Abstract
Despite decades-long bans on the production and use of certain chemicals, many halogenated organic compounds (HOCs) are persistent and can bioaccumulate in the marine environment with the potential to cause physiological harm to marine fauna. Highly lipid-rich tissue (e.g., marine mammal blubber) functions as a reservoir for HOCs, and selecting ideal indicator species is a priority for retrospective and proactive screening efforts. We selected five marine mammal species as possible indicators for the Southern California Bight (SCB) and applied a non-targeted analytical method paired with an automated data reduction strategy to catalog a broad range of known, known but unexpected, and unknown compounds in their blubber. A total of 194 HOCs were detected across the study species (n = 25 individuals), 81% of which are not routinely monitored, including 30 halogenated natural products and 45 compounds of unknown structure and origin. The cetacean species (long-beaked common dolphin, short-beaked common dolphin, and Risso’s dolphin) averaged 128 HOCs, whereas pinnipeds (California sea lion and Pacific harbor seal) averaged 47 HOCs. We suspect this disparity can be attributed to differences in life history, foraging strategies, and/or enzyme-mediated metabolism. Our results support proposing (1) the long- and short-beaked common dolphin as apex marine predator sentinels for future and retrospective biomonitoring of the SCB ecosystem and (2) the use of non-targeted contaminant analyses to identify and prioritize emerging contaminants. The use of a sentinel marine species together with the non-targeted analytical approach will enable a proactive approach to environmental contaminant monitoring.
Keywords: nontargeted mass spectrometry, halogenated organic contaminants, marine mammals, bioaccumulation
Graphical Abstract
1. INTRODUCTION
The Southern California Bight (SCB), in the eastern North Pacific Ocean, hosts notable biodiversity in its coastal and pelagic habitats. A multitude of species are exposed to legacy and ongoing contamination by bioaccumulative compounds such as dichlorodiphenyltrichloroethane (DDT) related compounds, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) (Dodder et al., 2012; Maruya and Schiff, 2009). Negative health effects such as endocrine disruption (Brouwer et al., 1989), reproductive impairment (Gilmartin et al., 1976; Roos et al., 2012; Schwacke et al., 2002), and immune system suppression (Hall et al., 1992; Ross, 2002; Schwacke et al., 2012) experienced by marine mammals in other geographic regions, but less studied in the SCB, have been attributed to chronic organic contaminant exposure, potentially increasing susceptibility to infectious disease. HOCs have also been speculated to enhance sensitivity of certain marine mammal species to poisoning by domoic acid (Tiedeken and Ramsdell, 2010), a potent neurotoxin produced by harmful algal blooms that are increasing in magnitude and frequency in the SCB (Gulland and Hall, 2007). Thus, apex predators (i.e., cetaceans and pinnipeds) in this region are integral to the detection of previously unrecognized environmental contaminants in addition to providing exposure data for possible contaminant-related health impacts.
A comprehensive assessment of contaminant load is required to evaluate the toxicological risk associated with contaminant exposure in wildlife, which may vary depending on the complexity of the contaminant mixture (Desforges et al., 2017). Marine mammals serve as effective indicators of marine pollution due to their high trophic position, large stores of lipid-rich blubber, and relatively long lifespans (Bossart, 2011; Ross, 2000). These characteristics can lead to relatively high concentrations and diverse classes of bioaccumulative HOCs from both anthropogenic and natural sources. Routine targeted screening for tissue HOCs consists of defined lists of compounds that may only account for a proportion of the contaminant load. As a result, many uncharacterized compounds with potential to cause physiological harm may be missed by routine research and monitoring (Shaul et al., 2015). A non-targeted analytical method employing comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GC×GC/TOF-MS) is capable of expanding the analysis to include thousands of both known and previously unidentified chemical constituents through enhanced chromatographic resolution and narrower chromatographic peak widths leading to enhanced sensitivity compared to single dimension GC systems (Hoh et al., 2012). The analytical method was combined with searches against reference mass spectra, de novo interpretation, and matches to authentic standards. The certainty was categorized based on the method of identification (e.g., matches to authentic standards had the highest confidence) (Hoh et al., 2012). Recent non-targeted studies of dolphins and seabirds have revealed that a substantial proportion of compounds are not routinely monitored (Alonso et al., 2017; Hoh et al., 2012; Millow et al., 2015; Shaul et al., 2015). Prior analyses of blubber samples from eight Pacific common bottlenose dolphins (Tursiops truncatus) identified 280 unmonitored and/or unknown compounds (86%) among 327 total HOCs (Shaul et al., 2015). The bottlenose dolphin study established the utility of this analytical method for detecting an expanded range of HOCs in wildlife tissues and identifying priority compounds for further investigation.
In this study, our primary objective was to evaluate apex marine sentinels for monitoring bioaccumulative HOCs using non-targeted analysis. We created an inventory of anthropogenic and naturally occurring bioaccumulative HOCs for each of five marine mammal species as candidate sentinels for the SCB: eastern North Pacific long-beaked common dolphins (Delphinus bairdii), short-beaked common dolphins (Delphinus delphis), Risso’s dolphins (Grampus griseus), California sea lions (Zalophus californianus) and Pacific harbor seals (Phoca vitulina). A secondary technical objective of this study was the development of software to automatically identify HOCs from their fragmentation mass spectra, enabling data reduction and sample size expansion (Code 1 and 2, Supplemental Information). This was necessary in order to evaluate chemical accumulation trends across species. Additional objectives were to identify unexpected compounds that may pose an elevated risk based on abundance and frequency of detection, and prioritize unknowns for potential source and structure determination.
2. MATERIALS AND METHODS
Study species were selected based on established habitat range within the SCB, known availability of archived specimens, and documented susceptibility or suspected resistance to domoic acid toxicosis (Danil et al., 2010; Goldstein et al., 2008). Blubber samples were collected between 1990 and 2014, from individuals incidentally killed during fishing activities in the SCB or those that stranded dead. Five blubber samples from each of the five species were analyzed. Samples were restricted to males with priority given to mature (using length as a proxy) by-caught individuals (Table S2) to reduce the variability caused by 1) including females known to offload contaminants, and 2) age and health status (stranded individuals are generally sick). Blubber samples were archived at −20 °C. Approximately 20 g of frozen, full-depth blubber was sub-sampled and processed following protocols outlined by Shaul et al. (2015). Final extracts were analyzed on a Pegasus 4D GC×GC/TOF-MS equipped with an Agilent 6890 gas chromatograph using instrumental parameters optimized for marine mammal blubber by Hoh et al. (2012).
