Abstract
Background:
Acute promyelocytic leukemia (APL) is a unique leukemia subtype requiring specialized treatment including all-trans retinoic acid (ATRA). A prior report demonstrated worse outcome among young children <5 years old compared to older children.
Methods:
We evaluated outcomes for pediatric patients (<18 years old; N=83) with APL treated on North American Intergroup study CALGB 9710 at Children’s Oncology Group sites. Induction and consolidation included ATRA, cytarabine and anthracyclines. Patients ≥15 years old were randomized to addition of arsenic trioxide (ATO) consolidation. All patients were randomized to ATRA maintenance with vs without oral chemotherapy.
Results:
The estimated 5-year overall survival (OS) was 82% and event-free survival (EFS) was 54%. Seven patients (8.4%) died during induction due to coagulopathy. Maintenance randomization demonstrated that addition of oral chemotherapy to ATRA significantly reduced relapse rate, but difference in EFS did not reach statistical significance (P=0.12; 5-year rates [95% CI]: 41% [17–64%] ATRA only vs 72% [56–88%] ATRA plus chemotherapy). There was no difference (P=0.93) in EFS for age <5 years vs. 5–12.99 years vs. 13–17.99 years (5-year rates: 56%, 47% and 45%, respectively). Among adolescents 15–17.99 years old in the ATO randomization, there was a significantly lower relapse risk at 5 years for those receiving ATO (0% ATO vs. 44% no ATO; P=0.02).
Conclusion:
Our data demonstrate that intensified ATRA, cytarabine and anthracycline chemotherapy is effective for pediatric APL including very young patients, but early deaths and relapses remain barriers to cure. Further improvements are likely with incorporation of ATO into pediatric APL regimens.
Keywords: APL, Pediatric, ATRA, Arsenic Trioxide
Introduction
In the United States, acute promyelocytic leukemia (APL) in children constitutes 5–10% of acute myeloid leukemia (AML).[1] Molecularly, this disease is characterized by a fusion protein, PML/RARα from translocation of the PML gene on chromosome 15 and the retinoic acid receptor α gene on chromosome 17. Severe bleeding diathesis is a clinical hallmark of APL. With traditional chemotherapy treatments, induction mortality rates as high as 10–30% have been reported secondary to hemorrhage or thrombosis from coagulopathy.[2–4]
Treatment with all-trans retinoic acid (ATRA) induces differentiation of leukemic promyelocytes into mature granulocytes.[1,5–8] More recently arsenic trioxide (ATO) has been incorporated in the treatment of APL, first for relapse and subsequently for de novo APL.[9–14] The first North American intergroup trial (INT0129) showed improved disease-free survival among pediatric APL patients treated with ATRA during induction and/or maintenance.[1] The design of the second North American intergroup trial for APL, Cancer and Leukemia Group B (CALGB) 9710, was developed to improve outcomes through treatment intensification. CALGB is now part of the Alliance for Clinical Trials in Oncology. On CALGB 9710, compared to the INT0129 trial, daunorubicin was dose intensified and cytarabine was included at a higher dose for induction therapy. ATRA was introduced into consolidation, and chemotherapy (mercaptopurine and methotrexate) was added to maintenance. For patients age ≥15 years, ATO was evaluated in a randomized early consolidation phase. The outcomes for adult patients age ≥18 years on CALGB 9710 have been previously reported.[12]
Clinical trials in APL have generally reported similar outcomes for children and adults. However, sub-group analysis of 26 children treated on 2 consecutive trials of the European APL group compared outcomes for children age <5 years (N=12) to children age 5–12 years (N=14), and demonstrated that the children age <5 years had increased risk of relapse.[15] We report here the results for a larger cohort of 83 children age <18 years, including 41 children age <13 years, treated on CALGB 9710 and provide an analysis of outcomes in very young children.
Methods
The Children’s Oncology Group (COG) portion of CALGB 9710 opened in June 1999 and closed in March 2005. The trial was approved by the IRB at each participating COG institution and patients’ legal guardians provided informed consent. Eligibility required morphologic diagnosis of APL and molecular testing confirmation (PML-RARα or RARα-PML positive by RT-PCR in a central laboratory). Patients found to be negative by molecular testing were removed from study. Lumbar punctures were not required at diagnosis. Prior therapy with hydroxyurea, corticosteroids or leukapheresis for elevated white blood cell (WBC) count was permitted. Prior use of systemic chemotherapy or retinoids was not allowed. There were no performance status or organ function eligibility criteria. This trial was registered at ClinicalTrials.gov (NCT00003934).
Patient Population
The CALGB 9710 Intergroup study enrolled patients through four North American adult cooperative groups and the Children’s Oncology Group (COG). In order to control for differences in pediatric versus adult treatment center, only patients registered at COG treatment sites were included in our analysis. Patients age ≥15 years were randomly assigned to consolidation courses with ATO. Thus, we report outcomes for all patients age <18 years (N=83) as well as the outcomes for those patients age <18 years who did not receive ATO (N=67). Among patients who did not receive ATO, we also present a sub-analysis comparing young children (age <0–4.99 years, N=16) and those of older age (5–12.99 years, N=25; and 13–17.99 years, N=26).
