Abstract
Background:
Fetal origin of adult cardiovascular disease is one of the most pressing public concerns and economic problem in modern life. Maternal cigarette smoking/nicotine abuse increases the risk of cardiovascular disease in offspring. However, the underlying mechanisms and theranostics remain unclear. We hypothesized that fetal and neonatal nicotine exposure enhances microRNA-181a (miR-181a) which targets large-conductance Ca2+-activated K+ (BKCa) channels, resulting in increased coronary vascular tone in adult offspring.
Methods:
Nicotine or saline was administered to pregnant rats via subcutaneous osmotic minipumps from gestational day 4 until postnatal day 10. Experiments were conducted in adult (~ 6 month old) male offspring.
Results:
Nicotine enhanced pressure-induced coronary vascular tone, which was abrogated by BKCa channel blocker. Nicotine selectively attenuated coronary BKCa β1 but not α subunit expression. Functionally, nicotine suppressed BKCa current density and inhibited BKCa activator NS1619-induced coronary relaxations. Furthermore, activation of BKCa increased coronary flow and improved heart ischemia/reperfusion-induced infarction. Nicotine selectively enhanced miR-181a expression. MiR-181a mimic inhibited BKCa β1 expression/channel current and decreased NS1619-induced coronary relaxation. Antioxidant eliminated the difference of BKCa current density between the saline and nicotine-treated groups and partially restored NS1619-induced relaxation in nicotine group. MiR-181a antisense decreased vascular tone and eliminated the differences between nicotine exposed and control groups.
Conclusion:
Fetal and neonatal nicotine exposure-mediated miR-181a overexpression plays an important role in nicotine-enhanced coronary vascular tone via epigenetic down-regulation of BKca channel mechanism, which provides a potentially novel therapeutic molecular target of miR-181a/BKca channels for the treatment of coronary heart ischemic disease.
Keywords: maternal nicotine, miR-181a, BKCa channel, coronary tone, ischemic heart
1. Introduction
Fetal and neonatal nicotine exposure, either from maternal smoking or nicotine use during pregnancy, has become one of the most pressing public concerns in modern life [1, 2]. Epidemiologic studies suggest that maternal cigarette smoking is associated with increased risk of cardiovascular disease in offspring [3, 4], and clinical studies have suggested that cigarette smoking-induced myocardial ischemia is mediated by nicotine [5, 6]. Recently, studies in different animal models from our and other laboratories have demonstrated that perinatal nicotine exposure causes a cardiovascular dysfunction and develops coronary heart ischemia-sensitive phenotype in offspring [7–9]. However, the underlying epigenetic mechanisms remain largely unknown.
MicroRNAs (miRNAs) belong to a family of small noncoding RNAs with a single strand of 18–25 nucleotides that regulate multiple target genes at the post-transcriptional level. Increasing evidence supports the pivotal role of miRNAs in the pathophysiological processes in the cardiovascular development and in the setting of coronary heart ischemic disease [10–12]. Recent studies indicate that miR-181a was down-regulated in obese patients with cardiovascular disease [13]. However, an up-regulation of miR-181a has also been shown in patients with cardiovascular disease [14, 15]. These findings suggest that miR-181a may play a key role in the development of cardiovascular disease either through compensatory or pathologic mechanisms. As a pivotal epigenetic mechanism, miRNAs are also sensitive to various prenatal insults and contribute to the programming of cardiovascular disease in late life [16–18]. Furthermore, previous studies have shown that nicotine selectively regulates specific miRNA expression [19–21]. These novel findings suggest that miRNA-mediated signaling may be a novel therapeutic molecular target and a new mechanistic association between nicotine exposure and development of coronary heart ischemia-sensitive phenotype.
Ischemic heart disease is induced by insufficient coronary blood flow to the myocardium, typically due to exaggerated coronary artery constriction or a narrowed vascular wall with the condition of atherosclerosis. Large-conductance Ca2+-activated K+ (BKCa) channels are abundantly expressed in coronary vascular smooth muscle cells (SMCs). Activation of the channel induces vascular relaxation. Therefore, the BKCa channels play a key role in regulating coronary vascular tone and coronary blood flow [22, 23]. BKCa channel in vascular SMCs mainly consists of a pore-forming α subunit and regulatory β1 subunits [24]. Decreased BKCa β1 subunit expression has been observed in coronary heart ischemic disease and other cardiovascular disease [26, 27].
Given the fact that our recent studies in nicotine-exposed pregnant rat model have demonstrated an aberrant development of heart ischemia-sensitivity phenotype in adult offspring [8, 9, 28], we first evaluated in the present study whether nicotine exposure altered coronary reactivity. Next, we examined whether nicotine exposure epigenetically down-regulated BKCa channel expression/activity. Finally, we investigated whether nicotine exposure selectively enhanced miR-181a expression and tested the specific hypothesis that therapeutic inhibition of miR-181a could rescue nicotine-induced exaggerated coronary vascular tone via epigenetic down-regulation of BKCa channel expression/activity in offspring.
2. Materials and Methods
The full details of the methods are presented in the Supplementary Material Section online.
2.1. Experimental animals
The procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University. All animal studies followed the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Time-dated (day 2 of gestation) pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI). On day 4 of gestation, the rats were randomly divided into two groups: saline control and nicotine-treated groups. Saline or nicotine (at 4 μg/kg/min) was individually administered to pregnant rats through osmotic minipumps from day 4 of pregnancy to 10 days after birth, as described in detail previously [8, 9, 28, 29]. The dose of nicotine resulted in blood levels closely resembling those of moderate human smokers [30]. Our previous studies have shown similar effects of perinatal nicotine on cardiac function in both male and female offspring [8, 9], therefore, the adult male offspring (~ 6 month-old) were kept to use for the underlying mechanism studies.
