Abstract
IL-1 signaling pathway has been shown to play a critical role in the pathogenesis of chronic, autoinflammatory skin diseases such as psoriasis. However, the exact cellular and molecular mechanisms have not been fully understood. Here, we show that IL-1β is significantly elevated in psoriatic lesional skin and imiquimod (IMQ)-treated mouse skin. In addition, IL-1R signaling appears to correlate with psoriasis disease progression and treatment response. IL-1 signaling in both dermal γδ T cells and other cells such as keratinocytes is essential to an IMQ-induced skin inflammation. IL-1β induces dermal γδ T cell proliferation and IL-17 production in mice. In addition, IL-1β stimulates keratinocytes to secrete chemokines which preferentially chemoattract peripheral CD27− CCR6+IL-17 capable producing γδ T cells (γδT17). Further studies reveal that endogenous IL-1β secretion is regulated by skin commensals to maintain dermal γδT17 homeostasis in mice. Mouse skin associated with corynebacterium, bacterial enriched in human psoriatic lesional skin has increased IL-1β and dermal γδT17 cell expansion. Thus, IL-1β-IL-1R signaling pathway may contribute to skin inflammation and psoriasis pathogenesis via the direct regulation of dermal IL-17-producing cells and stimulation of keratinocytes for amplifying inflammatory cascade.
Introduction
Psoriasis is an autoinflammatory skin disease affecting 2-3% of all individuals in the United States (Rachakonda et al., 2014) and is a serious global problem with at least 100 million individuals affected worldwide. Recent studies have revealed that cytokines such as TNF-α/IL-23/IL-17 play critical roles in psoriasis pathogenesis (Lowes et al., 2014). Antibodies against TNF-α/IL-23/IL-17 have been approved for the treatment of patients with moderate-to-severe plaque psoriasis. Cytokine IL-1 family has also been implicated to be a key player in psoriasis pathogenesis (Renne et al., 2010). Previous studies have shown that IL-1 is a dominant cytokine in patients with generalized pustular psoriasis (Johnston et al., 2017). In addition, whole-exosome SNP array has identified IL1RL1 gene as one of the new susceptibility loci for psoriasis (Zuo et al., 2015). Further genetic studies reveal that polymorphisms in IL-1B gene can be used to differentiate patients with psoriasis of early and late onset (Hebert et al., 2014). The IL-1 cytokine family is a growing group of cytokines including IL-1α, IL-1β, and IL-1 receptor antagonist (IL-1RN) (Jensen, 2010). IL-1α has been shown to be essential in the induction of Munro’s microabscess formation in imiquimod (IMQ)-induced murine psoriasis-like model (Uribe-Herranz et al., 2013). IL-1α also stimulates human keratinocytes to induce potent proinflammatory responses (Yano et al., 2008). However, IL-1α mRNA level is decreased in psoriatic skin (Cooper et al., 1990) and IL-1α levels in plasma and skin show an inverse correlation with Psoriasis Area Severity Index (PASI) score (Tamilselvi et al., 2013) before and after treatment with Methotrexate, posing a question about the importance of IL-1α in human psoriasis pathogenesis. IL-1β has a well-documented role in the pathogenesis of psoriasis (Lowes et al., 2014). IL-1β inhibitors have been tested in clinical trials for the treatment of psoriasis. Recently there was a case report demonstrating that a patient with severe pustular psoriasis treated with anti-IL-1β canakinumab had complete skin clearance with no recurrent systemic manifestations (Skendros et al., 2017). IL-1β is known to be critical in IL-17-producing T cell differentiation and activation (Cai et al., 2014, Ghoreschi et al., 2010, Sutton et al., 2009). Despite these studies, how IL-1β production is regulated in healthy skin and psoriatic lesional skin and the exact cellular and molecular mechanisms by which the IL-1β-IL-1R signaling pathway regulates skin inflammation have not been fully understood.
In the current study, we show that IL-1β mRNA and protein expression levels are significantly elevated in psoriatic lesional skin and predominately secreted by macrophages, dendritic cells (DCs), and keratinocytes in human. Neonatal thymocytes/bone marrow chimeric mouse studies reveal that IL-1 signaling in γδ T cells and other radio-resistant cells is required to induce IMQ-mediated skin inflammation. IL-1β not only activates dermal γδ T cells for IL-17 secretion but also stimulates keratinocytes to secrete chemokines which chemoattract IL-17-capable producing T cells. Notably, endogenous IL-1β secretion in the skin is regulated by skin commensals in mice and is associated with dermal IL-17-producing γδ T (γδ T17) cell homeostatic expansion and activation. Altered skin microbiome dysbiosis in psoriasis patients may result in an exaggerated IL-1β production thus priming skin for inflammation. These findings identify pivotal roles of the IL-1β-IL-1R signaling pathway in maintaining skin homeostasis in the steady state as well as in the induction of inflamed skin such as psoriasis.
Results
IL-1β is significantly elevated in human psoriatic skin and IMQ-treated mouse skin.
It is debatable whether IL-1β protein is produced in the skin (Mizutani et al., 1991). We first examined the mRNA level of IL-1β in psoriatic lesional skin and skin tissues from healthy individuals. Consistent with previous studies (Dombrowski et al., 2011), IL-1β mRNA level was significantly increased in psoriatic lesional skin compared to that in normal skin (Figure 1A). To further determine IL-1β protein expression in skin tissues, immunofluorescent staining was performed. IL-1β protein expression was enhanced in psoriatic lesional skin although healthy control skin did express low level of IL-1β (Figure 1B). IL-1β protein was largely co-localized with keratinocytes in psoriatic lesional skin (Figure 1C). CD11c+DCs and CD163+ macrophages also secreted IL-1β (Figure 1C). We next used RNA-based next-generation sequencing (RNA-seq) to analyze lesional skin samples from patients initially effectively treated with glucocorticoid but disease recurred after stopping treatment. Gene-set-enrichment analysis (GSEA) revealed that IL-1R signaling pathway-related genes were downregulated in patients effectively treated with glucocorticoid whereas these genes were upregulated when the disease recurred (Figure 1D). This was confirmed by real-time PCR analysis. These data suggest that the IL-1β-IL-1R signaling pathway is associated with disease progression and treatment response.