Study design
All patients were treated with the same induction and consolidation regimens. Patients age ≥15 years were randomized to receive vs not receive 2 cycles of ATO consolidation. At the time of study development, younger patients were not eligible for the ATO randomization due to unknown cognitive effects in that age group. Patients in remission after consolidation were randomized to maintenance therapy. CALGB 9710 began with a maintenance randomization to observation versus ATRA. The protocol was soon amended to randomize between maintenance with ATRA alone or ATRA plus chemotherapy based on early results from the AIDA0493 study suggesting a benefit to ATRA maintenance.[16] The treatment regimen is detailed in Table 1. There was no intrathecal chemotherapy in this trial. Obese patients were to be dosed using actual weight and not an adjusted or ideal weight.
TABLE 1.
Treatment Regimen
| Induction | ||
| ATRA | 45 mg/m2/day PO divided BID | Day 1 to CR or 90 days maximum |
| Cytarabine | 200 mg/m2/day IV continuous infusion | Days 3–9 |
| Daunorubicin | 50 mg/m2/dose IV daily (1.5 mg/kg/day continuous infusion for age <3 years) | Days 3–6 |
| ATO Consolidation (2 cycles): Only Patients ≥15 years were eligible to be randomized to receive or not receive ATO | ||
| Arsenic Trioxide | 0.15 mg/kg/dose IV | 5 days/week for 5 weeks/cycle (2 week rest between Cycles) |
| Chemotherapy Consolidation (2 cycles): All Patients | ||
| ATRA | 45 mg/m2/day PO divided BID | Days 1–7 |
| Daunorubicin | 50 mg/m2/dose IV daily (1.5 mg/kg continuous infusion for age <3 years) | Days 1–2 for age <15 years and Days 1–3 for age ≥15 years |
| Maintenance Randomization* | ||
| ATRA Only Maintenance: | ||
| ATRA | 45 mg/m2/day PO divided BID | 7 days repeated every other week for 1 year |
| ATRA Plus Chemotherapy Maintenance: | ||
| ATRA | 45 mg/m2/day PO divided BID | 7 days repeated every other week for 1 year |
| Mercaptopurine | 60 mg/m2/day PO daily | Daily for 1 year |
| Methotrexate | 20 mg/m2/dose PO once weekly | Once weekly for 1 year |
Early in the trial, a small number of patients were randomized to ATRA only maintenance vs observation (no maintenance).
Supportive care
Allopurinol (300 mg/day for patients age >6 years; 150 mg/day for younger patients) was recommended. Management of coagulopathies, transfusions and antibiotics was at the discretion of the treating physician. Coagulation testing three times weekly until normalization was recommended, but use of heparin was discouraged. If differentiation syndrome was suspected, ATRA was held and dexamethasone (10 mg IV or PO twice daily for children weighing >40 kg or 0.25 mg/kg IV or PO twice daily for smaller children) begun promptly and continued for 3 days. ATRA was resumed once the signs and symptoms of this syndrome had resolved. For patients with proven pseudotumor cerebri (PTC), ATRA was to be held until the toxicity grading improved to grade 0 or 1 (mild headache), then restarted at 75% dosing. If PTC recurred, then ATRA was to be managed as above and then restarted at 50% dosing. Modifications of ATO dosing because of toxicity were as previously reported.[12]
Statistical Analysis
All analyses were based on the study database frozen on October 9, 2014. Patient characteristics and clinical outcomes were summarized and compared between age groups of interest. Response and relapse were defined by 1990 NCI criteria. Toxicity was graded using the NCI Common Toxicity Criteria version 2.0. Overall survival (OS) was defined as the time from study entry to death due to any cause. Event-free survival (EFS) was defined as the time from study entry to first event, where an event was defined as failure to achieve a CR, relapse after achieving a CR, or death. Patients who were event-free were censored at the time of their last follow-up or at initiation of subsequent therapy. Disease-free survival (DFS) was defined as time from attainment of a CR to relapse or death after achieving CR. Patients who were alive and relapse free were censored at the time of their last follow-up or at initiation of subsequent therapy. Fisher’s exact tests were used to compare categorical variables between age groups.[17] Kaplan-Meier methods and logrank tests were used to evaluate and compare distributions of OS and EFS overall and between age groups.[18,19] Estimated 5-year survival rates with 95% confidence limits (95% CI) were calculated using these methodologies. Cumulative incidence of relapse (CIR) was calculated using the time from study entry to the time of relapse, where death without relapse was a competing event and those who were alive and relapse-free were censored at their last evaluation time point. The methods of Fine and Gray were used to assess and graphically and quantitatively compare CIR in the presence of competing risks between groups.[20,21] Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. The CALGB 9710 trial accrued pediatric patients while completing the primary objective of a randomized comparison on ATO consolidation in adult patients. The trial’s statistical design did not include pre-specified analyses of the pediatric cohort, and thus this report represents a retrospective analysis of trial data.
