Abstract
In the United States, end-stage renal disease patients receiving hemodialysis have an exceedingly high risk of sudden cardiac death (SCD), accounting for 29% of death events, likely relating to their uremic milieu, recurring exposure to fluid and electrolyte fluxes, and underlying cardiovascular pathology. Furthermore, epidemiologic studies have shown that SCD events, as well as mortality and hospitalizations, occur most frequently on the first dialysis day following the long interdialytic gap, suggesting that abrupt fluctuations in the accumulation and removal of electrolytes, fluid, and uremic toxins over the dialysis cycle may be contributory. Some population based observational studies have suggested that lower dialysate potassium concentrations appear associated with heightened risk of post-dialysis cardiac arrest in hemodialysis patients, although the optimal serum-to-dialysate potassium gradient remains unclear. Some observational studies suggests that low dialysate calcium concentrations and high serum-to-dialysate calcium gradients may predispose to SCD. There is ongoing controversy about an association betweenhigher dialysate bicarbonate concentrations and higher risk of cardiac arrest, which is likely due to confounding by indication. Some observational studies have also shown that large interdialytic weight gains, fluid retention, and high ultrafiltration (UFR) rates are linked with higher risk of SCD and mortality. However, there remains considerable controversy regarding the pros and cons of designating a specific upper UFR limit with extended treatment times as a clinical practice measure, and further studies are needed to define the optimal tools, metrics, targets, and implementation measures for volume control in the hemodialysis population. In this Review, we highlight the epidemiology and pathophysiology of how specific aspects of the hemodialysis procedure may relate to the risk of SCD, as well as preventative strategies and future research directions that can address this risk.
Keywords: Sudden cardiac death, hemodialysis, volume, electrolytes
Introduction
In the United States (US), there are currently more than 550,000 patients with end-stage renal disease (ESRD) receiving maintenance dialysis, among whom there is an exceedingly low five-year survival (42%) and heightened risk of sudden cardiac death (SCD).1 The US Renal Data System (USRDS) analyses suggest that as many as 29% of deaths among dialysis patients may be attributed to arrhythmias and cardiac arrest.1 Rigorously-adjudicated data from some hemodialysis trials (e.g., Hemodialysis [HEMO] Study,2 Die Deutsche Diabetes Dialyse Studie,3 Evaluation of Cinaclcet HCl Therapy to Lower Cardiovascular Events [EVOLVE] Study4) also show that ~22 to 26% of deaths are due to SCD.5,6 It is estimated that the rate of SCD is 49.2 events per 1000 person-years, which is more than 25-fold greater that of the general population.7 Furthermore, international data from an observational study center known as the Dialysis Outcomes and Practice Patterns (DOPPS) registry has shown that in the United States, SCD may account for greater proportion of deaths in which the cause is known (33.4%) vs. other participating countries (i.e., lowest prevalence observed in Sweden at 6.8%).8
ESRD patients receiving hemodialysis may be more uniquely predisposed to SCD owing to their uremic milieu, recurring exposure to fluid and electrolyte fluxes, and pre-existing cardiovascular pathology (e.g., structural heart disease, vascular calcification).6,9–11 Indeed, observational studies suggest that hemodialysis-related factors may have a greater bearing upon their risk of cardiac arrest than underlying cardiovascular comorbidities.12 Hence, it is possible that cautious attention to and adjustment of the dialysis prescription may provide opportunity to attenuate the enormous cardiovascular morbidity and mortality experienced by ESRD patients. In this Review, we examine the epidemiology and pathophysiology of how specific aspects of the hemodialysis procedure relate to the risk of SCD, cardiovascular morbidity, and mortality, as well as preventative strategies and future research directions that can address this risk.
Hemodialysis Schedule and the Long Interdialytic Interval
Among ESRD patients without substantial residual kidney function (i.e., renal urea clearance ≤3 ml/min/1.73m2), hemodialysis is typically prescribed as a thrice-weekly regimen with two one-day and two one-day interdialytic intervals between treatment sessions.9,10 A number of epidemiologic studies have demonstrated that SCD events, as well as mortality and hospitalizations, occur more frequently on the first dialysis day following the long interdialytic gap but likely after the hemodialysis session and less likely before or during the hemodialysis (Table 1).9,10,13 In a study that examined 375,482 death events among US dialysis patients over the period of 1977 to 1997, Bleyer et al. reported that SCD and cardiac death events most commonly occurred on Mondays and Tuesdays among hemodialysis patients; in contrast, an even distribution of events was observed across all weekdays among peritoneal dialysis patients.14 A subsequent study of over 77,000 US hemodialysis patients from a national large dialysis organization (LDO) similarly observed that in-center cardiac arrest events most frequently occurred on Mondays vs. other days of the week among patients on a Monday-Wednesday-Friday (MWF) treatment schedule; however, there did not appear to be a day-of-the week association with mortality among patients on a Tuesday-Thursday-Saturday (TTS) treatment schedule, possibly due to receipt of dialysis after the first weekend night (i.e., Friday) mitigating fluid and electrolyte accumulation over the long interval.15 It was also found that incenter cardiac arrests occurred throughout the peridialytic interval (i.e., 7%, 81%, and 12% events occurred immediately preceding, during, and following treatments prior to leaving dialysis unit). Further advancing our understanding of the time course of SCD was an analysis of 80 US hemodialysis patients by Bleyer et al., which showed that events were somewhat more frequent in the last 12 hours of long interdialytic gap prior to dialysis and in particular during the first 12 hours immediately after dialysis (i.e., bimodal death distribution).16 Large population-based studies using data from the ESRD Clinical Performance Measures Project,17 international DOPPS cohort,18 and United Kingdom Renal Registry19 have corroborated that hemodialysis patients have a heightened risk of mortality and hospitalization as well as a trend towards higher risk of SCD following the Mondays and Tuesdays after the long interdialytic gap. Most recently, among patients who underwent implantable loop recorders for continuous cardiac rhythm monitoring in the prospective Monitoring in Dialysis study, the rate of clinically significant arrhythmias (defined as those likely to be associated with sudden death, i.e., ventricular tachycardia with rate ≥130 beats per minute [BPM], bradycardia with rate ≤40 BPM for at least six seconds, asystole for at least three seconds, symptomatic events with electrocardiography-confirmed clinically relevant arrhythmia) was higher during the first weekly dialysis session than during the final 12 hours of the long interdialytic gap.5,20
Table 1.
