Abstract
Thiamine is an essential micronutrient that plays a key role in energy metabolism. Many populations worldwide may be at risk of clinical or subclinical thiamine deficiencies, due to famine, reliance on staple crops with low thiamine content, or food preparation practices, such as milling grains and washing milled rice. Clinical manifestations of thiamine deficiency are variable; this, along with the lack of a readily accessible and widely agreed upon biomarker of thiamine status, complicates efforts to diagnose thiamine deficiency and assess its global prevalence. Strategies to identify regions at risk of thiamine deficiency through proxy measures, such as analysis of food balance sheet data and month‐specific infant mortality rates, may be valuable for understanding the scope of thiamine deficiency. Urgent public health responses are warranted in high‐risk regions, considering the contribution of thiamine deficiency to infant mortality and research suggesting that even subclinical thiamine deficiency in childhood may have lifelong neurodevelopmental consequences. Food fortification and maternal and/or infant thiamine supplementation have proven effective in raising thiamine status and reducing the incidence of infantile beriberi in regions where thiamine deficiency is prevalent, but trial data are limited. Efforts to determine culturally and environmentally appropriate food vehicles for thiamine fortification are ongoing.
Keywords: thiamine deficiency, beriberi, LMIC, nutrition, erythrocyte transketolase, thiamine diphosphate
Purpose statement
In January and March 2017, the Sackler Institute for Nutrition Science at the New York Academy of Sciences and the Bill & Melinda Gates Foundation convened a technical consultation to explore the global prevalence and disease burden of thiamine deficiency. Participants with expertise in micronutrient malnutrition, pediatrics, biochemistry, neurology, and public health supplementation and fortification programs presented research findings and participated in discussion groups with the goal of determining the best biomarkers of thiamine status, estimating the global prevalence of thiamine deficiency, devising strategies for increasing thiamine status, and identifying research needs. The group's longer term objective is to improve the control of thiamine deficiency, including achieving consensus on diagnostic criteria, establishing better data on the global burden of disease, and expanding effective intervention strategies.
The following paper describes the current state of thiamine research and the significance of thiamine deficiency in low‐ and middle‐income countries (LMIC) from public health and clinical perspectives, as well as a summary of the consultation group's discussions and conclusions.
Thiamine biology
Thiamine, also called vitamin B1, is an essential micronutrient. The human body's supply of thiamine depends almost entirely on dietary intake; there is no endogenous synthesis, though some forms of bacteria in the intestine can produce a small amount of thiamine.1 Thiamine has a short half‐life (1−12 h) and body stores are limited; thus, a regular dietary supply is required to maintain tissue thiamine levels.2, 3 The richest food sources of thiamine are whole grains, yeasts, meats, legumes, and nuts. In many high‐income countries, wheat flour, cereals, and infant formulae are fortified with thiamine; thiamine‐fortified foods contribute about half of the total amount of the vitamin consumed in these settings.4 In LMIC where thiamine fortification is less common, a lack of dietary diversity and reliance on low‐thiamine staple foods are leading causes of thiamine deficiency.
Thiamine is present in the body as free thiamine, as well as in several phosphorylated forms: thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), and thiamine triphosphate (ThTP) (reviewed in Refs. 5, 6, 7). The different forms of thiamine detectable in plasma or whole blood are shown in Table 1.8 ThDP, also called thiamine pyrophosphate, is the metabolically active form, constituting some 80% of total body thiamine. ThDP is an essential cofactor in multiple enzyme complexes involved in the metabolism of carbohydrates and amino acids (Fig. 1).9 These enzyme complexes include the pyruvate dehydrogenase complex (which converts pyruvate to acetyl‐CoA), the α‐ketoglutarate dehydrogenase complex (which converts α‐ketoglutarate to succinyl‐CoA), and the branched chain α‐keto acid dehydrogenase complex (which converts branched chain α‐keto acids to the corresponding acyl‐CoAs).
Table 1.
Distribution of thiamine derivatives in human whole blood and plasma in nmol/L ± standard deviation with the percent of total thiamine derivatives in each specimen type
| Specimen (n) | Thiamine (nmol/L) | ThMP (nmol/L) | ThDP (nmol/L) | ThTP (nmol/L) |
|---|---|---|---|---|
| Whole blood (7) | 4 ± 3 (2.4%) | 10 ± 4 (6.1%) | 138 ± 33 (83.6%) | 13 ± 4 (7.9%) |
| Plasma (3) | 11 ± 3 (68.7%) | 5 ± 2 (31.3%) | n.d. | n.d. |
ThMP, thiamine monophosphate; ThDP, thiamine diphosphate; ThTP, thiamine triphosphate; n.d., not detectable.
Adapted from Gangolf et al.8
Figure 1.
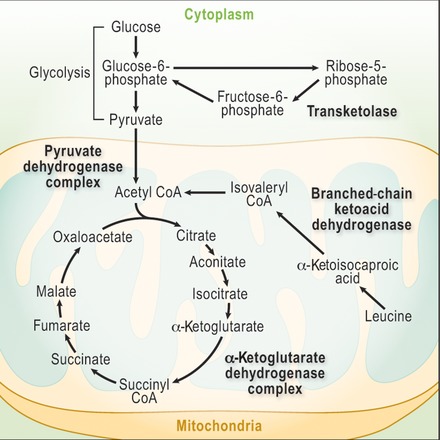
Thiamine diphosphate is a cofactor required for several metabolic processes, shown in bold text. Adapted from Thurnham.9
ThDP is also a cofactor of cytosolic transketolase in the pentose phosphate pathway and of 2‐hydroxyacyl‐CoA lyase, a peroxisomal enzyme involved in α‐oxidation of phytanic acid. It seems that the α‐ketoglutarate dehydrogenase is most sensitive to thiamine deficiency, and reduced activity of this enzyme complex can quickly lead to reduced ATP synthesis, oxidative damage, and, ultimately, cell death (Fig. 2).10 Thiamine circulates in the blood primarily in erythrocytes and is delivered to cells with high metabolic need, notably cells in the brain, liver, pancreas, heart, and skeletal and smooth muscles, including cardiac myocytes.
Figure 2.
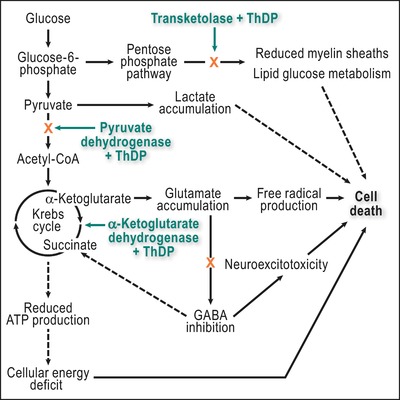
The three thiamine‐dependent enzymes and their role in the pathogenesis of cell death in thiamine deficiency. Adapted from Fattal‐Valevski.10 Dashed lines represent indirect pathways. ThDP, thiamine diphosphate.
The recommended nutrient intake (RNI) of thiamine is 1.2 mg/day for men and 1.1 mg/day for women, and increases to 1.4 mg/day for pregnant and 1.5 mg/day for lactating women. In infancy, the adequate intake is set at 0.2 mg/day (0−6 months) and 0.3 mg/day (7−12 months). The RNI gradually increases to 0.5 mg/day for children ages 1−3 years, 0.6 mg/day 4−6 years, and 0.9 mg/day by ages 7−9 years. After age 10, children's thiamine requirement is the same as for adults. There are no known adverse effects of high thiamine intakes; and there is no upper intake level for thiamine.11
Biomarkers for assessing thiamine status
Thiamine status can be evaluated in two ways, by assessing the degree of ThDP‐saturation of a thiamine‐dependent enzyme (erythrocyte transketolase (ETK) assay), and by measuring thiamine metabolites in accessible tissues. The ETK assay is considered to be more informative, as it demonstrates actual functionality of the vitamin. Table 2 lists the most relevant thiamine biomarkers with their advantages and disadvantages. Both methods show thiamine status to be lower in regions of the world where beriberi occurs than where it does not occur. However, in populations with beriberi, both affected and nonaffected individuals may have similarly low values.12, 13 Thiamine esters and other metabolites are found in blood and urine. Whole blood total thiamine (i.e., free thiamine plus its esters) typically ranges from 75 to 195 nmol/L, most of which is present as ThDP (70−180 nmol/L).14 The rate of ThDP depletion in erythrocytes is similar to that of other organ tissues, and it is well correlated with dietary intake.9, 15
Table 2.
Thiamine biomarkers used to measure recent intake and thiamine status
| Biomarker | Specimen | Advantages | Disadvantages |
|---|---|---|---|
| Direct measurement | |||
| Thiamine | Plasma | Indicates recent intake | Not an indicator of thiamine status |
| ThMP | Plasma | Indicates recent intake | Not an indicator of thiamine status |
| ThDP | Whole blood; erythrocytes | Biologically active vitamer and indicator of thiamine status | Unstable if specimen is not properly handled |
| Indirect/functional measurement | |||
| ETK activity coefficient | Washed erythrocytes | Functional assay of biological activity | Assay is not readily available |
ThMP, thiamine monophosphate; ThDP, thiamine diphosphate; ETK, erythrocyte transketolase.
Analysis of thiamine diphosphate
Analysis of ThDP in whole blood or erythrocytes can be a useful biomarker of thiamine status.14 The majority (∼80%) of the total thiamine content of whole blood is present in erythrocytes as ThDP. Whole blood or erythrocyte ThDP (eThDP) concentration is reflective of body stores and provides a better measure of thiamine status than total thiamine. However, direct measurement of ThDP does not assess thiamine metabolic function.
The expected concentration of ThDP in whole blood is approximately 70−180 nmol/L for healthy individuals, though there is no universally accepted cutoff value for thiamine deficiency, and a wide range of cutoffs have been used.14, 16 Thiamine metabolites can be determined by high‐performance liquid chromatography (HPLC) with ultraviolet‐visible spectroscopy (UV/VIS) or fluorescence detection.14, 17, 18 Thiamine is excreted in urine, chiefly as free thiamine and ThMP, with smaller amounts of ThDP and more than 20 other metabolites, including the oxidation product thiochrome. Urinary losses of thiamine metabolites vary with plasma thiamine concentrations, increasing markedly when renal tubular reabsorption is saturated, which occurs in healthy adults at intakes of 0.3−0.4 mg thiamine per 1000 kcal.19 Above that threshold, excretion of the vitamin exceeds 100 μg/day, whereas urinary excretion in deficient individuals is <25 μg/day. Increased concentrations of both pyruvate and α‐ketoglutarate in whole blood or plasma, and of methylglyoxal in the urine and cerebrospinal fluid, also occur in thiamine deficiency.
Sample type
ThDP can be measured in erythrocytes; however, whole blood is a more practical specimen. Whole blood ThDP concentration has been shown to correlate with eThDP concentration and obviates the need for separating and washing erythrocytes.17 ThDP can be measured in venous blood collected into ethylenediaminetetraacetic acid (EDTA)– or heparin‐containing specimen tubes. When erythrocytes are analyzed instead of whole blood, the cells must be washed with saline before testing in a process that is cumbersome and time‐consuming, and may decrease ThDP concentration.20 Thus, using whole blood as a sample matrix shortens analysis time and simplifies sample preparation.
Analytical methods
Individual thiamine species, including ThDP, can be measured directly using several techniques. HPLC with either pre‐ or postcolumn derivatization coupled with fluorescence detection has been available for several decades and is the most common method in current use.21 In this method, samples are prepared by removal of proteins and derivatization to produce fluorescent thiochrome compounds that are separated on a reverse phase analytical column, then detected and quantified.14
Methods using mass spectrometry have also been developed.18 Liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) allows sensitive and selective measurement of underivatized ThDP. LC–MS/MS methods require smaller sample volumes and are faster, but the instruments are more expensive than an HPLC with an optical detector.
Data analysis and presentation
Since there are no standard reference ranges currently accepted for ThDP, the data should be presented with the reference interval used by the reporting laboratory. For erythrocytes, ThDP concentrations should be reported in nmol/L red blood cells (RBCs). For whole blood, there is a need to normalize to RBC volume or hemoglobin concentrations.22 For these data, best practice would be to present both the measured ThDP (nmol/L whole blood) and the ThDP normalized to hematocrit (nmol/L RBC) or hemoglobin (nmol/gram hemoglobin). This will help ensure that data from different studies are comparable, and hematocrit or hemoglobin normalized whole blood data could be directly compared with eThDP concentrations. Historically, some data have been presented as μg/L but to make data between studies more readily comparable, it is suggested to use nmol/L.
Challenges and limitations of ThDP assessment
Preanalytical: Specimens should be protected from light and stored cold. Thiamine is stable for a few hours refrigerated and for several months stored frozen (–20 to –80 °C).14
Thiamine compounds are photosensitive, and must be stored in the dark;
They are also labile compounds requiring careful procedures for sample collection, transport, and storage at least –20 °C to avoid spontaneous hydrolysis;
Specimens of whole blood must be frozen to ensure lysis of erythrocytes.
Analytical:
Chromatographic separation of thiamine metabolites is necessary for analysis by HPLC with optical detection; this is not necessary for determination by mass spectrometry.
Calibration using an internal standard to account for losses during sample preparation, while desirable, is rarely used due to the lack of appropriate thiamine standards useful for HPLC/fluorescence methods. Isotopically labeled internal standards are available for LC–MS/MS methods.
Analytical methods have not been standardized. Considerable variation has been observed between laboratories.23
Analysis of erythrocyte transketolase activity
The functional assessment of thiamine status was developed more than 50 years ago, using the response of the ThDP‐dependent enzyme transketolase in erythrocyte hemolysates to exogenous ThDP.15 Erythrocyte transketolase (ETK) activity is suboptimal when thiamine intake, and hence, ThDP supply, is low. Therefore, the change in ETK activity upon the addition of exogenous saturating amounts of ThDP is indicative of thiamine functional status.
Sample type
Washed, anticoagulated (lithium heparin– or EDTA‐containing tubes) erythrocytes are used for this assay. Erythrocytes are washed with isotonic saline solution (0.9% NaCl) to avoid osmotic damage to the cells; standard procedure involves three cycles of washing, centrifugation, removal of supernatant and the top few mm of cells, and resuspension in saline. The washed cells, without supernatant, are frozen at –70 °C or colder and are osmotically lysed after thawing by resuspending in water before analysis. The assay requires only a few microliters of washed erythrocytes; sample volume requirement is governed by the amounts needed to complete the washing, lysis, and subsequent dilution steps.
Analytical methods
The assay of ETK activity coefficient (Fig. 3) can be performed conveniently in a UV‐transparent 96‐well microplate, with parameters matched to the Cobas Bio assay.24 All wells must be held at the same temperature, 37 °C, and all reagents must be in excess throughout the temperature equilibration and reading phases to ensure linearity. Precise measurement of the high absorbance is necessary, demanding good optics and a flat meniscus. Because ribose‐5‐phosphate is converted to xylulose‐5‐phosphate by endogenous pentose phosphate isomerase and pentose phosphate epimerase, which are present in erythrocytes in nonlimiting amounts, it is not necessary to add xylulose‐5‐phosphate to perform the assay.25, 26 The assay does not require a calibrant. Quality control specimens can be prepared from bulk samples from single donors and stored at –70 °C; the between‐assay coefficient of variation (SD*100/mean) of controls in the “adequate status” range is typically 3–5 percent.
Figure 3.
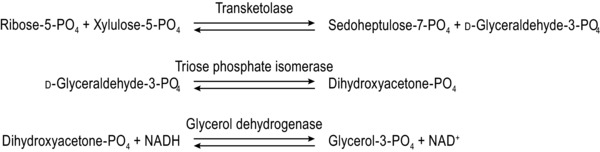
The sequential reactions involved in the erythrocyte transketolase (ETK) assay.
Data analysis and presentation
ETK activity is expressed in terms of the rate of decrease of absorbance at 340 nm, corrected for any changes in the reagent blank. But, as ETK activity does not directly indicate the portion of the enzyme actually bound to ThDP, the enzyme activity should be measured in the presence and absence of exogenous ThDP. The ratio of activities under these conditions, that is, the ETK activation coefficient (ETKAC = ETK activity with added ThDP/baseline ETK activity) should be reported. This is sometimes expressed as the percentage activation α. Thiamine‐adequate subjects typically have ETKAC values ≤1.15 (α ≤ 15%), indicating that they have >85% of ETK bound to ThDP. Subjects with ETKAC values <1.15 (α < 15%) are considered to be at low risk of clinical thiamine deficiency; those with ETKAC values 1.15–1.25 (α 15–25%) or >1.25 (α > 25%) are considered to be at moderate and high risks, respectively.27 Symptoms of beriberi, the primary disease of thiamine deficiency, have generally been associated with ETKAC values >1.4 (α > 40%). Alternatively, basal ETK activity per unit mass of hemoglobin may be reported.
Challenges and limitations of ETKAC assessment
Preanalytical:
As freezing causes erythrocyte lysis, erythrocyte washing must be completed before freezing.
Fresh‐frozen specimens must be used; freeze thaw cycles can diminish the transketolase activity. Thus, multiple aliquots should be prepared and stored in the event that a sample needs to be reanalyzed.
Analytical:
There is a requirement for identical temperature for each enzyme assay procedure; if the assay is performed in a microplate, this requires a microplate reader‐incubator that achieves equal temperature in all wells.
There is no gold‐standard assay against which to standardize the ETKAC assay.
The assay can be difficult to standardize, and interassay precision can be poor unless careful analytical procedures are followed.
Interpretational:
ETK activity can be influenced by factors other than ThDP concentration, such as age, genetics, and variability in binding of the apoenzyme.17
A summary of the analytical requirements for the ThDP and ETK assays can be found in Table 3.
Table 3.
Analytical requirements for thiamine biomarkers
| ThDP | ETKAC | |
|---|---|---|
| Assay type | Direct measurement | Indirect/functional assay |
| Analytical instrument | HPLC or LC–MS/MS | UV spectrophotometer |
| Specimen type | Whole blood or washed erythrocytes | Washed erythrocytes |
| Collection tube | Heparin or EDTA | Heparin or EDTA |
| Sample processing | 3× saline wash to purify erythrocytes or hemolyzed whole blood | 3× saline wash to purify erythrocytes |
| Minimum volume |
|
30 μL |
| Storage | Room temperature for a few hours, store at –20 °C for a few months, –70 °C for several months/years | Room temperature for a few hours, store at –20 °C for a few weeks, –70 °C for several months/years |
| Shipping | Dry ice | Dry ice |
ThDP, thiamine diphosphate; ETKAC, erythrocyte transketolase activation coefficient; HPLC, high‐performance liquid chromatography; LC–MS/MS, liquid chromatography coupled to tandem mass spectrometry; EDTA, ethylenediaminetetraacetic acid.