Results
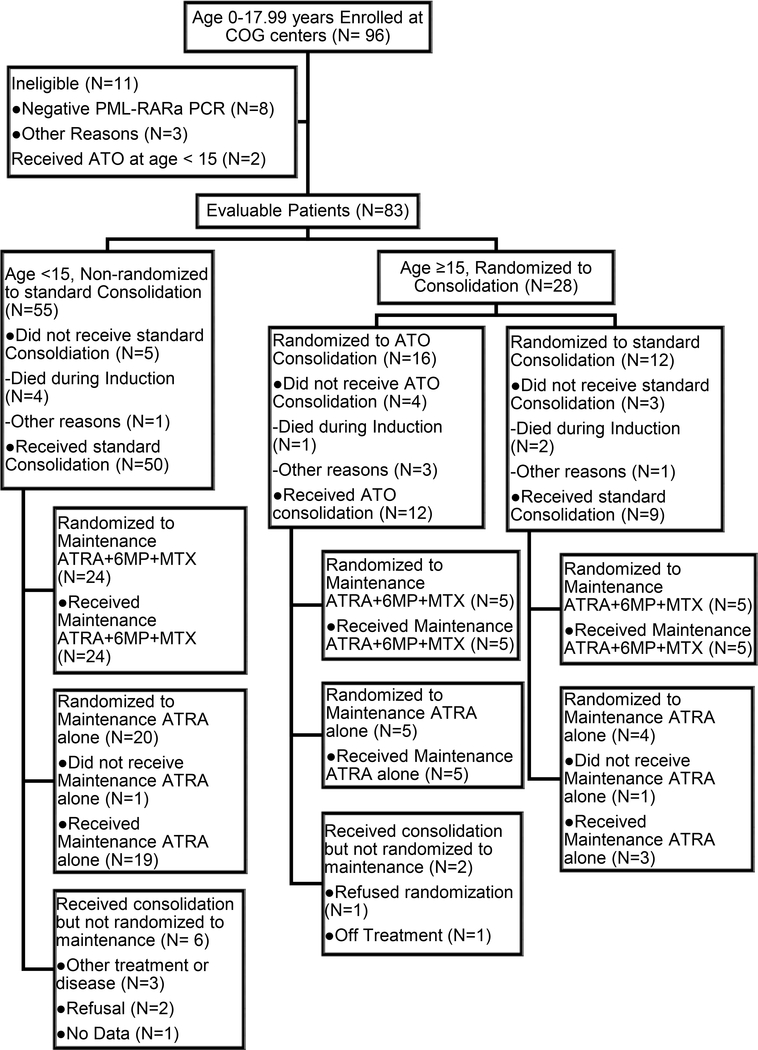
Ninety-six patients age <18 years at study entry enrolled between June 1999 and March 2005 from 60 COG treatment centers. Eleven patients were deemed ineligible with most (N=8) ineligible due to negative PCR testing for PML-RARα. In addition, two patients age <15 years who received ATO were excluded from all analyses. Thus 83 patients were eligible and evaluable from 53 COG treatment centers. Fifty-five patients age <15 years received standard consolidation. Twenty-eight patients age ≥15 years were randomized to consolidation, including 16 patients randomized to ATO and 12 patients randomized to standard consolidation. Early in the study, 1 patient was randomized to observation without maintenance and 2 patients were randomized to ATRA alone maintenance. Following an amendment to change the maintenance randomization, 29 patients were randomized to ATRA alone and 34 patients were randomized to ATRA plus chemotherapy (Fig. 1).
FIGURE 1.
CONSORT diagram
Due to a previously reported increased rate of relapse in patients age <5 years, we performed an analysis across ages 0–4.99 years (N=16), 5–12.99 years (N=25) and 13–17.99 years (N=26 not receiving ATO). Clinical characteristics for the whole cohort and by age group were examined (Supplementary Table S1). There was no significant difference in gender, race, ethnicity, Sanz risk group, diagnostic WBC, M3v variant, or CD56 expression prevalence between the age groups.[22]
Induction Response
The CR rate was 83% (69/83) for all patients age <18 years. Five patients were classified as partial response (PR) and 2 patients failed to achieve CR or PR. However, this study did not include central pathology review of remission status and these non-CR responses could not be confirmed. No data on flow cytometry based minimal residual disease (MRD) was collected on this study. Seven patients (8.4%) died during induction, and 6 deaths occurred within one week of registration on study. All patients died from complications related to coagulopathy (Supplementary Table S2).
