Abstract
We have recently developed a therapeutic platform – drug-free macromolecular therapeutics (DFMT) that induces apoptosis in B cells by crosslinking of CD20 receptors, without the need for low molecular weight cytotoxic drug. In this report a DFMT system was synthesized and evaluated based on human serum albumin (HSA) and two complementary coiled-coil forming peptides, CCE and CCK. Fab’ fragment of anti CD20 monoclonal antibody rituximab (RTX) was attached to CCE (Fab’-CCE); multiple grafts of CCK were conjugated to HSA (HSA-(CCK)7). Confocal fluorescence microscopy demonstrated colocalization of both nanoconjugates at the surface of non-Hodgkin’s lymphoma (NHL) Raji cells. The colocalization led to coiled-coil formation, CD20 crosslinking and apoptosis induction. The apoptotic levels were evaluated by Annexin V, Caspase 3 and TUNEL assays. Selective surface binding of DFMT to CD20+ cells was validated in experiments on a coculture of CD20+ (Raji) and CD20- (DG-75) cells. DMFT could trigger calcium influx only in Raji cells, but not in DG-75 cells. This HSA-based DFMT system presents a highly specific treatment for NHL and other B cell malignancies with considerable translational potential.
Keywords: Drug-free macromolecular therapeutics, CD20, Coiled coils, Human serum albumin, Lymphoma
Graphical Abstract

CD20 crosslinking as a result of two biorecognition events initiates apotosis in Raji cells. First, birecognition of Fab’ fragment by CD20 decorates the cells with CCE, second, biorecognition of CCE and CCK results in coiled-coil formation and receptor crosslinking.
1. Introduction
In 2017 in the United States, there were an estimated 72,240 new cases of Non-Hodgkin lymphoma (NHL) and 20,140 deaths in both males and females.[1] Of the heterogeneous group of NHLs the majority (85–90%) derive from B lymphocytes and the remaining develop from T lymphocytes or natural killer cells.[2] RTX (a chimeric anti-CD20 monoclonal antibody (mAb)) combined with low molecular weight drugs remains a mainstay in fit patients.[3,4] Clinical experience indicates that a large fraction of patients have a poor response and/or demonstrate resistance to treatment.[5] The unresponsiveness and/or resistance resulted from inefficient crosslinking of CD20 receptors by effector cells via Fc fragments of ligated RTX due to reduced expression of CD20 and hyperactivation of the antiapoptotic signaling pathways.[6,7] Additionally, Fc receptor-mediated endocytosis[8] and trogocytosis of CD20 receptors contribute to the weak response.[9]
CD20 is a slowly internalizing receptor, expressed on more than 95% of B cell lymphomas.[10–13] It functions as a store-operated calcium channel and regulator of cell cycle.[14–16] The suitability of CD20 as a target for NHL treatment has been validated.[17,18] CD20 is expressed on both, normal and NHL B cells; however, it is not expressed on stem cells, progenitor cells, and mature or activated plasma cells.[10] Thus treatment results in a temporary decrease of B cell count that can be restored in a relatively short period.[3,4,19] There are three main mechanisms of apoptosis induction in NHL cells: antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and CD20-mediated apoptosis.[20–22]
The coiled-coil is one of the basic folding patterns of native proteins.[23] It forms by self-assembly of two or more α-helices coiling together into a left-handed super-helix. Depending on the primary structure, individual helices may associate as homodimers, heterodimers in parallel or antiparallel alignments, or form higher order aggregates.[24] The attractiveness of coiled-coils for the design of self-assembling systems is the fact that higher order structures may be predicted based on the primary sequence.[25]
Hybrid copolymers composed of a synthetic backbone and multiple peptide grafts self-assemble into 3D hydrogels mediated by biorecognition of complementary peptide sequences. One of such hybrid systems is composed of N-(hydroxypropyl)methacrylamide (HPMA) copolymer backbone and coiled-coil forming peptides, CCE and CCK. These peptides associate by forming antiparallel coiled-coil heterodimers.[26,27] When individually grafted to HPMA copolymers they are soluble in aqueous media. However, equimolar mixtures of P-(CCK)x and P-(CCE)y (P is the HPMA copolymer backbone) spontaneously self-assemble into hydrogels even at very low concentrations.[26,27]
The excellent biorecognition of CCE and CCK was an inspiration for the design of a hybrid system to mediate a biological process; the success of this approach would demonstrate the similarity between the design of biomaterials and the design of nanomedicines. The biorecognition of CCE and CCK at cell surface should result in CD20 crosslinking and apoptosis initiation. Ultimately, B cell non-Hodgkin lymphoma (NHL) was chosen as a suitable first target. The direct apoptosis of B cells is mediated by crosslinking of CD20 bound antibodies via their Fc fragment by immunocompetent cells.[28] Inspired by the self-assembly of hybrid graft copolymers we developed a new therapeutic paradigm – drug-free macromolecular therapeutics (DFMT).[29] The original coiled-coil based DFMT system is composed of two nanoconjugates: a) bispecific enganger - conjugate of anti-CD20 antibody Fab’ fragment with CCE (Fab’-CCE); b) crosslinking effector - HPMA copolymer grafted with multiple copies of CCK (P-(CCK)x). Exposure of B cells to Fab’-CCE decorates the cells with the CCE motif due to the first biorecognition event – binding of Fab’ to CD20. Subsequent exposure of decorated cells to P-(CCK)x results in the formation of antiparallel coiled-coils at the cell surface, crosslinking of CD20 receptors and initiation of apoptosis. This second biorecognition event is mediated by CCE/CCK heterodimer formation (Figure 1). The system was efficient in inducing apoptosis in Burkitt’s lymphoma Raji B cells in vitro,[30] in an animal model of NHL in vivo,[31] and on patient cells.[32] Interestingly, in contrast to traditional NHL treatment, DFMT triggered direct and specific apoptosis of B cell lymphomas without the help of effector cells. This is achieved by the design of synthetic effectors that mimic the crosslinking function of immune effector cells such as natural killer (NK) cells and macrophages.[29,30] Recently, a pair of phosphorodiamidate morpholino (MORF) oligomers, MORF1 and MORF2, was designed and Fab’-MORF1 and P-(MORF2)x[29] as well as HSA-(MORF2)x nanoconjugates were synthesized and are being evaluated. Previously we have proved multivalent polymer-Fab’ conjugates could also crosslink CD20 and induce apoptosis in NHL cells.[33,34] However, it is difficult to prepare such constructs with high valence due to steric factors.[29] Moreover, the main advantage of the current binary system is the opportunity of pre-targeting.[29] Within the time lag following the administration of the bispecific engager (Fab’-CCE), the blood concentration of unbound engager decreases dramatically. Moreover, off-target bound enganger internalizes and is deactivated (degraded) in the lysosomes. When the CCK containing crosslinking effector is administered, CCK is exposed to CCE predominantly on target B cells; this results in high efficiency of apoptosis initiation and minimal adverse effects.