2.2. Measurement of coronary artery myogenic tone
To measure coronary vascular myogenic tone, septal coronary arteries were isolated as we described previously [31]. The detail methods were presented the Supplementary Material Section online.
2.3. Relaxation Studies
For relaxation studies, the pressurized arteries were pre-contracted with the sub-maximal concentration of norepinephrine (3 μM), followed by increasing concentrations of BKCa channel activator NS1619. Arterial diameter data were recorded using the SoftEdge Data Acquisition Subsystem, as described previously [31].
2.4. Measurement of BKCa channel current
Coronary arterial SMCs were isolated and enzymatically dissociated from both nicotine-treated and control offspring, and whole-cell K+ currents were recorded using an EPC 10 patch-clamp amplifier with Patchmaster software, as we previously described [32]. The detail methods were presented the Supplementary Material Section online.
2.5. Measurement of heart ischemia/reperfusion (I/R) injury
A heart ischemia/reperfusion (I/R) Langendorff preparation system was used, as we previously described [8, 9, 28]. The detail methods were presented the Supplementary Material Section online.
2.6. MicroRNA Transfection
The method of microRNA transfection has been described in our previous studies [33]. The detail methods were presented the Supplementary Material Section online.
2.7. Real-Time Reverse Transcription PCR (RT-PCR) analysis
Total RNA was isolated from coronary arterial segments using TRIzol reagent (Invitrogen, CA) and subjected to reverse transcription with miScript cDNA Synthesis system (Bio-Rad, Hercules, CA). Quantification of mature miRNAs was performed using the miScript II RT kit and the miScript SYBER Green PCR kit with miScript Primer Assay kit (Qiagen) according to the manufacturer’s instructions as described previously [33, 34]. SNORD61 was used as the internal control.
2.8. Western Immunoblotting
Protein abundance of BKCa channel in coronary arteries was measured as described previously [9, 32]. The detail methods were presented the Supplementary Material Section online.
2.9. Statistical analysis
All data are expressed as the mean ± SEM obtained from the number (n) of experimental animals given. Differences between the groups were compared by Student’s t-test or analysis of variance (ANOVA) using the GraphPad Prism software, where appropriate. For all comparisons, P-values less than 0.05 indicated statistical significance.
3. Results
3.1. Effect of nicotine exposure on pressure-dependent myogenic tone and BKCa channel-mediated relaxation in coronary arteries.
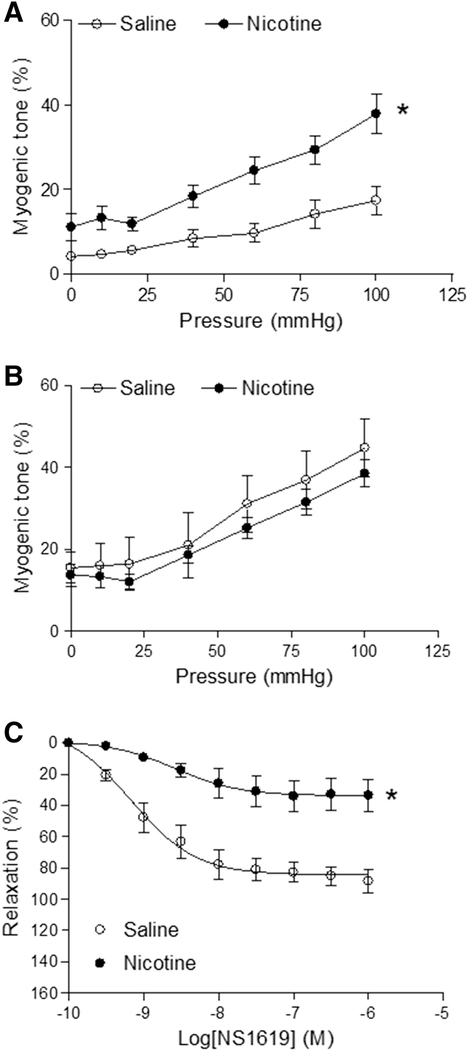
As shown in Figure 1A, the pressure-induced vascular tone in the nicotine-treated group was significantly higher than in saline control group. Pretreatment with BKCa channel inhibitor TEA (1.0 mM) increased the pressure-induced vascular myogenic tone in saline control group and eliminated the difference between the saline control and nicotine-treated groups (Figure 1B). In pressurized coronary arteries, BKCa channel opener NS1619 induced dose-dependent relaxations in both saline control and nicotine-exposed offspring (Figure 1C). However, NS1619-induced vascular relaxations in nicotine-exposed group were lower than in the saline control group (maximal relaxation: 86.8 ± 4.9% vs. 34.0 ± 4.8%; P < 0.05).
Figure 1. Effect of fetal and neonatal nicotine on myogenic tone and NS1619-induced relaxation in coronary arteries.
Pressure-dependent myogenic tone was determined in the absence (A) or presence (B) of TEA (1.0 mM, 20 min) in coronary arteries isolated from saline control or nicotine-exposed offspring (6 month old age). (C) The coronary arteries were placed in the chamber of a pressure myograph and were pressurized to 45 mmHg. Then, the pressurized arteries were pre-contracted with the sub-maximal concentration of norepinephrine (3 μM), followed by increasing concentrations of NS1619. The relaxation (%) is expressed as the percentage of the change in diameter in each tissue-induced by NS1619. Data of pressure-induced vascular tone are means ± SEM of animals from each group (n = 4) were analyzed by 2-way ANOVA. *P < 0.05 versus saline control. The values of the NS1619-induced maximal relaxation response were calculated by the GraphPad Prism software and presented in the text.