Selected studies of the day-of-week dialysis schedule and outcomes in hemodialysis patients.
| Author (Year) | Study Population (N) | Outcome | Findings |
|---|---|---|---|
| Bleyer et al. (1999)14 | HD and PD patients from USRDS. (N=375,482 death events; US) | Sudden death, cardiac death. | • Sudden and cardiac deaths most common on Mondays and Tuesdays in HD patients. • Higher rate of deaths on Mondays among patients on MWF schedule vs. Tuesdays among patients on TTS schedule. • Even distribution of events across weekdays amongst PD patients. |
| Karnik et al. (2001)15 | HD patients from national LDO. (N >77,000; US) | In-center cardiac arrest. | • Cardiac arrest most frequent on Monday vs. other days of week (among patients on MWF schedule). • No day of week effect observed among patients on TTS schedule. • 7%, 81%, and 12% events occurred immediately preceding, during, and following treatments prior to leaving dialysis unit. |
| Bleyer et al. (2006)16 | HD patients from five centers in SE US. (N=80 patients with sudden cardiac death; US) |
Sudden cardiac death. | • Death events more common in the last 12 hours of long interdialytic gap prior to dialysis and during the first 12 hours immediately after dialysis (bimodal death distribution). |
| Foley et al. (2011)17 | HD patients from the ESRD Clinical Performance Measures Project. (N= 32,065; US) |
Mortality due to all-cause, CV, infection, CV arrest, and MI. Hospitalization due to MI, CHF, CVA, dysrhythmia, and any CV event. |
• Higher mortality and hospitalization (all causes) the day after the long interdialytic gap. • Subgroup of patients with less than 1 year of prior dialysis treatment, unlike the overall study population, did not have higher-than expected mortality after the long interdialytic interval. |
| Zhang et al. (2012)18 | HD patients from DOPPS cohort. (N=22,163; US, Japan, Europe) | Mortality. | • Higher mortality after the long interdialytic gap among US, Japanese, and European patients. • Highest mortality seen on last HD session of the week: ▪ Japanese patients on MWF schedule had highest risk of non-CV mortality on Friday. ▪ European patients on TTS schedule had highest risk of CV mortality on Saturday. |
| Fotheringham et al. (2015)19 |
HD patients from UK Renal Registry. (N=5864; UK) |
Mortality, hospitalizations. | • Higher hospitalization rates the day after the long interdialytic gap across all treatment schedules (MWF, TTS, TTSun). • Higher mortality rates that day after the long interdialytic gap across all treatment schedules, largely due to out-of-hospital deaths. |
| Roy- Chaudhury et al. (2018)5,20 |
HD patients from the Monitoring in Dialysis study. (N=66; US, India) |
Clinically significant arrhythmias. | • Highest rates of clinically significant arrhythmias during the first dialysis session of the week and the last 12 hours of the long interdialytic interval. |
Abbreviations: HD, hemodialysis; PD, peritoneal dialysis; USRDS, United States Renal Data System; US, United States; MWF, Monday-Wednesday-Friday; TTS, Tuesday-Thursday-Saturday; PD, peritoneal dialysis; LDO, large dialysis organization; SE, southeast; ESRD, end-stage renal disease; CV, cardiovascular; MI, myocardial infarction; CHF, congestive heart failure; CVA, cerebrovascular accident; DOPPS, Dialysis Outcomes Practice Patterns; UK, United Kingdom; TTSun, Tuesday-Thursday-Sunday.
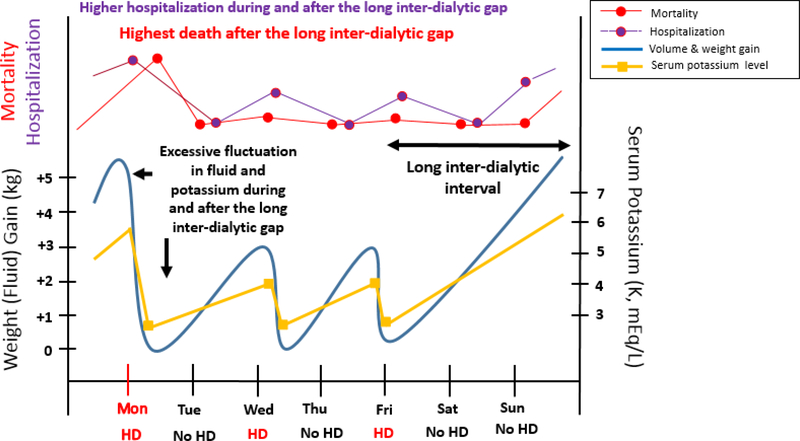
Multiple coincident factors contributing to the precarious peridialytic period (i.e., before, during, and after the interdialytic gap) are hypothesized to intensify risk of SCD, including: 1) abrupt fluctuations in electrolyte (e.g., potassium, calcium, magnesium) accumulation and removal over the dialysis cycle, 2) excessive sodium, fluid, and interdialytic weight gains (IDWGs), resulting in ventricular remodeling and high rates of ultrafiltration, leading to intradialytic hypotension, myocardial stunning, ischemia of other end-organs, and presyncope/syncope, and 3) accrual of uremic toxins that promote inflammation, oxidative stress, endothelial dysfunction, and downstream atherosclerosis and cardiovascular disease (Figure 1).9,10 In the remainder of the Review, we discuss how specific aspects of the hemodialysis procedure may contribute to the heightened morbidity and mortality of the long interdialytic interval, as well as modifications of the dialysis prescription that may mitigate this risk.
Figure 1. Fluid and electrolyte fluctuations in a hypothetical patient receiving thrice-weekly hemodialysis treatment on Monday, Wednesday, and Friday.
