Abstract
The FRUVEDomics study investigates the effect of a diet intervention focused on increasing fruit and vegetable intake on the gut microbiome, and cardiovascular health of young adults with/at risk for Metabolic Syndrome (MetS). It was hypothesized the recommended diet would result in metabolic and gut microbiome changes. The 9-week dietary intervention adhered to the USDA Dietary Guidelines for Americans and focused on increasing fruit and vegetable intake to equal half of the diet. Seventeen eligible young adults with/or at high risk of MetS, consented and completed preintervention and postintervention measurements, including anthropometric, body composition, cardiovascular, complete blood lipid panel, and collection of stool sample for microbial analysis. Participants attended weekly consultations to assess food logs, food receipts, and adherence to the diet. Following intention-to-treat guidelines all 17 individuals were included in the dietary, clinical, and anthropometric analysis. Fruit and vegetable intake increased from 1.6 to 3.4 cups of fruits and vegetables (P < .001) daily. Total fiber (P = .02) and insoluble fiber (P < .0001) also increased. Clinical laboratory changes included an increase in sodium (P = .0006) and low-density lipoprotein cholesterol (P = .04). In the fecal microbiome, Erysipelotrichaceae (phylum Firmicutes) decreased (log2 fold change: −1.78, P = .01) and Caulobacteraceae (phylum Proteobacteria) increased (log2 fold change = 1.07, P = .01). Implementing a free living 9-week diet, with intensive education and accountability, gave young adults at high risk for/or diagnosed with MetS the knowledge, skills, and feedback to improve diet. To yield greater impact a longer diet intervention may be needed in this population.
Keywords: Metabolic syndrome, Young adults, Healthy diet, MyPlate diet, Fruits and vegetables
1. Introduction
Individuals with Metabolic Syndrome (MetS) have variable combinations of at least 3 of the following 5 components: increased waist circumference, blood pressure, triglycerides, and blood glucose and decreased high-density lipoprotein (HDL). A MetS diagnosis indicates overall increased chronic disease burden throughout the lifespan [1], most specifically increasing rates of heart disease and diabetes [2, 3]. Unfortunately, MetS is often undiagnosed in the young adult population [4], missing the opportunities to treat current comorbidities and prevent future diseases [2].
Hypercholesterolemia, enteral inflammation, and other cardiovascular risks [5, 6] associated with MetS are related to a gut microbiome with low diversity [7]. The gut microbiome has been described as an organ with cells that communicate and are involved in energy distribution and storage [8]. The diversity and bacterial content of an individual’s gut microbiome is dependent on host genotype and age, as well as environmental factors, including diet composition [6]. Diet-induced modifications in the intestinal microbiome have been established in both human and animal models of obesity [9]. Diet is a modifiable risk factor [10] and an effective target for lifestyle interventions in young adults [11] to both improve gut microbiome health and decrease risk of MetS.
Currently, many areas of the world are adopting the Western diet, which is high in total fat, saturated fat, and refined carbohydrate intake, but low in fruits, vegetables, and other plantbased foods, resulting in low dietary fiber, nonstarchy polysaccharides, and resistant starch [12]. This diet contributes to increased inflammation and decreases gut microbiome diversity [13–15]. Diets high in fruits and vegetables that also increase fiber intake, for example, the Mediterranean [16–20] and Dietary Approach to Stop Hypertension [21] diets, have been shown to decrease risk of MetS and improve the gut microbiome diversity [22]. Prevalence of MetS decreased from 61.4% to 13.7% in a group of adults eating a Mediterranean diet for 1 year, [18]. Panunzio et al. observed similar improvements in MetS risk factors (lower body weight, C-reactive protein (CRP), and fasting insulin) in adults after 25 weeks of a Mediterranean diet [23]. Similarly, metabolic parameters improved in participants after an 8-week energy-restricted Mediterranean diet [24]. We previously reported our results of an 8-week dietary intervention focused on adopting the 2010 Dietary Guidelines for Americans that improved metabolic and cardiovascular measures in young adults without MetS [25]. Altogether, these results indicate an intensive 9-week diet intervention can improve metabolic parameters. However, little research has demonstrated whether a similar diet produces the same outcomes in a young adult population with high-risk and/or overt MetS.
Young adults are an optimal population to target for an education-based diet intervention [26] focused on improving dietary behaviors to reduce chronic disease risk [27]. Implementing dietary modifications can reduce long- and short-term risk of coronary heart disease events [28], and improve individual health outcomes [2]. Diet-induced change in the intestinal microbiome can contribute to overall health by modulating immune-mediated interactions and food metabolism [9] in the host. In this study, young adults at high-risk for and/or having MetS were recruited to determine the behavioral and gut microbial changes that would occur given a dietary intervention focused on the MyPlate guideline to increase fruits and vegetables to half of their dietary intake. The primary research hypothesis was that the educational intervention with intense (weekly) personalized monitoring and feedback would increase fruit and vegetable intake in young adults. The secondary hypothesis was that the improvements in dietary habits would improve metabolic and gut microbiome health.
2. Methods and materials
2.1. Research design
Ethical approval was obtained from the West Virginia University (WVU) Institutional Review Board and informed consent was collected from each subject prior to enrollment in the study. Young adults completed the diet intervention at WVU in the fall of 2016 (Clinical Trials Record NCT03115866). The research presented here is a subset of the registered clinical trial. The results from the other arms of the study are presented in other research articles either published or currently in review. Recruitment occurred through word of mouth, flyers posted around campus, announcements in classrooms, and e-mails to the student body.
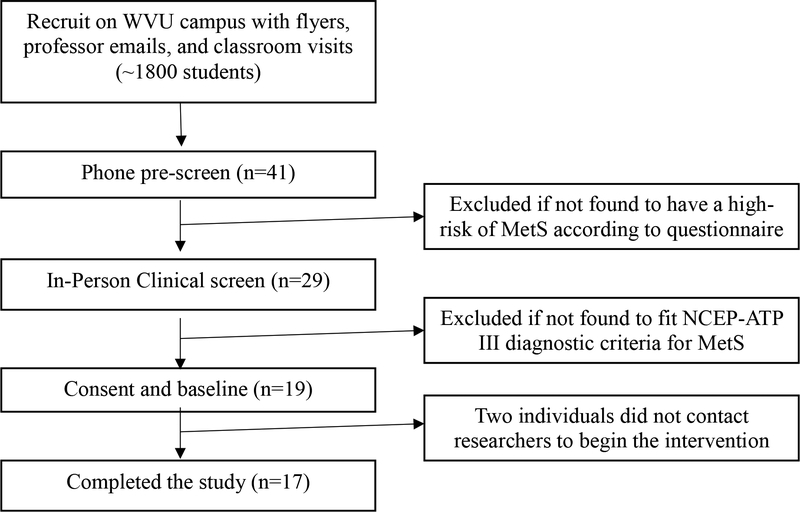
Eligibility criteria included to be diagnosed with MetS at the in-person screening (Figure 1). The National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III MetS criteria was used to diagnose the participants, where subjects needed to have 3 of the following components: waist circumference >102cm (men), >88cm (women), serum triglycerides >150mg/dL, serum HDL <40mg/dL (men), <50mg/dL (women), blood pressure ≥130/85mm Hg, and fasting blood glucose ≥ 100mg/dL [29]. Exclusion criteria were diagnosis or treatment of a serious behavioral disorder within the past year, pregnancy, use of antibiotics in the past 6 months or presence of another chronic disease. Prescreening involved a phone interview, followed by an in-person anthropometric assessment and confirmation of MetS clinical laboratory components. After consent was obtained participants were scheduled for a preintervention (week 0) assessment to determine baseline measures (Fig. 1). The same measurements were taken 9 weeks later at the postintervention assessment (week 9).