3.2. Effect of nicotine exposure on coronary vascular BKCa channel expression and activities.
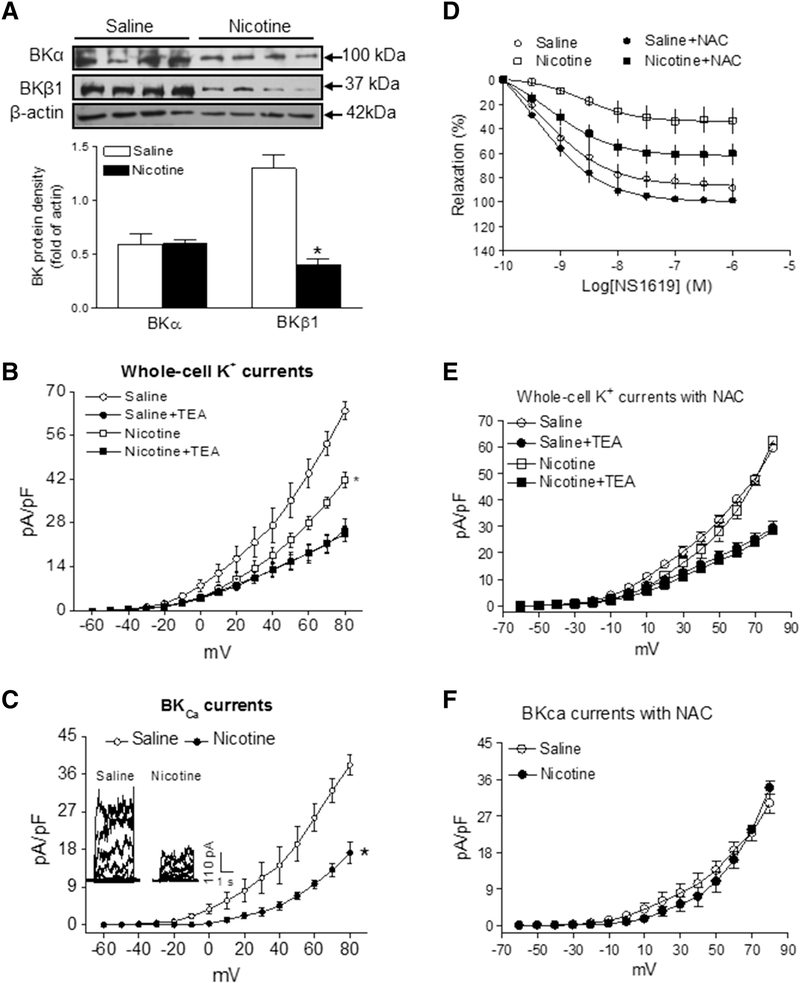
Nicotine exposure had no effect on protein abundance of BKCa α subunit but decreased the abundance of BKCa β1 subunit in coronary arteries of adult offspring as compared to the controls (Figure 2A). The whole-cell K+ current densities in coronary arterial myocytes in the voltage range of −60 mV to +80 mV were significantly higher in control than in nicotine-exposed offspring (at +80 mV: control, 63.8 ± 0.9 pA/pF; nicotine, 41.7 ± 2.4 pA/pF; P < 0.05; Figure 2B). As shown in Figure 2B, whole-cell K+ currents were sensitive to blockade by BKCa channel inhibitor TEA (1.0 mM). Treatment with TEA eliminated the differences of whole-cell K+ currents between the control and nicotine-exposed animals. As shown in Figure 2C, BKCa current densities, determined as the TEA-sensitive portion of the whole-cell K+ currents, were suppressed by nicotine exposure. The BKCa currents in nicotine exposed coronary arterial myocytes were lower than in control groups (at +80 mV: control, 38.2 ± 2.5 pA/pF; nicotine, 17.2 ± 2.5 pA/pF; P < 0.05) (Figure 2C). Similar TEA-insensitive portion of the whole-cell K+ currents in coronary myocytes of control and nicotine-exposed offspring suggests that the currents primarily mediated by voltage-gated potassium (Kv) channels were not altered by nicotine exposure.
Figure 2. Effect of fetal and neonatal nicotine on BKCa channel abundances/current and the role of ROS.
For measurement of BKCa channel protein level (A), coronary arteries were collected from both control and nicotine-treated adult offspring. The protein abundances of both BKCa α and β1 subunits in the coronary artery tissues were determined by Western blot analysis. For measurement of the whole-cell K+ channel currents (B) and BKCa channel currents (C), coronary arterial SMCs were isolated and enzymatically dissociated from both nicotine-exposed and control offspring. The BKCa channel current was determined as the difference between the whole-cell K+ current in the absence and presence of BKCa channel inhibitor TEA (1.0 mM). To see the effect of ROS on BKCa channel, an antioxidant N-acetyl-cysteine (NAC) was added in the coronary arteries or coronary arterial SMCs isolated from saline control or nicotine-exposed offspring. NS1619-induced relaxations (D) in the pressurized coronary arteries were determined in the absence or presence of 1 mM NAC. Whole-cell K+ channel currents (E) and BKCa channel currents (F) in the coronary arterial SMCs were recorded in the presence of 1 mM NAC. The data were expressed as the means ± SEM of animals from each group (n = 3~5). The BKCa channel protein comparison was determined between the nicotine-treated and saline control groups by Student’s t-test. The BKCa channel current comparison was determine by two-way ANOVA statistical analysis for. *P < 0.05 versus saline control. The values of the maximal relaxation response and their differences among groups were presented in the text.
3.3. Effect of reactive oxygen species (ROS) on nicotine-mediated BKCa channel activities.
Our previous studies have shown that perinatal nicotine exposure enhances ROS production in vasculatures [35]. To determine the role of ROS in BKCa channel activator NS1619-induced vascular tone, we measured the NS1619-induced coronary vascular relaxation with or without of antioxidant N-acetyl-cysteine (NAC) treatment. As shown in Figure 2D, NS1619-induced coronary vascular relaxations were not significantly altered by antioxidant in saline control offspring (maximal relaxation: 86.8 ± 4.9% vs. 99.7 ± 2.5%; P > 0.05). However, treatment with NAC reduced the effect of nicotine and partially restored the NS1619-induced relaxations in nicotine-exposed offspring (maximal relaxation: 34.0 ± 4.8% vs. 62.2 ± 4.6%; P < 0.05).
