Abstract
Terrestrial biogeochemical feedbacks to the climate are strongly modulated by the temperature response of soil microorganisms. Tropical forests, in particular, exert a major influence on global climate because they are the most productive terrestrial ecosystem. We used an elevation gradient across tropical forest in the Andes (a gradient of 20°C mean annual temperature, MAT), to test whether soil bacterial and fungal community growth responses are adapted to long‐term temperature differences. We evaluated the temperature dependency of soil bacterial and fungal growth using the leucine‐ and acetate‐incorporation methods, respectively, and determined indices for the temperature response of growth: Q 10 (temperature sensitivity over a given 10oC range) and T min (the minimum temperature for growth). For both bacterial and fungal communities, increased MAT (decreased elevation) resulted in increases in Q 10 and T min of growth. Across a MAT range from 6°C to 26°C, the Q 10 and T min varied for bacterial growth (Q 10–20 = 2.4 to 3.5; T min = −8°C to −1.5°C) and fungal growth (Q 10–20 = 2.6 to 3.6; T min = −6°C to −1°C). Thus, bacteria and fungi did not differ significantly in their growth temperature responses with changes in MAT. Our findings indicate that across natural temperature gradients, each increase in MAT by 1°C results in increases in T min of microbial growth by approximately 0.3°C and Q 10–20 by 0.05, consistent with long‐term temperature adaptation of soil microbial communities. A 2°C warming would increase microbial activity across a MAT gradient of 6°C to 26°C by 28% to 15%, respectively, and temperature adaptation of microbial communities would further increase activity by 1.2% to 0.3%. The impact of warming on microbial activity, and the related impact on soil carbon cycling, is thus greater in regions with lower MAT. These results can be used to predict future changes in the temperature response of microbial activity over different levels of warming and over large temperature ranges, extending to tropical regions.
Keywords: bacteria, climate warming, fungi, Q10, Ratkowsky equation, soil carbon cycle, tropical forest
1. INTRODUCTION
Soil microorganisms regulate terrestrial biogeochemical cycles, and their response to temperature is a critical factor in regulating feedbacks associated with climate warming (Davidson & Janssens, 2006). Models have demonstrated that the nature of temperature‐adaptive responses in soil microbial physiology, community composition, enzyme function or growth, may have major influences on atmospheric CO2 accumulation in the 21st century (Wieder, Bonan, & Allison, 2013). Of all terrestrial ecosystems, tropical forests exert the largest influence on global climate because they are the most productive and have the highest respiration rates (Beer et al., 2010; Pan et al., 2011), in addition to containing the highest biomass of soil microorganisms (Serna‐Chavez, Fierer, & Bodegom, 2013). It is surprising, therefore, that we have a limited understanding of the temperature response of soil microbial communities in these ecosystems.
Research on the temperature response of soil organic matter cycling has been extensive, albeit concentrated outside the tropics, but a consensus remains elusive (Conant et al., 2011; Karhu et al., 2014; Kirschbaum, 2006). The focus of this work has often been on the temperature response of respiration, in the context of its potential impact as a positive feedback on climate warming (Davidson & Janssens, 2006) and the potential for a temperature‐adaptive response of the microbial community in affecting this feedback (Bradford et al., 2008; Karhu et al., 2014). Such an adaptation response has been defined as a change in microbial community composition, physiology or enzyme function, which has a net result of metabolism being better‐optimized to a given temperature (Bárcenas‐Moreno, Gomez‐Brandon, Rousk, & Bååth, 2009; Bradford, 2013). The lack of consensus among studies on the temperature response of microbial activity arises partly because respiration, a commonly measured index of microbial activity, has an “apparent” temperature sensitivity that is influenced by multiple environmental variables (“indirect effects”) that vary among soils (Nottingham, Whitaker, et al., 2015). It will also be affected indirectly by factors other than the temperature regime, such as substrate availability and moisture (Davidson & Janssens, 2006; Nottingham, Whitaker, et al., 2015). To isolate the direct effect of temperature, one must estimate the “intrinsic” temperature sensitivities of specific processes such as carbon‐assimilation, enzyme activities and growth, which are independent of these indirect effects. These intrinsic temperature sensitivities can be assessed in controlled short‐term incubation experiments (Kirschbaum, 2006), providing standard reproducible information on microbial temperature responses which are then comparable across biomes.
Another reason for the lack of consensus is due to the different ways in which temperature responses are modelled. The temperature sensitivity of microbial processes, such as growth and respiration, has been described using various metrics. A commonly used parameter is the Q 10 value, which represents the ratio of a process at (T + 10°C)/T, where T = standard reference temperature. However, comparison of Q 10 among studies requires careful consideration of the differences in temperature range and reference temperatures used for its calculation, because Q 10 is not constant over a given temperature range. The Q 10 of respiration and microbial growth is higher when determined at lower temperatures (Bååth, 2018; Kirschbaum, 2006; Lloyd & Taylor, 1994) and models often incorporate a higher Q 10 for lower temperatures (Del Grosso et al., 2005; Jenkinson, Adams, & Wild, 1991). This dependency of Q 10 on the measurement temperature range makes it difficult to compare Q 10 among studies that use different temperature ranges in their calculations. The measurement of Q 10 over a large temperature range, assuming constant Q 10, can also introduce problems in predicting the effects of temperature on respiration and growth (Bååth, 2018). Temperature dependency is also often modelled using an Arrhenius relationship, , where k = rate, A = constant, E a = activation energy, R = universal gas constant, and T = absolute temperature. Here, the activation energy (E a) determines the temperature sensitivity. However, since there is a close relationship between E a and Q 10 within the range of temperatures normally found in soils (Raven & Geider, 1988), E a has the same problems in interpretation and determination as Q 10.
An alternative approach that can be used to characterize respiration and growth, while below its temperature optimum (T opt), is the use of the square root relationship: A 0.5 = a × (T–T min), where A is activity (e.g., growth or respiration), T min the apparent minimum temperature for activity (°C), and a is a slope parameter related to absolute activity (Ratkowsky, Olley, Mcmeekin, & Ball, 1982) (Figure 1). The square root relationship, also called the Ratkowsky equation, has been widely used to model the rate of bacterial growth in water (Bell & Ahlgren, 1987; Li & Dickie, 1987) and bacterial and fungal growth in soil (Dıaz‐Raviña, Frostegård, & Bååth, 1994; Pietikäinen, Pettersson, & Bååth, 2005; Rinnan, Rousk, Yergeau, Kowalchuk, & Bååth, 2009; van Gestel, Reischke, & Bååth, 2013). It has also been shown to be an adequate representation of the temperature responses of respiration and decomposition (Bååth, 2018; Kätterer, Reichstein, Andren, & Lomander, 1998; Pietikäinen et al., 2005), and it has been shown that T min increases and decreases following community temperature adaptation to the thermic environment (Bååth, 2018; Bárcenas‐Moreno et al., 2009). Because it is independent of the temperature range at which it is calculated, it can be compared more easily among studies than E a or Q 10. By determining T min following the square root model, we can obtain information on the temperature responses of microbial growth or respiration, which can be related with other process rates such as enzyme kinetics (E a, Q 10). The T min metric, therefore, provides information on the community‐level adaptation to temperature, which can be easily compared across biomes and can be used to predict future effects of climate change.
Figure 1.
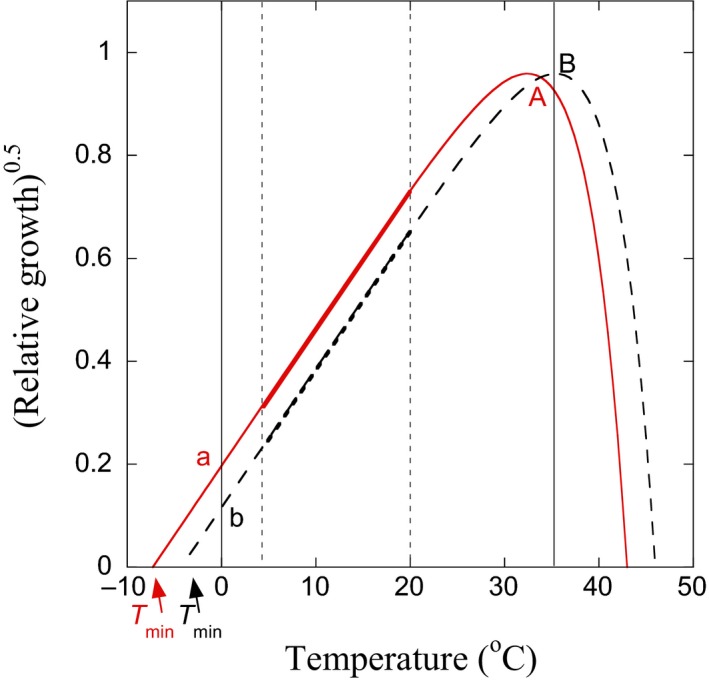
A comparison of the temperature sensitivity of growth for two hypothetical microbial communities, plotted with a square root transformation. Community A (full line, red) is low temperature‐adapted (T min = −7.3°C), and community B (dashed line, black) is high temperature‐adapted (T min = −4.3°C). Three indices of temperature sensitivity are shown. T min was determined by linear regression (thick lines) using measurements at 20 and 4°C (thin stippled vertical lines) and extrapolation, where T min for community A < T min for community B. Q 10–20 (see Methods) was calculated using the same regression, where Q 10–20 for community A <Q 10–20 for community B. A temperature sensitivity index (log 35/0) was determined by the log of the ratio of growth at 35°C and 0°C (thin vertical lines), that is log [(A/a)2] for community A and log [(B/b)2] for community B. The temperature sensitivity index log 35/0 for community A <log 35/0 for community B [Colour figure can be viewed at wileyonlinelibrary.com]
Biogeographic variation in the T min for microbial growth and respiration in soils from different ecosystems has been tentatively estimated as −10 to −15°C in Arctic/Antarctic regions, −5 and −10°C in temperate regions and 0 to −5°C in tropical regions (Pietikäinen et al., 2005; Rinnan et al., 2009; van Gestel et al., 2013). Furthermore, by combining several studies on the effect of mean annual temperature (MAT) on T min for bacterial growth, it has been predicted that a 1°C increase in MAT would result in an increased T min of between 0.2 and 0.3°C (Rousk, Frey, & Bååth, 2012). Bååth (2018) also predicted, using a tentative global envelope of T min in soils, that a 1°C increase in MAT would result in an increased T min of around 0.3°C. However, the temperature sensitivity of soil microbial growth has been studied across only a limited MAT range. For example, Rinnan et al. (2009) studied soils ranging from −4°C to +9°C, while Rousk et al. (2012) compared a MAT of 7°C with an artificially heated treatment with a MAT of 12°C. To be able to predict changes in temperature sensitivity over a major part of the global MAT variation (i.e., −15 to 30°C), a larger range needs to be tested, of course also including tropical regions.