Fig. 1 -.
Consort diagram depicting the flow of participants from recruitment to statistical analysis. Prescreening determined risk of MetS, then the in-person clinical screen where participants were screened for the 5 different components of MetS according to the NCEP-ATP III. Nineteen individuals then consented to participate, but 2 never began the intervention. This left a total of 17 individuals who started and completed the study.
2.2. Diet intervention
Participants were expected to be willing to adhere to a diet of 50% fruit and vegetable intake (4–5 daily cups) and energy recommendations based on weight maintenance, age, and physical activity in accordance with the Dietary Guidelines for Americans for 9 weeks. Their willingness was determined by an affirmative answer to the following question: “Are you willing and able to adhere to a nutritional intervention consisting of half your plate fruits and vegetables for eight weeks?” The recommended portions for each food group were calculated for each participant using US Department of Agriculture MyPlate prior to the start of the intervention from the computer software Nutritionist Pro (Axxya Systsems LLC, Redmond, WA, USA). Each participant attended a 2-hour education session prior to the start of the intervention to receive materials and tools ($230) to help achieve their personal diet recommendations.
During the intervention, participants were required to attend weekly, 1-hour counseling sessions with a trained nutrition researcher. These counseling sessions utilized motivational interviewing techniques to encourage client-led goal setting. Participants provided daily food and activity logs, food receipts, and food pictures at this time. These were used to determine diet compliance and aid in developing strategies to improve their diets. Each week participants were asked, “Which day depicts the most common eating habits from the week?” The day reported was used to analyze food components for macronutrients, fiber, sugar, empty calorie, and fruit and vegetable consumption using the Nutritionist Pro analysis software. During these weekly sessions participants also received financial compensation, totaling $230 for participating in all components of the study.
Compliance with the diet was based on participants consuming at least 50% fruits and vegetables, or 4–5 daily cups. Participants were asked, “Rate yourself on a scale of 0–100, how often did you maintain the prescribed diet?” The researcher would also rate the participant based on their dietary food log and counseling session. A combined and averaged score of 75 or higher, as well as the Nutritionist Pro report of 4–5 fruit and vegetable cups a day was determined to be diet compliant each week. According to intention-to-treat guidelines all 17 recruited individuals were included for the dietary and anthropometric data analysis, although 2 of the participants had significant changes in sleep, stress, physical activity, or medication;, used antibiotics or steroids; and were noncompliant to the dietary recommendations.
2.3. Outcome measures
2.3.1. Nutrition and behavior
Clinical and nutrition history was obtained by a trained researcher at a face-to-face interview. A survey was distributed to participants prior to beginning the 9-week diet intervention, and at the end of the intervention to collect data on sleep hours and stress using the Pittsburgh Sleep Quality Index [30] and the Cohen Perceived Stress Score [31], respectively.
Physical activity was measured by participants wearing an accelerometer (ActiGraph GT3X, Actilife 6.0 Software, Pensacola, FL,USA) around their waist for 1 week preintervention and postintervention. Instructions were provided based on manufacturer’s guidelines. Non-wear time was defined as ≥60 repeated minutes of zero activity counts. Average steps per day were calculated using age specific cut points by Freedson et al. [32]. Participants were instructed to keep physical activity, stress, and sleep hours consistent throughout the study to ensure that the diet was the main intervention.
2.3.2. Anthropometrics and cardiovascular measurements
Anthropometric measurements were taken by a trained researcher while the participant was wearing minimal, tight-fitting clothing, with no shoes using standard procedures and outlined in the Supplementary Material.
Brachial (b) systolic and diastolic blood pressures (SBP and DBP) were measured with an automated, oscillometric sphygmomanometer (Critikon Dinamap Compact BP monitor, GE Medical Tampa, FL, USA), and pulse pressure was calculated from systolic bSBP-bDBP. Pulse wave analysis was performed noninvasively on the radial artery (SphygmoCor System, ATCor Medical, Sydney, NSW, Australia). All measurements were made in triplicate and the mean values were used for subsequent analysis. The SphygmoCor system synthesizes a central (ascending aortic) pressure wave form from the radial pressure wave form that does not differ from that of an intra-arterially recorded wave [33] using a validated generalized transfer function [34] that has good reproducibility under major hymodynamic changes [35]. Pulse wave velocity is measured using the same tonometry unit, alternating the placement of the probe on the carotid and femoral pulses. Velocity is determined by the difference in arrival time of the pulse wave between sits. In the supine position, B-mode ultrasound (GE Vivid i) 2-dimensional images of the right common carotid artery were obtained 1–2 cm proximal to the carotid bifurcation to measure maximal lumen diameter, and carotid intima-mediathickness (IMT) following standard procedures [36]. Cross-sectional area of the carotid artery was calculated as [(maximal lumen diameter/2)2 × π] - [(maximal lumen diameter/2 − IMT)2 × π]. The wall-to-lumen ratio (W/L) of the right CCA was calculated as 2 × IMT/lumen diameter in diastole [37]. A blinded expert then assessed the quality for each measure to make the determination if the value was valid.
2.3.3. Blood and stool sample collection/processing
Fasting blood was collected by venipuncture prior to the intervention, and after the 8-week intervention. Samples were analyzed by Ruby Memorial Hospital Clinical laboratory for basic chemistry analysis, complete lipid panel (i.e., cholesterol, HDL, low-density lipoprotein [LDL]) and selected endocrine analysis (i.e., insulin, high-sensitive CRP). We report data collected preintervention (week 0) and postintervention (week 9).