To determine the role of ROS in the nicotine-mediated effect on BKCa channel activities, whole-cell K+ and BKCa channel currents were determined in the presence of NAC in myocytes freshly isolated from coronary arteries of control and nicotine-exposed animals. In the presence of NAC treatment, there was no significant difference of whole-cell K+ current densities between the control and nicotine-exposed offspring (at +80 mV: control, 59.7 ± 1.5 pA/pF; nicotine, 62.5 ± 1.3 pA/pF; P > 0.05; Figure 2E). Similarly, there was also no difference of BKCa current densities between the control and nicotine-exposed offspring (at +80 mV: control, 30.2 ± 2.4 pA/pF; nicotine, 34.0 ± 1.5 pA/pF; P > 0.05; Figure 2F).
3.4. Effect of nicotine exposure on miRNAs expression and the role of miR-181a on nicotine-mediated coronary vascular tone.
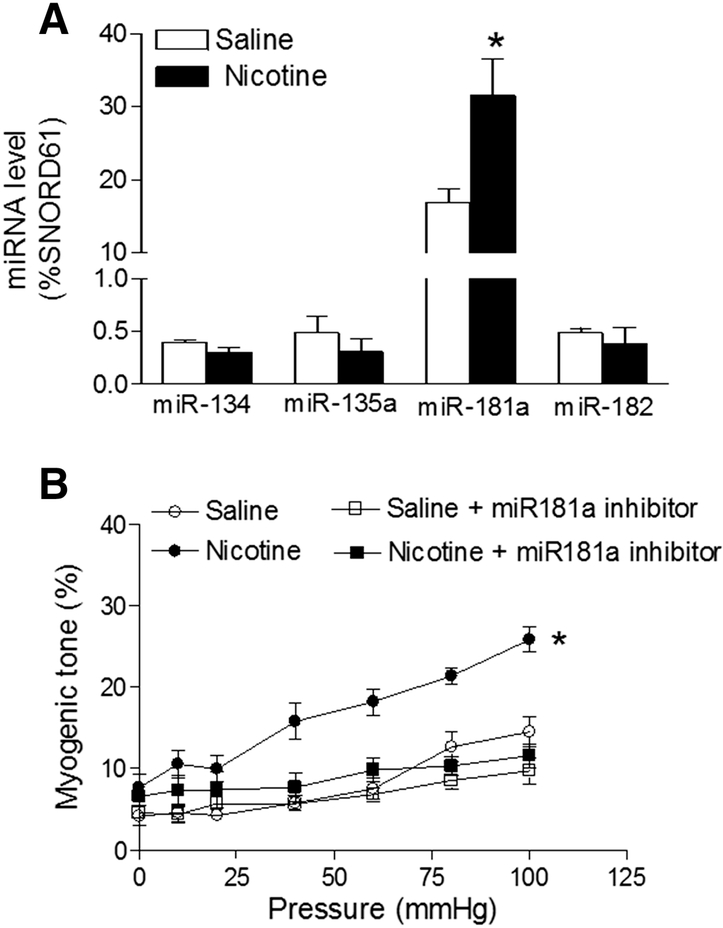
As shown in Figure 3A, nicotine exposure selectively enhanced the expression levels of miR-181a in coronary arteries as compared to the controls (16.96 ± 1.84% vs. 31.52 ± 4.99%; P < 0.05). To see whether inhibition of miR-181a attenuates nicotine-mediated coronary vascular tone, coronary arteries isolated from both control and nicotine-exposed offspring were transfected with anti-miR181a. As shown in Figure 3B, miR-181a-LNA significantly inhibited pressure-induced coronary vascular tone in the nicotine-exposed offspring and eliminated the differences of vascular tone between the control and nicotine-exposed offspring. To see whether the nicotine-mediated changes of miR-181a start in the neonatal period and continuing to adulthood, we measured the miR-181a levels in neonatal hearts (because of the technique limitation, we cannot isolate coronary arteries in early age of offspring). As shown in Figure 1S, nicotine exposure also enhanced the levels of miR-181a in neonatal hearts as compared to the controls (58.33 ± 3.63% vs. 89.98 ± 6.83%; P < 0.05).
Figure 3. Effect of fetal and neonatal nicotine on miRNAs levels and the effect of miR-181a inhibitor on perinatal nicotine-mediated coronary vascular tone.
(A) Coronary arteries were isolated from control or nicotine-exposed offspring. MicroRNAs levels in the coronary arteries were measured by qRT-PCR analysis. The expression levels of miRNAs are expressed as percentage of SNORD61. Data are means ± SEM of animals from each group (n = 4~6) and compared between the nicotine-treated and saline control groups by Student’s t-test. *P < 0.05 versus saline control. (B) Coronary arteries were freshly isolated from both saline control and nicotine-exposed offspring, and then treated ex vivo with miR-181a-LNA or negative control for 48 hours. After treatment, the pressure-induced myogenic tone was determined in the coronary arteries. Data are means ± SEM of animals from each group (n = 4~5). *P < 0.05 versus saline control, as determined by two-way ANOVA Statistical analysis.
