Abstract
Objectives
Autologous nerve grafting remains the gold standard for repair of peripheral nerve injuries. Its use, however, is limited by donor nerve availability and donor site morbidity. This is especially problematic after failure of an initial autograft that requires a repeat nerve graft, resulting in a second surgical site with associated morbidity. Based on the molecular differences in nerve degeneration in the proximal and distal segments after transection, we hypothesized that a chronically-denervated proximal stump may be viable for autologous nerve repair.
Methods
20 Sprague-Dawley rats underwent right sciatic nerve excision and sural nerve transection. After 8 weeks, nerve repair was performed by harvesting the proximal segment of the sural nerve (n=10) or a fresh sural nerve (n=10) from the contralateral hind limb. Electrophysiological changes were analyzed to compare the fresh and denervated grafts.
Results
Electrophysiological testing demonstrated higher compound motor action potential in the denervated group compared to the fresh autograft group, however this difference was not statistically significant (p=0.117).
Conclusion
The proximal segment of a chronically-denervated sural nerve can be as effective as a fresh sural nerve for autologous repair of peripheral nerve injuries in a rodent model.
Keywords: Delayed nerve repair, denervated graft, nerve autograft, proximal nerve stump
Introduction
Peripheral nerve injuries (PNI) are commonly associated with limb trauma and can be a significant cause of morbidity and permanent disability [1–5]. A previous study at a U.S. Level 1 trauma center identified a nearly 3% prevalence of peripheral nerve injuries in trauma patients [3]. Despite general medical advances and improvements in microsurgical techniques, these injuries continue to cause persistent motor and sensory deficits and chronic pain, leading to permanent disability [6]. Simple nerve transections can be repaired for the most part under minimal tension [7,8]. For segmental nerve defects, nerve allograft repair is an option but is limited by its high cost and the lack of cellular support and a suitable microenvironment [9]. Repair using autologous nerve graft, most commonly the sural nerve, remains the gold standard [10,11]. However, its use is restricted by the risk of donor site complications and, more importantly, the lack of available and expendable donor nerves [12–15]. This is especially problematic after failure of an initial autograft that requires a repeat nerve graft, resulting in a second surgical site with associated morbidity.
After nerve injury, Wallerian degeneration is initiated within 2 to 3 days and involves the breakdown of axons distal to the nerve lesion [16,17]. The same process occurs following harvesting of a donor nerve autograft distal to the graft site. Proximally, axonal degeneration takes place within the zone of injury and extends only to the next node of Ranvier [18]. The timing of these processes allow Schwann cells to proliferate, align, and form bands of Büngner, longitudinal columns of Schwann cells that induce and guide axonal extension from the proximal stump to the distal stump. The goal of microsurgical repair of an injured nerve, whether via autograft, allograft, or nerve conduit, is to provide the proximal axonal stump a pathway for this antegrade regeneration. The proximal stump may confer a regenerative advantage over the distal stump because of greater preservation of trophic support. Previous studies have revealed chronically-denervated nerves distal to the zone of injury are inferior to fresh donor nerves for nerve grafting [19]. However, the utility of the nerve segment proximal to the zone of injury has not been explored in animal or clinical studies.
The purpose of this study was to evaluate the viability of the proximal stump of a previously transected nerve. We hypothesized that the proximal segment of a chronically injured nerve can be as effective as a fresh donor autograft for nerve repair in rats. Translational implications include the potential to avoid complications associated with a new donor site and the loss of an intact, functional nerve in patients. This study was designed to assess neuromuscular recovery comparing a chronically-denervated versus freshly-harvested sural nerve autograft in a rodent model.
Materials and Methods
Animal model
20 male Sprague-Dawley rats weighing approximately 250 g were randomly assigned to either the denervated autograft group (DA, n=10) or the fresh autograft group (FA, n=10). They were housed in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care and were maintained on 12-hour light/dark cycles and supplied rat chow and water ad libitum. All experimental procedures were performed with the approval of the Institutional Animal Care and Use Committee.
