32):4831–4841. [DOI] [PubMed] [Google Scholar]
- (32).Ellis SJ, Wakefield CE, McLoone JK, Robertson EG, Cohn RJ. Fertility concerns among child and adolescent cancer survivors and their parents: A qualitative analysis. J Psychosoc Oncol 2016;34(5):347–362. [DOI] [PubMed] [Google Scholar]
- (33).Barlevy D, Elger BS, Wangmo T, Ravitsky V. Adolescent oncofertility discussions: Recommendations from a systematic literature review. AJOB Empirical Bioethics 2017;8(2):106–115. [DOI] [PubMed] [Google Scholar]
- (34).The National Center for Ethics in Health Care. “Teach Back” A Tool for Improving Provider-Patient Communication. 2006; Available at: https://www.ethics.va.gov/docs/infocus/InFocus_20060401_Teach_Back.pdf, 2017.
- (35).Landier W, Chen Y, Namdar G, Francisco L, Wilson K, Herrera C, et al. Impact of tailored education on awareness of personal risk for therapy-related complications among childhood cancer survivors. J Clin Oncol 2015;33(33):3887–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]