Abstract
Introduction:
Rett syndrome (RTT) is a complex neurodevelopmental disorder with known behavioral abnormalities, both internalizing (e.g., anxiety, social withdrawal) and externalizing (e.g., aggression, self-abuse). However, a broad evaluation of behavioral abnormalities in a large cohort is lacking.
Objective:
In this report, we describe profiles of internalizing and externalizing behavior in individuals evaluated in the multi-center U.S. Rett Natural History Study.
Methods:
Cross-sectional and longitudinal data were collected from 861 females with RTT and from 48 females who have MECP2 mutations without meeting criteria for RTT. Standard statistical methods including linear regression evaluated internalizing behavioral components from the Child Health Questionnaire (CHQ-PF50) and externalizing components from the Motor Behavioral Assessment (MBA).
Results:
We found mildly to moderately severe internalizing behaviors in nearly all individuals with RTT, while externalizing behaviors were mild and uncommon. Internalizing behavior in RTT was comparable to groups with psychiatric disorders. Participants with mixed (internalizing and externalizing) behaviors were younger and less affected overall, but showed prominent self-injury and worsening internalizing behaviors over time.
Conclusions:
This study revealed that internalizing behaviors are common at a clinically significant level in RTT. Understanding clinical features associated with behavioral profiles could guide treatment strategies.
Keywords: Rett syndrome, behavior and behavior mechanisms, problem behavior, observational study
1. Introduction
Rett syndrome (RTT; OMIM 312750) is a severe neurodevelopmental disorder associated with intellectual disability (ID) and communication and motor deficits. Diagnosed in approximately 1 in 10,000 females [1], the vast majority of cases are linked to a mutation in the gene encoding the methyl-CpG-binding protein 2 (MeCP2), a transcriptional regulator involved in synaptic development and maintenance [2,3]. Independent of molecular findings, the main diagnostic criteria for RTT include a period of regression with recovery or stabilization plus 4 characteristic neurologic features (loss of acquired hand skills, loss of acquired spoken language, gait abnormalities, and hand stereotypies) [4]. Those with classic (or typical) RTT meet all 4 main criteria while individuals with variant (or atypical) RTT meet at least 2 of the 4 main criteria plus additional supportive criteria. The clinical presentation of individuals with RTT is highly variable, but general correlations with disease severity have been reported for specific genotypes [2]. While not included in the diagnostic criteria for RTT, behavioral abnormalities are a commonly observed component of the RTT phenotype, though precise estimates of their prevalence are not available [5, 6, 7, 8].
Initial reports of behavioral abnormalities focused on autistic-like features in RTT, including social withdrawal and loss of communication skills during the regression period [9]. In the last two decades, and particularly since the publication of the Rett Syndrome Behaviour Questionnaire (RSBQ), the range of behavioral abnormalities in RTT has been greatly expanded. Unfortunately, terminology and methodology used in these reports has been inconsistent and sometimes confusing. Problematic behaviors have been labeled descriptively or in terms of their resemblance to psychiatric diagnostic features (e.g., anxiety, mood disorders) but without a clear relationship with Diagnostic and Statistical Manual of Mental Disorders [10] diagnoses per se. Moreover, most of these studies have involved relatively small cohorts. Therefore, a need exists in RTT for delineating a broad view of abnormal behaviors independent of diagnostic classifications. A well-established approach is the characterization of two major categories of problem behaviors, internalizing and externalizing, as a foundation for determining diagnostic and therapeutic targets in a specific population. This type of abnormal behavioral survey has been carried out for a variety of neurologic and psychiatric conditions, including neurodevelopmental disorders [11, 12, 13, 14, 15].
Recent literature has reported on internalizing behaviors in RTT, such as anxiety, depression, and abrupt mood changes, which seem to be frequent [5, 6, 7, 8]. Symptoms compatible with generalized anxiety that are commonly observed in RTT include worsening of hyperventilation and breath-holding, panic attacks, inability to relax, inconsolable crying, nervousness, tenseness, trembling in the absence of frightening situations, worry, or screaming episodes [7, 8, 16, 17, 18, 19, 20, 21, 22, 23]. Social anxiety appears to be particularly prominent and may present with avoidance of eye contact, avoidance of others, lack of emotional facial expressions, difficulty initiating communication, shyness, and withdrawal from social contact [6, 17]. Depression in individuals with intellectual disability and limited communication is difficult to diagnose, but often manifests as social withdrawal, abnormally low mood and/or lack of interest [7, 24, 25]. Externalizing behaviors, such as impulsivity, hyperactivity, aggression, self-abuse, inconsolable crying and screaming episodes are less frequently reported in RTT. One previous study reported low levels of overactivity, impulsivity and self-mutilation in RTT when compared to a contrast group matched for age, gender, language, self-help skills, and intellectual ability [7]. Another recent study of epilepsy from the U.S. RTT Natural History Study (RNHS) found that aggressive behavior was associated with lower likelihood of seizures [26].
The present study aims to provide a survey of behavioral abnormalities in RTT, within a framework of internalizing and externalizing behaviors rather than based on clinical diagnoses. For this purpose, we primarily used data collected with two instruments in the protocol of the RNHS: the Motor Behavioral Assessment (MBA), a RTT-oriented clinical assessment developed for the delineation of the natural history of the disorder; and the Child Health Questionnaire - Parent Form 50 (CHQ-PF50), a widely used measure of quality of life that includes a psychosocial/mental health component. We estimated prevalence of internalizing and externalizing behaviors, characterized their associated features, and determined changes over time of abnormal behavioral profiles in the RNHS. The latter is the largest cohort of participants with RTT analyzed in terms of behavioral abnormalities to date. Understanding the characteristics and natural history of behavioral difficulties in RTT is critical for the diagnosis and management of these impairing clinical manifestations of the disorder.
2. Methods and Materials
2.1. Participants
Participants were recruited via the multicenter RNHS from 2006 to 2015 for longitudinal analysis in a cohort study as a precursor for conducting clinical trials. Individual assessments were conducted by a clinician every six to 12 months at one of the eight sites in the United States. The RNHS consortium is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, National Institutes of Health.