From Rhee CM and Kalantar-Zadeh K, Kidney International 2015; 88(3): 442–444. Reproduced with permission.10
Potassium Accumulation and Removal and the Dialysate Potassium Concentration
The accrual of excess potassium over the long interdialytic interval results in hyperkalemia, and has been associated with malignant ventricular or atrial arrhythmias, and SCD among hemodialysis patients.7,9,21–23 Conversely, potassium removal and large potassium fluxes ensuing from the hemodialysis procedure may also theoretically lead to arrhythmias. USRDS data collected from US hemodialysis patients over 2007 to 2010 has shown that the prevalence of hyperkalemia (defined as serum potassium ≥5.5 meq/L) is 2.0- to 2.4-fold higher on the day after the long (two-day) vs. short (one-day) interdialytic interval.24 However, international data from the DOPPS cohorts has shown that serum potassium levels measured prior to the first hemodialysis session of the week were modestly higher than mid-week levels across 20 participating countries (ranging from a Δ of 0.01 meq/L in China to 0.19 meq/L in Germany).25 While some experts suggest that hemodialysis patients may have greater “tolerance” of hyperkalemia, multiple observational studies show that modestly elevated (≥5.6 meq/L)24–26 as well as lower levels of serum potassium within the reference range (<4.0 meq/L)26 are associated with higher death risk, presumably due to arrhythmogenic pathways. In the largest study of US hemodialysis patients conducted to date by Kovesdy et al., pre-dialysis serum potassium levels of 4.6 to 5.3 meq/L were associated with the greatest survival.26
Selecting the appropriate dialysate potassium concentration is a mainstay of maintaining hemodialysis patients within this precise serum potassium range. However, there remains uncertainty regarding the optimal dialysate potassium concentration, particularly amongst hyperkalemic patients. As a result, there are large variations in the prescription of dialysate potassium concentrations worldwide. For example, DOPPS data has shown that in Spain the prevalence of low dialysate potassium concentration (<2 meq/L) administration is quite high (62%), whereas utilization in the US is markedly low (3%).25
Through the years, a large body of evidence has shown that use of low dialysate potassium concentrations (<2 meq/L) are linked with higher risk of SCD (Table 2). In a case-cohort study of US hemodialysis patients, Karnik et al. first showed that those who experienced in-center cardiac arrest were two times more likely to have been dialyzed against a dialysate potassium concentration of 0 or 1.0 meq/L on the day of arrest; notably, pre-dialysis serum potassium levels were lower among cases vs. controls (4.78 vs. 4.93 meq/L, respectively).15 A subsequent study of 80 cases of SCD by Bleyer et al. showed that, while ~25% and ~50% of patients had pre-dialysis serum potassium levels of <4.0 meq/L and 4.0-<5.0 meq/L, respectively, all patients were prescribed a dialysate potassium concentration of 2.0 meq/L, also signaling potential inattention and/or infrequent adjustment of the dialysate potassium prescriptions.16 More recently, in a rigorous study of 502 SCD cases matched to 1632 hemodialysis controls by Pun et al., receipt of a dialysate potassium concentration of <2.0 meq/L was associated with a two-fold higher risk of SCD compared to a concentration of ≥2.0 meq/L; however, among patients whose pre-dialysis serum potassium was ≥6.5 meq/L, dialysate potassium concentrations <2.0 meq/L trended towards lower risk of SCD.12 A subsequent study of 37,765 hemodialysis patients across 12 countries from the DOPPS cohort indicated that dialysate potassium concentrations of ≤1.5 meq/L and 2–2.5 meq/L were associated with higher SCD risk compared to concentrations ≥3.0 meq/L,8 while a case-control analysis of 924 cases and 75,538 control patients from a US national LDO found that dialysate potassium concentrations of 1.0 meq/L were linked with higher risk of peridialytic cardiopulmonary arrest.27
Table 2.
Selected studies of dialysate prescription characteristics and outcomes in hemodialysis patients.
| Author (Year) | Study Population (N) | Exposure | Findings |
|---|---|---|---|
| Dialysate Potassium Concentration | |||
| Karnik et al. (2001)15 | HD patients from national LDO. (N >77,000; US) | Dialysate K 0, 1, 2, 2.5, 3, and 4 meq/L. | • Dialysate K of 0 or 1 meq/L higher risk of cardiac arrest. |
| Bleyer et al. (2006)16 | HD patients from five centers in SE US. (N=80 patients with sudden cardiac death; US) |
N/A. | • All patients treated with dialysate K of 2 meq/L. |
| Kovesdy et al. (2007)26 | HD patients from national LDO. (N=81,013; US) | Dialysate K ≤1, 2, 3, and >3 meq/L. | • Dialysate ≥3 meq/L higher risk of death among patients with serum K >5.0 meq/L. |
| Pun et al. (2011)12 | HD patients from national LDO. (N=2132; US) | Dialysate K ≤1, 2, 3, and 4 meq/L. | • Dialysate K <2 meq/L higher risk SCD. |
| Jadoul et al. (2012)8 | HD patients from DOPPS Phases 1- 3. (N=37,765; 12 countries) |
Dialysate K ≤1.5, 2–2.5, and ≥3 meq/L. | • Dialysate K <3 meq/L associated with higher risk SCD (using patient-level approach). |
| Flythe et al. (2014)27 | HD patients from national LDO. (N=76.462; US) | Dialysate K 1, 2, and 3 meq/L. | • Dialysate K of 1 meq/L associated with higher peridialytic cardipulmonary arrest. |
| Karaboyas et al. (2017)25 |
HD patients from DOPPS Phases 1- 5. (N=55,183; 20 countries) |
Dialysate K 2 vs. 3 meq/L. | • No difference in all-cause mortality or arrhythmia composite outcome (arrhythmia related hospitalization or sudden death). |
| Brunelli et al. (2017)22 | Medicare Parts A/B enrollees from national LDO. (N=62,388; US) | Dialysate K ranging 1 to <4 meq/L; Serum-to-dialysate gradient ranging <0 to ≥5 meq/L. | • Incremental association between higher serum-to-dialysate gradient ≥3 meq/L associated with higher risk of hospitalizations and ED visits. • Most patients prescribed dialysate K 2-<4 meq/L; none prescribed dialysate K <1 meq/L. |
| Ferrey et al. (2017)28 | HD patients from 16 centers in Southern CA. (N=624; US) |
Dialysate K ≤1, 2, and 3 meq/L. | • Dialysate K of 1 meq/L associated with higher death in patients with serum K ≥5.0 meq/L. |
| Dialysate Bicarbonate or “Total Buffer” Concentration | |||
| Tentori et al. (2013)38 | HD patients from DOPPS Phases 2- 4. (N=17,031; 11 countries) |
Dialysate bicarbonate (Δ4 meq/L increase). | • Higher dialysate bicarbonate associated with higher all-cause and infection-related mortality, as well as all-cause and CV hospitalization, likely confounding by indication. • Trend towards association between higher dialysate bicarbonate and SCD, CV death, arrhythmia-related hospitalization, and infection-related hospitalization. • Trend towards association between higher dialysate bicarbonate and intradialytic hypotension and IDWG. |
| Flythe et al. (2014)27 | HD patients from national LDO. (N=76,462; US) | Dialysate buffer (bicarbonate + acetate) of <41, 41–45 (ref), vs. >45meq/L | • No association of “total buffer” with peridialytic cardiopulmonary arrest. |
| Dialysate Calcium Concentration | |||
| Pun et al. (2011)12 | HD patients from national LDO. (N=2132; US) | Dialysate Ca 1.0, 2.0, 2.5, 3.0, and 3.5 meq/L. | • Dialysate Ca <2.5 meq/L higher risk SCD. |
| Pun et al. (2013)41 | HD patients from national LDO. (N=2070; US) | Dialysate Ca <2.5, 2.5, >2.5meq/L; Serum-to-dialysate Ca gradient (Δ1 meq/L). |
