Abstract
Background
Whether omega-3 fatty acid supplementation reduces risk of cardiovascular disease or cancer remains unclear.
Methods
The VITamin D and OmegA-3Trial (VITAL) was a randomized, placebo-controlled, 2X2 factorial trial of vitamin D3 (2000IU/day) and marine omega-3 fatty acids (1 g/day) in the primary prevention of cardiovascular disease and cancer among 25,871 U.S. men aged ≥50 and women aged >55, including 5,106 African Americans. Primary endpoints were major cardiovascular events (myocardial infarction, stroke, and cardiovascular mortality) and total invasive cancer. Secondary outcomes included individual components of the cardiovascular composite, the composite plus coronary revascularization, site-specific cancers, and cancer mortality. This paper reports the results of omega-3 and placebo.
Results
During a median 5.3 years, rates of the primary outcomes did not differ between the omega-3 and placebo groups -- 805 participants had a major cardiovascular event, hazard ratio [HR]= 0.92; 95% confidence interval [CI], 0.80–1.06, p= 0.24. Invasive cancer was diagnosed in 1,617 participants, HR 1.03 (0.93-1.13, p=0.56). In the analysis of key secondary endpoints, hazard ratios and 95% CIs comparing omega-3 to placebo were: expanded cardiovascular events, HR 0.93 (0.82-1.04); total myocardial infarction HR 0.72 (0.59-0.90); total stroke, HR 1.04 (0.83-1.31); cardiovascular mortality HR 0.96 (0.76-1.21); and cancer deaths (n=341, HR 0.97 (0.79-1.20). For all-cause mortality (n=978), the HR was 1.02 (0.90-1.15). No excess risks of bleeding or other serious adverse events were observed.
Conclusions
Omega-3 fatty acid supplementation did not reduce major cardiovascular events or cancer incidence.
Marine-derived long-chain omega-3 ( also called n-3) fatty acids have shown promise for the primary prevention of cardiovascular disease in animal studies, small randomized trials designed with intermediate cardiovascular endpoints, and observational epidemiologic investigations.1 However, mid-sized to large trials testing the effect of n-3 fatty acid supplements on clinical cardiovascular outcomes in secondary prevention or high-risk settings have shown inconsistent results.1, 2 Large trials of n-3 supplements for primary prevention of cardiovascular disease in a general population selected only on age and not on vascular risk factors such as diabetes or dyslipidemia are lacking. Data on n-3 fatty acids and cancer risk have also been inconsistent.3 Given the popularity of fish oil as a strategy to reduce chronic disease,4 clarifying the relation between supplemental n-3 fatty acids and risks of cardiovascular disease and cancer and obtaining more definitive data on the benefit-risk balance of these supplements is a high priority. The VITamin D and OmegA-3 TriaL (VITAL) was conducted to address these knowledge gaps in a diverse U.S. cohort.
METHODS
Study Design
This randomized, double-blind, placebo-controlled, 2×2 factorial trial tested the benefits and risks of vitamin D3 (2000 IU/day) and n-3 fatty acids (1 g/day fish-oil capsule containing 840 mg of n-3 fatty acids including eicosapentaenoic acid [EPA, 460 mg] + docosahexaenoic acid [DHA, 380 mg]) in the primary prevention of cardiovascular disease and cancer among 25,871 men aged ≥50 and women aged ≥55, including 5,106 African Americans. The results are presented in two papers, with details of the full design in the accompanying paper containing the vitamin D3 data, the Supplementary Appendix, and also published earlier.5, 6 The protocol is posted at NEJM.org. The n-3 fatty acid dose chosen was that recommended by the American Heart Association for cardioprotection7 and demonstrated as beneficial in a secondary prevention population.8 The recruitment flow diagram is presented in Figure S1 in the Supplementary Appendix. Randomization to n-3 fatty acids, vitamin D, both active agents, or both placebos was completed in March 2014. Study medication ceased as planned on December 31, 2017, yielding a median intervention period of 5.3 years (range 3.8-6.1 years).