Figure 1. IL-1β expression was significantly increased in both murine and human psoriatic lesions.
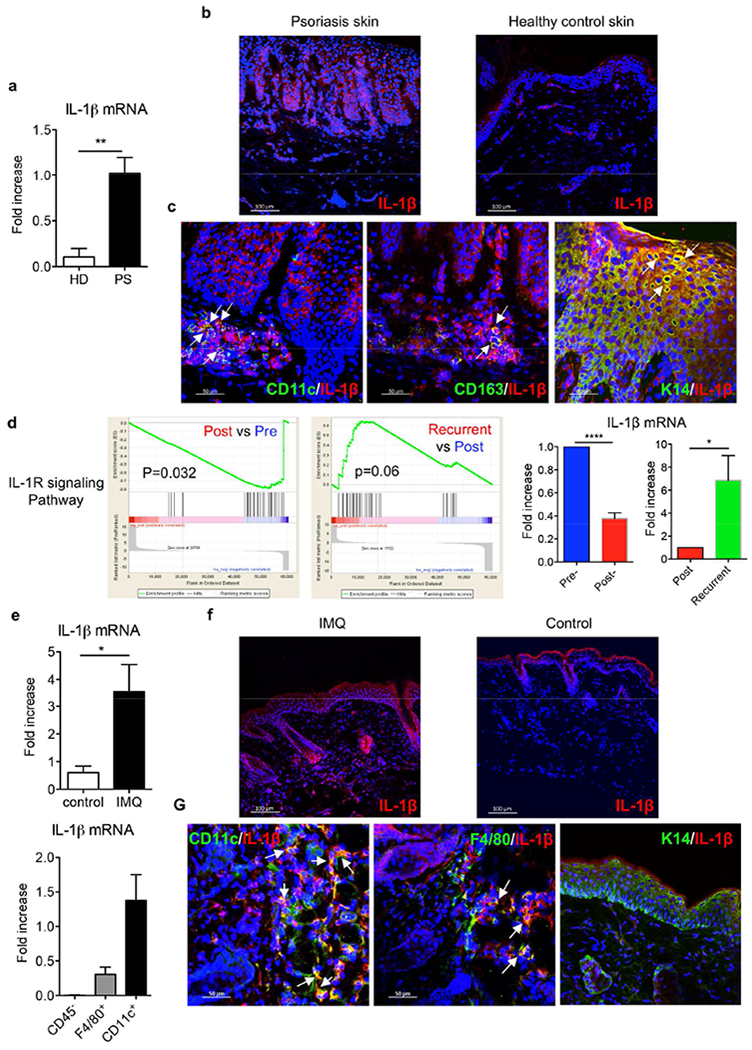
(a) IL-1β mRNA levels from skin biopsies collected from psoriasis patients (PS, n=5) and healthy donors (HD, n=4). (b) Frozen sections were stained with IL-1β mAb (red) and DAPI (blue). Scale bar =100 μm. (c) Frozen sections from psoriatic lesional skin were stained with IL-1β mAb (red) and human CD11c mAb (green) or human CD163 mAb (green) or keratin 14 mAb (green), and DAPI (blue). Scale bar =50 μm. (d) Gene set enrichment analysis identifies significant transcriptional downregulation of IL-1R signaling pathway in psoriasis patients initially effectively treated with glucocorticoid while upregulation in recurrent patients after stopping treatment (NCBI GEO with the accession number GSE114729). The mRNA expression of IL-1β by real-time PCR analysis was calculated using Pre- or Post-treatment level as base level. (e) C57BL/6 mice received daily topical application with IMQ or vehicle control for 3-5 days. IL-1β mRNA levels from IMQ-treated or vehicle control mouse skin were measured. CD11c+ cells, F4/80+ cells and CD45− cells were sorted from 3 days of IMQ-treated mouse skin and IL-1β mRNA level was measured. (f) Skin frozen sections from IMQ-or vehicle control-treated mice were stained with IL-1β mAb (red) and DAPI (blue). Scale bar =100 μm. (g) Skin frozen sections from IMQ-treated mice were stained with IL-1β mAb (red) and mouse CD11 c mAb (green), or mouse F4/80 mAb (green), or keratin 14 mAb (green), and DAPI (blue). Scale bar =50 μm. *p< 0.05, **p< 0.01, ***p< 0.001.
We next examined IL-1β expression levels in an IMQ-induced psoriasis-like mouse model. Similarly, the mRNA and protein expression level of IL-1β was significantly increased in IMQ-treated skin (Figure 1E, F). However, IL-1β mRNA was mainly expressed in DCs and macrophages. CD45 negative cells such as keratinocytes did not express appreciable level of IL-1β mRNA (Figure 1E). This was confirmed by immunofluorescent staining showing IL-1β protein was co-localized with DCs and macrophages but not with keratinocytes (Figure 1G), highlighting the differences between mouse and human skin.
IL-1R on γδ T cells is essential to induce skin inflammation in mice.
Cytokine IL-1β exhibits its biological functions through IL-1 receptor type 1 (IL-1R) and IL-1R is expressed on many types of cells in the skin. Our previous studies have shown that IMQ- or IL-23-induced skin inflammation is significantly decreased in IL-1R KO mice (Cai et al., 2014). In addition, dermal γδ T cells play a critical role in these two models (Cai et al., 2011, Pantelyushin et al., 2012). We hypothesized that IL-1R expression on dermal γδ T cells is necessary to mediate skin inflammation. We established neonatal thymocytes/bone marrow chimeric mice in which IL-1R was only deficient in γδ T cells to test this hypothesis. As additional controls to verify the chimeric mice, chimeric mice from IL-1R KO mice (Figure S1A) indeed showed a reduced erythema and scales (Figure S2A). Histopathologically, these mice had decreased epidermal thickness and neutrophil infiltration as compared to chimeric mice from WT mice (Figure 2A). We then used chimeric mice in which IL-1R is deficient in γδ T cells (Figure S1B). Upon IMQ topical treatment, these mice showed a reduced erythema and scales (Figure S2B). In addition, epidermal thickness from γδ T IL-1R KO chimeric mice was significantly decreased compared to that in IL-1R intact mice (Figure 2B). The mRNA expression levels of IL-17, TNF-α, and IL-6 were also significantly decreased. These data suggest that IL-1R expression on γδ T cells is essential in IMQ-induced skin inflammation.