The temperature sensitivity of bacterial growth in soil has been reasonably well studied (Rinnan et al., 2009; Rousk et al., 2012; van Gestel et al., 2013), but this is not the case for fungal growth. Only two earlier studies, in soils and only from the temperate zone, have compared T min for fungi and bacteria (Birgander, Olsson, & Rousk, 2018; Pietikäinen et al., 2005). Similar but slightly lower T min for fungal compared to bacterial growth was found, suggesting fungi to be better adapted to low temperature conditions. However, more studies are needed to test this further, covering a larger variation in MAT.
Elevation gradients on mountainsides have been used to understand plant biogeography by ecologists since the 18th century (Linnaeus, 1781; von Humboldt & Bonpland, 1805), but more recently they have been used as powerful tools to understand how climate change affects plant and microbial ecology (Nottingham, Whitaker, et al., 2015; Sundqvist, Sanders, & Wardle, 2013), by revealing the long‐term temperature acclimation or adaptive changes in plant physiology, soil processes and soil microbial composition (Giardina, Litton, Crow, & Asner, 2014; Girardin et al., 2014; Nottingham, Fierer, et al., 2018). Here, we used a 3.5 km elevation gradient in Peru to explore the long‐term temperature adaptation of bacterial and fungal growth to a 20°C gradient in MAT. We tested the following hypotheses: (a) Increasing MAT (i.e., decreasing elevation) will increase the temperature optima for bacterial growth, resulting in higher T min, temperature sensitivity index (growth at 35°C/0°C) in lower elevation sites with higher MAT; (b) T min will increase around 0.2 to 0.4 per degree Celsius increase in MAT, equivalent to around 0.05 higher Q 10–20 per degree increase in MAT; and (c) based on earlier results in temperate‐zone studies (Pietikäinen et al., 2005), we hypothesize a lower temperature sensitivity index for fungi than bacteria. Since our results cover a gradient in MAT from 6 to 26°C, our data can be used to predict future changes in the temperature sensitivity of microbial growth and activity in soil over a large range of MAT.
2. MATERIALS AND METHODS
2.1. Study sites
The elevation transect under study lies on the Eastern flank of the Andes in southeastern Peru, in the upper Madre de Dios/Madeira watershed. The transect is approximately 270 km in length and spans 3,450 m in elevation from 194 m to 3,644 m above sea level (asl). The transect consists of 14 sites, each with a 1 ha permanent sampling plot, all in old growth tropical forest except for one site on high elevation grassland (Supporting Information Table S1).
Mean annual temperature (MAT) decreases with increasing elevation across the transect (dropping from 26 to 6°C; Supporting Information Figure S1). There is little variation in seasonal temperature across the gradient, with mean daily air temperature differing only by about 4°C between warmest and coolest month, irrespective of elevation, although diurnal variation can increase this range slightly (Rapp & Silman, 2012). Mean annual precipitation (MAP) is consistently high and does not vary consistently with elevation, ranging from 1,506 to 5,302 mm/year among the sites (Nottingham, Whitaker, et al., 2015).
The plots are situated on predominantly Palaeozoic (~450 Ma) meta‐sedimentary mudstone (~80%), with plutonic intrusions (granite) underlying the sites between 1,500 and 2,020 m asl. The soils at the sites above 2,520 m are Umbrisols (Inceptisols), while the soils from 1,000 to 2,020 m are Cambisols (Inceptisols). The soils below 1,000 m, at the two lowland sites, are HaplicAlisols (Ultisols) (194 m asl) and Haplic Cambisols (Inceptisols) (210 m asl) (according to FAO, with USDA Soil Taxonomy in parentheses). Further descriptions of soil, climate and floristic composition of these sites are reported elsewhere (Nottingham, Fierer, et al., 2018; Fyllas et al., 2017; Rapp et al., 2012; Whitaker et al., 2014; van de Weg, Meir, Grace, & Atkin, 2009).
2.2. Soil sampling and analyses
For all sites, soil samples were collected during November 2011. These ecosystems are highly aseasonal, with no significant intra‐annual variation in mean monthly temperature (Rapp & Silman, 2012) and no evidence of seasonal soil or plant moisture constraints (van de Weg et al., 2014; Zimmermann, Meir, Bird, Malhi, & Ccahuana, 2010); therefore, the comparison of soil properties for these sites at a single time point was approximated as representative of patterns likely to be found throughout the year. Furthermore, temperature seasonality has earlier been shown to have no or very little effect on the temperature‐growth response of bacterial communities, even in soils with a large amplitude in temperature over the year (Birgander et al., 2018; van Gestel et al., 2013). We collected soil from four corner subplots and a central subplot, within each of the 1 ha permanent study plots at each elevation site, with soil from these subplots used as five individual replicates. For each subplot, the upper 10 cm surface soil was collected using a soil auger and stored in sealed plastic bags. Soil samples were stored for 1–2 months at approximately 17°C until analysis. Earlier studies have shown that storing soil samples at <25°C for up to 2 months does not affect the temperature characteristics of microbial communities (Bárcenas‐Moreno et al., 2009; Birgander, Reischke, Jones, & Rousk, 2013).
2.3. Bacterial and fungal growth
Temperature sensitivity of microbial growth was determined by measuring instantaneous growth of bacteria and fungi at different temperatures, as earlier used by Pietikäinen et al. (2005). Bacterial growth was estimated using the leucine (Leu) incorporation method, while fungal growth was estimated using the acetate‐in‐ergosterol (Ac‐in‐erg)‐incorporation method (Bååth, 2001; Rousk & Bååth, 2011). Since many samples were processed (14 soils × 5 replicates = 70 soil samples), microbial growth was measured for all soils at one temperature on separate days. This experimental design was suitable to determine relative changes in temperature sensitivity with differences in MAT between sites.
The growth rate of bacteria was estimated using the leucine incorporation method, following Bååth, Pettersson, and Söderberg (2001). Briefly, soil samples (1 g fresh weight) were vortexed with 20 ml distilled H2O for 3 min. and then centrifuged at 1,000 g for 10 min. Aliquots (1.5 ml) of the resulting suspension were transferred to 2 ml tubes and 2 µl [3H]Leu (37 MBq/ml and 5.74 TBq/mmol) combined with unlabelled Leu, resulting in 275 nM Leu in the bacterial suspensions. After incubation at the desired temperature (in water baths), the reaction was terminated with 75 µl 100% trichloroacetic acid. Incubation time was modified according to the incubation temperature to compensate for lower incorporation at low temperatures (Pietikäinen et al., 2005), with 24 hr for 0 and 4°C, and 2 hr for 20 and 35°C. Washing of the samples and measurement of incorporated radioactivity was performed following Bååth et al. (2001).
The growth rate of fungi was estimated using the acetate‐in‐ergosterol‐incorporation method (Newell & Fallon, 1991) adapted for soil (Bååth, 2001), with modifications. Briefly, soil samples (1 g fresh weight) were transferred to test tubes to which 20 µl [1–14C] acetic acid (sodium salt; 7.4 MBq/ml and 2.04 GBq/mmol) with unlabelled sodium acetate, and 1.5 ml distilled H2O, resulting in a final acetate concentration of 220 µM. The resulting soil slurry was then incubated in the dark (for twice as long as the corresponding samples used for bacterial growth), after which 1 ml formalin (5%) was added to terminate the reaction. Ergosterol was then extracted, separated and quantified using high‐performance liquid chromatography and a UV detector (282 nm). The ergosterol peak was collected, and the amount of incorporated radioactivity was determined.