2.2. Diagnoses and Genetic Testing
Diagnoses of RTT or other related phenotypes were made by a RNHS neurologist or geneticist (D.G.G., W.E.K., J.L.N., A.K.P., and S.A.S) with extensive clinical experience in RTT, applying published diagnostic guidelines [4]. All participants had MECP2 testing by a qualified laboratory. Participants with clinical RTT were included regardless of mutation status. Those with a diagnosis of atypical RTT were divided into two categories based on scores on the Clinical Severity Scale (CSS): atypical severe patients (score >20) and atypical mild (score <20), as previously described [27]. The CSS is a global clinical severity scale that covers key features of RTT. Non-RTT patients with a MECP2 mutation were included to confirm the specificity of behavioral profiles to RTT. MECP2 mutations were categorized based on average phenotypical severity as mild (R133C, R294X, R306C, 3’ truncations and other point mutations), moderate (T158M) or severe (R106W, R168X, R255X, R270X, insertions, deletions, large deletions and splice site) [2, 27]. Participants without one of these specific mutations were coded as missing data for the mutation severity category.
2.3. Measures
2.3.1. Clinical Severity Score (CSS)
The CSS is a clinician-completed questionnaire that uses a Likert-type scale to rank statements in fairly broad categories of features of RTT: Onset, Growth, Motor, Communication, and Rett Behaviors/Other neurologic. Each item is ranked from either 0–4 or 0–5, with higher scores indicating greater severity and a maximum total score of 58.
2.3.2. Motor behavioral assessment (MBA)
The MBA is a clinician-completed questionnaire that uses a Likert-type scale to score 34 items based on severity from 0–4 (None, 25% of time, 50% of time, 75% of time, 100% of time), with a maximum total score of 136. In addition to total and subscale scores, the following items were selected for analysis of externalizing behaviors: Irritability, crying tantrums (“Irritability”), Aggressive behavior (head banging, throwing, spitting, etc.), Self-mutilation/pulling hair or ears, scratching, etc. (“Self-mutilation”), and Biting self & others. Any participant with a result of “25% of the time” (equivalent to score ‘1’) or more for any of those 4 MBA items was considered as having an “Externalizing profile” for our behavioral cohort analysis.
2.3.3. Child Health Questionnaire - Parent Form 50 (CHQ-PF50)
The CHQ-PF50 is a validated and generic quality of life measure. It was designed for caregivers to complete in reference to their children, ages 5 to 18; however, in RTT the CHQ-PF50 has been applied to the entire age range of the RNHS cohort that includes participants younger than 5 years and older than 18 years [6, 28, 29, 30]. In this study, the same caregiver was expected to complete the form prior to each study visit. The questionnaire is comprised of 12 scales (Physical Functioning, Role/Social Limitations – Emotional/Behavioral, Role/Social Limitations – Physical, Bodily Pain/Discomfort, Behavior, Mental Health/Well-being, Self Esteem, General Health Perceptions, Parental Impact – Emotional, Parental Impact – Time, Family Activities and Family Cohesion) and 2 stand-alone items (Global Health and Global Behavior Items). The Mental Health/Well-being (MH) scale from the CHQ-PF50 was analyzed to quantify internalizing behavior. Each item was scored using a 5-level Likert-type scale: “All of the time”, “Most of the time”, “Some of the time”, “A little of the time”, and “None of the time”. The raw scores from the 5 items that make up the MH specific questions were converted into a 0 to 100 scale. A lower MH score indicates that the child is displaying more prominent internalizing behaviors (e.g., feeling anxious, depressed). Below are the 5 items (a-e) that generate the MH scale:
During the past 4 weeks, how much of the time do you think your child:
Felt like crying?
Felt lonely?
Acted nervous?
Acted bothered or upset?
Acted cheerful?
Any participant with a response of “a little of the time” (equivalent to score ‘1’) or more for any item a-d was considered to have an “Internalizing profile” for our behavioral cohort analysis.
2.3.4. Current History and Medication Log
Clinicians recorded observation and parental report of numerous developmental skills, clinical features, and treatments at each visit that could be related to or influence behavior. Medication logs were also updated at each RNHS visit, including indication of medication entered as either free text or as a SNOMED code.
2.4. Statistical Analyses
As an initial study of behavioral profiles in RTT, we employed a combination of descriptive and comparative analyses. Descriptive analyses included a variety of indices of variability as well as frequency histograms. Due to the non-normal distribution and ordinal nature of the behavioral data, we used nonparametric tests for comparisons (i.e., Kruskal-Wallis test, Mann-Whitney) and correlations (i.e., Spearman’s rho correlation coefficient; Chi-square test). Considering that this was an exploratory study, tables present all analyses. We have emphasized in the text significant results that survived multiple comparison corrections by the Bonferroni method. Changes in behavioral items were examined through linear regression analyses with age as an independent variable. Analyses were performed using IBM SPSS 24.0.
2.5. Human Studies Approval
Parental consent for study conduct and publication of results was obtained prior to entry into the study for all participants. Each participating institution retained institutional review board approval for the implementation of this study protocol and consent form (ClinicalTrial.gov; Identifier: NCT00299312).
3. Results
3.1. Characteristics of the Study Population
861 females with RTT over the age of 3 years with baseline MBA results were included in this study. We restricted the lower age limit to evaluate predominantly individuals at post-regression stages, with relatively stable behavioral profiles. At baseline, the mean age was 11.25 9.0 years with a range of 3 to 66 years. 85.5% (n=736) of participants were diagnosed with classic RTT, 7.7% (n=66) atypical mild, and 6.9% (n=59) atypical severe. MECP2 mutations were found in 93.4% (n=804) of all participants with RTT: this included 96.3% with classic RTT, 83.3% with atypical mild RTT, and 67.8% with atypical severe RTT. 82.2% (n=711) of participants with RTT had baseline CHQ data and therefore could be assigned a behavioral cohort for this study. Participants with RTT had a total of 4,755 visits over a maximum of 9.5 years. Non-RTT females (n=48) were analyzed as a comparison cohort: 100% had MECP2 mutations, 72.9% had both CHQ and MBA data at baseline. Additional demographic information has been reported previously [26, 29].
3.2. Prevalence and Severity of Internalizing and Externalizing Behaviors
Internalizing behaviors were assessed using the 5 items of the CHQ Mental Health/Well-being (MH) subscale. Each internalizing behavior was present in over half of the participants with RTT, with severity in the mild to moderate range (most occurring “A little of the time” or “Some of the time”) (Fig. 1). However, score distribution of the “Acted upset” was shifted towards higher severity with relatively fewer participants scored as “None of the time” and more with “Some of the time”. Contrasting with the “negative” items, the only “positive” (non-problematic) behavior, “Acted cheerful”, was scored predominantly as “Most of the time”. While CHQ MH subscale scores and most individual item scores were similar among all diagnostic groups, atypical mild participants did have worse scores for “Acted nervous” (Table 1). Internalizing behaviors were also found to be more severe in participants with RTT and mild MECP2 mutations compared to participants with either moderate or severe mutations (Table 2).
