To the Editor
Allergen-specific immunotherapy (AIT) is a clinically and cost-effective allergy treatment that modifies the course of the disease and has long-lasting effects.1,E7,E8 However, allergen extract–based forms of AIT require administration of multiple doses, which makes treatment cumbersome and leads to poor compliance in patients.2 A number of approaches were proposed to address this issue, including use of AIT materials with higher safety, such as allergoids, recombinant allergen derivatives, and allergen-derived peptides, which allow shortening of the buildup phase.E9
In this study we have investigated the ability of the recombinant B cell epitope–based allergy vaccine BM32 to induce allergen-specific IgG antibodies and the ability of these antibodies to inhibit allergic patients’ IgE binding to grass pollen allergens, as well as allergen-induced T-cell proliferation. BM32 is a recombinant grass pollen (Phleum pratense) allergy vaccine based on 4 fusion proteins consisting of peptides from the 4 major timothy grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Phl p 6) fused to the PreS carrier protein from hepatitis B, which lacks relevant allergenic activityE10 and therefore can be injected into allergic patients without need for updosing.3,4 For our immunogenicity studies, we have used a dose (ie, 20 μg of each of the 4BM fusion proteins) that has been safely administered to allergic patients (ClinicalTrials.gov identifiers NCT01445002, NCT01538979, and NCT02643641).4
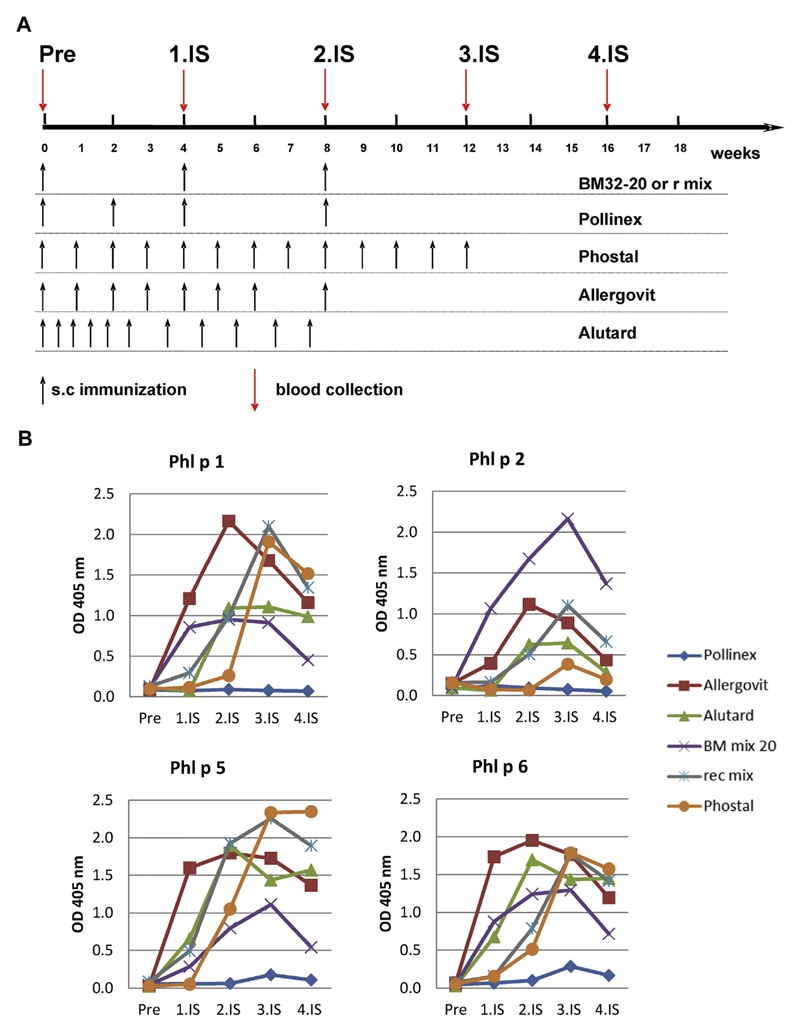
The main finding of our current study was that 3 monthly subcutaneous injections of aluminum hydroxide–adsorbed BM32 induced IgG antibody levels to the major grass pollen allergens Phl p 1, Phl p 5, and Phl p 6 in rabbits, which were comparable with natural allergen extract–based registered grass pollen allergy vaccines requiring more than 8 injections (Allergovit grass; Allergopharma, Reinbek, Germany; Alutard SQ grass mix; ALK-Abelló, Hørsholm, Denmark; and Phostal grasses + rye; Stallergenes, Antony, France; see the Methods section in this article’s Online