2.4. Calculation of T min, temperature sensitivity index and Q 10
T min was calculated using growth at 4°C and 20°C, assuming a straight‐line relationship for the squared growth rates versus temperature (Figure 1), according to the Ratkowsky equation (Ratkowsky et al., 1982):
where T min (°C) is the apparent minimum temperature for growth, T is the measurement temperature (in our case 4 and 20°C), and a is a slope parameter related to the absolute growth rate. Since T min will always be determined by extrapolation, an alternative temperature sensitivity index, log 35/0 was defined as the log ratio of growth at 35°C and 0°C (Figure 1). A similar ratio was suggested by Bárcenas‐Moreno et al. (2009) as a rapid and sensitive way to study changes in temperature sensitivity and has been shown to correlate with T min (Rinnan et al., 2009). We chose a large temperature range for the temperature sensitivity index (i.e., 35°C and 0°C), to accommodate the variation expected by the large range in MAT between sites. Means and SE were calculated for each site (n = 5 replicate soil samples per site). Regressions against temperature for the different growth indices were then made using mean values per site (n = 14), since they were the independent samples. For indices of fungal growth, sample size (n) was 13 because one site (TC, high elevation grassland) had activity values which were too low to be able to calculate temperature sensitivity.
We calculated Q 10 for the 10 to 20°C range (Q 10–20) using the mean T min values for each site, according to the equation:
where R = the temperature range (for Q 10–20, R = 10) and T L is the lowest temperature in the range (e.g., for Q 10–20 T L = 10) (Bååth, 2018). To calculate Q 10 at MAT±5°C (Q MAT±5), we modified the equation where T L = (MAT−5), with MAT from Supporting Information Table S1. To estimate activity with an increase in MAT of 2°C (representing the lowest end of the range of predicted global MAT increase by 2100; 2–6°C; IPCC, 2013), we used R = 2 and T L = MAT. We further modified Equation ([Link]) to account for temperature adaptation—according to our finding that thermal adaptation of growth led to an increase in T min by 0.6°C per 2°C increase in MAT—by replacing T min with (T min + 0.6).
3. RESULTS
3.1. Soil properties
Increased elevation was highly negatively correlated with a decrease in MAT (R 2 = 0.99, p < 0.001; Supporting Information Figure S1), such that elevation and MAT were interchangeable as explanatory variables. Increased elevation was associated with an increase in total C (R 2 = 0.46, p = 0.008) and total N (R 2 = 0.51, p = 0.004); small but nonsignificant increases in total P (R 2 = 0.18, p = 0.13) and extractable P (R 2 = 0.22, p = 0.09). Soil pH did not vary with elevation (R 2 = 0.001, p = 0.90) (Supporting Information Table S1). Further detail on soil and microbial community properties for these sites, including in organic horizons, is provided elsewhere (Nottingham, Turner, et al., 2015; Whitaker et al., 2014).
3.2. Bacterial growth
Higher MAT (decreased elevation) resulted in bacterial communities with growth adapted to higher temperatures. All three methods of expressing temperature adaptation of bacterial growth were highly positively correlated with MAT (Figure 2). T min varied with MAT according to the equation T min = −10.0 + 0.33*MAT (R 2 = 0.89, p < 0.001, Figure 2a). T min increased from −8°C at the highest elevation sites with MAT around 6°C to a T min of −1.5°C in the lowland sites with MAT around 26°C. This is equivalent to an increased T min of 0.33 ± 0.035°C per 1 degree of increase in MAT in this temperature range. In addition, the temperature sensitivity index log 35/0 was highly positively correlated with MAT (R 2 = 0.88, p < 0.001), also indicating consistent changes in temperature adaptation with changes in MAT (Figure 2b). Given that Q 10 varies with the temperature range used for its calculation (Bååth, 2018; Kirschbaum, 2000), we calculated Q 10 only for one intermediate range of temperatures (between 10 and 20°C). Q 10–20 increased from approximately 2.4 at a MAT of 6°C to almost 3.5 at a MAT of 26°C (R 2 = 0.93, Figure 2c). This translates to an increase in Q 10–20 of 0.055 ± 0.004 per 1 degree of increase in MAT for this temperature range.
Figure 2.
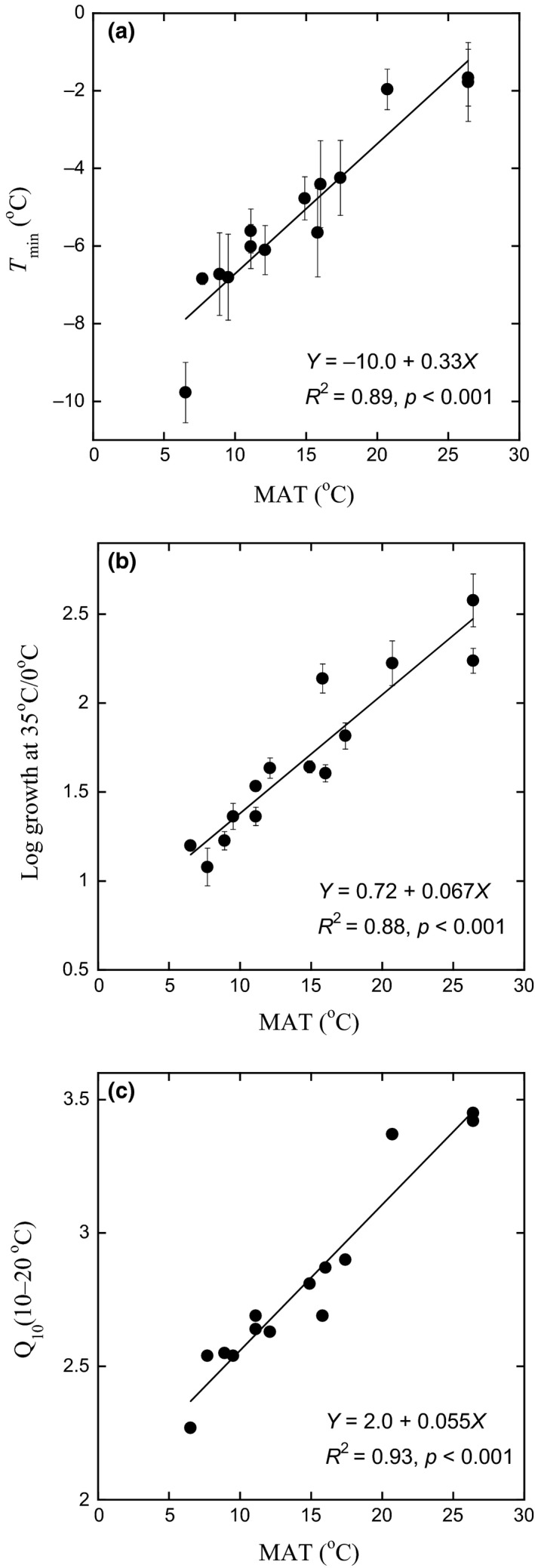
Bacterial community growth response to differences in mean annual temperature (MAT) along an elevation gradient in the Andes. The temperature sensitivity was expressed using three different metrics. (a) Temperature sensitivity expressed by T min as affected by MAT, calculated from the Ratkowsky model, (b) temperature sensitivity expressed by the log of the ratio of instantaneous growth at 35/0°C as affected by MAT, (c) temperature sensitivity expressed by Q 10–20 (see Methods) as affected by MAT, calculated from T min values. Bars indicate SE (n = 5). Regressions were calculated with mean values for each site (n = 14)
3.3. Fungal growth
The temperature sensitivity of fungal growth was affected by site MAT in a similar way as bacterial growth (Figure 3). For fungal growth, T min varied with MAT according to the equation T min = −7.8 + 0.25*MAT (R 2 = 0.54, p < 0.01, Figure 3a). T min increased from approximately −6°C in soil from high elevation (MAT ~6°C) to approximately −1°C in soil from low elevation (MAT ~26°C) (R 2 = 0.54, p < 0.01, Figure 3a). This translates to an increase in T min of 0.25 ± 0.069°C per 1 degree of increase in MAT, for this MAT range. The same pattern of fungal growth adapted to MAT was also shown by increases in the temperature sensitivity index (log growth 35/0) and Q 10–20 (R 2 = 0.67, p < 0.001, Figure 3c) with increased MAT (R 2 = 0.93, p < 0.001, Figure 3b). The Q 10–20 for fungal growth increased by 0.049 ± 0.0104 per 1 degree of increase in MAT.
Figure 3.
