Abstract
African Americans are 1.4 times more likely than European Americans to carry the APOE ε4 allele, a risk factor for Alzheimer’s disease (AD). However, little is known about the neural correlates of cognitive function in older African Americans and how they relate to genetic risk for AD. In particular, no past study on African Americans has examined the effect of APOE ε4 status on pattern separation—mnemonic discrimination performance and its corresponding neural computations in the hippocampus. Previous work using the mnemonic discrimination paradigm has localized increased activation in the DG/CA3 hippocampal sub-regions as being correlated with discrimination deficits. In a case-control high-resolution fMRI study of 30 healthy African Americans, ages 60 and older, we observed APOE ε4 related impairments in mnemonic discrimination, coincident with dysfunctional hyperactivation in the DG/CA3 and CA1 regions, despite no evidence of structural differences in the hippocampus between carriers and non-carriers. Our results add to the growing body of evidence that deficits in pattern separation may be an early marker for AD related neuronal dysfunction.
Keywords: APOE, Pattern Separation, High-resolution fMRI, Hippocampus, Alzheimer’s disease
1.0. INTRODUCTION
We applied task-activated functional magnetic resonance imaging (fMRI) to examine the effects of Apolipoprotein E (APOE) ε4 allele on medial temporal lobe (MTL) dysfunction in a population of cognitively healthy African Americans. The APOE ε4 allele is the strongest identified genetic risk factor for Alzheimer’s disease (AD) (Potter and Wisniewski, 2012). Its presence has been reported to be associated with heightened episodic memory related dysfunction in the MTL (Bookheimer et al., 2000; Dennis et al., 2010; Filippini et al., 2009; Michaelson, n.d.) which is one of the earliest pathological changes that occur in AD (Gomez-Isla et al., 1996; Price et al., 2001). Despite the fact that African Americans are at elevated risk for AD (Alzheimer’s Association, 2018; Tang et al., 2001) and have a higher frequency of the APOE ε4 allele compared to European Americans (Logue et al., 2011), the neural changes that occur in older African Americans and how they relate to genetic risk factors for AD remain unclear. In particular, no previous study on African Americans has examined the effect of APOE ε4 status on the neural computations for “pattern separation,” that is, separating similar representations into distinct, non-overlapping representations while encoding and retrieving episodic memories. This neural computation depends on hippocampal circuitry as demonstrated by a number of empirical reports across species and approaches (Leal and Yassa, 2018).
Our study examined this by comparing a group of healthy African American APOE ε4 carriers (ε4+) to age and education matched same-race non-carriers (ε4-) using high-resolution fMRI during a mnemonic discrimination task where participants were asked to distinguish between novel, repeated (old), and similar (lure) information. The neurocomputational mechanism underlying this paradigm is pattern separation, which functions to reduce the mnemonic interference by encoding distinctive representations for similar input patterns (Leal and Yassa, 2018). We therefore tested the hypothesis that APOE ε4 genetic risk is associated with impairments in pattern separation, that is, behavioral discrimination deficits and the corresponding neural dysfunction in the hippocampus.
1.1. Background
An estimated 5.5 million Americans aged 65 and older are living with AD as of 2018 (Alzheimer’s Association, 2018). In particular, African Americans are at elevated risk for age-related cognitive decline and memory loss, with double the prevalence of AD compared to European Americans (Alzheimer’s Association, 2018; Tang et al., 2001). The causes of this health disparity in AD are not sufficiently understood. Furthermore, little is known about the neural correlates of cognitive function in older African Americans and how they relate to genetic risk factors for AD.
The hippocampus is among the earliest loci for pathological changes in AD (Gomez-Isla et al., 1996; Price et al., 2001), with converging evidence suggesting that hippocampal dysfunction may be an early indicator of the neurodegenerative process associated with AD. Several studies have shown that patients with Mild Cognitive Impairment (MCI) exhibit increased activation, or hyperactivation, in the hippocampus during encoding and retrieval of episodic memories (Celone et al., 2006; Dickerson et al., 2005, 2004; Hämäläinen et al., 2007; Kircher et al., 2007; Miller et al., 2008b; Yassa et al., 2010). Similar patterns of hyperactivation have also been observed in individuals at genetic or familial risk for AD (Bassett et al., 2006; Bondi et al., 2005; Bookheimer et al., 2000; Quiroz et al., 2010), healthy older adults who perform poorly on the task (Miller et al., 2008a), and amyloid positive, mildly impaired older adults (Sperling et al., 2009).
An accelerated rate of AD related pathology in the hippocampus has been associated with the inheritance of the APOE ε4 allele, the strongest identified genetic risk factor for Alzheimer’s disease (AD) (Potter and Wisniewski, 2012). One ε4 allele can increase the risk of AD 2–3 times and two ε4 alleles can increase the risk 12 times (Michaelson, n.d.). Furthermore, it confers greater AD risk in women compared to men (Altmann et al., 2014). Healthy APOE ε4 carriers show heightened age-related decreases in MTL cortical thickness and hippocampal volume even decades before the onset of AD (Michaelson, n.d.). Even young APOE ε4 carriers show hyperactivation in the MTL (Dennis et al., 2010), specifically in the hippocampus (Bookheimer et al., 2000; Filippini et al., 2009) during an encoding task, indicating that the APOE-related functional changes in the hippocampus can manifest even decades before cognitive decline.
Growing evidence suggests that the hippocampus possesses a unique circuitry that is computationally capable of resolving mnemonic interference during the encoding and retrieval of episodic memories by using pattern separation, the ability to independently represent and store similar experiences (Leal and Yassa, 2018). Hence, mnemonic discrimination paradigms that are sensitive to the functional changes related to pattern separation can be used to detect alterations in the hippocampus and surrounding medial temporal cortices that may confer vulnerability to AD. Impaired mnemonic discrimination is associated with aberrant hyperactivation in the dentate and CA3 subfields of the hippocampus in non-demented older adults (Dickerson et al., 2005; Reagh et al., 2017; Yassa et al., 2011a, 2011b) as well as in individuals with MCI (Bakker et al., 2015, 2012; Tran et al., 2017; Yassa et al., 2010), the extent of which predicts discrimination deficits.