Data were processed using the LECO ChromaTOF mass spectrometer data system (version 4.51.6.0 optimized for Pegasus) and an automated data handling procedure. Details are described in SI-1 and Figure S4. Briefly, custom data reduction software was developed based on the algorithm described by Pena-Abaurrea et al., 2014, which examined mass spectra for ion intensity ratios characteristic of halogenation. Additional rules and a cross-checking procedure were applied to reduce the false positive rate. If the same mass spectrum was present in > 2 samples, the cross-checking procedure required a manual search for the compound in the remaining 23 samples.
On average, 479 halogenated mass spectra per sample were selected by the algorithm (out of an average of 9210 chromatographic features per sample), rate of 80% to 85% compared to a fully manual process (Table S3). Core components of the R script are provided in SI-1, and the full R script is provided at https://github.com/OrgMassSpec/IdentifyHalogenatedSpectra. Chromatographic peaks and the corresponding mass spectra selected by the algorithm were manually reviewed, and confirmed halogenated compounds were structurally identified through searches against existing mass spectral libraries generated from prior analysis of marine mammals using the same method (Mackintosh et al., 2016; Shaul et al., 2015) , searches against the NIST 2014 Electron Impact Mass Spectral Library, matches to authentic reference standards, or manual interpretation following the earlier methods. The mass spectra of unknown compounds are provided in SI-2. All identified compounds were also identified in the aforementioned prior studies and are provided in their publications (Mackintosh et al., 2016; Shaul et al., 2015).
3. RESULTS AND DISCUSSION
3.1. Species Contaminant Profile Comparisons.
Cetacean blubber contained markedly more compounds than pinniped blubber, with the long-beaked common dolphin, short-beaked common dolphin, and Risso’s dolphin averaging 133, 128, and 124 HOCs, respectively. These averages were two- to three-fold higher than average number of HOCs detected in the California sea lion (53 HOCs) and harbor seal (40 HOCs; Table 1).
Table 1.
Number of detected compounds (and number not typically monitored) in each designated structural class, and average number detected in each study species.
| Long-beaked common dolphin (D. capensis, n=5) | Short-beaked common dolphin (D. delphls, n=5) | Risso’s dolphin (G. griseus, n=5) | California sea lion (Z. califomlanus, n=5) | Harbor seal (P. vitulina, n=5) | ||||
|---|---|---|---|---|---|---|---|---|
| Structural class | No. HOCs in class | (No. not typically monitored) | Source | Average no. HOCs | Average no. HOCs | Average no. HOCs | Average no. HOCs | Average no. HOCs |
| B/CDE | 1 | 1 | unknown | 0 | 0 | 0 | 0 | 0 |
| Brominated anisole | 1 | 1 | mixed | 1(1) | 0 | 0 | 0 | 0 |
| Chlordane-related | 14 | 9 | anthropogenic | 8(3) | 8(4) | 7(3) | 5(2) | 1(0) |
| Chlorinated benzene | 1 | 1 | anthropogenic | 1(1) | 1(1) | 1(1) | 0 | 1(1) |
| Chlorinated styrene | 1 | 1 | anthropogenic | 1(1) | 0 | 0 | 0 | 0 |
| DDT-related | 24 | 16 | anthropogenic | 13(7) | 12(5) | 10(4) | 4(2) | 3(1) |
| Dichlorobenzophenone | 1 | 1 | anthropogenic | 0 | 0 | 0 | 0 | 0 |
| Dieldrin | 1 | 0 | anthropogenic | 0 | 1(0) | 0 | 0 | 0 |
| DMBP | 16 | 16 | natural | 8(8) | 12 (12) | 13(13) | 2(2) | 0 |
| HCH-related | 2 | 0 | anthropogenic | 1(0) | 1(0) | 1(0) | 1(0) | 0 |
| Heptachlor-related | 3 | 2 | anthropogenic | 1(1) | 2(1) | 1(0) | 0 | 0 |
| MBP | 6 | 6 | natural | 4(4) | 4(4) | 3(3) | 2(2) | 0 |
| MeO-B/CDE | 1 | 1 | natural | 0 | 0 | 1(1) | 0 | 0 |
| MeO-BDE | 3 | 3 | natural | 2(2) | 2(2) | 2(2) | 0 | 0 |
| MeO-CDE | 1 | 1 | unknown | 0 | 0 | 0 | 0 | 0 |
| MeO-PBB | 1 | 1 | natural | 1(1) | 1(1) | 1(1) | 0 | 0 |
| Methylenebistrichloroanisole | 1 | 1 | anthropogenic | 1(1) | 1(1) | 1(1) | 0 | 0 |
| Methylsulfonyl-PCB | 7 | 7 | anthropogenic | 2(2) | 1(1) | 1(1) | 1(1) | 3(3) |
| Mirex-related | 5 | 4 | anthropogenic | 3(2) | 3(2) | 2(1) | 1(0) | 1(0) |
| PBB | 10 | 9 | anthropogenic | 4(3) | 4(3) | 4(3) | 1(0) | 2(2) |
| PBDE | 16 | 6 | anthropogenic | 10(1) | 9(2) | 9(1) | 8(0) | 6(0) |
| PBHD | 3 | 3 | natural | 1(1) | 1(1) | 2(2) | 0 | 0 |
| PCT | 6 | 6 | anthropogenic | 2(2) | 1(1) | 3(3) | 0 | 0 |
| Pyrrolidinecarbonyl chloride | 1 | 1 | anthropogenic | 0 | 0 | 0 | 0 | 0 |
| TCPM | 8 | 8 | anthropogenic | 6(6) | 6(6) | 6(6) | 2(2) | 1(1) |
| TCPMOH | 2 | 2 | anthropogenic | 1(1) | 1(1) | 1(1) | 0 | 0 |
| Toxaphene | 13 | 5 | anthropogenic | 4(0) | 7(0) | 5(0) | 0 | 0 |
| Unknown | 22 | 22 | unknown | 5(5) | 5(5) | 6(6) | 0 | 0 |