Figure 1.
Induction of apoptosis in human Burkitt’s NHL Raji B cells by crosslinking of its CD20 antigens mediated by antiparallel coiled-coil formation at the cell surface. The simplified schematic diagram is not drawn to scale.
Human serum albumin (HSA) is a (non-glycosylated) soluble protein (mol. wt. 66,500); it constitutes about half of the serum proteins (35–50 g/L human serum). It is composed of 585 amino acid residues with 17 disulfide bridges and one free cysteine in position 34. It is a heart-shaped molecule with 67% α-helical content and no β-sheets; it folds into three domains connected via long flexible loops.[35] HSA is stable in the pH range of 4 to 9 and is resistant to denaturation. It was used frequently as a drug carrier.[36,37] HSA has a serum half-life of 21 days. The neonatal FcRn receptor plays a key role in maintaining high levels of HSA in the circulation. FcRn prevents HSA from degradation by recycling the FcRn-HSA complex back from endosomes to the cell surface.[35,38,39]
This study focuses on the synthesis and evaluation of an HSA-based DFMT, replacing the synthetic polymer backbone with a natural polymer in the multivalent CCK-containing nanoconjugate. HSA has been used in FDA approved therapeutics.[36,37] The multivalent CCK-containing HSA nanoconjugate should possess enhanced intravascular half-life and easier scalable synthesis than conjugates based on synthetic macromolecules. The aim of this study is to evaluate the impact of the new nanoconjugate design on the efficiency of CD20 antigen crosslinking and apoptosis initiation. To this end we synthesized two nanoconjugates: the bispecific enganger - a RTX-derived Fab’ fragment conjugated to CCE (Fab’-CCE); the crosslinking effector - HSA modified with multiple copies of CCK (HSA-(CCK)7). We determined apoptotic initiation in Raji B cells following exposure to these nanoconjugates using Annexin V/propidium iodide, caspase 3, and TUNEL assays. The CCE and CCK peptides do not have distinct secondary structure at pH 7.[26] They unite by hydrophobic and electrostatic interactions first. Once in contact, they quickly fold into antiparallel coiled-coils.[27] Consequently, the in vitro apoptosis induction of malignant B cells was evaluated at different concentration ratios of HSA-(CCK)7 to Fab’-CCE.
2. Experimental Section
2.1. Materials
Common reagents and solvents were purchased from Sigma-Aldrich and Fisher Scientific and used as received unless otherwise specified. N-α-Fmoc protected amino acids were purchased from P3 Biosystems and AAPPTEC. SM(PEG)2 (succinimidyl-[(N-maleimidopropionamido)-diethyleneglycol] ester) was from Thermo Fisher Scientific. Tris(2-carboxyethyl)phosphine (TCEP) was from Thermo Scientific. Cyanine monosuccinimidyl esters, Cy5-NHS and Cy3-NHS, were from Lumiprobe. Human serum albumin (HSA) and pepsin were from Sigma. Rituximab (Genentech) was obtained from Huntsman Cancer Hospital, University of Utah at a stock concentration of 10 mg mL−1.
2.2. Synthesis and Characterization of Nanoconjugates
2.2.1. Peptide Synthesis
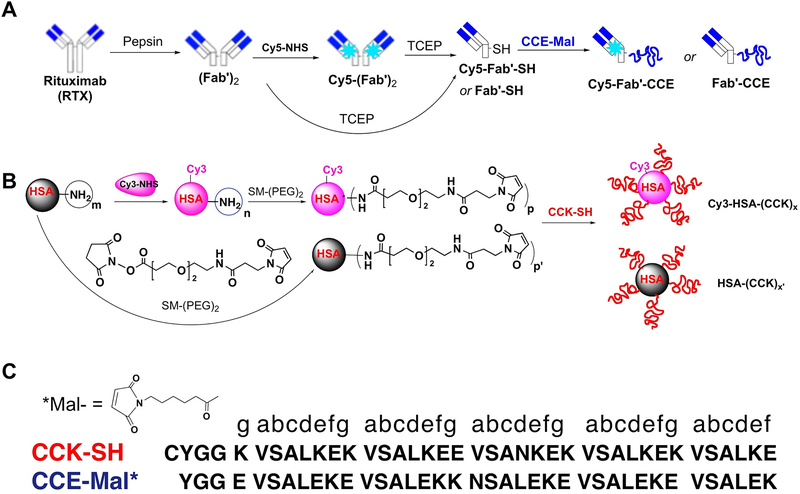
The peptides CCE and CCK were synthesized using Fmoc/tBu strategy on 2-chlorotrityl resin using a peptide synthesizer PS3 (Protein Technologies) as previously described.[40] Three amino acid residues YGG were added to each N terminus as spacer. Then the N terminus of CCE was capped with 6-maleimidocaproic acid (denoted as ‘CCE-Mal’), whereas CCK peptide with N-terminus thiol modification (denoted as “CCK-SH”) was obtained by addition of a cysteine residue. Crude peptides were purified by RP-HPLC equipped with a preparative C18 column (Zorbax 250 × 21.2 mm) from Agilent Technologies (Santa Clara, CA). CCE was eluted with a linear gradient at a flow rate of 5 mL/min, where Buffer A was 0.1% ammonium acetate (pH 6.5) in water and Buffer B was 0.1% trifluoroacetic acid (TFA) in acetonitrile. CCK was purified with a linear gradient where buffer A was 0.1% TFA in water.