The Easy Sampler stool kits (ALPCO, Salem, NH, USA) were used to collect fecal samples from each participant. The samples were frozen within 2 hours of collection, and stored at −80° C for later processing. A direct polymerase chain reaction (PCR) approach was used on isolated stool DNA to amplify bacterial DNA for sequencing using the Extract-N-Amp Plant PCR kit (Sigma-Aldrich, Darmstadt, Germany St Louis, MO, USA) as previously described [38]. In short, DNA from fragments of fecal samples (100mg) was diluted 1/600 in RNAse DNase free water for PCR amplification. The PCR primers for the V3 to V4 regions of the 16S ribosomal RNA were used as described [39]. Bacterial DNA was amplified on a Techne Genius Model FGEN02TP Thermal Cycler using the Extract-N-Amp kit following manufacturer’s instructions with the following modifications: The PCR conditions were: 95°C for 6 minutes to denature; 95°C for 2 minutes, 50°C for 2 minutes, 72°C for 2 minutes for 30 cycles; 72°C for 4 minutes; 0.5 μmol/L forward primer, 0.5 μmol/L reverse primer and DNA in a total volume of 60-μL samples, amplified in triplicate, and reaction products were pooled prior to purification. PCR products were purified with Ampure XP beads (Beckman Coulter Life Sciences, Indianapolis, IN, USA) per manufacturer’s instructions.
Paired end sequencing was performed using an Illumina MiSeq (San Diego, CA, USA) in the Genomics Core Facility at WVU and then paired ends were merged with FLASH (fast length Magoc and Slazberg Bioinformatics 27:21). Quantitative Insights Into Microbial Ecology (QIIME v1.9.1 http://qiime.org) [40] was used to split the libraries and pick open-reference taxonomic units (OTUs) at 97% similarity. OTUs were first filtered by excluding those below a minimum threshold count of 500.
2.4. Statistical analyses
All continuous outcome distributions were examined for goodness of fit by the Shapiro-Wilk W test. When lack of normality was found, those variables were transformed. All analyses were completed using JMP 13.0 (JMP, Version Pro 13.2, SAS Institute Inc., Cary, NC, USA, Copyright 2016), SAS 9.3 (SAS, Version 9.3, SAS Institute Inc., Cary, NC, Copyright 2002–2012), or R (R Core Team [2013], Vienna, Austria. URL: http://www.R-project.org). A p value less than or equal to 0.05 was considered significant. Benjamini-Hochberg was used to control type I error rate during multiple analyses (with 20 or more items), using false discovery rate at 10%.
2.5. Diet and anthropometrics
Log transformation was used for fiber, sugar, carbohydrate grams, fat grams, dietary cholesterol, monounsaturated fat, polyunsaturated fat, CRP, and LDL. A repeated-measures analysis of variance was used to assess differences in dietary data collected weekly over the 9-week study. Week (referred to as intervention, accrued over the 9 weeks) was used as repeated effect (week 0–9) in the model. Dietary values were also tested with a specific contrast (week 0 vs. week 9, representing preintervention and postintervention). Matched-pairs t test was used to determine differences in survey, clinical, and anthropometric measures between preintervention and postintervention. Soluble, insoluble, and CRP data had unusual distributions difficult to normalize with transformations. Soluble and insoluble fiber were analyzed using Mantel-Haenszel for nonparametric repeated measures [41]. CRP analysis utilized the Wilcoxon Signed Rank test, which is a nonparametric matched-pairs test.
2.6. Microbiome changes
Microbiome taxa were excluded from this analysis if the total counts of all samples were less than 500. Changes in the microbiome from preintervention to postintervention were assessed using a generalized linear model, DESeq2, where counts were modeled using a negative binomial distribution with a fitted mean, an OTU-specific dispersion parameter, and a Benjamini-Hochberg correction.
2.7. Diet and microbiome
An arcsine square root transformation of the microbiome abundance was used for the linear regression and statistical test modeling of diet, anthropometrics, and demographic variables. Gut bacteria were further filtered out for this analysis and if >17% of the samples had an abundance of <0.005 of total microbiome with that particular OTU (similar filtering method [42]). Relationships of microbial phyla and families with dietary and anthropometric variables, indicated by a p value ≤0.05 were further assessed using a stepwise multiple linear regression to determine how dietary changes were influencing the gut microbiome. Influential observations were examined by studentized residuals, with values greater than 2 being eliminated from the particular model in 1 round of screening. Below are the general equations used, and the order of statistical analyses.
- Relationships of dietary and anthropometric measures with each microbial (OTU) were initially screened by stepwise regression:
where p_OTUi is a proportion of specific OTU examined, μ is the mean response, and ε represents random error term.(1)
- Specific dietary and anthropometric factors found significant in primary regression analysis (1) for each OTU were entered into the secondary model to examine their effect and the effect of intervention (pre and post) and their interaction by analysis of covariance:
where D and A * Intervention represent the interactions of specific dietary variable (D) with intervention and of specific anthropometric (A) variable with intervention respectively.(2)
2.8. Power analysis
A post hoc power analysis was completed for a 1-sided, paired-sample t test with 15 participants using the data from the current study to determine the statistical power for the fruit and vegetable intake (from food log dietary analysis), and number of MetS risk factors changed throughout the intervention. The power for fruit and vegetable intake change was computed to 66.5%, whereas the power of detecting change in the number of MetS risk factors from screening to postintervention was approaching 100%. The effect size, calculated by Cohen d for fruit and vegetable intake was 0.81, while number of MetS risk factors was 1.41, which are both suitable effect sizes [43].
3. Results
3.1. Diet and clinical
Two participants were found to have dietary and medication noncompliance (i.e. antibiotic use), although all individuals were included in the analysis (Fig. 1). Most of the participants identified as white (76.5%), female (64.7%) and Appalachian (52.9%), with an average age of 22.2 ± 3.4 years old. Average body mass index identified participants in the morbid obesity class II (37.9 ± 5.0), and the average hemoglobin A1C was within normal limits (5.3 ± 0.4) (Table 1). There were no significant changes in sleep, stress, and physical activity throughout the intervention (Supplemental Table S1). There was a significant increase in fruit and vegetable (preintervention: 1.6 ± 1.4 cups; postintervention 3.4 ± 2.7 cups, P < .001), total fiber (preintervention: 16.1 ± 12.9 g, postintervention 20.0 ± 5.4 g, P = .022), soluble fiber (preintervention: 0.3 ± 0.63 g, postintervention 0.26 ± 0.4 g, P = .044) and insoluble fiber intake (preintervention: 1.0 ± 1.8 grams; postintervention 1.0 ± 1.5, P < .0001) during the 9-week intervention (Table 2 and Supplemental Figure S1A–D). Similarly, considering specific contrast of preintervention (week 0) and postintervention (week 9),there was an increase in fruit and vegetable cups (P = .006) and protein percent (P = .003), with a decrease in empty energy (P = .003).