Patients with an early (induction) death (N=7) were compared to those without early death (N=76) for the following clinical features: WBC >10,000 cells/μL (100% vs 24%, P=0.001), percent with M3v histology (50% vs 14%, P=0.003), and ECOG performance score ≥3 (17% vs 5%, P=0.22). Observed differences in WBC and M3v histology achieved statistical significance, although we recognize the limitations given the small numbers for these comparisons. We have previously reported the high prevalence of FLT3 mutations among pediatric patients on CALGB 9710 who had specimens available for FLT3 mutation testing. Among 50 patients tested, 12 (24%) had FLT3/ITD and 9 (18%) had FLT3 point mutations in the tyrosine kinase domain. The induction death rate for CALGB 9710 patients was significantly higher in patients with FLT3 mutations compared to FLT3 wild type (33% vs. 0%, P=0.0012).[23]
Treatment Toxicities
While 7 patients died during induction, there were no deaths during consolidation or maintenance phases. Toxicities during each phase of treatment of grades 3–5 were collected (Supplementary Table S3). Differentiation syndrome occurs early in treatment and may include respiratory distress, hypoxemia, fever, erythematous rash, pulmonary infiltrates, pleural and pericardial effusions, weight gain, and congestive heart failure with impaired myocardial contractility and episodic hypotension.[24] No deaths were attributed to differentiation syndrome on this study; however, 37% of patients were reported as experiencing this syndrome.
ATRA associated pseudotumor cerebri (PTC) manifests as headaches and/or visual disturbance due to increased intracranial pressure. The rates of PTC are reported to be higher in children than adults. Data on PTC were not systematically collected on this study, but descriptive comments on toxicity reporting forms indicated that at least 5 patients (6%) developed this complication. Additionally, headache of grade 3 or 4 (although not specifically linked to a diagnosis of PTC) was reported with some frequency in each course: induction 11%, ATO consolidation 16.7%, ATRA/daunorubicin consolidation 6.8%, and maintenance 8.5%.
Among 12 patients treated with ATO consolidation cycles, grade 3–4 non-hematologic toxicities occurring in greater than 10% of patients included headache (16.7%), infection with neutropenia (16.7%), and pain (16.7%). While ATO is known to cause prolongation of the corrected QT interval (QTc), only 1 patient was reported to have a prolonged QTc toxicity which was grade 3. During maintenance, 9.8% (N=6) of patients had grade 3 or 4 elevation of ALT/SGPT. There were no grade 3–4 non-hematologic toxicities that occurred in greater than 10% of patients during ATRA/daunorubicin consolidation or maintenance (Supplementary Table S2).
Survival Outcomes for Patients Age <18 Years
At the time of analysis, 69 (83%) patients were alive with a median follow-up time for survivors of 6.3 years (range 0.4–11.9). In the overall cohort, the 5-year survival estimate for OS was 82% (95% CI: 74%−91%) and for EFS was 54% (95% CI: 42–65%) (Supplementary Figures S1a and S1b). The majority of relapses occurred in the first 2 years following diagnosis, and the cumulative incidence of relapse was 25% at 2 years, 30% at 3 years and 36% at 5 years (Supplementary Figure S1c).
WBC count at diagnosis is a previously described predictor for outcome. Five-year survival for patients with WBC >10,000 vs WBC ≤10,000 was OS 67% (95%CI: 48–86) vs 88% (95%CI: 80–97), EFS 50% (95%CI: 30–71) vs 55% (95%CI: 41–69) and DFS 73% (95%CI: 50–96) vs 52% (95%CI: 37–67) (Fig. 2). The estimated relapse risk at 5 years was 39% for WBC ≤10,000 vs 26% for WBC >10,000. Thus, while those with higher WBC had a higher rate of early events, surprisingly those with lower WBC had a greater relapse rate and hence the EFS was not different.
FIGURE 2.
Survival for all patients <18 years stratified by presenting WBC. Kaplan-Meier plots for overall survival (A), event free survival (B), and disease-free survival (C) demonstrate that high presenting WBC is a risk marker for worse overall survival.
While FLT3 mutations were prevalent (42%) in the CALGB 9710 cohort and associated with early death as noted above, there was no association with survival. For FLT3 mutant vs. wild type, the 5 year OS was 62% vs. 68% (log rank P=0.057) and 5 year EFS was 43% vs. 42% (log rank P=0.23).[23]
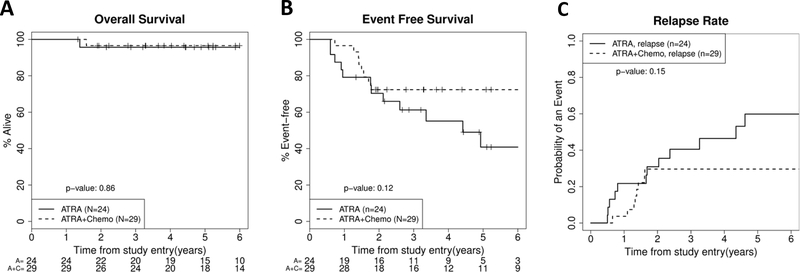
Patients were randomized to 1 year of maintenance therapy with ATRA alone or ATRA with chemotherapy (oral mercaptopurine and methotrexate). Even when restricting the analysis to patients who did not receive ATO in consolidation, the OS was not significantly different (P=0.86) with 5-year OS rates of 96% (95%CI: 87–100) for ATRA alone (N=24) and 97% (95%CI: 90–100) for ATRA plus chemotherapy (N=29) (Fig. 3a). However, patients who received ATRA only maintenance did have more relapses and a trend toward lower EFS compared to ATRA plus chemotherapy maintenance (41% (95%CI: 17–64) vs 72% (95%CI: 56–88), log-rank P=0.12; Figs. 3b and 3c). These survival curves diverge after 2 years post-diagnosis, which coincides with the completion of maintenance therapy.