Figure 2. IL-1R signaling in γδ T cells is essential to induce skin inflammation.
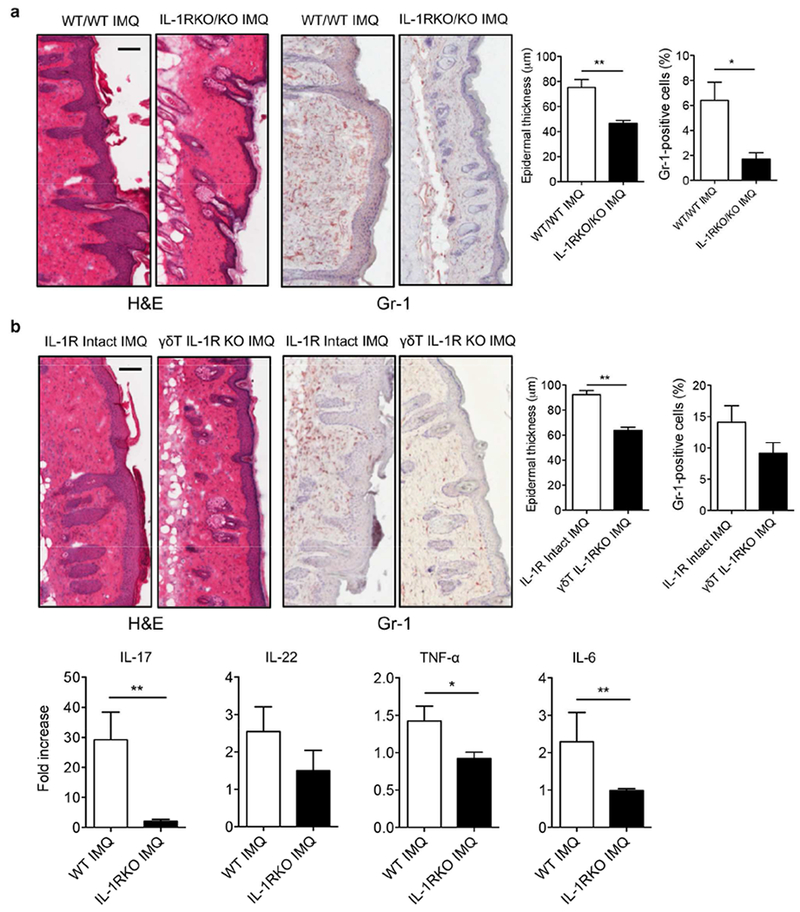
(a) Reconstituted WT or IL-1R KO chimeric mice (n=3) were treated daily for 5 days with IMQ or vehicle control. Representative H&E-stained sections and frozen sections stained with Gr-1 are shown. Gr-1 positive cells are brown. Skin tissues were also stained with CD45 and Gr-1 assessed by flow cytometry. Epidermal thickness and percentage of CD45+Gr-1+ cells were measured. Scale bar =100 μm. Data are representative of two independent experiments with similar results. (b) Reconstituted mice (n=3) with WT γδ T cells or IL-1R deficiency in γδ T cells were treated daily for 5 days with IMQ or vehicle control. Representative H&E-stained sections and frozen sections stained with Gr-1 are shown. Gr-1 positive cells are brown. Skin tissues were also stained with CD45 and Gr-1 assessed by flow cytometry. Epidermal thickness and percentage of CD45+Gr-1+ cells were measured. Scale bar =100 μm. Data are representative of two independent experiments with similar results. The mRNA levels of IL-17, IL-22, TNF-α and IL-6 were measured by real-time PCR analysis. *p< 0.05, **p< 0.01.
IL-1R on other cells also contributes to IMQ-induced skin inflammation.
We next examined whether IL-1R on other cells, particularly radio-resistant cells such as keratinocytes is also necessary for IMQ-induced skin inflammation. We established chimeric mice in which all other cells do not express IL-1R except γδ T cells (Figure S1C). Upon IMQ topical treatment, these mice showed a reduced erythema and scales (Figure S2C). Histopathologically, these mice also showed decreased epidermal thickness as compared to IL-1R intact mice although infiltrated neutrophils and the mRNA expression levels of IL-17, IL-22, TNF-α, and IL-6 were not significantly altered (Figure 3). These data suggest that IL-1R signaling on other cells, possibly keratinocytes also contributes to IMQ-induced skin inflammation, particularly for epidermal hyperplasia.
Figure 3. IL-1R signaling in other cells is important to induce full-fledged skin inflammation.