Table 1.
Demographic and health-related characteristics of the study population (n = 17)
| Agea | 22.2 ± 3.4 |
| Sex (% male) | 6 (35.3) |
| Race/ethnicity (%) | |
| White | 13 (76.5) |
| African American | 3 (17.6) |
| Asian | 1 (5.9) |
| Hispanic | 0 |
| Other | 0 |
| Body mass index | |
| Total | 37.95 ± 5.04 |
| Male | 36.52 ± 4.5 |
| Female | 38.73 ± 5.3 |
| Hemoglobin A1C (%) | 5.3 ± 0.4 |
| From Appalachia (%) | 9 (52.9) |
Data presented as means ± SD or number (%).
Table 2.
Intervention effects on daily dietary factors across the duration of the study
| Dietary factor | Preinterventiona | Postinterventiona | Diet effect (P value) | Pre vs post (P value) |
|---|---|---|---|---|
| Kilocalories | 9,408.0 ± 5,088.2 | 6823.3 ± 2,124.5 | .556 | .024 |
| Carbohydrate (%) | 48.1 ± 15.8 | 47 ± 6.6 | .428 | .784 |
| Fat (%) | 36.9 ± 12.4 | 32.3 ± 5.1 | .268 | .156 |
| Protein (%) | 15.2 ± 6.3 | 20.7 ± 5.4 | .236 | .003* |
| Fiber (g) | 16.1 ± 12.9 | 20.0 ± 9.2 | .022* | .137 |
| Insoluble fiber (g) | 0.96 ± 1.8 | 1.03 ± 1.5 | .008b* | .46c |
| Soluble fiber (g) | 0.3 ± 0.63 | 0.26 ± 0.4 | .044b* | .5c |
| Total Sugar (g) | 92.0 ± 67.5 | 81.3 ± 43.5 | .736 | .707 |
| Empty energy (kJ) | 4,221.1 ± 2,754.1 | 1,962.2 ± 1,054.1 | .129 | .003* |
| Monounsaturated fat (g) | 28.7 ± 25.8 | 14.6 ± 10.2 | .367 | .031 |
| Polyunsaturated fat (g) | 15.1 ± 15.4 | 8.0 ± 6.1 | .774 | .1 |
| Saturated fat (g) | 30.4 ± 21.1 | 20.6 ± 8.3 | .331 | .06 |
| Cholesterol (mg) | 265.5 ± 269.2 | 280.4 ± 192.2 | .200 | .544 |
| Fruit & vegetables (cups) | 1.6 ± 1.4 | 3.4 ± 2.7 | <.001* | .006* |
Repeated-measures analysis of variance testing the effect of intervention was completed on variables with weekly measures (week 0–9); however, only preintervention and postintervention means ± standard deviation are reported in the table (N = 17). A specific contrast between pre and post is also reported in this table.
Denotes significance with an α = .05.
Data presented as means ± SD.
Mantel-Haenszel was used for nonparametric repeated measures.
Nonparametric Wilcoxon Signed Rank test was used for these values.
The MetS components in the NCEP ATP III guidelines [28] at in-person screening, (Table 3) were as follows (most to least prevalent): increased waist circumference (100% of participants), low HDL (76.5%), high serum triglycerides (64.7%), high fasting blood glucose (47.1%), and high blood pressure (11.8%). At the in-person screening, 15 individuals qualified for MetS with 3 risk factors, and 2 with either 4 or 5 risk factors. Within the next 2 weeks when preintervention measurements were taken, 2 individuals demonstrated 3 risk factors, 11 had 2 risk factors, and 4 had 1 risk factor; none had more than 3 of the MetS components. Eight weeks later, at postassessment, 3 participants had 3 risk factors, 5 had 2 risk factors, and 9 had 1 risk factor. Overall, the number of MetS components decreased from screening (3.2 ± 0.6 per person) to preintervention (1.9 ± 0.6 per person) to postintervention (1.7 ± 0.8 per person), although this was not significant from preintervention to postintervention (P = .43). Clinical measures (Table 4) indicated an increase in sodium (P = .0006) and LDL (P = .04) preintervention to postintervention. We also noted a 3% decrease in body fat percentage (P = .04), although this was not statistically significant. Participant arterial function was assessed (Table 4), and 10 individuals were found to have valid measurements (per expert blinded review) to be used in the analysis with no significantly different measures preintervention and postintervention.
Table 3.
MetS risk factors of participants at the screening, preintervention, and postintervention
| Criteriaa | Sex | Screening (%) | Preintervention (%) | Postintervention (%) |
|---|---|---|---|---|
| Waist circumference | Male | 6 (100) | 6 (100) | 6 (100) |
| Female | 11 (100) | 11 (100) | 11 (100) | |
| Serum HDL | Male | 6 (100) | 2 (33.3) | 3 (50) |
| Female | 7 (63.6) | 6 (54.5) | 5 (45.5) | |
| Fasting serum triglycerides | Male | 6 (100) | 2 (33.3) | 1 (16.7) |
| Female | 5 (45.5) | 3 (27.3) | 2 (18.2) | |
| Fasting blood glucose | Male | 5 (55.6) | 0 (0) | 0 (0) |
| Female | 3 (27.3) | 1 (9.0) | 0 (0) | |
| Blood pressure | Male | 1 (16.7) | 0 (0) | 0 (0) |
| Female | 1 (9.0) | 0 (0) | 0 (0) | |
| Total number | Total (means ± SD) | 51 (3.2 ± 0.6) | 31 (1.9 ± 0.6) | 28 (1.7 ± 0.8) |
Values for 17 subjects: female n = 11 and male n = 6.
Number and percentage of individuals meeting the following MetS criteria:
waist circumference >102 cm (men), or >88 cm (women); serum HDL <40 mg/dL (men) or <50 mg/dL (women); fasting blood glucose (women) ≥100 mg/dL; fasting serum triglycerides ≥150 mg/dL; blood pressure ≥130/85 mm Hg.
Table 4.