Fig. 1.

Frequency of internalizing behaviors from the CHQ Mental Health Subscale in RTT (all participants).
Note. CHQ = Child Health Questionnaire. RTT= Rett syndrome.
Table 1.
Scores of Internalizing Behaviors from the CHQ Mental Health Subscale in RTT
| Internalizing Behaviors | ||||||
|---|---|---|---|---|---|---|
| Diagnosis Group | CHQ Mental Health Subscale (Range 5– 100) |
CHQ Individual Items (Range 1–5) |
||||
| Felt lonely | Felt like crying |
Acted nervous | Acted bothered or upset |
Acted cheerful |
||
| Classic (n=610) |
68.73 ± 15.0 | 4.09 ± 1.0 | 3.69 ± 0.8 | 3.84 ± 1.0 | 3.45 ± 0.8 | 3.69 ± 0.8 |
| Atypical Mild (n=55) |
64.91 ± 16.5 | 4.07 ± 1.0 | 3.55 ± 0.9 | 3.24 ± 1.1***↓ | 3.22 ± 0.8 | 3.91 ± 0.6 |
| Atypical Severe (n=46) |
70.22 ± 13.9 | 4.04 ± 1.0 | 4.00 ± 0.9 | 4.14 ± 0.9 | 3.51 ± 0.7 | 3.42 ± 1.0 |
| Non-RTT (n=48) | 62.57 + 14.1 | 3.83 ± 1.0 | 3.40 ± 0.8 | 3.54 ± 1.1 | 3.11 ± 0.8*↓ | 3.63 ± 0.6 |
Note. All values are reported as mean ± SD. Lower scores indicate more severe internalizing behaviors. All score comparisons are in reference to classic RTT cohort using the non-parametric Independent Samples Kruskal-Wallis Test. Bolded values are significantly ↓ lower than classic RTT;
p <.05,
p <.001.
Significance values have been adjusted by the Bonferroni correction for multiple comparisons. CHQ = Child Health Questionnaire. RTT= Rett syndrome.
Table 2.
Behavior scores based on MECP2 mutation severity categories
| Mutation severity | Internalizing score (range 5– 100) † |
Externalizing scores (range 0–4) | |||
|---|---|---|---|---|---|
| CHQ MH Subscale |
Irritability | Self- mutilation |
Aggressive behavior |
Biting self & others |
|
| Mild (n= 360) | 66.15 ± 15.6 | 0.51 ± 0.9 | 0.33 ± 0.8 | 0.34 ± 0.8 | 0.33 ± 0.8 |
| Moderate (n=7) | 72.79 ± 13.3**↓ | 0.47 ± 0.8 | 0.25 ± 0.7 | 0.15 ± 0.4 | 0.28 ± 0.6 |
| Severe (n= 348) | 69.48 ± 14.6*↓ | 0.43 ± 0.8 | 0.20 ± 0.6**↓ | 0.11 ± 0.4***↓ | 0.16 ± 0.5**↓ |
Note. Analyses of entire RTT cohort, regardless of clinical presentation. All values are reported as mean ± SD. Severe mutations = R106W, R168X, R255X, R270X, insertions, deletions, large deletions, and splice site; Moderate mutation = T158M; Mild mutations = R133C, R294X, R306C, 3’ truncations, and other point mutations.
Lower scores indicate more severe internalizing behaviors; higher scores indicate greater impairment for externalizing items. Values in bold are significantly different from the mild group based on non-parametric IndependentSamples Kruskal-Wallis Test;
p <0.05,
p <0.01,
p <0.001.
=more severe
=less severe.
CHQ MH= Child Health Questionnaire Mental Health Subscale.
In addition, internalizing behaviors were evaluated in the population expected to be more affected due to reported medication use for anxiety or reported use of an SSRI. Individuals with RTT who were being treated for anxiety with any medication had lower CHQ MH scores (i.e., more severe internalizing behaviors) than their untreated counterparts (Table 3). Similarly, those participants with RTT on an SSRI for anxiety or for any indication had worse internalizing behavior.
Table 3.
Scores of Internalizing Behaviors based on SSRI Use or Any Medication Use for Anxiety in RTT
| Clinical Feature | Present | CHQ MH (mean ± SD) |
|---|---|---|
| Anxiety – on any medication | Yes (n=118) No (n=590) |
64.83 ± 16.5** 69.27 ± 14.6 |
| Anxiety – on an SSRI | Yes (n=81) No (n=627) |
63.40 ± 17.5**
69.19 ± 14.6 |
| Any SSRI Use | Yes (n=106) No (n=602) |
62.55 ± 17.5***
69.58 ± 14.3 |
Note. Sample: All RTT participants. Non-Parametric Independent Sample Mann-Whitney U Test
p <0.05
p <0.01
p <0.001.
Bolded values are more severe than untreated counterparts. CHQ MH = Child Health Questionnaire Mental Health Subscale. RTT= Rett syndrome.
Externalizing behaviors were assessed using 4 selected items from the MBA. Contrasting with internalizing behaviors, externalizing behaviors were reported in only a minority of participants with RTT and when one was reported, it was usually infrequent (e.g., observed “25% of the time”) (Fig. 2). Score distributions were similar for the 4 items and severity scores for each externalizing behavior were similar among all of the RTT diagnostic groups, with the exception of more “Aggressive behavior” in the atypical mild cohort (Table 4). Participants with mild MECP2 mutations had more severe scores for 3 of the 4 externalizing items (“Self-mutilation”, “Aggressive behavior”, and “Biting self & others”) compared to those with severe mutations; externalizing scores were similar between the mild and moderate mutation groups (Table 2). Neither anxiety nor SSRI use was associated with differences in externalizing behaviors in RTT (p >.05).
Fig. 2.