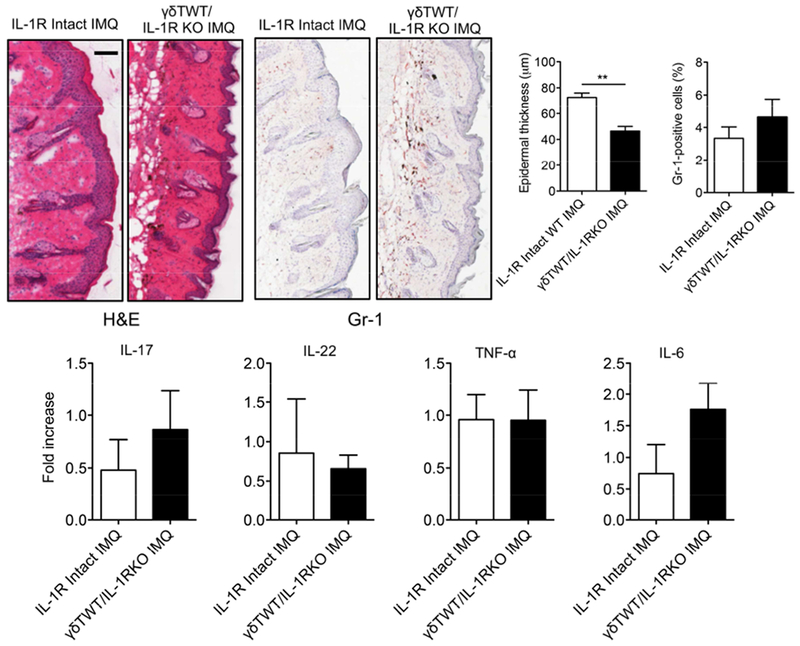
Reconstituted mice (n=3) with WT γδ T cells and IL-1R intact or deficiency in other cells were treated daily for 5 days with IMQ or vehicle control. Representative H&E-stained sections and frozen sections stained with Gr-1 are shown. Gr-1 positive cells are brown. Skin tissues were also stained with CD45 and Gr-1 assessed by flow cytometry. Epidermal thickness and percentage of CD45+Gr-1+ cells were measured at day 5. Scale bar =100 μm. Data are representative of two independent experiments with similar results. The mRNA levels of IL-17, IL-22, TNF-α and IL-6 were measured by qPCR analysis. *p < 0.05.
IL-1β stimulates dermal γδT cell proliferation and IL-17 production as well as stimulates keratinocytes to secrete chemokines.
IL-1β is critical in IL-17-producing T cell differentiation and activation. Consistent with previous studies (Cai et al., 2014), IL-1β induced dermal γδ T cell proliferation and synergized with IL-23 for IL-17 production (Figure 4A). In contrast, IL-23 induced minimal γδ T cell proliferation (Figure 4B). However, IL-23-induced dermal γδ T cell IL-17 production was abrogated in IL-1R KO mice (Figure 4A, B), suggesting that IL-1R signaling is required for IL-17 production in dermal γδ T cells.
Figure 4. IL-1R signaling is essential in dermal γδ T cell proliferation and IL-17 production.
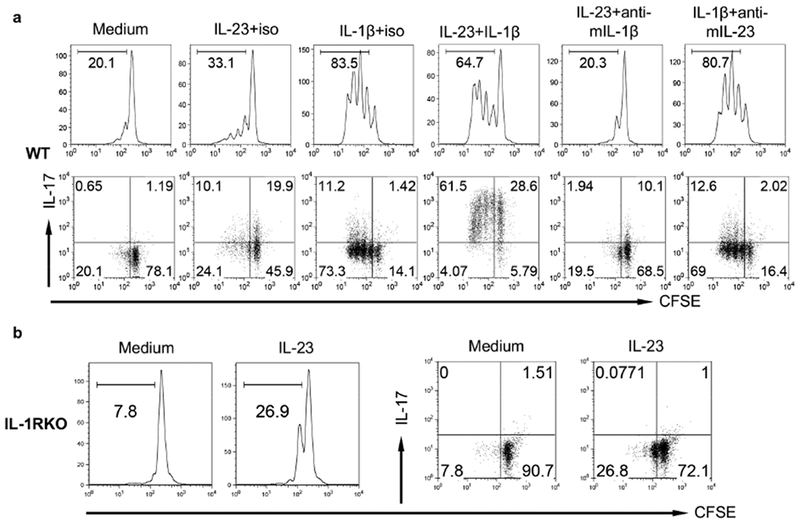
(a) Whole skin cell suspensions from C57BL/6 WT mice were labeled with CFSE and then stimulated with IL-23, IL-1β, IL-23 plus IL-1β for 3 days. CFSE dilution and intracellular IL-17 production by dermal γδ T cells were determined by flow cytometry. Flow plots gated on CD3int γδTCRint cells are representative of at least three independent experiments with similar results. (b) Whole skin cell suspensions from IL-1R KO mice labeled with CFSE were stimulated with IL-23 for 3 days. CFSE dilution and intracellular IL-17 production by dermal γδ T cells were determined by flow cytometry. Flow plots gated on CD3int γδTCRint cells are representative of at least three independent experiments with similar results.
Since our studies showed that IL-1R on other cells, such as radio-resistant cells is also critical in skin inflammation, particularly for epidermal thickness (Figure 3), we examined whether IL-1β functions on other cells leading to such effect. Keratinocytes have been shown to produce IL-1β. IL-1β also functions on keratinocytes in an autocrine fashion (Kupper et al., 1988). IL-1β stimulated primary murine keratinocytes to produce chemokines (Cai et al., 2011) including CCL20 (Figure 5A). IL-1β-induced CCL-20 production in mouse keratinocytes was dependent on the STAT3 and NF-kB pathway but independent of the MAPK and mTOR pathways. CD27 can be used as a functional marker to differentiate IL-17-producing versus IFN-γ-producing γδ T cells (Ribot et al., 2009). IL-17-producing γδ T cells do not express CD27 and constitutively express CCR6 which is the receptor for chemokine CCL20. In contrast, IFN-γ-producing γδ T cells express CD27 but not CCR6. As shown in Figure 5B, supernatants from IL-1β-stimulated keratinocytes chemoattracted CD27− but not CD27+ γδ T cells. We also noticed that IL-1β slightly stimulated mouse KC for proliferation (Figure 5C). To examine whether human IL-1β has similar function, we stimulated human keratinocytes with IL-1β. Similarly, IL-1β stimulated human keratinocytes to produce chemokines including CCL20 (Figure 5D). This was also associated with STAT3 and NF-kB pathway. Further chemotaxis assay revealed that supernatants from IL-1β-stimulated keratinocytes chemoattracted CCR6+ T cells (Figure 5E). However, IL-1β did not enhance human keratinocyte proliferation (Figure 5F). Taken together, these data suggest that IL-1β can stimulate keratinocytes to secrete chemokines which chemoattract IL-17 capable-producing CCR6+ T cells.
Figure 5. IL-1β stimulates keratinocytes (KCs) to secrete chemokines, which are capable of chemoattracting γδT17 cells.