The relationship between ETKAC and whole blood or erythrocyte ThDP concentrations
Threshold ETKAC values indicative of thiamine deficiency or adequacy are based largely on results of dated studies in animal models. No cutoff values are available for erythrocyte or whole blood ThDP concentrations, which to date have been studied predominantly in thiamine‐adequate adults from high‐income countries.16 Limited data are available from two different clinical series of European patients with either chronic alcoholism28 or other illnesses17 to compare blood ThDP levels and ETKAC values in the same individuals. For example, one study of 63 medical and surgical patients at risk of thiamine deficiency (e.g., with chronic renal failure) found that the inflection point in the ETKAC−ThDP curve occurred at an ETKAC value of approximately 1.25 (i.e., 25% activation over baseline), which corresponded to a blood ThDP concentration of 280 ng/g hemoglobin (Fig. 4). Similar results were reported from the study of patients with chronic alcoholism.28 However, it is not certain if the same results would occur in populations with endemic thiamine deficiency.
Figure 4.
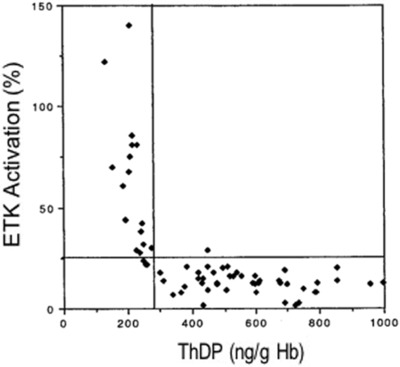
Comparison of the erythrocyte ThDP assay and ETKAC values from medical and surgical patients who were determined to be at risk of thiamine deficiency (from Talwar et al.17). ThDP, thiamine diphosphate; ETKAC, erythrocyte transketolase activation coefficient.
Therefore, there is a need to generate cutoff values for erythrocyte or whole blood ThDP concentrations indicative of both clinical thiamine deficiency and adequacy in at‐risk populations, such as pediatric populations—who are especially vulnerable—in countries where beriberi is reported. Such studies should assess the relationships of ETKAC values and whole blood or eThDP concentrations and compare both to measures of thiamine intake and clinical parameters of health.
Point‐of‐care diagnostics for rapid assessment of thiamine status
The assessment of biomarkers of thiamine status is not widely practiced, particularly in LMIC. It appears that only one laboratory, in Cambridge, UK, currently determines ETKAC, and that only a few laboratories currently measure ThDP and/or ThMP in erythrocytes. The cold chain required to transport the samples to laboratories adds costs and challenges to assessment of thiamine status. As clinical thiamine deficiency may present suddenly and be rapidly fatal, especially in infants, clinicians are advised to treat suspected beriberi patients empirically with thiamine. A rapid, positive response to thiamine is conventionally interpreted as confirmation of deficient thiamine status. A rapid test of thiamine status that avoids the need for a cold chain would be useful both in clinical practice and for nutritional surveillance.
Point‐of‐care (PoC) diagnostics have recently emerged as an analytical alternative for remote settings. Microfluidic devices are attractive candidates for use with PoC applications for diagnostics.29, 30 Alternatively, a PoC device could detect ThDP (or chemical analogs) based on electrochemical properties. Portable electrochemical analyzers have been recently developed by the Plaxco,31 Whitesides,32 and Wheeler laboratories.33 New methods are also being developed using a modified thiamine binding protein, which recognizes ThDP and ThMP, produced in Escherichia coli. Based on the initial report, this fluorescence‐based assay has a lower limit of detection of 0.5 nM, and range between 1 and 370 nM, which puts it well within the physiological concentrations of thiamine in human blood and provides a relatively rapid (∼30 min) and high throughput method for thiamine analysis.34
Dried blood spot (DBS) samples, which are already used worldwide for newborn screening, coupled with a microfluidic paper‐based analytical device (μPAD) could potentially make population assessment easier, more cost‐effective, and faster for the detection of thiamine deficiency. Shih et al. have demonstrated DBS analysis by digital microfluidics.35 Two companies, Diagnostics For All (DfA) and MC10, have also developed a μPAD for the detection of vitamin A.36 The device has been used to detect vitamin A colorimetrically using a competitive assay for retinol‐binding protein at a cost of $1 per test (including the integrated reader). Developing DBS‐based techniques to analyze thiamine content could potentially simplify the cold‐chain and make assessment more readily available. The above‐mentioned devices, as well as a myriad of low‐cost PoC solutions presented by other groups and organizations, are still under development. Extensive field testing and evaluation against gold standards will be required before applying these devises for routine use in thiamine status assessment.
Thiamine deficiency and its consequences
Risk factors for thiamine deficiency
Thiamine deficiency is rare in healthy individuals in food‐secure settings, where access to thiamine‐rich foods ensures adequate intakes.9 Regions where diets are monotonous and the primary sources of energy are starchy, low‐thiamine staples, such as polished rice or cassava, are likely to be at high risk of thiamine deficiency.37, 38, 39 Regular consumption of foods containing thiamine antagonists, such as betel nut or tea leaves, and thiaminases in foods such as raw fish and African silkworm larvae, have also been implicated as precipitants of thiamine deficiency.9, 12, 40 Some bacteria (e.g., Clostridium botulinum) are also capable of producing thiaminases.41 Conditions that lead to food insecurity, including drought, conflict, displacement due to war, famine, or natural disaster, as well as severe acute malnutrition (SAM), raise the risk of deficiency.42, 43, 44, 45 Abnormal gut microbiota, as occurs in SAM, may also play a role in reduced thiamine uptake.46, 47
Infants are particularly vulnerable to the effects of thiamine deficiency in the first months of life, and exclusively breastfed infants of thiamine‐deficient mothers are at highest risk.16, 48 Thiamine deficiency is also frequently observed in patients who are critically ill or in intensive care because of increased demand for thiamine (hypermetabolism).49, 50, 51, 52, 53, 54 In clinical practice, associated comorbidities and other risk factors, such as SAM with fever and shock and use of dextrose‐based intravenous fluid, further increase the risk of thiamine deficiency.42 Most other cases of thiamine deficiency occur among alcoholics,28 postoperative bariatric surgery patients,55 or patients with advanced HIV infection/AIDS,56, 57 who tend to have low thiamine intakes or absorption and impaired thiamine utilization.
Children with SAM often have multiple risk factors for thiamine deficiency. Upon admission to a hospital, these patients often receive several days of a low‐protein 75 kcal/100 mL refeeding milk formula (F75), which provides a possibly insufficient amount of thiamine (0.5–1.7 mg/day depending on the total daily F75 intake). Several experts have recently recommended increasing the thiamine content of this formula to align with therapeutic amounts.58
Diseases associated with thiamine deficiency
Overt thiamine deficiency syndromes represent a spectrum of clinical presentations that have been historically divided into two categories based on symptomology: (1) beriberi, which can be “wet” if it predominantly affects the cardiovascular system and is accompanied by edema, or “dry” if it predominantly affects the peripheral nervous system; and (2) Wernicke's encephalopathy or Wernicke‐Korsakoff syndrome.5, 59 Beriberi affects the cardiovascular system (e.g., acute cardiologic form), nervous system (e.g., pseudo meningitic form), or both; whereas Wernicke's encephalopathy primarily presents with neurological signs (e.g., encephalopathy and peripheral neuropathy forms),39 as described in more detail in Figure 5. The most important pediatric presentation—usually referred to as infantile beriberi—has a wide range of nonspecific clinical features and organ involvement, including central nervous system abnormalities, mainly characterized by heart failure. Infantile beriberi sometimes presents in the context of an acute infectious illness, further complicating the diagnosis.39, 42 The consultation group suggested adopting the umbrella term thiamine deficiency disorders (TDDs) to describe the spectrum of overlapping clinical presentations attributable to thiamine deficiency across the life course. However, the term beriberi (without wet/dry subtype disaggregation) remains commonly used in clinical pediatrics, so it is retained here to refer generally to TDD presenting in infancy.
Figure 5.
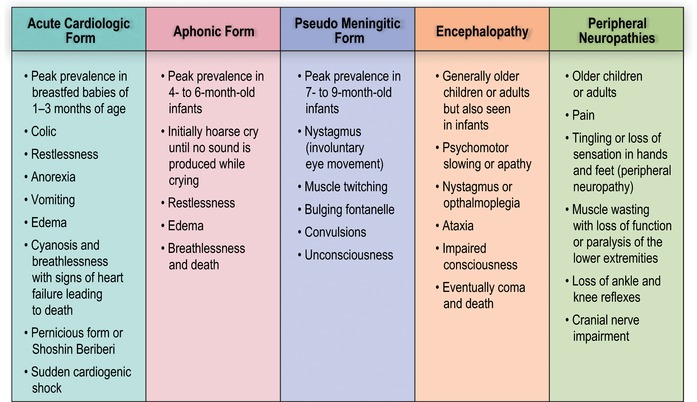
Clinical spectrum of thiamine deficiency disorders. Adapted from the WHO, 1999.39
TDDs primarily occur in low‐income countries as a result of low dietary thiamine intake. The risk of TDD is highest in the first year of life, especially during the period of exclusive breastfeeding, as mothers in regions where the disease is prevalent are thiamine deficient and thiamine content of breastmilk is related to maternal thiamine status.48 Infantile beriberi is rare in the first 2 months of life, as thiamine levels are higher in newborns, and then falls rapidly in the third month of life, likely due to changes in metabolic activity.60 It has been recognized in areas of Central and Southeast Asia,61 especially in Myanmar,44, 62 Laos,63, 64, 65 and Cambodia;13, 66 but, TDDs have also been observed in West Africa,67 Angola,45 Mayotte Island,68 Kiribati,69 Cuba,70 and parts of the Caribbean, and may go unrecognized in other settings.221
Infantile beriberi in children younger than 4 months of age typically presents first with nonspecific signs, including irritability, refusal to breastfeed, tachycardia and tachypnea, vomiting and incessant crying that is often described as “loud” or “piercing” and in some cases evolves into a “silent cry,” or aphonia.39 As the disease progresses, signs and symptoms of congestive heart failure begin to appear, such as tachypnea, tachycardia, pulmonary edema, hepatomegaly, and, sometimes, cyanosis; at this point, deterioration of the clinical condition is often rapid. As beriberi may be misdiagnosed as a viral infection, pneumonia, typhus, or malaria, beriberi patients may die before the correct diagnosis of thiamine deficiency is made.42, 44, 71 Prompt recognition of beriberi and immediate administration of thiamine produces rapid recovery. Within hours, even infants with advanced beriberi return to breastfeeding and, within days, their cardiac function returns to normal. Shoshin beriberi is a fulminant form of wet beriberi (acute cardiogenic shock with lactic acidosis multiorgan failure and no edema) and can easily mimic sepsis.72, 73, 74, 75, 76 Because of uncertainties regarding the diagnostic criteria of TDDs, it may only be possible to diagnosis beriberi after a patient demonstrates significant improvement following treatment with thiamine.
Beriberi in older infants and children may present with predominant neurological symptoms, including loss of appetite, nystagmus, ophthalmoplegia, bulging fontanelle, and loss of consciousness.59, 77, 78, 79 In areas with low awareness of thiamine deficiency, symptoms are easily mistaken for meningitis. In some cases, these signs may overlap with cardiac symptoms of beriberi. Older children, adolescents, and adults with TDD may present only with peripheral neuropathy—tingling or loss of sensation in the feet and hands, pain, and abnormal tendon reflexes—or with more severe signs of peripheral nervous system involvement, such as paralysis or sensory deficits.45, 80
Wernicke's encephalopathy, the most common form of thiamine deficiency in adults and older children, is characterized by a triad of signs and symptoms: abnormal eye movement, gait ataxia, and cognitive impairment. In younger children, though, the triad may not include ataxia.81, 82 The most severe form of Wernicke's encephalopathy can include a condition called Korsakoff's psychosis, in which patients are amnesiac, profoundly confused, and display confabulation with little or no working memory.83 This condition is more common in individuals with hereditary anomalies of transketolase in which its binding to ThDP is weak. In higher income countries, Wernicke's encephalopathy is most common in alcoholics, as chronic alcoholism impairs both intestinal thiamine absorption and increases thiamine requirements for metabolism. Wernicke's encephalopathy can also occur in HIV‐infected patients, pregnant women suffering from hyperemesis gravidarum, and postoperative bariatric patients.84, 85 If thiamine deficiency is corrected before the development of significant brain damage, the neurological symptoms may be completely reversible. However, if thiamine deficiency persists, it can result in permanent brain damage.83, 86 As Wernicke's encephalopathy in adults—especially in the nonalcoholic or chronically ill population—is less often fatal than infantile beriberi, it will not be extensively discussed here; readers interested in Wernicke's encephalopathy are referred to several excellent reviews of the disease.59, 81, 83, 85, 86
Some children with beriberi and adults with Wernicke's encephalopathy display similar brain changes on magnetic resonance imaging, specifically, bilateral, symmetric hyperintensity signals in the mammillary bodies, thalamic, and periaqueductal areas; however, pediatric brain neuroimaging may also show lesions in the basal ganglia and the frontal lobes. In a recent study of infants with thiamine deficiency encephalopathies, cranial ultrasound revealed hyperechoic lesions of the basal ganglia.73, 77, 79, 87
Additional putative TDDs found in Africa
Tropical ataxic neuropathy (TAN) is characterized by sensory polyneuropathy, gait ataxia, bilateral optic atrophy, and deafness. TAN is endemic in Nigeria, Ghana, and other West African nations where cassava root is a primary source of energy.88, 89 The etiology of TAN is not well understood and was thought to be related to the consumption of cyanogenic glycosides naturally present in cassava. However, this hypothesis has not been confirmed experimentally. On the other hand, in a placebo‐controlled trial, TAN patients have shown significant improvements with thiamine administration, suggesting that the condition may be due to thiamine deficiency.89
Epidemic spastic paraparesis, also known as konzo, is an upper motor neuron disease associated with conditions of food shortage (e.g., famine and drought) in Central and East Africa, where it has been found to be a leading cause of disability in young children.90, 91 Like TAN, konzo has been associated with consumption of cyanogenic glycosides from under‐processed cassava. As konzo patients display some overlapping symptoms of dry beriberi, thiamine deficiency has been suspected to play some role in this disease. However, there are as yet no clear data implicating thiamine deficiency in the pathogenesis of konzo.91
The Nigerian rainy season also produces epidemics of seasonal ataxia associated with consumption of the roasted larvae of the African silk worm, Anaphe venata, which contains thiaminases.92 Patients initially present with nonspecific signs such as nausea, vomiting, and dizziness. They rapidly develop nystagmus, tremor, dysarthria, ataxia, confusion, and coma. Early thiamine administration can reverse symptoms within 72 hours. Health education campaigns focused on raising awareness among hospital workers, hospitalized patients, and their relatives have been effective in reducing the prevalence of seasonal ataxia.40, 93
Variable presentations
As mentioned above, thiamine is a cofactor of several key cellular enzyme complexes found in mitochondria; thiamine deficiency, therefore, may be considered to be an acquired mitochondrial disease, which explains the multiple organ involvement, including the nervous and cardiac systems.42 Owing to the highly variable clinical presentations of TDD (Fig. 5) and the lack of consensus on clinical case definitions or biomarkers, these conditions are often misdiagnosed, possibly leading to gross underestimation of the prevalence of TDDs in many parts of the world. It is not clear why some thiamine‐deficient children present with only cardiac signs, while others present only with neurological signs. However, it is suspected that overall nutritional status and the presence or absence of comorbidities and multiple micronutrient deficiencies may contribute to the variable clinical presentations of thiamine deficiency.
Long‐term and subclinical consequences
As thiamine administration rapidly alleviates signs and symptoms of deficiency, patients with TDD can apparently make a full physical recovery if treated early. It was long believed that subclinical thiamine deficiency had no long‐term sequelae. However, persistent cognitive and motor deficits are now being recognized among children who experienced infantile thiamine deficiency. Longitudinal studies of Israeli children who survived a 2003 outbreak of thiamine deficiency after consuming an infant formula in which thiamine was erroneously omitted have shown long‐term medical, neurodevelopmental, and gross motor impairments.94, 95
The most severely affected survivors demonstrated marked intellectual disabilities, seizures, motor disabilities, microcephaly, auditory dysfunction, and complete heart block.94 Not all infants who received thiamine‐deficient formula were initially symptomatic; but even those who initially appeared unaffected subsequently displayed delayed language acquisition, persistent lexical and syntactical language impairments,96 gross motor delays,95 poor fine motor and coordination skills,97 dyslexia, and learning disabilities (unpublished data). It is assumed that these effects are the result of neuronal insult during a critical period in brain development. Considering the Israeli experience, one could hypothesize that it is possible that negative cognitive and developmental outcomes resulting from early and/or lifelong subclinical thiamine deficiency may also occur in regions where dietary thiamine intake is low.
More research is needed to understand the long‐term developmental effects of subclinical thiamine deficiency and to identify the factors that may trigger overt clinical disease in both infants and adults. Studies of Cambodian children have shown that blood ThDP levels of beriberi patients are often the same as their asymptomatic peers, which has led experts to believe that asymptomatic or subclinical thiamine deficiency may quickly shift to acute beriberi in the presence of other physiological stressors (e.g., acute infection, diarrhea, and parasitic infection).13 Additionally, there have been no long‐term intervention trials examining the impacts of maternal/infant thiamine supplementation on developmental outcomes—a critical research gap that the consultation group suggests should be urgently filled.
Cohorts of children treated for thiamine deficiency should also be followed in longitudinal prospective studies and evaluated for possible short‐ and long‐term sequelae of thiamine deficiency with serial neuropsychological testing and neurophysiological tests, such as nerve conduction studies, electroencephalography, and visual evoked potential testing. To facilitate neuropsychological studies in resource‐poor settings, simple, neurocognitive and neurodevelopmental‐testing instruments need to be developed and validated for use in local communities.
Despite a lack of consensus on the case definition of thiamine deficiency, clinical experiences have led experts to believe that many individuals in populations where thiamine intake is low teeter on the brink of clinical deficiency, and can be quickly pushed into overt deficiency by factors such as acute infections or other illnesses. The case studies of Israeli children exposed to a thiamine‐deficient formula diet are among the only studies of beriberi in children who were otherwise well nourished.98 The long‐term follow‐up of that cohort will be important for understanding the delayed effects of isolated thiamine insufficiency on neurobehavioral development.