3.5. The role of miRNA in regulation of BKCa channel.
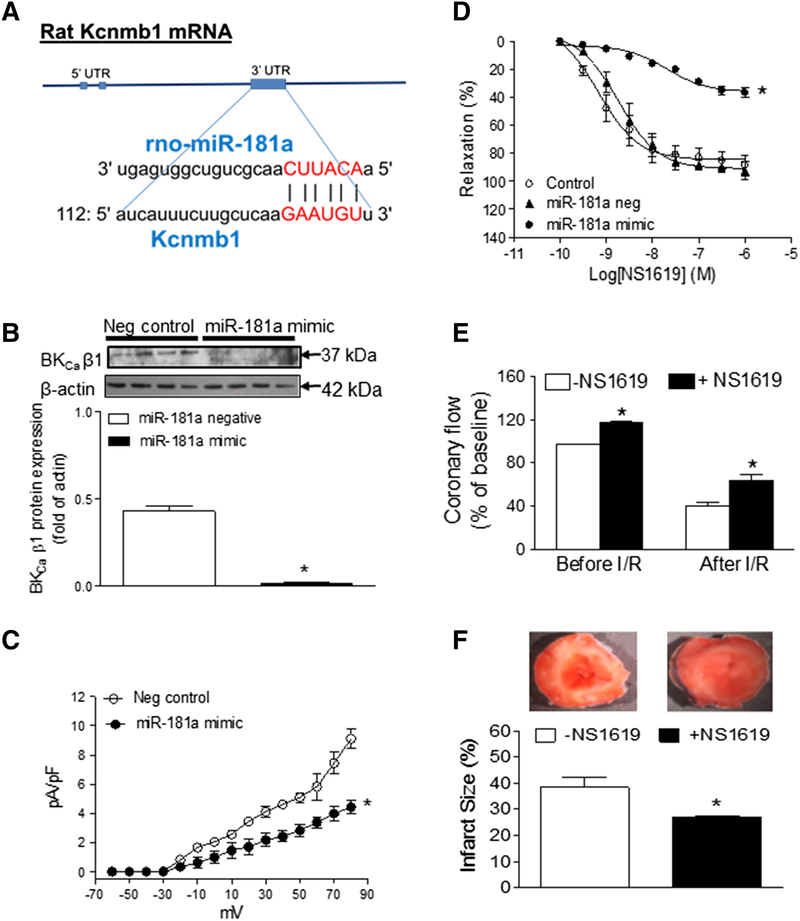
From the gene bank of rodent BKCa β1 encoded by Kcnmb1 mRNA sequence and the database on target miRNAs, we have identified that miR-181a is one of the key miRNAs which has predictive binding site at BKCa β1 mRNA 3’UTR region and can potentially regulate BKCa β1 gene expression (Figure 4A). To determine whether miR-181a directly regulates BKCa channel expression and activity, miR-181a mimic was transfected into the coronary arterial myocytes. As shown in Figure 4B, treatment with miR-181a mimic significantly knocked down BKCa β1 protein expression in coronary arteries. In addition, miR-181a mimic also attenuated BKCa current density in coronary SMCs (Figure 4C). Furthermore, miR-181a mimic (Figure 4D) decreased NS1619-induced coronary vascular relaxation as compared to the control (maximal relaxation: 36.04 ± 1.66% vs. 84.54 ± 3.21%; P < 0.05). However, miR-181a scramble (negative control) had no effects on NS1619-induced relaxation as compared to the control (maximal relaxation: 90.97 ± 3.11% vs. 84.54 ± 3.21%; P > 0.05).
Figure 4. Direct effect of miR-181a mimic on BKCa β1 expression and channel function and the effect of NS1619 on coronary flow and ischemic heart injury.
(A) Showing a potential binding site of miR-181a at 3’-UTR gene encoding BKCa β1 region (Kcnmb1). (B) Coronary arteries were freshly isolated from normal adult rats, and then treated with miR-181a mimic or negative control for 48 hours. After treatment with miR-181a mimic, BKCa β1 protein levels were determined in the coronary arteries by Western blot analysis. (C) BKCa channel current density was determined in the smooth muscle cells which were isolated from the miR-181a mimic-treated coronary arteries. (D) After treatment with miR-181a mimic or negative control, NS1619-induced relaxations were determined in the pressurized coronary arteries. (E) Hearts were isolated from adult rats and perfused in a Langendorff preparation. After the baseline recording, hearts are subjected to 30 minutes of global ischemia, followed by 60 minutes of reperfusion (I/R) in the absence or presence of NS1619 (10 μM) 10 minutes before ischemia and throughout the period of ischemia and reperfusion. The coronary flow rate was determined in the absence or presence of NS1619 both before and after I/R. (F) The I/R-induced infarct sizes in the left ventricle tissues were determined in the absence or presence of NS1619. Data are means ± SEM of animals from each group (n = 3~5). *P < 0.05 versus saline control, as determined by Student’s t-test or two-way ANOVA Statistical analysis.
3.6. Effect of NS1619 on coronary flow and ischemic heart injury.
To see whether BKCa channel-mediated coronary vascular tone plays a key role in coronary perfusion and heart ischemic injury, we performed experiments in ischemia/reperfusion (I/R) Langendorff preparation system [8, 9]. As shown in Figure 4E, the coronary flows both before and after I/R were significantly increased by infusion of the BKCa channel opener NS1619 as compared to the infusion of vehicle control. Furthermore, infusion with NS1619 also decreased I/R-induced cardiac injury (Figure 4F).
4. Discussion
Myogenic tone is an intrinsic property of vasculature, particularly small arteries. Pressure-induced myogenic tone is an important mechanism in the autoregulation of coronary blood flow [36]. Cardiac function is dependent on a constant oxygen supply from coronary circulation. The present study has shown increased coronary myogenic responses in the nicotine exposed offspring. Similarly, previous studies have also shown an increase in myogenic tone of systemic or coronary vessels in offspring in response to adverse perinatal stresses [31, 37]. Given the fact that perinatal nicotine exposure leads to a development of heart ischemia-sensitive phenotype in adult offspring [8, 9, 28], the present findings suggest that increased coronary vascular tone may be one of the key mechanisms underlying nicotine-mediated decrease in coronary flow and increase in heart ischemic injury. These findings are also consistent with epidemiological studies showing an association of adverse intrauterine environmental exposure and an increased risk of coronary heart disease in adult life [38].