Baseline questionnaires collected data on clinical and lifestyle risk factors and included a dietary questionnaire that ascertained self-reported intake of fish and other foods. Annual questionnaires assessed adherence to and potential side effects of randomized treatments, incident major illnesses, and risk factor updates. Baseline blood samples were collected from all willing participants (n=16,956 of 25,871 [66%]) and were assayed for plasma omega-3 index (EPA+DHA as a percent of total fatty acids9) by Quest Diagnostics using liquid chromatography-tandem mass spectrometry.
Study Endpoints
Primary endpoints were major cardiovascular events (composite of myocardial infarction, stroke, and cardiovascular mortality) and total invasive cancer. Secondary cardiovascular endpoints were major cardiovascular events plus coronary revascularization [coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI)] and individual components of the primary endpoint. Secondary cancer endpoints were incident colorectal, breast, and prostate cancers, and total cancer mortality. Medical records of those reaching endpoints were reviewed by an Endpoints Committee of physicians blinded to treatment assignment. Myocardial infarction and stroke were confirmed using established criteria.10, 11 Cancer was confirmed with histologic or cytologic data.12 Additional details regarding endpoint confirmation can be found in the preceding paper and the Supplementary Appendix.
Statistical Analysis
Treatment-effect analyses were based on the intention-to-treat principle, as described in the companion paper on vitamin D. Primary analyses were based on Cox proportional hazards models controlling for age, sex, and randomization to vitamin D.
Possible variations in treatment effect by age, sex, baseline cardiovascular risk factors, baseline dietary fish intake and plasma omega-3 index, and concurrent randomization to vitamin D were specified a priori. Because vitamin D was also studied, treatment effects in racial/ethnic groups were of interest. Aspirin and statin use were additional stratification variables. There was no control for multiple hypothesis testing, with no formal adjustment to the p-values or confidence intervals. Thus, results for exploratory outcomes and subgroups should be interpreted with caution. Additional details regarding the analyses are in the Supplementary Appendix.
RESULTS
Baseline characteristics of study participants are in Table 1 (with further details in Table S1 of the Supplementary Appendix). Of the 25,871 participants, 51% were women and the mean age was 67.1 years. The cohort was racially diverse, including 20% African Americans. Randomization balanced characteristics between groups. The questionnaire response rate averaged 93.1%, and self-reported treatment adherence rates (percent taking ≥2/3 of the study capsules) in the active and placebo groups averaged 81.6% and 81.5%, respectively, over 5-year follow-up (Table S2, Supplementary Appendix). The prevalence of outside use of fish-oil supplements was below 3.5% in both groups throughout follow-up. Among the 15,535 participants with analyzable baseline blood samples (60%), the mean plasma omega-3 index was 2.67% (SD, 0.9%) in both groups. Among the 1,583 participants who also provided an analyzable one-year blood sample, the mean omega-3 index rose to 4.13% (54.7% increase) in the active group and changed <2% in the placebo group.
Table 1.
Baseline Characteristics of the 25,871 VITAL Participants, According to Randomized Assignment to Marine Omega-3 Fatty Acids.a
| Baseline Characteristic |
All Participants | Treatment Group | |
|---|---|---|---|
| Omega-3s | Placebo | ||
| N | 25,871 | 12,933 | 12,938 |
| Sex, % female | 13085 (50.6) | 6547 (50.6) | 6538 (50.5) |
| Mean age ± SD, years | 67.1 ± 7.1 | 67.2 ± 7.1 | 67.1 ± 7.1 |
| Race/ethnicity, % | |||
| Non-Hispanic White | 18046 (71.3) | 9044 (71.5) | 9002 (71.2) |
| African American | 5106 (20.2) | 2549 (20.1) | 2557 (20.2) |
| Hispanic (not African American) | 1013 (4.0) | 491 (3.9) | 522 (4.1) |
| Asian/Pacific Islander | 388 (1.5) | 200 (1.6) | 188 (1.5) |
| American Indian/Alaskan Native | 228 (0.9) | 120 (0.9) | 108 (0.9) |
| Other/unknown | 523 (2.1) | 249 (2.0) | 274 (2.2) |
| Mean body mass index ± SD, kg/m2 | 28.1 (5.7) | 28.1 ± 5.7 | 28.1 ± 5.8 |
| Current smoking, % | 1836 ( 7.2) | 920 ( 7.2) | 916 ( 7.2) |
| Hypertension, treated with medication, %, | 12791 (49.8) | 6338 (49.3) | 6453 (50.2) |
| Cholesterol-lowering medication (current use), % | 9524 (37.5) | 4788 (37.7) | 4736 (37.2) |
| Diabetes, % | 3549 (13.7) | 1799 (13.9) | 1750 (13.5) |
Abbreviations: SD = standard deviation. There were no significant differences in the baseline characteristics between the groups.