Past studies examining the relationship between mnemonic discrimination of objects, neural pattern separation, and APOE ε4 status have yielded mixed results. In MCI patients, one study reported no differences in hippocampal hyperactivation or mnemonic discrimination between carriers and non-carriers (Tran et al., 2017). Another study reported a link between APOE ε4 homozygotes and performance on a brief mnemonic discrimination task in AD patients (Wesnes et al., 2014). A group of cognitively intact older adult carriers of APOE ε4 was found to perform worse than non-carriers on a spatial mnemonic discrimination task (Sheppard et al., 2016). African Americans are 1.4 times more likely than European Americans to carry the APOE ε4 gene variant (Logue et al., 2011); however, to date, no study has examined whether APOE carrier status in cognitively healthy older African Americans may be associated with impaired pattern separation, involving discrimination deficits, coincident with hippocampal hyperactivation.
1.2. Current Study
In the current study, we directly test the hypothesis that the APOE ε4 allele is associated with impaired mnemonic discrimination performance as well as hyperactivation of hippocampal dentate and CA3 subfields in older non-demented African Americans. To ensure that the differences are attributable to APOE genetic risk, and not due to other health and lifestyle factors, subjects also underwent a battery of standardized neuropsychological assessments, physical fitness tests, and reported daily habits.
2.0. METHODS
2.1. Participants
Participants in this study were recruited through the African-American Brain Health Initiative: A University-Community Partnership (AABHI) at Rutgers University-Newark (see www.brainhealth.rutgers.edu). From a larger parent study of 60 individuals, participants in the current study were selected for analysis in a case-control matched design. Of the parent sample, 15 individuals were homozygous or heterozygous for APOE ε4. We then matched these 15 APOE ε4+ individuals (ε4/ε4: n = 2; ε2/ε4: n = 3; ε3/ε4: n = 10) with 15 individuals who were APOE ε4- (ε2/ε3: n = 2; ε2/ε3: n = 3; ε3/ε3: n = 10) based on age and years of education. Similar to the methodology of Foster et al. (2017), we retained all ε4+ heterozygotes, including ε2/ε4 individuals, as any individual with an ε4 allele is at greater risk of AD than individuals without ε4 alleles (Liu et al., 2013). Therefore, the current study included 30 healthy adults, aged 60 to 90 years, with an average age of 69 years (Table 1).
Table 1.
Demographics, Neuropsychological Tests, Fitness, and Lifestyle Measures.
| Measure | APOE ε4+ | APOE ε4− | Difference (t-test) |
|---|---|---|---|
| Sample Size | 15 | 15 | |
| Age | 69.5 (6.74) | 69.2 (8.0) | |
| Education (years) | 14.7 (1.98) | 14.67 (2.38) | |
| BMI | 31.98 (7.38) | 30.89 (6.27) | p=0.667 |
| BP - Diastole | 80.4 (10.99) | 83.47 (11.32) | p=0.458 |
| BP - Systole | 148.53 (29.0) | 141.07 (20.9) | p=0.426 |
| Heart Rate (bpm) | 73.27 (16.09) | 68.87 (10.03) | p=0.376 |
| BDI | 7.33 (4.64) | 8.4 (5.43) | p=0.568 |
| Social Support | 66.07 (8.98) | 68.07 (14.23) | p=0.653 |
| MMSE | 27.87 (1.77) | 28.20 (1.9) | p=0.622 |
| DigitSpan | 23.27 (4.03) | 23.60 (3.9) | p=0.820 |
| NAART | 35.13 (9.94) | 36.27 (14.66) | p=0.812 |
| RAVLT – Immediate | 11.27 (2.02) | 12.93 (2.22) | p=0.040 |
| RAVLT – Delayed | 9.33 (2.22) | 12.73 (2.91) | p=0.001 |
| Exercise | 2.17 (2.04) | 1.66 (1.97) | p=0.495 |
| TV (hours/day) | 4.13 (1.32) | 4.97 (1.96) | p=0.184 |
| Sitting (hours/day) | 4.46 (2.24) | 5.30 (2.0) | p=0.3 |
| Sleep quality | 3.47 (0.92) | 3.07 (1.03) | p=0.271 |
| Gait Speed Test | 4.76 (1.20) | 4.86 (1.42) | p=0.834 |
| Repeated Chair Stand (s) | 12.43 (8.83) | 17.74 (12.4) | p=0.193 |
| TUG (s) | 11.16 (2.10) | 10.8 (3.41) | p=0.732 |
| VO2 | 12.62 (4.15) | 15.26 (3.45) | p=0.065 |
Data are presented as mean (standard deviation). Sleep Quality: 1=very poor, 2=poor, 3=satisfactory, 4=good, 5=excellent. Group differences were assessed via simple pairwise t-tests. Any statistically significant differences (p<0.05) are bolded.
Participants exhibiting any signs of dementia as revealed in the standardized neuropsychological assessments (detailed below), and those who took medications that could affect cognition were excluded from the study. Other exclusion criterion included history of excessive alcohol intake and/or drug use, psychiatric disorders (including Bipolar Disorder and Schizophrenia), Epilepsy or related seizure disorders, and significant cardiovascular and cerebrovascular diseases. Participants were also required to be native English speakers. All participants completed written informed consent prior to participation in the study.
2.2. Physical Fitness Assessment
In addition to measuring blood pressure, heart rate, and body mass index (BMI), a battery of physical assessments was administered to characterize fitness. Aerobic fitness (VO2 max) was assessed using the Six Minute Walk Test (SMWT). Participants were instructed to walk a pre-measured length on a flat surface for 6 minutes, covering as much ground as possible (McGavin et al., 1978, 1976); total distance was recorded (Noonan and Dean, 2000). Participants’ maximal oxygen consumption was approximated using:
VO2 max = MAX[4.948 + (0.023 * Distance (in meters)), (0.03 * Distance (in meters)) + 3.98]
This measure of maximal oxygen consumption (VO2 max) is widely recognized as both a representation of the functional limitations of the cardiovascular system as well as a measure of aerobic fitness (Taylor et al., 1955).