Intervention effects on clinical measures at preintervention and postintervention on all subjects
| Domain | Preinterventiond | Postinterventiond | Diet effect (P value) |
|---|---|---|---|
| Anthropometrics | |||
| Weight (kg) | 110.4 ± 18.9 | 110.1 ± 19.1 | .74 |
| Waist circumference (cm) | 108.1 ± 8.6 | 107.2 ± 8.0 | .15 |
| Hip circumference (cm) | 123.2 ± 12.0 | 122.0 ± 10.8 | .13 |
| Neck circumference (cm) | 40.2 ± 2.9 | 39.9 ± 3.1 | .53 |
| Body fat (%) | 44.1 ± 7.4 | 41.2 ± 8.6 | .17a |
| Arterial functionb | |||
| SBP (mm Hg) | 112.5 ± 9.6 | 115 ± 9.7 | .17 |
| DBP (mm Hg) | 69.5 ± 8.6 | 73.0 ± 6.2 | .04c |
| PWVcf (m/s) | 5.5 ± 0.8 | 5.9 ± 0.7 | .25 |
| Augmentation pressure | 2.7 ± 3.4 | 1.5 ± 2.3 | .23 |
| Augmentation index | 8.9 ± 12.0 | 5.8 ± 9.8 | .3 |
| Augmentation index @ 75 HR | 7.0 ± 9.8 | 2.9 ± 8.1 | .12 |
| IMT | 0.5 ± 0.05 | 0.5 ± 0.04 | .67 |
| Blood measures | |||
| Sodium (mmol/L) | 137.1 ± 1.8 | 139.1 ± 1.4 | .0006* |
| Potassium (mmol/L) | 4.1 ± 0.2 | 3.9 ± 0.2 | .09 |
| Glucose (mg/dL) | 90.1 ± 5.8 | 89.4 ± 6.6 | .61 |
| Insulin (uLU/mL) | 18.6 ± 9.8 | 19.6 ± 13.6 | .88 |
| Total cholesterol (mg/dL) | 171.1 ± 28.3 | 175.7 ± 27.4 | .33 |
| HDL (mg/dL) | 46.4 ± 11.4 | 44.8 ± 10.5 | .3 |
| LDL (mg/dL) | 99.0 ± 22.2 | 106.0 ± 21.7 | .04* |
| Triglycerides (mg/dL) | 128.5 ± 80.2 | 124.8 ± 87.5 | .73 |
| CRP (mg/dL) | 9.7 ± 10.9 | 10.2 ± 11.7 | .54a |
Values from N = 17 subjects.
HR, heart rate (beats/min); PWVcf, pulse wave velocity at the carotid and femoral artery.
Matched-pairs t test was used to examine preintervention vs postintervention survey measures and clinical measure differences.
Denotes significance with an α set at .05.
Nonparametric Wilcoxon signed rank test was used for these values.
Ten individuals were found to have valid arterial function measurements (per expert blinded review) to be used in the analysis.
Not significant after Benjamini-Hochberg test was completed using a false discovery rate of 10%.
Data presented as means ±SD.
3.2. Microbiome
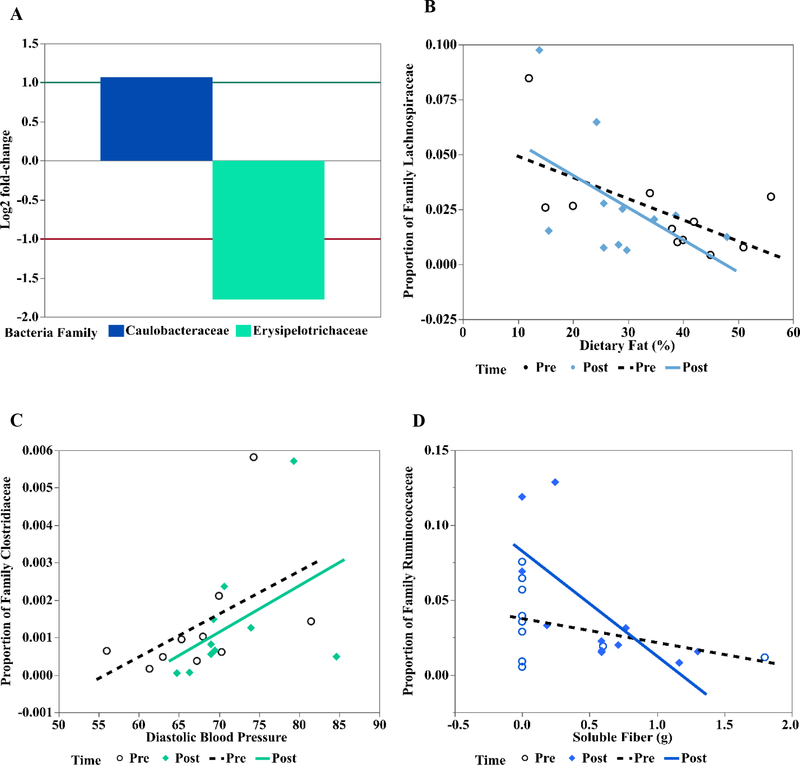
Of the 17 participants used for analysis in the diet and clinical variables, 5 of them did not have pre and post stool samples; thus, the microbiome analysis included 12 participants (Supplemental Fig. S2 A, B). Bacteroidetes and Firmicutes phyla of Bacteria were the most prevalent; on average 56% Bacteroidetes preintervention and after intervention, and 37% and 31% of Firmicutes pre and post, respectively, were estimated (Supplemental Fig. S2A). Seven main families of Bacteroidetes were identified (Supplemental Fig. S2B; Bacteroidaceae, Barnesiellaceae, Paraprevotellaceae, Porphyromonadaceae, Prevotellaceae, Rikenellaceae, and Odoribacteriaceae). Seventeen main families in the Firmicutes were identified (Bacillaceae, Carnobacteriaceae, Christensenellaceae, Clostridiales cluster, Clostridiaceae, Erysipelotrichaceae, Eubacteriaceae, Lachnospiraceae, Leuconostococaceae, Mogibacateriaceae, Peptostreprococaceae, Planococcaceae, Ruminococcaceae, Staphylococcaceae, Streptococcaceae, Turicibacteraceae, and Veillonellaceae). Additional detected phyla included Actinobacteria (with 4 families; Actinomycetaceae, Coriobacteraceae, Bifidobacteriaceae and Micrococcaceae), Proteobacteria (11 families; refer to Supplemental Fig. S2 Legend), phylum TM7, and Verrucomicrobia (1 family; Verrucomicrobiaceae). No changes were detected in the microbiomes when comparing samples from preintervention and postintervention at the phylum level. There were significant changes from preinterventionto postintervention in 2 groups at the family level (Fig. 2A). Specifically, Erysipelotrichaceae (phylum Firmicutes) decreased (log2 fold change: −1.78, P = .01) and Caulobacteraceae (phylum Proteobacteria) increased (log2 fold change= 1.07, P = .01) after intervention.
Fig. 2 -.