The structures were ascertained by MALDI/ToF mass spectrometry (UltrafleXtreme, Bruker Daltonics). CCK-SH: found (m/Z): 4180.6 (calcd M+ 4179.89), CCE-Mal: found: (m/Z): 4289.79, (calculated M+ 4288.7, Figure S1 A, B). The purity of the peptides was verified with analytical RP-HPLC. Results (Figure S2 C, D) indicated that the purity of peptides was > 95%.
2.2.2. Synthesis of Fab’-CCE conjugate labeled with Cy5
Rituximab was digested into F(ab′)2 with 10% (w/w) pepsin in citric buffer (pH 4.0) at 37 °C for 90 min.[42] F(ab′)2 was then labeled with (Cy5-NHS,) to introduce a fluorophore that facilitated the fluorescence microscopy investigation. Briefly, 2 eq. of Cy5-NHS in DMSO (2 mg mL−1 × 13 μL) were added into 2 mL of F(ab′)2 solution (1 mg mL−1) in PBS (pH 8.5) and incubated at r.t. for 2 h. The labeled F(ab′)2 was then purified with PD10 column (GE Healthcare, Buckinghamshire, UK) and ultrafiltration (30,000 Da cut-off) with PBS (pH 6.5) twice. To estimate the degree of labeling, the sample was scanned from 200–800 nm on Varian UV-Vis spectrophotometer (Varian Inc., Palo Alto, CA). The concentration of Cy5 was calculated using the absorbance at 645 nm with an extinction coefficient of 250 000 cm−1 M−1 in PBS. The concentration of F(ab′)2 was calculated by bicinchoninic acid (BCA) assay. The ratio of these two molar concentrations gave a labeling degree of 1.6 Cy5 per F(ab′)2 molecule.
Fab’-CCE was obtained by conjugation of freshly reduced Fab’-SH with CCE-mal using maleimide-thiol chemistry. Immediately prior to use, the labeled F(ab′)2 was reduced to Fab’ with 10 mM tris(2-carboxyethyl) phosphine hydrochloride (TCEP) in PBS (pH 6.5) containing 5 mM EDTA for 2 h at 37 °C in the dark. After removing excess of TCEP by ultrafiltration (30,000 Da cut-off) with PBS (pH 6.5) three times, CCE (10× in excess to Fab’) was added and the coupling reaction proceeded at 4 °C in the dark overnight. The excess CCE was removed by ultrafiltration (30,000 Da cut-off) with PBS (pH 7.4) four times. As reported[30,40] this coupling reaction follows a 1:1 stoichiometry. Thus the resulting conjugate was named Fab’-CCE. The digestion and conjugation were confirmed by size exclusion chromatography (SEC).
2.2.3. Synthesis of HSA-(CCK)x and Cy3-labeled HSA-(CCK)x conjugate
The number of reactive lysine residues of HSA was first determined using ninhydrin assay. The amount of amino group ([NH2]) in 1 mg HSA was 778 nmoL, suggesting ~ 52 conjugation sites available in one HSA molecule.
HSA (5 mg; 3.9 μmol NH2) was dissolved in 400 μL PBS (pH 7.5) and mixed with 18.3 mg SM(PEG)2 (~10 eq) in 150 μL DMSO. The mixture was stirred at r.t. for 2 h. Afterwards, the excess SM(PEG)2 was removed by ultrafiltration (30,000 cut-off) four times to yield HSA-Mal. The mole ratio of maleimide groups to HSA was determined by modified Ellman’s assay and BCA assay (Mal/HSA = 28/1).
To synthesize nanoconjugate HSA-(CCK)x, 2 mg HSA-mal (0.75 μmol [mal]) in 4 mL PBS (6.8) was mixed with CCK-SH (10 mg/ml, 0.9 mL, 2.15 μmol) and incubated at 4 °C in the dark overnight. The reaction was quenched with 2 eq. of 2-mercaptoethanol at 4 °C for 2 h. The excess CCK-SH and 2-mercaptoethanol were removed by ultrafiltration (30,000 cut-off). Size-exclusion chromatography (SEC) analysis confirmed the peak shifed to high Mw area, indicating successful synthesis of HSA-CCK conjugate (Figure S3). The number of CCK attached to HSA was estimated by MALDI-ToF-MS[41] which showed that Mw of the conjugate was about 104 kDa, suggesting the ratio of CCK to HSA was 6.8.
To prepare Cy3-labeled conjugate, 2 mg HSA (75 nmol, Mw 66.5 kDa) was first dissolved in fresh prepared 0.1 M NaHCO3 (pH 8.2). The 20 μL stock solution of Cy3-NHS in DMSO ([Cy3]:[HSA]=1:1.6) was added and stirred in dark at r.t. for 2 h. After reaction, the sample was purified using PD-10 column (GE Healthcare) to remove free dye. To quantify Cy3 substitution, the absorbance of the solution at 547 nm was determined on Varian Cary 400 Bio UV-visible spectrophotometer. The amount of Cy3 per HSA molecule was calculated based on the Cy3 standard curve and the concentration of HSA that was determined by BCA assay.
The Cy3-labeled HSA continued to be modified with SM(PEG)2 followed by reaction with CCK as described above. The conjugate was analyzed as 7 substitutions per HSA molecule.