Repository at www.jacionline.org; Fig 1), whereas almost no response was observed with Pollinex (Pollinex Quattro Plus grasses + rye; Bencard Allergie GmbH, Munich, Germany), a vaccine based on 4 injections. Importantly, BM32 induced higher levels of Phl p 2–specific IgG antibodies than any of the registered allergen extract–based vaccines (Fig 1). We consider this an important finding because it has been shown that group 2 allergens are major grass pollen allergens recognized by more than 60% of patients with grass pollen allergy and, when compared with the other grass pollen allergens by using skin testing, were found to show high allergenic potency.5 Thus our results indicate that it should be possible to build up sufficient levels of grass pollen allergen–specific IgG responses with only few injections (ie, 3-5) of BM32, whereas traditional allergy vaccines require more than double the number of updosing injections. Sublingual treatment even requires daily administration. Therefore we think that the treatment schedules based on BM32 will be more convenient for patients and should increase their compliance.
Fig 1.
Induction of grass pollen allergen–specific IgG responses in rabbits by means of immunization with BM32, recombinant grass pollen allergens, and natural grass pollen AIT extracts. A, Scheme of rabbit immunizations. Rabbits were immunized with 3 monthly injections of BM32 or a mix of recombinant grass pollen allergens. In parallel, rabbits were immunized with registered grass pollen extract–based AIT vaccines (Allergovit, Alutard, Phostal, and Pollinex), as recommended by the manufacturer. Serum samples were collected from all rabbits shortly before the first immunization (Pre) and in monthly intervals (1.IS, 2.IS, 3.IS, and 4.IS). B, Comparisons and time courses of allergen-specific IgG responses of rabbits. Shown are mean OD values corresponding to allergen-specific IgG levels (y-axes) at different time points (x-axes). s.c, Subcutaneous.
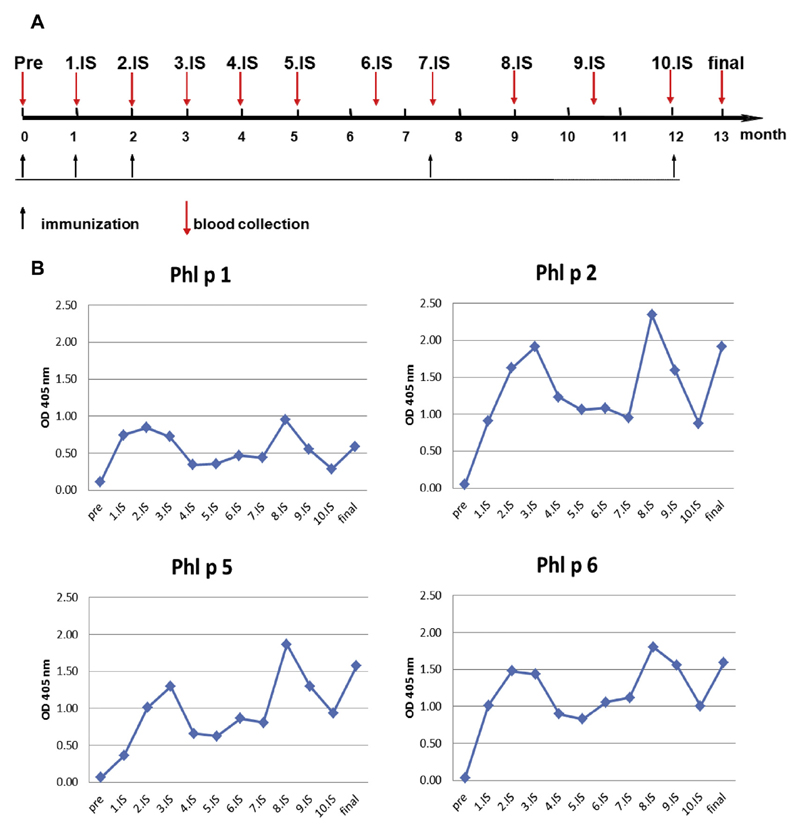
