Abstract
Objective:
To compare physical activity energy expenditure (PAEE) and total daily energy expenditure (TDEE) in successful weight loss maintainers (WLM) to normal weight controls (NC) and controls with overweight/obesity (OC).
Methods:
Participants were recruited in three groups: WLM (n=25, body mass index, BMI 24.1±2.3 kg/m2; maintaining ≥13.6 kg weight loss for ≥1 year), NC (n=27, BMI 23.0±2.0 kg/m2; similar to current BMI of WLM), and OC (n=28, BMI 34.3±4.8 kg/m2; similar to pre-weight loss BMI of WLM). TDEE was measured using the doubly labeled water method. Resting energy expenditure (REE) was measured using indirect calorimetry. PAEE was calculated as [TDEE – (0.1×TDEE) – REE].
Results:
PAEE in WLM (812±268 kcal/d, mean±SD) was significantly higher compared to both NC (621±285 kcal/d, p<0.01) and OC (637±271 kcal/d, p=0.02). As a result, TDEE in WLM (2495±366 kcal/d) was higher compared to NC (2195±522 kcal/d, p=0.01) but not significantly different from OC (2573±391 kcal/d).
Conclusion:
The high levels of PAEE and TDEE observed in individuals maintaining a substantial weight loss (−26.2±9.8 kg maintained for 9.0±10.2 years) suggest that this group relies on high levels of energy expended in physical activity to remain in energy balance (and avoid weight regain) at a reduced body weight.
Keywords: energy metabolism, physical activity, obesity, energy balance, weight maintenance
Introduction
Changes in energy expenditure that occur with weight loss have been suggested to contribute to the propensity for weight regain after weight loss. Total daily energy expenditure (TDEE) declines with weight loss due to decreases in both resting energy expenditure (REE) and physical activity energy expenditure (PAEE) that result primarily from the reduction in body mass (1). Some evidence suggests substantial weight loss may also generate additional decreases in REE and TDEE beyond that expected based on changes in body weight and/or composition alone (1–6). To prevent weight regain, a permanent behavior change that leads to either a lower energy intake (EI) and/or a higher level of physical activity (PA) must occur to compensate for the reduction in TDEE. While caloric restriction is effective for weight loss, it appears to be relatively ineffective as a sole strategy for long-term weight loss maintenance (7–9). The energy gap theory proposed by Hill et al. (10) states in part that the decline in TDEE with weight loss creates an “energy gap” that must be filled in order to remain in energy balance (and avoid weight regain) at a reduced body weight. Because high levels of PA are consistently associated with successful long-term weight loss maintenance (11–19), it is possible that successful weight loss maintainers (WLM) sustain high levels of PAEE to fill this gap. As a consequence of these high levels of TDEE, the EI required to match energy expenditure may be more feasible for weight-reduced individuals to comply with long-term.
Much of the evidence demonstrating that successful WLM sustain high levels of PA is based on studies that have used self-reported measures (20–22) or activity monitors (16, 17, 19). Few studies have quantified PAEE in weight-reduced individuals who were previously overweight/obese using the gold standard doubly labeled water (DLW) method. In weight-reduced women, Schoeller et al. demonstrated that higher physical activity levels (PAL) measured at the end of the weight loss period were associated with less weight regain one year later (11). However, PAL was only measured at baseline. Kerns et al. evaluated 14 contestants who completed “The Biggest Loser”, a U.S. televised weight loss competition, and found that 6 years after the competition, the successful weight loss maintainers demonstrated a higher PAEE (12.2±1.3 kcal/kg/d) compared to those who regained weight (8.0±1.4 kcal/kg/d), while changes in EI from baseline were not different between groups (23). However, that study involved a small sample of individuals with severe obesity (class III) who underwent an extreme diet and PA intervention in the context of a reality weight loss competition, which significantly limits the generalizability of these results.
Although successful WLM sustain high levels of PA, how this impacts PAEE and TDEE is not known. The goal of this study was to measure PAEE and TDEE in a sample of successful WLM, and compare these measures to two control groups; 1) normal weight controls (NC) whose BMI was similar to the current BMI of the WLM; and 2) controls with overweight/obesity (OC) whose current BMI was similar to the pre-weight loss maximum BMI of the WLM. Our global hypothesis was that WLM would sustain higher levels of PAEE compared to both control groups. Moreover, we hypothesized that because of the high levels of PAEE, WLM would sustain a TDEE that was higher than NC, but not different than OC.