2.3. Biorecognition of Fab’-CCE and HSA-(CCK)7 in solution
To demonstrate the biorecognition between Fab’-CCE and HSA-(CCK)7, circular dichroism (CD) spectrometry was employed. The following samples were prepared for these measurements: CCE (45 μM), CCK (45 μM), Fab’-CCE ([CCE]=45 μM), and HSA-(CCK)7 ([CCK]=45 μM)). CD spectra of individual samples (CCE, CCK, Fab’-CCE and HSA-(CCK)7) and equimolar mixtures (CCE+CCK, and Fab’-CCE + HSA-(CCK)7 were acquired at 25 °C on an Aviv 62DS CD spectrometer with a thermoelectric temperature control system (Aviv Biomedical, Lakewood, NJ). Wavelength scans were recorded at 2 nm intervals with a 5 s averaging time at each step from 250 to 200 nm using a 0.1 cm path length quartz cuvette. The spectra obtained were averaged from three consecutive scans and subtracted from the background.
2.4. Visualization of biorecognition on Raji B cell surface
Biorecognition between the components of the binary system was visualized by confocal fluorescence microscopy. After incubation with Fab’-CCE and HSA-(CCK)7, using either consecutive addition or premixture, cells were washed twice with PBS (pH 7.4). Then the cell suspensions were dropped onto glass bottom microwell dish (MetTek, Ashland, MA). FluoView1000-XY confocal Olympus IX81 microscope (Olympus America, Center Valley, PA) was employed to visualize the cells.
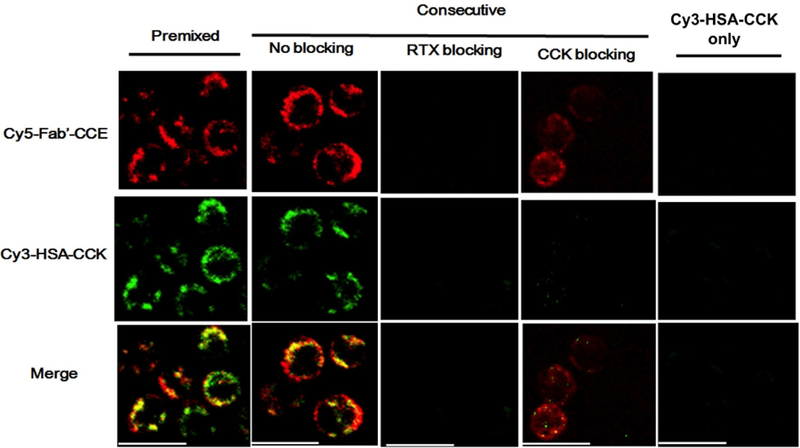
Raji cells were treated with two fluorescently labeled conjugates (Cy5-Fab′-CCE and Cy3-HSA-CCK) using “Premixed” and “Consecutive” strategies, respectively. For the “Premixed” treatment, Cy5-Fab′-CCE (0.2 nmol) and Cy3-HSA-CCK (1 nmol) were initially mixed in 50 μL culture medium at 37 °C for 0.5 h and then 2 × 105 Raji cells were incubated in 0.4 mL of medium containing the mixture for 0.5 h. For the “Consecutive” treatment, 2 × 105 Raji cells were first exposed to Cy5-Fab′-CCE (0.2 nmol) in medium (0.4 mL) at 37 °C for 0.5 h; then, the cells were washed twice with PBS to remove unbound Cy5-Fab′-CCE and treated with Cy3-HSA-CCK (1 nmol) in medium (0.4 mL) for another 0.5 h. In addition, an excess amount of RTX antibody (50 times excess, prior to Cy5-Fab′-CCE) or CCK (50 times excess, prior to Cy3-HSA-CCK) was added into “Consecutive” treatment for blocking. The cells treated with Cy3-HSA-CCK only (without pre-exposure of Cy5-Fab′-CCE) served as a control. The cell samples were transferred onto glass bottom microwell dishes (MatTek) and visualized under Nikon A1R confocal fluorescence microscope equipped with a Cy3 filter (excitation/emission = 552/565 nm) and a Cy5 filter (excitation/emission = 646/665 nm).
2.5. Apoptosis determination
Apoptosis induction, mediated by coiled-coil formation at the cell surface with concomitant crosslinking of CD20 receptors, was evaluated by three methods: Annexin V/propidium iodide assay, caspase 3 activation, and the TUNEL assay. For experimental details of these assays, please refer to the standard protocols from corresponding vendors: Annexin V/propidium iodide assay (Annexin V-FITC apoptosis detection kit; EMD Chemicals, Gibbstown, NJ), caspase 3 activity assay (employing a cell permeable fluorogenic caspase 3 substrate PhiPhiLuxR; OncoImmunin, Gaithersburg, MD), and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay (APO-BRDUTM TUNEL assay kit; Phoenix Flow Systems, San Diego, CA). 2×105 cells in 400 μL medium for both caspase 3 activity and Annexin V/propidium iodide binding assays, and 1×106 cells in 500 μL medium for terminal deoxynucleotide mediated-dUTP nick end labeling (TUNEL) assay.
Conjugates were added into cell culture by two different approaches: either premixing or consecutive addition. Consecutive exposure: the cells were incubated with Fab’-CCE conjugate first. After 1 h the cells were washed twice with PBS to remove unbound Fab′-CCE then exposed to HSA-(CCK)7. Premixed exposure: Fab’-CCE and HSA-(CCK)7 were mixed at 37 °C and after 1 h Raji cell were exposed to a preformed, self-assemble multivalent conjugate. In all experiments, Rituximab (RTX) was used as a positive control. Non-treated cells (in culture media) were used as negative controls. Different molar ratios of Fab’-CCE and HSA-(CCK)7 including 1:1, 1:10 and 1:25 ([CCE] was chosen as 1 μM, thus [CCK] = 1, 10, and 25 μM, respectively) were studied to achieve the maximum efficiency of apoptosis induction. To examine the cytoxicity of the individual components, cells were incubated with only Fab’-CCE (1 μM) or only HSA-(CCK)7 (1, 10, or 25 μM). The percentage of apoptotic cells was quantified with Annexin V/propidium iodide binding assays at various duration (6, 12, and 24 h), caspase 3 activity assay at 12 h and TUNEL assay at 24 h.