Myogenic tone is regulated by many different vasoconstrictor and vasodilator influences acting on the blood vessel. These influences can be separated into extrinsic factors that originate from outside of the blood vessel, and intrinsic factors that originate from the vessel itself. As an intrinsic factor, BKCa channel is predominately located in arterial SMCs. Activation of the channel induces vascular relaxation. Previous studies have demonstrated that BKCa channels play a key role in regulating coronary vascular tone and coronary blood flow [22, 23]. Our present findings that inhibition of BKCa channel by TEA (1 mM) enhanced coronary vascular tone and activation of BKCa channels by NS1619 induced dose-dependent coronary relaxation in control animals, suggest that BKCa channels play a key role in the regulation of coronary vascular tone. Furthermore, in present studies we found that TEA treatment eliminated the differences of pressure-induced myogenic tone between the control and nicotine-exposed offspring and NS1619-induced coronary relaxations were attenuated in nicotine-exposed offspring as compared to the controls. This data suggests that nicotine-enhanced coronary vascular tone is regulated by BKCa channel signaling. The BKCa channel in SMCs consists of a pore-forming α subunit and regulatory β1 subunits [24]. The present findings that nicotine selectively decreased BKCa β1 but not α subunit protein expression and attenuated BKCa channel current density, suggest that down-regulation of BKCa β1 expression may be one of the key mechanisms underlying nicotine-mediated heightened coronary vascular tone. Similarly, decreased BKCa β1 subunit expression has been observed in coronary heart ischemic disease and other cardiovascular disease [26, 27].
The present finding that nicotine exposure down-regulated BKCa β1 subunit expression, suggests that epigenetic mechanisms are involved. There are at least three major epigenetic regulatory pathways including DNA methylation, histone modification, and non-coding RNAs. As a pivotal epigenetic mechanism, miRNAs are sensitive to various prenatal insults [16, 17]. Recent studies have shown that prenatal stress, including maternal cigarette smoke, modifies epigenetic signatures through miRNA signaling linked to disease during critical periods of fetal development [17, 18]. From the gene bank of rodent BKCa β1 (KCNMB1) mRNA sequence and the database on target miRNAs, we have identified that many miRNAs, including miR-134, miR-135a, miR-181a, and miR-182, can potentially bind at 3’-UTR of gene encoding BKCa β1 mRNA region. Of interest, the present data indicates that nicotine exposure selectively enhanced miR-181a expression in coronary arteries in adult offspring. In addition, our data also showed a similar pattern of increase in miR-181a expression in neonatal hearts, which suggests that nicotine-mediated changes of miR-181a could program from the neonatal period to adulthood. Furthermore, we found that treatment of miR-181a mimic knocked down BKCa β1 expression, inhibited BKCa channel current and attenuated NS1619-induced coronary relaxation. These findings validate that miR-181a could directly down-regulate BKCa channel. To see whether the enhanced miR-181a expression plays a key role in nicotine-mediated up-regulation of coronary vascular tone, we treated the arteries with the miR-181a antisense. The present findings that inhibition of miR-181a eliminated the differences of pressure-induced coronary vascular tone between the control and nicotine-exposed offspring, suggest that increased miR-181a is at least one of the important mechanisms underlying nicotine-mediated exaggerated vascular tone. Indeed, previous studies have shown that multiple members of miRNAs are consistently up-regulated in coronary heart ischemic injury [10–12, 39]. Therapeutic treatment with anti-miRNAs has been reported in different animal models, where inhibition of the specific miRNA led to a reduced heart infarct size and enhanced heart function after ischemic cardiac injury [10–12, 39]. These novel findings provide a solid basis for our future studies to test whether inhibition of miR-181a restores the nicotine-mediated increased heart ischemic injury in offspring.
ROS are important biomarkers that regulate diverse biological responses. Maternal smoking and nicotine use during pregnancy result in increased ROS in fetal, neonatal and adult tissues [40, 41]. Recently, we have demonstrated that nicotine exposure induces a fetal programming of adult cardiovascular dysfunction associated with an enhanced ROS production in cardiovascular tissues [28, 35]. A novel finding in the present study is that antioxidant (NAC) treatment increased NS1619-induced coronary vascular relaxation in nicotine-exposed but not in control offspring, which provides direct evidence that nicotine-induced programming of vascular dysfunction is, at least, partly mediated by oxidative stress. Similar studies have reported that antioxidant treatment prevents pancreas β-cell apoptosis observed in nicotine-exposed offspring [42]. In addition, previous studies have demonstrated that antioxidant prevents nicotine-mediated cardiovascular dysfunction in offspring [28]. The present finding that antioxidant eliminates the differences of BKCa channel current density between the control and nicotine-exposed offspring, suggests that oxidative stress plays a causal role in nicotine-mediated suppression of BKCa channel function. Consistent with our finding, previous studies have also provided direct evidence of a causative role of oxidative stress in regulation of BKCa channel in vasculatures [32]. However, there is a debate whether ROS directly regulates BKCa channel function. Recent studies have shown that ROS directly increases miR-181a expression in cardiomyocytes and the inhibition of miR-181a confers cardiac protection against oxidative stress-induced apoptosis [43]. From those findings, we can speculate that nicotine-induced oxidative stress leads to up-regulation of miR-181a expression, and consequently down-regulation of BKCa channel and coronary dysfunction.