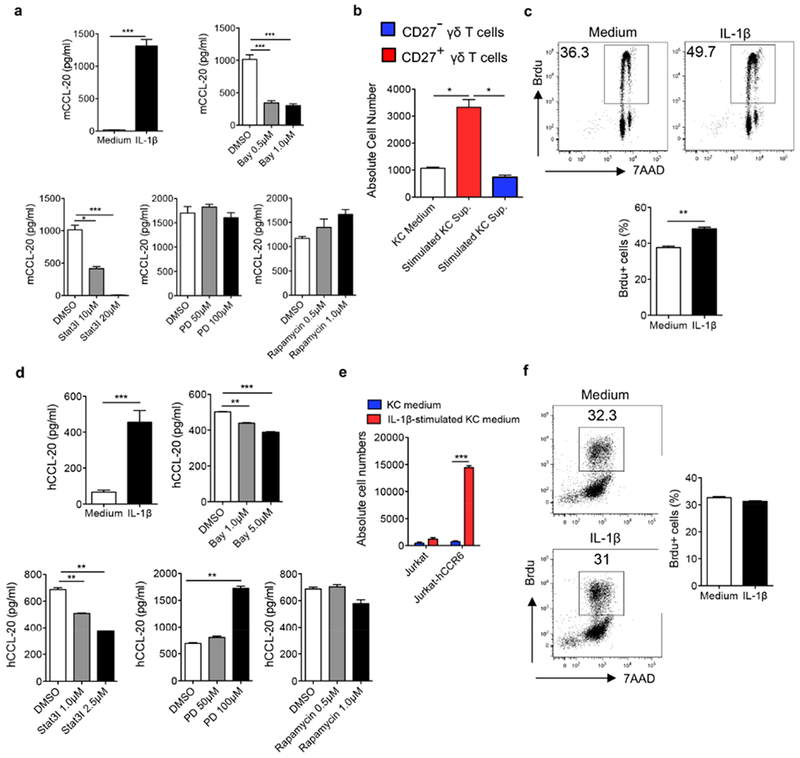
(a) Primary mouse KCs were stimulated with IL-1β in the absence or presence of signaling pathway inhibitors at indicated concentrations. CCL20 level was measured by ELISA. (b) Sorted CD27−/CD27+ γδ T cells were added on the top of insert and incubated with supernatants from mouse KCs stimulated with or without IL-1β. Cells were harvested and quantified by flow cytometry. (c) Primary mouse KCs were stimulated with IL-1β for 24 hours. Brdu was added in the culture for the last 2.5 hours. Cells were stained with anti-Brdu Ab and 7-AAD and analyzed by flow cytometry. Representative dot plots and summarized Brdu+ cells are shown. (d) Primary human KCs were stimulated with IL-1β in the absence or presence of signaling pathway inhibitors. Human CCL20 level was measured by ELISA. (e) Cultured Jurkat or Jurkat-hCCR6 cells were added on the top of insert and incubated with supernatants from human KCs stimulated with or without IL-1β. Cells were harvested and quantified by flow cytometry. (f) Primary human KCs were cultured and stimulated with IL-1β for 24 hours. Brdu was added in the culture for the last 2.5 hours. Cells were stained with anti-Brdu Ab and 7-AAD and analyzed by flow cytometry. Representative dot plots and summarized Brdu+ cells are shown. *p< 0.05, **p< 0.01, ***p< 0.001.
Skin commensals endow IL-1β production in health and psoriasis.
Having demonstrated critical roles of IL-1β in skin inflammation, one question that remains unanswered is how IL-1β secretion is regulated in the skin. We found that the mRNA and protein levels of IL-1β were significantly lower in germ-free (GF) mice as compared to those in specific-pathogen free (SPF) mice (Figure 6A). In contrast, IL-23 mRNA expression level was not affected (data not shown). Correspondingly, dermal γδ T cells and IL-17-producing γδ T cells in the skin were significantly lower in GF mice (Figure 6B). Since IL-23-induced dermal γδ T cell IL-17 production is dependent on IL-1β, we stimulated dermal γδ T cells with IL-23 and found that dermal γδ T cell IL-17 production from GF mice was significantly lower than those from SPF mice (Figure S3A). Addition of exogenous IL-1β did not fully rescue IL-23-induced IL-17 production from dermal γδ T cells in GF mice. Notably, dermal γδ T cells from GF mice showed lower IL-1R expression level than SPF mice (Figure S3B). These data suggest that skin commensals play a critical role in skin immune homeostasis, particularly for dermal γδ T17 cells via an endogeous IL-1β production.
Figure 6. Skin commensals or altered skin microbiota contribute to IL-1β-mediated dermal γδT17 expansion.