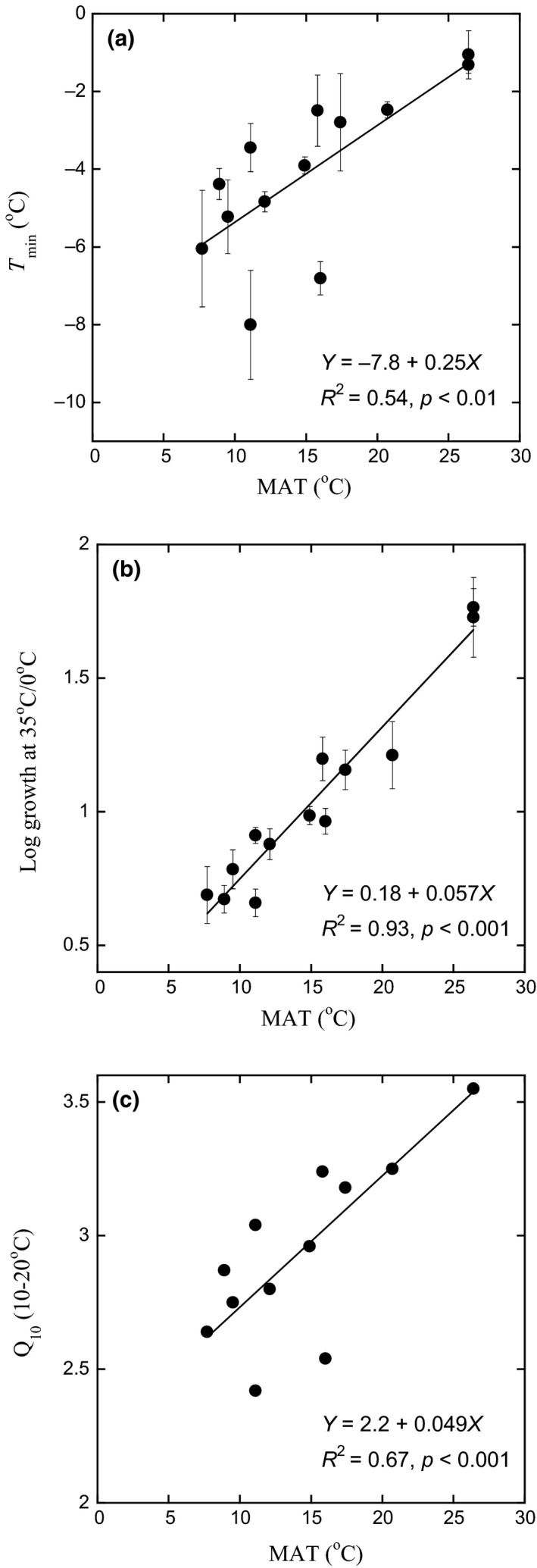
Fungal community growth response to differences in mean annual temperature (MAT) along an elevation gradient in the Andes. The temperature sensitivity was expressed using three different metrics. (a) Temperature sensitivity expressed by T min as affected by MAT, calculated from the Ratkowsky model, (b) temperature sensitivity expressed by the log of the ratio of instantaneous growth at 35/0°C as affected by MAT, (c) temperature sensitivity expressed by Q10–20 (see Methods) as affected by MAT, calculated from T min values. Bars indicate SE (n = 5). Regressions were calculated with mean values for each site (n = 13)
3.4. Fungal/bacterial relationships
There was a significant decrease in the log ratio of bacterial to fungal growth with increased MAT (R 2 = 0.53, p < 0.01, Figure 4a), with the ratios approximately five times lower in soil from low elevation (high MAT) compared to soil from high elevation (low MAT). The T min for fungal and bacterial growth was linearly related with no significant difference from a 1:1 line (R 2 = 0.47, p < 0.05, Figure 4b), indicating that fungal and bacterial community responses were similar over the gradient in MAT studied here (6–26°C). This similarity was further indicated by nonsignificant differences between bacteria and fungi for changes in T min and Q10–20 per 1 degree of increase in MAT.
Figure 4.
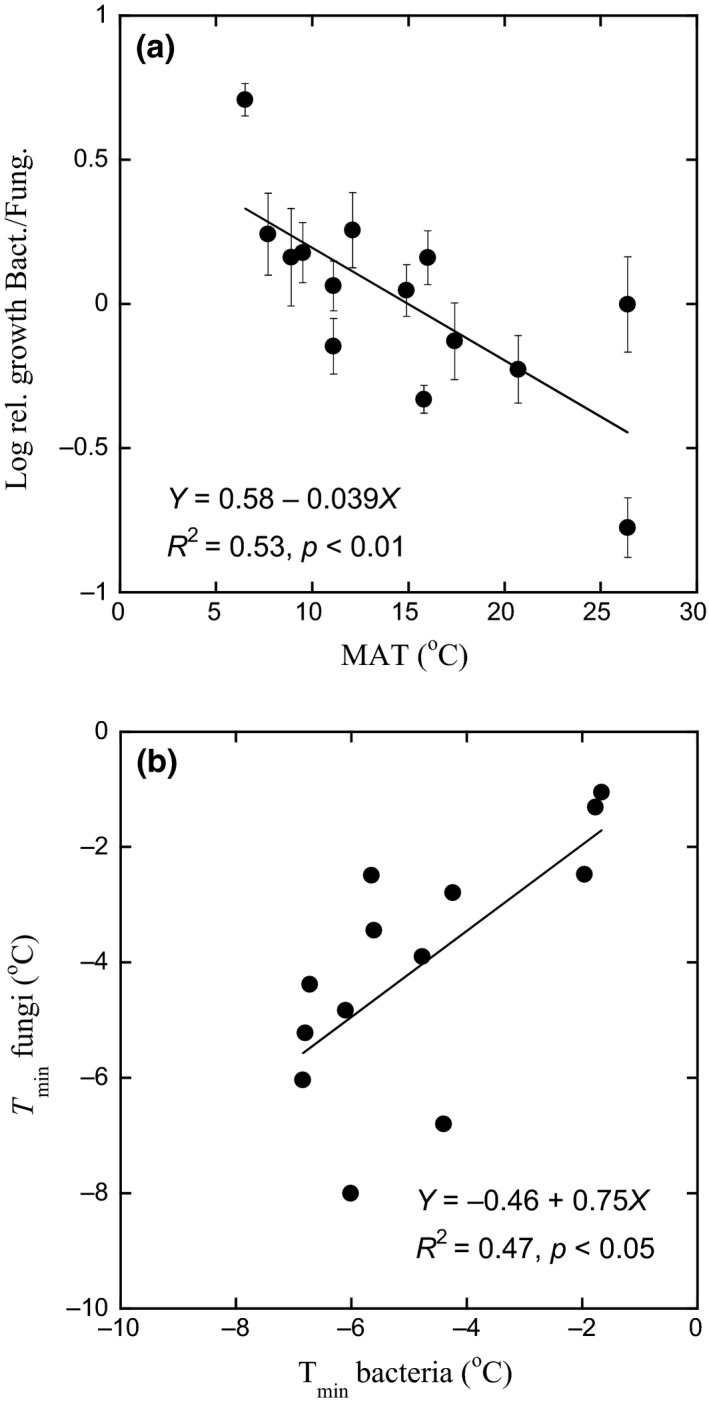
Comparison of the temperature responses of bacterial and fungal growth along an elevation gradient in the Andes, as affected by differences in mean annual temperature (MAT). (a) Relative bacterial to fungal growth. Growth of bacteria was estimated as leucine incorporation and for fungi as acetate‐in‐ergosterol‐incorporation method at 15°C. The data were normalized to a log value of 0 at MAT of 15°C. Bars indicate SE (n = 5). Regressions were calculated with mean values for each site (n = 14). (b) Correlation between T min for bacterial and fungal growth
3.5. Predicting future changes
We compared Q 10 for the three temperature intervals 5–15°C, 10–20°C and 15–25°C, by using the variation in T min for bacterial growth and the square root equation. While Q 10 for 15–25°C only varied between 2.0 and 2.6, Q 10 for 5–15°C varied between 2.8 and 6.3 (Figure 5a) and Q 10 for 10–20°C varied between 2.3 and 3.4 (Figure 2c). Thus, the estimated Q 10 value varied both according to the temperature interval used in the calculation and according to differences in MAT. This was illustrated by comparing Q 10 calculated for a fixed interval (Q 10–20) with Q 10 calculated for MAT±5°C (Q MAT±5). When calculated over fixed interval, Q 10–20 increased linearly with MAT, indicative of increased temperature sensitivity with increased MAT (Figure 5b using the line in Figure 2c). However, Q MAT±5 followed the opposite pattern and decreased with increased MAT. For example, for the four highest elevations (MAT ranging from 6.5 to 9.5°C), Q 10–20 values were approximately 2.5, while Q MAT±5 values were much higher, ranging from 3.5 to 4 (Figure 5b). The opposite pattern was found at lower elevations with higher MAT: In the two lowland forest sites (MAT = 26°C), Q 10–20 values (~3.5) were higher than Q MAT±5 values (~2). A Q 10 value calculated over a fixed interval therefore gave opposing results when compared to a Q 10 relevant for MAT (e.g., Q MAT±5).
Figure 5.