| Unknown-3 | 2 | 2 | unknown | 1(1) | 1(1) | 0 | 0 | 0 |
| Unknown-4 | 12 | 12 | unknown | 6(6) | 4(4) | 4(4) | 1(1) | 0 |
| Unknown-5 | 2 | 2 | unknown | 1(1) | 1(1) | 2(2) | 0 | 0 |
| Unknown-6 | 3 | 3 | unknown | 1(1) | 1(1) | 0 | 0 | 0 |
| Unknown-7 | 2 | 2 | unknown | 1(1) | 1(1) | 1(1) | 0 | 1(1) |
| Unknown-8 | 2 | 2 | unknown | KD | KD | KD | 0 | 0 |
| Average PCBs | - | - | anthropogenic | 43 | 39 | 35 | 24 | 17 |
| Average Total HOCs (excluding PCBs) | 194 | - | mixed | 90 | 89 | 89 | 29 | 22 |
| (Range) | (77 – 98) | (77 – 102) | (70 – 108) | (14 – 55) | (11 – 32) | |||
As a structural class, PCBs had the largest number of individual compounds, and were between 28% to 32% of the total number of identified compounds in cetaceans, and 43% to 45% in pinnipeds (Table 1). For the purpose of identifying which species had the highest number of individual compounds, PCBs are included in the discussion below. However, parent PCB compounds are not included in contaminant profile comparisons because they are well-characterized in the literature and frequently monitored. We included PCB metabolites (methylsulfonyl-PCBs) in profile comparisons because they are typically unmonitored. A total of 194 different HOCs, excluding PCBs, from various anthropogenic, natural, mixed, and unknown sources were identified across all 25 samples, 157 (or 81% of total) of which are not included in routine environmental monitoring surveys.
Hierarchical clustering of samples based on similarity in anthropogenic HOC prevalence identified two primary, separate clusters comprising cetacean and pinniped samples, revealing that cetacean contaminant profiles were more diverse compared to pinnipeds (Figure S1). The heat map shows this difference is not limited to a few compounds, but is consistent across the majority of individual contaminants. The most frequently detected compounds included p,p’-DDE, trans nonachor, tris(4-chlorophenyl)methane (4,4’,4”-TCPM), BDE-47, BDE-100, and BDE-99, all of which were detected in all 25 samples. Two potential confounding factors influencing contaminant accumulation were animal length (as a proxy for age) and collection year (Table S2). Contaminant profiles within species were not associated with either of these two factors (data not shown).
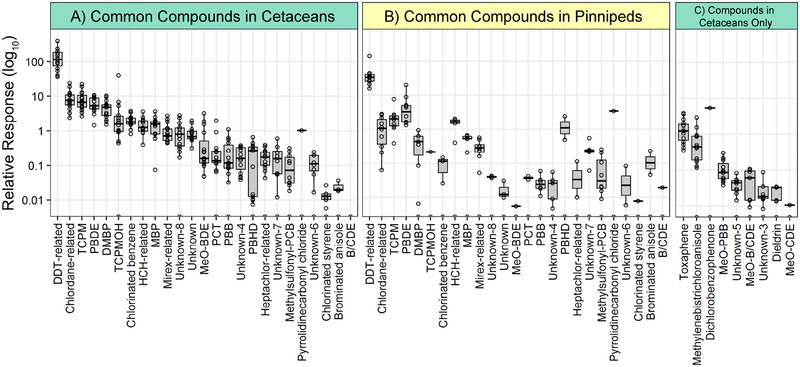
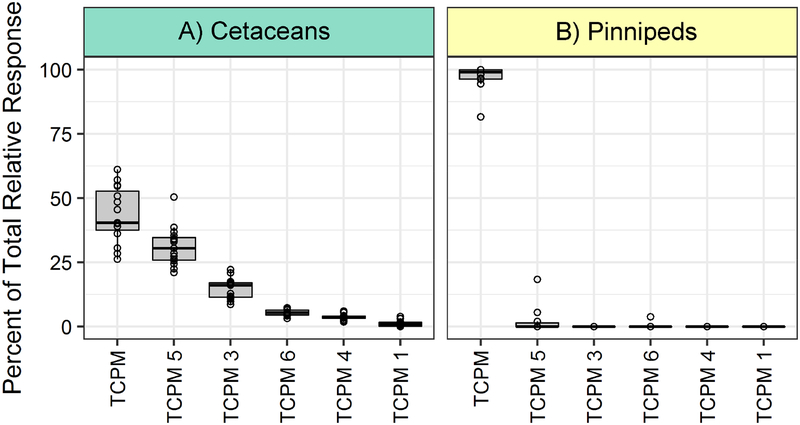
The HOCs detected in this study were organized into 35 classes based on similarity of molecular structure (Table 1; structural class descriptions in Table S1). DDT-related compounds constituted the most abundant structural class in both of the cetacean and pinniped groups (Figure 1), as well as for each species (Figure S2), which is consistent with the established contaminant signature found in marine mammals from this region (Blasius and Goodmanlowe, 2008; Shaul et al., 2015). The TCPM structural class consisted of eight isomers and was the second-most abundant. It was detected at levels comparable to the chlordane-related and PBDE classes, both of which are legacy HOCs of concern in the SCB (Dodder et al., 2012; Maruya and Schiff, 2009). Following its discovery in Puget Sound and Baltic Sea seals around 1990 (Walker et al., 1989; Zook et al., 1992), 4,4’,4’’-TCPM was determined to be an impurity from the technical preparation of pesticides such as DDT (Buser, 1995) and dicofol (de Boer, 1997). TCPM and its derivative, tris(4-chlorophenyl)methanol (TCPMOH), were measured in marine mammals from North America and Asia in the early 2000s, and were determined to have high biomagnification potential (Kajiwara, K. Kannan, M. Muraoka, M., 2001; Kannan et al., 2004; Minh et al., 2000; Watanabe et al., 2000). Although previously found to be one to three orders of magnitude greater in sea lions off the Northern and Central California coast compared to species from other locations (Kajiwara, K. Kannan, M. Muraoka, M., 2001; Kannan et al., 2004), TCPM and TCPMOH appear to have lost recognition as a pervasive contaminant in the North Pacific over the past decade. The parent compound, 4,4’,4”-TCPM, was detected in every blubber sample in this study. Notably, five additional TCPM isomers were present in nearly all dolphin samples (Figure 2) and constitute a relevant portion of the accumulated structural class. However, within the SCB, these isomers have only been observed by prior non-targeted studies of bottlenose dolphins (Mackintosh et al., 2016; Shaul et al., 2015). The consistent ratio of TCPM isomers observed across dolphin samples suggests similar exposure to a consistent mixture of isomers, constituting a persistent and unmonitored chemical mixture that could permeate trophic levels throughout the region.