A case definition for TDDs
Although TDDs are well documented in case studies, epidemiological studies linking thiamine deficiency to specific clinical scenarios are rare. This may be attributable to the heterogeneities in disease presentation and difficulty of assessing thiamine status.
The consultation group agreed that developing case definitions of the various TDDs may reduce some of the confusion surrounding diagnosis, and that this endeavor is a worthwhile subject for future research. Prospective cohort studies of children with clinically suspected beriberi would be valuable in identifying which combinations of clinical features are most predictive of confirmed thiamine deficiency. Such studies may enable the development of consensus case definitions for thiamine deficiency that are based on presenting clinical findings without requiring biochemical testing and could be used effectively by researchers and clinicians.
Developing a case definition
Criteria for enrolment of sick infants and children in a cohort study should be broad and inclusive to account for the wide spectrum of signs and symptoms of beriberi reported by clinicians and healthcare staff in areas where TDDs are prevalent (Fig. 6). Within the cohort, a subset of children may be subsequently identified as having a confirmed TDD. The accepted standard for diagnosing TDD in the absence of biochemical analysis is “clinical response to thiamine” (50−100 mg dose).74 A case definition would be based on the combination of presenting clinical features that are most predictive of clinical response to thiamine. As the research and clinical surveillance evidence base expands, TDD definitions may be refined, but should still serve as a guide to indicate when empirical treatment with thiamine should be encouraged, particularly as there is no known risk to treatment and the cost of treatment is relatively low.
Figure 6.
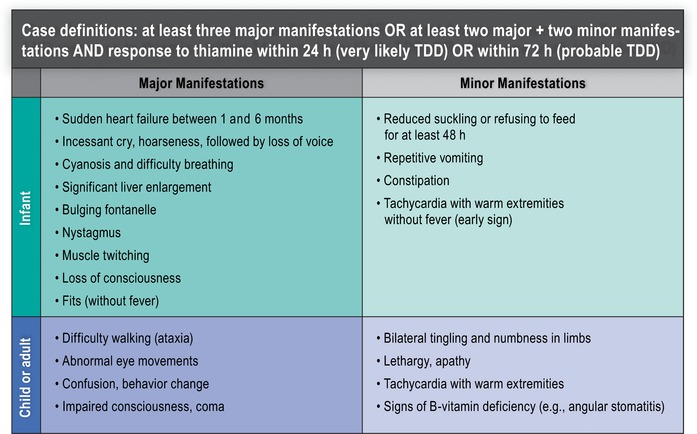
An example of a proposed case definition of thiamine deficiency disorder (TDD).
The initially proposed broad definition can be used to identify possible cases of thiamine deficiency, but confirmation of these conditions requires different protocols, including response to thiamine supplementation and biochemical indicators of thiamine status.
The following algorithm for thiamine therapeutic challenge includes the association of well‐identified risk factors and clinical conditions. Clinical manifestations in the presence of risk factors (Fig. 7) can be used to define the condition classified as “most likely TDD” when there is an expectation of significant clinical improvement within 24 h of thiamine therapeutic challenge or as “probable TDD” if significant clinical improvement is observed within 72 h of a thiamine therapeutic challenge.
Figure 7.
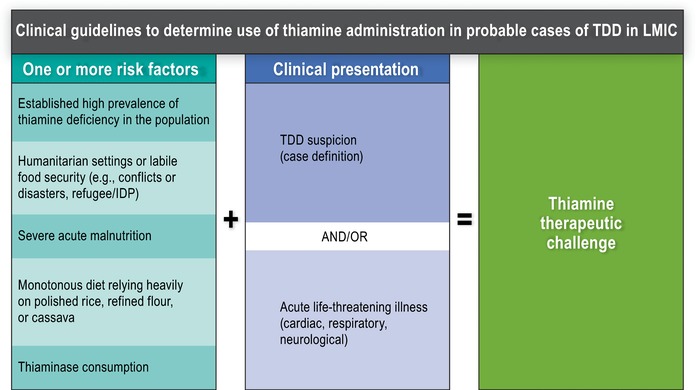
Clinical guidelines for empirical treatment of suspected thiamine deficiency disorders (TDDs). LMIC, low‐ and middle‐income countries; IDP, internally displaced person; TDD, thiamine deficiency disorder.
Prevalence of thiamine deficiency and burden of TDDs
Based on existing biomarker studies and case reports of thiamine‐responsive conditions, along with information on the use of polished rice or cassava as primary staples in the diets of many poor households in LMIC, risk of thiamine deficiency is thought to be of potential public health importance in many communities in Southeast Asia (e.g., Cambodia, Laos, and Myanmar), South Asia (e.g., Nepal and Northern India), and West Africa.63, 66, 75, 99
Experts agree that discrepancies in data produced by different laboratories and assay methods complicate assessment of the global prevalence of thiamine deficiency; and the dearth of population‐level biomarker data currently undermines efforts to determine the global and regional burden of disease. Increasing the number of countries that conduct thiamine status surveys using a biomarker such as ThDP or ETKAC would substantially improve global prevalence estimates. In regions such as sub‐Saharan Africa, where there may be an unrecognized problem, these status surveys could be especially important: if a problem is identified in one country, then the surrounding areas may also have thiamine deficiency.
Cambodia
Suboptimal thiamine status and TDDs have been recently documented in Cambodia.16 Verbal autopsy review suggests that approximately half of children dying after the neonatal weeks but during the first 6 months of life might have had beriberi.54 In 2012, Coats et al. published the results of a case–control study in which 27 infants presenting with clinical symptoms of beriberi (and their mothers) were compared with 27 infants and mothers without beriberi who also presented at a health clinic in Mesang District, Prey Veng, and 20 healthy American control dyads.13 Interestingly, there was no significant difference in the whole blood ThDP of Cambodian mothers and infants with or without beriberi.13 However, both sets of Cambodian mothers and infants had significantly lower ThDP than the Americans.13 Other cases of infantile beriberi have been reported in this same clinic in Mesang District in Prey Veng,66 as well as at a clinic in Siem Reap province.71
In 2013, Whitfield and colleagues conducted an exploratory cross‐sectional study to assess the thiamine status among women of reproductive age in rural and urban Cambodia, compared with women in Canada. Not surprisingly, they found significantly lower eThDP among nonpregnant, nonlactating women of reproductive age in both rural Prey Veng (mean ± SD, 149 ± 36 nmol/L RBC; n = 121) and urban Phnom Penh (156 ± 32 nmol/L RBC; n = 117), compared with women from Vancouver, Canada (179 ± 37 nmol/L RBC; n = 47; P < 0.05).37, 100 Although statistically significant, the authors note the relatively small absolute difference between women in Cambodia and Canada, which may be attributed to the small sample size and/or convenience sample of Vancouver women, the majority of whom were young university students.
The first‐ever Cambodian National Micronutrient Survey was conducted in conjunction with the 2014 Cambodian Demographic and Health Survey.101 This was a nationally representative survey of adults 15−49 years of age and their children (6–59 months) from 24 Cambodian provinces; eThDP was measured in 719 women and 761 children. The women's mean (95% CI) eThDP was 150 nmol/L RBC (146−153); the children's was 174 nmol/L RBC (171−179). As noted above, there are no widely accepted interpretative criteria for the classification of thiamine deficiency based on eThDP; nearly all of the cutoffs that have been used previously were derived from healthy populations, and most were calculated with fewer than the best practice of a minimum of 120 individuals.102 Two of the most extreme cutoffs from the literature define thiamine deficiency as <118.5 and <180 nmol/L RBC. The cutoff of <118.5 nmol/L RBC is the 95th percentile reference range among healthy black South African adults103 and was used in Bailey et al.;104 <180 nmol/L RBC is the 25th percentile of 103 healthy, asymptomatic adult employees of University “La Sapienza” Hospital in Rome.105 With a cutoff of eThDP <120 nmol/L RBC,104, 106, 107 27% of mothers and 15% of children in the Cambodian survey would be considered thiamine deficient; however, prevalence rates of deficiency were as high as 78% for mothers and 58% for children using the higher cutoff of <180 nmol/L RBC, highlighting the difficulties with establishing prevalence rates in the absence of clear cutoffs for thiamine deficiency.105
Finally, Cambodians have been responsive to increased thiamine intakes, both through diet, as from randomized controlled trials by Whitfield et al. of thiamine‐fortified fish sauce showing increases in eThDP100, 108 and breastmilk thiamine,100 and through thiamine supplementation, as shown in a recent pharmacokinetics study by Coats et al.109 Improvements to thiamine status upon supplementation are indicative of initial suboptimal status, as similar increases have not been observed among thiamine‐replete individuals. In part, this stems from the differential thiamine absorption depending on concentration: below 1 μmol/L, thiamine is transported through an active, carrier‐mediated, sodium‐dependent mechanism, whereas at higher concentrations thiamine is absorbed via passive diffusion.110 An experimental thiamine supplementation study among six healthy, thiamine‐replete Canadian males in the 1960s found 21% absorption of a 2.5‐mg thiamine dose, but decreased absorption of 9% and 4% at doses of 5 and 20 mg, respectively.111 Also, little thiamine is retained among thiamine‐replete individuals;112 for example, Australian researchers found rapid urinary excretion of thiamine among six volunteers (22−43 years; n = 3 men and 3 women) who consumed an 11‐mg dose of thiamine.110, 111, 112 In the study of thiamine‐fortified fish sauce, there was a statistically significant response between baseline and endline regardless of baseline thiamine status, except for the women who received the most heavily fortified fish sauce (where there was only a significant increase from baseline to endline among those women in the lowest tertile at baseline).108
Laos
There are several recent case reports of beriberi and suboptimal thiamine status among infants in Laos. A survey of 22 villages in northern Laos (various ethnic groups) reported a high infant (<6 months of age) mortality rate of 50 deaths among 468 live births.63 Of these 50 infant deaths, 36 occurred when infants were 1−3 months of age, and 17 were suspected to be caused by infantile beriberi.63 In the capital city of Vientiane, 13% of a cohort of 778 sick infants <1 year were found to have biochemical evidence of thiamine deficiency (defined as basal ETK activity <0.59, without clinical signs of beriberi).12
Another study of hospital records in Laos offered retrospective data on beriberi cases. Based on reports from 51 hospitals (including central, provincial, and district hospitals), about 60 infants under 12 months of age were treated with thiamine for beriberi each month, but biochemical measures were unavailable. In the catchment areas of these hospitals, there are an estimated 71,696 infants under 12 months old. Adult beriberi cases were much lower—an average of 20 men and 16 women were treated for thiamine deficiency per month. There was no seasonality associated with the caseload. In population centers like Luang Prabang, where health workers are reportedly more aware of the symptoms of beriberi, infant prevalence is highest at an estimated 2.5 per 1000 infants (Lao PDR Ministry of Health, unpublished data).
Myanmar
As described above, beriberi is the second leading cause of postneonatal death of children 29 to 365 days old in Myanmar. On a national level, this equates to 17% of infant (29 to 365 days old) deaths, but is more common in rural areas.113 Currently, Myanmar is combatting thiamine deficiency by providing 10 mg/day thiamine supplements to pregnant and lactating women up to 3 months postpartum.
Kashmir (India)
A number of reports of infantile beriberi have emerged from the northern region of India, where the diet consists largely of polished, unfortified rice.75, 77, 79, 114 Cases all occurred in infants who were exclusively breastfed, and the majority of mothers followed a customary dietary restriction. Postpartum food avoidances in this region lead to especially thiamine‐deficient diets consisting of polished rice and chicken soup.74 Nearly, 100 infants documented in these papers had either pulmonary hypertension or encephalopathy and nearly all responded to thiamine at the Government Medical College in Srinagar.
Outbreaks and individual case reports
Aside from the areas described above where thiamine deficiency is endemic, there have been a number of outbreaks reported in specific subpopulations within LMIC. Table 4 presents a list of the published outbreaks of TDDs in LMIC since 1980. As is the case in endemic areas, most of these cases of thiamine deficiency were caused by a limited diet that was high in polished rice. These recent outbreaks from LMIC suggest that thiamine deficiency can occur anywhere and under a number of circumstances. They also highlight the need for greater awareness of the clinical presentation of TDD in order to promptly diagnose and treat thiamine deficiency and prevent mortality associated with it.
Table 4.
Published outbreaks of thiamine deficiency (at least 15 people affected) since 1980 in low‐ and middle‐income countries where deficiency is not known to be endemic
| Continent | Country | Year | Population or location | Number of cases | Deaths | Age of cases | Notes | Refs. |
|---|---|---|---|---|---|---|---|---|
| Asia and Pacific | ||||||||
| Bhutan | 2015 | Female boarding school students | 17 (all females) | 0 | 9−18 years | Thiamine deficiency increased later in the school year | 174, 175 | |
| Papua New Guinea | 2009 | Boarding school students in Southern region | 6 severe deficiency, 24 marginal deficiency; 63% females | 0 | 14−22 years | 176 | ||
| Thailand | 1994−1995 | Rural Northeast | 31 thiamine deficient (thiamine effect >20%); 34 thiamine deficient 6 months later | 0 | 6−12 years | No association with parasitic infection | 177 | |
| Thailand | 2005 | Male fishermen in Maha Chai | 15 (12 probable, 3 confirmed) | 2 | 20−40 years | 2 months on a diet of fish and polished rice and 18 months at sea | 178 | |
| Thailand | 2012−2013 | Factory workers in Chachoengsao | 17 (suspected) | 3 (all males) | 20−30 years | Likely due to low thiamine and heavy physical activity | 179 | |
| Taiwan | 1999 | Chinese immigrants in a detention center | 34 probable and 70 possible cases out of 176 surveyed | 2 (all males) | 22−40 years | Average thiamine intake among the 15 hospitalized patients was 0.49 ± 0.1 mg/day | 180 | |
| Kiribati | 2012−2015 | Adult men, pregnant and lactating women, and infants | 34 confirmed (of 72 suspected cases) | 9 | <1 to >50 years | Majority of cases reported from September 2014–January 2015, but cases were still reported in 2017. Diet on Kuria Island is mainly imported unfortified white rice | 69 | |
| Africa | ||||||||
| South Africa | 1981−1985 | Patients admitted to King Edward VII Hospital in Durban | 41 (23 “fairly certain” beriberi) | Adult men | High consumption of Zulu beer | 181 | ||
| Somalia | 2009−2010 | Male African Union soldiers | 241 | 4 | 29 years (mean) | Restricted diet | 182 | |
| Gambia | 1990−1991 | Urban | 38; 27 men and 11 women | 4 | Men 36 years; women 35 years | Later half of rainy season | 183 | |
| Gambia | 1988 | North Bank region | 140 (suspected) | 22 | Adults and children | Twice the average rainfall | 67, 184 | |
| Côte d'Ivoire | 2002−2003 | Abidjan Prison | 597 definite cases; 115 probable cases (all males) | 7 | 15−73 years (28 mean) | Penal ration contained 1/5 of recommended dietary thiamine | 43 | |
| Côte d'Ivoire | 2008 | Abidjan prison (Maca) | 205 | 0 | 33 years (mean) | 185 | ||
| “West Africa” | 2002 | Prisoners | 211 | 25 | Adults | 186 | ||
| Guinea | 2015 | Prisoners | 618 | 1 | Adults | 187 | ||
| Mayotte and Reunion Islands (French Territories) | 1997−2005 | Mahoran or Comorian | 70 (67% females) (21 cases in 2004) | 27 years (mean) | Apparently healthy, young nonalcoholic adults | 188 | ||
| Mayotte Island | 2004 | Breastfed infants | 32 | 20 | 3 weeks–6 months | Occurred between April and July in children who were unsupplemented | 68 | |
| Angola | 2002 | Chipindo | 51 (suspected) | 10 | 4 months–86 years (21.3 years mean) | Extremely limited diet due to conflict; 32 cases were under 15 years old––most at risk | 45 | |
| Americas | ||||||||
| Brazil | 2006−2008 | Prospective study of PICU patients at the Hospital of São Paulo | 57 | 1.7 years (mean) | Magnitude of inflammatory response was a risk factor | 49 | ||
| Brazil | 2008 | Macuxi Amerindian communities in Roramia | 87 | 3 | 31 years (1−85 years) | 90% of cases had “normal” ThDP | 189 | |
| Brazil | 2006−2008 | Maranhão State | 1207 | 40 (1 female) | ∼20−40 years | Cases were largely from May to August, in young, low‐income men performing heavy labor | 191 | |
| French Guiana | 2013−2014 | Illegal gold miners | 42 (5 females) | 1 | 22−65 years | Cases still reported in 2016 | 190 | |
| Cuba | 1992−1993 | Pinar del Rio and Havana | 107 | 0 | >15 years | 70 | ||
| Colombia | 1991−1993 | Marines in the Naval School Almirante Padilla | 22 | 2 | 20−21 years | 192 | ||
There have also been clinical case reports of suboptimal thiamine status in various high‐income countries where beriberi was previously thought to be nonexistent or eradicated. Such reports include, for instance, a 24‐year‐old Japanese man presenting with beriberi after subsisting on rice balls for 4 years,76 and a 38‐year‐old Scottish man on an extreme weight‐loss diet.115 These isolated cases, where individual dietary or lifestyle habits were likely responsible for the case presentation, should not be considered as evidence for instigating a national public health intervention.
Methods to estimate the risk of thiamine deficiency in populations lacking information on thiamine status
The best indicators of risk of thiamine deficiency in a population are (1) clinical signs of beriberi that respond to thiamine treatment, and (2) a high prevalence of the population with biomarkers indicative of deficiency. While a definition of high prevalence currently does not exist, a cutoff of 20% has been used by the World Health Organization (WHO) for anemia. However, few countries have produced representative data on the population's thiamine status, and the interpretation of erythrocyte or whole blood ThDP remains uncertain (as discussed above). Several approaches using food availability or intake data and month‐specific infant mortality rates have been proposed to fill the gaps in population‐level indicators of thiamine status.
For the purposes of this technical consultation, two preliminary analyses were performed to assess the population risk of thiamine deficiency using these proxy measures. It should be noted that these provide a cursory overview of what could be done in greater detail to identify areas at risk of TDD. Because elevated ETKAC represents a functional abnormality due to thiamine deficiency, we suggest that this is currently the best biochemical evidence of deficiency. If more than 10% of the population has an ETKAC >1.2, this should be considered evidence of an elevated risk of thiamine deficiency in the population. When more than 20% of the population has an ETKAC above this threshold, this should be considered a public health problem and a large‐scale intervention should be considered. Once information is available on the whole‐blood or eThDP concentration that corresponds to elevated ETKAC, ThDP can be used as an alternative biomarker for population assessment, using the same prevalence cutoffs to indicate whether there is a problem of public health concern.