Methods
Participants
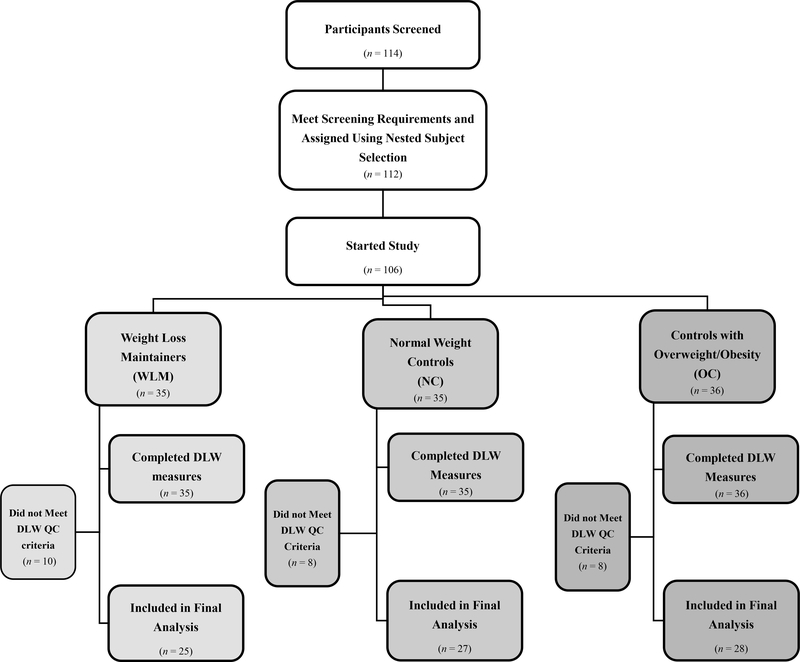
This case-control study was conducted at the University of Colorado Anschutz Medical Campus and approved by the Colorado Multiple Institutional Review Board. Participants included 106 adults (Figure 1) and were studied between October 2009 and August 2012. We recruited participants through campus wide flyers and email announcements. To enhance recruitment of WLM, a letter was sent to members of the National Weight Control Registry (NWCR) database living in the Denver Metro area. The NWCR was established in 1994 as a prospective cohort study to better understand behavioral patterns of individuals who are successfully maintaining weight loss; entry criteria includes maintenance of ≥13.6 kg weight loss for ≥1 year (24). Interested individuals then underwent preliminary telephone screening to determine if they met eligibility criteria for one of the three study groups: 1) Weight loss maintainers (WLM, maintaining ≥13.6 kg weight loss for ≥1 year); 2) normal weight controls (NC, BMI similar to current BMI of WLM) with no history of overweight/obesity, and 3) controls with overweight/obesity (OC, BMI similar to the maximum BMI of WLM). A nested subject selection procedure was used to obtain similar distributions for age (categories <36, 36–49, and ≥50 years) and sex (male vs. female) across all three groups. This design also ensured similar distribution between NC and WLM current BMI (categories <22, 23–25, and 26–30 kg/m2) and similar distribution between OC current BMI and WLM pre-weight loss maximum BMI (categories 26–30, 31–35, 36–40, and ≥41 kg/m2).
Figure 1: Study CONSORT Diagrama.
aConsolidated Standards of Reporting Trials (CONSORT); Doubly Labeled Water (DLW); Quality Control (QC).
Eligible individuals were invited to attend an in-person screening visit. After providing informed written consent, a health history and physical exam was completed. Individuals were excluded if they had any physical or medical condition that restricted PA (including diabetes, cardiovascular disease, cancer, and significant musculoskeletal, neurologic, or psychiatric disorders), had undergone weight loss surgery or were taking weight loss medications, were smokers, were not weight stable (>5 kg fluctuation in body weight over past 6 months), or were taking medications known to affect appetite or metabolism. Women who were pregnant or lactating were also excluded. Eligible individuals were then scheduled for a one-week free-living monitoring period.
Body Weight and Composition
Weight was measured with a calibrated digital scale (to the nearest 0.2 lbs; Tanita, BWB-800) and height with a wall-mounted stadiometer (to the nearest 0.1 cm). Weight was measured at the screening visit and on days 1 and 8 of the one-week free-living monitoring period. Waist circumference was measured just over the iliac crests at screening using a tape measure. Fat mass (FM) and fat free mass (FFM) were measured with the dual-energy X-ray absorptiometry (DXA, Delphi-W version 13.1.0; Hologic Inc., Bedford, MA) at screening. One OC participant’s supine body width exceeded scan window dimensions so FM and FFM were determined from bioelectrical impedance analysis (Tanita, TBF-105).
Resting Energy Expenditure
REE was measured using standard indirect calorimetry (Truemax 2400, ParvoMedics, Salt Lake City, UT) with the ventilated hood technique. Before each test was performed, the metabolic cart gas analyzers and flow meter were calibrated per manufacture recommendations. Participants were instructed to fast for 12 hours overnight, which was confirmed by study staff upon arrival in the clinic. Upon arrival (~7 AM), participants rested supine, awake, and lightly clothed in a thermoneutral (20–23 °C), dimly lit, quiet room for 30 minutes. REE was calculated using the Weir equation (25). Respiratory gas exchange was measured for 15 minutes, and the last 10 minutes were used to average REE. Criteria employed to determine if the REE measurement was acceptable included stability (coefficient of variance (CV) of the final 10 minutes <5%) and average metabolic equivalents (METs) <1.10, as previously described (26). REE measurements that did not meet these criteria were considered invalid and excluded from the analysis. REE was measured on days 1 and 8 of the free-living monitoring period, and averaged to produce a single value for REE. Intraclass correlation coefficient for day 1 and day 8 within-subject REE measures was high (0.96).