Cardiovascular Disease
During follow-up, there were 805 major cardiovascular events, including 386 in the n-3 and 419 in the placebo group (comparing omega-3 with placebo, HR=0.92; 95% confidence interval, 0.80-1.06; p=0.24) (Table 2). Results of analyses of prespecified secondary cardiovascular outcomes were as follows: total myocardial infarction (HR=0.72 [0.59-0.90]; cardiovascular mortality (HR=0.96 [0.76-1.21]); total stroke (HR=1.04 [0.83-1.31]); and expanded cardiovascular composite (HR=0.93 [0.82-1.04]). Additional vascular outcomes included coronary artery bypass graft (HR=0.99 [0.73-1.33]), percutaneous coronary intervention (HR=0.78 [0.63-0.95]); fatal myocardial infarction (HR=0.50 [0.26-0.97]); and total coronary heart disease (HR=0.83 [0.71-0.97]) (see Table 2, which also shows results for stroke subtypes and stroke death).
Table 2.
Hazard Ratios (HR) and 95% Confidence Intervals (CI) of the Primary, Secondary, and Other Outcomes by Randomized Assignment to Omega-3 (n-3) Fatty Acids in Intention-to-Treat Analyses
| No. of Events | ||||
|---|---|---|---|---|
|
Outcome |
n-3 (N = 12,933) |
Placebo (N = 12,938) |
HR |
95% CI |
| Cardiovascular disease, 1° and 2° Outcomes | ||||
| Major cardiovascular eventsa,b | 386 | 419 | 0.92 | 0.80-1.06 |
| Expanded cardiovascular eventsc | 527 | 567 | 0.93 | 0.82-1.04 |
| Total myocardial infarction | 145 | 200 | 0.72 | 0.59-0.90 |
| Total stroke | 148 | 142 | 1.04 | 0.83-1.31 |
| Cardiovascular mortality | 142 | 148 | 0.96 | 0.76-1.21 |
| Other vascular outcomesd | ||||
| Coronary artery bypass graft (CABG) | 85 | 86 | 0.99 | 0.73-1.33 |
| Percutaneous coronary intervention (PCI) | 162 | 208 | 0.78 | 0.63-0.95 |
| Total coronary heart diseasee | 308 | 370 | 0.83 | 0.71-0.97 |
| Ischemic stroke | 111 | 116 | 0.96 | 0.74-1.24 |
| Hemorrhagic stroke | 25 | 19 | 1.32 | 0.72-2.39 |
| Coronary heart disease death | 37 | 49 | 0.76 | 0.49-1.16 |
| Fatal myocardial infarction | 13 | 26 | 0.50 | 0.26-0.97 |
| Stroke death | 22 | 20 | 1.10 | 0.60-2.01 |
| Total invasive cancera | 820 | 797 | 1.03 | 0.93-1.13 |
| Breast | 117 | 129 | 0.90 | 0.70-1.16 |
| Prostate | 219 | 192 | 1.15 | 0.94-1.39 |
| Colorectal | 54 | 44 | 1.23 | 0.83-1.83 |
| Cancer death | 168 | 173 | 0.97 | 0.79-1.20 |
| All-cause mortality | 493 | 485 | 1.02 | 0.90-1.15 |
| Excluding the first two years of follow-up: | ||||
| Major cardiovascular events | 269 | 301 | 0.89 | 0.76-1.05 |
| Total myocardial infarction | 94 | 131 | 0.72 | 0.55-0.93 |
| Total invasive cancer | 536 | 476 | 1.13 | 1.00-1.28 |
| Cancer death | 126 | 135 | 0.93 | 0.73-1.19 |
| All-cause mortality | 371 | 381 | 0.97 | 0.84-1.12 |
Primary outcomes
A composite of myocardial infarction, stroke, and cardiovascular mortality
A composite of myocardial infarction, stroke, cardiovascular mortality, and coronary revascularization (CABG, PCI)
Not prespecified as 1° or 2° outcomes.