Effect of intervention and covariates on microbiome in 12 adult subjects. In GLM analysis, differences in 2 families between preintervention and postintervention were detected. A, Specifically, Erysipelotrichaceae (phylum Firmicutes) decreased (Log2 fold change: −1.78, p = .01) and so was about 0.29 of the values before intervention, and Caulobacteraceae (phylum Proteobacteria) increased (Log2 fold change = 1.07, p = .01), corresponding to 2.1 times higher abundance after the intervention. Analysis of covariance microbiome depicted relationship of the covariates (dietary or anthropometric, mostly continuous variables) on specific OTU. B, Specifically, increasing dietary fat percent inversely affected proportion of family Lachnospiraceae. C, Increased DBP increased proportion of family Clostridiaceae. D, Proportion of Family Ruminococaceae decreased with increased soluble fiber. For these relationships (B-D), no direct effect of intervention on microbiome was detected.
Results of stepwise regression (1) and ANCOVA (2) indicated (Supplemental Table S2) the abundance of three families of Firmicutes were associated with dietary and clinical variables, while unaffected by intervention. Decreasing calories from dietary fat were associated with decreasing Lachnospiraceae (P =0.028) (Figure 2B). Increasing diastolic blood pressure corresponded to increasing Clostridiaceae (P =0.029, Figure 2C). As soluble fiber intake increased Ruminococcaceae decreased (P =0.002, Figure 2D).
4. Discussion
4.1. Diet Intervention
This study was designed to increase the fruit and vegetable intake of young adults at high risk for/or having MetS, and then determine how the dietary changes impacted participants’ metabolic measures and gut microbiome composition over 8 weeks. The cohort significantly increased fruit and vegetable intake from 1.6 cups per day at preintervention, which is average for this age group [44], to 3.4 cups, which is significantly closer to the recommended 5 cups every day.
Because the primary null hypotheses of intervention not bringing dietary intake changes was rejected, this free living, education based, intensely monitored and personalized intervention was effective in increasing fruit and vegetable intake in this population. Previously, in a metaregression of 122 young adult diet interventions it was determined that the most effective interventions focused on a behavior change approach to help participants self-monitor [45] and remain motivated [46]. This study implemented those concepts in the design through counseling by motivational interviewing, daily tracking of food, weekly goal setting, and screening participants to ensure they were motivated at the beginning of the study to begin dietary changes to improve metabolic health.
The MetS components that this cohort exhibited at baseline varied slightly in prevalence from a previously reported pooled analysis of 34 studies on young adults with MetS [1]. Abdominal obesity, prevalent at preintervention and postintervention, and elevated fasting glucose and triglycerides, prevalent at screening, were found at higher rates in this cohort. All 17 participants qualified for MetS at the in-person screening and consent; however, only 2 participants met the diagnostic criteria for MetS at the time of preintervention measures that occurred up to 2 weeks later. Because of this gap between consent and the start of the study participants were instructed to not begin any lifestyle changes until the official start of the study. The changes that did occur could be explained by the Hawthorne Effect in research on human behavior [47]. This phenomenon indicates that discussion of the intervention with individuals diagnosed with a negative health outcome (such as MetS during the screening appointment) [48], can inherently cause participants to change behavior because they know they are being observed [49]. Alternatively, because the risk factor that declined the most from screening and consent to preintervention were glucose and triglyceride levels, it could also be that individuals did not fast for as long as instructed prior to the screening appointment.
Although number of MetS components varied at the preassessment, participants were still included in the study because MetS qualification at screening was the study entry criterion and it was not anticipated that clinical components would change between these 2 time points. This cohort all had at least 1 MetS component, which indicates they are at an increased risk of MetS. There was an overall decrease in total number of MetS components. On the other hand, sodium and LDL cholesterol increased, although according to clinical guidelines it was still within the reference range [50]. Therefore, examining the accepted secondary null hypothesis of our study, there were some clinical improvements in participants but not enough to yield significant improvements. One possible explanation for the lack of improvement in clinical outcomes is that a longer intervention may be needed in a group of individuals with overt MetS in comparison to a relatively healthy group. Similarly, Honrath et al. implemented a 10-week nutrition education intervention to increase fruit and vegetable intake and did not see significant improvements in anthropometric measurements: weight, body mass index, and body fat percentage [46].
The primary objective/hypothesis of the study was achieved with overall improvements in fruit and vegetable intake, total and insoluble fiber, and empty energy intake, although this did not result in overall metabolic improvements. Other studies that have yielded positive changes in metabolic health were based on improvements in other areas of the diet [25]. For example, in a 12-week study of 417 weightstable Europeans with MetS, it was found that an isoenergetic diet reducing only saturated fat had no effect on insulin sensitivity or any of the 5 components of MetS. The only improvement in metabolic health occurred in the group with low-fat, high-complex carbohydrates and supplemented with long-chain omega-3 polyunsaturated fatty acids [51]. A 10- to 12-week study by Klein et al, whose participants also had a decrease in body fat with equal energy intake, found that these changes did not significantly alter insulin sensitivity, blood pressure, CRP, or any other inflammatory markers [52]. In addition, the amount of protein during our intervention increased. Because it is not ensured in our analysis that this protein was healthy, lean proteins, we may speculate, as other studies have found that increased animal source protein could relate to this study population not having any clinical improvements [53–54]. Overall, it may take a diet that is focused on several dietary improvements and a negative energy balance, or a longer intervention to result in weight loss and the desired metabolic improvements in this population [55].
4.2. Microbiome
The gut microbiome can be affected by weight status [6, 9, 56, 57] and risk factors associated with MetS diagnosis [5, 6]. Among relationships with diet and disease, Firmicutes and Bacteroidetes are among the most commonly identified and studied in lean and obese individuals [56]. As expected, the current cohort’s fecal microbiomes consisted mainly of Firmicutes and Bacteroidetes. There was little change overall from preassessment to postassessment in the fecal microbiome. However, significant changes at the family level were a decrease in Erysipelotrichaceae (phylum Firmicutes) and an increase in Caulobacteraceae (phylum Proteobacteria). Other researchers have found a positive association between Erysipelotrichaceae and the proinflammatory cytokine interleukin-1β [58], dietary fat intake [59] and obesity [60]. The concurrent decrease in this family, dietary fat intake, and body fat percentage in the current study population aligns with this prior research.
Caulobacteraceae is a family of bacteria in the Proteobacteria phylum. Increased Proteobacteria has been associated with obesity and dysbiosis (reviewed by Shin et al [61]). Caulobacteraceae has not been studied in detail in intestinal dysbiosis in humans; however, an increase in Caulobacteraceae abundance was found in piglets fed lysinerestricted diets, resulting in decreased expression of lysine transporters, decreased expression of leptin in the blood, and decreased levels of ghrelin and CCK mRNA in the jejunum, all of which were associated with increased feed intake [62]. Projecting those findings to our study population, an increase in Caulobacteraceae could potentially contribute to physiologic responses that decrease satiety in the participants undergoing dietary intervention, which could be problematic for maintaining the diet long term and possibly contribute to failures in the interventional approach.