• Dialysate Ca <2.5 meq/L higher risk SCD. • Increasing serum-to-dialysate Ca gradient associated with higher risk of SCD. |
| Flythe et al. (2014)27 | HD patients from national LDO. (N=76,462; US) | Dialysate Ca <2.3, 2.3–2.5, and >2.5 meq/L. | • No association with peridialytic cardiopulmonary arrest. |
| Brunelli et al. (2015)42 | Medicare-eligible HD patients from national LDO. (N=79 converter facilities & N=control facilities; US) |
Facility conversion from predominant use (≥75% patients) of 2.50 meq/L dialysate Ca to predominant use of lower dialysate Ca (<2.5 meq/L) vs. maintenance of predominant use of 2.50 meq/L dialysate calcium. |
• Conversion to lower dialysate Ca <2.5 meq/L associated with increased and intradialytic hypotension, hypocalcemia, and CHF-related hospitalization. |
| Pun et al. (2016)43 | Secondary analysis HD patients from EVOLVE trial. (N=3883; international) | Dialysate Ca <2.5, 2.5, and ≥2.5 meq/L. | • No association between dialysate Ca nor serum-to-dialysate Ca gradient with primary composite endpoint, CV death, or SCD. |
| • No effect modification of cinacalcetoutcomes by dialysate Ca or serum-todialysate Ca gradient. | |||
| Roy- Chaudhury et al. (2018)5,20 |
HD patients from the Monitoring in Dialysis study. (N=66; US, India) |
Dialysate Ca >2.5 vs. 2.5 meq/L; Dialysate Ca ≤2 vs. 2.5 meq/L. | • Highest rates of clinically significant arrhythmias during the first dialysis session of the week and the last 12 hours of the long interdialytic interval. |
| Dialysate Magnesium Concentration | |||
| Lacson et al. (2015)46 |
HD patients from a national LDO. (N=23,574; US) | Dialysate Mg of 0.75, 0.75–0.99, 1.0, 1.01–1.49, and ≥1.50 mEq/L. | • No association with mortality. |
| Schmaderer et al. (2017)48 |
HD patients. (N=75; Germany) |
Dialysate Mg 0.75 mmol vs. 0.50 mmol (1.50 vs. 1.0 meq/L, respectively). | • High dialysate Mg associated with lower mortality risk. |
Abbreviations: HD, hemodialysis; LDO, large dialysis organization; US, United States; K, potassium; SE, southeast; SCD, sudden cardiac death; DOPPS, Dialysis Outcomes Practice Patterns; ED, emergency department; CA, California; CV, cardiovascular; IDWG, interdialytic weight gain; Ca, calcium; CHF, congestive heart failure; Mg, magnesium.
In recent years, greater attention has been placed on the prescription of the dialysate potassium concentration relative to the serum potassium concentration. For example, there is concern among some clinicians that rapid intradialytic changes in potassium due to a high serumto-dialysate potassium gradient (i.e., difference between serum and dialysate potassium concentrations) may result in cardiovascular instability and fatal arrhythmias. To date, studies of the serum-to-dialysate potassium gradient and mortality have shown mixed findings (Table 2). In the first study to examine the interaction between serum and dialysate potassium concentrations among US hemodialysis patients from a national LDO, Kovesdy et al. found that the highest three-year mortality rate was observed among the subgroup of patients with a high pre-dialysis serum potassium of >5.0 meq/L who were dialyzed against a high dialysate potassium of >3.0 meq/L.26 As noted above, Pun et al. found that, among patients with predialysis serum potassium levels <5.0 meq/L, low dialysate potassium concentrations of <2.0 vs. ≥2.0 meq/L were associated with incrementally higher SCD-risk with lower serum potassium levels, whereas among patients with higher pre-dialysis serum potassium levels >6.5 meq/L, there was a trend towards lower risk.12 It should be highlighted that among patients with higher serum potassium levels, low dialysate potassium concentrations did not per se show benefit (i.e., did not show lower mortality risk). Yet in a study of 55,183 DOPPS participants across 20 countries, dialysate potassium concentration was not associated with all-cause mortality or an arrhythmia composite outcome (arrhythmia related hospitalization or sudden death); however, it should be noted that the study cohort was restricted to patients receiving a narrow range of dialysate potassium concentrations (i.e., 2.0 vs. 3.0 meq/L only).25 Brunelli et al. subsequently examined 62,388 Medicare Part A and B enrollees from a national LDO and found an incremental association between higher serum-to-dialysate potassium gradients ≥3.0 meq/L (i.e., difference between serum and dialysate potassium concentration greater than or equal to 3.0 meq/L) with higher risk of hospitalizations and emergency department visits.22 Most recently, Ferrey et al. examined a prospective cohort of 624 hemodialysis patients across 16 outpatient dialysis units and similarly found that receipt of a low dialysate potassium concentration of 1.0 meq/L was associated with higher death risk in those with higher serum potassium levels (≥5.0 meq/L) but not in those with lower levels (<5.0 meq/L).28 One potential explanation for discrepant findings across studies may be that the serum-to- dialysate potassium gradient carries differential short-term vs. long-term risk. For example, it has been suggested that a large serumto-dialysate-potassium gradient may carry short-term risk (i.e., cardiac arrhythmias, rebound hypertension), whereas a small gradient among patients with higher serum potassium levels may bear long-term risk (i.e., inadequate potassium clearance leading to potassium overload and eventual death).22 Hence, rigorous prospective trials are needed to determine optimal the dialysate potassium concentration and serum-to-dialysate potassium gradient among hemodialysis patients.23
Dialysate potassium profiling in which the dialysate potassium concentration is altered over the course of a treatment session has been suggested as one method of avoiding suboptimal serum-to-dialysate potassium gradients.7,9,23 There has been one small study of 30 hemodialysis patients showing that dialysate potassium profiling (also known as potassium modeling) was associated with lower incidence of premature ventricular contractions during and after dialysis as compared with the use of fixed dialysate potassium concentrations.23,29 The advent of new oral potassium binding agents, sodium zirconium cyclosilate and patiromer,30,31 as well as a more balanced dietary potassium including high fiber diet and without strict restriction, may provide opportunity to avert the need for low dialysate potassium concentrations among patients with high pre-dialysis serum potassium levels.7,9,10,23 Moreover, while pre-dialysis serum potassium levels are typically measured on a monthly basis in outpatient hemodialysis units, more frequent routine measurement of serum potassium as well as quality of care protocols that support more timely adjustment/titration of the dialysate potassium concentration may attenuate risk of SCD.12,23
Acid-Base Status and the Dialysate Bicarbonate Concentration