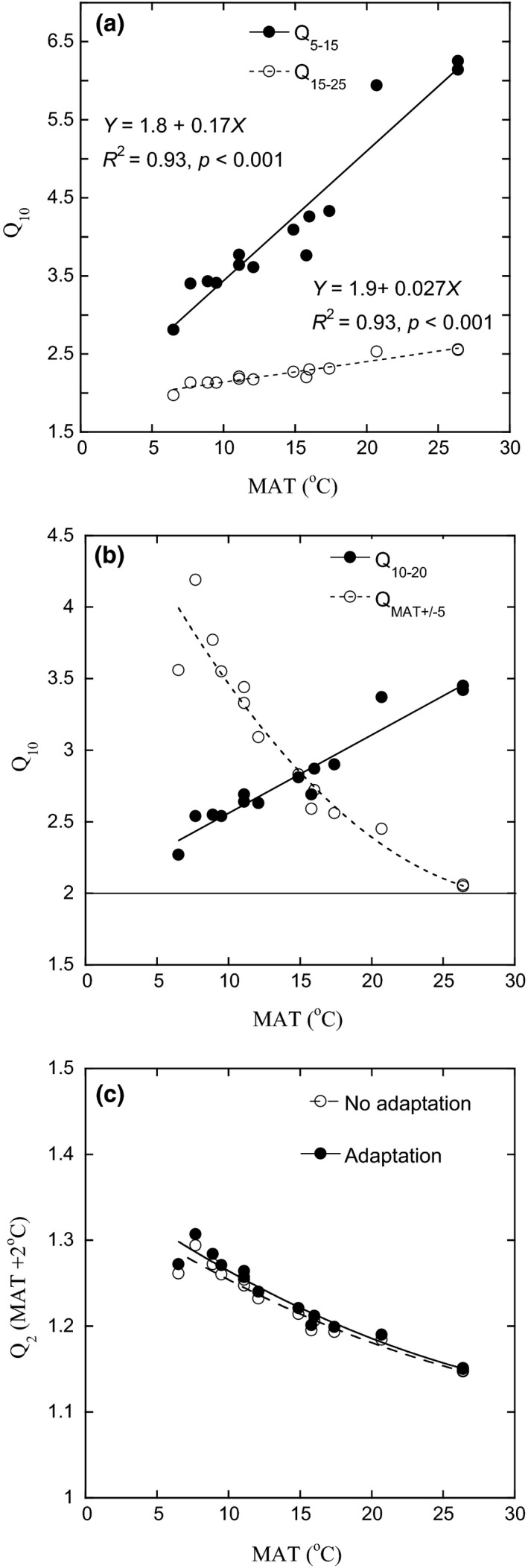
The effect of using different temperature ranges, according to MAT differences along an elevation gradient, to calculate Q 10 values for bacterial growth. (a) Q 10 calculated using T min from Figure 2a in the range 5–15°C and 15–25°C. (b) Standardized Q 10 (10–20°C) calculated using T min from Figure 2a, compared with in situ specific Q 10 (MAT ±5°C; Y = 5.2–0.21X + 0.0033X 2, R 2 = 0.92). The thin line at a value of 2 for Q 10 indicates an approximately upper limit for Q 10 for most enzyme activities (V max) for these sites (Nottingham et al., 2016). (c) Predicted increases in growth with a 2°C increase in MAT calculated using T min from Figure 2a. “No adaptation” was calculated using Equation ([Link]), while “adaptation” was calculated assuming a 0.6°C increase in T min
We used data from Figure 2a to predict the increase in microbial activity with 2°C of warming (Figure 5c). Assuming no adaptation, where T min does not respond to warming, the increase in microbial activity with warming was largest in soils from high elevation and low MAT. For example, microbial activity was predicted to increase by 27% in the four sites with lowest MAT and by 15% in the sites with highest MAT. When we accounted for an adaptation response, whereby T min increased by 0.6°C per 2°C increase in MAT (Figures 2 and 3a), these predicted increases in microbial activity with warming were slightly higher, with the largest increases for soils from high elevation (low MAT). For example, the predicted increase in “temperature‐adapted” soil microbial activity with a 2°C warming was 28% in the four sites with lowest MAT (an increase of 1.2% points compared to no adaptation), while at a MAT of 26°C, the predicted increase was only 15% (an increase of 0.3% points compared to no adaptation; Figure 5c).
4. DISCUSSION
4.1. Adaptation to MAT along the gradient
Our main finding, along the 3.5 km tropical elevation gradient with a 20°C change in MAT, was that the temperature response of microbial growth (T min) is determined by MAT. Our results suggest that an increase in MAT by 1°C will result in an increased T min by approximately 0.3°C (and Q 10–20 by 0.05 units) for bacteria and fungi. This outcome is consistent with our second hypothesis. This also provides further evidence for long‐term temperature adaptation of soil microbial growth (hypothesis 1) and the first information for both bacteria and fungi across such a large MAT range and in the tropical biome.
Our key finding that the long‐term temperature adaptation of microbial growth results in a 0.3°C increase in Tmin per 1 degree of increase in MAT is consistent with studies of the temperature response of bacteria performed in other ecosystems, although over much narrower ranges in MAT. For example, T min of bacterial growth increased by 0.24–0.38°C per 1°C MAT increase along a 13°C MAT gradient in Antarctica (−4 to 9°C) (Rinnan et al., 2009), while 3 years of experimental soil warming (+5°C) in a temperate forest with a MAT of 7°C increased the T min of bacterial growth by 0.19°C per 1°C warming (Rousk et al., 2012). A recent compilation of studies on the temperature adaptation of bacterial growth found the same pattern we show here: on average T min increased by approximately 0.3°C per 1 degree Celsius increase in MAT (Bååth, 2018). Thus, our findings extend previous observations for bacteria and fungi and across a large MAT range of 6–26°C; in particular, our data fill the gap in understanding for the 9–26°C MAT range, on the thermal adaptation of soil microbial growth to differences in MAT.
Although this is the first study in which the temperature adaptation (T min) of soil bacterial growth has been evaluated in tropical ecosystems, our estimates of the absolute value of T min are consistent with findings from other ecosystems with similar MAT. For example, we found a T min of bacterial growth of approximately −8°C at the highest elevation sites with MAT of 6.5°C, which is similar to a T min of bacterial growth of −5 to −8°C for several sites in southern Sweden with MAT of approximately 8°C (Dıaz‐Raviña et al., 1994; Pietikäinen et al., 2005; Rinnan et al., 2009), but lower than a T min for bacterial growth of −11°C for Antarctic soils with MAT of −4°C (Rinnan et al., 2009). In a desert soil with mean seasonal temperature of 27°C, the T min for bacterial growth ranged between −1 and 0°C (van Gestel et al., 2013), consistent with a T min of −1.5°C in our lowland forest sites with MAT of approximately 26°C. These consistencies among studies spanning humid tropical forest, dry desert, temperate and Antarctic ecosystems together suggest a very generally applicable finding: The T min of microbial growth is strongly determined by ambient temperature regimes and is not constrained by differences in other climatic or edaphic factors. This was also suggested in a tentative global envelope of T min for soil microbial activity and growth proposed by Bååth (2018), with cold, polar regions having T min between −10 and −15°C, temperate regions (including the high elevation sites in the present study) between −5 and −10°C and warm, tropical regions (including our low elevation sites) having T min between 0 and −5°C. Our results, covering such a large span of MAT, thus strongly corroborate the global variation in T min hypothesized by Bååth (2018).
4.2. Comparing temperature effects on bacterial and fungal growth
Our results showed that bacterial growth and fungal growth respond similarly to temperature differences, contrary to our third hypothesis that fungi would be better adapted to lower temperatures (have a lower T min). The ranges of T min for fungi (−1 to −6°C) and bacteria (−1.5 to −8°C) were similar, and the relationships between Tmin and MAT difference were not significantly different between the two microbial groups. This finding contrasts with the study by Pietikäinen et al. (2005), where a lower T min for fungi was found in comparison with bacteria in a study of two soil types, suggesting increased dominance of fungi during cold periods. Based on our more comprehensive data from 14 different soils, our results run counter to the hypothesis that fungi have a lower T min compared to bacteria. Our data on the ratio of bacterial/fungal growth (Figure 4a) showed relatively more bacterial than fungal growth at lower MAT in the highland soils. Our results might thus suggest that earlier studies indicating fungal dominance in cold environments may be explained by other environmental factors covarying with temperature (e.g., N availability; Nottingham, Hicks, et al. (2018); Nottingham, Turner, et al. (2015)). A further complicating factor is that the methodology provides proxies for bacterial and fungal growth and there may be small methodological errors when comparing results for bacterial growth (method reflects protein synthesis) with results for fungal growth (method reflects membrane synthesis). As discussed by Pietikäinen et al. (2005), these methodological differences could affect the determined T min of growth, but the effect is likely to be minor. Furthermore, it has been shown that small variations in T min have only minor effects on predicted yearly activities (Rousk et al., 2012).
4.3. Application of T min and the square root equation for respiration and growth
In studies of respiration along the same elevation transect, Q 10–20 of soil respiration varied between 2.1 and 6.9 (Zimmermann, Meir, Bird, Malhi, & Ccahuana, 2009), which is equivalent to a T min variation of −12.3 to +3.9°C, assuming a square root relationship (equation 4 in Bååth, 2018). This is a similar range, albeit slightly larger, to that found for microbial growth. However, the variation in Q 10 for respiration was not related to elevation (Zimmermann et al., 2009), suggesting no temperature adaptation of respiration. However, only four sites were studied in Zimmermann et al. (2009), with large variations in estimates of Q 10 of soil respiration, which likely reflected different temperature responses of soil and root‐derived respiration (Zimmermann et al., 2010). Bååth (2018) argued, using a compilation of a large number of respiration studies (Hamdi, Moyano, Sall, Bernoux, & Chevallier, 2013) and models used to predict respiration (Del Grosso et al., 2005; Jenkinson et al., 1991; Kätterer & Andren, 2001; Kirschbaum, 2000; Lloyd & Taylor, 1994; Svensson et al., 2008), that T min (and Q 10) for microbial growth and respiration should be very similar in soils globally, and that both could be described by the square root equation. Although more precise data on respiration–temperature relationships for the present elevation gradient are needed, we suggest that our data on the temperature sensitivity of soil microbial growth will also be relevant for respiration. Specifically, we hypothesize that our result showing that an increase in MAT by 1°C results in an increased T min by approximately 0.3°C (and Q 10–20 by 0.05 units) over the gradient of MAT between 6 and 26°C may also be applicable for soil heterotrophic respiration.