FIGURE 3.
Survival and relapse risk for patients <18 years stratified by maintenance therapy. Kaplan-Meier plots for overall survival (A), event free survival (B) and relapse risk (C). For figure 3C, death without relapse curves are not shown because there were none of these events for either group. These demonstrate no significant difference in outcome by maintenance arm, but of interest there were more relapses after 2 years (the end of therapy) in the non-chemotherapy maintenance group. These survival curves represent patients who completed induction and consolidation to be eligible for maintenance randomization and thus survival is higher than in the entire cohort represented in Figure 2.
Outcomes by Age Group including Young Children
A prior report from the European APL group demonstrated inferior outcomes in children age <5 years.[15] In a sub-group analysis, we excluded the adolescents who received ATO consolidation and compared outcomes across 3 age groups: age <5 years (N=16), age 5–12.99 years (N=25), and age 13–17.99 years (N=26). OS was not significantly different (P=0.65), with 5-year OS rates of 78% (95%CI: 55–100) for age <5 years, 80% (95%CI: 64–96) for age 5–12.99 years, and 87% (95%CI: 73–100) for age 13–17.99 years (Fig. 4a). Similarly, EFS was not significantly different (P=0.93), with 5-year EFS rates of 56% (95%CI: 32–81) for age <5 years, 47% (95%CI: 26–69) for age 5–12.99 years, and 45% (95%CI: 25–66) for age 13–17.99 years (Fig. 4b). Young children did not have a higher relapse rate; 5-year cumulative incidence of relapse (CIR) by age was: <5 years=29%, 5–12.99 years=49%, 13–17.99 years=48%, P=0.71 (Fig. 4c). However, we acknowledge the limited power available with these subgroup sample sizes.
FIGURE 4.
Survival and relapse risk for three age sub-groups among pediatric patients. Kaplan-Meier plots for overall survival (a), event free survival (b) and relapse risk following remission (c) demonstrate that young children have similar survival and relapse rate as older children and adolescents.
Effect of ATO Consolidation in Adolescents
We analyzed patients age 15–17.99 years who were eligible for randomization to ATO treatment. While randomization occurred at treatment initiation, a number of patients did not receive consolidation (Fig. 1). In a treatment-received analysis, 9 patients received standard consolidation (no ATO) and 12 patients received ATO consolidation. OS rate at 5 years was similar between the groups (no ATO 100%, ATO 92%; Fig. 5a). One patient who received ATO died without relapse. The 5-year EFS was 56% for those not receiving ATO compared to 92% for those who received ATO (Fig. 5b). Relapse risk at 5 years was 44% for those not receiving ATO but 0% for those who received ATO (P=0.02; Fig. 5c). None of these comparisons, except relapse risk, resulted in significant differences, although we recognize the inherent limitations associated with the small numbers in these subgroups.
FIGURE 5.
Survival and relapse risk for patients age 15–17.99 years stratified by ATO consolidation treatment. Kaplan-Meier plots for overall survival (a) and event free survival (b) are not significantly different, but relapse risk (c) was lower among patients who received ATO. In figure 5c, the relapse risk lines for both “ATO, relapse” and “No ATO, death without relapse” are overlapping on the 0% probability line.