Uncorrected acid-base derangements and in particular metabolic acidosis may have detrimental effects on the health and survival of hemodialysis patients. For example, acidemia may lead to 1) protein degradation and decreased albumin synthesis, culminating in protein-energy wasting, as well as 2) reduced bone density, osteopenia/osteoporosis, and subsequent fracture risk,11 both potent predictors of mortality in this population.32–34 It is less likely that alkalemia would lead to heightened morbidity and mortality although some authors have controversially claimed vis-à-vis 1) exacerbation of hypokalemia, 2) prolongation of the QT interval and arrhythmogenic risk, 3) cerebral vasoconstriction and decreased cerebral perfusion, and 4) respiratory suppression and hypoventilation.11 A large population-based study suggested that there was a U-shaped association between serum bicarbonate levels and mortality in hemodialysis patients but after multi-variate adjustment the consistent death predictability was with academic and not alkalemic range.35 In the study by Wu et al. of 56,835 hemodialysis patients, analyses that accounted for case-mix confounders showed that only low but not high pre-dialysis serum bicarbonate levels (serum bicarbonate levels <22 but not ≥26 meq/L, respectively) were consistently associated with higher death risk.35 Yet in an analysis of 15,132 hemodialysis patients from the Japanese Society of Dialysis Therapy Renal Registry, pre- and post-dialysis serum bicarbonate levels were not associated with mortality.36 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) clinical practice guidelines advise maintaining pre-dialysis serum bicarbonate levels >22 meq/L to avoid acidemia.11
In the 1950’s, bicarbonate was directly added as the principal source of alkali during hemodialysis treatments, but this approach was problematic due to requirement of frequent mixing and immediate use, bacterial contamination, and precipitation of calcium carbonate. This prompted its replacement with acetate-based solutions in the 1960’s (i.e., acetate converted into bicarbonate via the citric acid cycle).37 However, the use of acetate led to adverse events, including hemodynamic instability, nausea/vomiting, and headaches. This resulted in the reemergence of bicarbonate as the main alkali source of the dialysate, while acetate-based solutions serve as acid concentrate to attenuate the alkalotic effect of the bicarbonate solution. The latter is the foundation of the contemporary practice, in that outpatient dialysis units utilize a three-stream (acid-base-water) proportioning system, which includes administration of purified water, bicarbonate concentrate, and an acid concentrate (e.g., acetic acid, citric acid, sodium diacetate) that is kept separate from the bicarbonate concentrate and prevents a rapid rise in pH from the addition of bicarbonate. As such, these historical developments have led to the emergence of an unfortunate misunderstanding pertaining to the determinants of the pre-dialysis serum bicarbonate level and the choice of dialysate bicarbonate concentration, along with the wrong and misleading concept of “total buffer” about the contribution of dialysate acetate (i.e., converted into bicarbonate in the liver), whereas acetate in the acid concentrate should not be considered when prescribing dialysate bicarbonate concentrations.11
To date, there have been few studies that have examined dialysate bicarbonate concentrations with respect to SCD-risk, showing mixed findings and likely related to residual confounding (Table 2). In a study of 17,031 hemodialysis patients across 11 countries from the DOPPS cohort, there was a trend towards higher dialysate bicarbonate concentrations (Δ4 meq/L) and risk of SCD, arrhythmia-related hospitalizations, and cardiovascular deaths,38 which was likely related to confounding by indication in that higher risk patients are more likely to receive this prescription secondarily. Higher dialysate bicarbonate concentrations were not associated with higher mortality, but all-cause and cardiovascular hospitalizations appeared higher. There was also a trend towards an association between higher dialysate bicarbonate levels and intradialytic hypotension, with the latter as a risk factor for SCD events. However, a subsequent study of 76,462 US hemodialysis patients from an LDO which examined “total dialysate buffer” levels (bicarbonate + acetate) categorized as <41, 41–45 (reference) vs. >45 meq/L did not show associations with peridialytic cardiopulmonary arrest.27 While optimal dialysate bicarbonate prescribing practices require further study, we recommend titration of dialysate bicarbonate concentrations to a target of serum bicarbonate levels >24 mEq/L and avoidance of the “total buffer” calculation as wrong and misleading.
Dialysate Calcium Concentrations
Calcium balance in hemodialysis patients is largely dictated by dietary intake, medications (e.g., calcium-based phosphorus binders, vitamin D analogues, calcimimetics), and the dialysis procedure, including the serum-to-dialysate calcium gradient and clearance by diffusion and convection.11 Among hemodialysis patients without residual kidney function, the dialysis procedure is the primary means of calcium removal. Epidemiologic data show that both low and high serum calcium levels are associated with higher mortality risk in hemodialysis patients. In a study of 107,200 hemodialysis patients from a national LDO by Miller et al., both lower and higher pre-dialysis serum calcium levels (<9.0 and >10.0 mg/dl, respectively) were associated with higher mortality risk.39 Furthermore, it was also shown that patients who experienced a rise and decline in serum calcium over a six-month period had heightened mortality compared to those whose levels remained stable.
In order to maintain normal serum calcium levels, Kidney Disease Improving Global Outcomes guidelines recommend a dialysate calcium concentration of 2.5 to 3.0 meq/L (1.25 to 1.50 mmol/L), although based on 2D (i.e., weak) evidence.40 Over time, clinical practice guidelines have advised lowering dialysate calcium concentrations to achieve a neutral or negative calcium balance, given potential risk of vascular calcification, ectopic calcium deposition, and cardiovascular mortality.11,41 Conversely, there is also trepidation that excessive lowering of dialysate calcium concentrations may promote 1) decreased vascular smooth muscle cell and cardiac myocyte contractility, decreased system vascular resistance and cardiac output, and hypotension, as well as 2) QT-interval prolongation and SCD.41
These latter concerns are supported by several recent studies. In a case-control study of 2132 prevalent hemodialysis patients from a national LDO that examined a wide spectrum of dialysate calcium concentrations (ranging from 1.0 to 3.5 meq/L), Pun et al. found that dialysate calcium concentrations of <2.5 meq/L were associated with higher risk of SCD.12 In a subsequent case-control study of hemodialysis patients from a national LDO, Pun et al. again observed that dialysate calcium concentrations of <2.5 meq/L as well as higher serum-todialysate calcium gradients (Δ1 meq/L) were associated with higher risk of witnessed cardiac arrests.41 In a facility-level analysis by Brunelli et al. that compared outpatient dialysis units which maintained dialysate calcium concentrations at 2.5 meq/L vs. those that lowered levels from 2.5 meq/L to <2.5 meq/L, facilities that lowered dialysate calcium concentrations experienced a higher incidence of intradialytic hypotension and heart failure hospitalizations.42 However, three recent studies have not corroborated these findings. Among 76,462 hemodialysis patients from a national LDO, dialysate concentrations categorized as <2.3, 2.3–2.5, and >2.5 meq/L were not associated with cardiopulmonary arrest.27 Similarly, a secondary analysis of 3883 hemodialysis patients from the EVOLVE trial did not observe an association between dialysate calcium concentrations (categorized as <2.5, 2.5, vs. ≥2.5 meq/L) nor the primary composite endpoint (death or first non-fatal myocardial infarction, hospitalization for unstable angina, heart failure or peripheral vascular event), cardiovascular death, nor SCD.43 There was also no difference in the impact of cinacalcet upon outcomes by the dialysate calcium concentration nor serum-to-dialysate calcium gradient. Most recently, among the prospective Monitoring in Dialysis Study cohort, dialysate calcium concentrations >2.5 meq/L were associated with higher risk of clinically significant arrhythmias compared to concentrations of 2.5 meq/L.5,20
