Abstract
CD8+ tissue-resident memory T cells (TRM cells) in two mucosal tissues, the skin and the female reproductive tract, proliferate in situ to generate a secondary pool of TRM cells that does not exit into the circulation.
An effective memory T cell response is based on tiered layers of defense that often begin with locally poised CD8+ TRM cells. In this issue of Nature Immunology, Beura et al.1 and Park et al.2 describe the early and late effects of secondary infection on pre-existing TRM cell pools in two mucosal tissues, the skin and the female reproductive tract (FRT), and show that CD8+ TRM cells proliferate in situ to generate a secondary pool of TRM cells that does not exit into the circulation. Although CD8+ memory T cells recruited from the circulation (TCIRC cells) may also take up residence in the reinfected tissue, they do not outcompete the pre-existing TRM cell population1,2.
CD8+ memory T cells are a heterogeneous population of cells that can be conceptually segregated on the basis of their functional and spatial properties. Three memory T cell populations have been shown to circulate between the blood and organs: central memory T cells (TCM cells), characterized by their high expression of lymph node (LN)-homing receptors, regenerative potential and interleukin 2 (IL-2) production; effector memory T cells (TEM cells), distinguished by low expression of LN-homing receptors and high expression of CX3CR1, and which predominantly survey the blood and exert immediate cytotoxic functions; and peripheral memory T cells (TPM cells), which are CX3CR1int and preferentially patrol peripheral tissues via a distinct pattern of migration from blood to tissue to lymph. A fourth population of CD8+ memory T cells, the TRM cells, derived from CX3CR1lo/int and KLRG1lo effector T cells and commonly distinguished by their increased expression of CD69 and CD103, do not recirculate in the host, but rather dwell long-term within tissues to provide rapid local protection. Such spatial compartmentalization of memory T cells provides a shield of protective immunity acting both outside-in and inside-out.
Poised at the front lines of defense, often within epithelial barriers, CD8+ TRM cells serve as motile sentinels that patrol the tissue microenvironment for reinfection3 (Fig. 1). Intravital imaging by Park et al.2 and Beura et al.1 revealed different rates of motility of TRM cells in the skin and FRT, with mean velocities of 2 μm min–1 and 10 μm min–1, respectively. Notably, in the FRT, CD8+ T cell velocity is slower down in the collagen-rich perimetrium than in the myometrium1, possibly because of the unique tissue architectures and densities of the two regions. This is akin in some ways to the situation in the skin, where CD8+ T cell velocities are significantly faster in the dermis than in the epidermis3. Given the seemingly random motion of these motile CD8+ TRM cells, it will be informative to learn whether they preferentially interact with cells that express survival-cue proteins such as IL-15 and TGF-β.
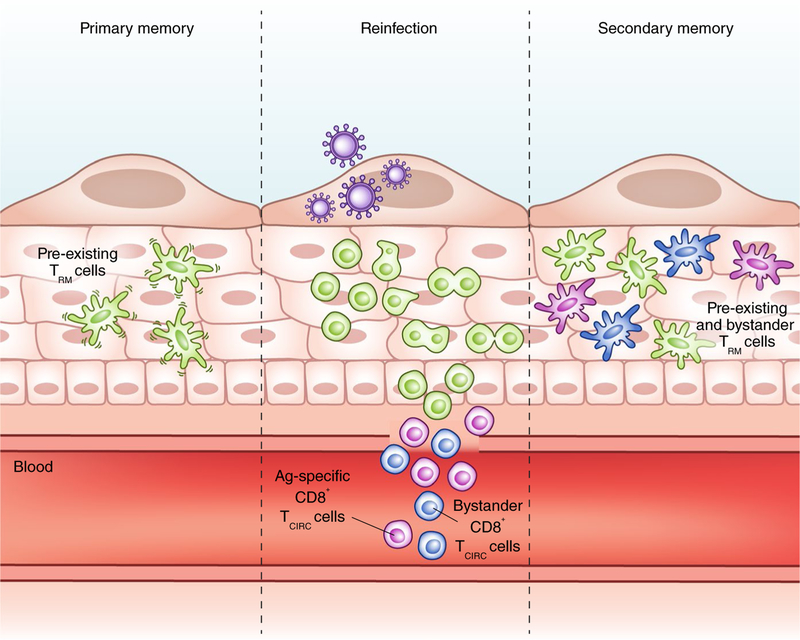
Fig. 1 |. Effects of secondary infection on a pre-existing TRM cell population.