Total Daily Energy Expenditure, Physical Activity Energy Expenditure, and Physical Activity Level
TDEE over days 1–8 was determined using the DLW method (27). On the dosing day, participants arrived at the testing center following a 12-hour overnight fast. Upon arrival, participants voided their bladder and provided a baseline urine sample for determination of background abundances of 2H and 18O. Participants then consumed an oral dose of DLW containing 2.05 g/kg total body water (TBW, estimated as 73% of fat free mass determined from DXA) of 10 atom percent excess (APE) 18O and 0.14 g/kg TBW of 99.8 APE 2H (ISOTEC, Sigma Aldrich, Miamisburg, OH). The dosing cup was rinsed twice with 30 mL of water and the rinsing dose consumed. Exact time of dosing was recorded. Participants were instructed to void their bladder 1–3 hours after dosing, and additional urine samples were collected 4 and 5 hours after the dosing. On day 8, participants returned to the testing center. They were instructed to void upon waking, and the 2nd and 3rd voids were obtained at the same times as the 4- and 5hour post-dose samples on the dosing day. The urine samples were analyzed for 18O enrichment by isotope ratio mass spectrometry after equilibration with carbon dioxide. 2H was reduced with a platinum catalyst and the deuterium enrichment was determined by isotope ratio mass spectrometry (Delta V Advantage, Thermo Electron North America LLC, West Palm Beach, FL). Each sample was analyzed in a duplicate. If the difference between duplicate runs exceeded 2 δ‰ for 2H:1H or 1 δ ‰ for 18O:16O for a given sample, then that sample was run again and only duplicate values that fell within this range were used. TBW was calculated as the average of the dilution spaces of 2H and 18O (28), and the rate of carbon dioxide production was calculated using the equation A6 from Schoeller et al. (27). To be included in the analysis, data had to meet the following quality control criteria: 1) valid dilution space ratio, 2) similar TBW estimates from 2H and 18O, 3) similar slopes of elimination using the 4- and 5-hour urine collections on day 1 and day 8, and 4) sufficient abundance of 18O above background at day 8. TDEE was then estimated using the Weir equation, assuming a respiratory quotient of 0.86 (25). PAEE was calculated as [TDEE – (.1×TDEE) – REE], which assumes that the thermic effect of feeding is 10% of TDEE. Because the energy cost of PA is proportional to body weight for a given intensity and duration (29), PAEE was also calculated as relative to body weight (kg). PAL was calculated as [TDEE/REE].
Steps
Steps were assessed using the activPAL™ activity monitor (PAL Technologies, Glasgow, Scotland) to provide an additional objective measure of PA. The activPAL™ is a small (23×43×5 mm) and lightweight (10 g) device that uses accelerometer-derived information about thigh position to estimate time spent sitting/lying, standing, and stepping. The device is attached to the anterior thigh and is waterproofed by wrapping it in a nitrile sleeve, allowing for 24-hour measurement. The activPAL™ is a valid and reliable device for measuring steps per day (30). Participants were asked to wear the device continuously for seven consecutive days. Data were considered valid and used for analysis if the device was worn for >10 hours/d of time spent awake on ≥4 days (including ≥1 weekend and ≥2 weekdays) as previously described (16).
Statistical Analysis
Statistical analyses were performed with SAS version 9.4 (SAS System for Microsoft, SAS Institute Inc., Cary, NC, USA), with the type I error rate fixed at 0.05 (two tailed). Fisher’s exact tests were used to compare categorical demographic characteristics across the three groups. Normality of outcome measures was checked with the Shapiro-Wilk test. For variables where the Shapiro-Wilk test p<0.05, data transformations were used. A square root transformation was used for PAEE and FM. A log (base 10) transformation was used for minimum weight, maximum weight ever lost, FFM, REE, and PAL. A log (natural base) transformation was used for TDEE. Data are presented as mean±SD or mean (95% CI) unless otherwise stated. A one-way analysis of variance (ANOVA, PROC GLM, SAS) was used to estimate between-group differences in all outcome variables. A one-way analysis of covariance (ANCOVA) was used to estimate between-group differences in REE, adjusted for fat free mass only as well as FM and FFM. A Pearson’s correlation coefficient (PROC CORR, SAS) was used to examine the correlation between steps and TDEE, PAEE (unadjusted, and relative to body weight in kg), and PAL. Power was estimated using PASS (power and Sample Size, NCSS, Kayesville, UT) software. Using the most conservative assumptions, we estimated a clinically meaningful difference in TDEE between WLM and controls (NC and OC) would be ≥5% (130 kcal/d). Therefore, using a two-sample t-test, it was estimated that we would need 35 subjects per group to have 80% power to detect this difference. Although <35 subjects per group were included in the analysis, we retained statistical power to observe a between-group difference of 130 kcal/d as evidenced by the 95% CIs of the between-group difference in TDEE (WLM:NC 60–538 kcal/d, WLM:OC −316–158 kcal/d) (31). Results are reported as mean±SD unless otherwise stated.
Results
The REE and step data from this cohort have been previously published (16, 26). Of the 106 participants who completed the DLW measurements, TDEE data from 26 (WLM=10, NC=8, OC=8) was excluded from the analysis based on the quality control criteria outlined in the methods resulting in a final sample size of 80 participants (25 WLM, 27 NC, 28 OC) (Figure 1). Of these 80 participants, 4 did not have a valid REE (1=NC, 3=OC), and 12 did not have valid activPAL data (4=WLM, 8=OC). The nested subject selection procedures successfully achieved similar group means for age and sex (Table 1). The current BMI of WLM (24.1±2.3 kg/m2) was not different from NC (23.0±2.0 kg/m2), and maximum BMI of WLM (32.9±4.4 kg/m2) was not different from current BMI of OC (34.3±4.8 kg/m2). WLM were maintaining a weight loss of 26.2±9.8 kg for 9.0±10.2 years. There was no significant change in weight during the free-living week in WLM (0.22±0.67 kg), NC (−0.07±0.59 kg), or OC (0.39±1.16 kg).