2.6. Apoptosis induction in CD20 negative DG-75 cells
2×105 DG-75 cells were consecutively treated with 1 μM of Fab’-CCE for 1 h and 1 μM of HSA-(CCK)7 for 24 h. Then cells were washed and apoptosis was analyzed using Annexin V-FITC/PI double staining protocol. Due to absence of CD20 expression on DG-75 cell surface, Fab’-CCE/HSA-(CCK)x treatment did not trigger any apoptosis, as compared with untreated cells.
3. Results and Discussion
3.1. Preparation of Fab’-CCE and HSA-CCK conjugates
The Fab’-CCE and HSA-CCK conjugates were synthesized in a multistep procedure as shown in Figure 2. The peptides CCE and CCK were prepared by solid-phase peptide synthesis as previously reported.[26] The primary structure of the coiled-coil forming peptides CCE and CCK is shown in Figure 2C. The N-termini of CCE and CCK were modified with maleimide and thiol groups for conjugation, respectively. The purity and identity of peptides were confirmed by reversed-phase HPLC and MALDI-TOF mass spectrometry (Figure S1). The formation of coiled-coils by CCE/CCK peptides was determined using circular dichroism (CD) spectroscopy.[26,27] The Fab’ fragment from a humanized anti CD20 mAb RTX was tethered to CCE-Mal via a thioether bond to produce a Fab’-CCE conjugate with molecular weight of ~55 kDa (Figures 2 and S2). To prepare the HSA-(CCK)x conjugate, we first modified HSA with succinimidyl-[(N-maleimidopropionamido)-diethyleneglycol] ester (SM(PEG)2) to convert the amino groups to maleimide groups on the surface of HSA. Then, CCK-SH peptide was grafted via a stable thioether linkage to the surface of HSA. SEC analysis confirmed the successful synthesis of HSA-(CCK)x conjugate (Figure S3). The final product HSA-(CCK)7 had 7 CCK grafts per HSA (x=7), which was estimated by MALDI-TOF MS analysis (Figure S4). To visualize the in vitro biorecognition of Fab’-CCE and HSA-(CCK)7 conjugates, we fluorescently labeled Fab’-CCE with Cy5 (Cy5-Fab’-CCE) and HSA-(CCK)7 with Cy3 (Cy3-HSA-(CCK)7), as shown in Figure 2.
Figure 2.
(A) Synthesis of Fab’-CCE. (B) Synthesis of HSA-(CCK)7. (C) CCE, CCK peptide sequences. NHS-Cy5: Cyanine 5 monosuccinimidyl ester. NHS-Cy3: Cyanine 3 monosuccinimidyl ester.
3.2. Biorecognition of Fab’-CCE and HSA-(CCK)7
The biorecognition of Fab’-CCE and HSA-(CCK)7 was assessed by CD spectrometry. A pronounced coiled-coil signal (minima at 208 and 222 nm) was observed upon the mixing of Fab’-CCE and HSA-(CCK)7) (Figure S5). Confocal fluorescence microscopy was used to demonstrate the biorecognition and colocalization of Fab’-CCE and HSA-(CCK)7 at the surface of human NHL Raji B cells (CD20+). First, exposure of Raji cells to Cy5-labeled Fab’-CCE resulted in cell surface with Cy5 signal (red), because of the decoration of CD20 with Fab’-CCE; cells exposed to only Cy3-labeled HSA-(CCK)7 did not show significant fluorescent signal (Figure 3). Second, when Raji cells were exposed to fluorescently-labeled conjugates (Fab’-CCE and HSA-(CCK)7), with either premixed or consecutive treatments, red and green (Cy3) signals appeared at the surfaces of B cells (Figure 3). This result is in agreement with our studies on the interaction of CD20 Fab’-CCE and P-(CCK)x (HPMA copolymer grafted with multiple copies of CCK).[30,40,42] Finally, when the Raji cells were consecutively treated with an excess amount of RTX antibody or CCK peptide, the signal of Cy5 or Cy3 was decreased, respectively, due to a blocking effect (Figure 3). The 3-dimensional confocal images of Raji cells (Figure S6) also confirmed the biorecognition of Cy5-Fab’-CCE and Cy3-HSA-(CCK)7 at the surface of B cells. These results implied that coiled-coil heterodimers were formed at the cell surface.
Figure 3.
Visualization of colocalization between Cy5-Fab’-CCE and Cy3-HSA-(CCK)x on Raji cell surface. Representative confocal images of Raji cells treated with Cy5-Fab’-CCE and Cy3-HSA-(CCK)x. The Raji lymphoma B cells were incubated with the premixture of two conjugates, or consecutively exposed to Cy5-Fab’-CCE and then (after 0.5 h) to Cy3-HSA-(CCK)x. In consecutive treatment, for blocking purpose, excess (50-fold) RTX antibody (Ab) was added prior to Cy5-Fab’-CCE, or excess (50-fold) CCK was added prior to Cy3-HSA-(CCK)x. The cells treated with Cy3-HSA-(CCK)x only (without Cy5-Fab’-CCE) served as a control. Cy3, green; Cy5, red. Scale bar, 20 μm.
3.3. Selective surface binding of Fab’-CCE and HSA-(CCK)7
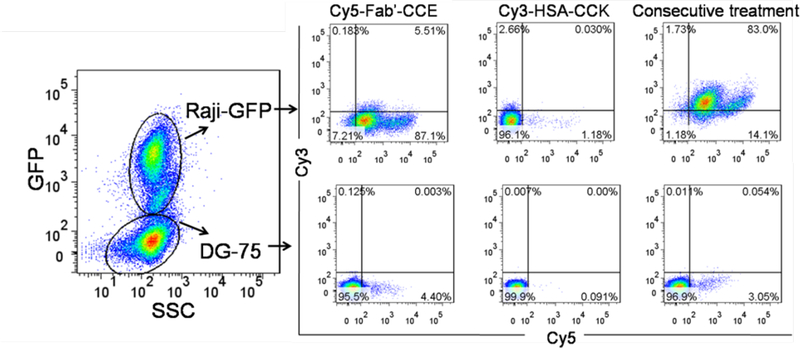
For the investigation of Fab’-CCE/HSA-(CCK)7 specificity, 8×104 Raji-GPF cells (CD20 positive) were mixed and co-cultured with a higher number of 12×104 DG-75 cells (CD20 negative). Then 1 μM of Cy5-Fab’-CCE was added and incubated with cells in 400 μL cell culture medium for 1 h. Afterwards, cells were washed with 400 μL cold PBS twice and further incubated with 1 μM of Cy3-HSA-(CCK)x for 1 h. Then cells were washed with 400 μL cold PBS twice and flow cytometry was used to detect the fluorescence of GPF, Cy5 and Cy3 within the cells. The binding of Cy5-Fab’-CCE/Cy3-HSA-(CCK)7 in Raji-GPF and DG-75 cells were distinguished by GFP expression. Co-cultured cells treated with Cy5-Fab’-CCE or Cy3-HSA-(CCK)7 alone were set as controls.