A composite of myocardial infarction, coronary revascularization (CABG, PCI), and coronary heart disease death.
From Cox regression models controlling for age, sex, and vitamin D randomization group. The 95% CIs have not been adjusted for multiple comparisons.
Cumulative incidence rates of major cardiovascular events are shown in Figure 1. For major cardiovascular events, the curves did not differ significantly between groups. After excluding the first 2 years of follow-up, the HR for major cardiovascular events was 0.89 (0.76-1.05), and the reduction in myocardial infarction persisted (Table 2).
Figure 1.
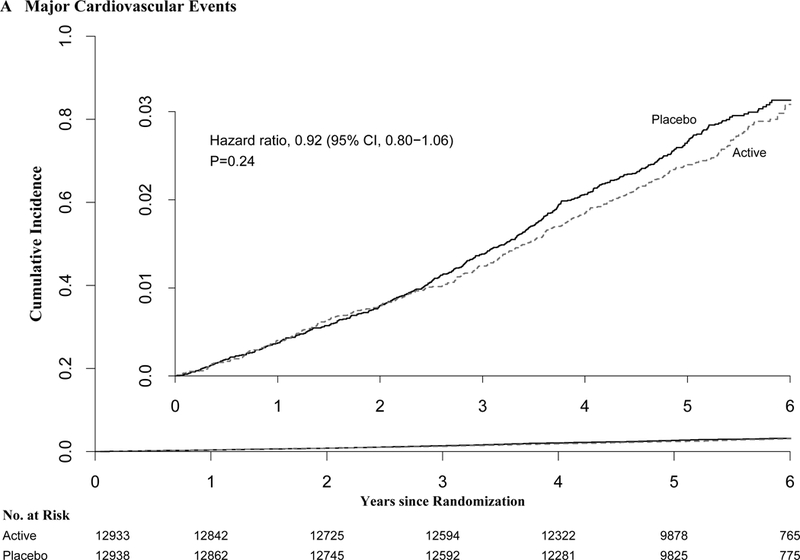
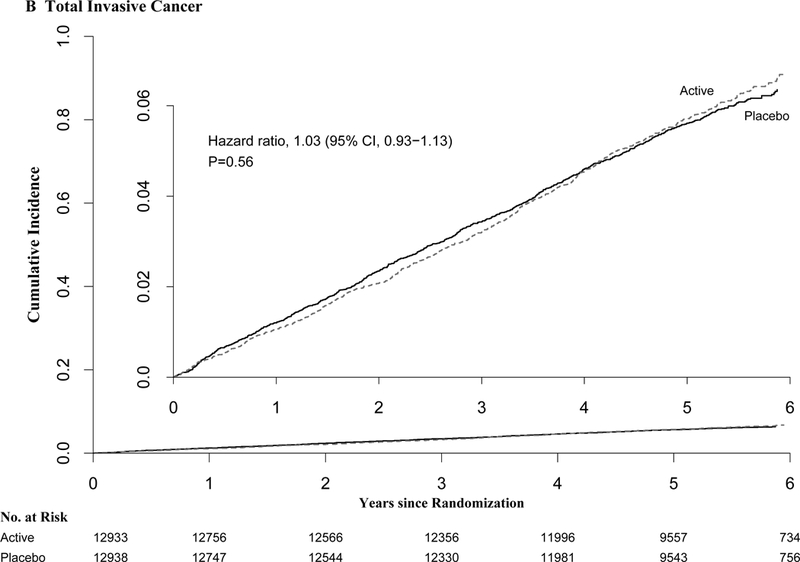
Cumulative Incidence Rates of A) Major Cardiovascular Events, B) Total Invasive Cancer, by Year of Follow-up. From Cox regression models controlling for age, sex, and vitamin D randomization group (intention-to-treat analyses).