Whereas the safety of lower vs. higher dialysate calcium concentrations renders further study, it may be most prudent to utilize interventions such as pharmacotherapies (e.g., phosphate binders, vitamin D analogues, calcimimetics) and dietary adjustments in lieu of abrupt titration of the dialysate calcium concentration.11
Dialysate Magnesium Concentrations
Previously deemed a “neglected cation,” an increasing body of evidence has demonstrated the importance of serum magnesium levels with respect to dialysis patient outcomes.23,44 While high serum magnesium levels carry risk of 1) oversuppression of parathyroid hormone levels and adynamic bone disease, 2) hypotension, 3) bradycardia and heart block, and 4) neuromuscular toxicity, lower magnesium levels may also result in 1) QT-interval prolongation and atrial ventricular arrhythmias, as well as 2) seizures.11,23 Among 142,555 Japanese in-center hemodialysis patients, Sakaguchi et al. was the first to show a J-shaped relationship between serum magnesium levels and mortality risk such that the first, second, third, and sixth sextiles were associated with higher mortality, with optimal level at ~2.8 mg/dl.45 In a more recent study of 23,574 hemodialysis patients from a national LDO by Lacson et al., there was an inverse linear association between serum magnesium levels and mortality risk, such that levels <1.30 meq/L were linked with higher death.46 A national study of 9359 incident hemodialysis patients also showed that lower serum magnesium levels <2.0 mg/dl were associated with higher mortality.47
To maintain normal-range serum magnesium levels, a dialysate magnesium concentration of 1.0 meq/L (0.5 mmol/L) is recommended, and oral supplementation or higher dialysate magnesium concentrations may be needed among patients with increased gastrointestinal losses (i.e., diarrhea), malnutrition, or use of proton pump inhibitors.11,23 Contemporary cross-sectional data from a national LDO show that a dialysate magnesium concentration of 1.0 meq/L is most commonly prescribed (50%) among hemodialysis patients (i.e., 15%, 16%, 16%, and 3% of patients prescribed dialysate magnesium concentrations of <0.75, 0.75–0.99, 1.01–1.49, and ≥1.50 meq/L, respectively).46
Few studies to date have examined dialysate magnesium concentrations and outcomes in hemodialysis patients. However, in a secondary analysis of the aforementioned study by Lacson et al., dialysate magnesium concentrations (categorized as 0.75, 0.7–0.99, 1.0, 101–1.49, and 1.50 meq/L) were not linked with mortality risk.46 In a small prospective study of 75 German hemodialysis patients among whom 25 patients receiving higher dialysate magnesium concentrations (0.75 mmol/L or 1.0 meq/L) were matched to 50 patients receiving lower/normal concentrations (0.50 mmol/L or 1.0 meq/L), higher dialysate magnesium levels were associated with lower mortality; however, as analyses were only adjusted for age and Charlson Comorbidity Index, interpretation of these findings are limited by residual confounding.48
Fluid Accumulation and Removal and Intradialytic Hypotension
The paramount importance of adequate volume control in hemodialysis patients has catalyzed a “Volume First” initiative among leaders in the field.49 Indeed, fluid accumulation and excess removal result in substantial morbidity and mortality among hemodialysis patients. In terms of the former, fluid accumulation leads to 1) higher blood pressures, left ventricular hypertrophy, and cardiac modeling which will lead to 2) supply-demand mismatch, impaired coronary perfusion, and subendocardial ischemia, as well as 3) an arrhythmogenic cardiac substrate at risk for SCD events.10,50,51 As one of the first studies to highlight the toxicity of fluid overload, in an analysis of 34,107 hemodialysis patients by Kalantar-Zadeh et al., higher IDWGs ≥3.0 kg were identified as a potent predictor of mortality (reference: 1.5-<2.0 kg).52 Recently, Zoccali et al. reported that very severe pulmonary congestion ascertained by lung ultrasound was associated with a 4.2-fold higher death risk among 293 Italian hemodialysis patients.53
Conversely large amounts of fluid removal over a relatively short duration of time may lead to intradialytic hypotension and myocardial stunning and fibrosis.10 In 1999, Karnik et al. showed that a drop in systolic blood pressure of ≥30 mmHg portended subsequent cardiac arrest, an early signal of the link between intradialytic hypotension and SCD. Intradialytic hypotension is indeed a frequent occurrence (~20%) in outpatient hemodialysis units,51 and Chou et al. and others have shown a direct linear relationship between the frequency of intradialytic hypotension and mortality among hemodialysis patients.54
Multiple studies have demonstrated a link between higher ultrafiltration rates (UFRs) and mortality in hemodialysis patients. Among prevalent hemodialysis patients from the DOPPS cohort, Saran et al. showed that UFRs >10 ml/kg/hour were associated with higher all-cause mortality risk.55 Among a cohort of Italian hemodialysis patients, Movilli et al. showed that an UFR >12.4 ml/kg/hour was associated with higher death risk,56 and Flythe et al. observed a similar threshold (>13 ml/kg/hr) for all-cause and cardiovascular mortality in a secondary analysis of the HEMO trial.57 Among incident hemodialysis patients who may bear residual kidney function, Kim et al. has shown that a UFR >10 ml/kg/hour is associated with higher allcause and cardiovascular death risk.58 With respect to SCD, Jadoul et al. has shown that an ultrafiltration volume that is >5.7% of post-dialysis weight was associated with greater risk.8
These data have prompted policy-makers (e.g., Kidney Care Quality Alliance, Centers for Medicare and Medicaid Services, National Quality Forum) to adopt UFR (i.e., <13 ml/kg/hour among patients with a dialysis session length <240 minutes) as a clinical performance measure.59 While there remains considerable controversy regarding the pros and cons of designating a specific upper UFR limit with extended treatment times as a quality measure, this initiative has brought volume control to the forefront of discussions about optimal dialysis management among clinicians, researchers, LDO’s, and regulatory bodies while small sized women are more likely to be penalized with longer dialysis treatment time of 4 hours as a consequence of these unfair policies since they are more likely to exhibit values >13 ml/kg/hour given the smaller denominator values (weight) I these women who indeed would not benefit from longer dialysis treatment time.