Food availability and intake data
It is well documented that diets high in polished, unfortified rice can lead to low thiamine intake in a population.63 National food balance sheets (FBSs) provide information on the annual per capita availability of different food commodities in a given country, which can be used to estimate the risk of inadequate intake of a nutrient based on its availability in the food supply.116 As expected, the results of this analysis indicate that there is low availability of thiamine in the food supply of several countries in Southeast Asia, including Cambodia, Myanmar, and Laos. Some LMIC elsewhere in Asia also had low thiamine availability, including Sri Lanka, Bangladesh, Mongolia, and Tajikistan. As described previously, there may be an underappreciated burden of thiamine deficiency in Africa. FBS data suggest inadequate availability of thiamine in the national food supply of Botswana, Somalia, Libya, Guinea Bissau, and the Gambia. More work is needed in these countries and others in the African regions to assess the possible extent of thiamine deficiency. While there is some co‐occurrence with riboflavin deficiency, it is not well correlated with thiamine deficiency.
These analyses considered the fact that rice and wheat fortification programs may be in place in a given country, as indicated in Figure 8. Countries highlighted in turquoise are those where the estimated mean thiamine availability is lower than the RNI for men (1.2 mg/day). Countries shaded in yellow have rice or wheat flour fortification programs in place that include thiamine; but the coverage of these programs is not known, so their impact on the population risk of thiamine deficiency is uncertain. Countries shaded in gray have an estimated mean thiamine availability above the RNI for men. In the Americas, only the Dominican Republic, Haiti, Ecuador, and Uruguay had inadequate thiamine availability. However, all of these countries except Uruguay fortify wheat and/or maize with thiamine.
Figure 8.
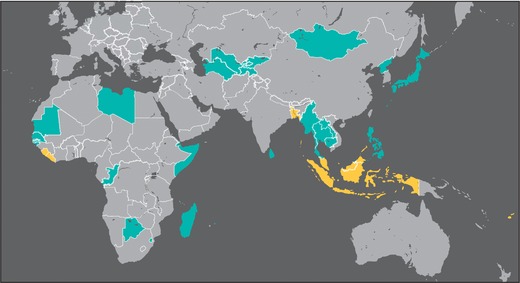
Map depicting countries where the estimated per capita availability of thiamine in the national food supply (as per food balance sheets) is below the recommended nutrient intake for men of 1.2 mg/day (in turquoise), and countries where rice or wheat flour fortification is in place to address low thiamine availability (in yellow). Countries where the estimated mean thiamine availability is greater than or equal to 1.2 mg/day are in gray.
Thiamine intake has also been assessed by micronutrient status surveys that use 24‐h recalls or food frequency questionnaires (FFQs) to estimate intake. Table 5 shows the results of published studies of micronutrient intakes, including thiamine intakes, of pregnant and lactating women in LMIC. These studies suggest the need to assess thiamine status in a number of areas outside of Southeast Asia, including parts of Africa (e.g., Burkina Faso) and South America (e.g., Colombia and Peru).
Table 5.
List of estimated thiamine intakes from studies assessing micronutrient intakes of pregnant and lactating women in low‐ and middle‐income countries since 1980
| Continent | Country | Location | Number of cases | Method | Mean ± SD (mg/day) | Refs. |
|---|---|---|---|---|---|---|
| Asia | ||||||
| Thailand | Songkhla (Southern) | 236 | 24‐h diet record and FFQ | 0.46 ± 0.14 | 193 | |
| Thailand | Pattani | 166 | FFQ | 0.45 (0.1–1.9 min–max) | 194 | |
| Thailand | Narathiwat (Southern) | 400 | FFQ | 0.7 (0.1–2.5 min–max) | 195 | |
| China | Wenzhou | 20 | 7‐day diet record | 0.87 ± 0.32 | 196 | |
| Changzhou | 82 | 7‐day diet record | 0.81 ± 0.26 | |||
| China | Rural Southern Mountain (Li ethnicity) | 189 | 5‐day diet record | 0.98 ± 0.38 | 197 | |
| Rural Southern Coast (Li ethnicity) | 76 | 5‐day diet record | 0.80 ± 0.41 | |||
| China | 163 | 24‐h recall | 1.77 ± 0.79 | 198 | ||
| China | Urban | 479 | 24‐h recall and FFQ | 1.2 ± 0.9 | 199 | |
| Indonesia | Central Java | 122 | 6−24 h recalls | First trimester: 0.66 ± 0.28 | 200 | |
| 406 | 6−24 h recalls | Second trimester: 0.77 ± 0.24 | ||||
| 356 | 6−24 h recalls | Third trimester: 0.82 ± 0.33 | ||||
| Lao PDR | Vientiane | 300 | 24‐h recall | 0.8 ± 0.5 | 162 | |
| Nepal | Bhaktapur | 466 | Multiple pass 24‐h recall | 0.78 ± 0.23 | 201 | |
| India | Delhi | 178 | 2−24 h recalls | 1.1 ± 0.6 | 202 | |
| India | Haryana/Hisar (semiarid) | 30 | 3−24 h recalls | 0.89 ± 0.2 | 203 | |
| Haryana/Bhiwani (arid) | 30 | 3−24 h recalls | 0.79 ± 0.2 | |||
| Haryana/Kurukshetra (wet) | 30 | 3−24 h recalls | 0.89 ± 0.1 | |||
| India | Ludhiana | 66 | Dietary survey | 1.3 ± 0.2 | 204 | |
| India | Coimbatore | 316 | 1.2 | 205 | ||
| India | Haryana/Hisar | 120 | 24‐h recall | 1.77 ± 0.76 | 206 | |
| India | Farming | 45 | 3‐day food record and diet habit survey | 1.3 ± 0.4 | 207 | |
| Nonfarming | 45 | 3‐day food record and diet habit survey | 1.3 ± 0.3 | |||
| Palau | Koror | 25 | 24‐h diet recall | 1.9 ± 1.0 | 208 | |
| Iran | Maku Urban | 142 | 2−24 h recalls and FFQ | 1.90 ± 0.73 | 209 | |
| Maku Rural | 142 | 2−24 h recalls and FFQ | 2.10 ± 0.42 | |||
| Africa | ||||||
| Burkina Faso | Hounde | 218 | 24‐h recall | 0.81 (P25 = 0.54; P75 = 1.12) | 210 | |
| Morocco | Rural | 63 | FFQ | 1.67 ± 0.62 | 211 | |
| Urban | 92 | FFQ | 1.46 ± 0.38 | |||
| Ghana | Rural | 15 | FFQ and 24‐h recall | 1.6 (0.7−2.4 range) | 212 | |
| Suburban | 15 | FFQ and 24‐h recall | 2.2 (1.3−4.6 range) | |||
| Egypt | Kalama | 50 | Self‐report and sample weighing | 1.1 ± 0.3 | 213 | |
| South and Central America | ||||||
| Colombia | Southeastern Cali | 381 | 24‐h recall | 0.58 (median) (0.42−0.85 IQR) | 214 | |
| Peru | Chanchamayo | 206 | Multiple pass 24‐h recall | 0.69 ± 0.47 | 215 | |
| Peru | Lima, Villa El Salvador | 168 | 24‐h recall | 10−24 weeks gestation: 0.8 (5th = 0.4; 95th = 1.8) | 216 | |
| 120 | 24‐h recall | 28–30 weeks gestation: 1.0 (5th = 0.4; 95th = 1.8) | ||||
| Mexico | Mexico City | 112 | 24‐h recall | 1.3 (1.1−1.5 min–max) | 217 | |
| Brazil | Sao Paulo | 72 | 24‐h recall | 1.1 (0.8−1.5 IQR) | 218 | |
| Chile | Concepcion | 214 | 24‐h recall | 1.5 (median) (1.3‐1.7 IQR) | 219 | |
| Ecuador | Quito | 74 | Survey of food patterns and nutrient intake | 1.9 | 220 | |
FFQ, food‐frequency questionnaire; IQR, interquartile range.
While the risk of inadequate thiamine intake has been assessed most frequently through the use of dietary intake surveys and national FBSs, household consumption and expenditure survey (HCES) data are now being used more frequently to estimate household‐level micronutrient intake. These surveys provide several advantages in that the data are collected routinely in many countries; and the results are easily subdivided by socioeconomic status and geographical area, both of which can affect the risk of inadequate thiamine intakes. For example, India, a large country with a diverse population, is not identified by FBS data as a country with thiamine deficiency. However, infantile beriberi cases have been reported in recent years in the northern region of Kashmir, where diets consist largely of polished rice.75, 79, 114 The ability to disaggregate dietary data for specific population subgroups can be useful in assessing the risk for thiamine deficiency.
Infant mortality data
Month‐specific infant mortality rates may also be used to identify regions at high risk of widespread thiamine deficiency. As noted in the 1999 WHO document on thiamine deficiency, “the typical feature of infantile beriberi is that instead of infant mortality decreasing after the first month, it remains high or even increases to a peak at about the third month.”39 A second peak in infant mortality may also be observed around the sixth month. Thus, plotting infant mortality rates by month of age can provide useful information as to whether thiamine deficiency may be a problem in a country.
To assess this in areas with a known problem, Demographic and Health Survey (DHS) data from Cambodia were examined, which shows an increase in infant mortality around 3 months of age and in some cases at 7 months (Fig. 9).
Figure 9.
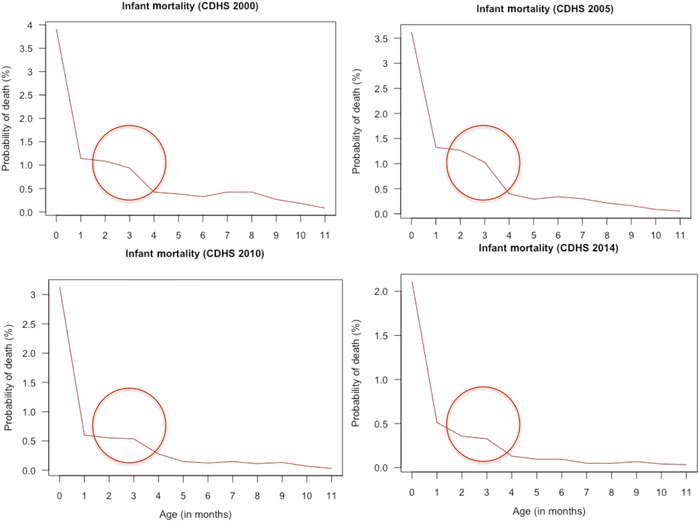
Comparison of infant mortality rates by month of age in Cambodia based on DHS data. CDHS, Cambodian Demographic and Household Survey.
While the methods described above can provide rough approximations of thiamine intake or the presence of TDD, more data are needed to quantify the prevalence and severity of thiamine deficiency. This would ideally be done through biomarker surveys of thiamine status in representative samples of the population and active surveillance for cases of beriberi.
Thiamine status assessment decision tree
Introduction and purpose of the decision tree
Suboptimal thiamine status has been called the “forgotten disease of Asia,”12 as reports of TDDs in the region are common despite the known etiology and relatively straightforward approaches to prevention and treatment.117 There is current evidence of suboptimal thiamine intake or status in Southeast Asia, notably in Cambodia,13, 37, 66, 71, 100, 108 Laos,12, 63, 118, 119 and Myanmar,113 parts of India,75, 79 and in recent reports of Karen refugees upon their arrival in the Mae La Camp on the Thai‐Myanmar border.44, 62
What is not currently clear is how widespread suboptimal thiamine status is and whether countries should be prioritizing thiamine as a micronutrient of concern for public health intervention programs. For instance, while there have been several reports of infantile beriberi in Laos, it is unclear whether the issue is localized or widespread, and whether it is a major contributor to the burden of infant mortality in the country. The consultation group has developed a decision tree to help guide countries on the best course of action for investigating thiamine status and/or implementing interventions to improve thiamine intake.
The decision tree consists of two paths: one for those countries where there is a known high risk of thiamine deficiency, based on beriberi or TDD case reports, and another for those where there may be some risk of thiamine deficiency in the absence of beriberi reports. For the former, the group suggests countries confirm with biomarkers if possible and engage with stakeholders to discuss possible interventions and monitoring programs. For the latter, we suggest both several methods for analyzing existing data to better understand the risk (prevalence and severity) of suboptimal dietary thiamine intake and implementing surveys and/or clinical surveillance of thiamine status. This roadmap is based on the current state of knowledge, and it serves as a guide for public health action: rapid assessment, diagnosis, and treatment should continue for any individual presenting at a health facility with clinical signs consistent with TDD. Figure 10 provides a decision tree for countries to consider when assessing the potential for thiamine deficiency.
Figure 10.
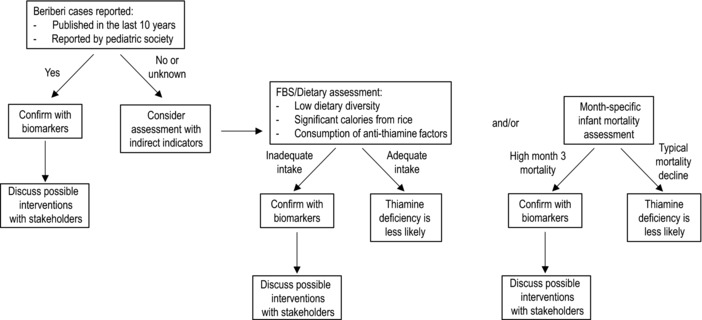
A proposed approach for assessing a country's risk of thiamine deficiency disorders (TDDs) and the actions the countries could consider taking to prevent or eliminate TDD cases.
Countries with known TDD cases
Countries with known TDD cases may consider proceeding directly to an intervention (described below). However, it would be helpful to confirm the presence of thiamine deficiency in the population by assessing biomarkers of thiamine status, especially among women of reproductive age, and establishing surveillance sites in hospitals and healthcare centers to monitor the prevalence of TDDs systematically and continuously. It is important to note that case reports of beriberi are often considered the tip of the iceberg in terms of suboptimal thiamine status and an indicator of a more widespread public health problem.
Biomarker status assessment
Biomarker status assessment can be used to improve estimates of severity and indicate at‐risk groups within the population. It is also useful to generate baseline data before interventions to assess an intervention's effectiveness. The data can also be used in conjunction with dietary intake data to help calibrate the dosage for potential fortification interventions.
Surveillance
For these countries, active hospital surveillance for infantile beriberi, possibly using sentinel surveillance sites, is an important aspect of determining prevalence of thiamine deficiency in regions with low thiamine intake. The consultation group suggested conducting a medical record review in major hospitals where TDD cases exist, or a prospective study in these settings in search of one or more key conditions associated with beriberi, such as congestive heart failure, and response to thiamine treatment, which may offer additional information about potential prevalence and underdiagnosis.
Countries without documented TDD cases
For those countries that do not have reports of documented TDD cases but where there is reason for concern about the adequacy of thiamine intake, the consultation group suggests that health authorities in regional and national governments tap existing dietary intake or food availability (FBS) data to determine the risk of inadequate thiamine intake in a given country. Reviewing large nationally or regionally representative dietary intake data can provide insight into a population's risk of thiamine deficiency. If the results of these analyses indicate that there is an elevated risk of thiamine deficiency, the country may consider a more thorough assessment and intervention, as discussed above for the countries with documented TDD cases.
Regions with low dietary thiamine intake are obvious targets for assessments and surveillance, and the consultation group suggested identifying regions at particular risk by considering those in which ≥50% of calories are derived from low‐thiamine staple crops like cassava and polished rice. For example, a relatively recent analysis of FBSs estimated that milled (white, polished) rice makes up 1520 of the 2411 kcal/day per capita in Cambodia, or 63% of daily energy supply.120 Such diets are likely to be limited in several essential nutrients, including thiamine. When considering the intake of “anti‐thiamine” factors, it is not known how much consumption is required to affect thiamine status. However, it is well known that countries with populations regularly consuming raw fish, betel nuts, or silkworm larvae (A. venata) are more likely to have TDDs, especially when combined with a diet containing relatively low amounts of thiamine.80 Surveillance techniques, including dietary recall, as well as thiamine biomarker testing, could provide greater insight into the prevalence of subclinical thiamine deficiency in these regions.
Individual quantitative dietary assessment
Usual thiamine intake can be assessed by quantitative dietary intake data collected in a representative sample of the population of interest using methods such as 24‐h recalls, estimated or weighed food records, or FFQs.107 The preferred assessment method is a multiple‐pass, interviewer‐administered, 24‐h recall, in which individuals are asked to recall intake over the previous 24‐h period with one or more nonconsecutive repeat days to determine the distribution of “usual intakes in the population” by removing intraindividual day‐to‐day variation.107, 121 After dietary intake data are collected, thiamine intake is determined using a food composition database, and then the dietary reference intakes can be used to assess dietary adequacy of the population. Specifically, the percent of individuals of a given lifestage group whose usual intake is less than the respective estimated average requirement (EAR) provides the prevalence of inadequate intake in that population subgroup.121 The EAR for thiamine for women of reproductive age and for pregnant/lactating women is 0.9 and 1.1 mg/day, respectively.122
Food balance sheets
As noted in Gibson and Cavalli‐Sforza's recent publication, while detailed dietary consumption data would be ideal to assess thiamine intake adequacy, FBSs can be a useful proxy in the absence of intake data.123 The Food and Agriculture Organization of the United Nations publishes annual FBSs to document the amounts of various food commodities available for dietary consumption.123 FBSs report national food availability, not individual or household food consumption, so regional differences in household food security status within the country and intrahousehold food distribution patterns may result in an over‐ or underestimation of thiamine adequacy as reflected in FBSs.124 Nevertheless, if the FBSs indicate that there is a shortfall in thiamine availability nationally, at least some individuals in the population are likely to have inadequate intakes. A recent analysis of FBSs from 17 countries in the Western Pacific highlighted potential dietary thiamine inadequacy in Cambodia and Mongolia.123 A global analysis of FBSs indicated that several countries outside Southeast Asia may also have significant inadequacies in thiamine intake.116 These include Bangladesh, Sri Lanka, Mozambique, Zimbabwe, Madagascar, Sierra Leone, Congo, and Liberia, where more than 40% of the population was estimated to have inadequate thiamine intake.
Household consumption and expenditure survey
A nationally representative dataset that could be harnessed for analysis is the World Bank HCES, which includes several subsurveys including the Household Income and Expenditure Survey.125 With modules investigating annual household expenditures on food and beverage commodities (and occasionally consumption data), analysis of this data can be useful in highlighting potential thiamine inadequacy. HCESs have also been used to identify potential fortification vehicles. A recent comparison of 24‐h recalls and HCES data in Uganda found that although the HCES modeling underestimated consumption, these models were able to successfully differentiate consumption patterns by life stage groups.124
Infant mortality
As described previously, an infant mortality rate that rises around 3 months of age rather than continuing to decline after the newborn period is characteristic of populations with thiamine deficiency.39 As part of an initial assessment of thiamine status, a country should consider examining available infant mortality data by month of age to aid in its evaluation. Because beriberi is not frequently observed until 3 months of age and is fatal if untreated, this mortality pattern seems to be specific to areas of thiamine deficiency.