The application of the square root model (using T min) was suggested as a simple method to quantify the growth or activity response of the microbial community to the temperature regime (Bååth, 2018). This approach is particularly useful because, unlike Q 10, T min values are not dependent on the temperature range used for their calculation. This was clear in the present study. Thus, the use of one Q10 value for the range of temperatures found in the present elevation gradient would result in bias when predicting growth and respiration. In contrast, T min as descriptor of temperature adaptation of the community and the square root model to estimate the temperature response can be used to calculate Q 10 for any temperature interval (Bååth, 2018). Thus, T min can be used to calculate a standardized Q 10 in each of the sites studied, for example, Q 10–20 in Figure 2c, but also to calculate a Q 10 related to the MAT at each specific site (Figure 5b).
4.4. Comparing temperature sensitivity of growth and enzyme activity
Before microorganisms can use soil organic matter for growth and respiration, macromolecules must be degraded by extracellular enzymes. The strong temperature‐adaptive responses of microbial growth we found across this elevation and MAT gradient (Figures 2 and 3c) occurred despite a largely insensitive temperature response of enzyme activities reported in a previous study of the same gradient (Nottingham et al., 2016). This previous study of enzyme temperature sensitivity found no elevation patterns in the Q 10 of the maximum enzymatic catalytic rate (V max) for 5 out of 7 soil enzymes, with only small increases in Q 10 for V max with increased elevation for 2 enzymes, ß‐glucosidase and ß‐xylanase (Nottingham et al., 2016). Studies from other sites are consistent with a general insensitivity of Q 10 of enzymatic V max to temperature. Nine years of experimental soil warming in a temperate forest increased enzymatic V max but did not affect its Q 10 response (Schindlbacher, Schnecker, Takriti, Borken, & Wanek, 2015); while a cross‐latitudinal study found no differences in the Q 10 of V max for five hydrolytic enzymes, although a relationship was observed between MAT and the Q 10 of the half‐saturation constant (K m) of ß‐glucosidase (German, Marcelo, Stone, & Allison, 2012). Thus, the temperature responses of growth do not appear to be principally the result of differences in enzyme function, and there appear to be differences both in the applicable model and in the adaptation responses, for enzyme activity, growth and respiration. Enzymatic activity usually follows a strict Arrhenius relationship with temperature (Davidson, Janssens, & Luo, 2006; German et al., 2012), with very little increase in Q 10 at decreasing temperature. Furthermore, Q 10 values of enzyme activity are often ≤2, irrespective of temperature (Nottingham et al., 2016; Schindlbacher et al., 2015). A comparison of the temperature sensitivities (Q 10) of MAT‐relevant microbial growth and enzyme activities (Figure 5b), indicates that enzyme activities have a lower Q 10 over the whole range of MAT, with the discrepancy increasing at lower temperatures. Overall, these results reinforce the need to understand intrinsic temperature responses of discrete biochemical processes—microbial growth, respiration and enzymatic activity—which together determine the temperature response of the overall C balance (Conant et al., 2011; Davidson et al., 2006).
4.5. Modelling adaptation: using Q 10 calculated over a fixed interval and at MAT
We show that a Q 10 value calculated using MAT (Q MAT±5) provides a robust metric to model temperature responses, but a Q 10 value calculated over a fixed interval (e.g., Q 10–20) gives misleading results when comparing sites with differences in MAT. The accurate estimation of Q MAT±5 was possible for the studied elevation gradient, because there is little annual and seasonal variation in temperature at each site (Rapp & Silman, 2012; Zimmermann et al., 2010). In sites with low MAT, Q MAT±5 was higher than Q 10–20 and vice versa in sites with high MAT (and Q MAT±5 = Q 10–20 where MAT = 15°C). This resulted in a Q 10–20 that increased with MAT (2.3 at 6°C to 3.4 at 26°C), but a Q MAT±5 that decreased with MAT (3.7 at 6°C to 2 at 26°C). Thus, Q 10–20 (or determined across any fixed temperature interval) is useful to compare the relative temperature responses of different processes among studies across the same temperature range, while Q MAT±5 is useful for modelling temperature responses across gradients in MAT. However, Q 10–20 is misleading where temperature ranges differ among studies, and it is difficult to use this information to infer general responses to future climate warming across different biomes.
Thus, using this model based on Q MAT±5, we can conceptualize the microbial growth response to warming as the result of two counteracting effects: the direct temperature effect according to the Q 10 trajectory at a fixed T min and the adaptation effect in changing the T min (and thereby altering the Q 10 trajectory; Figure 6). Using data from Figure 2a for bacterial growth, a soil with MAT of 6°C will have a T min of −8°C and Q 10 will vary with temperature according to the −8°C trajectory: decreasing with increasing temperature. However, we can include our results for long‐term temperature adaptation of microbial growth, 0.3°C increase in T min per degree increase in MAT (Figures 2 and 3). By including temperature adaptation in this model, +6°C warming will increase T min by around 2°C and alter the Q 10 trajectory to one where microbial growth is slightly higher at the new temperature regime (dashed red arrow; Figure 6).
Figure 6.
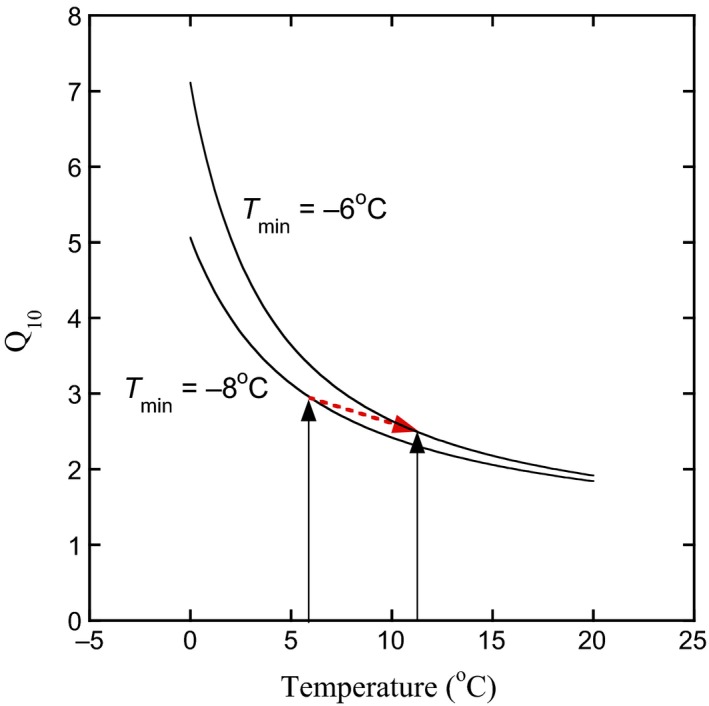
Conceptual figure on the effect of increasing MAT on Q 10, calculated using the square root relationship. A soil with MAT of 6°C, and a T min of −8°C, has Q 10 ~3 (left black arrow). By increasing MAT by 6°C to 12°C (right black arrow), there is a decrease in Q 10 (~2.7) but an increase in T min (by 2°C to −6°C). The new T min trajectory results in a decrease in Q 10 when calculated at the new temperature (Q MAT+2; red dashed arrow) but a higher Q 10 if calculated across a fixed temperature range (e.g., Q 0–10). The trajectories were calculated using the square root relationship for T min of −6 and −8°C (see Bååth, 2018) [Colour figure can be viewed at wileyonlinelibrary.com]
4.6. Predicting effects of future climate change scenarios
The use of T min and the square root equations will enable simple estimation of the temperature sensitivity across the MAT range relevant for future predicted climate change scenarios. Cramer et al. (2004) predicted a warming in the tropics of 4° by 2100; this could be modelled by calculating Q 4 for MAT+4°C. A similar calculation was made for heterotrophic respiration at site‐specific MATs by Zimmermann et al. (2009) for four of the sites (at 3,030, 1,500, 1,000 and 200 m elevation equivalent to 11°C to 26°C MAT), with Q 4 relevant to climate change predictions estimated to be 1.66, 1.29, 1.27 and 1.0 (i.e., with an increasing MAT of 4°C). Calculating similar Q 4 values for bacterial growth and MAT +4°C resulted in very similar predictions, 1.55, 1.40, 1.38 and 1.31. These similarities are thus further indications that our estimates of temperature sensitivity of microbial growth are also relevant for heterotrophic respiration.
The predicted global warming by 2100 ranges from 1.4 to 5.8°C, based on a range of emission scenarios (IPCC, 2013). Thus, considering a conservative 2°C increase in global MAT, the relative impact on microbial activity (growth and respiration) will be stronger in ecosystems with lower MAT (28%) than with higher MAT (15%), suggesting that with the same predicted increase in MAT, the relative effect will be stronger at lower temperatures. The relatively greater impact at lower MAT may be further exacerbated because greater warming is predicted in higher‐latitude ecosystems (IPCC, 2013), although significant impacts in tropical regions could occur if MAT exceeds thermal optima for activity (T opt).
Because the elevation gradient under study here is largely aseasonal in temperature (Rapp & Silman, 2012), we could use MAT and one single Q MAT±5 to characterize the temperature adaptation of microbial growth. This will not be the case in ecosystems with large seasonal temperature variation, including deserts (van Gestel et al., 2013) or temperate and continental climates, where Q 10 will vary seasonally (Bååth, 2018). However, by determining T min and using the square root equation, it is straightforward to model the effect of seasonal temperature variation, as shown by Rousk et al. (2012). Similar to our study, their data also suggested that the effect of temperature adaptation was minor compared to the effect of seasonal temperature variation, in determining the Q 10 of microbial activity (cf Figure 5c,6).