Ischemic heart is associated with an increased coronary vascular tone and a reduction of coronary reserve which is mainly regulated by BKCa channel [23]. Our present data show that activation of BKCa channel by NS1619 increases coronary flow and decreases ischemia/reperfusion-induced heart injury, which are consistent with previous reports that administration of BKCa channel opener NS1619 increases coronary flow, decreases heart ischemic injury and improves heart function [44]. These findings suggest that BKCa channel plays a significant physiological role in maintaining coronary perfusion and in protecting against heart ischemic injury. In addition, our recent studies have shown increased heart vulnerability to ischemic injury in perinatal nicotine exposed offspring [8, 9]. Of importance, our present finding indicates that nicotine exposure causes a decrease in BKCa β1 subunit expression/channel activity and increase in coronary arterial vascular tone in adult rat offspring. These observations suggest a novel mechanism that nicotine-induced repression of BKCa β1 subunit may account for the reduction in coronary reserve and development of heart ischemic disease. In our future study, we will further investigate whether miR-181a/BKCa channel signaling plays an important role in nicotine-mediated ischemic heart injury and dysfunction in offspring.
4.1. Study limitations
Although nicotine-mediated miR-181a over expression is one of the important molecular mechanisms underlying nicotine-induced coronary heart dysfunction in offspring, one of the limitations is that we don’t know how nicotine exposure induces miR-181a over expression. Recent studies have shown that miR-181a gene can be regulated by DNA methylation mechanism [45, 46], and our recent studies have also demonstrated that nicotine exposure alters DNA methylation patterns in offspring [9]. Therefore, in our future studies we will investigate whether nicotine-mediated DNA methylation plays a key role in regulation of miR-181a expression. Another limitation for present study is the selectivity of BKCa channel inhibitor (TEA) and activator (NS1619). Although previous studies have demonstrated that TEA (1 mM) and NS1619 are predominately acting on BKCa channel in vasculatures [32, 33], in our future studies, we will use some other specific BKCa channel inhibitor (such as iberiotoxin) and activator (NS11021) to validate our present results. In addition, cross-fostering experiments will be performed in our future study to determine whether the programming effect was driven from the mother or fetus/neonates.
4.2. Conclusions and future directions
Our present studies demonstrate that perinatal nicotine exposure selectively enhances BKCa-targeting miR-181a expression which directly cause an epigenetic repression of the BKCa β1 gene and a decrease in BKCa channel function, leading to heightened coronary vascular tone in offspring. Furthermore, inhibition of miR-181a and BKCa channel can reverse nicotine-mediated exaggerated coronary vascular tone in offspring. These findings have significant implications for our understanding of the epigenetic molecular mechanisms underlying fetal programming of coronary heart disease. Furthermore, this study suggests that modulation of the BKca channels by specific miRNA may be a novel therapeutic target for coronary heart ischemic diseases. Recent studies have shown that paternal nicotine exposure can cause behavioral disorder in multiple generations, which are associated with changes of certain gene (such as miR-15b gene) expression through DNA methylation mechanism [47, 48]. These novel evidences provide a molecular basis for our animal model that perinatal nicotine exposure could program miR-181a/BKCa β1 gene persisting in adulthood. Therefore, in our future studies, we will determine nicotine-mediated cardiac function and miR-181a/BKCa β1 gene expression patterns in different developing ages, even in the next generation.
Supplementary Material
Highlights.
Perinatal nicotine induces fetal programming of adult coronary heart dysfunction.
Perinatal nicotine selectively increases miR-181a in coronary arteries in offspring.
The enhanced miR-181a epigenetically represses BKCa β1 gene and channel function.
The decreased BKCa channel leads to development of coronary dysfunctional phenotype.
BKCa targeting miR-181a may be a novel therapeutic target for coronary heart ischemic diseases associated with fetal stress exposure.
Acknowledgments
This work was supported by National Institutes of Health Grants HL135623 (DX), DA041492 (DX), HD088039 (DX), HL118861 (LZ), and Loma Linda University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing of interests
The authors have declared that no competing interest exist in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000; 5: 231–41. [DOI] [PubMed] [Google Scholar]
- [2].Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010; 116: 364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alverson CJ, Strickland MJ, Gilboa SM, Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics. 2011; 127: e647–53. [DOI] [PubMed] [Google Scholar]
- [4].Blake KV, Gurrin LC, Evans SF, Beilin LJ, Landau LI, Stanley FJ, et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000; 57: 137–47. [DOI] [PubMed] [Google Scholar]
- [5].Schror K, Zimmermann KC, Tannhauser R. Augmented myocardial ischemia by nicotine-mechanisms and their possible significance. Br J Pharmacol. 1998; 125: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Winniford MD, Wheelan KR, Kremers MS, Ugolini V, van den Berg E Jr, Niggemann EH, et al. Smoking induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alteration in coronary artery tone. Circulation 1986; 73: 662–7. [DOI] [PubMed] [Google Scholar]
- [7].Gao YJ, Holloway AC, Zeng ZH, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008; 590: 264–68. [DOI] [PubMed] [Google Scholar]
- [8].Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther. 2008; 324: 331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ke J, Dong N, Wang L, Li Y, Dasgupta C, Zhang L, et al. Role of DNA methylation in perinatal nicotine-induced development of heart ischemia-sensitive phenotype in rat offspring. Oncotarget. 2017; 8: 76865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. 2012; 109: 17615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009; 324: 1710–3. [DOI] [PubMed] [Google Scholar]
- [12].Hinkel R, Penzkofer D, Zuhlke S, Fischer A, Husada W, Xu QF, et al. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a larger-animal model. Circulation. 2013; 128: 1066–75. [DOI] [PubMed] [Google Scholar]
- [13].Hulsmans M, Sinnaeve P, Van der Schueren B, Mathieu C, Janssens S, Holvoet P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J Clin Endocrinol Metab. 2012; 97: E1213–8. [DOI] [PubMed] [Google Scholar]
- [14].Marques FZ, Romaine SP, Denniff M, Denniff M, Eales J, Dormer J, et al. Signature of miR-181a on the renal transcriptome and blood pressure. Mol Med. 2015; 21: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhu J, Yao K, Wang Q, Guo J, Shi H, Ma L, et al. Circulating miR-181a as a potential novel biomarker for diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016; 40: 1591–1602. [DOI] [PubMed] [Google Scholar]
- [16].Khorram O, Han G, Bagherpour R, Magee TR, Desai M, Ross MG, et al. Effect of maternal undernutrition on vascular expression of micro and messenger RNA in newborn and aging offspring. Am J Physiol Regul Integr Comp Physiol. 2010; 298: R1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, et al. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS One 2013; 8: e56967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maccani MA, Knopik VS. Cigarette smoke exposure-associated alterations to non-coding RNA. Front Genet. 2013; 3: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ng TK, Carballosa CM, Pelaez D, Wong HK, Choy KW, Pang CP, et al. Nicotine alters microRNA expression and hinders human adult stem cell regenerative potential. Stem Cells Dev. 2013; 22:781–90. [DOI] [PubMed] [Google Scholar]
- [20].Taki FA, Pan X, Zhang B. Chronic nicotine exposure systemically alters microRNA expression profiles during post-embryonic stage in Caenorhabditis elegans. J Cell Physiol. 2014; 229:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Pan T, Zhong X, Cheng C. Nicotine upregulates microRNA-21 and promotes TGF-β-dependent epithelial-mesenchymal transition of esophageal cancer cells. Tumour Biol. 2014; 35: 7063–72. [DOI] [PubMed] [Google Scholar]
- [22].Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Sciences. 1992; 256: 532–35. [DOI] [PubMed] [Google Scholar]
- [23].Han JG, Yang Q, Yao XQ, Kwan YW, Shen B, He GW. Role of large-conductance calcium-activated potassium channels of coronary arteries in heart preservation. J Heart Lung Transplant. 2009; 28: 1094–101. [DOI] [PubMed] [Google Scholar]
- [24].Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997; 502: 545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]..