Table 1:
Characteristics of Study Participantsa
| Characteristic | WLM (n = 25) | NC (n = 27) | OC (n = 28) | Overall P value | P value, WLM:NC | P value, WLM:OC |
|---|---|---|---|---|---|---|
| Age (y) [Mean ± SD] | 44.6 ± 12.2 | 49.4 ± 12.7 | 47.2 ± 11.5 | 0.37 | 0.16 | 0.44 |
| Anthropometric Measures [Mean ± SD] | ||||||
| Weight (kg) | 67.8 ± 9.4 | 63.7 ± 10.8 | 96.7 ± 17.8 | <0.01 | 0.27 | <0.01 |
| BMI (kg/m2) | 24.1 ± 2.3 | 23.0 ± 2.0 | 34.3 ± 4.8 | <0.01 | 0.22 | <0.01 |
| Waist Circumference (cm) b | 83.6 ± 7.3 | 83.7 ± 6.9 | 107.0 ± 13.1 | <0.01 | 1.00 | <0.01 |
| Maximum Weight (kg) c | 92.4 ± 14.7 | 67.9 ± 11.9 | 103.9 ± 21.5 | <0.01 | <0.01 | 0.01 |
| Minimum Weight (kg) d, e | 62.4 ± 9.9 | 56.4 ± 9.4 | 67.2 ± 16.2 | 0.01 | 0.05 | 0.25 |
| Maximum BMI (kg/m2) d | 32.9 ± 4.4 | 24.5 ± 2.3 | 36.8 ± 5.9 | <0.01 | <0.01 | <0.01 |
| Maximum Weight Ever Lost (kg) e | 26.2 ± 9.8 | 6.3 ± 5.6 | 12.8 ± 7.9 | <0.01 | <0.01 | <0.01 |
| Weight Loss Maintenance Duration (y) | 9.0 ± 10.2 | n/a | n/a | n/a | - | - |
| Sex, Male [n, (%)] | 5 (20%) | 7 (26%) | 6 (21%) | 0.87 | - | - |
| Ethnicity [n, (%)] | 0.69 | - | - | |||
| Hispanic/Latino | 1 (4%) | 3 (11%) | 3 (11%) | |||
| Not Hispanic/Latino | 24 (96%) | 24 (89%) | 25 (89%) | |||
| Race [n, (%)] | 0.04 | - | - | |||
| White | 25 (100%) | 24 (89%) | 23 (82%) | |||
| Black/African American | 0 (0%) | 1 (3.7%) | 5 (18%) | |||
| Asian | 0 (0%) | 1 (3.7%) | 0 (0%) | |||
| Not Reported | 0 (0%) | 1 (3.7%) | 0 (0%) | |||
| Respiratory Quotient [Mean ± SD] f | 0.81 ± 0.04 | 0.81 ± 0.03 | 0.80 ± 0.04 | 0.33 | 0.58 | 0.14 |
| Body Composition [Mean ± SD] | ||||||
| Fat Mass (kg) g | 18.8 ± 4.6 | 18.7 ± 4.1 | 38.7 ± 9.6 | <0.01 | 0.99 | <0.01 |
| Fat Mass (%) | 28.4 ± 6.5 | 30.1 ± 5.7 | 40.5 ± 5.1 | <0.01 | 0.29 | <0.01 |
| Fat Free Mass (kg) e | 47.6 ± 8.7 | 44.0 ± 9.4 | 56.4 ± 10.8 | <0.01 | 0.11 | <0.01 |
| Fat Free Mass (%) | 71.6 ± 6.5 | 69.9 ± 5.7 | 59.5 ± 5.1 | <0.01 | 0.29 | <0.01 |
Fisher’s Exact Test used for categorical variables; Continuous variables analyzed using one-way ANOVA. Significant P values (alpha <0.05) indicated in bold. Body Mass Index (BMI); Weight loss maintainers (WLM); Normal weight controls (NC); Controls with overweight/obesity (OC).
n = 27 for OC and n = 26 for NC. One OC participant had an invalid waist circumference, and one NC participant was missing waist circumference.
Excluding pregnancy.
After age 18 and excluding illness.
Analyzed using log (base 10) transformation, but untransformed mean±SD presented.
n = 26 for NC and n = 25 for OC.
Analyzed using square root transformation, but untransformed mean±SD presented.
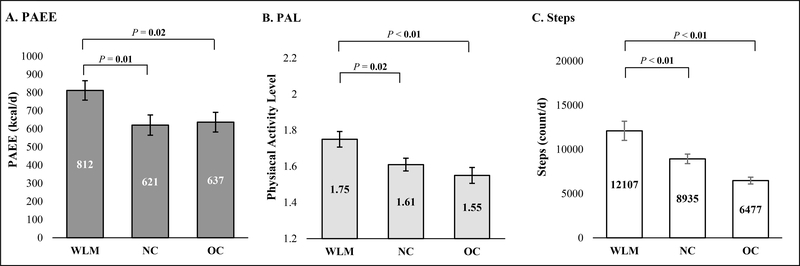
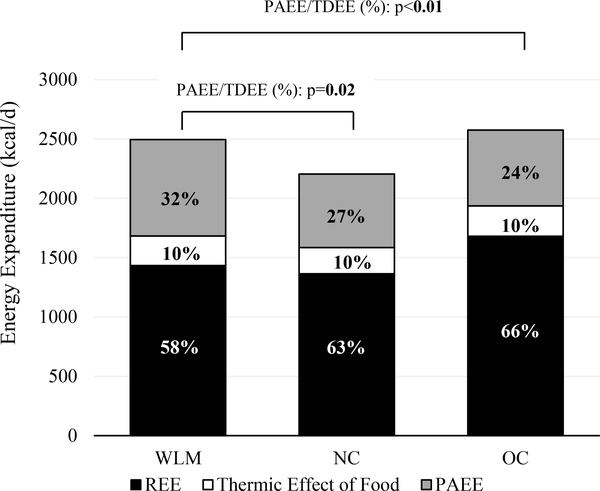
PAEE, TDEE, and REE data are presented in Table 2. Total PAEE in WLM (812±268 kcal/d) was significantly higher compared with both NC (621±285 kcal/d) and OC (637±271 kcal/d; Figure 2A). Similar results were obtained when PAEE was expressed relative to body weight (kcal/kg/d). In addition, PAEE comprised a significantly greater proportion of TDEE in WLM compared to both NC and OC, despite the similar body mass of NC and the greater body mass of OC (Figure 3). As a result, TDEE in WLM (2495±366 kcal/d) was significantly higher than NC (2195±522 kcal/d), but not different from OC (2573±391 kcal/d). Unadjusted REE (kcal/d) of WLM was not different compared with NC, but was significantly lower compared to OC. However, after adjusting for differences in FM and FFM, there were no between-group differences in REE.