If a country suspects that its population may be at risk, but lacks the above data, health authorities could consult with the relevant pediatric society and/or primary healthcare providers in pediatric hospitals to determine if cases exist but have not been reported to higher level authorities.
Assistance from the global community
There are also a number of actions from the global community that may aid countries in assessing the prevalence of thiamine deficiency. For example, the creation of a standing thiamine technical committee could provide technical assistance to countries in assessing the prevalence of thiamine deficiency. If a standing committee were created, it could also educate decision makers more broadly about the potential value of thiamine status assessment and control programs. Additionally, developing a network of resource laboratories would improve the standardization of thiamine assessment across laboratories, as has been done successfully for other micronutrients, such as vitamin D.126 This network could also support the preparation of certified reference materials for thiamine status biomarkers and laboratory‐quality assurance schemes.
Strategies to improve thiamine status
Food fortification, supplementation, optimizing thiamine intake from the diet, health education, and behavior change are all important strategies for increasing thiamine intake in regions where both clinical and subclinical deficiencies are likely to be widespread. This section describes the programmatic approaches to fortification, supplementation, dietary modification and education, and training of healthcare workers, for countries that are aiming to improve their population's thiamine status.
Food fortification
Large‐scale, mandatory food fortification has several advantages: it has potential to be a sustainable and cost‐effective intervention,38, 127, 128, 129 and it requires little or no behavior change on the part of the consumer, as the fortificant is added to a commonly consumed food vehicle (i.e., staple food or condiment). Moreover, food fortification can restore nutrients such as thiamine, riboflavin, and iron lost during processing.130 As a population‐wide intervention, fortification offers the potential to reach large sections of the population with relatively low cost and effort.131 Large‐scale fortification holds considerable promise for increasing thiamine status in women (and thus in breastfeeding infants). Fortification is a sustainable, long‐term solution for combating suboptimal thiamine status and related TDDs. Thus, the consultation group suggests that countries with cases of TDD consider developing a mandatory thiamine fortification program in situations where the food system is compatible with this approach. Most often this has been done successfully in combination with other fortification efforts, such as cofortification of thiamine along with other micronutrients in wheat flour in several countries, but it has also been done as a sole fortificant in experimental fish sauce.108, 132, 133
Two forms of thiamine are used in fortification: thiamine hydrochloride, which is the fortificant of choice for liquid vehicles, and thiamine mononitrate, which is preferred for dry applications. When fortifying with thiamine, it is important to consider that both forms of thiamine are heat labile, are destabilized in the presence of sulfites, and are somewhat light sensitive. The WHO has estimated that thiamine is one of the lowest cost fortificants; for example, $0.018 (USD) provides 100% of the EAR for a male per day for 1 year.38
Identifying a food vehicle for fortification
After determining that micronutrient intake is low enough to merit a food fortification program, national governments or regional organizations must determine the best vehicle for delivering that micronutrient to at‐risk groups.38 Several important considerations inform the choice of an appropriate fortification vehicle, including dietary consumption, technical aspects of fortification, and scale and location of production; specific fortification vehicles will be discussed below. Fortification rapid assessment tool surveys and dietary intake surveys can be used to identify potential vehicles for fortification and appropriate levels of fortification.134
Consumption
The vehicle must be consumed regularly throughout the year by a large proportion of the population at risk of deficiency.38 While unintended consequences, such as overconsumption by segments of the population—men tend to have higher dietary micronutrient consumption due to unequal household food distribution—should be taken into account, this is less relevant for thiamine fortification as no adverse events have been reported from excess thiamine intake, and hence, no upper intake levels exist.122, 127
Technical considerations
Choice of fortificant, including cost and stability (degradation due to heat, light, oxidation, moisture, and pH) of the premix, must be considered. Ensuring there are no adverse reactions between the fortificant and the vehicle is also essential. Organoleptic properties must be considered, as any alteration to the taste, smell, texture, or appearance of the food raises the risk of consumer rejection.38, 135 Fortunately, thiamine has no safety or sensory limitations. 136
Role of industry
Vehicles that are processed at a centralized facility are ideal, as the presence of multiple food production sites can complicate the distribution of premix and quality control of final products.38, 137 “Buy in” from the relevant food industry groups helps ensure successful fortification with sufficient quality control.137
Implementation
There are significant costs associated with implementing a mandatory fortification program, beyond simply purchasing the premix. Initial start‐up costs of fortification equipment and establishment of quality control and monitoring programs must be considered.38 If possible, cofortifying an established vehicle may be an ideal means of decreasing some of these costs.
Rice may seem to be an obvious choice of a staple for thiamine fortification in Southeast Asia, as a recent FBS analysis from several countries in the region indicates that rice makes up a sizeable proportion of the dietary energy supply.120 However, rice fortification would be challenging and costly for several reasons. Traditional cultural practices dictate that rice is typically washed several times before cooking, which removes the micronutrients added through powdering techniques, although extruded or waxed fortified rice kernels are not affected by washing.61, 138 Potentially more importantly, there is limited consolidation of rice millers in many countries. For example, nearly, every village throughout Cambodia mills rice locally, and there are about 16,000 rice millers in Myanmar. Thus, fortification would be highly decentralized, increasing costs and complicating adequate quality control measures.139
Determining the level of fortification
The EAR is the daily intake value that is estimated to meet the physiological needs of half the healthy individuals in a particular sex and life‐stage category.140 The WHO recommends using the EAR cut‐point method to determine optimal levels of fortification38 to provide most individuals (97.5%) in a population group at the greatest risk of deficiency with a total intake that exceeds the EAR.140 The EAR cut‐point approach requires knowing the target population's usual intakes of both the fortification vehicle and the micronutrient of interest.38 At the moment, there are limited detailed dietary data available for any of the countries where TDDs are well documented. Before fortification programs are introduced, detailed data on usual consumption of both potential food vehicles and thiamine are required to optimize the level of fortification, ensure safety, and minimize cost.
Food vehicles for thiamine fortification
Wheat flour fortification
Wheat flour is fortified with thiamine in many countries, including the United States and Canada, the UK, Indonesia, Jordan, South Africa, and some Central Asian countries.132, 141, 142 Despite thiamine's sensitivity to heat and light, breads and pasta made with fortified wheat flour retain more than 70% of thiamine after baking or cooking.38 Wheat flour fortification with thiamine is feasible in areas of South Asia where thiamine deficiencies are prevalent, particularly because costs are low. However, if a local population consumes very little wheat overall, as in many Southeast Asian countries, or wheat milling is decentralized, fortification of other staple food vehicles or condiments may be necessary to assure adequate thiamine consumption. Wheat consumption should be more closely monitored in future fortification efforts, especially in Southern Asia.
Fish and soy sauces
Fish sauce has shown promise as a vehicle for thiamine fortification. In parts of Southeast Asia, the condiment is widely used and readily consumed by young children and adults alike at most meals. Any bacterial thiaminases associated with fish gastrointestinal tracts appear to be denatured in the pasteurization processes. Thiamine added after pasteurization causes no organoleptic changes, and a thiamine‐fortified fish sauce has been tested in Cambodia. Two small, concurrent randomized controlled trials of thiamine‐fortified fish sauces (one sauce fortified at 2 g/L, one at 8 g/L, and one control sauce) were conducted over 6 months in Prey Veng province, Cambodia. After perinatal consumption of thiamine‐fortified fish sauce, mothers and their exclusively breastfed infants showed improvements in eThDP and breastmilk thiamine levels over a 6‐month period.100 In a second cohort of rural Cambodian mothers and their children <5 years, those in the thiamine‐fortified groups had significantly higher eThDP compared with the control group.108 Fish and soy sauces have been established as vehicles for iron fortification in Cambodia,143, 144 Vietnam,145, 146 and Thailand.147 It is likely that thiamine could also be added to these condiments at low additional cost, but further investigation is needed to determine the most regionally relevant vehicle for each country, with considerations for consumption and centralized production. Decentralized production may be the major barrier; for instance, as February 2014, there were 42 registered producers fortifying fish and soy sauce with iron in Cambodia.148
Salt
Salt fortification with iodine has been implemented in more than 120 countries, and its use has dramatically reduced iodine deficiency disorders in many parts of the world.149 Salt is under consideration as a vehicle for additional micronutrient fortification and could be a promising vehicle for thiamine fortification.150 A trial of multiple micronutrient‐fortified salt (including 2 mg thiamine/10 g salt and 10 other microencapsulated micronutrients) in Indian school children showed that the multifortified salt retained adequate nutrient content after 20 min of cooking time and lost only 0.52% of thiamine. After 6 months of storage at 30 °C and 45% relative humidity, the fortified salt retained 96% of the thiamine original content.151 While no assessment of thiamine status was performed, indicators of vitamin A, iodine, and riboflavin (vitamin B2) deficiency were significantly reduced after 12 months of consuming the multifortified salt. Despite this promising evidence, salt requires more investigation as a vehicle for fortification. Recent data from Cambodia show that a lack of donor support for iodine premix and/or a suboptimal monitoring and quality control program have resulted in a decrease in iodized salt use (38% of salt samples in 2014, down from 78% in 2011).152 This highlights the importance of continued government and donor support to ensure the sustainability of any fortification program.
Rice fortification
Rice is the dietary staple in many of the countries where TDDs are known to be prevalent, and as such should be considered as a potential fortification vehicle. The suggested approaches for rice fortification per the Food Fortification Initiative are:
Coated rice kernels, accomplished by applying a thiamine‐enriched mixture of waxes to rice kernels and mixing fortified kernels with polished rice in a ratio of 1:50−1:200; or
Mixing extruded, enriched, rice‐shaped kernels (made by hot extrusion from a rice flour and vitamin/mineral premix) with typical polished rice in a similar ratio.
Both approaches maintain thiamine content through cooking; however, fortified rice kernels produced by powdering techniques appear to be more vulnerable to washing.153
Rice fortification faces several challenges in LMIC. Rice is often sourced from multiple local millers, and fortification efforts that rely on many different producers are generally viewed as less viable. Rice fortification also requires specialized equipment, which limits industry uptake. Indeed, data from the Fortified Rice Project, a pilot fortification program in Myanmar, show that only one local miller was equipped with an extruder to produce fortified rice‐shaped kernels, and only eight (of some 16,000) millers are fortifying rice. Further, the fortificants used to enrich rice may be destroyed if rice is stored at high temperatures. Despite these concerns about using rice as a vehicle, rice fortification may still be practical in some situations, such as when food is distributed during emergencies or through food aid programs, where the challenges of decentralized milling do not apply.
Monitoring and quality control is key
It is essential to address monitoring and quality assurance from the earliest stages of program planning, including measures to take appropriate actions on low compliance food producers. Several recent programs from various LMIC indicate that a lack of regular quality assurance measures to ensure adequate fortification and/or monitoring and follow‐up of health outcomes can undermine the success of the programs in the long term.137, 152 Attention should also be paid in the presence of multiple producers of food vehicles in the country, on how to maximize the monitoring and quality control of adequate fortificant level.
Biofortification
Biofortification—use of conventional plant breeding methods, agronomic approaches, or genetic engineering to increase the micronutrient content of a plant—is another method of increasing dietary intake of a micronutrient.154 Goyer recently developed a rice cultivar that overexpresses the genes THIC and THI1, which results in a fivefold higher thiamine content.155 However, the thiamine is found primarily in the husk, which, as stated above, is removed during polishing. There was also a small increase in endosperm thiamine, which contained 1.3 times higher thiamine compared with wild‐type rice. Thiamine biofortification is not a viable public health approach to TDD prevention at the moment, however. Research in this area is ongoing.
Supplementation
Thiamine supplementation guidelines have focused on emergency situations, most often when conflict or famine results in food insecurity for large numbers of refugees or displaced persons. Infantile beriberi outbreaks are well documented in such populations, for example, in the Karen refugee population in Thailand.44 In these cases, the addition of thiamine‐rich foods to emergency aid rations or the delivery of thiamine supplements is recommended and has proven effective in reducing or eliminating cases of infantile beriberi.44 In the clinical setting, patients treated for beriberi are given thiamine supplements for approximately 1 month following recovery. Few nonemergency thiamine supplementation programs have been implemented. One such program, the Infantile Beriberi Project in Myanmar, offers 10 mg of daily thiamine supplements to pregnant and lactating women from the final month of pregnancy through the first 3 months of lactation. It is essential to monitor and evaluate the programs on an ongoing basis, both for program coverage and recipient adherence to the supplementation regimen.
Supplementation of pregnant and lactating women
Based on current knowledge, infantile beriberi is the most severe adverse outcome of suboptimal thiamine status; clinical symptoms develop more quickly among infants than adults due to the rapid growth and development that occurs at this life stage relative to dietary intake.156 Without rapid thiamine administration, infants can die of infantile beriberi within hours of clinical presentation.44
Infantile beriberi presents during the exclusive breastfeeding period as a result of consuming thiamine‐deficient breastmilk from mothers with low thiamine status.48, 156 Since breastmilk is recommended as the sole source of nutrition for infants under 6 months,157 maternal dietary thiamine intake must be improved to prevent infantile beriberi and related mortality.61
Maternal thiamine supplementation has been shown to successfully prevent suboptimal thiamine status among Karen refugees in the Mae La camps44, 158 and has also been shown to improve breastmilk thiamine concentrations during research projects in rural Cambodia,100, 109 the Gambia,159 and India.160 Results of a recent study in Prey Veng, Cambodia, suggest that improved maternal thiamine intake in the third trimester of pregnancy allows for fetal uptake in utero,100, 161 highlighting the importance of adequate status in both pregnancy and lactation. This is further supported by a thiamine pharmacokinetics study that found significant increases in breastmilk thiamine after 5 days of maternal supplementation, although this was not sufficient time to increase infant whole blood ThDP or plasma thiamine concentrations.109
Countries with reports of infantile beriberi or evidence of low biochemical thiamine status should consider either the addition of a thiamine supplement to complement the current perinatal iron‐folic acid supplementation program, or should introduce a multiple micronutrient supplement containing thiamine, such as the UNICEF/WHO/UNU international multiple micronutrient preparation (UNIMMAP). With any indication that dietary thiamine intake is low in a given population, the consultation group suggests considering switching from the standard iron‐folic acid supplementation regimen in pregnancy and lactation to one including thiamine or the UNIMMAP formulation to reduce the risk of infantile beriberi, a practice that was adapted by Myanmar in 2016.
Thiamine status of a breastfed infant is closely linked to that of the mother, so improving thiamine status of lactating women is a logical approach to prevent infantile beriberi. However, additional research is needed to establish the optimal timing, dose, and duration of maternal supplementation. Adding thiamine to an existing supplementation program could be an easy and cost‐effective method to improve thiamine status of pregnant and lactating women.
Multiple micronutrient powders
In many countries, infants older than 6 months of age receive multiple micronutrient powders (MNP) that include thiamine, and could be used as a method to increase thiamine status. While the impact of this intervention on thiamine specifically has not been documented, it is likely to improve status and functional outcomes. A straightforward intervention that may be tested to lower the prevalence of thiamine deficiency in children is the use of MNP for home fortification.
Behavior change
Changing dietary patterns to increase the consumption of thiamine‐rich foods, and limit the intake of known thiamine antagonists and thiamine‐poor foods, could improve thiamine intake. Nutrition education and behavioral change communication on promoting dietary diversity with nutrient‐rich foods are essential to changing dietary patterns. In some countries like Myanmar, ideas about avoiding specific foods are still common for pregnant and lactating women that result in limited intake of nutrient‐rich foods and increase the risk of deficiencies. In a cross‐sectional survey in Vientiane, Lao, Barennes et al. found that 93% of women (n = 274/300) practiced postpartum food restriction (phit kam). It was common for women to consume only a traditional herbal tea for the first 15 days postpartum (n = 285/300; 95%), which translated to an estimated mean (95% CI) thiamine intake of 0.4 (0.1−0.7 mg/day) intake during this period (dietary intake n = 31, days 1−15, 24 h recall).163 Raising awareness of the consequences of local food avoidances in pregnant and lactating women and promoting thiamine‐rich alternatives can improve maternal thiamine intake.
As noted above, in populations where white, polished rice is the main dietary staple, thiamine is lacking not only because white rice is thiamine poor, but also because the sheer volume consumed limits the consumption of thiamine‐rich foods. Parboiling rice can increase thiamine content of polished rice by leeching thiamine from the bran into the endosperm. Additionally, traditional food processing techniques such as fermentation can also be promoted in the nutrition education message, as it can increase the level of thiamine and other B vitamins in the plant‐based diets.164
Providing nonspecific nutrition education messages will likely not have a significant effect on the population's thiamine intake or status. Rather in LMIC, the promotion of locally available nutrient‐rich foods could be considered as an alternative to optimize thiamine intake as well as to tackle the coexistence of multiple micronutrient deficiencies. Identifying context‐specific nutrient‐rich foods and supporting their intake may be useful, especially in resource‐poor countries as a long‐term and sustainable strategy. An approach known as Linear Programming is available to identify the problem nutrients in the diet of the population, to formulate the food‐based recommendations that resemble the current dietary practices, and to fill the nutrient gaps. This approach considers multiple factors, such as existing dietary pattern, nutrient content of foods, food cost, and cultural factors, and hence reduces the challenge for the population to totally change their current dietary practices.165, 166, 167
Food processing and consumer behaviors
Food processing changes also have the potential to affect the thiamine content of various foods. Rice processing and consumption is particularly important because, as detailed above, TDD often appears in regions where polished rice is a dietary staple.
In most rice‐consuming countries, well‐polished white rice is preferred for various reasons, including cultural (e.g., white rice is a status symbol,61 it has optimal organoleptic properties) and economic (e.g., removal of the lipid‐rich outer bran increases shelf‐life,168 and less fuel and water is required for cooking).168, 169 There are also economic incentives for rice millers to polish rice thoroughly, as rice bran is a major animal feed product in Southeast Asia.170, 171 Anecdotal evidence from rural Cambodia suggests that local rice millers do not charge famers to mill rice, but rather are allowed to keep the rice polishings, which they sell as animal feed. With this model, it is in the best interest of the rice miller to fully mill the husk and bran of the rice to maximize the weight of animal feed they can sell.