The kinetics of allergen-specific IgG antibodies induced with BM32 were similar to those induced by natural allergen extract–based vaccines in terms of increases and decreases (Fig 1). According to the hapten carrier principle described by the Nobel laureate B. Benacerraf,E11 a robust antibody response can be obtained against a haptenic structure lacking T-cell epitopes if this structure is covalently bound to an unrelated carrier molecule, such as the PreS that contains T-cell epitopes. Carrier-specific T cells support antibody production against the carrier and the hapten. Therefore the question was whether an allergen-specific IgG immune response initiated with BM32 can be boosted by repeated immunization with BM32. To address this question, we kept rabbits that had received a first course of 3 monthly injections with BM32 for 5.5 months without immunization and then applied 1 booster injection; after an additional 4.5 months, we administered another booster injection (see Fig E1 in this article’s Online Repository at www.jacionline.org). BM32-induced grass pollen allergen–specific IgG production could be readily boosted after it had decreased, indicating that it will be possible to boost allergen-specific IgG levels in several consecutive pollen seasons (see Fig E1). We also found that rabbits immunized with BM32 produced PreS-specific antibodies (data not shown), and PreS-specific antibodies were also induced in patients who were treated with BM32.4,6 Interestingly, PreS-specific antibodies induced through vaccination with BM32 also protected liver cells from hepatitis B infections.6
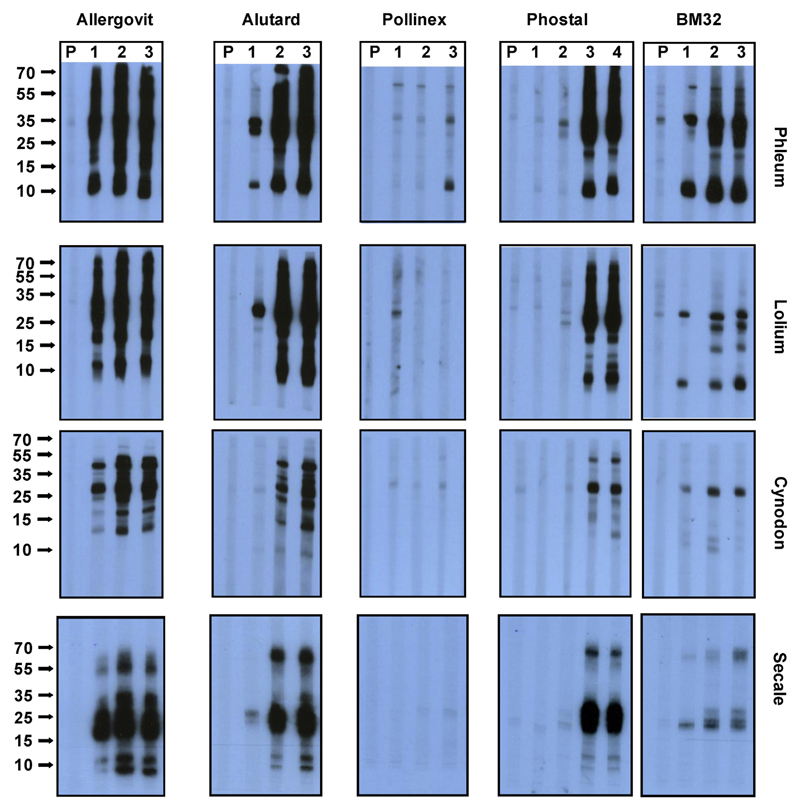
Another important observation was that BM32-induced IgG antibodies not only reacted with the 4 recombinant timothy grass pollen allergens but also with natural timothy grass pollen allergens, and we observed cross-reactivity with the major allergens in other grass species belonging to the Pooideae and Chloridoideae using immunoblotting (see the Methods section and Fig E2 in this article’s Online Repository at www.jacionline.org). This finding suggests that the BM32-induced antibodies might also recognize allergens from other grass species.