Figure 1.
Summed relative abundance of HOC structural classes. The three panels show A) the compounds in the cetaceans (n=15) that were detected in both the cetaceans and pinnipeds, B) the compounds in the pinnipeds (n=10) that were detected in both the cetaceans and pinnipeds, and C) the compounds detected in the cetaceans only. The panels have identical y-axis scales. For each structural class, the bold horizontal line corresponds to the median, the box corresponds to the interquartile range (IQR), and the whiskers flag potential outliers and extend to the smallest and largest values that are < 1.5 × IQR from the 25th and 75th percentiles, respectively.
Figure 2.
Percent total relative abundance of the detected TCPM isomers in cetaceans and pinnipeds. TCPM is the parent compound 4,4’,4”-TCPM that was previously targeted in marine mammal contaminant surveys (see text). The other five TCPM isomers were not included in prior targeted surveys. Two additional TCPM related isomers were detected in two or fewer samples and are not included in the figure. Figure 1 describes the box plot components.
3.2. Halogenated Natural Products.
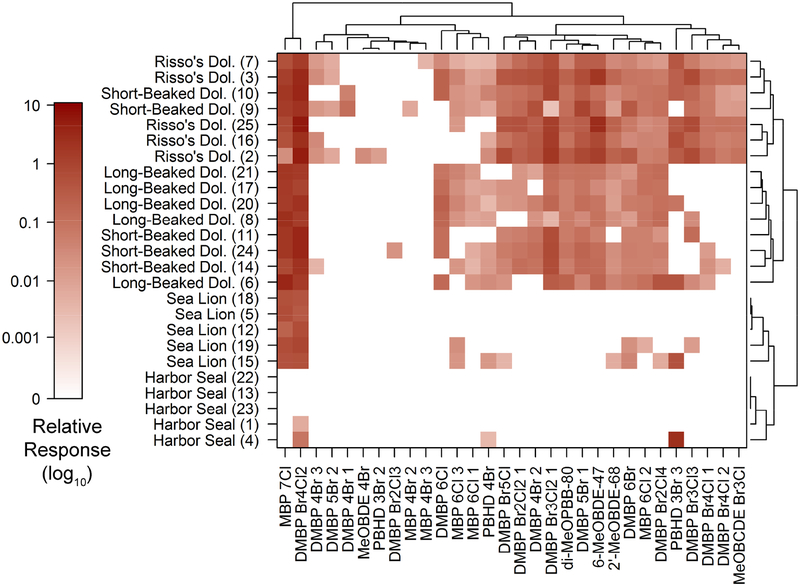
The 30 identified halogenated natural products (HNPs) belonged to six different structural classes and accounted for 15% of the HOCs. These compounds are produced by algae or sponges and have a biomagnification potential similar to anthropogenic HOCs (Pangallo and Reddy, 2010). Despite their global distribution and profusion in marine food webs and biota, the health implications of exposure to HNPs are largely unknown (Shaul et al., 2015). We observed a more diverse array of HNPs in the dolphin species compared to the pinnipeds, with dimethyl bipyrroles (DMBPs) the most prevalent and abundant class (Table 1, Figure 1). 1,1’-Dimethyl-tetrabromo-dichloro-2,2’-bipyrrole (DMBP Br4Cl2) was the most common individual natural product, and was detected in all but one sea lion and four harbor seal samples (Figure S2). This compound was previously detected in California sea lion blubber at concentrations rivaling that of BDE 47 (Stapleton et al., 2006). Heptachlorinated 1-methyl 1’,2-bipyrrole (MBP 7Cl) was the second-most common HNP and was found in all cetacean samples and three sea lion samples. This widespread natural product, also referred to as Q1, has been identified in Atlantic marine mammals (Teuten et al., 2006).
HNP profiles have been shown to be unique to different marine species and ecotypes within a geographic region (Dorneles et al., 2010; Hauler et al., 2013; Pangallo and Reddy, 2010; Shaul et al., 2015). The species in this study displayed nearly unique HNP profiles (Figure 3). California sea lions clustered separately from harbor seals, and all common dolphins grouped separately from the Risso’s dolphins (with the exception of two short-beaked common dolphins), suggesting that foraging strategies and locations may be discernable using HNP profiles. In contrast, anthropogenic profiles were unique between cetaceans and pinnipeds, but species within these taxonomic groups were not different (Figure S1).
Figure 3.
Hierarchical clustering heat map of the halogenated natural product profiles among all 25 samples. The number in parenthesis indicates the sample number.
The difference between HNP and anthropogenic contaminant clustering may be due to the origin of these compounds to the local marine environment. Legacy anthropogenic compounds originated from terrestrial sources and now contaminate broad areas of marine sediment in the SCB (Dodder et al., 2012; Maruya and Schiff, 2009). HNPs may have spatially distinct ecological profiles based on algal and sponge community composition, leading to unique bioaccumulation patterns based on the habitats and foraging strategies of the different species. HNPs have important potential applications beyond toxicological assessments, including the differentiation of ecotypes or populations within species (Dorneles et al., 2010; Shaul et al., 2015), evaluating trophic relationships (Pangallo and Reddy, 2010), and could enhance indirect chemical methods for determining diet composition of free-ranging marine mammal species (Bowen and Iverson, 2012).