- [26].Amberg GC, Santana LF. Downregulation of the BK channel betal 1 subunit in genetic hypertension. Circ Res. 2003; 93: 965–71. [DOI] [PubMed] [Google Scholar]
- [27].Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel betal 1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004; 559: 849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xiao D, Wang L. Huang X, Li Y, Dasgupta C, Zhang L Protective effect of antenatal antioxidant on nicotine-induced heart ischemia-sensitive phenotype in rat offspring. PLoS One. 2016; 11: e0150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fuller DD, Dougherty BJ, Sandhu MS, Doperalski NJ, Reynolds CR, Hayward LF. Prenatal nicotine exposure alters respiratory long-term facilitation in neonatal rats. Respir Physiol Neurobiol. 2009; 169: 333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fewell JE, Smith FG, Ng VK. Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J Appl Physiol (1985). 2001; 90: 1968–76. [DOI] [PubMed] [Google Scholar]
- [31].Xiao D, Yang S, Zhang L. Prenatal cocaine exposure causes sex-dependent impairment in the myogenic reactivity of coronary arteries in adult offpring. Hypertension. 2009; 54: 1123–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hu XQ, Huang X, Xiao D, Zhang L. Direct effect of chronic hypoxia in suppressing large conductance Ca2+-activated K+ channel activity in ovine uterine arteries via increasing oxidative stress. J Physiol. 2016; 594: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hu XQ, Dasgupta C, Xiao J, Yang S, Zhang L. Long-term high altitude hypoxia during gestation suppresses large conductance Ca2+-activated K+-channel function in uterine arteries: a causal role for microRNA-210. J Physiol. 2018. June 5 Doi: 10.1113/JP276058. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang L, Ke J, Li Y, Ma Q, Ma Q, Dasgupta C, Huang X, et al. Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int J Biol Sci. 2017; 13: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011; 164: 1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev. 2006; 86: 1263–1308. [DOI] [PubMed] [Google Scholar]
- [37].Hemmings DG, William SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005; 289: H674–82. [DOI] [PubMed] [Google Scholar]
- [38].Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002; 13: 364–68. [DOI] [PubMed] [Google Scholar]
- [39].Hullinger TG, Montgometry RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012; 10: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Noakes PS, Thomas R, Lane C, Mori TA, Barden AE, Devadason SG, et al. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax. 2007; 62: 714–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bruin JE, Petre MA, Lehman MA, Raha S, Gerstein HC, Morrison KM, et al. Maternal nicotine exposure increases oxidative in the offspring. Free Radic Biol Med. 2008; 44: 1919–25. [DOI] [PubMed] [Google Scholar]
- [42].Bruin JE, Woynillowicz AK, Hettinga BP, Tarnopolsky MA, Morrison KM, Gerstein HC, et al. Maternal antioxidants prevent β-cell apoptosis and promote formation of dual hormone-expressing endocrine cells in male offspring following fetal and neonatal nicotine exposure. J Diabetes. 2012; 4: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang L, Huang H, Fan Y, Kong B, Hu H, Hu K, et al. Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via the mitochondrial apoptotic pathway. Oxid Med Cell Longev. 2014; 2014:960362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cao CM, Xiao Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther. 2005; 312: 644–50. [DOI] [PubMed] [Google Scholar]
- [45].Mei Q, Li X, Zhang K, Wu Z, Li X, Meng Y, et al. Genetic and methylation-induced loss of miR-181a2/181b2 within chr9q33.3 faciliyayes tumor growth of cervical cancer through the PIK3R3/Akt/FoxO signaling pathway. Clin Cancer Res. 2017; 23:575–586. [DOI] [PubMed] [Google Scholar]
- [46].Han L, Witmer PD, Casey E, Valle D, Sukumar S. DNA methylation regulated microRNA expression. Cancer Biol Ther. 2007; 6:1284–8. [DOI] [PubMed] [Google Scholar]
- [47].McCarthy DM, Morgan TJ Jr, Lowe SE, Williamson MJ, Spencer TJ, Biederman J, et al. Nicotine exposure of male mice produces hehavioral impairment in multiple generations of descendants. PLoS Biol. 2018; 16:e2996497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, et al. Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b. Sci Rep. 2017; 7:7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.