Subgroup analyses showed a possible reduction in the primary cardiovascular outcome with omega-3 supplementation among those with low fish consumption (Figure 2). Additional subgroup analyses are presented in the Supplementary Appendix, Tables S3-S4 and Figure S2, with a focus on exploring differences according to race/ethnicity, diabetes status, number of traditional cardiovascular risk factors, dietary fish intake, and other variables for the primary endpoint of major cardiovascular events and the secondary endpoint of total myocardial infarction. For myocardial infarction, these are presented as explanatory analyses to assess whether the risk reduction was similar across subgroups. The suggestion of greater risk reductions for myocardial infarction among African Americans and those with lower fish intake, comparing the omega-3 group with the placebo group, is discussed in the Supplementary Appendix. For the other secondary cardiovascular endpoints of stroke, cardiovascular mortality, and the expanded composite of major cardiovascular events plus coronary revascularization, no appreciable effect modification was found (data not shown).
Figure 2:
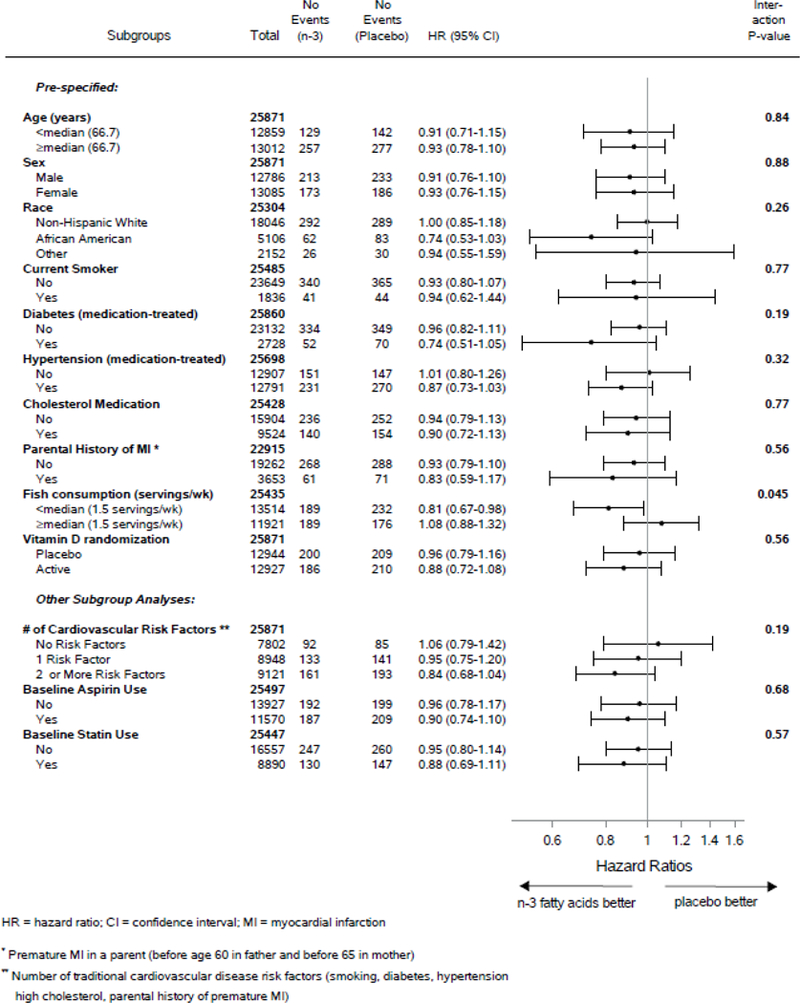
Hazard Ratios of Major Cardiovascular Events by Subgroups, comparing Omega-3 Fatty Acids (n-3) and Placebo Groups. From Cox regressions models controlling for age, sex, and vitamin D randomization group (intention-to-treat analyses). Analyses have not been adjusted for multiple comparisons.
Cancer and All-Cause Mortality
During follow-up, 1,617 participants developed invasive cancer, with similar rates in the n-3 and placebo groups (820 vs. 797 cancers; HR=1.03 [0.93-1.13]; p=0.56) (Table 2). No significant differences between randomized groups were observed for breast, prostate, or colorectal cancer; cancer mortality (RR=0.97 [0.79-1.20]); or all-cause mortality (HR=1.02 [0.90-1.15]).