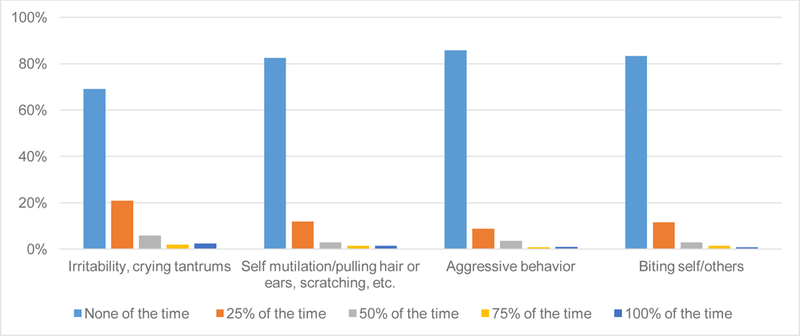
Frequency of externalizing behaviors from the MBA in RTT (all participants).
Note. MBA = Motor Behavioral Assessment. RTT= Rett syndrome.
Table 4.
Scores of Externalizing Behaviors from the MBA in RTT
| Externalizing Behaviors |
||||
|---|---|---|---|---|
| MBA Individual Items (Range 0–4) |
||||
| Diagnosis Group | Irritability | Self-mutilation | Aggressive behavior |
Biting self & others |
| Classic (n=736) | 0.46 ± 0.9 | 0.26 ± 0.7 | 0.21 ± 0.6 | 0.24 ± 0.6 |
| Atypical Mild (n=66) | 0.53 ± 0.9 | 0.36 ± 0.8 | 0.52 ± 1.0**↑ | 0.30 ± 0.8 |
| Atypical Severe (n=59) | 0.63 ± 1.0 | 0.34 ± 0.8 | 0.10 ± 0.4 | 0.31 ± 0.7 |
| Non-RTT (n=48) | 0.25 ± 0.7 | 0.38 ± 1.0 | 0.08 ± 0.3 | 0.02 ± 0.1 |
Note. All values are reported as mean ± SD. Scores are directly related to severity. All score comparisons are in reference to classic RTT cohort using the non-parametric Independent Samples Kruskal-Wallis Test. Bolded values are significantly ↑ higher than classic RTT;
p-value<.001.
Significance values have been adjusted by the Bonferroni correction for multiple comparisons. MBA = Motor Behavioral Assessment. RTT= Rett syndrome.
We confirmed the expected inverse relationship between Internalizing and Externalizing items as well as the positive relationship among Externalizing items (Table 5). Although these findings were statistically significant, the magnitude of the correlations were low or negligible.
Table 5.
Spearman’s Rho Correlations of Internalizing and Externalizing Behavior Scores in RTT
| Internalizing Externalizing |
||||||
|---|---|---|---|---|---|---|
| Behavior | Subscale or Item | CHQ MH | Irritability | Self- mutilation |
Aggressive behavior |
Biting self & others |
| Internalizing | CHQ MH | 1 | −.141** | −.086* | −.080* | −.126** |
| Externalizing | Irritability | −.141** | 1 | .228** | .220** | .238** |
| Self-mutilation | −.086* | .228** | 1 | .293** | .468** | |
| Aggressive behavior |
−.080* | .220** | .293** | 1 | .308** | |
| Biting self & others | −.126** | .238** | .468** | .308** | 1 | |
Note. p <0.05,
p <0.01;
− 0.3 to 0.3 = negligible correlations, 0.3 to 0.5 = low positive correlations (also shown in bold print); no moderate or high correlations were found. CHQ MH = Child Health Questionnaire Mental Health Subscale. RTT= Rett syndrome.
3.3. Behavioral profiles
Using the selected CHQ and MBA items, participants were separated into four distinct cohorts based on behaviors displayed at baseline: Internalizing-only, Externalizing-only, Mixed (Internalizing and Externalizing), or Neither. Overwhelmingly in RTT, participants displayed internalizing behaviors; about half (52.0%) had only internalizing behaviors and half (45.1%) had concurrent externalizing behaviors. Behavioral profiles did not vary by RTT diagnosis, but a relationship with mutation severity was confirmed. Externalizing behaviors were overrepresented in the mild MECP2 mutation group as compared to the severe mutation group (p <.05); the mild mutation group had more participants profiled as Mixed (49.8% vs. 37.2%) and fewer as Internalizing-only (48.0% vs. 61.0%). Because of the small number of participants with RTT in the Neither (n=15) and Externalizing-only (n=7) categories, we excluded them from additional analyses.
The Internalizing-only and Mixed behavioral profiles were compared in terms of a variety of characteristics, including key features of RTT. The Mixed behavioral cohort was younger and had lower (i.e., less severe) CSS total scores than the Internalizing-only cohort (Table 6). The behavioral profiles showed no difference in MBA total scores (adjusted to remove externalizing items), another indicator of overall severity. These findings held true in the classic RTT cohort, but no significant differences in age or overall severity were noted within the two atypical groups.
Table 6.
Differences in age and clinical severity between behavioral cohorts in RTT overall and by diagnosis group.
| Diagnosis Group |
Behavioral Profile | Age (Range 3–49 years) |
CSS Total (Range 1–47) |
MBA Total [excluding externalizing items] (Range 7–92) |
|---|---|---|---|---|
| All RTT | Internalizing-only (n=368) Mixed (n=319) |
12.03 ± 8.9 9.86 ± 7.8**↓ |
23.61 ± 8.4 20.84 ± 8.1***↓ |
47.03 ± 15.3 46.92 ± 15.1 |
| Classic | Internalizing-only (n=319) Mixed (n=271) |
12.06 ± 8.9 10.12 ± 8.1**↓ |
24.11 ± 7.9 21.27 ± 7.6***↓ |
48.13 ± 14.5 50.47 ± 15.1 |
| Atypical Mild | Internalizing-only (n=27) Mixed (n=28) |
10.09 ± 6.6 8.65 ± 5.2 |
11.78 ± 4.4 10.86 ± 4.8 |
25.65 ± 10.9 28.78 ± 11.2 |
| Atypical Severe | Internalizing-only (n=22) Mixed (n= 20) |
13.92 ± 11.4 8.02 ± 6.2 |
31.00 ± 5.8 29.05 ± 5.2 |
56.32 ± 11.1 56.35 ± 11.5 |
Note. All values reported as mean ± SD. Bolded values are significantly different from Internalizing-only cohort based on non-parametric Independent Samples t-test;
p-value<.05
p-value<.01
p-value<.001;
=higher
=lower.
CSS = Clinical Severity Score. MBA = Motor Behavioral Assessment. RTT= Rett syndrome.