Table 2:
Comparison of Energy Expenditure across Subject Groupa
| Energy Expenditure Outcome, [Least Squares Means (95% CI)] | WLM (n = 25) | NC (n = 27) | OC (n = 28) | P value, Omnibus F test | WLM:NC |
WLM:OC |
||
|---|---|---|---|---|---|---|---|---|
| Difference, (WLM-NC) | P value | Difference, (WLM-OC) | P value | |||||
| PAEE | ||||||||
| PAEE (kcal/d) b, c | 812 (703 – 922) | 621 (513 – 728) | 637 (528 – 747) | 0.02 | 192 (38 – 345) | 0.01 | 175 (20 – 330) | 0.02 |
| PAEE (kcal/body weight kg/d) b | 12.1 (10.6 – 13.5) | 9.6 (8.1 – 11.0) | 7.0 (5.5 – 8.4) | <0.01 | 2.5 (0.5 – 4.6) | 0.02 | 5.1 (3.1 – 7.2) | <0.01 |
| REE | ||||||||
| REE (kcal/d) b, d | 1433 (1338 – 1528) | 1365 (1272 – 1458) | 1680 (1585 – 1774) | <0.01 | 68 (−65 – 200) | 0.21 | -247 (−380 - −113) | <0.01 |
| REE (kcal/d) b, d, adjusted for fat free mass | 1465 (1422 – 1508) | 1468 (1425 – 1512) | 1539 (1494 – 1586) | 0.03 | -3 (−64 – 57) | 0.65 | -74 (−139 - −10) | 0.03 |
| REE (kcal/d) b, d, adjusted for fat mass and fat free mass | 1489 (1441 – 1537) | 1490 (1442 – 1538) | 1493 (1430 – 1556) | 0.61 | -1 (−60 – 59) | 0.62 | -4 (−96 – 89) | 0.51 |
| TDEE | ||||||||
| TDEE (kcal/day) e | 2495 (2322 – 2667) | 2195 (2029 – 2361) | 2573 (2411 – 2736) | <0.01 | 299 (60 – 538) | <0.01 | -78 (−316 – 158) | 0.55 |
Results from one-way ANOVA. Significant P values (alpha <0.05) indicated in bold. Weight Loss Maintainers (WLM); Normal weight controls (NC); Controls with Overweight/Obesity (OC); Physical Activity Energy Expenditure (PAEE); Total Daily Energy Expenditure (TDEE); Resting Energy Expenditure (REE).
n = 26 for NC and n = 25 for OC.
Analyzed using square root transformation, but untransformed mean (95% CI) presented.
Analyzed using log (base 10) transformation, but untransformed mean (95% CI) presented.
Analyzed using log (natural base) transformation, but untransformed mean (95% CI) presented.
Figure 2A-C: Physical Activity Energy Expenditure (A), Physical Activity Level (B) and Steps (C) across Subject Groupa-b.
aComparison of PAEE (A), PAL (B) and steps (C) across subject group. Results from one-way ANOVA and presented as mean±SE. Significant P values (alpha < 0.05) indicated in bold. PAEE analyzed using square root transformation, but untransformed mean±SE presented. PAL, caluclated as TDEE/REE, was analyzed using log (base 10) transformation, but untransformed mean±SE are presented; Weight Loss Maintainers (WLM); Normal Weight Controls (NC); Controls with Overweight/Obesity (OC); Physical Activity Energy Expenditure (PAEE), Physical Activity Level (PAL).
bPAL data are as follows: WLM: n = 25, 1.75±0.04; NC: n = 26, 1.61±0.04; OC: n = 25, 1.55±0.04; WLM:NC P = 0.02, WLM:OC P < 0.01; Steps data (count/d) are as follows: WLM: n = 21, 12107±1085; NC: n = 27, 8935±539; OC: n = 20, 6577±385; WLM:NC P < 0.01, WLM:OC P < 0.01.
Figure 3: Proportion of Physical Activity Energy Expenditure out of Total Daily Energy Expenditure across Subject Groupa-b.
aResults are from one-way ANOVA and presented as untransformed means (kcal/d). Significant P values (alpha < 0.05) indicated in bold. Labels represent the mean proportion of each component out of the mean total daily energy expenditure for each subject group. Weight Loss Maintainers (WLM); Normal Weight Controls (NC); Controls with Overweight/Obesity (OC); Physical Activity Energy Expenditure (PAEE); Resting Energy Expenditure (REE).
bMean±SD of PAEE/TDEE (%): WLM 32±7%, NC 27±7%, OC 24±8%; WLM:NC P = 0.02, WLM:OC P < 0.01.
The PAL of WLM was significantly higher than both NC and OC (Figure 2B). Similarly, daily step counts in WLM were higher than both NC and OC (Figure 2C). For correlation analyses, a smaller sample was used due to invalid REE and/or activPAL data (21 WLM, 26 NC, 20 OC). In WLM, daily step counts were strongly and positively correlated with total PAEE (kcal/d, r=0.78, p<0.01), relative PAEE (kcal/kg/d, r=0.85, p<0.01), and PAL (r=0.89, p<0.01), and there was a trend for a moderate, positive correlation with TDEE (r=0.41, p=0.07). In NC, daily step counts were moderately, positively correlated with relative PAEE (kcal/kg/d, r=0.45, p=0.02) and PAL (r=0.48, p=0.01), but not with total PAEE. In OC, there were no significant correlations between daily step counts and PAEE (total or relative), PAL, or TDEE.