Parboiled rice
While rice naturally contains thiamine in its outer husk and bran (aleurone layers), the practice of milling away those layers, or “polishing,” to produce shelf‐stable white rice renders it very low in thiamine. Parboiling rice before milling reduces this loss, allowing some migration of thiamine from the aleurone layer to the endosperm. It has been estimated that approximately 50% of rice produced is parboiled, but with very inhomogeneous global coverage.162 Unfortunately, parboiling rice is not commonplace in Southeast Asia, as this process traditionally resulted in a musty aftertaste caused by mold growth during long‐term solar drying. Today, parboiled rice is dried in commercial driers; and, while taste is unaffected, this process causes a darkening of the endosperm, which decreases consumer acceptability.
Thiamine antagonists
Consumption of foods containing thiamine antagonists, such as certain fish species and larvae of the African silk worm (as described above), is also thought to contribute to the development of TDD. Thiaminases are heat‐liable, so recommendations to consume thiaminase‐containing foods cooked rather than raw should ameliorate deficiency.156 The effects of gallic acid‐containing tannins, such as those found in tea and betel nuts,172 can be prevented by delaying contact between thiamine and tannins (e.g., consuming tea hours after a thiamine‐containing meal, rather than during the meal).173
Interventions to alter current food processing practices and consumption patterns may improve the thiamine content of diets. For instance, incentivizing rice millers to remove less rice bran, or developing improved storage technologies to increase the shelf life of brown rice, could improve the thiamine intake of rice consumers. Consumer education is also important: addressing and explaining the benefits of parboiled rice could increase acceptability despite darkened kernels, or simply advising not to drink tea with meals could help prevent thiamine degradation.
Education of healthcare providers
As noted above, TDDs are often under‐recognized by clinicians, leading to missed or delayed diagnosis. In addition, it is not uncommon for infants to present with beriberi while their mothers remain asymptomatic.13, 119, 156
We suggest that countries with known cases of TDD consider developing culturally relevant healthcare provider education tools to help identify, treat, and prevent suboptimal thiamine status. A recent trial in Cambodia employed a local artist to develop a poster outlining the common signs of infantile beriberi for study participants and to raise awareness of infantile beriberi symptoms (Fig. 11). While this tool was originally designed to educate study participants about the signs of thiamine deficiency, it was also shared with local village health volunteers and village chiefs to increase awareness of infantile beriberi. Alternatively, video clips showing the appropriate assessment, diagnoses, treatment, and importantly the recovery of infants presenting with beriberi may be extremely helpful training tools for healthcare practitioners. Professional training and in‐service refresher courses for health‐care providers in settings where thiamine deficiency occurs should provide information on promoting appropriate dietary habits and counseling pregnant and lactating women not to avoid thiamine‐rich foods.
Figure 11.
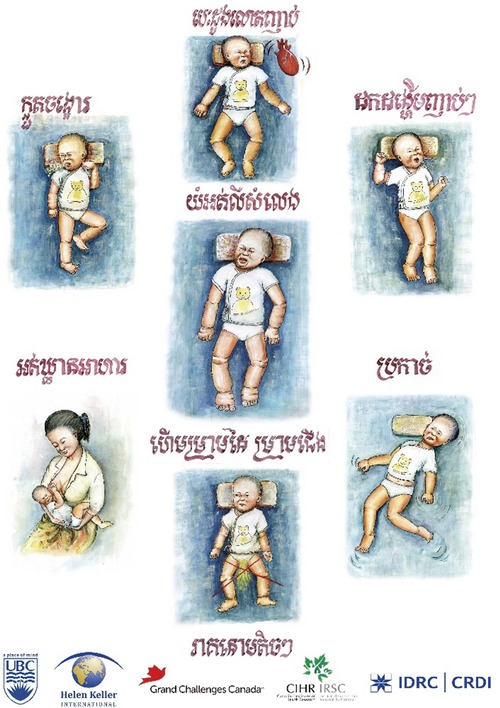
Educational poster outlining common signs of infantile beriberi (in Khmer).
Research opportunities
As described throughout this report, there are a number of unknowns regarding assessment, prevalence, treatment, and the health effects of thiamine deficiency. These are summarized in Table 6 as a list of thiamine “research opportunities” elaborated by the consultation group during a series of discussions. Resolving these issues will increase understanding of TDDs and improve the ability to identify and prevent them.
Table 6.
Thiamine research opportunities
| Biomarkers |
|
|
|
| Thiamine deficiency disorders |
|---|
|
|
|
| Thiamine deficiency prevalence |
|---|
|
| Thiamine deficiency prevention |
|---|
|
|
|
Conclusions
Thiamine deficiency and its clinical manifestations as TDDs may be quite common in many regions of the world, especially in LMIC. A number of populations around the world are at risk of thiamine deficiency based on low dietary intakes, resulting primarily from subsistence on low‐thiamine staple grains. Assessing the thiamine status of populations in these areas should be a priority. With new research suggesting that even subclinical deficiency may impair neurocognitive development, there is reason to be highly concerned about thiamine insufficiency, even in areas where clinical beriberi may be uncommon. While treatment of confirmed or suspected beriberi with thiamine is rapid and safe, the heterogeneity of symptoms makes thiamine deficiency difficult to identify, suggesting that the prevalence of TDDs may be vastly underestimated. The use of available biomarkers needs to be expanded to provide better information on the prevalence of deficiency and determine if interventions are necessary. However, reported cases of TDD is a strong indication of thiamine deficiency among the population, and an intervention is likely required. In such cases of known or suspected thiamine deficiency, there are a number of possible interventions, including food fortification and supplementation of pregnant and lactating women and of young children.
Competing interests
The authors declare no competing interests.
Acknowledgments
This paper was developed with the support of the technical consultation on “Global Prevalence and Disease Burden of Thiamin Deficiency,” convened by the Sackler Institute for Nutrition Science, a program of the New York Academy of Sciences, in January and March 2017. The consultation and the development of this paper were supported by funding from the Bill & Melinda Gates Foundation. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors thank Tiffany Bowman for her assistance in creating many of the figures. L.B. is Research Director of the F.R.S.‐FNRS (Belgium).
Footnotes
[Correction added on August 28, 2018, after first online publication: The word “agonists” has been replaced with “antagonists” throughout.]
References
- 1. Najjar, V.A. & Holt E.. 1943. The biosynthesis of thiamine in man and its implications in human nutrition. JAMA 123: 683–684. [Google Scholar]
- 2. Tallaksen, C.M.E. , Sande A., Bøhmer T., et al 1993. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur. J. Clin. Pharmacol. 44: 73–78. [DOI] [PubMed] [Google Scholar]
- 3. Bettendorff, L. , Wins P. & Lesourd M.. 1994. Subcellular localization and compartmentation of thiamine derivatives in rat brain. Biochim. Biophys. Acta 1222: 1–6. [DOI] [PubMed] [Google Scholar]
- 4. Berner, L.A. , Keast D.R., Bailey R.L., et al 2014. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J. Acad. Nutr. Diet. 114: 1009–1022.e8. [DOI] [PubMed] [Google Scholar]
- 5. Lonsdale, D. 2006. A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives. Evid. Based Complement. Alternat. Med. 3: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bettendorff, L. & Wins P.. 2009. Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 276: 2917–2925. [DOI] [PubMed] [Google Scholar]
- 7. Manzetti, S. , Zhang J. & Van Der Spoel D.. 2014. Thiamin function, metabolism, uptake, and transport. Biochemistry 53: 821–835. [DOI] [PubMed] [Google Scholar]
- 8. Gangolf, M. , Czerniecki J., Radermecker M., et al 2010. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One 5: e13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thurnham, D.I. 2013. Thiamin: physiology. Encycl. Hum. Nutr. 4: 274–279. [Google Scholar]
- 10. Fattal‐Valevski, A. 2011. Thiamine (vitamin B1). J. Evid. Based Complement. Alternat. Med. 16: 12–20. [Google Scholar]
- 11. FAO & World Health Organization . 2005. Vitamin and Mineral Requirements in Human Nutrition. 2nd ed.: 1–20. Geneva: World Health Organization. [Google Scholar]
- 12. Khounnorath, S. , Chamberlain K., Taylor A.M., et al 2011. Clinically unapparent infantile thiamin deficiency in Vientiane, Laos. PLoS Negl. Trop. Dis. 5: e969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coats, D. , Shelton‐Dodge K., Ou K., et al 2012. Thiamine deficiency in Cambodian infants with and without beriberi. J. Pediatr. 161: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu, J. & Frank E.L.. 2008. Rapid HPLC measurement of thiamine and its phosphate esters in whole blood. Clin. Chem. 54: 901–906. [DOI] [PubMed] [Google Scholar]
- 15. Brin, M. 1964. Erythrocyte as a biopsy tissue for functional evaluation of thiamine adequacy. JAMA 187: 762–766. [DOI] [PubMed] [Google Scholar]
- 16. Whitfield, K.C. , Smith G., Chamnan C., et al 2017. High prevalence of thiamine (vitamin B1) deficiency in early childhood among a nationally representative sample of Cambodian women of childbearing age and their children. PLoS Negl. Trop. Dis. 11: e0005814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talwar, D. , Davidson H., Cooney J., et al 2000. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin. Chem. 46: 704–710. [PubMed] [Google Scholar]
- 18. Puts, J. , de Groot M., Haex M., et al 2015. Simultaneous determination of underivatized vitamin B1 and B6 in whole blood by reversed phase ultra high performance liquid chromatography tandem mass spectrometry. PLoS One 10: e0132018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Interdepartmental Committee on Nutrition for National Defense. 1963. Manual for Nutrition Surveys 2nd ed Washington D.C.: US Department of Health, Education and Welfare. Public Health Services. NIH, US Governemnt Printinting Office. [Google Scholar]
- 20. Ihara, H. , Matsumoto T., Shino Y., et al 2005. Assay values for thiamine or thiamine phosphate esters in whole blood do not depend on the anticoagulant used. J. Clin. Lab. Anal. 19: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collie, J.T.B. , Greaves R.F., Jones O.A.H., et al 2017. Vitamin B1 in critically ill patients: needs and challenges. Clin. Chem. Lab. Med. 55: 1652. [DOI] [PubMed] [Google Scholar]
- 22. Floridi, A. , Pupita M., Palmerini C.A., et al 1983. Thiamine pyrophosphate determination in whole blood and erythrocytes by high performance liquid chromatography. Int. J. Vitam. Nutr. Res. 54: 165–171. [PubMed] [Google Scholar]
- 23. Hoad, K.E. , Johnson L.A., Woollard G.A., et al 2013. Vitamin B1 and B6 method harmonization: comparison of performance between laboratories enrolled in the RCPA Quality Assurance Program. Clin. Biochem. 46: 772–776. [DOI] [PubMed] [Google Scholar]
- 24. Vuilleumie, J.P. , Keller H.E. & Keck E.. 1990. Clinical chemical methods for the routine assessment of the vitamin status in human populations. Part 3: the apoenzyme stimulation tests for vitamin B1, B2 and B6 adapted to the Cobas‐Bio analyser. Int. J. Vitam. Nutr. Res. 60: 126–135. [PubMed] [Google Scholar]
- 25. Bayoumi, R.A. , Rosalki S.B., A.Bayoumi R., et al 1976. Evaluation of methods of coenzyme activation of erythrocyte enzymes for detection of deficiency of vitamins B B2 and B6. Clin. Chem. 22: 327–335. [PubMed] [Google Scholar]
- 26. Warnock, L.G. 1970. A new approach to erythrocyte transketolase measurement. J. Nutr. 100: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 27. Turck, D. , Bresson J.‐L., Flynn A., et al 2016. EFSA scientific opinion on dietary reference values for thiamin. EFSA J. 14: e04653. [Google Scholar]
- 28. Herve, C. , Beyne P., Lettéron P., et al 1995. Comparison of erythrocyte transketolase activity with thiamine and thiamine phosphate ester levels in chronic alcoholic patients. Clin. Chim. Acta 234: 91–100. [DOI] [PubMed] [Google Scholar]
- 29. Sia, S.K. & Kricka L.J.. 2008. Microfluidics and point‐of‐care testing. Lab Chip 8: 1982–1983. [DOI] [PubMed] [Google Scholar]
- 30. Nayak, S. , Sridhara A., Melo R., et al 2016. Microfluidics‐based point‐of‐care test for serodiagnosis of Lyme disease. Sci. Rep. 6: 35069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rowe, A.A. , Bonham A.J., White R.J., et al 2011. CheapStat: an open‐source,“Do‐It‐Yourself” potentiostat for analytical and educational applications. PLoS One 6: e23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nemiroski, A. , Christodouleas D.C., Hennek J.W., et al 2014. Universal mobile electrochemical detector designed for use in resource‐limited applications. Proc. Natl. Acad. Sci. USA 111: 11984–11989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dryden, M.D.M. & Wheeler A.R.. 2015. DStat: a versatile, open‐source potentiostat for electroanalysis and integration. PLoS One 10: e0140349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Edwards, K.A. , Seog W.J., Han L., et al 2016. High‐throughput detection of thiamine using periplasmic binding protein‐based biorecognition. Anal. Chem. 88: 8248–8256. [DOI] [PubMed] [Google Scholar]
- 35. Shih, S.C.C. , Yang H., Jebrail M.J., et al 2012. Dried blood spot analysis by digital microfluidics coupled to nanoelectrospray ionization mass spectrometry. Anal. Chem. 84: 3731–3738. [DOI] [PubMed] [Google Scholar]
- 36. Lee, S. , Aranyosi A.J., Wong M.D., et al 2016. Flexible opto‐electronics enabled microfluidics systems with cloud connectivity for point‐of‐care micronutrient analysis. Biosens. Bioelectron. 78: 290–299. [DOI] [PubMed] [Google Scholar]
- 37. Whitfield, K.C. , Karakochuk C.D., Liu Y., et al 2015. Poor thiamin and riboflavin status is common among women of childbearing age in rural and urban Cambodia. J. Nutr. 145: 628–633. [DOI] [PubMed] [Google Scholar]
- 38. Allen, L.H. , de Benoist B., Dary O., et al 2006. Guidelines on food fortification with micronutrients. WHO, Geneva. [Google Scholar]
- 39. World Health Organization . 1999. Thiamin deficiency and its prevention and control in major emergencies. WHO, Geneva. [Google Scholar]
- 40. Moyo, A.A. , Bimbo F.M., Adeyoyin K.M., et al 2014. Seasonal ataxia: a case report of a disappearing disease. Afr. Health Sci. 14: 769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ringe, H. , Schuelke M., Weber S., et al 2014. Infant botulism: is there an association with thiamine deficiency? Pediatrics 134: e1436–e1440. [DOI] [PubMed] [Google Scholar]
- 42. Hiffler, L. , Rakotoambinina B., Lafferty N., et al 2016. Thiamine deficiency in tropical pediatrics: new insights into a neglected but vital metabolic challenge. Front. Nutr. 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahoua, L. , Etienne W., Fermon F., et al 2007. Outbreak of beriberi in a prison in Côte d'Ivoire. Food Nutr. Bull. 28: 283–290. [DOI] [PubMed] [Google Scholar]
- 44. Luxemburger, C. , White N.J., ter Kuile F., et al 2003. Beri‐beri: the major cause of infant mortality in Karen refugees. Trans. R. Soc. Trop. Med. Hyg. 97: 251–255. [DOI] [PubMed] [Google Scholar]
- 45. Duce, M. , Escriba J.M., Masuet C., et al 2003. Suspected thiamine deficiency in Angola. F. Exch. 26–28. [Google Scholar]
- 46. Monira, S. , Nakamura S., Gotoh K., et al 2011. Gut microbiota of healthy and malnourished children in Bangladesh. Front. Microbiol. 2: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Million, M. , Diallo A. & Raoult D.. 2017. Gut microbiota and malnutrition. Microb. Pathog. 106: 128–138. [DOI] [PubMed] [Google Scholar]
- 48. Allen, L.H. 2012. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv. Nutr. 3: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peixoto de Lima, L.F. , Leite H.P. & AC Taddei J.A.de. 2011. Low blood thiamine concentrations in children upon admission to the intensive care unit: risk factors and prognostic significance. Am. J. Clin. Nutr. 93: 57–61. [DOI] [PubMed] [Google Scholar]
- 50. McConachie, I. & Haskew A.. 1988. Thiamine status after major trauma. Intensive Care Med. 14: 628–631. [DOI] [PubMed] [Google Scholar]
- 51. Krishna, S. , Taylor A.M., Supanaranond W., et al 1999. Thiamine deficiency and malaria in adults from southeast Asia. Lancet 353: 546–549. [DOI] [PubMed] [Google Scholar]
- 52. Rosner, E.A. , Strezlecki K.D., Clark J.A., et al 2015. Low thiamine levels in children with type 1 diabetes and diabetic ketoacidosis: a pilot study. Pediatr. Crit. Care Med. 16: 114–118. [DOI] [PubMed] [Google Scholar]
- 53. Donnino, M.W. , Andersen L.W., Chase M., et al 2016. Randomized, double‐blind, placebo‐controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit. Care Med. 44: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kauffman, G. , Coats D., Seab S., et al 2011. Thiamine deficiency in ill children. Am. J. Clin. Nutr. 94: 616–617. [DOI] [PubMed] [Google Scholar]
- 55. Becker, D.A. , Balcer L.J. & Galetta S.L.. 2012. The neurological complications of nutritional deficiency following bariatric surgery. J. Obes. 2012: 608534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luong, K.V.Q. & Nguyễn L.T.H.. 2013. The role of thiamine in HIV infection. Int. J. Infect. Dis. 17: e221–e227. [DOI] [PubMed] [Google Scholar]
- 57. Mouly, S. 1996. Beri‐beri and thiamine deficiency in HIV infection. AIDS 10: 931. [DOI] [PubMed] [Google Scholar]
- 58. Hiffler, L. , Adamolekun B., Fischer P.R., et al 2017. Thiamine content of F‐75 therapeutic milk for complicated severe acute malnutrition: time for a change? Ann. N.Y. Acad. Sci. 1404: 20–26. [DOI] [PubMed] [Google Scholar]
- 59. Thurnham, D.I. 2013. Thiamin: beriberi. Encycl. Hum. Nutr. 4: 264–273. [Google Scholar]
- 60. Wyatt, T. , Nelson D. & Hillman E.. 1991. Age‐dependent changes blood and cerebrospinal in thiamin concentrations in whole fluid in infants and children. Am. J. Clin. Nutr. 53: 530–536. [DOI] [PubMed] [Google Scholar]
- 61. Carpenter, K.J. 2000. Beriberi, White Rice, and Vitamin B: A Disease, A Cause, A Cure. Berkeley, CA: University of Califormia Press. [Google Scholar]
- 62. McGready, R. , Simpson J.A., Cho T., et al 2001. Postpartum thiamine deficiency in a Karen displaced population. Am. J. Clin. Nutr. 74: 808–813. [DOI] [PubMed] [Google Scholar]
- 63. Barennes, H. , Sengkhamyong K., René J.P., et al 2015. Beriberi (thiamine deficiency) and high infant mortality in Northern Laos. PLoS Negl. Trop. Dis. 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soukaloun, D. , Kounnavong S., Pengdy B., et al 2003. Dietary and socio‐economic factors associated with beriberi in breastfed Lao infants. Ann. Trop. Paediatr. 23: 181–186. [DOI] [PubMed] [Google Scholar]
- 65. Khounnorath, S. , Chamberlain K., Taylor A.M., et al 2011. Clinically unapparent infantile thiamin deficiency in Vientiane, Laos. PLoS Negl. Trop. Dis. 5: 19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Porter, S.G. , Coats D., Fischer P.R., et al 2014. Thiamine deficiency and cardiac dysfunction in Cambodian infants. J. Pediatr. 164: 1456–1461. [DOI] [PubMed] [Google Scholar]
- 67. Tang, C.M. , Wells J.C., Rolfe M., et al 1989. Outbreak of beri‐beri in The Gambia. Lancet 334: 206–207. [DOI] [PubMed] [Google Scholar]
- 68. Tajahmady, A. , Quatresous I., Sissoko D., et al 2004. Une épidémie de béribéri infantile à Mayotte, avril–juillet 2004. Bull. Epidemiol. Hebdo. 45: 213–214. [Google Scholar]
- 69. Niles, E.J. , Huppatz C. & Beck K.. 2015. WHO report for Kiribati Ministry of Health & medical services outbreak of thiamine deficiency disease.