Discussion
With 83 eligible patients, this trial represents one of the largest treatment series reported for pediatric APL, but a limitation of the age-based analysis is its retrospective nature. The CALGB 9710 trial was not initially designed to compare outcomes differences by age group. However, results of this trial confirm the findings from the first North American intergroup trial (INT0129), which demonstrated improved outcomes with ATRA in induction and/or maintenance.[1] Despite enrolling a higher percentage of high risk patients with WBC >10,000 (30% vs 21%), this trial had a superior 5-year OS of 82% compared to 69% on INT0129. These trials had differences in duration of ATRA treatment and intensities of anthracycline and cytarabine treatment. On INT0129, no ATRA doses were given during consolidation cycles, only a minority of patients received ATRA during both induction and maintenance (9/53) and some patients received no ATRA (7/53). The AIDA0493 trial for pediatric APL included ATRA in induction and maintenance but no ATRA during consolidation, and OS at over 10 years was 89%.[25] The current study included ATRA throughout all treatment cycles. This approach was also utilized on the German AML-BFM trials −93/−98/−2004, and that group reported a 5-year OS of 89%.[26]
Multiple expert guidelines recommend early treatment with ATRA to diminish the risk of early death. Despite including ATRA in induction, there were 7 early deaths (8.4% early death rate) on this trial and all were due to hemorrhagic or ischemic events. Leukocytosis at diagnosis (WBC >10,000/μL) was a significant risk for early death. The M3v subtype had an increased risk for early death, and we have previously reported that FLT3 mutations were a risk for early death.[23] There is correlation between leukocytosis, FLT3 mutations and M3v. A recent retrospective study of early death in pediatric APL (which did not include FLT3 mutation data) suggested leukocytosis was more predictive than M3v.[27] The lower CR rate on the current trial (83%) was impacted by the early death rate. There were also a number of patients (6%) classified as PR at end of induction and yet achieved CR after the next cycle. Eligible patients came from 53 treatment centers and there was no central review of pathology. This may have contributed to a higher PR rate because interpretation of maturing promyeloblasts in bone marrow at the end of induction therapy in APL can be challenging.[28]
The cumulative dose of daunorubicin on the current trial was 400mg/m2 for age <15 years and 500mg/m2 for age ≥15 years which is higher than the 225–360mg/m2 on INT0129 (dose dependent on induction allocation). Even higher anthracycline doses were used on AIDA0493 which included 80mg/m2 of idarubicin and 50mg/m2 of mitoxantrone (total 650mg/m2 daunorubicin equivalents assuming a 5:1 conversion), but AML-BFM trials achieved OS 89% with 350mg/m2 of anthracyclines.[26] While anthracyclines are highly effective therapy for APL, children are especially susceptible to cardiac toxicity. On the current study, 2 patients died of cardiac complications while in remission after completion of therapy. This highlights the need for decreasing anthracycline dose through use of other effective therapies. Indeed, anthracycline exposure was significantly decreased on the COG trial AAML0631 along with incorporation of ATO consolidation which resulted in excellent outcomes (3 year OS 94% and EFS 91%).[29]
Several studies have demonstrated reduced relapse risk, especially in higher risk patients, following treatment with high dose cytarabine.[30–32] INT0129, AIDA0493 and the AML-BFM trials all included high dose cytarabine in consolidation while the current study did not. It is possible that less cytarabine exposure contributed to this trial’s higher relapse rate of 36% at 5 years. Results of the maintenance randomization suggested some benefit to oral mercaptopurine and methotrexate. While the results did not reach statistical significance, patients treated with ATRA only maintenance had an EFS of 41% (95% CI: 17–64%) compared to 72% (95% CI: 56–88%) for those treated with ATRA plus chemotherapy. This potential benefit, however, must be interpreted in the context of a treatment regimen that did not include any cytarabine in consolidation nor ATO for the majority of patients. In evaluating relapse data from this trial, it is also very informative that despite no intrathecal therapy nor high dose cytarabine, there were no isolated CNS relapses and only one combined relapse with CNS, bone marrow and skin. The patient with combined relapse had a subdural hemorrhage during induction and demonstrated CD2 expression (87%) at relapse, features which have been suggested to predispose APL patients to extramedullary relapse and worse prognosis, respectively.[33,34] The finding of low CNS relapse risk on this study agrees with previous reports in pediatric APL.[35]
While the higher relapse rate resulted in an EFS of 54% at 5 years, the OS was 82%. It is likely that many of these relapsed patients were successfully treated with ATO (although subsequent treatment data after relapse were not consistently collected). The report on ATO randomization for adults patients (age >18 years) on this study clearly demonstrates the efficacy of ATO.[12] Only patients age ≥15 years were eligible for the ATO randomization, and there were no relapses among adolescents age 15–17.99 years who received ATO while those age 15–17.99 years randomized to no ATO had a relapse rate of 44%. The COG AAML0631 trial has now shown that a regimen including ATO consolidation for all children with APL results in a low relapse risk (4% at 3 years).[29] The recent Italian-German APL0406 randomized trial has further demonstrated excellent survival for adult patients with standard risk APL receiving ATRA and ATO without any doses of anthracycline or cytarabine.[14]
The role of age in relapse risk remains an outstanding question in pediatric APL. European APL trial data had suggested a higher relapse risk among young children (age <5 years).[15] Our analysis included a larger cohort than that studied by the European APL group, and our data do not show a difference in outcome. Trials utilizing new treatment regimens (including ATO) should continue to assess risk by age, but there are not sufficient data currently to recommend risk-adapted therapies based on young age.