Discussion
Study results suggest that PAEE of individuals maintaining a substantial weight loss (WLM, 26.2±9.8 kg maintained for 9.0±10.2 years) was significantly higher than PAEE of both non-reduced normal weight controls of similar BMI (NC), as well as controls with overweight/obesity of significantly higher BMI (OC). As a result of these high levels of PAEE, the TDEE in WLM was significantly higher than NC, but not different from the TDEE in OC, whose BMI was similar to the pre-weight loss maximum BMI of WLM. WLM also demonstrated significantly higher levels of objectively measured steps/d as compared to non-reduced controls of both types. The high levels of PAEE and TDEE observed in successful WLM suggest that this group relies on high levels of energy expended in PA to remain in energy balance (and avoid weight regain) at a reduced weight.
We observed high levels of PAEE relative to body size in our sample of WLM. Mean relative PAEE in WLM was ~12 kcal/kg/d as compared to ~10 kcal/kg/d in NC and ~7 kcal/kg/d in OC. These results are consistent with two previous studies that used DLW to assess energy expenditure in weigh-treduced individuals (11, 23). Schoeller et al. followed women for one year after weight loss and suggested that a PAEE of ~11 kcal/kg/d may be required to prevent weight regain (11). Kerns et al. (23) found a PAEE of ~12 kcal/kg/d in contestants from “The Biggest Loser” televised weight loss competition who maintained weight loss of ≥13% at 6 years follow up, as compared to a PAEE of ~8 kcal/kg/d in contestants who regained weight. PAL was also higher in WLM (~1.75) as compared to both NC (~1.61) and OC (~1.55). The PAL of ~1.75 observed in our sample of WLM is consistent with recommendations from the IASO 1st Stock Conference, which recommended a PAL of ≥1.70–1.75 to prevent weight regain (32). These estimates of PAL equate to approximately 60–90 minutes/d of moderate-intensity PA, such as walking, or 30–45 minutes/d of vigorous-intensity activity, such as running (32). Combined, these data suggest that high levels of PAEE may be critically important for successful long-term weight loss maintenance.
The differences in PAEE across our study groups are reflected in a similar pattern observed in objectively measured steps/d: WLM exhibited significantly higher steps/d (~12,100) compared to both NC (~8900) and OC (~6500). The daily step counts observed in our sample of WLM is higher than 10,000 steps/d, a common public health recommendation for PA. These results are consistent with prior studies demonstrating higher levels of objectively measured PA in WLM compared to controls of both types (16, 17). Further, in our study, objectively measured steps/d were significantly and positively correlated with relative PAEE in WLM and NC, but this correlation was not observed in OC. This finding suggests that in individuals of a normal body weight, actual PA performed (steps/d) may be driving PAEE, whereas in individuals with overweight/obesity, factors other than steps/d (such as the energy cost of moving a higher body mass) may play a stronger role in determining PAEE.
At weight maintenance, TDEE is equivalent to total daily EI. Participants in this study were weight stable during the 7 day DLW measurement period, thus TDEE can be interpreted to represent EI during the measurement period. In WLM, TDEE was ~2500 kcal/d, indicating that total daily EI was likely ~2500 kcal/d during the measurement period. This estimate of EI is substantially higher than previous studies that report a relatively restricted level of EI in successful WLM (~1370 to 1440 kcal/d) (24, 33). However, these studies relied on self-reported estimates of EI, which suffer from significant limitations and biases (34). If the short-term DLW measurements are reflective of participants’ habitual energy expenditure and intake patterns, our results indicate that EI in WLM was significantly higher than that of NC (of a similar BMI) and not significantly different from that of OC (of a substantially higher BMI). While recognizing the limitations of the cross-sectional data collection, the implications are that WLM filled the “energy gap” that resulted from weight loss (10) with high levels of PA rather than with reduced EI. Several studies have documented that long-term adherence to calorie restricted diets is difficult (7–9). In contrast, results from observational studies (16–18, 20) as well as randomized controlled trials (14, 19, 22, 35, 36) support the view that PA is critically important for successful weight loss maintenance. The high levels of PAEE and TDEE observed in our sample of WLM suggest they rely on high levels of energy expended in PA (rather than chronic restriction of EI) to achieve energy balance at a reduced body weight.
Our energy expenditure and EI findings are consistent with results from the longitudinal study of “The Biggest Loser” contestants which indicate that changes in EI (DLW intake-balance method) from baseline to 6 years after the competition were not correlated with amount of weight loss or weight regained at 6 years, whereas changes in PAEE were strongly correlated with weight loss and weight regained (23). Our findings are also consistent with Drenowatz et al. (37) who conducted a 2 year observational study to assess changes in weight, EI (DLW intake-balance method), and PA (SenseWear armband) in 195 young adults (age 20–35 years, BMI 20–35 kg/m2). In a subset who lost >5% body weight over the 2 years, EI did not change significantly from baseline, while bouts ≥10 minutes of moderate-to-vigorous PA significantly increased (by ~35 minutes/d) (37). Taken together, these results suggest PA may play a relatively greater role in weight loss maintenance rather than chronic restriction of EI.