- 70. Macias‐Matos, C. , Rodriguez‐Ojea A., Chi N., et al 1996. Biochemical evidence of thiamine depletion during the Cuban neuropathy epidemic, 1992–1993. Am. J. Clin. Nutr. 64: 347–353. [DOI] [PubMed] [Google Scholar]
- 71. Keating, E.M. , Nget P., Kea S., et al 2014. Thiamine deficiency in tachypnoeic Cambodian infants. Paediatr. Int. Child Health 35: 312–318. [DOI] [PubMed] [Google Scholar]
- 72. Saya, R.P. , Baikunje S., Prakash P.S., et al 2012. Clinical correlates and outcome of shoshin beriberi. N. Am. J. Med. Sci. 4: 503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xin, Y. , Wan D.H., Chu Q., et al 2011. Severe sepsis as an initial presentation in children with Wernicke's encephalopathy: report of a case and literature review. Chin. J. Pediatr. 49: 612–616. [PubMed] [Google Scholar]
- 74. Bhat, J.I. , Rather H.A., Ahangar A.A., et al 2017. Shoshin beriberi–thiamine responsive pulmonary hypertension in exclusively breastfed infants: a study from northern India. Indian Heart J. 69: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Qureshi, U.A. , Sami A., Altaf U., et al 2016. Thiamine responsive acute life threatening metabolic acidosis in exclusively breast‐fed infants. Nutrition 32: 213–216. [DOI] [PubMed] [Google Scholar]
- 76. Murase, C. , Otowa N., Minami J., et al 2013. Shoshin beirberi in a young man living on Japanese rice balls. J. Gen. Intern. Med. 28: S390. [Google Scholar]
- 77. Wani, N.A. , Qureshi U.A., Ahmad K., et al 2016. Cranial ultrasonography in infantile encephalitic beriberi: a useful first‐line imaging tool for screening and diagnosis in suspected cases. Am. J. Neuroradiol. 37: 1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Narasimha Rao, S. , Mani S., Madap K., et al 2008. High prevalence of infantile encephalitic beriberi with overlapping features of Leigh's disease. J. Trop. Pediatr. 54: 328–332. [DOI] [PubMed] [Google Scholar]
- 79. Qureshi, U.A. , Wani N.A., Ahmad K., et al 2015. Infantile Wernicke's encephalopathy. Arch. Dis. Child. 100: 648. [DOI] [PubMed] [Google Scholar]
- 80. McCandless, D.W. 2009. Thiamine Deficiency and Associated Clinical Disorders In Contemporary Clinical Neuroscience Series. pp. 31–46. New York, NY: Humana Press. [Google Scholar]
- 81. Lallas, M. & Desai J.. 2014. Wernicke encephalopathy in children and adolescents. World J. Pediatr. 10: 293–298. [DOI] [PubMed] [Google Scholar]
- 82. Kesler, A. , Stolovitch C., Hoffmann C., et al 2005. Acute ophthalmoplegia and nystagmus in infants fed a thiamine‐deficient formula: an epidemic of Wernicke encephalopathy. J. Neuro ophthalmol. 25: 169–172. [DOI] [PubMed] [Google Scholar]
- 83. Isenberg‐Grzeda, E. , Kutner H.E. & Nicolson S.E.. 2012. Wernicke–Korsakoff‐syndrome: under‐recognized and under‐treated. Psychosomatics 53: 507–516. [DOI] [PubMed] [Google Scholar]
- 84. Lavoie, J. & Butterworth R.F.. 1995. Reduced activities of thiamine‐dependent enzymes in brains of alcoholics in the absence of Wernicke's encephalopathy. Alcohol. Clin. Exp. Res. 19: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 85. Butterworth, R.F. , Gaudreau C., Vincelette J., et al 1991. Thiamine deficiency and Wernicke's encephalopathy in AIDS. Metab. Brain Dis. 6: 207–212. [DOI] [PubMed] [Google Scholar]
- 86. Ogershok, P.R. , Rahman A., Nestor S., et al 2002. Wernicke encephalopathy in nonalcoholic patients. Am. J. Med. Sci. 323: 107–111. [DOI] [PubMed] [Google Scholar]
- 87. Kornreich, L. , Bron‐Harlev E., Hoffmann C., et al 2005. Thiamine deficiency in infants: MR findings in the brain. Am. J. Neuroradiol. 26: 1668–1674. [PMC free article] [PubMed] [Google Scholar]
- 88. Adamolekun, B. 2011. Neurological disorders associated with cassava diet: a review of putative etiological mechanisms. Metab. Brain Dis. 26: 79–85. [DOI] [PubMed] [Google Scholar]
- 89. Monekosso, G.L. , Annan W.G.T. & Ashby P.H.. 1964. Therapeutic effect of vitamin B complex on an ataxic syndrome in western Nigeria. Trans. R. Soc. Trop. Med. Hyg. 58: 432–436. [DOI] [PubMed] [Google Scholar]
- 90. Adamolekun, B. 2010. Etiology of Konzo, epidemic spastic paraparesis associated with cyanogenic glycosides in cassava: role of thiamine deficiency? J. Neurol. Sci. 296: 30–33. [DOI] [PubMed] [Google Scholar]
- 91. Nzwalo, H. & Cliff J.. 2011. Konzo: from poverty, cassava, and cyanogen intake to toxico‐nutritional neurological disease. PLoS Negl. Trop. Dis. 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Adamolekun, B. , McCandless D.W. & Butterworth R.F.. 1997. Epidemic of seasonal ataxia in Nigeria following injestion of the African Silkworm Anaphe venata: role of thiamine deficiency? Metab. Brain Dis. 12: 251–258. [DOI] [PubMed] [Google Scholar]
- 93. Adamolekun, B. & Ndububa D.A.. 1994. Epidemiology and clinical presentation of a seasonal ataxia in Western Nigeria. J. Neurol. Sci. 124: 95–98. [DOI] [PubMed] [Google Scholar]
- 94. Mimouni‐Bloch, A. , Goldberg‐Stern H., Strausberg R., et al 2014. Thiamine deficiency in infancy: long‐term follow‐up. Pediatr. Neurol. 51: 311–316. [DOI] [PubMed] [Google Scholar]
- 95. Fattal‐Valevski, A. , Bloch‐Mimouni A., Kivity S., et al 2009. Epilepsy in children with infantile thiamine deficiency. Neurology 73: 828–833. [DOI] [PubMed] [Google Scholar]
- 96. Fattal, I. , Friedmann N. & Fattal‐Valevski A.. 2011. The crucial role of thiamine in the development of syntax and lexical retrieval: a study of infantile thiamine deficiency. Brain 134: 1720–1739. [DOI] [PubMed] [Google Scholar]
- 97. Harel, Y. , Zuk L., Guindy M., et al 2017. The effect of subclinical infantile thiamine deficiency on motor function in preschool children. Matern. Child Nutr. 13 10.1111/mcn.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fattal‐Valevski, A. , Kesler A., Sela B.‐A., et al 2005. Outbreak of life‐threatening thiamine deficiency in infants in Israel caused by a defective soy‐based formula. Pediatrics 115: e233–e238. [DOI] [PubMed] [Google Scholar]
- 99. Nosten, F.H. 2015. Beriberi in Cambodia. Paediatr. Int. Child Health 34: 283–284. [DOI] [PubMed] [Google Scholar]
- 100. Whitfield, K.C. , Karakochuk C.D., Kroeun H., et al 2016. Perinatal consumption of thiamine‐fortified fish sauce in rural Cambodia: a randomized clinical trial. JAMA Pediatr. 170: e162065. [DOI] [PubMed] [Google Scholar]
- 101. National Institute of Statistics, Directorate General for Health & ICF International . 2015. Cambodia demographic and health survey 2014. Phnom Penh, Cambodia and Rockville, Maryland.
- 102. Raghavan, R. , Ashour F.S. & Bailey R.. 2016. A review of cutoffs for nutritional biomarkers. Adv. Nutr. 7: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. van der Westhuyzen, J. , Steyn N.P., Icke G.C., et al 1988. Thiamin intakes and erythrocyte thiamin levels in eleven‐year‐old children in the Western Cape. Trop. Geogr. Med. 40: 218–222. [PubMed] [Google Scholar]
- 104. Bailey, A.L. , Finglas P.M., Wright A.J., et al 1994. Thiamin intake, erythrocyte transketolase (EC 2.2.1.1) activity and total erythrocyte thiamin in adolescents. Br. J. Nutr. 72: 111–125. [DOI] [PubMed] [Google Scholar]
- 105. Mancinelli, R. , Ceccanti M., Guiducci M.S., et al 2003. Simultaneous liquid chromatographic assessment of thiamine, thiamine monophosphate and thiamine diphosphate in human erythrocytes: a study on alcoholics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 789: 355–363. [DOI] [PubMed] [Google Scholar]
- 106. Bailey, A.L. & Finglas P.M.. 1990. A normal phase high performance liquid chromatographic method for the determination of thiamin in blood and tissue samples. J. Micronutr. Anal. 7: 147–57. [Google Scholar]
- 107. Gibson, R.S. 2005. Principles of Nutritional Assessment. 2nd ed New York, NY: Oxford University Press. [Google Scholar]
- 108. Whitfield, K.C. , Karakochuk C.D., Kroeun H., et al 2017. Household consumption of thiamin‐fortified fish sauce increases erythrocyte thiamin concentrations among rural Cambodian women and their children younger than 5 years of age: a randomized controlled efficacy trial. J. Pediatr. 181: 242–247.e2. [DOI] [PubMed] [Google Scholar]
- 109. Coats, D. , Frank E.L., Reid J.M., et al 2013. Thiamine pharmacokinetics in Cambodian mothers and their breastfed infants. Am. J. Clin. Nutr. 98: 839–844. [DOI] [PubMed] [Google Scholar]
- 110. Rindi, G. & Laforenza U.. 2000. Thiamine intestinal transport and related issues: recent aspects. Proc. Soc. Exp. Biol. Med. 224: 246–255. [DOI] [PubMed] [Google Scholar]
- 111. Morrison, A.B. & Campbell J.A.. 1960. Vitamin absorption studies. 1. Factors influencing the excretion of oral test doses of thiamine and riboflavin by human subjects. J. Nutr. 72: 435–440. [DOI] [PubMed] [Google Scholar]
- 112. Davis, R.E. , Icke G.C., Thom J., et al 1984. Intestinal absorption of thiamin in man compared with folate and pyridoxal and its subsequent urinary excretion. J. Nutr. Sci. Vitaminol. 30: 475–482. [DOI] [PubMed] [Google Scholar]
- 113. The Republic of the Union of Myanmar Ministry of Health & UNICEF . 2014. Study on cause of under‐five mortality.
- 114. Wani, N.A. , Qureshi U.A., Jehangir M., et al 2016. Infantile encephalitic beriberi: magnetic resonance imaging findings. Pediatr. Radiol. 46: 96–103. [DOI] [PubMed] [Google Scholar]
- 115. McKenna, L.A. , Drummond R.S., Drummond S., et al 2013. Seeing double: the low carb diet. Br. Med. J. 346: f2563. [DOI] [PubMed] [Google Scholar]
- 116. Beal, T. , Massiot E., Arsenault J.E., et al 2017. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS One 12: e0175554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Butterworth, R.F. 2001. Maternal thiamine deficiency: still a problem in some world communities. Am. J. Clin. Nutr. 74: 712–713. [DOI] [PubMed] [Google Scholar]
- 118. Mayxay, M. , Taylor A.M., Khanthavong M., et al 2007. Thiamin deficiency and uncomplicated falciparum malaria in Laos. Trop. Med. Int. Health 12: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Soukaloun, D. , Lee S.J., Chamberlain K., et al 2011. Erythrocyte transketolase activity, markers of cardiac dysfunction and the diagnosis of infantile beriberi. PLoS Negl. Trop. Dis. 5: e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. FAO. FAOSTAT . Food balance sheet Cambodia. 2011. Accessed July 8, 2018. http://www.fao.org/faostat/en/#country/115.
- 121. Carriquiry, A.L. 1999. Assessing the prevalence of nutrient inadequacy. Public Health Nutr. 2: 23–34. [DOI] [PubMed] [Google Scholar]
- 122. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline. 1998. Thiamin In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. pp. 58–86. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- 123. Gibson, R.S. & Cavalli‐Sforza T.. 2012. Using reference nutrient density goals with food balance sheet data to identify likely micronutrient deficits for fortification planning in countries in the Western Pacific region. Food Nutr. Bull. 33: 214–220. [DOI] [PubMed] [Google Scholar]
- 124. Dary, O. & Jariseta Z.R.. 2013. Validation of dietary applications of Household Consumption and Expenditures Surveys (HCES) against a 24‐hour recall method in Uganda. Food Nutr. Bull. 33: S190–S198. [DOI] [PubMed] [Google Scholar]
- 125. Coates, J. , Bell W.F.L., Bermudez O.I., et al 2017. Getting the food list “right”: leveraging household consumption and expenditure surveys for food security and nutrition. FASEB J. 31: 756–786. [Google Scholar]
- 126. Binkley, N. , Sempos C.T. & V.D.S.P. (VDSP) . 2014. Standardizing vitamin D assays: the way forward. J. Bone Miner. Res. 29: 1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dwyer, J.T. , Wiemer K.L., Dary O., et al 2015. Fortification and health: challenges and opportunities. Adv. Nutr. 6: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Smith, G. 2015. Micronutrient fortification of food: issues for Asia. J. Nutr. Sci. Vitaminol. 61(Suppl.): S183–S185. [DOI] [PubMed] [Google Scholar]
- 129. Hurrell, R. 1999. The Mineral Fortification of Foods. Surrey: Leatherhead Publishing. [Google Scholar]
- 130. Rosenberg, I.H. , Abrams S.A., Beecher G.R., et al 2004. Dietary reference intakes: guiding principles for nutrition labeling and fortification. Nutr. Rev. 62: 73–79. [DOI] [PubMed] [Google Scholar]
- 131. Dijkhuizen, M.A. , Wieringa F.T., Soekarjo D., et al 2013. Legal framework for food fortification: examples from Vietnam and Indonesia. Food Nutr. Bull. 34: S112–S123. [DOI] [PubMed] [Google Scholar]
- 132. Harper, C.G. , Sheedy D.L., Lara A.I., et al 1998. Prevalence of Wernicke–Korsakoff syndrome in Australia: has thiamine fortification made a difference? Med. J. Aust. 168: 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Swaminathan, S. , Thomas T. & Kurpad A.V.. 2015. B‐vitamin interventions for women and children in low‐income populations. Curr. Opin. Clin. Nutr. Metab. Care 18: 295–306. [DOI] [PubMed] [Google Scholar]
- 134. Hess, S.Y. , Brown K.H., Sablah M., et al 2013. Results of Fortification Rapid Assessment Tool (FRAT) surveys in sub‐Saharan Africa and suggestions for future modifications of the survey instrument. Food Nutr. Bull. 34: 21–38. [DOI] [PubMed] [Google Scholar]
- 135. Khanh Van, T. , Burja K., Thuy Nga T., et al 2014. Organoleptic qualities and acceptability of fortified rice in two Southeast Asian countries. Ann. N.Y. Acad. Sci. 1324: 48–54. [DOI] [PubMed] [Google Scholar]
- 136. Institute of Medicine . 1998. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic acid, Biotin, and Choline. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 137. Wirth, J.P. , Laillou A., Rohner F., et al 2012. Lessons learned from national food fortification projects: experiences from Morocco, Uzbekistan, and Vietnam. Food Nutr. Bull. 33: S281–S292. [DOI] [PubMed] [Google Scholar]
- 138. Montgomery, S. , Rosenzweig J. & Smit J.. 2014. Technology for rice fortification. Accessed: July 8, 2018. https://sightandlife.org/wp-content/uploads/2017/07/SAL_WFP_RiceFort_LatinAm-Technology.pdf.
- 139. Wieringa, F.T. , Laillou A., Guyondet C., et al 2014. Stability and retention of micronutrients in fortified rice prepared using different cooking methods. Ann. N.Y. Acad. Sci. 1324: 40–47. [DOI] [PubMed] [Google Scholar]
- 140. Otten J., Hellwig J. & Meyers L., Eds. 2006. Dietary Reference Intakes: the Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press. [Google Scholar]
- 141. Preedy, V.R. , Srirajaskanthan R. & Patel V.B.. 2013. Handbook of Food Fortification and Health. 1st ed New York, NY: Humana Press. [Google Scholar]
- 142. Steyn, N.P. , Wolmarans P., Nel J.H., et al 2008. National fortification of staple foods can make a significant contribution to micronutrient intake of South African adults. Public Health Nutr. 11: 307–313. [DOI] [PubMed] [Google Scholar]
- 143. Cambodia Ministry of Planning . National Council of Nutrition. 2012. Proclamation for the production and consumption of iron fortified fish sauce and soy sauce. No.048 NCN. Phnom Penh.