Future pediatric APL treatment strategies should minimize anthracycline dose to protect children from cardiac toxicity while still striving for a low relapse rate. The high rate of early death on our study also highlights the need to more fully understand APL-related coagulopathy and implement prevention strategies.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA031946, U10CA033601, U10CA180821 and U10CA180882 to the Cancer and Leukemia Group B and Alliance for Clinical Trials in Oncology, U10CA003927, U10CA041287, and U10CA098543, U10CA180836 and U10CA180886 to the Children’s Oncology Group. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations Key
- 6MP
6-Mercaptopurine
- APL
Acute promyelocytic leukemia
- ANC
Absolute neutrophil count
- ATO
Arsenic trioxide
- ATRA
All trans retinoic acid
- CALGB
Cancer and Leukemia Group B
- CI
Confidence interval
- COG
Children’s Oncology Group
- CR
Complete remission
- DFS
Disease free survival
- EFS
Event free survival
- IV
Intravenous
- PCR
Polymerase chain reaction
- PR
Partial remission
- OS
Overall Survival
- PTC
Pseudotumor cerebri
- WBC
White blood cell
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Gregory J, Kim H, Alonzo T, Gerbing R, Woods W, Weinstein H, Shepherd L, Schiffer C, Appelbaum F, Willman C, Wiernik P, Rowe J, Tallman M, Feusner J. Treatment of children with acute promyelocytic leukemia: results of the first North American Intergroup trial INT0129. Pediatr Blood Cancer 2009:53(6):1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, Tallman MS. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood 2011:118(5):1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Mollgard L, Derolf AR, Stockelberg D, Tidefelt U, Wahlin A, Wennstrom L, Hoglund M, Juliusson G. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia 2011:25(7):1128–1134. [DOI] [PubMed] [Google Scholar]
- 4.de la Serna J, Montesinos P, Vellenga E, Rayon C, Parody R, Leon A, Esteve J, Bergua JM, Milone G, Deben G, Rivas C, Gonzalez M, Tormo M, Diaz-Mediavilla J, Gonzalez JD, Negri S, Amutio E, Brunet S, Lowenberg B, Sanz MA. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood 2008:111(7):3395–3402. [DOI] [PubMed] [Google Scholar]
- 5.Mandelli F, Diverio D, Avvisati G, Luciano A, Barbui T, Bernasconi C, Broccia G, Cerri R, Falda M, Fioritoni G, Leoni F, Liso V, Petti MC, Rodeghiero F, Saglio G, Vegna ML, Visani G, Jehn U, Willemze R, Muus P, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood 1997:90(3):1014–1021. [PubMed] [Google Scholar]
- 6.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, Wiernik PH. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 1997:337(15):1021–1028. [DOI] [PubMed] [Google Scholar]
- 7.Sanz MA, Martin G, Rayon C, Esteve J, Gonzalez M, Diaz-Mediavilla J, Bolufer P, Barragan E, Terol MJ, Gonzalez JD, Colomer D, Chillon C, Rivas C, Gomez T, Ribera JM, Bornstein R, Roman J, Calasanz MJ, Arias J, Alvarez C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood 1999:94(9):3015–3021. [PubMed] [Google Scholar]
- 8.Burnett A, Grimwade D, Solomon E, Wheatley K, Goldstone A. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the Randomized MRC Trial. Blood 1999:93(12):4131–4143. [PubMed] [Google Scholar]
- 9.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutre S, Dahlberg S, Ellison R, Warrell RP Jr., United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 2001:19(18):3852–3860. [DOI] [PubMed] [Google Scholar]
- 10.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 1997:89(9):3354–3360. [PubMed] [Google Scholar]
- 11.Mathews V, George B, Chendamarai E, Lakshmi KM, Desire S, Balasubramanian P, Viswabandya A, Thirugnanam R, Abraham A, Shaji RV, Srivastava A, Chandy M. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol 2010:28(24):3866–3871. [DOI] [PubMed] [Google Scholar]
- 12.Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, Couban S, Appelbaum FR, Tallman MS, Larson RA. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 2010:116(19):3751–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravandi F, Estey E, Jones D, Faderl S, O’Brien S, Fiorentino J, Pierce S, Blamble D, Estrov Z, Wierda W, Ferrajoli A, Verstovsek S, Garcia-Manero G, Cortes J, Kantarjian H. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol 2009:27(4):504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013:369(2):111–121. [DOI] [PubMed] [Google Scholar]
- 15.Bally C, Fadlallah J, Leverger G, Bertrand Y, Robert A, Baruchel A, Guerci A, Recher C, Raffoux E, Thomas X, Leblanc T, Idres N, Cassinat B, Vey N, Chomienne C, Dombret H, Sanz M, Fenaux P, Ades L. Outcome of acute promyelocytic leukemia (APL) in children and adolescents: an analysis in two consecutive trials of the European APL Group. J Clin Oncol 2012:30(14):1641–1646. [DOI] [PubMed] [Google Scholar]
- 16.Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, Latagliata R, Specchia G, Baccarani M, Di Bona E, Fioritoni G, Marmont F, Rambaldi A, Di Raimondo F, Kropp MG, Pizzolo G, Pogliani EM, Rossi G, Cantore N, Nobile F, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood 2011:117(18):4716–4725. [DOI] [PubMed] [Google Scholar]