To further investigate these characteristics in classic RTT, specific developmental and clinical features along with therapies commonly used in the disorder were compared between the Internalizing-only and Mixed behavioral profile cohorts (Table 7). All developmental features were more advanced in the Mixed behavioral cohort, with significant differences found in more complex gross and fine motor skills (“Holds cup/bottle to drink”, “Walks unsupported”, “Hand use to feed self”, “Standing unsupported”). “Self-Abuse” was strongly related to behavioral profile, with a higher rate in the Mixed behavioral cohort. While this association is expected based on the definition of the Mixed behavioral profile, this provides consistent evidence from separate data collection points within the RNHS (i.e., MBA and Current History). Although no other clinical feature or treatment was significantly different between Internalizing-only and Mixed behavioral profiles after stringent correction for multiple comparisons, a few approached significance. Specifically, seizures, constipation, and frequent daytime naps were more prevalent in the Internalizing-only behavioral cohort.
Table 7.
Relationship between Behavioral Profile, Developmental and Clinical Features, and Treatments for Participants with Classic RTT.
| Category | Total % (n=687) |
Internalizing- only Behavioral Cohort % (n=368) |
Mixed Behavioral Cohort % (n=319) |
χ2 | Fisher’s Exact p-value |
|
|---|---|---|---|---|---|---|
| Developmental Features | ||||||
| Holds cup/bottle to drink | 43.2 | 35.4 | 52.4 | 17.205* | 0.000 | |
| Walking unsupported | 52.4 | 45.5 | 60.5 | 13.326* | 0.000 | |
| Hand use to feed self | 46.6 | 40.1 | 54.2 | 11.736* | 0.001 | |
| Standing unsupported | 55.1 | 48.9 | 62.4 | 10.727* | 0.001 | |
| Walking supported | 73.6 | 68.7 | 79.3 | 8.599 | 0.004 | |
| Pincer grasp | 21.0 | 16.9 | 25.8 | 6.995 | 0.009 | |
| Verbalize with meaning | 23.7 | 20.4 | 27.7 | 4.313 | 0.042 | |
| Standing supported | 88.3 | 85.9 | 91.1 | 3.911 | 0.054 | |
| Manipulates objects with hands | 44.4 | 44.2 | 44.6 | 0.012 | 0.934 | |
| Clinical Features | ||||||
| General | Self-Abuse | 35.4 | 25.4 | 47.2 | 30.554* | 0.000 |
| Seizures | 60.0 | 65.2 | 53.9 | 7.836 | 0.005 | |
| Cool hands | 49.3 | 48.9 | 49.8 | 0.049 | 0.869 | |
| Cool feet | 80.0 | 82.4 | 77.1 | 2.595 | 0.121 | |
| Gastrointestinal | Constipation | 82.0 | 86.2 | 77.1 | 8.206 | 0.005 |
| Gastroesophageal reflux |
44.2 | 41.1 | 48.0 | 2.832 | 0.097 | |
| Gallbladder dysfunction |
1.9 | 2.5 | 1.1 | 1.571 | 0.210 | |
| Diarrhea | 9.0 | 8.2 | 10.0 | 0.589 | 0.472 | |
| Sleep | Frequent daytime naps |
37.1 | 42.3 | 31.0 | 8.049 | 0.005 |
| Difficulty arousing in the morning |
13.6 | 12.2 | 15.1 | 1.054 | 0.335 | |
| Difficulty going to sleep |
23.9 | 22.3 | 25.8 | 1.029 | 0.333 | |
| Wakes frequently during night |
41.5 | 39.8 | 43.5 | 0.840 | 0.402 | |
| Breathing | Hyperventilation | 54.9 | 53.3 | 56.8 | 0.740 | 0.407 |
| Breath holding | 72.7 | 71.5 | 74.2 | 0.537 | 0.516 | |
| Puffing air or saliva |
51.4 | 50.2 | 52.8 | 0.400 | 0.563 | |
| Aerophagia | 48.5 | 48.3 | 48.7 | 0.011 | 0.934 | |
| Oromotor | Drooling | 76.9 | 77.7 | 76.0 | 0.247 | 0.625 |
| Chokes easily | 42.4 | 41.7 | 43.2 | 0.132 | 0.739 | |
| Effective chewing |
55.9 | 56.1 | 55.7 | 0.009 | 0.934 | |
| Bruxism | 78.0 | 78.1 | 77.9 | 0.003 | 1.000 | |
| Treatments | ||||||
| Occupational therapy | 77.5 | 74.3 | 81.2 | 3.979 | 0.048 | |
| Attends school/adult program | 90.3 | 88.4 | 92.6 | 2.988 | 0.094 | |
| Music therapy | 16.1 | 14.4 | 18.1 | 1.454 | 0.261 | |
| Hippotherapy | 15.9 | 17.6 | 14.0 | 1.365 | 0.260 | |
| Speech therapy | 76.1 | 74.3 | 78.2 | 1.247 | 0.287 | |
| Any SSRI Use | 15.6 | 14.7 | 16.6 | 0.390 | 0.570 | |
| Anxiety – on any medication | 17.3 | 16.0 | 18.8 | 0.822 | 0.383 | |
| Physical therapy | 81.5 | 82.4 | 80.4 | 0.390 | 0.595 | |
| Swimming therapy | 16.3 | 15.7 | 17.0 | 0.182 | 0.737 | |
| Other therapy | 13.1 | 12.5 | 13.7 | 0.160 | 0.714 | |
| Anxiety – on an SSRI | 11.9 | 12.2 | 11.4. | 0.870 | 0.799 | |
| No therapy | 8.8 | 9.1 | 8.5 | 0.066 | 0.884 | |
Note. p <.00125 after Bonferroni corrections for multiple comparisons. RTT = Rett syndrome.
3.4. Comparison of RTT with other cohorts
Compared to participants with classic RTT, individuals with MECP2 mutations who did not meet diagnostic criteria for RTT had similar internalizing and externalizing item scores for nearly all items; only “Acted bothered” was more common in this population (Table 1, Table 4). Non-RTT participants were categorized into Internalizing-only (65.7%) or Mixed (34.3%) behavioral profiles with rates similar to participants with RTT (p=0.376).
CHQ MH subscale scores from other reported cohorts were also compared to the classic RTT group (Fig. 3). Classic RTT participants had more frequent internalizing behavior than the general pediatric population, the general population of females, and an epilepsy group; on the other hand, participants with RTT had scores similar to individuals with psychiatric disorders [31].
Fig. 3.