As shown in Figure 4, when the co-cultured cells were treated with Cy5-Fab’-CCE alone, Cy5-Fab’-CCE only targeted to CD20 positive Raji-GFP cells but not CD20 negative DG-75 cells, thus confirming the selective biorecognition between CD20 receptors and anti-CD20 Fab’. When the co-cultured cells were treated with Cy3-HSA-(CCK)7 alone, the majority of both cells were not stained with Cy3. Thus most of Cy3-HSA-(CCK)7 were precluded from entering the cells, which might be due to the macromolecular size of HSA and its poor membrane affinity. Of note, when co-cultured cells were consecutively exposed to Cy5-Fab’-CCE and Cy3-HSA-(CCK)7, Raji-GPF cells were dual Cy5 and Cy3 positive while DG-75 cells were dual negative. These results validated the specificity of two biorecognition events in DFMT.
Figure 4.
The mixture of CD20 positive Raji-GFP cells (expressing green fluorescence proteins) and CD20-negative DG-75 cells were consecutively exposed to Cy5-Fab′-CCE for 1 h and Cy3-HSA-(CCK)7 for 1 h. Then the binding of Cy5-Fab’-CCE/Cy3-HSA-(CCK)7 in different cells (distinguished by GFP expression) were analyzed by flow cytometry. Co-cultured cells treated with Cy5-Fab’-CCE or Cy3-HSA-(CCK)7 alone were also compared.
3.4. Apoptosis induction of human NHL B-cells by Fab’-CCE and HSA-(CCK)7 nanoconjugates
Apoptosis induction of human NHL B-cells mediated by coiled-coil formation at the cell surface, with concomitant crosslinking of CD20 receptors, was evaluated by three methods: Annexin V/propidium iodide assay, caspase 3 activation, and terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay. Throughout these studies, anti-CD20 rituximab mAb was used as a positive control. The apoptotic activity induced with Fab’-CCE and HSA-(CCK)7 by ether consecutive or premixed methods at different incubation times was first evaluated by Annexin V/propidium iodide assay. The exposure of cells to the individual components Fab’-CCE or HSA-(CCK)7 at the concentration of 1 μM showed a very low percentage of cell death, independent of the incubation interval. In contrast, co-treatment with Fab’-CCE and HSA-(CCK)7, ether consecutively or as a premixture, effectively induced apoptosis of Raji B cells at the concentration of 1 μM for both Fab’-CCE and HSA-(CCK)7 conjugates (Figure 5A).
Figure 5.
Apoptosis induction of Raji cells analyzed by Annexin V/PI assay (A), Caspase 3 (B) and TUNEL assays (C). Untreated: cells in culture medium; Fab’–CCE: single-component control at 1 μM; HSA-(CCK)7 1, 10 and 25 eq: single-component control ([CCK] = 1, 10 and 25 μM); Pre 1, 10 and 25 eq: premixture of Fab’–CCE (1 μM) and HSA-(CCK)7 ([CCK]= 1,10 and 25 μM); Con 1, 10 and 25 eq: consecutive addition of Fab’–CCE (1 μm) followed (1 h later) by HSA-(CCK)7 ([CCK] = 1, 10 and 25 μM). The apoptotic cells include early, late and dead cells. Statistics (Student’s t-test) was performed by comparing each treatment group with the corresponding untreated group at the same treatment time (6, 12 or 24 h) (***p < 0.0001, **p < 0.005, *p < 0.05, n.s.: no significant difference). Results are presented as mean values ± standard deviation (n=3).
Furthermore, we examined the influence of the ratio between CCE and CCK on apoptosis induction of Raji B cells. Using a constant concentration of Fab’-CCE (1 μM), a 10-time or 25-time folds of HSA-(CCK)7 were used (CCE : CCK = 1 : 10 or 1 : 25); excess of CCK significantly enhanced apoptotic levels when compared to the treatment with equimolar CCE/CCK (Figure 5). The consecutive treatment of Fab’-CCE with 25 times excess of HSA-(CCK)7 induced the highest apoptotic levels to Raji B cells in all three assays. Similar but less pronounced results were observed with the linear HPMA copolymer-based DFMT system.[30] The increased apoptotic level with increased excess of HSA-(CCK)7 has two reasons. As mentioned above the biorecognition of CCE and CCK is built on electrostatic and hydrophobic interactions[26] followed by fast folding into coiled-coil heterodimers. The other probable reason is the restricted flexibility of the HSA heart shape conformation when compared to linear HPMA copolymer. Consequently, not all CCK peptides attached to HSA can participate in CD20 crosslinking. Another factor that would impact the apoptotic levels, not evaluated here, is the CCK valence (number of CCK covalently attached on per molecule of HSA). The importance of this factor on apoptotic levels was demonstrated in the DFMT system composed of HPMA copolymer and a pair of complementary morpholino oligonucleotides.[43,44]
There was no significant difference in apoptosis induction between consecutive and premixed exposure of Raji B cells to Fab’-CCE and HSA-(CCK)7. In addition, similar levels of apoptosis induction were observed at different exposure intervals (6, 12 and 24 h) in all three different ratios of HSA-(CCK)7 to Fab’-CCE (1:1; 10:1; and 25:1) treatments. The extent of apoptosis of Raji cells induced by RTX increased with increasing incubation time. HSA-(CCK)7 administered alone showed minor time-dependent in vitro cytotoxicity at the concentration of 25 μM most probably due to the positive charge of CCK peptide (Figure 5A).