Surgical procedure
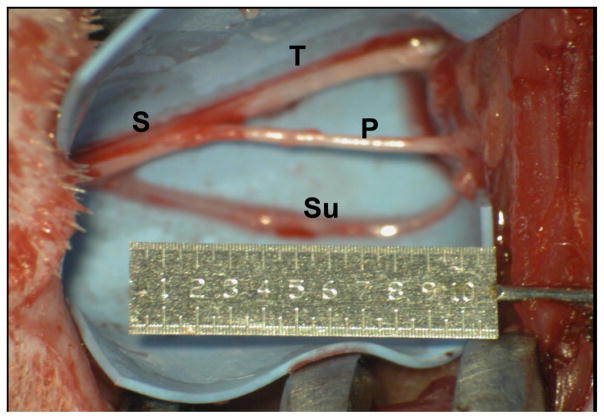
Surgeries were performed under aseptic conditions with isoflurane anesthesia. Using a standard biceps femoris-splitting approach, the right sciatic nerve was exposed along its course in the posterior thigh (Figure 1), and a 10 mm segment was excised. Next, the right sural nerve was dissected free 20 mm distal to its branching from the sciatic nerve and transected. Free nerve stumps were buried into the surrounding muscle and fixed with a single 10-0 monofilament nylon suture to minimize neuroma formation. Surgical wounds were thoroughly irrigated and closed with sutures. Postoperatively, the animals received buprenorphine for analgesia.
Figure 1.
Surgical exposure of the sciatic (S), tibial (T), common peroneal (P), and sural (Su) nerves.
After 8 weeks, the surgical site was re-opened. The sciatic nerve stumps were resected until healthy fascicles were reached. In the DA group, the proximal stump of the previously transected right sural nerve was dissected free, and a 20 mm segment was harvested to fashion a 10 mm cable graft. Additionally, a sham exposure of the left sural nerve was performed. In the FA group, an incision in the left hindlimb was created to expose the sural nerve. The same 20 mm segment was harvested to create the donor cable graft. In both groups, the cable graft was sutured into the 10 mm right sciatic nerve defect using three 10-0 monofilament nylon sutures in a radial fashion. The wound was irrigated and closed with sutures, and buprenorphine was administered for postoperative analgesia.
Compound motor action potential (CMAP)
8 weeks after nerve repair, each animal was anesthetized using ketamine injection, and the sciatic nerve was exposed bilaterally. The nerves were kept moist and warm. The compound motor action potential (CMAP) was measured using a Sierra® Wave system (Cadwell Industries, Inc., Kennewick, WA). Stimulating electrodes were inserted into the right sciatic nerve proximal to the graft site and in the tibial and common peroneal nerves distal to the graft site. A recording electrode was inserted into the gastrocnemius muscle. The nerves were stimulated with a constant current of 0.5 mA for 0.1 ms, repeated 3 times and the average amplitude was recorded. The protocol was similarly tested on the contralateral uninjured sciatic nerve to serve as an internal reference. The measured amplitude from the right hind limb was normalized to the measurement in the left hind limb for each rat.
Statistical analysis
SigmaStat 3.11 (Systat Software Inc., San Jose, CA) was used for data analysis. The data are presented as mean ± standard deviation. Direct pairwise comparisons were performed to compare function and CMAP between the animal groups. Statistical significance was set at p<0.05.
Results
All animal subjects survived for the duration of the study. At the time of CMAP measurement, the two strands of the sural nerve cable graft had scarred sufficiently to form a one-fascicled graft. In both the DA and FA groups, CMAP indicated functional recovery of the right sciatic nerve. Normalized mean amplitude was higher in the DA group (0.14 ± 0.092) versus the FA group (0.0582 ± 0.092). However, direct pairwise comparison demonstrated that this difference was not statistically significant (p=0.117) (Figure 2).
Figure 2.
Comparison of normalized CMAP amplitude through the graft site in the right sciatic nerve.
Discussion
Healthy donor nerves for autologous repair continue to be the gold standard and treatment of choice for surgeons to ensure the best outcomes. However, when there is a previously damaged nerve or a segment of a previously harvested nerve available, debate exists as to the possibility of that nerve serving as a donor autograft. The aim of our study was to assess the viability of the proximal stump of a chronically-denervated nerve as a potential nerve graft. Our work here demonstrated no significant differences in electrophysiological outcomes between denervated and fresh sural nerve autografts in rats.
Classically, denervated nerve tissue has been utilized to treat various defects. For example, irreversibly damaged ulnar nerves after substantial trauma have been harvested as vascularized nerve grafts [20,21]. With associated injury to the lower trunk of the brachial plexus, the ulnar nerve has also been harvested to treat injuries to other parts of the brachial plexus [19]. The advantages to this strategy include the harvesting of an expendable nerve in the same surgical field and the avoidance of morbidity associated with a separate harvest site. The ulnar nerve is indeed a viable option as studies have demonstrated survival of the entire length of the ulnar nerve with intact vascular supply from the superior ulnar collateral artery and its venae comitantes despite the poor likelihood of ulnar nerve recovery after direct repair [20,22]. However, in the clinical literature, positive results are limited to case reports and small case series. The indications for using this technique remain restricted to repair of the radial and median nerves in brachial plexus trauma. Whether the theoretical use of this technique can be applied to the sural nerve or other expendable nerves often harvested for autografts is still unknown. Moreover, the impact of the chronicity of denervation and the prolonged lack of axonal contact on nerve regenerative outcomes requires further studies in greater detail.