The Short Physical Performance Battery (SPPB) was used to evaluate static balance when standing, gait speed at a regular pace, and movements consisting of sitting down and standing up. To assess static balance, the participant is asked to maintain up to 3 hierarchical standing postures for up to 10 seconds. First, the participant stands with feet together. If the participant can maintain this posture for 10 seconds, they then perform a semi-tandem stance position. If semi-tandem is held for 10 seconds, it is followed by a tandem stance posture. For the gait speed test, the participant is asked to walk at his or her comfortable speed across a 4-meter distance. Finally, the participant is asked to stand from a standard chair without upper extremity assistance. If they can stand 1 time, they then are instructed to complete 5 sit-to-stands as quickly as possible without upper extremity assistance. Performance was measured by the time spent during each test. This battery is a reliable measure to assess general physical performance in older adults (ages 60 and above), and has been found to be predictive of future decline in health status and function (Studenski et al., 2003).
The Timed Up and Go (TUG) test was used to assess functional mobility and dynamic balance. In this test, participants are seated and then stand and walk 3-meters before turning around and walking back to sit. In a 2-year study, TUG completion time predicted future disability for basic activities of daily living (ADL) in older adults free of disability at baseline (Donoghue et al., 2014).
2.3. Standardized Neuropsychological Assessments and Self-Reported Measures
Prior to MRI scanning, a neuropsychological battery consisting of the Mini Mental State Exam (MMSE) (broad assay of cognitive impairment), Rey Auditory Verbal Learning Test (RAVLT) Immediate and Delayed Recall (sensitive to verbal memory), North American Adult Reading Test (NAART35) (sensitive to verbal intellectual ability), and Wechsler Adult Intelligence Scale (WAIS-IV) Digit Span (sensitive to working memory) was administered to characterize cognition (Table 1). The Beck Depression Inventory (BDI) was administered to measure characteristic attitudes and symptoms of depression. Participants also reported health and lifestyle factors such as sleep quality, daily exercise and activity levels, and, answered a social support questionnaire.
2.4. fMRI Behavioral Paradigm: Mnemonic Discrimination Task
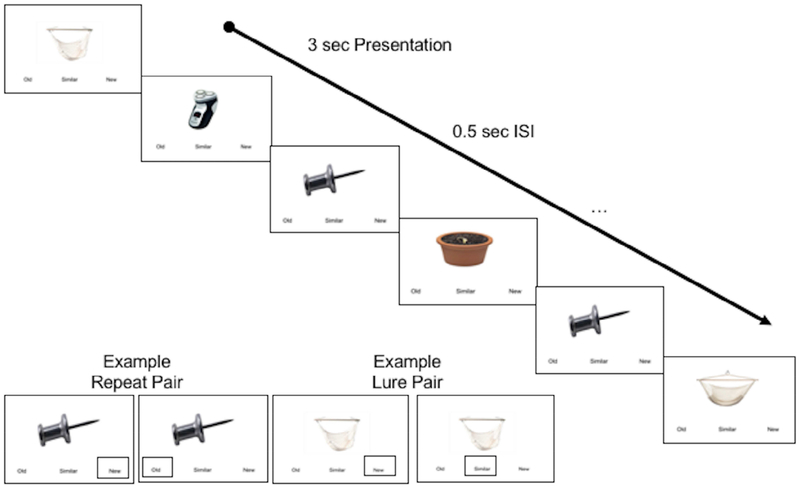
Participants were given a verbal explanation of the task, and, completed pre-training with mock stimuli outside the scanner. As shown in Figure 1, the task consisted of an explicit 3-alternative forced choice task (for more details see Kirwan and Stark (Kirwan and Stark, 2007)), in which participants viewed novel (new), repeated (old), and lure (similar) stimuli. Stimuli were colored photographs of common objects. Each participant completed a single run consisting of 96 similar pairs, 96 identical pairs and 192 unrelated novel items (foils), totaling 576 trials. All trial types were fully randomized throughout the run. Each stimulus was presented for 3 seconds with a 0.5 second inter-stimulus-interval. The number of trials separating similar and identical pairs randomly varied between 10 and 40 trials. Participants were instructed to make a judgment as to whether the object seen was new (i.e., novel items), old (i.e., repeated items) or similar but not identical (i.e., lure items). Of critical interest were the participants’ responses on the lure items. A response of “old” to a lure (i.e., similar) item would constitute a failure of discrimination (possibly indicative of reduced capacity for pattern separation), whereas an accurate response of “similar” to a lure would constitute a successful discrimination (Yassa et al., 2010, 2011a). As in prior work, a Lure Discrimination Index (LDI) was calculated as p(“Similar”|Lure) – p(“Similar”|Foil)], which accounts for response bias.
Fig. 1.
An example of the mnemonic discrimination behavioral task. Each item was presented for 3 seconds with a 0.5 second inter-stimulus-interval. Novel (new), repeated (old), and lure (similar) items were fully randomized throughout the run. Examples of a repeat pair (left) and a lure pair (right) are shown.
2.5. MRI Data Acquisition
Magnetic resonance imaging (MRI) data was acquired on a 3T Siemens Allegra, using a 32-channel Multiband parallel encoding coil, at the Rutgers University Brain Imaging Center (RUBIC) at Rutgers University, Newark. If required, MRI-compatible glasses were used on the day of scanning. A high-resolution 3D magnetization-prepared rapid gradient echo (MP-RAGE) structural scan was acquired in the sagittal plane for each participant: repetition time (TR)=1900ms, echo time (TE)=2.52ms, 9° flip angle, 176 slices (no gap), voxel size 1.0 × 1.0 × 1.2 mm, field of view (FOV)=270 × 254 × 212, with a total acquisition time of 9 minutes. High-resolution Multiband echo-planar images were collected using a field of view (FOV) of 208 × 208 × 125, a repetition time (TR) of 664 milliseconds, an echo time (TE) of 30 milliseconds, a flip angle of 30°, an isotropic resolution of 1.8 mm with no gap, and, a Multiband acceleration factor of 5. Forty-five axial slices were acquired covering the entire brain. Multiband parallel imaging enabled the acquisition of high-resolution functional images, with large sampling rates for full-brain coverage, through the acquisition of multiple slices simultaneously. This resulted in significantly reduced acquisition time, which also limited distortion resulting from magnetic susceptibility. Furthermore, the high temporal efficiency has been shown to provide greater statistical power (Feinberg et al., 2010).