Apoptosis levels induced by Fab’-CCE and HSA-(CCK)7 conjugates were also evaluated by caspase 3 (Figure 5B) and TUNEL assays (Figure 5C). After incubation for 12 h, both consecutive and premixed treatment with Fab’-CCE (1 μM) and HSA-(CCK)7 (25 μM) induced about 34% of Raji B cells apoptosis as indicated by caspase 3 assay. The TUNEL assay experiments showed that 24 h consecutive exposure of Fab’-CCE (1 μM) and HSA-(CCK)7 (25 μM) induced 50% apoptosis of Raji B cells which was a little higher than premixed treatment. The dependence of apoptotic levels on the concentration ratio of nanoconjugates determined by caspase 3 and TUNEL assays showed similar results as obtained from Annexin V/propidium iodide assay (Figure 5).
3.5. Calcium influx investigation after treatment with Fab’-CCE and HSA-(CCK)7
Raji and DG-75 cells (2×105) were consecutively treated with 1 μM of Fab’-CCE for 1 h and 1 μM of HSA-(CCK)7 for 6 h. Then cells were loaded with Fluo-3AM (5 μM, Thermo Scientific) for 30 min. Afterward, flow cytometry was applied, and the fluorescent intensity of intracellular calcium was measured by exciting cells at 488 nm and detecting the emission at 530 nm. The results (Figure 6) showed consecutive treatment with Fab’-CCE and HSA-(CCK)7 could only trigger increase of intracellular calcium levels in Raji cells but not DG-75 cells. As we have shown previously, the major contribution to enhanced calcium concentration is influx of extracellular calcium.[45]
Figure 6.
Intracellular calcium levels in Raji and DG-75 cells following DFMT treatment. Statistics (Student’s t-test) was performed by comparing each treatment group with the corresponding untreated group (*p < 0.05, n.s.: no significant difference). Results are presented as mean values ± standard deviation (n=3).
4. Conclusions
In summary, we have developed a new DFMT based on human serum albumin. Two nanoconjugates, Fab’-CCE and HSA-(CCK)7 colocalize at the cell surface mediated by the biorecognition of two coiled-coil forming peptides, CCE and CCK. Crosslinking of CD20 antigens induces apoptosis of B cells by calcium influx and mitochondrial signal pathway.[45] Increasing the excess of HSA-(CCK)7 over Fab’-CCE enhanced apoptotic levels due to enhanced coiled-coil formation resulting in more efficient crosslinking of CD20 antigens. This phenomenon was more pronounced in the HSA DFMT system when compared to the HPMA copolymer-based DFMT due to the rigidity of the HSA. The latter may have restricted the participation of all CCK grafts in CD20 crosslinking. The studies presented here together with the biocompatibility of CCE and CCK[46] bode well for the translational potential of the HSA-based DFMT system.
Supplementary Material
Acknowledgements:
The work was supported in part by NIH grant RO1 GM95606 (to J.K.) from the National Institute of General Medical Sciences, the University of Utah Research Foundation, and the Huntsman Cancer Institute. We acknowledge support of funds in conjunction with grant P30 CS042014 awarded to Huntsman Cancer Institute and to the ET Program at Huntsman Cancer Institute.
Abbreviations
- CCE
coiled-coil forming peptide (E VSALEKE VSALEKK NSALEKE VSALEKE VSALEK)
- CCK
coiled-coil forming peptide (K VSALKEK VSALKEE VSANKEK VSALKEK VSALKE)
- CD
circular dichroism
- Con
consecutive administration of nanoconjugates
- DMFT
drug-free macromolecular therapeutics
- HPMA
N-(2-hydroxypropyl)methacrylamide
- HSA
human serum albumin
- mAb
monoclonal antibody
- MALDI ToF
matrix-assisted laser desorption/ionization time of flight
- NHL
non-Hodgkin’s lymphoma
- P
HPMA copolymer backbone
- PI
propidium iodide
- Pre
administration of premixed nanoconjugates
- RTX
rituximab
- SM(PEG)2
succinimidyl-[(N-maleimidopropionamido)-diethyleneglycol] ester
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end-labeling
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest.
J.Y. and J.K. are co-inventors on a pending US patent application (PCT/US2017/37736; assigned to the University of Utah) related to this work.
Contributor Information
Libin Zhang, Department of Pharmaceutics and Pharmaceutical Chemistry/CCCD, University of Utah, Salt Lake City, UT 84112, USA.
Yixin Fang, Department of Pharmaceutics and Pharmaceutical Chemistry/CCCD, University of Utah, Salt Lake City, UT 84112, USA.
Lian Li, Department of Pharmaceutics and Pharmaceutical Chemistry/CCCD, University of Utah, Salt Lake City, UT 84112, USA.
Jiyuan Yang, Department of Pharmaceutics and Pharmaceutical Chemistry/CCCD, University of Utah, Salt Lake City, UT 84112, USA.