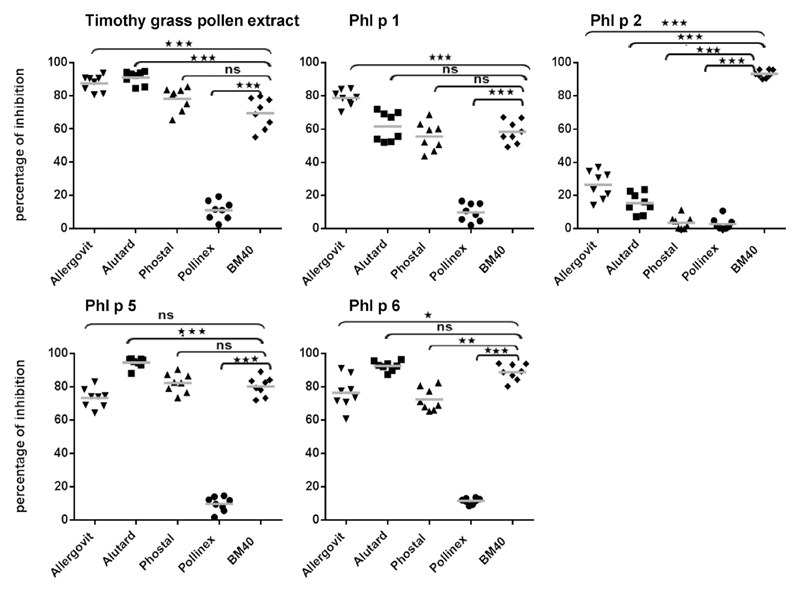
It is known that induction of allergen-specific IgG antibodies that block IgE binding to allergens in allergic patients is important for the clinical efficacy of AIT because such blocking IgG inhibits allergen-induced mast cell and basophil degranulation and IgE-facilitated allergen presentation and the consecutive T-cell activation.7 Therefore we compared BM32-induced IgG with IgG antibody levels induced with allergen extract–based vaccines regarding their ability to inhibit IgE binding to the major timothy grass pollen allergens and natural timothy grass pollen allergen extract in allergic patients using an IgE inhibition ELISA (see the Methods section and Table E1 in this article’s Online Repository at www.jacionline.org and Fig 2). These experiments showed that BM32-induced IgG inhibited IgE binding to Phl p 1, Phl p 5, and Phl p 6, as well as against timothy grass pollen extract, in allergic patients, although antibodies induced by Allergovit and Alutard inhibited IgE binding to timothy grass pollen extract significantly better than those induced by BM32 (P = .0008, Fig 2). Anti-Pollinex antibodies produced less than 10% inhibition of IgE binding to the allergens and timothy grass pollen extract and thus inhibited IgE binding to timothy grass pollen extract and the individual grass pollen allergens significantly less (P = .0008) than those induced by all the other vaccines. The induction of grass pollen allergen–specific IgG by 4 injections of Pollinex was also modest in allergic patients and only significant after the second treatment year.8,9
Fig 2.
Inhibition of IgE binding in allergic patients to grass pollen allergens with rabbit antibodies induced by vaccination. Shown are the percentages of inhibition of IgE binding (y-axes) to timothy grass pollen extract, Phl p 1, Phl p 2, Phl p 5, and Phl p 6 by anti-Allergovit, anti-Alutard, anti-Phostal, anti-Pollinex, or anti-BM32 rabbit antibodies (x-axes). Significant differences between BM- and extract-induced antibodies regarding inhibition of IgE binding are indicated. *P < .05, **P < .01, and ***P < .001. ns, Nonsignificant.