2.6. fMRI Data Analysis
2.6.1. Preprocessing
Analysis of imaging data was conducted using FSL (FMRIB Software Library; www.fmrib.ox.ac.uk/fsl). Skull stripping was conducted using the FSL brain extraction (BET) (Smith, 2002) with the center of gravity of each image as a reference point. Functional images were motion corrected using MCFLIRT (FMRIB’s motion correction linear image registration tool) (Jenkinson et al., 2002), smoothed using a 5.0 mm Gaussian FWHM kernel, and co-registered to their skull-stripped structural images (degrees of freedom, 9; cost function, normalized mutual information; interpolation, sinc function) using FSL’s linear registration tool (Jenkinson et al., 2002; Jenkinson and Smith, 2001). We used Advanced Normalization Tools (ANTs) (Avants et al., 2011) to warp each individual participant’s structural scan into an in-house high-resolution 0.65 mm isotropic template using a diffeomorphic nonlinear registration algorithm (SyN) (Klein et al., 2009). The transformation parameters were then applied to the coplanar functional data in order to align them to the custom template, for individual and group level general linear model (GLM) analyses.
2.6.2. Analysis
Behavioral vectors based on trial type and behavioral responses were used to model the data in a GLM analysis conducted using the fMRI Expert Analysis Tool (FEAT) utility. A trial averaging window of 3.5 s was used beginning from trial onset. The novel foils that were not subsequently tested served as an arbitrary baseline, against which other conditions were compared. The resultant fit coefficients (betas) therefore estimated activity versus baseline (novel foils) for a given trial type. Based on Yassa et al. (2011a, 2010) our critical contrasts of interest were encoding: the first presentation of lures subsequently called “similar” compared with lures subsequently called “old,” and retrieval: lures called “similar” compared with lures called “old.” For these contrasts, the resultant fit coefficients estimated an increase in the difference between correct rejections and false alarms. At the group level, differences in separation-related activity were examined, by conducting a whole-brain GLM analysis comparing APOE ε4+ risk group versus ε4-group during our critical encoding and retrieval contrasts. The FLAME 1 (FMRIB’s Local Analysis of Mixed Effects) mixed-effects model was used and group level Z statistic maps were generated for each contrast with the FSL cluster correction at Z = 1.65 and a family-wise error threshold of p = 0.05.
2.6.3. Extracting Region of Interest (ROI) Voxels
Based on our a priori hypothesis, the effects of the contrasts were examined in the medial temporal lobe (MTL) cortex and hippocampal subfields. Voxels were selected for subsequent analyses based on combining the voxels that showed group differences during encoding and/or retrieval, with anatomical ROIs that were based on manual delineations of the subfields and regions of interest on the custom template. ROIs in the MTL were segmented based on published protocols (Reagh et al., 2017). Voxel Z statistics from the resulting hybrid functional/structural ROIs were averaged and all subsequent statistical analyses were conducted on these averages.
2.7. Genetic Data Collection and Processing
Saliva samples were collected using Oragene kits during the neuropsychological testing visit before MRI scanning. DNA extraction and genotyping were conducted at the Rutgers University Human Genetics Institute. APOE SNP genotyping (rs429358 and rs7412) was carried out by real-time PCR on an Eppendorf Mastercycler thermal cycler, using TaqMan SNP Genotyping assays (C_3084793_20 and C_904973_10 for rs429358 and rs7412, respectively).
3.0. RESULTS
3.1. Behavioral Results
All participants underwent an extensive battery of standardized neuropsychological, health, fitness, and lifestyle assessments as well as measures of education and verbal fluency. Participants included in our analyses were within the age and education adjusted norms (Table 1). No differences were observed on measures of cognitive intactness (MMSE, Digit Span, NAART), physical health/fitness (BMI, Blood Pressure, Heart Rate, Gait Speed, Repeated Chair Stand, Timed Up and Go, VO2 max), depression (BDI), social support, or self-reported lifestyle measures (exercise, time spent in a sedentary state, and sleep quality). As seen in Table 1, there was a significant difference in short-term (RAVLT – Immediate, p=0.04) and long-term auditory-verbal memory (RAVLT – Delayed, p=0.001). Participants who were in the APOE ε4− group exhibited stronger immediate (M=12.93, SD=2.22) and delayed recall (M=12.73, SD=2.91) than those in the APOE ε4+ risk group (M 11.27, SD=2.02; M=9.33, SD=2.22).
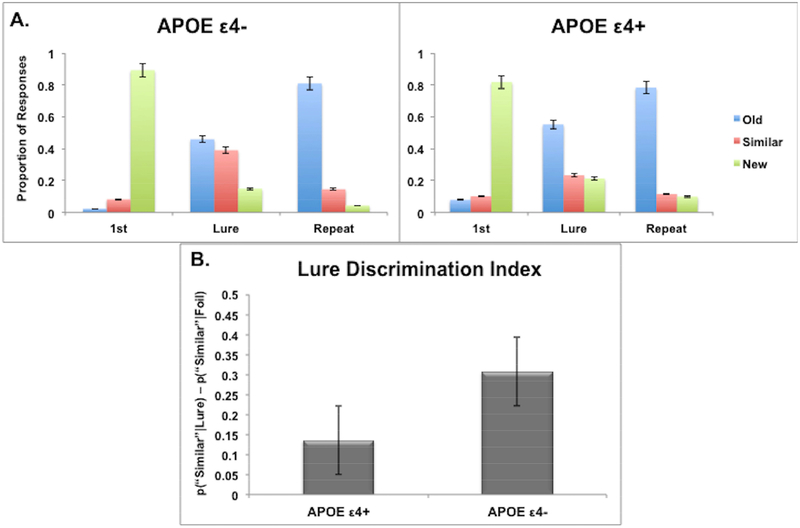
The mnemonic discrimination task is depicted in Figure 1 and described in Methods. Briefly, participants were shown a series of photographs of common objects, which were old targets (previously seen images), similar lures (images that were similar but not identical to ones previously seen) and dissimilar foils (never before seen images). For each image, participants were instructed to indicate if it was new (i.e., novel items), old (i.e., repeated items) or similar but not identical (i.e., lure items). Those in the APOE ε4+ risk group were much more likely to generate “false alarms” to items that were similar (i.e., “lures”) than the APOE ε4-group (Figure 2). The ε4+ risk group successfully labeled 23.4% (SD = 15) of the lure trials as “similar,” whereas ε4-group did so on 39.1% (SD = 19.5) of the lure trials; t(28) = 2.49, p = 0.019. The ε4+ risk group demonstrated a significant impairment on the key LDI measure t(28) = 2.53, p = 0.018. Furthermore, there was a significant positive correlation between LDI scores and RAVLT immediate (r(30) = 0.435, p = 0.016) and delayed (r(30) = 0.532, p = 0.002) recall.