3.3. Unknown Contaminants.
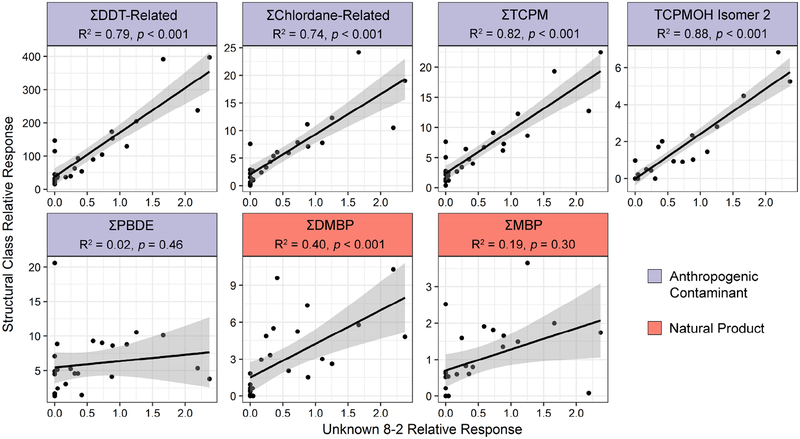
Based on isotopic profiles in their mass spectra, 45 observed compounds were determined to contain halogens, but their complete structural identities could not be determined through mass spectral searches or de novo interpretation (mass spectra in SI-2 and accessible online at https://github.com/OrgMassSpec/SpecLibMarineUnknown2018). Ten of the halogenated unknown compounds have not, to our knowledge, been previously identified in marine biota; they are first 10 spectra described in SI-2. The number of unknowns accounted for 24% of the total number of identified HOCs and outnumbered each of the DDT-related (n=24), chlordane-related (n=14) and PBDE (n=16) structural classes. These unidentified compounds could be emerging contaminants from current anthropogenic activities, previously undiscovered legacy pollutants, or halogenated natural products. For example, the unknown-8–2 isomer (SI-2, page 46) was relatively abundant in cetacean blubber and a significant regression was found with ƩDDT-Related (R2=0.79, p<0.001), ƩChlordane-Related (R2=0.74, p<0.001), ƩTCPM (R2=0.82, p<0.001, and the TCPMOH Isomer 2 metabolite (R2=0.88, p<0.001); Figure 4), indicating the unknown compound may have a similar history of anthropogenic use in this region. Unknown-8–2 did not have a significant regression with ƩPBDE, a compound class with a different history compared to the other anthropogenic contaminants, including later peak use (Dodder et al., 2012). There was a significant, but less robust regression with ƩDMBP (R2=0.4, p<0.001), indicating unknown-8–2 may not be a natural product.
Figure 4.
Linear regressions of the relative abundance of Unknown-8–2 (an unknown halogenated compound) against the relative abundance of the five most abundant anthropogenic structural classes and two most abundant natural product classes. TCPME (a second TCPMOH isomer) was observed in one sample; therefore, a regression was made against TCPMOH Isomer 2 only.
3.4. Differences in Cetacean vs. Pinniped Profiles.
Pinnipeds appear to accumulate fewer compounds across a smaller number of structural classes, however the compounds that do accumulate in pinniped blubber are found at similar abundance to those in cetaceans (Figure 1). Although California sea lions feed on the same pelagic schooling fish as common dolphins, and even regularly feed in industrialized embayments (Meng et al., 2009), the multitude of HOCs in their contaminant profile is reduced (53 average total HOCs in sea lions versus 133 and 128 average total HOCs in long- and short-beaked common dolphins, respectively; Table 1). Possible explanations for this discrepancy are likely physiological, such as differences in life history strategies and enzyme-mediated metabolism. Pinnipeds undergo periods of fasting during annual breeding and molting seasons (Ling, 1970). These processes are energetically costly, enhancing blubber turnover and mobilizing contaminants to the bloodstream (Peterson et al., 2014). Small cetacean species maintain a homogenous HOC profile across blubber depth indicating more stable concentrations over time (Méndez-Fernandez et al., 2016). Cetaceans also have a lower capacity for metabolizing PCBs compared to other terrestrial and marine mammals, such as seals (Routti et al., 2008; Tanabe et al., 1988), due to lower or absent CYP2B liver enzyme activity (Boon et al., 1997). An enhanced capacity for metabolizing and excreting certain organic contaminants was proposed to explain the much lower levels of TCPM and TCPMOH observed in pinnipeds compared to cetaceans (Minh et al., 2000). Enhanced metabolism has also been proposed as an explanation for the absence of HNPs from pinniped blubber despite presence in prey items (Pangallo and Reddy, 2010) and the lack of methoxy-BDEs detected in California sea lions (Stapleton et al., 2006). The expanded range of HOCs evaluated in this study revealed additional structural classes that appear to align with this phenomenon (Figure 1).
3.5. Best Sentinel Determination.
Based upon the multitude and abundance of detected HOCs, cetacean species are more effective regional bioindicators for cataloging emerging and unknown HOCs. The following discussion excludes PCBs because they were not previously included in the associated bottlenose dolphin study (Shaul et al., 2015). Whereas 103 anthropogenic and naturally occurring unmonitored compounds, and 45 unknowns, were detected in the cetacean samples from the current study (94% of all unmonitored compounds from both cetaceans and pinnipeds), only 46 typically unmonitored HOCs from anthropogenic and natural sources, and 12 unknowns, were detected in pinniped blubber. Within the cetaceans, the long-beaked common, short-beaked common, and Risso’s dolphin samples averaged 90, 89, and 89 HOCs per sample, respectively. In contrast, an average of 209 HOCs per sample (n=8) was detected in Pacific common bottlenose dolphins in the prior study using the same blubber sampling and instrumental method (Shaul et al., 2015). The bottlenose dolphins were collected from the same region, during approximately the same timeframe (1995–2010). The data processing methods were different. Halogenated compounds in the bottlenose dolphins were identified by a fully manual process. For the current project, as described in detail in SI-1, we used an automated process (with manual cross-checking between samples as a final step). Direct comparison of the two processes determined the automated process detection rate of halogenated compounds was 80% to 85% of the manual process. The difference in detection rates is not large enough to account for the difference in the number of HOCs detected in bottlenose dolphins compared to the other species.