Isaacs et al. previously compared fresh and chronically-denervated autograft in a rodent nerve repair model [19]. However, their harvest was from the distal stump of a previously transected peroneal nerve. Histologically, longer periods of denervation led to decreased number of axons but increased axon diameter. Muscle belly diameter and weight was significantly decreased in the rats with the longest durations of denervation. Direct measurement of functional recovery using the sciatic functional index (SFI) was not performed as muscle diameter and weight were used for indirect characterization. Similarly in our study, we could not use SFI as a measure of functional recovery as both hind limbs of the rat underwent surgery. The lack of a normal extremity for print length and toe spread precludes the use of SFI calculations as previously described [23,24].
The results from Isaacs et al. are consistent with other animal studies on prolonged denervation and delayed nerve repair [25,26]. Physiologically, diminished muscle force after nerve injury is linked to a reduced number of axons [27]. With delayed primary nerve repair via ligation, the distal stumps demonstrated a significant decline in the number of axons and Schwann cell markers. Conversely, three months after nerve transection, axon numbers in the proximal stump increased two- to three-fold [28]. Vuorinen et al. examined the histological regeneration of axons after suturing a freshly axotomized tibial nerve to a chronically denervated (3 to 16 months) common peroneal nerve in rats [29]. Remnants of the bands of Büngner were discovered in areas of thin collagen fibrils within the endoneurial matrix. Axonal regeneration was demonstrated even in nerves that had been denervated for 16 months. However, regenerative success was negatively correlated with prolonged denervation. Using a similar rat model of anastomosing freshly-transected tibial nerves to chronically denervated common peroneal nerves, Sulaiman et al. supported these findings by showing that chronically denervated Schwann cells maintained the ability to re-myelinate axons but were unable to provide evidence for axonal regeneration [30,31]. In a separate study, Sulaiman et al. demonstrated superior nerve regeneration using FK506 (Tacrolimus) for chronically-axotomized nerves (2 month injured proximal tibial nerve) in comparison to chronically-denervated nerves (2 month injured distal common peroneal nerve) [32].
In combination with what has been put forth here, the proximal stump of a transected nerve may continue to serve a functional purpose after prolonged axotomy. The variability in the regenerative potential of the proximal and distal stumps of a chronically-denervated nerve suggests that Schwann cell denervation as a consequence of Wallerian degeneration plays a significant role. Our study is limited by the lack of functional recovery analysis and histological evaluation of the number and diameter of myelinated axons after repair. Nevertheless, our results further demonstrate that the proximal end of the previously axotomized nerve might carry on to serve an important function. Sulaiman et al. further indicated that its use in nerve repair may be augmented at the molecular level with gene and protein regulation to improve outcomes [32]. Whether its use as a nerve autograft outweighs the potential morbidity associated with harvesting a fresh autograft from another site is difficult to answer. We believe that more substantial research in animal models may be necessary before implementing this technique clinically.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- 1.Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, et al. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst. 2010;15:120–7. doi: 10.1111/j.1529-8027.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 2.Kouyoumdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006;34:785–8. doi: 10.1002/mus.20624. [DOI] [PubMed] [Google Scholar]
- 3.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–22. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Bekelis K, Missios S, Spinner RJ. Falls and peripheral nerve injuries: an age-dependent relationship. J Neurosurg. 2015;123:1223–9. doi: 10.3171/2014.11.JNS142111. [DOI] [PubMed] [Google Scholar]
- 5.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–5. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 6.Rivera JC, Glebus GP, Cho MS. Disability following combat-sustained nerve injury of the upper limb. Bone Joint J. 2014;96-B:254–8. doi: 10.1302/0301-620X.96B2.31798. [DOI] [PubMed] [Google Scholar]
- 7.Mavrogenis AF, Spyridonos SG, Antonopoulos D, Soucacos PN, Papagelopoulos PJ. Effect of sensory re-education after low median nerve complete transection and repair. J Hand Surg Am. 2009;34:1210–5. doi: 10.1016/j.jhsa.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Mailander P, Berger A, Schaller E, Ruhe K. Results of primary nerve repair in the upper extremity. Microsurgery. 1989;10:147–50. doi: 10.1002/micr.1920100218. [DOI] [PubMed] [Google Scholar]