In the IgE inhibition experiments BM32-induced IgG blocked IgE binding to Phl p 2 significantly better than IgG antibodies induced by the commercial vaccines (P = .0008, Fig 2). Therefore one might assume that BM32 could be superior to the extract-based vaccines regarding protection of group 2 allergen–induced symptoms. Finally, we also could demonstrate that BM32-induced antibodies inhibited specifically grass pollen allergen–induced T-cell proliferation (see the Methods section and Fig E3 in this article’s Online Repository at www.jacionline.org), which suggests that BM32 might also have beneficial effects on T cell–mediated late-phase symptoms of grass pollen allergy. However, a limitation of our study is that we performed the immunogenicity study in naive animals, and therefore there might be differences regarding already sensitized patients.
In summary, our immunogenicity studies indicate that induction of grass pollen allergen–specific protective antibody responses requires considerably fewer injections of BM32 compared with natural allergen extract–based vaccines and that the use of carrier proteins in the vaccine helps to overcome the poor immunogenicity of group 2 allergens. Given the strong reduction of allergenic activity of BM32, one might expect that BM32 represents a grass pollen allergy vaccine that could be superior to allergen extract–based vaccines.
Methods
Recombinant allergens, allergen extracts, and products for subcutaneous AIT of grass pollen allergy
The purified recombinant grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6 were obtained from Biomay AG (Vienna, Austria). Grass pollen from Phleum pratense, Lolium perenne, Cynodon dactylon, and Secale cereale were purchased from Allergon (Välinge, Sweden), and aqueous pollen extracts were prepared.E1
Products for subcutaneous AIT of grass pollen allergy were purchased from 4 companies. The registered vaccines were obtained from the pharmacy of the Vienna General Hospital. The following products were used: Allergovit 006 (grasses, 100%; article no. 12142; expiration date, October 2011; reference no. 100904579; Charge batch: J0653373; Allergopharma, Reinbek, Germany); Alutard SQ L299 grass mix (batch: 008342708001; expiration date, November 2011; ALK-Abelló, Hørsholm, Denmark); Phostal 671 (rye 50% + 688 5 grasses 50%; batch: AT0071486/1490588; expiration date, July 2011; Stallergenes, Antony, France); and Pollinex Quattro Plus (1 mL [4130 grasses + rye], batch: 127758; reference no. 2010/050/003325; Bencard Allergie GmbH, Munich, Germany).
The products were stored at +4°C, as recommended by the manufacturers, and used during 2010, long before the end of the expiration dates. Treatment was performed exactly according to the recommendations of the manufacturers, as shown also in Fig 1, A.
Alutard SQ contains a mix of 6 natural grass pollen extracts (Phleum pratense, Poa pratensis, Festuca pratensis, Lolium perenne, Dactylis glomerata, and Avena elatior) adsorbed to aluminum hydroxide. Phostal is a vaccine composed of a 6-grass extract mix (Phleum pratense, Poa pratensis, Lolium perenne, Dactylis glomerata, Anthoxanthum odoratum, and Secale cereale) absorbed onto calcium phosphate. Allergovit contains a grass pollen allergoid composed of 6 different grass pollen species (Phleum pratense, Poa pratensis, Festuca pratensis, Lolium perenne, Dactylis glomerata, and Holcus lanatus) adsorbed to aluminum hydroxide. Pollinex is based on an allergoid made from extracts of 12 grass pollens and rye cereal (Agrostis tenuis, Dactylis glomerata, Cynosurus cristatus, Arrhenatherum elatius, Poa pratensis, Festuca pratensis, Alopecurus pratensis, Lolium perenne, Bromus species, Anthoxanthum odoratum, Phleum pratense, Holcus lanatus, and Secale cereale), which is adsorbed onto L-tyrosine with addition of the immunostimulatory adjuvant monophosphoryl lipid A.E2
Formulation of aluminum hydroxide–adsorbed vaccines containing BM32 fusion proteins or recombinant grass pollen allergens
An aluminum hydroxide–adsorbed vaccine containing 20 μg of each of the purified BM32 fusion proteins (BM321, BM322, BM325, and BM326) or each of the purified timothy grass pollen allergens (rPhl p 1, 20 μg; rPhl p 2, 10 μg; rPhl p 5, 20 μg; rPhl p 6, 10 μg) in a dose volume of 200 μL was obtained by using the following procedure: in a first step the individual fusion proteins were adsorbed to aluminum hydroxide (Alhydrogel; Brenntag Biosector, Ballerup, Denmark; 10 mg of Al3+/mL), resulting in 4 intermediate adsorbates containing 0.4 mg/mL of the single fusion protein and 3 mg/mL Al3+ in 2 mmol/L phosphate buffer (pH 7.4). These 4 intermediate adsorbates were then mixed in a 1:1:1:1 fashion, resulting in the BM32 vaccine mix containing 0.1 mg/mL of each BM32 fusion proteins or wild-type allergens and 3 mg/mL Al3+ in 2 mmol/L phosphate buffer (pH 7.4).