This indicates the bottlenose is the most contaminated marine mammal among the six species studied in the SCB. However, bottlenose dolphins do not often strand, limiting availability of full-depth blubber samples from this species, and hampering ongoing alternate research that relies on live biopsy samples (Figure S3). Archived specimens are also exceptionally rare for the Risso’s dolphin, which typically range offshore. Therefore, based on similar HOC profiles and readily available sample material, it is recommended that long- and short-beaked common dolphins (which contained 80% of the unmonitored compounds found among all species in this study) serve as effective sentinels for fulfilling the two main priorities of the non-targeted work in the SCB: retrospective evaluation of contaminant trends and proactive screening for emerging contaminants of concern.
3.6. Domoic Acid Susceptibility.
Species with observed sensitivity to domoic acid toxicosis (i.e., long-beaked common dolphins, short-beaked common dolphin, and California sea lions) did not contain unique contaminant signatures compared to those that have not been documented to experience mass mortality events following blooms (i.e., common bottlenose dolphins, Risso’s dolphins, and harbor seals (Gulland and Hall, 2007; Lefebvre et al., 1999). In fact, domoic acid-related stranding events were not observed for the most contaminated species, the Pacific common bottlenose dolphin. This suggests contaminants do not play a profound role in the domoic acid sensitivity exhibited by particular SCB marine mammal populations at the species level. This result reinforces existing speculation that prey preference likely has the greatest impact on the risk of poisoning by domoic acid (Lefebvre et al., 1999). The Northern anchovy has been implicated as the primary vector of exposure and is a preferred prey item for California sea lions, long-, and short-beaked common dolphins (Lowry and Carretta, 1999), all of which experience domoic acid toxicosis (Danil et al., 2010).
3.7. Implications for Contaminant Monitoring.
The population of the five coastal counties bordering the SCB is projected to increase to over 20 million people by 2020 (Maruya and Schiff, 2009), thus there is a demand for vigilance in monitoring of both legacy and emerging contaminants in this ecologically and economically valuable coastal zone. Therefore, as new compounds are released into the environment, non-targeted contaminant analysis allows researchers and managers to comprehensively screen for emerging compounds of concern, fast-tracking them for possible source, structure, and toxicity testing. Further evaluation and characterization of the unknown compounds highlighted in this study is imperative for assessing seafood safety and possible distribution of such contaminants on a global scale, particularly when the abundance of these compounds rivals that of many legacy pollutants. Additionally, chemicals identified in this study may serve as unique tracers to gather information about the physiology and ecology of marine mammal species that serve as sentinels of marine environmental quality.
Supplementary Material
Highlights:
organic contaminants were identified in Southern California marine mammal blubber
cetaceans accumulated more contaminants than pinnipeds
common dolphins are proposed as sentinels for emerging contaminants in this region
ACKNOWLEDGEMENTS
We are grateful to Wayne Lao and David Tsukada (SCCWRP) for performing the blubber oil extraction and to Kayo Watanabe and Susan Mackintosh (SDSU) for training on instrumental and software analysis. We also thank the many California/Oregon Gillnet Fishery Observers and California Marine Mammal Stranding Network participants who collected the samples used in this study, and the SWFSC for establishing and maintaining an invaluable archive of marine mammal tissues. Funding from National Oceanic and Atmospheric Administration (NOAA) Prescott Award NA14NMF4390177, the Scripps Center for Ocean and Human Health Program through the National Science Foundation (OCE-1313747) and National Institute of Environmental Health Sciences (P01-ES021921), and the San Diego State University Division of Research Affairs supported this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTARY DATA
The following is the supplementary data related to this article:
Supporting Information Part 1 (SI-1) and Supporting Information Part 2 (SI-2).
The authors declare no conflict of interest.
REFERENCES
- Alonso MB, Maruya KA, Dodder NG, Lailson-Brito J, Azevedo A, Santos-Neto E, Torres JPM, Malm O, Hoh E, 2017. Nontargeted Screening of Halogenated Organic Compounds in Bottlenose Dolphins (Tursiops truncatus) from Rio de Janeiro, Brazil. Environ. Sci. Technol 51, 1176–1185. 10.1021/acs.est.6b04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius ME, Goodmanlowe GD, 2008. Contaminants still high in top-level carnivores in the Southern California Bight: Levels of DDT and PCBs in resident and transient pinnipeds. Mar. Pollut. Bull 56, 1973–1982. 10.1016/j.marpolbul.2008.08.011 [DOI] [PubMed] [Google Scholar]
- Boon JP, Van der Meer J, Allchin CR, Law RJ, Klungsøyr J, Leonards PEG, Spliid H, Storr-Hansen E, Mckenzie C, Wells DE, 1997. Concentration-dependent changes of PCB patterns in fish-eating mammals: structural evidence for induction of cytochrome P450. Arch. Environ. Contam. Toxicol 33, 298–311. [DOI] [PubMed] [Google Scholar]