Fig. 2.
Performance on the mnemonic discrimination task based on APOE ε4 status. Panel (A) shows response proportions on different trial types in APOE ε4 carriers (ε4+) and non-carriers (ε4-). There was a significant difference between groups on the critical lure items, where APOE ε4+ group were more likely to generate false alarms to the lure items, mischaracterizing them as “old” items instead of “similar”. Panel (B) shows the difference between the two groups on the Lure Discrimination Index. Those in the APOE ε4+ group had a significantly lower discrimination index score.
3.2. Functional Neuroimaging Results
To examine group differences in separation-related activity, we compared ε4+ risk group vs. ε4-group activity during our critical contrast (lures called “similar” minus lures called “old”) during both the initial presentation and subsequent presentations. As detailed in the Methods section, the first contrast was based on the first presentation of items that were subsequently tested with a lure, whereas the second contrast was based on the actual lure presentation. Given that our hypotheses were specific to MTL regions, we conducted region-of-interest (ROI) analyses in MTL cortex and hippocampal subfields; As shown in Figure 3, during both encoding (initial presentation) and retrieval (subsequent presentations), the APOE ε4+ risk group showed increased activity in the left DG/CA3 hippocampal subfield (encoding: t(28) = 2.453, p = 0.021; retrieval: t(28) = 2.236, p = 0.033). Increased activation was also observed bilaterally in the CA1 region of the hippocampus (encoding: t(28) = 3.122, p = 0.004; retrieval: t(28) = 3.012, p = 0.005). Similar to the methodology of Yassa et al. (2011a), the contrasts used in the above analyses are not relative to baseline but rather relative to a zero difference between false alarms and correct rejections. An increase in activation therefore represents an increase in the difference between correct rejections and false alarms.
Fig. 3.
Activation level during the critical contrast (lures called “similar” minus lures called “old”) based on hybrid anatomical/functional ROIs, for both the initial presentation (encoding) and subsequent presentations (retrieval) based on APOE ε4 carrier status. During both encoding and retrieval, the APOE ε4+ risk group showed increased hippocampal activity bilaterally in the CA1 (A, B) and, in the left DG/CA3 (C).
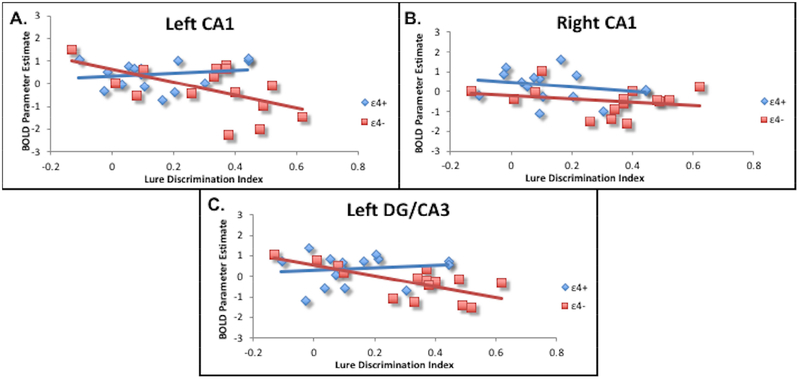
To investigate the potential link between the behavioral ability to pattern separate and observed hyperactivity in the DG/CA3 and CA1 regions, we performed a correlational analysis between each participant’s LDI and activity during the encoding (lures subsequently called “similar” minus lures subsequently called “old”) and retrieval (lures called “similar” minus lures called “old”) contrasts. Correlations were assessed at the level of ε4+ risk group, ε4-group, and collapsed across the entire sample (Figure 4). During the retrieval contrast, LDI scores across groups were negatively correlated with activity in left DG/CA3 for the ε4-group (r = − 0.683, p = 0.005) and the entire sample (r = −0.451, p = 0.012). A similar negative correlation was observed in the left CA1 for the ε4-group (r = − 0.545, p = 0.036) and the entire sample (r = −0.430, p = 0.018). In the right CA1, we found a significant negative correlation between LDI and activation across the entire sample (r = −0.381, p = 0.038), but neither group featured a significant correlation individually. No significant correlations were found at the individual group level or across the entire sample during encoding.
Fig. 4.
Correlations between Lure Discrimination Index and activation in the CA1 (A, B) and left CA3/DG during retrieval. Across groups, there was a significant negative correlation between behavioral discrimination and functional activation.
Furthermore, a hierarchical linear regression (HLR) revealed that during retrieval, APOE ε4 status significantly modulates the association between LDI and activity in the left DG/CA3 (R2 change = 0.116, F(1,26) = 4.74, p = 0.038) and left CA1 (R2 change = 0.112, F(1,26) = 4.4, p = 0.046).
3.3. Structural MRI Results
Volumetric analyses across MTL regions and hippocampal subfields showed no significant group differences in either volume (Figure 5, A) or surface area (Figure 5, B).
Fig. 5.
Volumetric analyses of volume (A) and surface area (B) of MTL regions and hippocampal subfields for the two groups, APOE ε4+ versus APOE ε4-. There were no significant group differences in either volume or surface area.