- 9.Tang P, Chauhan A. Decellular Nerve Allografts. J Am Acad Orthop Surg. 2015;23:641–7. doi: 10.5435/JAAOS-D-14-00373. [DOI] [PubMed] [Google Scholar]
- 10.Spinner RJ, Shin AY, Bishop AT. Advances in the Repair of Peripheral Nerve Injury. Neurosurgery. 2015;62(Suppl 1):146–51. doi: 10.1227/NEU.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 11.Andersen CR, Schmidt AH, Fitzgerald CB, Tintle LS, Helgeson MM, Lehman LR, et al. Extremity War Injuries IX: Reducing Disability Within the Military. J Am Acad Orthop Surg. 2015;23:e13–26. doi: 10.5435/JAAOS-D-15-00205. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Chung MS, Gong HS, Chung JY, Park JH, Baek GH. Sural nerve autografts for high radial nerve injury with nine centimeter or greater defects. J Hand Surg Am. 2008;33:83–6. doi: 10.1016/j.jhsa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Hallgren A, Bjorkman A, Chemnitz A, Dahlin LB. Subjective outcome related to donor site morbidity after sural nerve graft harvesting: a survey in 41 patients. BMC Surg. 2013;13:39. doi: 10.1186/1471-2482-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FFIJ, Nicolai JP, Meek MF. Sural nerve donor-site morbidity: thirty-four years of follow-up. Ann Plast Surg. 2006;57:391–5. doi: 10.1097/01.sap.0000221963.66229.b6. [DOI] [PubMed] [Google Scholar]
- 15.Martins RS, Barbosa RA, Siqueira MG, Soares MS, Heise CO, Foroni L, et al. Morbidity following sural nerve harvesting: a prospective study. Clin Neurol Neurosurg. 2012;114:1149–52. doi: 10.1016/j.clineuro.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron. 2014;83:331–43. doi: 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–33. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 18.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–52. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs J, Adams S, Mallu S, Loveland K, Sandbulte Z. Comparison of the performance of chronically versus freshly denervated autograft in nerve repair. J Hand Surg Am. 2010;35:2001–7. doi: 10.1016/j.jhsa.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Birch R, Dunkerton M, Bonney G, Jamieson AM. Experience with the free vascularized ulnar nerve graft in repair of supraclavicular lesions of the brachial plexus. Clin Orthop Relat Res. 1988;237:96–104. [PubMed] [Google Scholar]
- 21.Hattori Y, Doi K, Ikeda K, Pagsaligan JM. Vascularized ulnar nerve graft for reconstruction of a large defect of the median or radial nerves after severe trauma of the upper extremity. J Hand Surg Am. 2005;30:986–9. doi: 10.1016/j.jhsa.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Bonney G, Birch R, Jamieson AM, Eames RA. Experience with vascularized nerve grafts. Clin Plast Surg. 1984;11:137–42. [PubMed] [Google Scholar]
- 23.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–38. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G. Is the Sciatic Functional Index always reliable and reproducible? J Neurosci Methods. 2008;170:255–61. doi: 10.1016/j.jneumeth.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Midha R, Munro CA, Chan S, Nitising A, Xu QG, Gordon T. Regeneration into protected and chronically denervated peripheral nerve stumps. Neurosurgery. 2005;57:1289–99. doi: 10.1227/01.neu.0000187480.38170.ec. discussion 1289–99. [DOI] [PubMed] [Google Scholar]
- 26.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15:3876–85. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cederna PS, Youssef MK, Asato H, Urbanchek MG, Kuzon WM., Jr Skeletal muscle reinnervation by reduced axonal numbers results in whole muscle force deficits. Plast Reconstr Surg. 2000;105:2003–9. doi: 10.1097/00006534-200005000-00014. discussion 2010–1. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson S, Wiberg R, McGrath AM, Novikov LN, Wiberg M, Novikova LN, et al. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS One. 2013;8:e56484. doi: 10.1371/journal.pone.0056484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vuorinen V, Siironen J, Roytta M. Axonal regeneration into chronically denervated distal stump. 1. Electron microscope studies. Acta Neuropathol. 1995;89:209–18. doi: 10.1007/BF00309336. [DOI] [PubMed] [Google Scholar]
- 30.Sulaiman OA, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32:234–46. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Sulaiman OA, Midha R, Munro CA, Matsuyama T, Al-Majed A, Gordon T. Chronic Schwann cell denervation and the presence of a sensory nerve reduce motor axonal regeneration. Exp Neurol. 2002;176:342–54. doi: 10.1006/exnr.2002.7928. [DOI] [PubMed] [Google Scholar]
- 32.Sulaiman OA, Voda J, Gold BG, Gordon T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp Neurol. 2002;175:127–37. doi: 10.1006/exnr.2002.7878. [DOI] [PubMed] [Google Scholar]