Cumulative incidence curves for cancer and all-cause mortality did not differ significantly between treatment groups at any year of follow-up (Fig. 2). Tests for proportionality over time suggested violation for cancer (p=0.075). In analyses excluding the first two years of follow-up, the HRs were 1.13 (1.00-1.28) for cancer incidence and 0.93([0.0.73-1.19) for cancer mortality in the n-3 group (Table 2).
In subgroup analyses, sex may have modified the results for cancer incidence (p-interaction=0.024) (Table S5). Fish intake at baseline may have modified the intervention’s effects on all-cause mortality, (p-interaction=0.017; Supplementary Appendix, Table S6). There were no other significant interactions for cancer endpoints or all-cause mortality.
Adverse Events
The intervention was not associated with significant differences in gastrointestinal symptoms, major bleeding episodes, or other serious side effects, compared to placebo (Supplementary Appendix, Table S7).
DISCUSSION
In this 5.3-year primary prevention trial, n-3 supplementation (1 g/day) as compared to placebo did not lead to a significant reduction in the primary endpoints of major cardiovascular events (a composite of myocardial infarction, stroke and cardiovascular mortality) or invasive cancer. Analyses of the components of the primary composite cardiovascular endpoint suggested a reduction in myocardial infarction and no change in cardiovascular mortality or stroke. Exploratory analyses excluding the first two years of follow-up suggested a nonsignificant increase in cancer incidence, but no increase in cancer mortality.
Meta-analyses of n-3 supplementation trials in adults with or at high risk for cardiovascular disease have concluded that supplementation has no, or at most a weak, preventive effect on cardiovascular outcomes, including major vascular events, major coronary events, myocardial infarction, stroke, and revascularization.13–15 The recently completed ASCEND trial,16 which tested n-3 supplementation (1 g/day) in U.K. adults with diabetes, also reported generally null results. Thus, the possible reduction in the secondary outcomes of myocardial infarction and PCI in our trial, which tested n-3 fatty acids for primary prevention in a usual-risk population, raises the question of potential differences in results between primary and secondary prevention settings. Notably, neither our trial nor the secondary prevention trials indicate benefit of n-3 supplementation on stroke or composite cardiovascular endpoints. Our finding of a possible reduction with n-3 supplementation for the primary cardiovascular outcome among those with low fish consumption -- a characteristic that has rarely been examined as an effect modifier in previous trials—is hypothesis-generating and requires further study.
Two early large (n≥10,000) open-label trials8, 17 testing doses of 1 g/day or higher found significant coronary protection, but all but one18 of the subsequent placebo-controlled trials16, 18–23 (some with smaller sample sizes18–21 and lower doses19, 21) did not. The finding of a possible reduction in our trial may be partly attributable to these design differences. Also, the prevalence of use of cardiovascular medications, including statins, β-blockers, and anticoagulants, was higher in recent trials, perhaps reducing the opportunity to detect incremental benefit. Although a recent meta-analysis14 of n-3 trials found no variation in results by statin use, dilution of a potential n-3 effect by other medications cannot be ruled out. Such a dilution would likely be greater in secondary prevention settings, in which medication use is more prevalent. In addition, participants in secondary prevention trials generally have more advanced atherosclerosis, which may require more powerful interventions than n-3 fatty acids or higher n-3 doses to avert clinical events. Indeed, a greater n-3 benefit on major vascular events was observed among participants without prior stroke in a recent meta-analysis14 and in those without prior vascular disease in a trial among patients with macular degeneration.24 Differences in fish consumption across study populations may have also influenced findings. Finally, there were few black participants in the secondary prevention trials, and our trial suggest a greater coronary benefit of supplemental n-3 fatty acids in this racial group.
The finding in a subgroup analyses of the secondary outcome of MI suggesting possible greater cardiovascular benefits of n-3 supplementation for African Americans than for non-Hispanic whites, was unexpected, especially given that both racial/ethnic groups had similar baseline EPA+DHA blood levels and fish intake, and may be a chance finding that requires corroboration in future trials. Recent observational studies find racial variation in associations of both marine- and plant-derived n-3 biomarkers with incident coronary disease.25 Gene variants influence metabolism and bioavailability of n-3 fatty acids, as demonstrated in Greenland Inuits,26 and may influence coronary risk.27 Other racial/ethnic differences in clinical, dietary, or environmental factors may also account for this finding.28 Finally, African Americans have a higher prevalence of comorbidities such as diabetes and hypertension. However, treatment-associated HRs for myocardial infarction remained reduced across cardiovascular-risk strata in African Americans, with greater risk reductions than in non-Hispanic whites (Table S3).