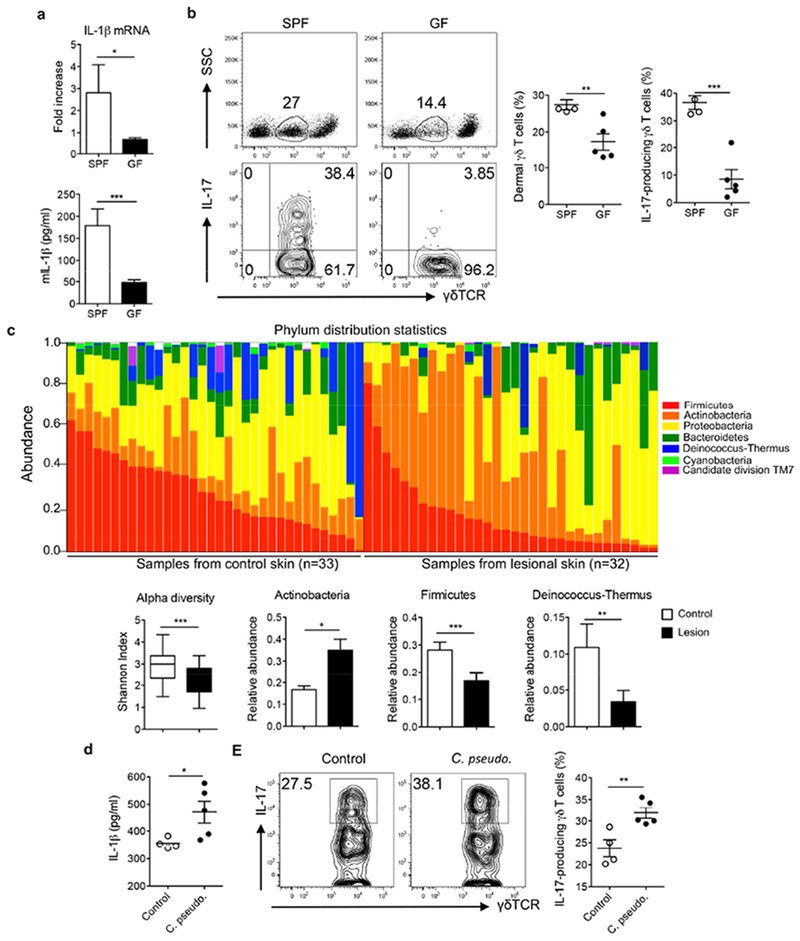
(a) The mRNA and protein levels of IL-1β in C57BL/6 SPF mice versus GF mice. (b) The frequency of dermal γδ T cells and γδT17 cells in C57BL/6 SPF and GF mice are shown. Flow plots gated on CD3+ cells (top) or CD3intγδTCRint cells (bottom) are representative of two independent experiments with similar results. (c) Skin swabs from psoriasis patients and healthy individuals were sequenced for skin microbiota analysis. Bacterial abundance at the phylum level, the Shannon index, and relative abundances of Actinobacteria, Firmicutes, and Deinococcus-Thermus between lesional skin and healthy control skin are shown. (d) The IL-1β protein levels from C57BL/6 mice associated with or without Corynebacterium pseudodiptheriticum were measured by ELISA. (e). Representative dot plots and summarized dermal γδT17 cells in mice associated with or without Corynebacterium pseudodiptheriticum are shown. Flow plots gated on CD3intγδTCRint cells are representative of two independent experiments with similar results. *p< 0.05, **p< 0.01, ***p< 0.001.
Previous studies have shown that psoriatic skin has substantially altered skin commensals (Alekseyenko et al., 2013, Gao et al., 2008). In our studies, swabs from psoriatic lesional skin and skin of healthy individuals (controls) were collected. Using high-throughput 16S rRNA gene sequencing, we assayed the cutaneous bacterial communities and characterized these samples. As shown in Figure 6C, skin microbiota from psoriasis patients had altered bacterial abundance at the phylum distribution level. Shannon index decreased from control to lesion, suggesting healthy skin has higher alpha diversity compared to lesions (Figure 6C). The five most common phyla: Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes and Deinococcus-Thermus with relative abundance more than 5% were shown. Comparison of the relative abundance of Actinobacteria, Firmicutes and Deinococcus-Thermus showed significant difference between control and lesional skin tissues.
To test the hypothesis that altered skin commensals lead to increased IL-1β production thus expanding IL-17-producing dermal γδ T cells, mice were applied with Corynebacterium pseudodiptheriticum. Indeed, the IL-1β protein level was increased in these mice (Figure 6D). Correspondingly, dermal γδT17 cells were also significantly increased in Corynebacterium pseudodiptheriticum associated mice (Figure 6E). Thus skin commensals contribute to both skin immune homeostasis and dysregulated immune responses such as psoriasis.
Discussion
The IL-1β-IL-1R signaling pathway has been shown previously to play critical roles in psoriasis pathogenesis (Lowes et al., 2014). However, many questions remain unanswered. Despite elevated IL-1β mRNA expression levels in psoriatic skin, it is not conclusive whether IL-1β protein expression is also elevated. In addition, it is not fully understood how IL-1β secretion is regulated in normal skin and psoriatic skin. Here, we show that IL-1β mRNA expression level is significantly elevated in human psoriatic lesional skin and also in IMQ-treated mouse skin, which is consistent with previous studies (Debets et al., 1997, Dombrowski et al., 2011). In addition, we show that the IL-1β-IL-1R signaling pathway is associated with disease progression and treatment response. This finding is important and suggests that this pathway may serve not only as targets for psoriasis treatment but also as biomarkers for disease progression and treatment response. Furthermore, elevated IL-1β protein is observed in psoriatic lesional skin as assessed by immunofluorescent staining. Biologically active IL-1β protein expression requires proteolytic activation of pro-IL-1β by protease caspase-1 (Lopez-Castejon and Brough, 2011). Previous studies demonstrate that normal human keratinocytes produce but do not process pro-IL-1β to be a mature functional IL-1β (Mizutani et al., 1991). However, caspase-1 activity is significantly increased in psoriatic skin (Dombrowski et al., 2011), suggesting that pro-IL-1β could be processed to secrete mature IL-1β, at least in an inflammatory condition. In our study, we use IL-1β antibody which recognizes mature form of IL-1β (17 kDa) and demonstrate that IL-1β protein expression is elevated in human psoriatic skin as well as in IMQ-treated mouse skin. These data suggest that under inflammatory conditions, pro-IL-1β can be processed to become an active, functional IL-1β. We also analyze the cellular origins of IL-1β in the skin. In human psoriatic lesional skin, keratinocytes are the major IL-1β producer as revealed by immunofluorescent staining. Macrophages and DCs also produce IL-1β. In contrast, macrophages and DCs in the IMQ-treated skin are the major IL-1β producer. Keratinocytes do not secrete appreciable level of IL-1β. These findings highlight the differences between mouse models and human disease.