Strategies for preventing high IDWG’s resulting in volume overload and necessitating high UFR’s include 1) prescription of diuretics to reduce IDWG amongst patients with residual kidney function and urine output, 2) routine counseling with respect to dietary salt and fluid restriction, 3) prescribing additional dialysis sessions, 4) extending treatment times, and 5) reducing exposure to high dialysate sodium concentrations.6,10,50,51 As the average dialysate sodium concentration has increased over the past four decades (from 135 meq/L in the 1970’s to 140 meq/L today) and sodium profiling may still be used to prevent intradialytic hypotension and cramping, these practices lead to a net sodium gain that may increase thirst and IDWG’s.59 It should also be noted that in the Monitoring in Dialysis study, higher pre-dialysis serum sodium levels were linked with clinically significant arrhythmias.5,20 Yet the Achilles’ heel of optimal volume management, particularly among US hemodialysis patients, is the absence of a practical, efficient, and accurate tool that can reliably measure extracellular volume and dry weight among hemodialysis patients. Hence, further research studies are needed to identify optimal instruments, metrics, and implementation measures in successfully achieving a “Volume First” approach.49
Future Directions and Conclusion
An important strategy in preventing the risk of SCD is customizing and unvulgarizing the dialysis procedure to the individual patient according to Precision Medicine, in lieu of a “one-size-fits-all” approach. Indeed, multiple factors should be considered in prescribing and adapting patients’ dialysis prescriptions, including their underlying residual kidney function,61 comorbidities, symptoms, and lifestyle patterns. As the first month of hemodialysis is the highest-risk period for SCD,5 incident ESRD patients transitioning to dialysis require particular attention and vigilant modification of their prescription. By applying a personalized or precision medicine strategy in defining patients’ hemodialysis schedules, dialysate concentrations, and fluid removal targets, there may be opportunity to ameliorate their exceedingly high risk of SCD and to improve their overall well-being and patient satisfaction.63
Table 3.
Strategies for the prevention of sudden cardiac death and future research directions.
| Risk Factor | Strategies to Prevent Sudden Cardiac Death | Gaps in Knowledge & Future Research Areas |
|---|---|---|
| Potassium (K) accumulation and removal | Frequent serum K assessment and dialysate K adjustment. Avoid dialysate K <2 meq/L. Oral K binding resins. |
Optimal dialysate-to-serum-K gradient. Safety and effectiveness of dialysate K profiling. |
| Bicarbonate homeostasis | Avoidance of low dialysate bicarbonate. Do not account for other perceived sources of base/buffer (e.g., acetate). Avoid using the wrong concept of “total buffer” |
Optimal dialysate bicarbonate concentration. Better education and understanding of the role of acetate in acid concentrate, which should not be added to bicarbonate in the dialysate |
| Calcium (Ca) homeostasis | Avoid low dialysate Ca <2.5 meq/L. Avoid high dialysate-to-serum Ca gradient. Use vitamin D analogs, Ca-based binders, calcimimetics to optimize serum Ca. |
Optimal dialysate-to-serum-Ca gradient. |
| Magnesium (Mg) homeostasis | Avoid low dialysate Mg. Titrating dialysate Mg in Mg-losing states (i.e., GI losses/diarrhea, PPI use, malnutrition). |
Optimal dialysate Mg concentration. |
| Fluid accumulation and removal | Reduce salt and fluid intake. Diuretics among pts with RKF. Longer HD time. More frequent HD sessions. Nocturnal HD. Smaller dialysate-to-Na-gradient. Avoid intradialytic hypotension. |
Practical bedside tools/devices to ascertain estimated dry weight and volume status. Optimal UFR for specific patient populations. Role of dialysate cooling. |
Abbreviations: K, potassium; Ca, calcium; Mg, magnesium; GI, gastrointestinal; PPI, proton pump inhibitor; RKF, residual kidney function; HD, hemodialysis; Na, sodium; UFR, ultrafiltration rate.
Acknowledgements:
Funding/Support:
The authors are supported by the research grants from the NIH/NIDDK including K23-DK102903 (CMR), R03-DK114642 (CMR), K24-DK091419 (KKZ), R01-DK096920 (KKZ), U01-DK102163 (KKZ); National Kidney Foundation (CMR); American Thyroid Association (CMR); and philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, and Dr. Joseph Lee. The sponsors did not have a role in the writing of the report, nor in the decision to submit the article for publication.
Footnotes
Conflicts of Interest:
None.
References:
- 1.US Renal Data System. USRDS 2015 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD; 2015. [Google Scholar]
- 2.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney international. 2004;65(6):2380–2389. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. The New England journal of medicine. 2005;353(3):238–248. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler DC, London GM, Parfrey PS, et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc. 2014;3(6):e001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charytan DM, Foley R, McCullough PA, et al. Arrhythmia and Sudden Death in Hemodialysis Patients: Protocol and Baseline Characteristics of the Monitoring in Dialysis Study. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(4):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makar MS, Pun PH. Sudden Cardiac Death Among Hemodialysis Patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69(5):684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung AM, Hakim RM. Dialysate and serum potassium in hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(1):125–132. [DOI] [PubMed] [Google Scholar]
- 8.Jadoul M, Thumma J, Fuller DS, et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(5):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee CM. Serum Potassium and the Long Interdialytic Interval: Minding the Gap. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;70(1):4–7. [DOI] [PubMed] [Google Scholar]
- 10.Rhee CM, Kalantar-Zadeh K. Implications of the long interdialytic gap: a problem of excess accumulation vs. excess removal? Kidney international. 2015;88(3):442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornley-Brown D, Saha M. Dialysate content and risk of sudden cardiac death. Curr Opin Nephrol Hypertens. 2015;24(6):557–562. [DOI] [PubMed] [Google Scholar]
- 12.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney international. 2011;79(2):218–227. [DOI] [PubMed] [Google Scholar]
- 13.Passman RS, Herzog CA. Bad things come to those who wait: Dialysis, sudden death, and the long interdialytic period. Heart Rhythm. 2015;12(10):2056–2057. [DOI] [PubMed] [Google Scholar]
- 14.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney international. 1999;55(4):1553–1559. [DOI] [PubMed] [Google Scholar]
- 15.Karnik JA, Young BS, Lew NL, et al. Cardiac arrest and sudden death in dialysis units. Kidney international. 2001;60(1):350–357. [DOI] [PubMed] [Google Scholar]
- 16.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney international. 2006;69(12):2268–2273. [DOI] [PubMed] [Google Scholar]
- 17.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. The New England journal of medicine. 2011;365(12):1099–1107. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Schaubel DE, Kalbfleisch JD, et al. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney international. 2012;81(11):1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fotheringham J, Fogarty DG, El Nahas M, Campbell MJ, Farrington K. The mortality and hospitalization rates associated with the long interdialytic gap in thrice-weekly hemodialysis patients. Kidney international. 2015;88(3):569–575. [DOI] [PubMed] [Google Scholar]
- 20.Roy-Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients. Kidney International. 2018;93(4):941–951. [DOI] [PubMed] [Google Scholar]