That supplemental n-3 fatty acids confer coronary protection is biologically plausible. Data from laboratory and animal studies, as well as small trials of intermediate cardiovascular endpoints in humans, support mechanisms including anti-thrombotic, hypotriglyceridemic, blood pressure lowering, and anti-inflammatory effects; impeded growth of atherosclerotic plaques; slowing of heart rate; reduced susceptibility to cardiac arrhythmias; and promotion of nitric-oxide induced endothelial relaxation whereby n-3 fatty acids may reduce risk.1, 5 Experimental studies support relevant molecular and gene-regulatory effects.1 The dose-response curve for most effects plateaus at 1 g/day or lower.29 Observational studies suggest significant inverse associations between fish intake or n-3 fatty acid biomarkers and coronary outcomes—findings compatible with these mechanisms.25, 30–32
With regard to cancer, our findings are consistent with results of secondary cardiovascular disease prevention trials, which have mostly reported neutral effects or slight (but nonsignificant) elevations in cancer incidence with n-3 fatty acids.8, 16, 17, 22, 23, 33, 34 A 2014 meta-analysis of 10 n-3 fatty acid trials found a nonsignificant 10% increase in cancer risk (p=0.12).35 A 2018 meta-analysis of n-3 trials of cardiovascular disease14 also reported no significant association between supplementation and incident cancer but did not provide an effect estimate. Our finding of a more favorable effect on incident cancer in women contrasts with results of the SU.FOL.OM3 n-3 trial33 of an increased cancer risk in women but not men. Three trials have reported on cancer mortality, with two finding a neutral effect16, 18 and one a borderline significant reduction.34 The absence of a significant n-3 treatment effect on all-cause mortality in the present trial is consistent with results of meta-analyses of earlier trials13, 15 and ASCEND.16
The strengths of our trial include a large general population sample with racial/ethnic and geographic diversity; high follow-up rates and pill-taking adherence; high blood-collection rates; validated biomarkers of adherence; dietary assessments; and rigorously adjudicated endpoints. Ancillary studies examining diabetes, atrial fibrillation, cognition, autoimmune disorders, and other outcomes will inform the overall benefit-risk balance of n-3 supplementation.
Our study also has certain limitations. Median treatment duration was 5.3 years. The trial’s single n-3 dose did not permit exploration of dose-response relationships. However, the dose used has been recommended by the American Heart Association for cardioprotection in persons with prior coronary disease7, 36 and is at least twice the dose recommended for cardiovascular protection in healthy populations (1-2 servings of fish per week).30, 36 Results of ongoing trials37, 38 testing higher doses in high-risk populations will be informative but may not apply to primary prevention. Some of our subgroup analyses are based on small numbers of events.
In summary, omega-3 fatty acid supplementation did not reduce the primary outcomes of major cardiovascular events (myocardial infarction, stroke, and cardiovascular mortality) and total invasive cancer.
Supplementary Material
ACKNOWLEDGMENTS
VITAL Investigators, Staff, and Study Participants
The authors thank the VITAL investigators, staff, and the trial participants for their outstanding dedication and commitment.
Drs. Manson, Cook, Lee, and Buring had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Funding/Support: VITAL was supported by grants U01 CA138962 and R01 CA138962, which included support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple Institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others.
Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured the plasma omega-3 index at no cost to the study.
VITAL was approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration.
VITAL is registered at clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
(Funded by the National Institutes of Health; VITAL ClinicalTrials.gov number: NCT01169259.)
Role of the Sponsor: The National Institutes of Health sponsors of the trial had a collaborative role in the design and conduct of the study and interpretation of the data. Final decisions concerning the above, however, as well as data collection, management, analysis, review or approval of the manuscript, and decision to submit the manuscript for publication resided with study investigators and the study research group. The opinions expressed in the manuscript are those of the study authors and do not necessarily represent the views of the Department of Health and Human Services/National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
The VITAL investigator group is listed in the Supplementary Appendix, available at NEJM.org.