4.0. DISCUSSION
The present study tested the hypothesis that APOE ε4 genetic risk in non-demented older African Americans is associated with performance on a mnemonic discrimination task and its corresponding neural computations in the MTL. We observed APOE ε4-related impairments in mnemonic discrimination, coincident with specific hyperactivity in the left DG/CA3 and CA1. Moreover, activity in both DG/CA3 and CA1 was negatively correlated with discrimination performance, and this association was moderated by ε4 status. Importantly, there were no structural differences between carriers (ε4+) and non-carriers (ε4-) in volume and surface area of MTL regions and hippocampal subfields. There were also no group differences on standardized neuropsychological tests (with the exception of RAVLT), physical fitness assessments, or health and lifestyle measures.
Lure discrimination was found to be significantly decreased in the APOE ε4+ group compared with the non-carrier group, indicating that there is a behavioral episodic memory deficit associated with APOE ε4 that can be characterized as a shift in bias from pattern separation to pattern completion. Consistent with this, APOE ε4 carriers also showed lower scores on RAVLT immediate and delayed recall, another measure of episodic memory. Discrimination performance on the task was positively correlated with RAVLT scores, further validating pattern separation as a facet of episodic memory. Notably, in our study, no group differences were observed on broad measures of cognitive intactness, suggesting that the presence of an APOE ε4 allele disrupts episodic memory in older adults who are otherwise cognitively healthy. These results add to the growing body of evidence on the association between APOE ε4 and episodic memory in the elderly (Caselli et al., 2011; Liang et al., 2017; Mayeux et al., 2001; Nilsson et al., 2006). Moreover, the mnemonic discrimination paradigm is particularly sensitive to the core constructs taxed by MTL pathology, compared to other episodic memory tasks, such as RAVLT, as demonstrated by previous research showing strong correlations between behavioral discrimination with both preclinical hippocampal hyperactivity and perforant path integrity (Yassa et al., 2010, 2011b).
A similar discrimination deficit has been demonstrated in a previous study that examined the effects of APOE ε4 on spatial pattern separation (Sheppard et al., 2016). However, recent studies specifically investigating object pattern separation, as examined in the present study, found no differences based on ε4 status in aMCI patients (Tran et al., 2017), and, in AD patients, an impairment in difficult (but not easy) discrimination was found exclusively in ε4 homozygotes (Wesnes et al., 2014). We build significantly on these lines of research by providing evidence of object pattern separation deficits in cognitively healthy ε4 carriers, irrespective of whether they had one or two copies of the ε4 allele.
Commensurate with behavioral impairments, during task-activated fMRI, APOE ε4 carriers showed significantly increased activation in the hippocampus, localized to the left DG/CA3 and CA1 sub-regions. This hyperactivity was inversely associated with participants’ discrimination performance on the task, suggesting that the increased activation is maladaptive and is a marker for neuronal dysfunction. Pathological hippocampal hyperactivity, specific to the DG/CA3 subfield, is now well established in non-demented older adults (Reagh et al., 2017; Yassa et al., 2011a, 2011b) as well as MCI patients (Bakker et al., 2012, 2015; Tran et al., 2017; Yassa et al., 2010). However, we also found a moderating effect of APOE ε4 status on the negative correlation between performance on the mnemonic discrimination task, and, activation in the left DG/CA3 and left CA1 subfields, such that this association was significantly stronger in the non-carrier group. Further work is required to understand the significance of this result, but it could potentially indicate that the ε4-related pathology results in abnormal hippocampal recruitment that may not be linked with cognitive effort. As a result, APOE ε4 carriers show dysfunctional hippocampal hyperactivity that is not strongly inversely proportionate to discrimination performance, as observed in non-carriers. This interpretation requires further exploration, focusing particularly on whether the moderation effect may be driven by individuals who are homozygous for the ε4 allele.
Increased hippocampal activation in cognitively normal APOE ε4 carriers has been reported in a number of studies, but the imaging methods used in those previous studies had insufficient resolution to localize that activation to a specific hippocampal sub-region (Bookheimer et al., 2000; Burggren et al., n.d.). Our results are therefore well in line with these findings, and with high-resolution imaging, extend them to suggest a specific role for the DG/CA3 and CA1 subfields. This is also consistent with animal models of APOE ε4 (Andrews-Zwilling et al., 2010; Palop and Mucke, 2009), predicting that hippocampal hyperactivation, localized particularly to the DG/CA3 region would be observed in ε4 carriers. Furthermore, volume and surface area measures of the MTL regions and hippocampal subfields did not differ between APOE ε4 carrier and non-carrier groups, confirming that the observed functional decline in DG/CA3 and CA1 were not due to measurable structural differences. Therefore, our results provide compelling evidence for an APOE ε4 related deficit in mnemonic discrimination, which likely result from DG/CA3 and CA1 hyperactivation. This suggests that non-demented older persons with a genetic risk for AD have alterations in MTL function without obvious morphologic or behavioral indications of impending disease. Whether these results are specific to African Americans, who are at elevated risk for AD and have a higher frequency of the APOE ε4 allele, remains a significant question.
The present results stand in contrast to those of Tran et al. (Tran et al., 2017), who found that the presence of the APOE ε4 allele did not contribute to increased DG/CA3 activation during pattern separation. The discrepancy between our results and theirs likely arises from differences in the population studied; while our study examines cognitively healthy individuals, Tran et al. (Tran et al., 2017) compared APOE ε4 carriers versus non-carriers in patients with aMCI. As such, this difference has important implications, inviting the hypothesis that the presence of the APOE ε4 allele may initiate an earlier onset of AD or it may be associated with more widespread dysfunction during the preclinical stage of AD, but has little effect on the disease’s course once individuals progress to a clinical diagnosis of aMCI or AD. Thus, deficits in pattern separation (impaired mnemonic discrimination coupled with hyperactivation in DG/CA3 and CA1) may be an early marker for AD related neuronal dysfunction, and in conjunction with genetic risk, may enhance our ability to detect individuals likely to develop AD before actual disease onset.