We did not observe a significantly lower REE in WLM as compared to controls of both types, after adjusting for differences in FM and FFM. Some (but not all) studies have suggested that REE declines to a greater extent than expected from changes in body mass and/or body composition with weight loss (2, 38), and that this disproportionate reduction in REE may persist long-term (1–6 years) (38–40). For example, Fothergill et al. evaluated longitudinal changes in REE in 14 contestants, 6 years after completing “The Biggest Loser” televised weight loss competition. Using contestants’ baseline data to develop predictive equations for REE, mean REE after 6 years follow-up was ~500 kcal/d lower than predicted in that sample (38). Our group recently published a more in-depth examination of REE in the current study sample that compares measured REE to predicted REE, using several predictive equations. Our results indicated no consistent evidence of a lower than predicted REE in successful WLM (26). While results from this study suggest that our sample of WLM do not exhibit substantially lower REE (adjusted for FM and FFM) than controls, these data should be interpreted with caution as the lack of REE measurements prior to weight loss in this group does not allow us to determine whether REE may have decreased to a greater extent than expected for the amount of weight lost within a given individual.
Our study has several limitations. Due to the case-control study design, we were unable to assess longitudinal changes in PAEE or TDEE within subject groups. It is possible that our sample of successful WLM is inherently different than those who are not successful at weight loss or weight loss maintenance. Thus, future prospective, longitudinal studies of weight loss maintenance using objective measures of energy expenditure and EI are needed. Our WLM sample was relatively small and homogenous (predominantly female and Caucasian), therefore, results may not be generalizable to other populations. (31)Despite these limitations, this is the first study to compare objectively measured PAEE and TDEE, using the DLW method, in a group of adults who have successfully maintained their weight loss to non-reduced individuals with normal weight and individuals with overweight/obesity. Results from this study provide valuable insight into how individuals successfully achieve long-term weight loss maintenance.
Conclusion
Individuals maintaining a substantial weight loss (−26.2±9.8 kg maintained for 9.0±10.2 years) demonstrated significantly higher total PAEE (kcal/d), relative PAEE (kcal/kg/d), PAL, and objectively measured steps/d compared to controls of both types. As a result, TDEE in successful weight loss maintainers was significantly higher than non-reduced individuals of similar BMI, and not significantly different than TDEE in individuals with overweight/obesity. The TDEE observed in our weight stable weight loss maintainers (~2500 kcals/d) suggests habitual EI in this sample may be substantially higher than the level of EI reported in prior studies, which relied on self-reported EI measures in weight-reduced individuals. The high PAEE and TDEE observed in our sample of weight loss maintainers strongly supports the hypothesis that these individuals rely on increasing energy expended in activity (rather than chronic restriction of EI) to achieve energy balance at a reduced body weight; however, longitudinal studies are needed to further explore these findings.
STUDY IMPORTANCE QUESTIONS.
What is already known about this subject?
To prevent weight regain after weight loss, individuals must maintain a lower energy intake and/or a higher level of physical activity to compensate for the reduction in total daily energy expenditure (TDEE) that occurs with loss of body mass.
While caloric restriction is effective for weight loss, it appears to be relatively ineffective as a sole strategy for long-term weight loss maintenance. In contrast, high levels of physical activity are consistently associated with successful long-term weight loss maintenance.
Although high levels of physical activity are needed for weight loss maintenance, how this impacts physical activity energy expenditure (PAEE) and TDEE is unknown.
What does your study add?
Successful weight loss maintainers have significantly higher levels of PAEE as compared to both normal weight controls and controls with overweight/obesity.
As a result of these high levels of PAEE, the TDEE in weight loss maintainers is significantly higher than TDEE in normal weight controls of similar body mass index (BMI), but not different from TDEE in controls with overweight/obesity who have a substantially higher BMI.
The high levels of PAEE and TDEE observed in our sample of successful weight loss maintainers suggest that this group relies on high levels of energy expended in activity to remain in energy balance (and avoid weight regain) at a reduced weight.
Acknowledgements
We would like to thank all of our study participants as well as Dr. Rena Wing for providing access to the NWCR to assist with recruitment of study participants.
FUNDING: This work was supported by grants from the National Institutes of Health: NIH K23 DK078913, P30 DK048520, NIH UL1 TR002535, NIH T32 HL116276, NIH K99 DK100465, NIH R00 DK100465 as well as the American Heart Association: AHA 16PRE29660012. Dr. Melanson is supported by resources from the Geriatric Research, Education, and Clinical Center at the Denver VA Medical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government
Footnotes
CLINICAL TRIAL REGISTRATION: The trial has been registered at ClinicalTrials.gov (NCT03422380)
DISCLOSURE: JH and HW are partners in Shakabuku LLC, a company that provides weight management services, outside the submitted work; JH and HW have been issued a patent on the “Energy Gap” (United States Patent #7,949,506). JH and HW received clinical trial grant funding from Novo Nordisk and Gelesis. JH and HW received clinical trial grant funding and received payment for speaking for the Cattleman’s Beef Association. JH and HW report stock options from Retrofit, a company that provides weight management services. JH serves as a consultant for Zaluvida, Gelesis, and Livongo. HW accepted personal fees as an advisory board member for Atkins, a low-carbohydrate weight loss program during the time frame of the study presented. KL is a paid consultant for PAL Technologies, the company that manufactures the device used to measure physical activity in this study.