One critical question related to IL-1β production is how IL-1β is regulated. Previous studies have shown that cytosolic DNA fragments in psoriatic skin stimulate inflammasome AIM2 activation in keratinocytes leading to increased IL-1β production (Dombrowski et al., 2011). In our study, we observe a low but detectable level of IL-1β in normal human skin and naive mouse skin. Cytosolic DNA fragments could not be detected in healthy skin (Dombrowski et al., 2011). We thus hypothesize that skin commensals may be related to endogenous IL-1β production. Indeed, GF mice have significantly lower IL-1β mRNA and protein expression levels compared to SPF mice. This is also associated with lower frequency of dermal γδ T cells and IL-17 production in GF mice. These data suggest that skin commensals are important to maintain skin immune homeostasis which plays a critical role in local immunity and inflammation (Naik et al., 2012). These results also support the idea that IL-1 signaling is diminished in the absence of commensals as shown by lower IL-1R expression level on dermal γδ T cells from GF mice. Furthermore, it implies that resident commensals may provide a protective role in skin infection. Indeed, deficiency of IL-1R leads to severe cutaneous vaccinia virus infection (Tian et al., 2017). This notion is also supported by a recent study showing that an ocular commensal induces IL-17-producing γδ T cells thus protecting against corneal infection (St Leger et al., 2017). These data lead us to examine whether altered bacterial commensals in psoriatic skin are associated with increased IL-1β and IL-17 production in patients with psoriasis. In our study, Actinobacteria is the most common phylum in psoriasis while Firmicutes in controls, which is different from a previous study (Gao et al., 2008). The differences may be related to the different sampling and skin conditions or different ethnic groups. Nevertheless, Corynebacterium which belongs to Actinobacteria phylum is significantly elevated at the genus level in psoriatic lesional skin (Alekseyenko et al., 2013). Intriguingly, application of Corynebacterium pseudodiptheriticum in mouse skin increases IL-1β production along with increased dermal γδ17 cells. Increased IL-17 production may also stimulate keratinocytes to secrete more IL-1β (Cho et al., 2012), thus establishing an amplification loop of inflammatory responses. It will be interesting to determine whether increased Corynebacterium colonization in healthy normal skin also leads to elevated IL-1β production thus leading to priming of IL-17-producing cells in human.
IL-1β is known to be critical in inducing Th17 and γδT17 cell differentiation and effector function. Indeed, we show that IL-1β stimulates dermal γδ T cell for proliferation and synergizes with IL-23 for IL-17 production in mice. Interestingly, IL-23-induced IL-17 production is dependent on IL-1R signaling, suggesting critical role of IL-1R signaling in dermal γδ T cell activation. Our study shows that IL-1 signaling on γδ T cells is important in IMQ-induced epidermal hyperplasia. It is worth noting that IMQ-induced skin inflammation model although has been widely used in preclinical psoriasis studies, there are many limitations with this model such as untended consequences of topical treatment and limited aspects of human psoriasis (Hawkes et al., 2017). It thus needs to be determined whether IL-1R signaling is critical in activating human dermal IL-17-producing cells. In addition, it has been shown previously that dermal γδ T cells are the major cellular source of IL-17 in IMQ-induced skin inflammation (Cai et al., 2011). In contrast, both αβ and γδ T cells produce IL-17 in psoriatic lesional skin (Cai et al., 2011, Matos et al., 2017) and αβ T cells are considered to play a predominant role in disease initiation (Matos et al., 2017).
We show that IL-1β stimulates epidermal keratinocytes to secrete chemokines. Of interest, chemokine CCL20 is induced upon IL-1β stimulation. This is in both mouse and human primary keratinocytes. Notably, CCL20 is significantly increased in lesional psoriatic skin (Homey et al., 2000, Liu et al., 2010). CCR6 is expressed on mouse dermal γδT17 cells (Cai et al., 2011, Cai et al., 2014) and human Vδ1 and Vδ2 γδT17 cells as well as circulating Vδ2 γδT17 cells with a skin-homing CLA+ phenotype (Caccamo et al., 2011, Laggner et al., 2011, Wu et al., 2014). We observe that supernatants from IL-1β-stimulated keratinocytes preferentially chemoattract CD27− IL-17 capable of producing γδ T cells. These findings suggest that IL-1β may induce an amplified inflammatory cascade through recruiting γδT17 from the periphery.
In summary, we demonstrate that IL-1β-IL-1R signaling is essential in regulating skin immunosurveillance and inflammation. Endogenous low level of IL-1β stimulated by skin commensals maintains γδT17 homeostasis in mouse skin while microbiota dysbiosis upregulates IL-1β leading to expanded γδT17 cells which may prime skin for the induction of inflammation. IL-1β can directly activate γδT17 cells in mice and also stimulate keratinocytes for chemokine secretion which chemoattracts more IL-17 capable producing cells from the periphery, thus establishing an amplified inflammatory response, leading to skin inflammation such as psoriasis pathogenesis.
Material and Methods
Mice.
C57BL/6 WT, TCRδ−/−, and Illr1−/− mice were purchased from the Jackson Laboratory. GF mice were kindly provided by Dr. Yang-xin Fu (University of Chicago & UT Southwestern). All animals were housed and treated in accordance with institutional guidelines and approved by the IACUC at the University of Louisville.
Human Subjects:
See the supplemental material and methods.
Skin tissue preparation and cell stimulation.
Whole skin cells from mouse back skin were prepared as previously described (Cai et al., 2011). The detailed methods are described in the supplemental material and methods.
Flow cytometry analysis and intracellular cytokine staining.
See the supplemental material and methods.
T cell chemotaxis assay.
See the supplemental material and methods.
Generation of neonatal thymocytes/bone marrow chimeric mice.
The neonatal thymocytes/bone marrow chimeras were generated as previously reported (Cai et al., 2014). Briefly, recipient mice were lethally irradiated with 950 cGy and then were intravenously transferred with 5-10×106 thymocytes from neonatal mice 0–48 hours after birth. After 24 hours, the recipient mice received 5–10×106 bone marrow (BM) cells. All chimeric mice were allowed to reconstitute for at least 8 weeks before use in experiments.
Establishment of psoriasis-like mouse models.
Imiquimod-induced psoriasis-like mouse model was established as previously described (Cai et al., 2014). The detailed methods are described in the supplemental material and methods.
Topical association of bacteria.