- Bossart GD, 2011. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol 48, 676–690. 10.1177/0300985810388525 [DOI] [PubMed] [Google Scholar]
- Bowen WD, Iverson SJ, 2012. Methods of estimating marine mammal diets: A review of validation experiments and sources of bias and uncertainty. Mar. Mammal Sci. 29, 719–754. 10.1111/j.1748-7692.2012.00604.x [DOI] [Google Scholar]
- Brouwer A, Reijnders PJH, Koeman JH, 1989. Polychlorinated biphenyl (PCB)-contaminated fish induces vitamin A and thyroid hormone deficiency in the common seal (Phoca vitulina). Aquat. Toxicol 15, 99–106. [Google Scholar]
- Buser HR, 1995. DDT, a Potential Source of Environmental Tris(4-chlorophenyl)methane and Tris(4-chlorophenyl)methanol. Environ. Sci. Technol 29, 2133–2139. [DOI] [PubMed] [Google Scholar]
- Danil K, Chivers SJ, Henshaw MD, Thieleking JL, Daniels R, Leger JAS, 2010. Cetacean strandings in San Diego County, California, USA. J. Cetacean Res. Manag 11, 163–184. [Google Scholar]
- de Boer J, 1997. Environmental Distribution and Toxicity of Tris(4-Chlorophenyl)Methanol and Tris(4-Chlorophenyl)Methane, in: Reviews of Environmental Contamination and Toxicology. pp. 95–106. [Google Scholar]
- Desforges J-P, Levin M, Jasperse L, De Guise S, Eulaers I, Letcher RJ, Acquarone M, Nordøy E, Folkow LP, Hammer Jensen T, Grøndahl C, Bertelsen MF, St. Leger J, Almunia J, Sonne C, Dietz R, 2017. Effects of Polar Bear and Killer Whale Derived Contaminant Cocktails on Marine Mammal Immunity. Environ. Sci. Technol 51, 11431–11439. 10.1021/acs.est.7b03532 [DOI] [PubMed] [Google Scholar]
- Dodder NG, Maruya KA, Lauenstein GG, Ramirez J, Ritter KJ, Schiff KC, 2012. Distribution and sources of polybrominated diphenyl ethers in the southern california bight. Environ. Toxicol. Chem. SETAC 31, 2239–2245. 10.1002/etc.1957 [DOI] [PubMed] [Google Scholar]
- Dorneles PR, Lailson-Brito J, Dirtu AC, Weijs L, Azevedo AF, Torres JPM, Malm O, Neels H, Blust R, Das K, Covaci A, 2010. Anthropogenic and naturally-produced organobrominated compounds in marine mammals from Brazil. Environ. Int 36, 60–67. 10.1016/j.envint.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Gilmartin WG, Delong RL, Smith AW, Sweeney JC, LAPPE BWD, Risebrough RW, Griner LA, Dailey MD, Peakall DB, 1976. Premature parturition in the California sea lion. J. Wildl. Dis 12, 104–115. [DOI] [PubMed] [Google Scholar]
- Goldstein T, Mazet JAK, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FMD, 2008. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc. R. Soc. Lond. B Biol. Sci 275, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland FMD, Hall AJ, 2007. Is Marine Mammal Health Deteriorating? Trends in the Global Reporting of Marine Mammal Disease. EcoHealth 4, 135–150. 10.1007/s10393-007-0097-1 [DOI] [Google Scholar]
- Hall AJ, Law RJ, Wells DE, Harwood J, Ross HM, Kennedy S, Allchin CR, Campbell LA, Pomeroy PP, 1992. Organochlorine levels in common seals (Phoca vitulina) which were victims and survivors of the 1988 phocine distemper epizootic. Sci. Total Environ. 115, 145–162. [DOI] [PubMed] [Google Scholar]
- Hauler C, Martin R, Knölker H-J, Gaus C, Mueller JF, Vetter W, 2013. Discovery and widespread occurrence of polyhalogenated 1,1’-dimethyl-2,2’-bipyrroles (PDBPs) in marine biota. Environ. Pollut 178, 329–335. 10.1016/j.envpol.2013.03.025 [DOI] [PubMed] [Google Scholar]
- Hoh E, Dodder NG, Lehotay SJ, Pangallo KC, Reddy CM, Maruya KA, 2012. Nontargeted comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry method and software for inventorying persistent and bioaccumulative contaminants in marine environments. Environ. Sci. Technol 46, 8001–8008. 10.1021/es301139q [DOI] [PubMed] [Google Scholar]
- Kajiwara K Kannan M Muraoka MN, 2001. Organochlorine Pesticides, Polychlorinated Biphenyls, and Butyltin Compounds in Blubber and Livers of Stranded California Sea Lions, Elephant Seals, and Harbor Seals from Coastal California, USA. Arch. Environ. Contam. Toxicol 41, 90–99. 10.1007/s002440010224 [DOI] [PubMed] [Google Scholar]
- Kannan K, Kajiwara N, Le Boeuf B., Tanabe S, 2004. Organochlorine pesticides and polychlorinated biphenyls in California sea lions. Environ. Pollut 131, 425–434. 10.1016/j.envpol.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Powell CL, Busman M, Doucette GJ, Moeller PD, Silver JB, Miller PE, Hughes MP, Singaram S, Silver MW, others, 1999. Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Nat. Toxins 7, 85–92. [DOI] [PubMed] [Google Scholar]
- Ling JK, 1970. Pelage and molting in wild mammals with special reference to aquatic forms. Q. Rev. Biol 16–54. [DOI] [PubMed] [Google Scholar]
- Lowry MS, Carretta JV, 1999. Market squid (Loligo opalescens) in the diet of California sea lions (Zalophus californianus) in southern California (1981–1995). Calif. Coop. Ocean. Fish. Investig. Rep 196–207. [Google Scholar]
- Mackintosh SA, Dodder NG, Shaul NJ, Aluwihare LI, Maruya KA, Chivers SJ, Danil K, Weller DW, Hoh E, 2016. Newly Identified DDT-Related Compounds Accumulating in Southern California Bottlenose Dolphins. Environ. Sci. Technol 50, 12129–12137. 10.1021/acs.est.6b03150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruya KA, Schiff K, 2009. The extent and magnitude of sediment contamination in the Southern California Bight, in: Geological Society of America Special Papers. Geological Society of America, pp. 399–412. [Google Scholar]