References
- 1.Ballor DL, Poehlman ET. A meta-analysis of the effects of exercise and/or dietary restriction on resting metabolic rate. Eur J Appl Physiol Occup Physiol. 1995;71(6):535–542. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Astrup A, Gotzsche PC, van de Werken K, et al. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69(6):1117–1122. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Melby CL, Paris HL, Foright RM, Peth J. Attenuating the biologic drive for weight regain following weight loss: Must what goes down always go back up? Nutrients. 2017;9(5). doi: 10.3390/nu9050468. PubMed PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Baak MA. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol Behav. 2008;94(2):178–186. doi: 10.1016/j.physbeh.2007.12.020. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–R192. doi: 10.1152/ajpregu.00474.2002. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 7.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: Patterns of weight regain among men and women. Int J Obes. 1989;13(2):123–136. PubMed PMID: . [PubMed] [Google Scholar]
- 8.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119(7 Pt 2): 688–693. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Mark AL. Dietary therapy for obesity is a failure and pharmacotherapy is the future: A point of view. Clin Exp Pharmacol Physiol. 2006;33(9):857–862. doi: 10.1111/j.1440-1681.2006.04454.x. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Hill JO, Peters JC, Wyatt HR. Using the energy gap to address obesity: A commentary. J Am Diet Assoc. 2009;109(11):1848–1853. doi: 10.1016/j.jada.2009.08.007. PubMed PMID: ; PubMed Central PMCID: PMCPMC2796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66(3):551–556. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr. 2002;75(3):499–504. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 13.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: A randomized trial. JAMA. 1999;282(16):1554–1560. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 14.Borg P, Kukkonen-Harjula K, Fogelholm M, Pasanen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: A randomized trial. Int J Obes Relat Metab Disord. 2002;26(5):676–683. doi: 10.1038/sj.ijo.0801962. PubMed PMID: [DOI] [PubMed] [Google Scholar]
- 15.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: Does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78(4):684–689. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Ostendorf DM, Lyden K, Pan Z, et al. Objectively measured physical activity and sedentary behavior in successful weight loss maintainers. Obesity (Silver Spring). 2017. doi: 10.1002/oby.22052. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catenacci VA, Grunwald GK, Ingebrigtsen JP, et al. Physical activity patterns using accelerometry in the national weight control registry. Obesity (Silver Spring). 2011;19(6):1163–1170. doi: 10.1038/oby.2010.264. PubMed PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelan S, Roberts M, Lang W, Wing RR. Empirical evaluation of physical activity recommendations for weight control in women. Med Sci Sports Exerc. 2007;39(10):1832–1836. doi: 10.1249/mss.0b013e31812383c3. PubMed PMID: ; PubMed Central PMCID: PMCPMC2699680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakicic JM, Tate DF, Lang W, et al. Objective physical activity and weight loss in adults: The step-up randomized clinical trial. Obesity (Silver Spring). 2014;22(11):2284–2292. doi: 10.1002/oby.20830. PubMed PMID: ; PubMed Central PMCID: PMCPMC4225630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catenacci VA, Ogden LG, Stuht J, et al. Physical activity patterns in the national weight control registry. Obesity (Silver Spring). 2008;16(1):153–161. doi: 10.1038/oby.2007.6. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the national weight control registry. Am J Prev Med. 2014;46(1):17–23. doi: 10.1016/j.amepre.2013.08.019. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550–1559; discussion 1559–1560. doi: 10.1001/archinte.168.14.1550. PubMed PMID: ; PubMed Central PMCID: PMC2829743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerns JC, Guo J, Fothergill E, et al. Increased physical activity associated with less weight regain six years after “the biggest loser” competition. Obesity (Silver Spring). 2017;25(11):1838–1843. doi: 10.1002/oby.21986. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–246. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1–9. PubMed PMID: ; PubMed Central PMCID: PMCPMC1392602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostendorf DM, Melanson EL, Caldwell AE, et al. No consistent evidence of a disporportionately low resting energy expenditure in long-term successful weight loss maintainers. Am J Clin Nutr. 2018;108(4):659–666. doi: 10.1093/ajcn/nqy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: Validation in humans and proposed calculation. Am J Physiol. 1986;250(5 Pt 2):R823–830. doi: 10.1152/ajpregu.1986.250.5.R823. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2h- and 18o-labeled water in humans. Am J Physiol. 1994;267(4 Pt 1):E585–590. doi: 10.1152/ajpendo.1994.267.4.E585. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 29.Schoeller DA, Jefford G. Determinants of the energy costs of light activities: Inferences for interpreting doubly labeled water data. Int J Obes Relat Metab Disord. 2002;26(1):97–101. doi: 10.1038/sj.ijo.0801851. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Grant PM, Ryan CG, Tigbe WW, Granat MH. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med. 2006;40(12):992–997. doi: 10.1136/bjsm.2006.030262. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121(3):200–206. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the iaso 1st stock conference and consensus statement. Obes Rev. 2003;4(2):101–114. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Catenacci VA, Odgen L, Phelan S, et al. Dietary habits and weight maintenance success in high versus low exercisers in the national weight control registry. J Phys Act Health. 2014;11(8):1540–1548. doi: 10.1123/jpah.2012-0250. PubMed PMID: ; PubMed Central PMCID: PMCPMC4568993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhurandhar NV, Schoeller D, Brown AW, et al. Energy balance measurement: When something is not better than nothing. Int J Obes (Lond). 2015;39(7):1109–1113. doi: 10.1038/ijo.2014.199. PubMed PMID: ; PubMed Central PMCID: PMCPMC4430460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain--a systematic review. Obes Rev. 2000;1(2):95–111. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: A randomized trial. JAMA. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Drenowatz C, Hill J, Peters J, Soriano-Maldonado A, Blair S. The association of change in physical activity and body weight in the regulation of total energy expenditure. Eur J Clin Nutr. 2017;71(3):377–382. doi: 10.1038/ejcn.2016.228. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 38.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “the biggest loser” competition. Obesity (Silver Spring). 2016;24(8):1612–1619. doi: 10.1002/oby.21538. PubMed PMID: ; PubMed Central PMCID: PMCPMC4989512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97(5):990–994. doi: 10.3945/ajcn.112.050310. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–912. PubMed PMID: . [DOI] [PubMed] [Google Scholar]