Our results demonstrate consistent patterns of temperature adaptation (T min) in growth across a large temperature range. Our results also show how T min can be used as a single descriptor of temperature adaption of the microbial community, which together with the square root equation (Ratkowsky et al., 1982) can be used to predict temperature effects on microbial growth. However, in order to fully understand climate warming impacts on microbial communities and the carbon balance, further studies are required on the responses of microbial carbon use efficiency (carbon uptake, growth and respiration) (Bradford, 2013) and on the intrinsic temperature responses of, and the interactions between, different physical, biological and chemical components of the soil carbon cycle (Conant et al., 2011; Nottingham, Turner, et al., 2015). A major outstanding question is also whether these microbial growth responses to long‐term temperature differences observed along a tropical mountain elevation gradient, will shift either through acclimation or adaptation in response to short‐term climatic change.
Supporting information
ACKNOWLEDGEMENTS
This study was led with support from the UK Natural Environment Research Council (NERC), NE/G018278/1 and NE/N006852/1 to PM and also by an Australian Research Council (ARC) grant DP170104091 to PM and a European Union Marie‐Curie Fellowship FP7‐2012‐329360 to ATN. We thank the Asociación para la Conservación de la Cuenca Amazónica (ACCA) in Cusco and the Instituto Nacional de Recursos Naturales (INRENA) in Lima for access to the study sites. For their logistical support, we thank Dr. Eric Cosio and Eliana Esparza Ballón at Pontificia Universidad Católica del Perú (PUCP). For field support, we thank Walter H. Huasco and Adan J. Q. Ccahuana. This study an output of the Andes Biodiversity and Ecosystem Research Group consortium (www.andesconservation.org) and part of LUCCI (Lund University Centre for studies of Carbon Cycle and Climate Interactions).
Nottingham AT, Bååth E, Reischke S, Salinas N, Meir P. Adaptation of soil microbial growth to temperature: Using a tropical elevation gradient to predict future changes. Glob Change Biol. 2019;25:827–838. 10.1111/gcb.14502
REFERENCES
- Bååth, E. (2001). Estimation of fungal growth rates in soil using C‐14‐acetate incorporation into ergosterol. Soil Biology & Biochemistry, 33, 2011–2018. 10.1016/S0038-0717(01)00137-7 [DOI] [Google Scholar]
- Bååth, E. (2018). Temperature sensitivity of soil microbial activity modeled by the square root equation as a unifying model to differentiate between direct temperature effects and microbial community adaptation. Global Change Biology, 24, 2850–2861. 10.1111/gcb.14285 [DOI] [PubMed] [Google Scholar]
- Bååth, E. , Pettersson, M. , & Söderberg, K. H. (2001). Adaptation of a rapid and economical microcentrifugation method to measure thymidine and leucine incorporation by soil bacteria. Soil Biology & Biochemistry, 33, 1571–1574. 10.1016/S0038-0717(01)00073-6 [DOI] [Google Scholar]
- Bárcenas‐Moreno, G. , Gomez‐Brandon, M. , Rousk, J. , & Bååth, E. (2009). Adaptation of soil microbial communities to temperature: Comparison of fungi and bacteria in a laboratory experiment. Global Change Biology, 15, 2950–2957. 10.1111/j.1365-2486.2009.01882.x [DOI] [Google Scholar]
- Beer, C. , Reichstein, M. , Tomerlleri, E. , Ciais, P. , Jung, M. , Carvalhais, N. , … Bondeau, A. (2010). Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science, 329, 834–838. 10.1126/science.1184984 [DOI] [PubMed] [Google Scholar]
- Bell, R. T. , & Ahlgren, I. (1987). Thymidine incorporation and microbial respiration in the surface sediment of a hypereutrophic lake. Limnology and Oceanography, 32, 476–482. [Google Scholar]
- Birgander, J. , Olsson, P. A. , & Rousk, J. (2018). The responses of microbial temperature relationships to seasonal change and winter warming in a temperate grassland. Global Change Biology, 24, 3357–3367. 10.1111/gcb.14060 [DOI] [PubMed] [Google Scholar]
- Birgander, J. , Reischke, S. , Jones, D. L. , & Rousk, J. (2013). Temperature adaptation of bacterial growth and 14C‐glucose mineralisation in a laboratory study. Soil Biology & Biochemistry, 65, 294–303. 10.1016/j.soilbio.2013.06.006 [DOI] [Google Scholar]
- Bradford, M. A. (2013). Thermal adaptation of decomposer communities in warming soils. Frontiers in Microbiology, 4, 333 10.3389/fmicb.2013.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M. A. , Davies, C. A. , Frey, S. D. , Maddox, T. R. , Melillo, J. M. , Mohan, J. E. , … Wallenstein, M. D. (2008). Thermal adaptation of soil microbial respiration to elevated temperature. Ecology Letters, 11, 1316–1327. 10.1111/j.1461-0248.2008.01251.x [DOI] [PubMed] [Google Scholar]
- Conant, R. T. , Ryan, M. G. , Ågren, G. I. , Birge, H. E. , Davidson, E. A. , Eliasson, P. E. , … Hyvönen, R. (2011). Temperature and soil organic matter decomposition rates ‐ synthesis of current knowledge and a way forward. Global Change Biology, 17, 3392–3404. 10.1111/j.1365-2486.2011.02496.x [DOI] [Google Scholar]
- Cramer, W. , Bondeau, A. , Schaphoff, S. , Lucht, W. , Smith, B. , & Sitch, S. (2004). Tropical forests and the global carbon cycle: impacts of atmospheric carbon dioxide, climate change and rate of deforestation. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 359, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, E. A. , & Janssens, I. A. (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature, 440, 165–173. 10.1038/nature04514 [DOI] [PubMed] [Google Scholar]
- Davidson, E. A. , Janssens, I. A. , & Luo, Y. (2006). On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Global Change Biology, 12, 154–164. 10.1111/j.1365-2486.2005.01065.x [DOI] [Google Scholar]
- Del Grosso, S. J. , Parton, W. J. , Mosier, A. R. , Holland, E. A. , Pendall, E. , Schimel, D. S. , & Ojima, D. S. (2005). Modeling soil CO2 emissions from ecosystems. Biogeochemistry, 73, 71–91. 10.1007/s10533-004-0898-z [DOI] [Google Scholar]
- Dıaz‐Raviña, M. , Frostegård, A. , & Bååth, E. (1994). Thymidine, leucine and acetate incorporation into soil bacterial assemblages at different temperatures. FEMS Microbiology Ecology, 14, 221–231. 10.1016/0168-6496(94)90004-3 [DOI] [Google Scholar]
- Fyllas, N. M. , Bentley, L. P. , Shenkin, A. , Asner, G. P. , Atkin, O. K. , Díaz, S. , … Huasco, W. H. (2017). Solar radiation and functional traits explain the decline of forest primary productivity along a tropical elevation gradient. Ecology Letters, 20, 730–740. 10.1111/ele.12771 [DOI] [PubMed] [Google Scholar]
- German, D. P. , Marcelo, K. R. B. , Stone, M. M. , & Allison, S. D. (2012). The Michaelis‐Menten kinetics of soil extracellular enzymes in response to temperature: A cross‐latitudinal study. Global Change Biology, 18, 1468–1479. 10.1111/j.1365-2486.2011.02615.x [DOI] [Google Scholar]
- Giardina, C. P. , Litton, C. M. , Crow, S. E. , & Asner, G. P. (2014). Warming‐related increases in soil CO2 effux are explained by increased below‐ground carbon flux. Nature Climate Change, 4, 822–827. [Google Scholar]
- Girardin, C. A. , Farfan‐Rios, W. , Garcia, K. , Feeley, K. J. , Jørgensen, P. M. , Murakami, A. A. , … Maldonado, C. (2014). Spatial patterns of above‐ground structure, biomass and composition in a network of six Andean elevation transects. Plant Ecology & Diversity, 7, 161–171. 10.1080/17550874.2013.820806 [DOI] [Google Scholar]
- Hamdi, S. , Moyano, F. , Sall, S. , Bernoux, M. , & Chevallier, T. (2013). Synthesis analysis of the temperature sensitivity of soil respiration from laboratory studies in relation to incubation methods and soil conditions. Soil Biology & Biochemistry, 58, 115–126. 10.1016/j.soilbio.2012.11.012 [DOI] [Google Scholar]
- IPCC (2013). Climate change 2013: The physical science basis. Cambridge: Cambridge University Press. [Google Scholar]
- Jenkinson, D. S. , Adams, D. E. , & Wild, A. (1991). Model estimates of CO2 emissions from soil in response to global warming. Nature, 351, 304–306. 10.1038/351304a0 [DOI] [Google Scholar]
- Karhu, K. , Auffret, M. D. , Dungait, J. A. , Hopkins, D. W. , Prosser, J. I. , Singh, B. K. , … Hartley, I. P. (2014). Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature, 513, 81–84. 10.1038/nature13604 [DOI] [PubMed] [Google Scholar]