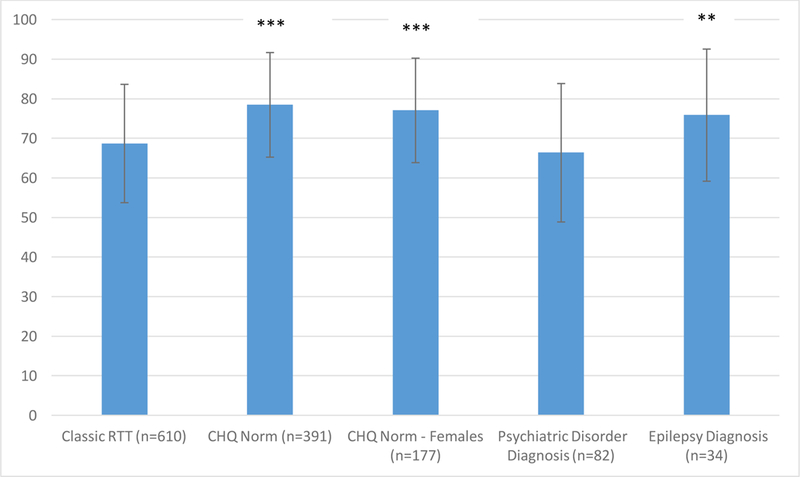
Mean CHQ Mental Health Subscale scores from the RNHS and other reported cohorts (HealthActCHQ, 2013). Note. *p <.05 **p <.01 ***p <.001 in comparison to the classic RTT group. Error bars indicate standard deviation of the mean. CHQ = Child Health Questionnaire. RNHS = Rett Natural History Study.
3.5. Behavioral profiles over time
Internalizing and externalizing behaviors were evaluated over time by combining all visits for participants with RTT in linear regression models using age as the independent variable (Table 8). Internalizing behaviors based on overall CHQ MH subscale scores did not change with age in the overall cohort. However, three CHQ MH items were influenced by age with variable magnitude and direction. Confirming the association of externalizing behaviors with younger age, three of the four externalizing items (“Irritability”, “Aggressive behavior”, and “Biting self & others”) decreased in frequency with age.
Table 8.
Linear regression analyses of behavioral items and profiles in RTT
| Cohort | Behavior item or subscale | R2 | F | Standardized β |
Sig. |
|---|---|---|---|---|---|
| All RTT | CHQ MH | 0.000 | 0.495 | −0.013 | 0.482 |
| Felt like crying↑ | 0.006 | 18.367 | 0.079 | 0.000*** | |
| Felt lonely↓ | 0.002 | 6.470 | −0.047 | 0.011* | |
| Acted nervous↑ | 0.003 | 9.257 | 0.056 | 0.002** | |
| Acted bothered or upset | 0.001 | 1.742 | 0.024 | 0.187 | |
| Irritability↓ | 0.011 | 54.597 | −0.107 | 0.000*** | |
| Self-mutilation | 0.000 | 0.497 | −0.010 | 0.481 | |
| Aggressive behavior↓ | 0.006 | 27.593 | −0.076 | 0.000*** | |
| Biting self & others↓ | 0.013 | 59.958 | −0.112 | 0.000*** | |
| Internalizing-only | CHQ MH | 0.003 | 3.699 | 0.052 | 0.055 |
| Felt like crying↑ | 0.015 | 20.208 | 0.121 | 0.000*** | |
| Felt lonely | 0.002 | 2.669 | 0.044 | 0.103 | |
| Acted nervous↑ | 0.010 | 14.463 | 0.102 | 0.000*** | |
| Acted bothered or upset↑ | 0.004 | 5.413 | 0.063 | 0.020* | |
| Irritability | 0.001 | 1.577 | −0.028 | 0.209 | |
| Self-mutilation↑ | 0.004 | 7.144 | 0.059 | 0.008** | |
| Aggressive behavior | 0.000 | 0.605 | −0.017 | 0.437 | |
| Biting self & others | 0.001 | 2.607 | −0.036 | 0.107 | |
| Mixed | CHQ MH↓ | 0.015 | 19.88 | −0.124 | 0.000*** |
| Felt like crying | 0.000 | 0.057 | 0.007 | 0.812 | |
| Felt lonely↓ | 0.173 | 39.174 | −0.173 | 0.000*** | |
| Acted nervous | 0.012 | 0.18 | −0.012 | 0.671 | |
| Acted bothered or upset | 0.002 | 2.414 | −0.043 | 0.121 | |
| Irritability↓ | 0.012 | 21.937 | −0.107 | 0.000*** | |
| Self-mutilation | 0.001 | 1.827 | −0.031 | 0.177 | |
| Aggressive behavior ↓ | 0.005 | 8.999 | −0.069 | 0.003** | |
| Biting self & others↓ | 0.015 | 28.546 | −0.122 | 0.000*** |
Note. Analyses of entire RTT cohort, regardless of clinical presentation. Bold text indicates significance.
p <.05
p <.01
p <.001;
= increase over time
= decrease over time for significant items.
When split by baseline behavioral profile, the Internalizing-only and Mixed cohorts had different trajectories over time (Table 8). The Internalizing-only cohort had no change in overall CHQ MH scores while the Mixed cohort scores decreased, indicating worsening of internalizing behaviors in the Mixed cohort over time. By definition, the Mixed cohort had higher initial externalizing scores, and three out of four items (“Irritability”, “Aggressive behavior”, and “Biting self & others”) decreased in frequency over time. “Self-mutilation” was the only externalizing behavior that changed in the Internalizing-only cohort, increasing in frequency over time.
Behavioral profile cohorts were also examined at all visits, which confirmed the high prevalence of internalizing behaviors over time (Table 9). Relative stability was noted in the participants who were categorized as Internalizing-only at baseline, with 83.4% of participants reporting only internalizing behaviors at subsequent visits. Externalizing behaviors were reported in 13.9% of subsequent visits from the initial Internalizing-only group, indicating that externalizing behaviors rarely develop if not present by age 3. In contrast, 42.2% of the Mixed cohort at baseline had continued reports of Mixed behaviors, but the majority (57.3%) of subsequent visits in this cohort actually had no externalizing behaviors reported.
Table 9.