The results of this study also advance our understanding of racial differences when examining genetic risk factors for cognitive decline to AD. Previous research has found that lower levels of education and socioeconomic status, limited physical activity, and a sedentary lifestyle are more common among African Americans and influence cognitive decline (Yaffe et al., 2013; Alzheimer’s Association, 2018). These factors not only place older African Americans at a heightened risk for AD, but, could potentially influence the predictive effect of APOE ε4 allele on cognition. The present study utilized a cross-sectional design that was restricted to African Americans living in and around Newark, New Jersey, thereby decreasing the between-group variability on the various environmental and health variables that may influence racial differences in the effect of APOE ε4 on cognitive decline. Our participants were demographically matched for age and education levels, and they come from urban areas and community dwellings that are fairly homogeneous for socioeconomic status. Furthermore, we did not find any differences on physical fitness, health, or lifestyle assessments between ε4 carriers and non-carriers, confirming that the observed impairment in pattern separation in older African Americans is not due to any of these factors, but, rather, attributable to genetic variations. We therefore expect these results to apply to other groups, such as Caucasians, but further investigation is required to elucidate the inter-racial generalizability of the relationship between APOE status and pattern separation.
There are several study limitations and specific future directions that should be acknowledged. First, the relatively small samples may not provide enough power to detect subtle effects of APOE genotype, particularly differences between carriers of one (heterozygotes) versus two (homozygotes) ε4 alleles. Additionally, there was a gender imbalance in our study with just one male participant. Although the association between AD and the APOE gene has been confirmed worldwide, it appears to differ by ancestral background, such that the overall effect of APOE on AD is lower in African Americans as compared to Caucasians (Tang et al., 2001). In comparison, the ABCA7 genetic variation is the strongest AD genetic risk factor for African Americans (outside of the APOE4 allele) with an odds ratio of 1.8 in African Americans (Reitz et al., 2013). Furthermore, APOE ε4 confers greater AD risk in women (Altmann et al., 2014), which may be driving the current results. Hence, future studies with a larger sample size are required to explore interactions between race and gender differences in the effects of APOE ε4 on pattern separation, as well as, the effects of ABCA7 variations.
5.0. CONCLUSIONS
The results of the study show that APOE ε4 contributes to maladaptive hyperactivation in DG/CA3 and CA1 hippocampal sub-regions during pattern separation in cognitively healthy subjects without any structural degradation or behavioral symptoms associated with the clinical diagnosis of aMCI or AD. This work has important implications for future assessments to understand how genetic risk may facilitate early biomarkers in uncovering neuronal dysfunction in the non-symptomatic, pre-clinical phase of the disease. Such research is necessary to develop more specific interventions targeting both older, non-demented individuals and younger individuals who are decades from the earliest symptoms of the disease. Further research is also necessary to be sure that findings that link genetics, neuroimaging, and AD risk are studied in different racial groups whose genetic risk factors for AD may differ.
APOE ε4 is associated with hippocampal hyperactivation during pattern separation
Hyperactivation is dysfunctional and correlated with impaired discrimination
No structural differences between ε4-carriers and non-carriers in MTL regions
No group differences observed on broad measures of cognitive intactness
Acknowledgements
This work was supported by a grant to Mark A. Gluck from the NIH/National Institute on Aging (R56-AG053961) and by support from the Chancellor and Provost’s offices at Rutgers University-Newark. Additional support came from grants to Michael A. Yassa from NIH/National Institute on Aging grants P50AG05146, R21AG049220 and R01AG053555. The authors thank Stephen Hanson for his guidance in in developing the Multiband fMRI acquisition protocol, and, the staff of the Rutgers University Brain Imaging Center (RUBIC), for their support in the brain imaging data collection.
Our ongoing research studies with older African Americans in the Greater Newark, New Jersey, area would not be possible without the guidance, input, and support of these members of the African American Brain Health Initiative’s Community Advisory Board: Tania Cajuste (East Orange Office of Senior Services), Margaret Cammarieri (American Heart Association | American Stroke Association), Honorable Mildred Crump (City of Newark City Council), Mary Dawkins (Hillside Senior Citizen Center), Mildred English (St. James AME Church), Jaklyn De Vore (Essex County Office of Senior Services), Deacon Francis Dixon (The New Hope Baptist Church), Deborah Flamengo (OUCP/AABHI Community Outreach Coordinator), Robin Lateef-Pharms (Bethany Senior Center), Louise Layton (Rutgers Aging Advisory Council), Yolanda Mack (Jehovah-Jireh Praise and Worship Center), Reverend Dr. Jacqueline Reeves (St. James AME Church), Joan Reeves (East Orange Office of Senior Services), Donna Sparks (Bethany Baptist Church), Sheltry Ward (New Jersey Black Nurses Association), Pastor Glenn Wilson (Pilgrim Baptist Church), Geri Woods-Coles (Bethany Baptist Church), and Glenda Wright (New Jersey Association of Public and Subsidized Housing).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The data used to support these findings are available from the co-first authors upon request.
Disclosure Statement
The authors confirm that there are no known conflicts of interest associated with the publication of this manuscript.