Along with few changes in metabolic outcomes, study length, the dietary variable changes, and the relatively small number of participants in the study could explain why few significant changes were observed in the gut microbiota. The 9-week length of dietary intervention in a free-living environment in this study may have not been sufficient time for significant changes. Similarly, in a 12-week intervention with obese individuals, Cotillard et al found significant improvements in body fat mass, adipocyte diameter, microbial gene richness, and biomarkers of insulin sensitivity, inflammation, and metabolism only when participants changed to an energy-restricted diet for the last 6 weeks of the study [63].
There are limitations in this study. First, with data from only 17 participants in the study and 12 used in the microbiome analysis, this limits the power needed to detect differences in dietary, behavioral, metabolic, and microbial health. However, we would note that we used a repeated measures design so that each subject served as their own control. This has the advantage of limiting treatment variability outcomes and improving statistical power with small population cohorts (compared to studies only using between subject comparisons). Additionally, we had limited attrition once subjects were consented which we believe is attributed to our attention to the stages of change that participants were in when screened for study eligibility. This, along with weekly counseling, may have increased the likelihood of participants being able to complete the study requirements and likely contributed to our high (15/17=88%) rate of diet-compliant individuals completing the study.
Another limitation is the study length of 9 weeks. Although this has been enough time to yield metabolic and microbial improvements in other studies [24], and in our previous study using a similar 8-week diet intervention [25], this cohort (with MetS components) did not see as many desired metabolic improvements, suggesting that as disease burden increases, longer diet interventions may be needed. Lengthening the study period may help give participants with lower baseline diet quality the extra time needed to improve their diet in a free-living intervention, which more closely resembles peoples’ lives and struggles. Lastly, the study participants were all largely white, and thus, future research should be expanded to include more diversity and geographic locations [64].
It has also been suggested that the 2-dimensional evaluation of the carotid (IMT), a hallmark of atherosclerosis and arterial remodeling, has been criticized for a lack of diagnostic accuracy as compared to the ultrasound assessment of carotid plaque. As such, our results reflecting no differences between preintervention and postintervention should be considered preliminary, and further work with a longer intervention is needed to examine whether a diet of fruit and vegetables alters plaque formation.
The average young adult college student eats only 1 serving of fruits and vegetables daily, which is contrary to the recommended 5 cups a day for the prevention of chronic diseases. In this study, a monitored, free-living diet intervention was found to be effective in increasing fruit and vegetable intake, and total fiber while decreasing empty energy in young adults with/or at high risk for MetS. This resulted in decreased body fat while decreasing Erysipelotrichaceae and increasing Caulobacteraceae. Currently a follow-up of this cohort is being conducted to determine if the educational intervention was able to yield any long-term health and diet improvements. Further research is needed to determine if a longer intervention using this diet will produce more success in improving metabolic and gut microbiome health. Additionally, inclusion and specific focus on other dietary components such as decreased dietary fat (especially saturated fat) and increased complex carbohydrates (especially foods rich in soluble fiber) will be needed to promote greater microbiome diversity and maximize changes in metabolic and cardiovascular health.
Supplementary Material
Acknowledgments
The authors declare that they have no competing interests. This work was supported by a research grant (#2014-67001-21851) from the US Department of Agriculture National Institute of Food and Agriculture, West Virginia Clinical Translational Science Institute (NIH P30 GM103488), West Virginia University Experimental Station Hatch WVA00627, WVA00641, WVA00689, and WVA00721, WVU Mountains of Excellence Flash Funding, WV INBRE P20 GM103434 for the Genomics core, and WVU Medicine. WVU Pediatrics grant; Obesity, Diabetes & Asthma Fund. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
List of Abbreviations
- b
brachial
- CRP
C-reactive protein
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- IMT
intima-medial thickness
- LDL
low-density lipoprotein
- MetS
Metabolic Syndrome
- NCEP ATP
National Cholesterol Education Program Adult Treatment Panel
- OTU
operational taxonomic unit
- PCR
polymerase chain reaction
- SD
standard deviation
- SBP
systolic blood pressure
- WVU
West Virginia University
Footnotes
Appendix A.
Supplemental materials
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nutres.2018.11.010.
References
- [1].Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med 2017;7:211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the united states, national health and nutrition examination survey, 1988–2012. Prev Chronic Dis 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- [4].Miller B, Fridline M, Liu PY, Marino D. Use of CHAID decision trees to formulate pathways for the early detection of metabolic syndrome in young adults. Comput Math Methods Med 2014;2014:242717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bo T, Shao S, Wu D, Niu S, Zhao J, Gao L. Relative variations of gut microbiota in disordered cholesterol metabolism caused by high-cholesterol diet and host genetics. MicrobiologyOpen 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen J, He X, Huang J. Diet effects in gut microbiome and obesity. J Food Sci 2014;79:R442–51. [DOI] [PubMed] [Google Scholar]
- [7].de Toro-Martín J, Arsenault BJ, Després J-P, Vohl M-C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017;9:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005;307:1915–20. [DOI] [PubMed] [Google Scholar]
- [9].John GK, Mullin GE. The Gut Microbiome and Obesity. Curr Oncol Rep 2016;18:45. [DOI] [PubMed] [Google Scholar]
- [10].Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA Study. J Am Diet Assoc 2007;107:979–87. [DOI] [PubMed] [Google Scholar]
- [11].Yosaee S, Erfani M, Bazrafshan M, Entezami N, Alinavaz M, Akbari M, et al. Correlation between diet quality and metabolic syndrome. JNFS 2017;2:213–20. [Google Scholar]
- [12].Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014;7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–72. [DOI] [PubMed] [Google Scholar]
- [14].Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell metab 2015;22:516–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 2010;4:232. [DOI] [PubMed] [Google Scholar]
- [16].Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004;292:1440–6. [DOI] [PubMed] [Google Scholar]
- [17].Kastorini CM. The effect of Mediterranean diet on metabolic syndrome and its components: a metaanalysis of 50 studies and 534906 individuals. J Am Coll Cardiol 2011;57:1299–313. [DOI] [PubMed] [Google Scholar]