Immunization of rabbits and detection of grass pollen allergen–specific rabbit antibodies
Rabbits were immunized subcutaneously 3 times in monthly intervals with aluminum hydroxide adsorbed BM32 containing 20 μg (n = 2) or 40 μg (n = 2) of each BM component or with an equimolar recombinant grass pollen allergen mix containing 20 μg of Phl p 1, 10 μg of Phl p 2, 20 μg of Phl p 5, and 10 μg of Phl p 6 (Charles River, Kisslegg, Germany).
For comparison, rabbits were immunized subcutaneously with AIT vaccines containing natural allergen extracts from 4 major companies (n = 2 per vaccine; Allergovit grass, Allergopharma; Alutard SQ grass mix, ALK-Abelló; Phostal grasses + rye, Stallergenes; and Pollinex Quattro Plus grasses + rye, Bencard Allergie GmbH), as recommended by the manufacturer. For Allergovit 7, injections of increasing strength were administered in weekly intervals, followed by one maintenance injection after 2 weeks (Fig 1, A). For Alutard, the shortened initial therapy schedule II was applied, consisting of 11 injections within the first 8 weeks (Fig 1, A). In the case of Phostal, 13 injections were given within 12 weeks, and for Pollinex, 3 injections were given in a 2-week interval, followed by a fourth injection after a further 4 weeks (Fig 1, A). Thus the preseasonal treatments were completed for BM32, the mix of recombinant allergens, Allergovit, Alutard, and Pollinex after 8 weeks and for Phostal after 12 weeks. Serum samples were taken from the rabbits on the day of first immunization (pre-serum) and in monthly intervals. Grass pollen allergen–specific IgG responses were measured by means of ELISA in sera obtained before and at 1-, 2-, 3-, and 4-month (Phostal) intervals (Fig 1, B).
The BM32-immunized rabbits continued to be monitored and vaccinated to study whether their immune response can be boosted. Their allergen-specific IgG response was measured in monthly intervals for a total duration of 13 months (Fig E1). Booster injections were administered after 7.5 and 12 months, and the final serum sample was obtained 4 weeks after the last immunization (Fig E1).
Measurement of grass pollen allergen–specific immune responses
ELISA plates (Thermo Scientific Nunc, Roskilde, Denmark) were coated with 3 μg/mL of each of the grass pollen allergens rPhl p 1, 2, 5, or 6 to determine grass pollen allergen–specific antibody responses. Allergen-specific rabbit IgG responses were measured by diluting sera 1:500 by means of ELISA. Bound rabbit IgG was detected with 1:2000 diluted donkey anti-rabbit horseradish peroxidase–coupled IgG antibodies (NA 934; GE Healthcare UK Limited, Chalfont St Giles, United Kingdom). Color development was done with 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid).E3
Analysis of cross-reactivity of rabbit sera to nitrocellulose-blotted grass pollen extracts
Grass pollen extracts (Phleum pratense, Lolium perenne, Secale cereale, and Cynodon dactylon) were separated by using 15% preparative SDS-PAGE (24 μg of extract/cm of gel) and blotted onto a nitrocellulose membrane.E4 Nitrocellulose strips were incubated with 1:1000 diluted rabbit sera and, for control purposes, with the corresponding preimmune sera. Bound rabbit antibodies were detected with a 1:3000 diluted iodine 125–labeled goat anti-rabbit antibody (PerkinElmer Life Science, Boston, Mass) and visualized by using autoradiography.E5
ELISA competition assay for analyzing inhibition of human IgE binding to natural and recombinant grass pollen allergens by BM32-specific IgG antibodies