Corynebacterium pseudodiphtheriticum (ATCC 10700) were cultured for 18 hours in brain heart infusion broth at 37°C. Bacteria were enumerated by assessing colony-forming units using traditional bacteriology techniques and by measuring optical density (OD) at 600nm using a spectrophotometer.
For topical association of bacteria, each mouse was associated with bacteria by applying bacterial suspension (approximately 1×109 c.f.u./ml, 0.5ml for two ears). Application of bacterial suspension was repeated every other day a total of four times. Mice were sacrificed at two weeks.
Skin histology, IHC staining, and Immunofluorescence staining.
See the supplemental material and methods.
Measurement of mouse IL-1β, mCCL-20, and hCCL-20 by ELISA.
See the supplemental material and methods.
Statistical analysis.
All quantitative data are shown as mean± s.e.m unless otherwise indicated. All samples were compared using two-tailed, unpaired Student’s T test. A P value less than 0.05 was considered significant. Statistical analysis was performed with GraphPad Prism software.
Supplementary Material
Acknowledgements:
This work was supported by the NIH R01AI128818 and the National Psoriasis Foundation (J.Y.) and by the NSFC 81761128008 and 91442123 (J.Z.). Bioinformatics support for this work was provided by the NIH grant P20GM103436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: YZ declared a conflict of interest and all other authors declared no conflict of interests in this study.
References:
- Alekseyenko AV, Perez-Perez GI, De Souza A, Strober B, Gao Z, Bihan M, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013;1(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood 2011;118(1): 129–38. [DOI] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal Role of Dermal IL-17-Producing gammadelta T Cells in Skin Inflammation. Immunity 2011;35(4):596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, et al. Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nature communications 2014;5:3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KA, Suh JW, Lee KH, Kang JL, Woo SY. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1beta by keratinocytes via the ROS-NLRP3-caspase-1 pathway. International immunology 2012;24(3):147–58. [DOI] [PubMed] [Google Scholar]
- Cooper KD, Hammerberg C, Baadsgaard O, Elder JT, Chan LS, Taylor RS, et al. Interleukin-1 in human skin: dysregulation in psoriasis. J Invest Dermatol 1990;95(5):24S–6S. [DOI] [PubMed] [Google Scholar]
- Debets R, Hegmans JP, Croughs P, Troost RJ, Prins JB, Benner R, et al. The IL-1 system in psoriatic skin: IL-1 antagonist sphere of influence in lesional psoriatic epidermis. J Immunol 1997;158(6):2955–63. [PubMed] [Google Scholar]
- Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Science translational medicine 2011;3(82):82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PloS one 2008;3(7):e2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 2010;467(7318):967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Gudjonsson JE, Ward NL. The Snowballing Literature on Imiquimod-Induced Skin Inflammation in Mice: A Critical Appraisal. J Invest Dermatol 2017;137(3):546–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert HL, Bowes J, Smith RL, McHugh NJ, Barker JN, Griffiths CE, et al. Polymorphisms in IL-1B distinguish between psoriasis of early and late onset. J Invest Dermatol 2014;134(5):1459–62. [DOI] [PubMed] [Google Scholar]
- Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol 2000;164(12):6621–32. [DOI] [PubMed] [Google Scholar]
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs 2010;11(11):1211–20. [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol 2017;140(1):109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper TS, Lee F, Birchall N, Clark S, Dower S. Interleukin 1 binds to specific receptors on human keratinocytes and induces granulocyte macrophage colony-stimulating factor mRNA and protein. A potential autocrine role for interleukin 1 in epidermis. J Clin Invest 1988;82(5):1787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol 2011;187(5):2783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lagowski JP, Gao S, Raymond JH, White CR, Kulesz-Martin MF. Regulation of the psoriatic chemokine CCL20 by E3 ligases Trim32 and Piasy in keratinocytes. J Invest Dermatol 2010;130(5):1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev 2011. ;22(4): 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annual review of immunology 2014;32:227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos TR, O’Malley JT, Lowry EL, Hamm D, Kirsch IR, Robins HS, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing alphabeta T cell clones. J Clin Invest 2017;127(11):4031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H, Black R, Kupper TS. Human keratinocytes produce but do not process prointerleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Invest 1991;87(3):1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science 2012;337(6098): 1115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest 2012;122(6):2252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014;70(3):512–6. [DOI] [PubMed] [Google Scholar]
- Renne J, Schafer V, Werfel T, Wittmann M. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. Br J Dermatol 2010;162(6): 1198–205. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 2009;10(4):427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skendros P, Papagoras C, Lefaki I, Giatromanolaki A, Kotsianidis I, Speletas M, et al. Successful response in a case of severe pustular psoriasis after interleukin-1beta inhibition. Br J Dermatol 2017;176(1):212–5. [DOI] [PubMed] [Google Scholar]
- St Leger AJ, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P, et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal gammadelta T Cells. Immunity 2017;47(1):148–58 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31(2):331–41. [DOI] [PubMed] [Google Scholar]
- Tamilselvi E, Haripriya D, Hemamalini M, Pushpa G, Swapna S. Association of disease severity with IL-1 levels in methotrexate-treated psoriasis patients. Scand J Immunol 2013;78(6):545–53. [DOI] [PubMed] [Google Scholar]
- Tian T, Jin MQ, Dubin K, King SL, Hoetzenecker W, Murphy GF, et al. IL-1R Type 1-Deficient Mice Demonstrate an Impaired Host Immune Response against Cutaneous Vaccinia Virus Infection. J Immunol 2017; 198(11):4341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Herranz M, Lian LH, Hooper KM, Milora KA, Jensen LE. IL-1R1 signaling facilitates Munro’s microabscess formation in psoriasiform imiquimod-induced skin inflammation. J Invest Dermatol 2013;133(6):1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014;40(5):785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Banno T, Walsh R, Blumenberg M. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin-1. J Cell Physiol 2008;214(1):1–13. [DOI] [PubMed] [Google Scholar]
- Zuo X, Sun L, Yin X, Gao J, Sheng Y, Xu J, et al. Whole-exome SNP array identifies 15 new susceptibility loci for psoriasis. Nature communications 2015;6:6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