Contributor Information
JoAnn E. Manson, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
Nancy R. Cook, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
I-Min Lee, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
William Christen, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Shari S. Bassuk, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Samia Mora, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Heike Gibson, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Christine M. Albert, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
David Gordon, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Trisha Copeland, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Denise D’Agostino, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Georgina Friedenberg, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Claire Ridge, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Vadim Bubes, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Edward L. Giovannucci, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA.
Walter C. Willett, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA.
Julie E. Buring, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
References
- 1.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–67. [DOI] [PubMed] [Google Scholar]
- 2.Rizos EC, Elisaf MS. Does supplementation with omega-3 PUFAs add to the prevention of cardiovascular disease? Curr Cardiol Rep. 2017;19:47. [DOI] [PubMed] [Google Scholar]
- 3.Gerber M Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012;107 Suppl 2:S228–39. [DOI] [PubMed] [Google Scholar]
- 4.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- 8.GISSI-Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 9.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive Medicine. 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35.22923432 [Google Scholar]
- 11.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 12.Fritz AG, Percy C, Jack A, et al. International Classification of Diseases for Oncology (ICD-O), Third Edition Geneva: World Health Organization; 2000. [Google Scholar]
- 13.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33. [DOI] [PubMed] [Google Scholar]
- 14.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7:CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018. [e-pub 2018 Aug 26]. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- 18.Tavazzi L, Maggioni AP, Marchioli R, et al. ; GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 19.Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26. [DOI] [PubMed] [Google Scholar]
- 20.Rauch B, Schiele R, Schneider S, et al. ; OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–9. [DOI] [PubMed] [Google Scholar]
- 21.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S; SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch J, Gerstein HC, Dagenais GR, et al. ; ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–18. [DOI] [PubMed] [Google Scholar]
- 23.Roncaglioni MC, Tombesi M, Avanzini F, et al. ; Risk and Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–8. [DOI] [PubMed] [Google Scholar]
- 24.Bonds DE, Harrington M, Worrall BB, et al. ; Writing Group for the AREDS Research Group. Effect of long-chain omega-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Internal Medicine. 2014;174:763–71. [DOI] [PubMed] [Google Scholar]
- 25.Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe). Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Internal Medicine. 2016;176:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–7. [DOI] [PubMed] [Google Scholar]
- 27.Martinelli N, Girelli D, Malerba G, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88:941–9. [DOI] [PubMed] [Google Scholar]
- 28.Dietary Tong H. and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta. 2016;1860:2891–8. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Appel LJ, Chiuve SE, et al. American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing and Council on Clinical Cardiology. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation. 2018;138:e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 32.Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15–29. [DOI] [PubMed] [Google Scholar]
- 33.Andreeva VA, Touvier M, Kesse-Guyot E, Julia C, Galan P, Hercberg S. B vitamin and/or omega-3 fatty acid supplementation and cancer: ancillary findings from the supplementation with folate, vitamins B6 and B12, and/or omega-3 fatty acids (SU.FOL.OM3) randomized trial. Arch Intern Med. 2012;172:540–7. [DOI] [PubMed] [Google Scholar]
- 34.Bordeleau L, Yakubovich N, Dagenais GR, et al. ; ORIGIN Trial Investigators. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care. 2014;37:1360–6. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YF, Gao HF, Hou AJ, Zhou YH. Effect of omega-3 fatty acid supplementation on cancer incidence, non-vascular death, and total mortality: a meta-analysis of randomized controlled trials. BMC Public Health. 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siscovick DS, Barringer TA, Fretts AM, et al. ; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing and Council on Clinical Cardiology. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation. 2017;135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt DL, Steg PG, Brinton EA, et al. ; REDUCE-IT Investigators. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol. 2017;40:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in Statin Treated Patients with High Levels of Triglycerides and Low Levels of High Density Lipoprotein Cholesterol: rationale and design of the STRENGTH Trial. Clin Cardiol. 2018. [e-pub 2018 Aug 19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