- 17.Altman DG. Practical statistics for medical research. London; New York: Chapman and Hall; 1991. xii, 611 p. p. [Google Scholar]
- 18.Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. J AmStat Assoc 1958:53(282):457–481. [Google Scholar]
- 19.Cox D Regression Models and Life-Tables. J Royal Stat Soc Series B (Methodoligical) 1972:34(2):187–220s. [Google Scholar]
- 20.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics 1988:16(3):1141–1154. [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999:94(446):496–509. [Google Scholar]
- 22.Sanz M, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, Díaz-Mediavilla J, Fioritoni G, González J, Liso V, Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F, Colomer D, Petti M, Ribera J, Mandelli F. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood 2000:96(4):1247–1253. [PubMed] [Google Scholar]
- 23.Kutny MA, Moser BK, Laumann K, Feusner JH, Gamis A, Gregory J, Larson RA, Powell BL, Stock W, Willman CL, Woods WG, Meshinchi S. FLT3 mutation status is a predictor of early death in pediatric acute promyelocytic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2012:59(4):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel SR, Eardley A, Heller G, Berman E, Miller WH Jr.,, Dmitrovsky E, Warrell RP Jr. All-trans retinoic acid for acute promyelocytic leukemia. Results of the New York Study. Annals of internal medicine 1994:120(4):278–286. [DOI] [PubMed] [Google Scholar]
- 25.Testi AM, Biondi A, Lo Coco F, Moleti ML, Giona F, Vignetti M, Menna G, Locatelli F, Pession A, Barisone E, De Rossi G, Diverio D, Micalizzi C, Arico M, Basso G, Foa R, Mandelli F. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood 2005:106(2):447–453. [DOI] [PubMed] [Google Scholar]
- 26.Creutzig U, Zimmermann M, Dworzak M, Urban C, Henze G, Kremens B, Lakomek M, Bourquin JP, Stary J, Reinhardt D. Favourable outcome of patients with childhood acute promyelocytic leukaemia after treatment with reduced cumulative anthracycline doses. Br J Haematol 2010:149(3):399–409. [DOI] [PubMed] [Google Scholar]
- 27.Abla O, Ribeiro RC, Testi AM, Montesinos P, Creutzig U, Sung L, Di Giuseppe G, Stephens D, Feusner JH, Powell BL, Hasle H, Kaspers GJL, Dalla-Pozza L, Lassaletta A, Tallman MS, Locatelli F, Reinhardt D, Lo-Coco F, Hitzler J, Sanz MA. Predictors of thrombohemorrhagic early death in children and adolescents with t(15;17)-positive acute promyelocytic leukemia treated with ATRA and chemotherapy. Ann Hematol 2017:96(9):1449–1456. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003:21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 29.Kutny MA, Alonzo TA, Gerbing RB, Wang YC, Raimondi SC, Hirsch BA, Fu CH, Meshinchi S, Gamis AS, Feusner JH, Gregory JJ Jr., Arsenic Trioxide Consolidation Allows Anthracycline Dose Reduction for Pediatric Patients With Acute Promyelocytic Leukemia: Report From the Children’s Oncology Group Phase III Historically Controlled Trial AAML0631. J Clin Oncol 2017:35(26):3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ades L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A, Stoppa AM, Lamy T, Rigal-Huguet F, Vekhoff A, Meyer-Monard S, Maloisel F, Deconinck E, Ferrant A, Thomas X, Fegueux N, Chomienne C, Dombret H, Degos L, Fenaux P, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol 2006:24(36):5703–5710. [DOI] [PubMed] [Google Scholar]
- 31.Sanz MA, Montesinos P, Rayon C, Holowiecka A, de la Serna J, Milone G, de Lisa E, Brunet S, Rubio V, Ribera JM, Rivas C, Krsnik I, Bergua J, Gonzalez J, Diaz-Mediavilla J, Rojas R, Manso F, Ossenkoppele G, Gonzalez JD, Lowenberg B, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood 2010:115(25):5137–5146. [DOI] [PubMed] [Google Scholar]
- 32.Lengfelder E, Haferlach C, Saussele S, Haferlach T, Schultheis B, Schnittger S, Ludwig WD, Staib P, Aul C, Gruneisen A, Kern W, Reichle A, Serve H, Berdel WE, Braess J, Spiekermann K, Wormann B, Sauerland MC, Heinecke A, Hiddemann W, et al. High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results of the German AMLCG. Leukemia 2009:23(12):2248–2258. [DOI] [PubMed] [Google Scholar]
- 33.Montesinos P, Diaz-Mediavilla J, Deben G, Prates V, Tormo M, Rubio V, Perez I, Fernandez I, Viguria M, Rayon C, Gonzalez J, de la Serna J, Esteve J, Bergua JM, Rivas C, Gonzalez M, Gonzalez JD, Negri S, Brunet S, Lowenberg B, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica 2009:94(9):1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin P, Hao S, Medeiros LJ, Estey EH, Pierce SA, Wang X, Glassman AB, Bueso-Ramos C, Huh YO. Expression of CD2 in acute promyelocytic leukemia correlates with short form of PML-RARalpha transcripts and poorer prognosis. American journal of clinical pathology 2004:121(3):402–407. [DOI] [PubMed] [Google Scholar]
- 35.Chow J, Feusner J. Isolated central nervous system recurrence of acute promyelocytic leukemia in children. Pediatr Blood Cancer 2009:52(1):11–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.