CD8+ TRM cells (green) are motile sentinels that patrol and survey their tissue microenvironments for reinfection. When they recognize their target antigen (Ag) during reinfection, CD8+ TRM cells arrest and undergo in situ proliferation to generate a secondary pool of TRM cells that does not exit into the circulation. Antigen-specific (purple) and bystander (blue) CD8+ TCIRC cells are also recruited into the infected tissue and adopt long-term tissue residency without displacing the pre-existing TRM cells, thereby increasing the number and repertoire of TRM cells in the tissue. Credit: Marina Spence/Springer Nature
A body of literature has informed on how TCM cells and TEM cells respond during secondary infection, but less is known about how TRM cells respond in situ. The prevailing idea in the field was that TRM cells had little self-renewal potential at steady state, and that upon reinfection TCM cells dominated the recall response owing to their increased proliferative capacity. By elegantly delineating the responses of TCIRC cells and TRM cells, Park et al.2 and Beura et al.1 demonstrate that pre-existing TRM cells undergo local proliferation in situ during reinfection. Whether there is a distinct subpopulation of TRM cells with regenerative capacity remains to be explored. Importantly, the secondary pool of TRM cells after reinfection is generated predominantly from the pre-existing, proliferating TRM cells, rather than from the circulating memory T cells recruited into the inflamed tissue. One notable finding by Park et al.2 is that homologous reinfection did not significantly boost the number of pre-existing TRM cells, which suggests a numerically stable pool of TRM cells that is robustly maintained, at least in the skin. Although there is evidence that CD103+ gut TRM cells can circulate and acquire properties of splenic memory when adoptively transferred and restimulated4, the present data suggest that when reactivated in situ, TRM cells maintain their cell fate identity and do not re-enter the circulation. Perhaps this occurs because the TRM cells further upregulate CD69 after reactivation, thereby preventing S1P-mediated egress into the blood or lymph. As prior work has demonstrated that secondary or tertiary ‘experienced’ TCIRC cells can become qualitatively different with each successive round of infection5,6, it will be interesting to further investigate the quality, longevity and function of these secondary TRM cells compared with those of primary TRM cells.
Comprehensive studies are beginning to discern the tissue-specific requirements for TRM cell formation and maintenance. For instance, TRM cells in the skin, salivary gland and kidney require IL-15 for their homeostatic maintenance, whereas those in the FRT or small intestine do not7,8. Additionally, TRM cells in the lung and brain require cognate antigen for their establishment9,10, unlike TRM cells in skin, FRT and nasal tissue, where topical application of the skin irritant DNFB2 and prime-and-pull strategies11 are sufficient to stimulate circulating CD8+ T cells to adopt TRM-cell-like phenotypes. Although Park et al. and Beura et al. both demonstrate that bystander CD8+ cells recruited during reinfection adopt tissue residency, the acquisition of CD103 expression on the infiltrating bystander cells occurred only in the skin1,2. Perhaps such tissue-specific effects stem from differences in the bioavailability of TGF-β, a hallmark cytokine for the induction of CD103 expression8,12.
The mucosal tissues are constantly exposed to different environmental challenges. Secondary heterologous infection in the lung can drive the migration of pre-existing CD8+ memory T cells into the bronchoalveolar spaces13. Whether these recruited memory T cells belong to lung TRM cells or TCIRC cells remains to be investigated, but this observation raises the question of whether successive waves of heterologous infection and inflammation can cause the pre-existing pool of TRM cells to shrink, expand or change qualitatively. In a series of heterologous rechallenges via varying infection routes, Park et al. observed that despite the influx and seeding of new TRM cells, the number of pre-existing TRM cells remained mostly unaltered2, which indicates that TRM cells persist long-term and that the entire TRM cell pool is expandable within tissues. This is consistent with previous observations that the memory TCIRC cell population is a flexible compartment that can accommodate increased numbers of CD8+ memory T cells after heterologous prime-boost vaccinations14. Together, these results imply that the encounter of various pathogens throughout life contributes to the development of a diverse repository of TRM cells, a goal that should be achieved by future vaccination strategies. ❐
Footnotes
Competing interests
The authors declare no competing financial interests.
References
- 1.Beura LK et al. Nat. Immunol 10.1038/s41590-017-0029-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SL et al. Nat. Immunol 10.1038/s41590-017-0027-5 (2018). [DOI] [Google Scholar]
- 3.Gebhardt T et al. Nature 477, 216–219 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Masopust D, Vezys V, Wherry EJ, Barber DL & Ahmed RJ Immunol 176, 2079–2083 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Nolz JC & Harty JT Immunity 34, 781–793 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser KA, Schenkel JM, Jameson SC, Vezys V & Masopust D Immunity 39, 171–183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenkel JM et al. J. Immunol 196, 3920–3926 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay LK et al. Nat. Immunol 14, 1294–1301 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Takamura S et al. J. Exp. Med 213, 3057–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakim LM, Woodward-Davis A & Bevan MJ Proc. Natl. Acad. Sci. USA 107, 17872–17879 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin H & Iwasaki A Nature 491, 463–467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammed J et al. Nat. Immunol 17, 414–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ely KH et al. J. Immunol 170, 1423–1429 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Vezys V et al. Nature 457, 196–199 (2009). [DOI] [PubMed] [Google Scholar]