- Méndez-Fernandez P, Galluzzi Polesi P, Taniguchi S, de O Santos MC, Montone RC, 2016. Validating the use of biopsy sampling in contamination assessment studies of small cetaceans. Mar. Pollut. Bull 107, 364–369. 10.1016/j.marpolbul.2016.04.021 [DOI] [PubMed] [Google Scholar]
- Meng X-Z, Blasius ME, Gossett RW, Maruya KA, 2009. Polybrominated diphenyl ethers in pinnipeds stranded along the southern California coast. Environ. Pollut 157, 2731–2736. 10.1016/j.envpol.2009.04.029 [DOI] [PubMed] [Google Scholar]
- Millow CJ, Mackintosh SA, Lewison RL, Dodder NG, Hoh E, 2015. Identifying bioaccumulative halogenated organic compounds using a nontargeted analytical approach: seabirds as sentinels. PloS One 10, e0127205 10.1371/journal.pone.0127205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh TB, Watanabe M, Tanabe S, Miyazaki N, Jefferson TA, Prudente MS, Subramanian A, Karuppiah S, 2000. Widespread contamination by tris (4-chlorophenyl) methane and tris (4-chlorophenyl) methanol in cetaceans from the North Pacific and Asian coastal waters. Environ. Pollut 110, 459–468. [DOI] [PubMed] [Google Scholar]
- Pangallo KC, Reddy CM, 2010. Marine Natural Products, the Halogenated 1′-Methyl-1,2′-bipyrroles, Biomagnify in a Northwestern Atlantic Food Web. Environ. Sci. Technol 44, 5741–5747. 10.1021/es101039d [DOI] [PubMed] [Google Scholar]
- Peterson SH, Hassrick JL, Lafontaine A, Thomé J-P, Crocker DE, Debier C, Costa DP, 2014. Effects of Age, Adipose Percent, and Reproduction on PCB Concentrations and Profiles in an Extreme Fasting North Pacific Marine Mammal. PLoS ONE 9, e96191 10.1371/journal.pone.0096191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos AM, Bäcklin B-MVM, Helander BO, Rigét FF, Eriksson UC, 2012. Improved reproductive success in otters (Lutra lutra), grey seals (Halichoerus grypus) and sea eagles (Haliaeetus albicilla) from Sweden in relation to concentrations of organochlorine contaminants. Environ. Pollut 170, 268–275. 10.1016/j.envpol.2012.07.017 [DOI] [PubMed] [Google Scholar]
- Ross PS, 2002. The Role of Immunotoxic Environmental Contaminants in Facilitating the Emergence of Infectious Diseases in Marine Mammals. Hum. Ecol. Risk Assess. Int. J 8, 277–292. 10.1080/20028091056917 [DOI] [Google Scholar]
- Ross PS, 2000. Marine Mammals as Sentinels in Ecological Risk Assessment. Hum. Ecol. Risk Assess. Int. J 6, 29–46. 10.1080/10807030091124437 [DOI] [Google Scholar]
- Routti H, Letcher RJ, Arukwe A, van Bavel B, Yoccoz NG, Chu S, Gabrielsen GW, 2008. Biotransformation of PCBs in Relation to Phase I and II Xenobiotic-Metabolizing Enzyme Activities in Ringed Seals (Phoca hispida) from Svalbard and the Baltic Sea. Environ. Sci. Technol 42, 8952–8958. 10.1021/es801682f [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Voit EO, Hansen LJ, Wells RS, Mitchum GB, Hohn AA, Fair PA, 2002. Probabilistic risk assessment of reproductive effects of polychlorinated biphenyls on bottlenose dolphins (Tursiops truncatus) from the Southeast United States coast. Environ. Toxicol. Chem 21, 2752–2764. 10.1002/etc.5620211232 [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Zolman ES, Balmer BC, De Guise S, George RC, Hoguet J, Hohn AA, Kucklick JR, Lamb S, Levin M, Litz JA, McFee WE, Place NJ, Townsend FI, Wells RS, Rowles TK, 2012. Anaemia, hypothyroidism and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus). Proc. Biol. Sci 279, 48–57. 10.1098/rspb.2011.0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul NJ, Dodder NG, Aluwihare LI, Mackintosh SA, Maruya KA, Chivers SJ, Danil K, Weller DW, Hoh E, 2015. Nontargeted biomonitoring of halogenated organic compounds in two ecotypes of bottlenose dolphins (Tursiops truncatus) from the Southern California Bight. Environ. Sci. Technol 49, 1328–1338. 10.1021/es505156q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG, Kucklick JR, Reddy CM, Schantz MM, Becker PR, Gulland F, Porter BJ, Wise SA, 2006. Determination of HBCD, PBDEs and MeO-BDEs in California sea lions (Zalophus californianus) stranded between 1993 and 2003. Mar. Pollut. Bull 52, 522–531. 10.1016/j.marpolbul.2005.09.045 [DOI] [PubMed] [Google Scholar]
- Tanabe S, Watanabe S, Kan H, Tatsukawa R, 1988. Capacity and Mode of PCB Metabolism in Small Cetaceans. Mar. Mammal Sci. 4, 103–124. [Google Scholar]
- Teuten EL, Pedler BE, Hangsterfer AN, Reddy CM, 2006. Identification of highly brominated analogues of Q1 in marine mammals. Environ. Pollut 144, 336–344. 10.1016/j.envpol.2005.10.052 [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS, 2010. Zebrafish Seizure Model Identifies p,p’-DDE as the Dominant Contaminant of Fetal California Sea Lions That Accounts for Synergistic Activity with Domoic Acid. Environ. Health Perspect. 118, 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W, Jarman WM, de Lappe BW, Tefft JA, 1989. Identification of Tris(chlorophenyl)methanol in Blubber of Harbor Seals from Puget Sound. Chemosphere 18, 1799–1804. [Google Scholar]
- Watanabe M, Kannan K, Takahashi A, Loganathan BG, Odell DK, Tanabe S, Giesy JP, 2000. Polychlorinated biphenyls, organochlorine pesticides, tris (4-chlorophenyl) methane, and tris (4-chlorophenyl) methanol in livers of small cetaceans stranded along Florida coastal waters, USA. Environ. Toxicol. Chem 19, 1566–1574. [Google Scholar]
- Zook DR, Buser HR, Bergqvist P-A, Rappe C, Olsson M, 1992. Detection of Tris(chlorophenyl)methane and Tris(4-chlorophenyl)methanol in Ringed Seal (Phoca hispida) from the Baltic Sea. Ambio 21, 557–560. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.