- 144. Theary, C. , Panagides D., Laillou A., et al 2013. Fish sauce, soy sauce, and vegetable oil fortification in Cambodia: where do we stand to date? Food Nutr. Bull. 34: S62–S71. [DOI] [PubMed] [Google Scholar]
- 145. Van Thuy, P. , Berger J., Nakanishi Y., et al 2005. The use of NaFeEDTA‐fortified fish sauce is an effective tool for controlling iron deficiency in women of childbearing age in rural Vietnam. J. Nutr. 135: 2596–2601. [DOI] [PubMed] [Google Scholar]
- 146. Van Thuy, P. , Berger J., Davidsson L., et al 2003. Regular consumption of NaFeEDTA‐fortified fish sauce improves iron status and reduces the prevalence of anemia in anemic Vietnamese women. Am. J. Clin. Nutr. 78: 284–290. [DOI] [PubMed] [Google Scholar]
- 147. Chavasit, V. , Tuntipopipat S. & Watanapaisantrakul R.. 2013. Fortification of fish sauce and soy sauce In Handbook of Food Fortification and Health. Preedy V., Srirajaskanthan R. & Patel V., Eds.: 113–125. New York, NY: Humana Press. [Google Scholar]
- 148. Laillou, A. , Pfanner S., Chan T., et al 2016. Beyond effectiveness—the adversities of implementing a fortification program. A case study on the quality of iron fortification of fish and soy sauce in Cambodia. Nutrients 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. World Health Organization . 2014. Guideline: fortification of food‐grade salt with iodine for the prevention and control of iodine deficiency disorders. World Health Organization, Geneva. [PubMed] [Google Scholar]
- 150. Sultan, S. , Anjum F.M., Butt M.S., et al 2014. Concept of double salt fortification; a tool to curtail micronutrient deficiencies and improve human health status. J. Sci. Food Agric. 94: 2830–2838. [DOI] [PubMed] [Google Scholar]
- 151. Vinodkumar, M. & Rajagopalan S.. 2009. Multiple micronutrient fortification of salt. Eur. J. Clin. Nutr. 63: 437–445. [DOI] [PubMed] [Google Scholar]
- 152. Laillou, A. , Mam B., Oeurn S., et al 2015. Iodized salt in Cambodia: trends from 2008 to 2014. Nutrients 7: 4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pachón, H. , Fabrizio C. & Rosenzweig J.. 2014. Addressing myths and misconceptions about rice fortification. Accessed: July 8, 2018. https://sightandlife.org/wp-content/uploads/2017/07/SAL_WFP_RiceFort_LatinAm-Addressing-Myths-and-Misconceptions.pdf.
- 154. Pourcel, L. , Moulin M. & Fitzpatrick T.B.. 2013. Examining strategies to facilitate vitamin B1 biofortification of plants by genetic engineering. Front. Plant Sci. 4: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Goyer, A. 2016. Thiamin biofortification of crops. Curr. Opin. Biotechnol. 44: 1–7. [DOI] [PubMed] [Google Scholar]
- 156. Bemeur, C. & Butterworth R.F.. 2014. Thiamin In Modern Nutrition in Health and Disease. 11th ed Ross A.C., Calallero B., Cousins R.J., et al Eds.: 317–324. Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- 157. Kramer, M.S. & Kakuma R.. 2004. The optimal duration of exclusive breastfeeding: a systematic review. Adv. Exp. Med. Biol. 54: 63–77. [DOI] [PubMed] [Google Scholar]
- 158. Stuetz, W. , Carrara V.I., McGready R., et al 2012. Micronutrient status in lactating mothers before and after introduction of fortified flour: cross‐sectional surveys in Maela refugee camp. Eur. J. Nutr. 51: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Prentice, A.M. , Roberts S.B., Prentice A., et al 1983. Dietary supplementation of lactating Gambian women. I. Effect on breast‐milk volume and quality. Hum. Nutr. Clin. Nutr. 37: 53–64. [PubMed] [Google Scholar]
- 160. Deodhar, A.D. , Rajalakshmi R. & Ramakrishnan C.V.. 1964. Studies on human lactation part III: effect of dietary vitamin supplementation on vitamin contents of breast milk. Acta Paediatr. 53: 42–48. [DOI] [PubMed] [Google Scholar]
- 161. Ortega, R.M. , Martínez R.M., Andrés P., et al 2004. Thiamin status during the third trimester of pregnancy and its influence on thiamin concentrations in transition and mature breast milk. Br. J. Nutr. 92: 129–135. [DOI] [PubMed] [Google Scholar]
- 162. Barennes, H. , Simmala C., Odermatt P., et al 2009. Postpartum traditions and nutrition practices among urban Lao women and their infants in Vientiane, Lao PDR. Eur. J. Clin. Nutr. 63: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Dewey, K.G. & Brown K.H.. 2003. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr. Bull. 24: 5–28. [DOI] [PubMed] [Google Scholar]
- 164. Ferguson, E.L. , Darmon N., Briend A., et al 2004. Food‐based dietary guidelines can be developed and tested using linear programming analysis. J. Nutr. 134: 951–957. [DOI] [PubMed] [Google Scholar]
- 165. Ferguson, E. , Darmon N. & Fahmida U.. 2008. Formulate and evaluate population‐specific complementary feeding recommendations using linear programming. Sight Life Mag. 3: 13–18. [Google Scholar]
- 166. Hlaing, L.M. , Fahmida U., Htet M.K., et al 2016. Local food‐based complementary feeding recommendations developed by the linear programming approach to improve the intake of problem nutrients among 12–23‐month‐old Myanmar children. Br. J. Nutr. 116: S16–S26. [DOI] [PubMed] [Google Scholar]
- 167. Vanier, N.L. , Paraginski R.T., Berrios J.D.J., et al 2015. Thiamine content and technological quality properties of parboiled rice treated with sodium bisulfite: benefits and food safety risk. J. Food Compos. Anal. 41: 98–103. [Google Scholar]
- 168. Khush, G.S. 1997. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 35: 25–34. [PubMed] [Google Scholar]
- 169. Bosma, R.H. , Udo H.M.J., Verreth J.A.J., et al 2005. Agriculture diversification in the Mekong Delta: farmers’ motives and contributions to livelihoods. Asian J. Agric. Dev. 2: 49–66. [Google Scholar]
- 170. Lemke, U. , Mergenthaler M., Rössler R., et al 2008. Pig production in Vietnam—a review. Pig News Inf. 29: 1R. [Google Scholar]
- 171. Pillaiyar, P. 1981. Household parboiling of parboiled rice. Kishan World 8: 20–22. [Google Scholar]
- 172. Janssen, M.M.T. 1997. Antinutritives In Food Safety and Toxicity. de Vries J., Ed.: 39–52. Boca Raton, FL: CRC Press. [Google Scholar]
- 173. Vimokesant, S. , Kunjara S., Rungruangsak K., et al 1982. Beriberi caused by antithiamin factors in food and its prevention. Ann. N.Y. Acad. Sci. 378: 123–136. [DOI] [PubMed] [Google Scholar]
- 174. Dzed, L. , Dorji T., Pelzom D., et al 2015. Status of thiamin deficiency in boarding school children from seven districts in Bhutan with previous history of peripheral neuropathy outbreaks: a cohort study. Bhutan Health J. 1: 49–56. [Google Scholar]
- 175. Dzed, L. , Thinley S., Tshomo D., et al 2016. Investigation of suspected peripheral neuropathy outbreak in Dechentsemo Central School, Thinleygang, Punakha. Public Health Indonesia 2: 118–124. [Google Scholar]
- 176. Temu, P. , Temple V.J., Saweri A., et al 2009. Thiamine (vitamin B1) status of boarding school students in the Southern Region of Papua New Guinea. P.N.G. Med. J. 52: 21–27. [PubMed] [Google Scholar]
- 177. Khampitak, T. , Knowles J., Yongvanit P., et al 2006. Thiamine deficiency and parasitic infection in rural Thai children. Southeast Asian J. Trop. Med. Public Health 37: 441–445. [PubMed] [Google Scholar]
- 178. Doung‐Ngern, P. , Kesornsukhon S., Kanlayanaphotporn J., et al 2007. Beriberi outbreak among commercial fishermen, Thailand 2005. Southeast Asian J. Trop. Med. Public Health 38: 130–135. [PubMed] [Google Scholar]
- 179. Nguyen, P.H. , Young M., Gonzalez‐Casanova I., et al 2016. Impact of preconception micronutrient supplementation on anemia and iron status during pregnancy and postpartum: a randomized controlled trial in rural Vietnam. PLoS One 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chen, K.T. , Twu S.J., Chiou S.T., et al 2003. Outbreak of beriberi among illegal mainland Chinese immigrants at a detention center in Taiwan. Public Health Rep. 118: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Naidoo, D.P. 1987. Beriberi heart disease in Durban A retrospective study. S. African Med. J. 72: 241–244. [PubMed] [Google Scholar]
- 182. Watson, J.T. , Bushra H., Lebo E.J., et al 2011. Outbreak of beriberi among African union troops in Mogadishu, Somalia. PLoS One 6: e28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rolfe, M. , Walker R.W., Samba K.N., et al 1993. Urban beri‐beri in The Gambia, West Africa. Trans. R. Soc. Trop. Med. Hyg. 87: 114–115. [DOI] [PubMed] [Google Scholar]
- 184. Thurnham, D.I. , Cathcart A.E. & Livingstone M.B.E.. 2011. A retrospective investigation of thiamin and energy intakes following an outbreak of beriberi in the Gambia. Nutrients 3: 135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Aké‐Tano, O. , Konan E.Y., Tetchi E.O., et al 2011. Le béribéri, maladie nutritionnelle récurrente en milieu carcéral en Côte‐d'Ivoire. Bull. Soc. Pathol. Exot. 104: 347–351. [DOI] [PubMed] [Google Scholar]
- 186. de Montmollin D., McMahon J., et al 2002. Outbreak of beri‐beri in a prison in West Africa. Trop. Doct. 32: 234–236. [DOI] [PubMed] [Google Scholar]
- 187. International Committee of the Red Cross . 2016. Guinea: Building better conditions in prisons; Accessed May 30, 2018. https://www.icrc.org/en/document/guinea-building-better-conditions-prisons. [Google Scholar]
- 188. Darcel, F. , Roussin C., Vallat J.‐M., et al 2009. Polyneuropathies in vitamin B1 deficiency in Reunion and Mayotte islands in 70 patients of Maori and Comorian descent. Bull. Soc. Pathol. Exot. 102: 167–172. [PubMed] [Google Scholar]
- 189. Cerroni, M.P. , Barrado J.C.S., Nobrega A.A., et al 2010. Outbreak of beriberi in an Indian population of the Upper Amazon Region, Roraima State, Brazil, 2008. Am. J. Trop. Med. Hyg. 83: 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Nacher, M. , Ville M., Guarmit B., et al 2017. A large outbreak of thiamine deficiency among illegal gold miners in French Guiana. Am. J. Trop. Med. Hyg. 96: 1248–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Padilha, E.M. , Fujimori E., Borges A.L.V., et al 2011. Perfil epidemiológico do beribéri notificado de 2006 a 2008 no Estado do Maranhão, Brasil. Cad. Saude Publica 27: 449–459. [DOI] [PubMed] [Google Scholar]
- 192. Martínez, M. , Román G., De La Hoz F., et al 1996. Estudio clínico y epidemiológico de un brote de beriberi húmedo en Cartagena de Indias, Colombia, 1992–1993. Biomedica 16: 41–51. [Google Scholar]
- 193. Jaruratanasirikul, S. , Sangsupawanich P., Koranantakul O., et al 2009. Influence of maternal nutrient intake and weight gain on neonatal birth weight: a prospective cohort study in southern Thailand. J. Matern. Neonatal Med. 22: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 194. Piammongkol, S. , Marks G.C., Williams G., et al 2004. Food and nutrient consumption patterns in third trimester Thai‐Muslim pregnant women in rural Southern Thailand. Asia Pac. J. Clin. Nutr. 13: 236–241. [PubMed] [Google Scholar]
- 195. Sukchan, P. , Liabsuetrakul T., Chongsuvivatwong V., et al 2010. Inadequacy of nutrients intake among pregnant women in the Deep South of Thailand. BMC Public Health 10: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Peng, Y. , Zhou T., Wang Q., et al 2009. Fatty acid composition of diet, cord blood and breast milk in Chinese mothers with different dietary habits. Prostaglandins Leukot. Essent. Fatty Acids 81: 325–330. [DOI] [PubMed] [Google Scholar]
- 197. Zhang, F. , Yi C., Fang G., et al 2010. Dietary intakes and behaviours in pregnant women of Li ethnicity: a comparison of mountainous and coastal populations in southern China. Asia Pac. J. Clin. Nutr. 19: 236–242. [PubMed] [Google Scholar]
- 198. Ma, A. , Chen X., Zheng M., et al 2002. Iron status and dietary intake of Chinese pregnant women with anaemia in the third trimester. Asia Pac. J. Clin. Nutr. 11: 171–175. [DOI] [PubMed] [Google Scholar]
- 199. Liu, F.‐L. , Zhang Y.‐M., Parés G.V., et al 2015. Nutrient intakes of pregnant women and their associated factors in eight cities of china: a cross‐sectional study. Chin. Med. J. 128: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Persson, V. , Winkvist A., Ninuk T., et al 2001. Variability in nutrient intakes among pregnant women in Indonesia: implications for the design of epidemiological studies using the 24‐h recall method. J. Nutr. 131: 325–330. [DOI] [PubMed] [Google Scholar]
- 201. Henjum, S. , Torheim L.E., Thorne‐Lyman A.L., et al 2015. Low dietary diversity and micronutrient adequacy among lactating women in a peri‐urban area of Nepal. Public Health Nutr. 18: 3201–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ghosh‐Jerath, S. , Devasenapathy N., Singh A., et al 2015. Ante natal care (ANC) utilization, dietary practices and nutritional outcomes in pregnant and recently delivered women in urban slums of Delhi, India: an exploratory cross‐sectional study. Reprod. Health 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jood, S. , Bishnoi S. & Khetarpaul N.. 2002. Nutritional status of rural pregnant women of Haryana state, Northern India. Nutr. Health 16: 121–131. [DOI] [PubMed] [Google Scholar]
- 204. Paramjit, Kla. & Puri R.. 1996. Impact of nutrition education on food and nutrient intake of pregnant women. Indian J. Matern. Child Health 7: 11–15. [PubMed] [Google Scholar]
- 205. Purushothaman, V. , Kupputhai U. & Meenakshi N.D.. 1988. Nutritional profile of selected expectant mothers and the cost of pregnancy. Indian J. Nutr. Diet. 25: 247. [PubMed] [Google Scholar]
- 206. Dahiya, S. 2002. Nutritional status assessment of pregnant women from Hisar city of Haryana. Nutr. Health 16: 239–247. [DOI] [PubMed] [Google Scholar]
- 207. Panwar, B. & Punia D.. 1998. Nutrient intake of rural pregnant women of Haryana State, Northern India: relationship between income and education. Int. J. Food Sci. Nutr. 49: 391–395. [DOI] [PubMed] [Google Scholar]
- 208. Pobocik, R.S. , Heathcote G.M., Spiers J.B., et al 2000. Nutritional and anthropometric assessment of a sample of pregnant women and young children in Palau. Asia Pac. J. Clin. Nutr. 9: 102–114. [DOI] [PubMed] [Google Scholar]
- 209. Esmaillzadeh, A. , Samareh S. & Azadbakht L.. 2008. Dietary patterns among pregnant women in the west‐north of Iran. Pak. J. Biol. Sci. 11: 793–796. [DOI] [PubMed] [Google Scholar]
- 210. Huybregts, L.F. , Roberfroid D.A., Kolsteren P.W., et al 2009. Dietary behaviour, food and nutrient intake of pregnant women in a rural community in Burkina Faso. Matern. Child Nutr. 5: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Belgnaoui, S. & Belahsen R.. 2006. Nutrient intake and food consumption among pregnant women from an agricultural region of Morocco. Int. J. Food Sci. Nutr. 57: 19–27. [DOI] [PubMed] [Google Scholar]
- 212. Nti, C.A. , Larweh P.M. & Gyemfua‐Yeboah Y.. 2002. Food consumption patterns, dietary quality and health status of expectant mothers: case studies in suburban and rural communities in Ghana. Int. J. Consum. Stud. 26: 7–14. [Google Scholar]
- 213. Kirksey, A. , Wachs T., Yunis F., et al 1994. Relation of maternal zinc nutriture to pregnancy and infant development in an Egyptian village. Am. J. Clin. Nutr. 60: 782–792. [DOI] [PubMed] [Google Scholar]
- 214. Suarez‐Ortegón, M.F. , Mosquera M., Caicedo D.M., et al 2013. Nutrients intake as determinants of blood lead and cadmium levels in Colombian pregnant women. Am. J. Hum. Biol. 25: 344–350. [DOI] [PubMed] [Google Scholar]
- 215. Gyorkos, T.W. , Shenker H., Larocque R., et al 2004. Sociodemographic and dietary correlates of anemia in pregnant women in Peru. Ecol. Food Nutr. 43: 497–516. [Google Scholar]
- 216. Sacco, L.M. , Caulfield L.E., Zavaleta N., et al 2003. Dietary pattern and usual nutrient intakes of Peruvian women during pregnancy. Eur. J. Clin. Nutr. 57: 1492–1497. [DOI] [PubMed] [Google Scholar]
- 217. Isela, R. , Hernández R., Quechol G.R., et al 2005. Alimentación y estado nutricio de mujeres embarazadas derechohabientes del Instituto Mexicano del Seguro Social en un área suburbana de la Ciudad de México. Ginecol. Obs. Mex. 73: 3–10. [PubMed] [Google Scholar]
- 218. Barbieri, P. , Nishimura R.Y., Crivellenti L.C., et al 2012. Relative validation of a quantitative FFQ for use in Brazilian pregnant women. Public Health Nutr. 16: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Eliana Duran, F. , Delia Soto A., Ana Maria Labrana T., et al 2007. Dietetic adequation of micronutrients in pregnant women. Rev. Chil. Nutr. 34: 321. [Google Scholar]
- 220. Weigel, M. , Narvaez W., Lopez A., et al 1991. Prenatal diet, nutrient intake and pregnancy outcome in urban Ecuadorian primiparas. Arch. Latinoam. Nutr. 41: 21–37. [PubMed] [Google Scholar]
- 221. Adamolekun, B. & Hiffler L.. 2017. A diagnosis and treatment gap for thiamine deficiency disorders in sub‐Saharan Africa? Ann. N.Y. Acad. Sci. 1408: 15–19. [DOI] [PubMed] [Google Scholar]