- Kätterer, T. , & Andren, O. (2001). The ICBM family of analytically solved models of soil carbon, nitrogen and microbial biomass dynamics descriptions and application examples. Ecological Modelling, 136, 191–207. 10.1016/S0304-3800(00)00420-8 [DOI] [Google Scholar]
- Kätterer, T. , Reichstein, M. , Andren, O. , & Lomander, A. (1998). Temperature dependence of organic matter decomposition: A critical review using literature data analyzed with different models. Biology and Fertility of Soils, 27, 258–262. 10.1007/s003740050430 [DOI] [Google Scholar]
- Kirschbaum, M. U. F. (2000). Will changes in soil organic carbon act as a positive or negative feedback on global warming? Biogeochemistry, 48, 21–51. [Google Scholar]
- Kirschbaum, M. U. F. (2006). The temperature dependence of organic‐matter decomposition—still a topic of debate. Soil Biology and Biochemistry, 38, 2510–2518. 10.1016/j.soilbio.2006.01.030 [DOI] [Google Scholar]
- Li, W. K. W. , & Dickie, P. M. (1987). Temperature characteristics of photosynthetic and heterotrophic activities ‐ seasonal variations in temperate microbial plankton. Applied and Environmental Microbiology, 53, 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnaeus, C. (1781). On the increase of the habitable earth. Amoenit. Acad., 2, 17–27. [Google Scholar]
- Lloyd, J. , & Taylor, J. A. (1994). On the temperature‐dependence of soil respiration. Functional Ecology, 8, 315–323. 10.2307/2389824 [DOI] [Google Scholar]
- Newell, S. Y. , & Fallon, R. D. (1991). Toward a method for measuring instantaneous fungal growth‐rates in field samples. Ecology, 72, 1547–1559. 10.2307/1940954 [DOI] [Google Scholar]
- Nottingham, A. T. , Fierer, N. , Turner, B. L. , Whitaker, J. , Ostle, N. J. , McNamara, N. P. , … Meir, P. (2018). Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology, 99, 2455–2466. 10.1002/ecy.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham, A. T. , Hicks, L. C. , Ccahuana, A. J. Q. , Salinas, N. , Bååth, E. , & Meir, P. (2018). Nutrient limitations to bacterial and fungal growth during cellulose decomposition in tropical forest soils. Biology and Fertility of Soils, 54, 219–222. 10.1007/s00374-017-1247-4 [DOI] [Google Scholar]
- Nottingham, A. T. , Turner, B. L. , Whitaker, J. , Ostle, N. , Bardgett, R. D. , McNamara, N. P. , … Meir, P. (2016). Temperature sensitivity of soil enzymes along an elevation gradient in the Peruvian Andes. Biogeochemistry, 127, 217–230. 10.1007/s10533-015-0176-2 [DOI] [Google Scholar]
- Nottingham, A. T. , Turner, B. L. , Whitaker, J. , Ostle, N. J. , McNamara, N. P. , Bardgett, R. D. , … Meir, P. (2015). Soil microbial nutrient constraints along a tropical forest elevation gradient: A belowground test of a biogeochemical paradigm. Biogeosciences, 12, 6071–6083. 10.5194/bg-12-6071-2015 [DOI] [Google Scholar]
- Nottingham, A. T. , Whitaker, J. , Turner, B. L. , Salinas, N. , Zimmermann, M. , Malhi, Y. , & Meir, P. (2015). Climate warming and soil carbon in tropical forests: Insights from an elevation gradient in the Peruvian Andes. BioScience, 65, 906–921. 10.1093/biosci/biv109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y. , Birdsey, R. A. , Fang, J. , Houghton, R. , Kauppi, P. E. , Kurz, W. A. , … Hayes, D. (2011). A large and persistent carbon sink in the world's forests. Science, 333, 988–993. 10.1126/science.1201609 [DOI] [PubMed] [Google Scholar]
- Pietikäinen, J. , Pettersson, M. , & Bååth, E. (2005). Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. Fems Microbiology Ecology, 52, 49–58. 10.1016/j.femsec.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Rapp, J. M. , & Silman, M. R. (2012). Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Climate Research, 55, 17–32. 10.3354/cr01127 [DOI] [Google Scholar]
- Rapp, J. M. , Silman, M. R. , Clark, J. S. , Girardin, C. J. , Galiano, D. , & Tito, R. (2012). Intra‐ and interspecific tree growth across a long altitudinal gradient in the Peruvian Andes. Ecology, 93, 2061–2072. 10.1890/11-1725.1 [DOI] [PubMed] [Google Scholar]
- Ratkowsky, D. A. , Olley, J. , Mcmeekin, T. A. , & Ball, A. (1982). Relationship between temperature and growth‐rate of bacterial cultures. Journal of Bacteriology, 149, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. A. , & Geider, R. J. (1988). Temperature and algal growth. New Phytologist, 110, 441–461. 10.1111/j.1469-8137.1988.tb00282.x [DOI] [Google Scholar]
- Rinnan, R. , Rousk, J. , Yergeau, E. , Kowalchuk, G. A. , & Bååth, E. (2009). Temperature adaptation of soil bacterial communities along an Antarctic climate gradient: Predicting responses to climate warming. Global Change Biology, 15, 2615–2625. 10.1111/j.1365-2486.2009.01959.x [DOI] [Google Scholar]
- Rousk, J. , & Bååth, E. (2011). Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiology Ecology, 78, 17–30. 10.1111/j.1574-6941.2011.01106.x [DOI] [PubMed] [Google Scholar]
- Rousk, J. , Frey, S. D. , & Bååth, E. (2012). Temperature adaptation of bacterial communities in experimentally warmed forest soils. Global Change Biology, 18, 3252–3258. 10.1111/j.1365-2486.2012.02764.x [DOI] [PubMed] [Google Scholar]
- Schindlbacher, A. , Schnecker, J. , Takriti, M. , Borken, W. , & Wanek, W. (2015). Microbial physiology and soil CO2 efflux after 9 years of soil warming in a temperate forest ‐ no indications for thermal adaptations. Global Change Biology, 21, 4265–4277. 10.1111/gcb.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna‐Chavez, H. M. , Fierer, N. , & Van Bodegom, P. M. (2013). Global drivers and patterns of microbial abundance in soil. Global Ecology and Biogeography, 22, 1162–1172. 10.1111/geb.12070 [DOI] [Google Scholar]
- Sundqvist, M. K. , Sanders, N. J. , & Wardle, D. A. (2013). Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annual Review of Ecology, Evolution, and Systematics, 44, 261–280. 10.1146/annurev-ecolsys-110512-135750 [DOI] [Google Scholar]
- Svensson, M. , Jansson, P. E. , Gustafsson, D. , Kleja, D. B. , Langvall, O. , & Lindroth, A. (2008). Bayesian calibration of a model describing carbon, water and heat fluxes for a Swedish boreal forest stand. Ecological Modelling, 213, 331–344. 10.1016/j.ecolmodel.2008.01.001 [DOI] [Google Scholar]
- Van De Weg, M. J. , Meir, P. , Grace, J. , & Atkin, O. (2009). Altitudinal variation in LMA, leaf tissue density and foliar nitrogen and phosphorus along an Andes‐Amazon gradient in Peru. Plant Ecology and Diversity, 2, 243–254. 10.1080/17550870903518045 [DOI] [Google Scholar]
- Van De Weg, M. J. , Meir, P. , Williams, M. , Girardin, C. , Malhi, Y. , Silva‐Espejo, J. , & Grace, J. (2014). Gross primary productivity of a high elevation tropical montane cloud forest. Ecosystems, 17, 751–764. 10.1007/s10021-014-9758-4 [DOI] [Google Scholar]
- Van Gestel, N. C. , Reischke, S. , & Bååth, E. (2013). Temperature sensitivity of bacterial growth in a hot desert soil with large temperature fluctuations. Soil Biology & Biochemistry, 65, 180–185. 10.1016/j.soilbio.2013.05.016 [DOI] [Google Scholar]
- Von Humboldt, A. , & Bonpland, A. (1805). Essai sur la géographie des plantes, Chez Levrault, Schoell et Compagnie, Libraries, Paris.
- Whitaker, J. , Ostle, N. , Nottingham, A. T. , Ccahuana, A. , Salinas, N. , Bardgett, R. D. , … Austin, A. (2014). Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes‐to‐Amazon elevation gradient. Journal of Ecology, 102, 1058–1071. 10.1111/1365-2745.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieder, W. R. , Bonan, G. B. , & Allison, S. D. (2013). Global soil carbon projections are improved by modelling microbial processes. Nature Climate Change, 3, 909–912. 10.1038/nclimate1951 [DOI] [Google Scholar]
- Zimmermann, M. , Meir, P. , Bird, M. I. , Malhi, Y. , & Ccahuana, A. J. Q. (2009). Climate dependence of heterotrophic soil respiration from a soil‐translocation experiment along a 3000 m tropical forest altitudinal gradient. European Journal of Soil Science, 60, 895–906. 10.1111/j.1365-2389.2009.01175.x [DOI] [Google Scholar]
- Zimmermann, M. , Meir, P. , Bird, M. I. , Malhi, Y. , & Ccahuana, A. J. Q. (2010). Temporal variation and climate dependence of soil respiration and its components along a 3000 m altitudinal tropical forest gradient. Global Biogeochemical Cycles, 24, GB4012 10.1029/2010GB003787 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