- [18].Salas-Salvadó J, Fernández-Ballart J, Ros E, et al. Effect of a mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the predimed randomized trial. Arch Intern Med 2008;168:2449–58. [DOI] [PubMed] [Google Scholar]
- [19].Steffen LM, Van Horn L, Daviglus ML, Zhou X, Reis JP, Loria CM, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br. J. Nutr 2014;112:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nunez-Cordoba JM, Martinez-Gonzalez MA. Mediterranean diet inversely associated with the incidence of metabolic syndrome: the SUN prospective cohort. Diabetes Care 2007;30:2957–9. [DOI] [PubMed] [Google Scholar]
- [21].Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial Effects of a Dietary Approaches to Stop Hypertension Eating Plan on Features of the Metabolic Syndrome. Diabetes care 2005;28:2823. [DOI] [PubMed] [Google Scholar]
- [22].Kovatcheva-Datchary P, Arora TS. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2013;27:59–72. [DOI] [PubMed] [Google Scholar]
- [23].Panunzio M, Caporizzi R, Antoniciello A, Cela E, Ferguson L, D’Ambrosio P. Randomized, controlled nutrition education trial promotes a Mediterranean diet and improves anthropometric, dietary, and metabolic parameters in adults. Ann Ig 2011;23:1325. [PubMed] [Google Scholar]
- [24].Bondia-Pons I, Martinez JA, la Iglesia R, Lopez-Legarrea P, Poutanen K, Hanhineva K, et al. Effects of short- and long- term Mediterranean- based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: The Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol Nutr Food Res 2015;59:711–28. [DOI] [PubMed] [Google Scholar]
- [25].Mathews AT, Famodu OA, Olfert MD, Murray PJ, Cuff CF, Downes MT, et al. Efficacy of nutritional interventions to lower circulating ceramides in young adults: FRUVEDomic pilot study. Physiol Rep 2017;5:e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lipsky LM, Nansel TR, Haynie DL, Liu D, Li K, Pratt CA, et al. Diet quality of US adolescents during the transition to adulthood: changes and predictors. Am J Clin Nutr 2017;105:1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morrell JS, Byrd-Bredbenner CP, Quick VP, Olfert MD, Dent A, Carey GB. Metabolic Syndrome: Comparison of Prevalence in Young Adults at 3 Land-Grant Universities. J Am Coll Health 2014;62:1–9. [DOI] [PubMed] [Google Scholar]
- [28].National Cholesterol Education Program Expert Panel. Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- [29].Miccoli R, Bianchi C, Odoguardi L, Penno G, Caricato F, Giovannitti MG, et al. Prevalence of the metabolic syndrome among Italian adults according to ATP III definition. NUMECD 2005;15:250–4. [DOI] [PubMed] [Google Scholar]
- [30].Smyth C. The Pittsburgh sleep quality index. Medsurg Nurs 2003;12:261–2. [PubMed] [Google Scholar]
- [31].Cohen S, Kamarck T, Mermelstein R. Perceived stress scale. Measuring stress: a guide for health and social scientists; 1994. [Google Scholar]
- [32].Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- [33].Chen C-H, Ting C-T, Nussbacher A, Nevo E, Kass DA, Pak P, et al. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension 1996;27:168–75. [DOI] [PubMed] [Google Scholar]
- [34].Chen C-H, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: validation of generalized transfer function. Circulation 1997;95:1827–36. [DOI] [PubMed] [Google Scholar]
- [35].Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006;47:1203–8. [DOI] [PubMed] [Google Scholar]
- [36].Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E. American society of echocardiography report: clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med 2006;11:201–11. [DOI] [PubMed] [Google Scholar]
- [37].Fournier SB, Reger BL, Donley DA, Bonner DE, Warden BE, Gharib W, et al. Exercise reveals impairments in left ventricular systolic function in patients with metabolic syndrome. Exp Physiol 2014;99:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Flores GE, Henley JB, Fierer N. A direct PCR approach to accelerate analyses of human-associated microbial communities. PLoS One 2012;7:e44563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bartram AK, Lynch MD, Stearns JC, Moreno-Hagelsieb G, Neufeld JD. Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end Illumina reads. Med Sci Sports Exerc 2011;77:3846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stokes ME, Davis CS, Koch GG. Categorical data analysis using SAS. SAS Institute; 2012. [Google Scholar]
- [42].Morgan XC, Kabakchiev B, Waldron L, Tyler AD, Tickle TL, Milgrom R, et al. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biol 2015;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cohen J. Statistical power analyses for the social sciences Hillsdale, NJ: Lawrence Erlbauni Associates; 1988. [Google Scholar]
- [44].American College Health Association. In: Association ACH, editor. American College Health Association-National College Health Assessment II: reference group executive summary fall 2016; 2017. [Hanover, MD: ] . [Google Scholar]
- [45].Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690. [DOI] [PubMed] [Google Scholar]
- [46].Honrath K, Wagner MG, Rhee Y. Does nutrition education with fruit and vegetable supplementation increase fruit and vegetable intake and improve anthropometrics of overweight or obese people of varying socioeconomic status? Ecol Food Nutr 2017:1–18. [DOI] [PubMed] [Google Scholar]
- [47].Gillespie R. Manufacturing knowledge: a history of the Hawthorne experiments Cambridge University Press; 1993. [Google Scholar]
- [48].MacNeill V, Foley M, Quirk A, McCambridge J. Shedding light on research participation effects in behaviour change trials: a qualitative study examining research participant experiences. BMC Public Health 2016;16:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;67:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015;372:55–65. [DOI] [PubMed] [Google Scholar]
- [51].Tierney AC, McMonagle J, Shaw D, Gulseth H, Helal O, Saris W, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond) 2011;35:800–9. [DOI] [PubMed] [Google Scholar]
- [52].Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350:2549–57. [DOI] [PubMed] [Google Scholar]
- [53].Esselstyn CB. A plant-based diet and coronary artery disease: a mandate for effective thereapy. J Geriatr Cardio 2017;14:317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Koeth RA, Zenang W, Levison BS, Buffa JA, Org E, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Med 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Klein S, Luu K, Gasic S, Green A. Effect of weight loss on whole body and cellular lipid metabolism in severely obese humans. Am J Physiol 1996;270:E739–45. [DOI] [PubMed] [Google Scholar]
- [56].Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010;18:190–5. [DOI] [PubMed] [Google Scholar]
- [57].Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- [58].Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 2015;211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci 2009;106:2365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shin N-R, Whon TW, Bae J-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015;33:496–503. [DOI] [PubMed] [Google Scholar]
- [62].Yin J, Han H, Li Y, Liu Z, Zhao Y, Fang R, et al. Lysine restriction affects feed intake and amino acid metabolism via gut microbiome in piglets. Cell Physiol Biochem 2017;44:1749–61. [DOI] [PubMed] [Google Scholar]
- [63].Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013;500:585. [DOI] [PubMed] [Google Scholar]
- [64].Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. Natl Health Stat Rep 2009;13:1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.