- 21.Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum Potassium and Short-term Clinical Outcomes Among Hemodialysis Patients: Impact of the Long Interdialytic Interval. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;70(1):21–29. [DOI] [PubMed] [Google Scholar]
- 22.Brunelli SM, Spiegel DM, Du Mond C, Oestreicher N, Winkelmayer WC, Kovesdy CP. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2018;33(7):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pun PH, Middleton JP. Dialysate Potassium, Dialysate Magnesium, and Hemodialysis Risk. Journal of the American Society of Nephrology : JASN. 2017;28(12):3441–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB. Serum Potassium Levels and Mortality in Hemodialysis Patients: A Retrospective Cohort Study. American journal of nephrology. 2016;44(3):179–186. [DOI] [PubMed] [Google Scholar]
- 25.Karaboyas, Zee J, Brunelli SM, et al. Dialysate Potassium, Serum Potassium, Mortality, and Arrhythmia Events in Hemodialysis: Results From the Dialysis Outcomes and Practice Patterns Study (DOPPS). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69(2):266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Regidor DL, Mehrotra R, et al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(5):999–1007. [DOI] [PubMed] [Google Scholar]
- 27.Flythe JE, Li NC, Lin SF, Brunelli SM, Hymes J, Lacson E, Jr. Associates of cardiopulmonary arrest in the perihemodialytic period. Int J Nephrol. 2014;2014:961978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrey A, You AS, Kovesdy CP, et al. Dialysate Potassium and Mortality in a Prospective Hemodialysis Cohort. American journal of nephrology. 2018;47(6):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buemi M, Aloisi E, Coppolino G, et al. The effect of two different protocols of potassium haemodiafiltration on QT dispersion. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(6):1148–1154. [DOI] [PubMed] [Google Scholar]
- 30.Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. The New England journal of medicine. 2015;372(3):222–231. [DOI] [PubMed] [Google Scholar]
- 31.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. The New England journal of medicine. 2015;372(3):211–221. [DOI] [PubMed] [Google Scholar]
- 32.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23(2):77–90. [DOI] [PubMed] [Google Scholar]
- 33.Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney international. 2013;84(6):1096–1107. [DOI] [PubMed] [Google Scholar]
- 34.Tentori F, McCullough K, Kilpatrick RD, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney international. 2014;85(1):166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clinical journal of the American Society of Nephrology : CJASN. 2006;1(1):70–78. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto T, Shoji S, Yamakawa T, et al. Predialysis and Postdialysis pH and Bicarbonate and Risk of All-Cause and Cardiovascular Mortality in Long-term Hemodialysis Patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(3):469–478. [DOI] [PubMed] [Google Scholar]
- 37.Kalantar-Zadeh K Moderator’s view: Higher serum bicarbonate in dialysis patients is protective. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;31(8):12311234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tentori F, Karaboyas A, Robinson BM, et al. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62(4):738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JE, Kovesdy CP, Norris KC, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. American journal of nephrology. 2010;32(5):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55(5):773–799. [DOI] [PubMed] [Google Scholar]
- 41.Pun PH, Horton JR, Middleton JP. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(5):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunelli SM, Sibbel S, Do TP, Cooper K, Bradbury BD. Facility Dialysate Calcium Practices and Clinical Outcomes Among Patients Receiving Hemodialysis: A Retrospective Observational Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(4):655–665. [DOI] [PubMed] [Google Scholar]
- 43.Pun PH, Abdalla S, Block GA, et al. Cinacalcet, dialysate calcium concentration, and cardiovascular events in the EVOLVE trial. Hemodialysis international International Symposium on Home Hemodialysis. 2016;20(3):421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhosaini M, Leehey DJ. Magnesium and Dialysis: The Neglected Cation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(3):523–531. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney international. 2014;85(1):174–181. [DOI] [PubMed] [Google Scholar]
- 46.Lacson E, Jr., Wang W, Ma L, Passlick-Deetjen J Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(6):1056–1066. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Streja E, Rhee CM, et al. Hypomagnesemia and Mortality in Incident Hemodialysis Patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(6):1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmaderer C, Braunisch MC, Suttmann Y, et al. Reduced Mortality in Maintenance Haemodialysis Patients on High versus Low Dialysate Magnesium: A Pilot Study. Nutrients. 2017;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiner DE, Brunelli SM, Hunt A, et al. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(5):685–695. [DOI] [PubMed] [Google Scholar]
- 50.Chou JA, Kalantar-Zadeh K. Volume Balance and Intradialytic Ultrafiltration Rate in the Hemodialysis Patient. Curr Heart Fail Rep. 2017;14(5):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou JA, Kalantar-Zadeh K, Mathew AT. A brief review of intradialytic hypotension with a focus on survival. Seminars in dialysis. 2017;30(6):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119(5):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zoccali C, Moissl U, Chazot C, et al. Chronic Fluid Overload and Mortality in ESRD. Journal of the American Society of Nephrology : JASN. 2017;28(8):2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou JA, Streja E, Nguyen DV, et al. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2018;33(1):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney international. 2006;69(7):1222–1228. [DOI] [PubMed] [Google Scholar]
- 56.Movilli E, Cancarini GC, Cassamali S, et al. Inter-dialytic variations in blood volume and total body water in uraemic patients treated by dialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(1):185–189. [DOI] [PubMed] [Google Scholar]
- 57.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney international. 2011;79(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim T, Kim T, Chang TI, Rhee CM, Streja E, Kalantar-Zadeh K. Longitudinal Changes of Ultrafiltration in a Cohort of 110,833 Incident Hemodialysis Patients. Abstract presented at the 2016 National Kidney Foundation Spring Clinical Meeting. [Google Scholar]
- 59.Kramer H, Yee J, Weiner DE, et al. Ultrafiltration Rate Thresholds in Maintenance Hemodialysis: An NKF-KDOQI Controversies Report. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;68(4):522–532. [DOI] [PubMed] [Google Scholar]
- 60.Gedney N, Kalantar-Zadeh K. Dialysis Patient-Centeredness and Precision Medicine: Focus on Incremental Home Hemodialysis and Preserving Residual Kidney Function. Semin Nephrol. 2018;38(4):426–432. [DOI] [PubMed] [Google Scholar]
- 61.Rhee CM, Obi Y, Mathew AT, Kalantar-Zadeh K. Precision Medicine in the Transition to Dialysis and Personalized Renal Replacement Therapy. Semin Nephrol. 2018;38(4):325–335. [DOI] [PubMed] [Google Scholar]
- 62.Wang AY, Kalantar-Zadeh K, Fouque D, et al. Precision Medicine for Nutritional Management in End-Stage Kidney Disease and Transition to Dialysis. Semin Nephrol. 2018;38(4):383–396. [DOI] [PubMed] [Google Scholar]
- 63.Rhee CM, Brunelli SM, Subramanian L, Tentori F. Measuring patient experience in dialysis: a new paradigm of quality assessment. Journal of nephrology. 2018;31(2):231240. [DOI] [PubMed] [Google Scholar]