REFERENCES
- Altmann A, Tian L, Henderson VW, Greicius MD, 2014. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol 75, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2018. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. J. Alzheimers Assoc 14, 367–429. [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Swilling D, Yan TX, Chen L, Huang Y, 2010. Apolipoprotein E4 causes age-and Tau-dependent impairment of GABAergic interneurons leading to learning and memory deficits in mice. J. Neurosci 30, 13707–13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC, 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M, 2015. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 7, 688–698. 10.1016/j.nicl.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M, 2012. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL, 2006. Familial risk for Alzheimer’s disease alters fMRI activation patterns. Brain 129, 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG, 2005. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64, 501–508. 10.1212/01.wnl.0000150885.00929.7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW, 2000. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med 343, 450–456. 10.1056/nejm200008173430701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY, n.d. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am. J. Geriatr. Psychiatry 10, 44–51. 10.1097/00019442-200201000-00006 [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DEC, Hoffman-Snyder CR, Woodruff BK, Rapcsak SZ, Reiman EM, 2011. Longitudinal modeling of frontal cognition in APOE ε4 homozygotes, heterozygotes, and noncarriers. Neurology 76, 1383–1388. 10.1212/WNL.0b013e3182167147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, 2006. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. J. Neurosci 26, 10222–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R, 2010. Temporal lobe functional activity and connectivity in young adult APOE ε4 carriers. Alzheimers Dement. J. Alzheimers Assoc 6, 303–311. 10.1016/j.jalz.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, 2004. Medial temporal lobe function and structure in mild cognitive impairment. Annu. Neurol 56, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, 2005. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue OA, Savva GM, Cronin H, Kenny RA, Horgan NF, 2014. Using timed up and go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch. Phys. Med. Rehabil 95, 1954–1961. 10.1016/j.apmr.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil K, Yacoub E, 2010. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLOS ONE 5, e15710 10.1371/journal.pone.0015710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, 2009. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci 106, 7209–7214. 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Kennedy KM, Rodrigue KM, 2017. Differential aging trajectories of modulation of activation to cognitive challenge in APOE ε4 groups: Reduced modulation predicts poorer cognitive performance. J. Neurosci 37, 6894–6901. 10.1523/jneurosci.3900-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DWJ, Morris JC, Growdon JH, Hyman BT, 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. Neuroscience 16, 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamäki M, Tanila H, Hänninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H, 2007. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging 28, 1889–1903. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S, 2001. A global optimisation method for robust affine registration of brain images. Med. Image Anal 5, 143–156. 10.1016/S1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT, 2007. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J. Neurol. Neurosurg. Psychiatry 78, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL, 2007. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem 14, 625–633. 10.1101/lm.663507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV, 2009. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802. 10.1016/j.neuroimage.2008.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Yassa MA, 2018. Integrating new findings and examining clinical applications of pattern separation. Nat. Neurosci 1–24. 10.1038/s41593-017-0065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Li Z, Wei J, Li C, Zhang X, Neuroimaging Initiative, A., #x, s Disease, 2017. Frequency specific effects of ApoE ε4 allele on resting-state networks in nondemented elders. BioMed Res. Int 2017, 1–8. 10.1155/2017/9823501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol 9, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Schu M, Vardarajan BN, Buros J, Green RC, Go RCP, Griffith P, Obisesan TO, Shatz R, Borenstein A, Cupples LA, Lunetta KL, Fallin MD, Baldwin CT, Farrer LA, 2011. A comprehensive genetic association study of alzheimer disease in african americans. Arch. Neurol 68, 1569–1579. 10.1001/archneurol.2011.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Small SA, Tang M-X, Tycko B, Stern Y, 2001. Memory performance in healthy elderly without Alzheimer’s disease: Effects of time and apolipoprotein-E. Neurobiol. Aging 22, 683–689. 10.1016/S0197-4580(01)00223-8 [DOI] [PubMed] [Google Scholar]
- McGavin CR, Artvinli M, Naoe H, McHardy GJ, 1978. Dyspnoea, disability, and distance walked: Comparison of estimates of exercise performance in respiratory disease. Br. Med. J 2, 241–243. 10.1136/bmj.2.6132.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin CR, Gupta SP, McHardy GJ, 1976. Twelve-minute walking test for assessing disability in chronic bronchitis. Br. Med. J 1, 822–823. 10.1136/bmj.1.6013.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson DM, n.d. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 10, 861–868. 10.1016/j.jalz.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA, 2008a. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc. Natl. Acad. Sci. U. S. A 105, 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC, 2008b. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry 79, 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L-G, Adolfsson R, Bäckman L, Cruts M, Nyberg L, Small BJ, Van Broeckoven C, 2006. The influence of APOE status on episodic and semantic memory: data from a population-based study. Neuropsychology 20, 645–65. [DOI] [PubMed] [Google Scholar]
- Noonan V, Dean E, 2000. Submaximal Exercise Testing: Clinical Application and Interpretation. Phys. Ther 80, 782–807. 10.1093/ptj/80.8.782 [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2009. Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol 66, 435–440. 10.1001/archneurol.2009.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H, Wisniewski T, 2012. Apolipoprotein E: Essential catalyst of the alzheimer amyloid cascade. Int. J. Alzheimers Dis 2012, 9 10.1155/2012/489428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC, 2001. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol 58, 1395–1402. [DOI] [PubMed] [Google Scholar]
- Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillón G, Lopera F, Stern CE, 2010. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol 68, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Noche JA, Tustison N, Delisle D, Murray EA, Yassa MA, 2017. Anterolateral entorhinal-hippocampal imbalance in older adults disrupts object pattern separation. bioRxiv. 10.1101/162925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, Evans D, 2013. Variants in the atp-binding cassette transporter (abca7), apolipoprotein e ϵ4, and the risk of late-onset alzheimer disease in african americans. JAMA 309, 1483–1492. 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Graves LV, Holden HM, Delano-Wood L, Bondi MW, Gilbert PE, 2016. Spatial Pattern Separation Differences in Older Adult Carriers and Non-Carriers for the Apolipoprotein E Epsilon 4 Allele. Neurobiol. Learn. Mem 129, 113–119. 10.1016/j.nlm.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp 17, 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA, 2009. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM, 2003. Physical performance measures in the clinical setting. J. Am. Geriatr. Soc 51, 314–322. 10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- Tang M-X, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R, 2001. Incidence of AD in African-Americans, Caribbean hispanics, and caucasians in northern Manhattan. Neurology 56, 49–56. 10.1212/wnl.56.1.49 [DOI] [PubMed] [Google Scholar]
- Taylor HL, Buskirk E, Henschel A, 1955. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J. Appl. Physiol 8, 73–80. 10.1152/jappl.1955.8.1.73 [DOI] [PubMed] [Google Scholar]
- Tran TT, Speck CL, Pisupati A, Gallagher M, Bakker A, 2017. Increased hippocampal activation in ApoE-4 carriers and non-carriers with amnestic mild cognitive impairment. Neuroimage Clin 13, 237–245. 10.1016/j.nicl.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes KA, Annas P, Basun H, Edgar C, Blennow K, 2014. Performance on a pattern separation task by Alzheimer’s patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E ∈4 status and cerebrospinal fluid amyloid-β42 levels. Alzheimers Res. Ther 6, 20 10.1186/alzrt250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E, 2013. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ 347, f7051 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL, 2011a. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21, 968–979. 10.1002/hipo.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL, 2011b. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl. Acad. Sci 108, 8873–8878. 10.1073/pnas.1101567108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL, 2010. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 51, 1242–1252. 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]