D. Christopher Radford, Department of Bioengineering, University of Utah, Salt Lake City, UT 84112, USA.
Jindřich Kopeček, Department of Pharmaceutics and Pharmaceutical Chemistry/CCCD, University of Utah, Salt Lake City, UT 84112, USA; Department of Bioengineering, University of Utah, Salt Lake City, UT 84112, USA.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017, CA. Cancer J. Clin 2017, 67, 7. [DOI] [PubMed] [Google Scholar]
- [2].Shankland KR, Armitage JO, Hancock BW, Lancet, 2012, 380, 848. [DOI] [PubMed] [Google Scholar]
- [3].Ujjani C, Cheson BD, Expert Rev. Hematol, 2013, 6, 191. [DOI] [PubMed] [Google Scholar]
- [4].Maloney DG, Engl N. J. Med, 2012, 366, 2008. [DOI] [PubMed] [Google Scholar]
- [5].Molina A, Annu. Rev. Med 2008, 59, 237. [DOI] [PubMed] [Google Scholar]
- [6].Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H, Blood, 2002, 99, 754. [DOI] [PubMed] [Google Scholar]
- [7].Seyfizadeh N, Seyfizadeh N, Hasenkamp J, Huerta-Yepez S, Crit. Rev. Oncol. Hemat, 2016, 97, 275. [DOI] [PubMed] [Google Scholar]
- [8].Dransfield I, Blood, 2014, 123, 606. [DOI] [PubMed] [Google Scholar]
- [9].Pham T, Mero P, Booth JW, PloS One, 2011, 6, e14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stashenko P, Nadler LM, Hardy R, Schlossman SF, J. Immunol, 1980, 125, 1678. [PubMed] [Google Scholar]
- [11].Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nader LM, Blood, 1984, 63, 1424. [PubMed] [Google Scholar]
- [12].Press OW, Farr AG, Borroz KI, Anderson SK, Martin PJ, Cancer Res, 1989, 49, 4906. [PubMed] [Google Scholar]
- [13].Michel RB, Mattes MJ, Clin. Cancer Res, 2002, 8, 2701. [PubMed] [Google Scholar]
- [14].Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF, J. Cell Biol, 1993, 121, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tedder TF, Engel P, Immunol. Today, 1994, 15, 450. [DOI] [PubMed] [Google Scholar]
- [16].Janas E, Priest R, Wilde JI, White JH, Malhotra R, Clin. Exp. Immunol, 2005, 139, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Armitage JO, Weisenburger DD, J. Clin. Oncol, 1998, 16, 2780. [DOI] [PubMed] [Google Scholar]
- [18].Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advan RH, Andreadis CB, Bartlett N, Byrd JC, Czuczman MS, Fayad LE, Fisher RI, Glenn MJ, Harris NL, Hoppe RT, Horwitz SM, Kelsey CR, Kim YH, Krivacic S, LaCasce AS, Nademanee A, Porcu P, Press OW, Rabinovitch R, Reddy N, Reid E, Saad AA, Sokol L, Swinnen LJ, Tsien C, Vose JM, Yahalom J, Zafar N, Dwyer M, Sundar H, Non-Hodgkin’s lymphomas, version 4.2014. J. Natl. Compr. Cancer Netw, 2014, 12, 1282–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheson BD, Leonard JP, Engl N. J. Med, 2008, 359, 613. [DOI] [PubMed] [Google Scholar]
- [20].Boross P, Leusen JHW, Am. J. Cancer Res, 2012, 2, 676. [PMC free article] [PubMed] [Google Scholar]
- [21].Okroj M, Österborg A, Blom AM, Cancer Treat. Rev, 2013, 39, 632. [DOI] [PubMed] [Google Scholar]
- [22].Shan D, Ledbetter JA, Press OW, Blood, 1998, 91, 1644. [PubMed] [Google Scholar]
- [23].Apostolovic B, Danial M, Klok HA, Chem. Soc. Rev, 2010, 39, 3541. [DOI] [PubMed] [Google Scholar]
- [24].Pechar M, Pola R, Biotechnol. Adv 2013, 31, 90. [DOI] [PubMed] [Google Scholar]
- [25].Kopeček J, Yang J, Angew. Chem. Int. Ed, 2012, 51, 7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang J, Xu C, Wang C, Kopeček J, Biomacromolecules, 2006, 7, 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang J, Wu K, Koňák Č, Kopeček J, Biomacromolecules, 2008, 9, 510. [DOI] [PubMed] [Google Scholar]
- [28].Deans JP,, Li H, Polyak MJ, Immunology, 2002, 107, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chu T-W, Kopeček J, Biomater. Sci, 2015, 3, 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu K, Liu J, Johnson RN, Yang J, Kopeček J, Angew. Chem. Int. Ed, 2010, 49, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu K, Yang J, Liu J, Kopeček J, Control J. Release, 2012, 157, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chu T-W, Kosak KM, Shami PJ, Kopeček J, Drug Deliv. Transl. Res 2014, 4, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johnson RN, Kopečková P, Kopeček J, Biomacromolecules 2012, 13, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chu T-W, Yang J, Kopeček J, Biomaterials 2012, 33, 7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bern M, Knutsen Sand KM, Nilsen J, Sandlie I, T Andersen J, J. Control Release, 2015, 211, 144. [DOI] [PubMed] [Google Scholar]
- [36].Kratz F, J. Control Release, 2008, 132, 171. [DOI] [PubMed] [Google Scholar]
- [37].Elsadek B, Kratz F, J. Control Release, 2012, 157, 4. [DOI] [PubMed] [Google Scholar]
- [38].Sockolosky JT, Szoka FC, Adv. Drug Deliv. Rev, 2015, 91, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Larsen MT, Kuhlmann M, Hvam ML, Howard KA, Mol. Cell. Ther, 2016, 4, 3; DOI: 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang R, Yang J, Chu T-W, Hartley JM, Kopeček J, Adv. Healthcare Mater, 2015, 4, 1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Torres OB, Jalah R, Rice KC, Li F, Antoline JFG, Iyer MR, Jacobson AE, Boutaghou MN, Alving CR, Matyas GR, Anal. Bioanal. Chem 2014, 406, 5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hartley JM, Zhang R, Gudheti M, Yang J, Kopeček J, J. Control Release, 2016, 231, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chu T-W, Yang J, Zhang R, Sima M, Kopeček J, ACS Nano, 2014, 8, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang L, Fang Y, Yang J, Kopeček J, J. Control Release, 2017, 263, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li L, Yang J, Wang J, Kopeček J, Macromol. Biosci, 2018, 18, DOI: 10.1002/mabi.201700196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kverka M, Hartley JM, Chu T-W, Yang J, Heidchen R, Kopeček J, Biomaterials 2014, 35, 5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.