Consistency of Behavioral Profiles over time in RTT
| Baseline Behavioral Profile |
Behavioral Profile at Subsequent Visits |
Total number of visits |
|||
|---|---|---|---|---|---|
| Internalizing-only | Externalizing-only | Mixed | Neither | ||
| Internalizing-only (n=368) |
833 | 1 | 138 | 27 | 999 |
| Mixed (n=319) |
532 | 5 | 403 | 16 | 956 |
Pearson Chi-Square = 200.812; p <.001
4. Discussion
This survey of internalizing and externalizing behaviors in the largest available RTT cohort, the U.S. RNHS, describes the main features and related parameters of these abnormal behaviors, along with their association in behavioral profiles that can be applied to the management of RTT. Using a validated pediatric measure of mental health and quality of life, which has already been applied to RTT, we found that the presence of internalizing behaviors in the mild to moderate range is nearly universal in individuals with RTT. In contrast, externalizing behaviors, evaluated with a clinical severity scale developed specifically for the RNHS and applied in many studies of RTT, are relatively rare and mild. These results suggest that, as in previous reports on anxiety and other behavioral abnormalities in RTT, externalizing or disruptive behaviors are uncommon because of the severe motor impairment. To gain further insight into problem behaviors in RTT, and with the goal of developing behavioral profiles that could be used clinically, we examined whether internalizing and externalizing behaviors associated in distinctive patterns. Indeed, two behavioral profiles emerged: Internalizing-only and Mixed (Internalizing and Externalizing). The two approximately equal-sized groups of individuals with RTT differed in age and clinical severity, with the Mixed cohort being younger and less affected clinically. This finding supported the notion that motor and overall clinical severity have an impact on the expression of abnormal behaviors in RTT, which could be reflected in the evolution of the disorder since clinical impairment worsens with age. This was confirmed by an evaluation of behavioral changes over time, which revealed a relatively more dynamic evolution in the Mixed cohort. This group had a worsening of internalizing behaviors and an improvement in scores on externalizing behaviors with age. These changes should be interpreted with caution, as the decline in externalizing behaviors may simply reflect progressive motor impairment in RTT. Follow up studies are needed to determine if the suggested greater similarity between the two behavioral profile groups over time is part of the natural history of RTT.
A detailed comparison of key features of RTT revealed that the distinction between Internalizing-only and Mixed behavioral profiles was related to certain clinical features. The Mixed group showed more advanced gross and fine motor skills at baseline. Supporting the relevance of two behavioral profiles, the Internalizing-only cohort had slightly greater severity of major clinical problems in RTT, namely constipation, seizures and daytime sleepiness. In contrast, the Mixed group had highly prevalent self-injurious behaviors. These initial clinical behavioral associations have implications in terms of RTT management, suggesting targets for prevention or more aggressive treatment.
While some differences exist in specific internalizing and externalizing behaviors among RTT subgroups, these appeared to be rather a reflection of clinical severity. Similarly, comparisons between RTT and non-RTT individuals with MECP2 mutation indicate that the reported behavioral profiles are present in general in individuals with MECP2 mutation and not exclusively in RTT. Comparisons between our RTT cohort and others previously characterized by the CHQ MH suggested that, regardless of diagnosis, the internalizing behaviors in RTT are clinically relevant. Scores on the CHQ MH in classic RTT are comparable to those in a group with a variety of psychiatric diagnoses and worse than in patients with epilepsy and in the general population. Emphasizing the clinical importance of internalizing behaviors in RTT is our exploratory analysis of individuals in the RNHS who were treated with medications for the indication of anxiety or were on medications usually prescribed for anxiety (i.e., SSRIs). These groups had more severe profiles of internalizing behaviors than their non-treated counterparts.
In conclusion, this survey of abnormal behaviors in RTT revealed that internalizing behaviors are common and at a clinically significant level. A combination of internalizing and externalizing behaviors is linked to younger age, better motor function, and mild MECP2 mutations, supporting previous studies that patients with RTT and less severe clinical presentation tend to have more prominent behavioral problems. Identification of developmental and clinical features associated with behavioral profiles could be used for developing prevention and treatment strategies in RTT. Despite its large cohort size and body of clinical data, the study presented several limitations. First, it employed two different measures for each category of abnormal behaviors and only the CHQ is a fully validated instrument. Nonetheless, the MBA has been used in more than a dozen studies of the natural history of RTT that have resulted in consistent profiles of clinical severity. This shortcoming could be addressed in the future by large-scale surveys with validated behavioral measures. Another limitation is that the examination of longitudinal changes in behavior used relatively simple statistical models; however, we considered it appropriate since this was a first general evaluation of these features. The analysis of the relationship between behavioral profiles and psychiatric diagnoses in RTT was restricted to anxiety and, in particular, to participants on medications for this indication. Psychiatric diagnoses are challenging in individuals with severe neurodevelopmental disorders such as RTT and diagnostic data are scarce. Nevertheless, as mental health issues continue to be a major concern in RTT, future research will need to identify which behaviors underlie specific psychiatric disorders. We hope the current study will serve as a catalyst for additional studies focused on behavioral and mental health issues, which substantially affect the quality of life of individuals with RTT.
Acknowledgments
The authors thank the caring participants, their families and Rettsyndrome.org for the support of the study.
Funding
This study was supported by National Institutes of Health (NIH) U54 grant HD061222, part of the NIH Rare Disease Clinical Research Network (RDCRN) and supported through a collaboration between the NIH Office of Rare Diseases Research (ORDR) at the NationalCenter for Advancing Translational Sciences (NCATS), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institutes of Health had no involvement in the study other than to promote the analyses of the Natural History Study dataset.
Footnotes
Authors’ disclosures
CBB received research support from Neuren and participates in clinical trials sponsored by Ovid. WEK is consultant to Anavex, Neuren, Edison, Newron, EryDel, Marinus, AveXis, Biohaven, Ovid, GW Pharmaceuticals, Zynerba, and Stalicla. DGG is a consultant to Newron Pharmaceuticals and participates in clinical trials sponsored by Neuren and Newron Pharmaceuticals. SAS is a consultant to AveXis and participates in clinical trials supported by Neuren and Ovid. JLN is a consultant to AveXis, Biohaven, Ovid, Teva, and Takada
Pharmaceuticals and participates in clinical trials sponsored by Neuren and Newron Pharmaceuticals. AKP is a consultant with Neuren Pharmaceuticals, Anavex, AveXis, and Teva and participates in clinical trials sponsored by Neuren and Newron Pharmaceuticals. The other authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
No authors have conflicts of interest to disclose. The authors agree to allow the journal to review their data if requested.
References
- [1].Fehr S, Bebbington A, Nassar N, Downs J, Ronen GM, N DEK, et al. Trends in the diagnosis of Rett syndrome in Australia. Pediatr Res 2011;70:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cuddapah VA, Pillai RB, Shekar KV, Lane JB, Motil KJ, Skinner SA, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J Med Genet 2014;51:152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaufmann WE, Johnston MV, Blue ME. MeCP2 expression and function during brain development: implications for Rett syndrome’s pathogenesis and clinical evolution. Brain Dev 2005;27. [DOI] [PubMed]
- [4].Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol 2010;68:944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anderson A, Wong K, Jacoby P, Downs J, Leonard H. Twenty years of surveillance in Rett syndrome: what does this tell us? Orphanet J Rare Dis 2014;9. [DOI] [PMC free article] [PubMed]
- [6].Barnes KV, Coughlin FR, O’Leary HM, Bruck N, Bazin GA, Beinecke EB, et al. Anxiety-like behavior in Rett syndrome: characteristics and assessment by anxiety scales. Journal of Neurodevelopmental Disorders 2015;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cianfaglione R, Clarke A, Kerr M, Hastings RP, Oliver C, Moss J, et al. A national survey of Rett syndrome: behavioural characteristics. Journal of Neurodevelopmental Disorders 2015;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mount RH, Charman T, Hastings RP, Reilly S, Cass H. The Rett Syndrome Behaviour Questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. J Child Psychol Psychiatry 2002;43:1099–110. [DOI] [PubMed] [Google Scholar]
- [9].Young DJ, Bebbington A, Anderson A, Ravine D, Ellaway C, Kulkarni A, et al. The diagnosis of autism in a female: could it be Rett syndrome? Eur J Pediatr 2008;167:661–9. [DOI] [PubMed] [Google Scholar]
- [10].American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5). Washington, D.C: American Psychiatric Publishing; 2013. [Google Scholar]
- [11].Buchanan-Pascall S, Gray KM, Gordon M, Melvin GA. Systematic Review and Meta-analysis of Parent Group Interventions for Primary School Children Aged 4–12 Years with Externalizing and/or Internalizing Problems. Child Psychiatry Hum Dev 2018;49:244–67. [DOI] [PubMed] [Google Scholar]
- [12].Dal Canto G, Pellacani S, Valvo G, Masi G, Ferrari AR, Sicca F. Internalizing and externalizing symptoms in preschool and school-aged children with epilepsy: Focus on clinical and EEG features. Epilepsy Behav 2018;79:68–74. [DOI] [PubMed] [Google Scholar]
- [13].Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: Cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet 2015;169:135–49. [DOI] [PubMed] [Google Scholar]
- [14].Stratis EA, Lecavalier L. Informant agreement for youth with autism spectrum disorder or intellectual disability: a meta-analysis. J Autism Dev Disord 2015;45:1026–41. [DOI] [PubMed] [Google Scholar]
- [15].Visootsak J, Sherman S. Neuropsychiatric and behavioral aspects of trisomy 21. Curr Psychiatry Rep 2007;9:135–40. [DOI] [PubMed] [Google Scholar]
- [16].Coleman M, Brubaker J, Hunter K, Smith G. Rett syndrome: a survey of North American patients. J Ment Def Res 1988;32:117–24. [DOI] [PubMed] [Google Scholar]
- [17].Esbensen AJ, Rojahn J, Aman MG, Ruedrich S. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. J Autism Dev Disord 2003;33. [DOI] [PubMed]
- [18].Halbach NS, Smeets EE, Schrander-Stumpel CT, van Schrojenstein Lantman de Valk HH, Maaskant MA, Curfs LM. Aging in people with specific genetic syndromes: Rett syndrome. Am J Med Genet A 2008;146A. [DOI] [PubMed] [Google Scholar]
- [19].Mount RH, Hastings RP, Reilly S, Cass H, Charman T. Behavioural and emotional features in Rett syndrome. Disabil Rehabil 2001;23. [DOI] [PubMed] [Google Scholar]
- [20].Naidu S, Murphy M, Moser HW, Rett A. Rett syndrome--natural history in 70 cases. Am J Med Genet Suppl 1986;1:61–72. [DOI] [PubMed] [Google Scholar]
- [21].Robertson L, Hall SE, Jacoby P, Ellaway C, de Klerk N, Leonard H. The association between behavior and genotype in Rett syndrome using the Australian Rett Syndrome Database. Am J Med Genet B Neuropsychiatr Genet 2006;141B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sansom D, Krishnan VH, Corbett J, Kerr A. Emotional and behavioural aspects of Rett syndrome. Dev Med Child Neurol 1993;35:340–5. [DOI] [PubMed] [Google Scholar]
- [23].Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, et al. Psychometric study of the Aberrant Behavior Checklist in Fragile X Syndrome and implications for targeted treatment. J Autism Dev Disord 2012;42:1377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ross E, Oliver C. The relationship between levels of mood, interest and pleasure and ‘challenging behaviour’ in adults with severe and profound intellectual disability. J Intellect Disabil Res 2002;46:191–7. [DOI] [PubMed] [Google Scholar]
- [25].Vos P, Cock P, Petry K, Noortgate W, Maes B. What makes them feel like they do? Investigating the subjective well-being in people with severe and profound disabilities. Res Dev Disabil 2010;31. [DOI] [PubMed]
- [26].Tarquinio DC, Hou W, Berg A, Kaufmann WE, Lane JB, Skinner SA, et al. Longitudinal course of epilepsy in Rett syndrome and related disorders. Brain 2017;140:306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Neul JL, Lane JB, Lee HS, Geerts S, Barrish JO, Annese F, et al. Developmental delay in Rett syndrome: data from the natural history study. J Neurodev Disord 2014;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Killian JT, Lane JB, Lee HS, Pelham JH, Skinner SA, Kaufmann WE, et al. Caretaker Quality of Life in Rett Syndrome: Disorder Features and Psychological Predictors. Pediatr Neurol 2016;58:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lane JB, Lee HS, Smith LW, Cheng P, Percy AK, Glaze DG, et al. Clinical severity and quality of life in children and adolescents with Rett syndrome. Neurology 2011;77:1812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tarquinio DC, Hou W, Neul JL, Kaufmann WE, Glaze DG, Motil KJ, et al. The Changing Face of Survival in Rett Syndrome and MECP2-Related Disorders. Pediatr Neurol 2015;53:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].HealthActCHQ. The CHQ Scoring and Interpretation Manual. Boston, MA: HealthActCHQ; 2013. [Google Scholar]