The ability of rabbit antibodies to inhibit the binding of IgE from patients with grass pollen allergy to timothy grass pollen extract or each of the recombinant timothy grass pollen allergens (rPhl p 1, 2, 5, and 6) was examined in ELISA competition assays, as described previously.E6 ELISA plates were coated with 0.2 μg per well of timothy grass pollen extract or 0.1 μg per well of each of the recombinant allergens preincubated with 1:4 diluted rabbit sera obtained 2 months after the last injection (ie, 3.IS for BM32, Allergovit, Alutard, and Pollinex and 4.IS for Phostal) and, for control purposes, with 1:4 diluted sera from rabbits without any immunization. After washing, plates were incubated with 1:5 diluted sera from patients with grass pollen allergy. Sera (n = 8) were residual serum samples from patients with grass pollen allergy who had been characterized by case history, skin prick test responses, and measurement of grass pollen–specific IgE antibodies (Table E1). Sera were anonymized and used with approval of the ethics committee of the Medical University of Vienna (EK1641/2014). Bound human IgE antibodies were detected with horseradish peroxidase–coupled goat anti-human IgE antibodies diluted 1:2500 (KPL, Gaithersburg, Md). The percentage of inhibition of IgE binding achieved by means of preincubation with anti-sera was determined as follows:
where ODI and ODP represent extinctions measured after preincubation with the rabbit immune and preimmune serum, respectively.
Inhibition of human allergen-specific T-cell proliferation by BM32-specific IgG or grass pollen allergen–specific antibodies
PBMCs were isolated from heparinized blood samples of patients with grass pollen allergy by means of Ficoll density gradient centrifugation (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). PBMCs (2 × 105) were cultured in triplicates in 96-well plates (Nunclone; Nalge Nunc International, Roskilde, Denmark) in 200 μL of serum-free Ultra Culture medium (BioWhittaker, Rockland, Me) supplemented with l-glutamine (10 mL of 200 mmol/L l-glutamine per liter; Sigma, St Louis, Mo), β-mercaptoethanol (5 mL of 50 mmol/L β-mercaptoethanol per liter, Sigma) and gentamicin (2 mL of 50 mg/mL per liter of medium, Sigma) at 37°C and 5% CO2 in a humidified atmosphere. PBMCs were stimulated with a mix of rPhl p 1, 2, 5, and 6 (0.5 μg per well of each allergen, recombinant mix) or rBet v 1 (2 μg per well) as a control in the presence of heat-inactivated (56°C for 30 minutes) 1:10 diluted sera obtained from rabbits 1 month after the last immunization with BM32 (40-μg dose), Alutard, or Allergovit or from normal rabbits. For control purposes, 4 U of IL-2 per well (Boehringer, Mannheim, Germany) or medium alone was used.
Statistical analysis
For statistical analyses, GraphPad Prism 6 software (GraphPad Software, La Jolla, Calif) was used. Differences between groups were compared by using the Wilcoxon signed-rank test. For multiple comparisons, the Bonferroni-Holm correction was applied. P values of less than .05 were considered significant.
Extended Data
Fig E1.
Boosting of BM32-induced allergen-specific IgG antibody responses. A, Scheme and time course of boosting experiment. Indicated are the time course (x-axis), injections, and dates of serum collection. B, Kinetics of allergen-specific IgG responses in BM32-immunized rabbits after initial immunization and 2 booster injections. Shown are mean OD values corresponding to allergen-specific IgG levels (y-axes) at different time points (x-axes).
Fig E2.
Cross-reactivity of BM32-induced antibodies with pollen extracts from different grasses. Nitrocellulose-blotted pollen extracts from Phleum pratense, Lolium perenne, Cynodon dactylon, and Secale cereale were reacted with sera obtained from rabbits immunized with Allergovit, Alutard, Pollinex, Phostal, or BM32 before immunization (P) and after 4 monthly intervals (1, 2, 3, and 4). Bound rabbit IgG was detected with iodine 125–labeled antibodies and visualized by means of autoradiography. Molecular weights (in kilodaltons) are indicated on the left margins.
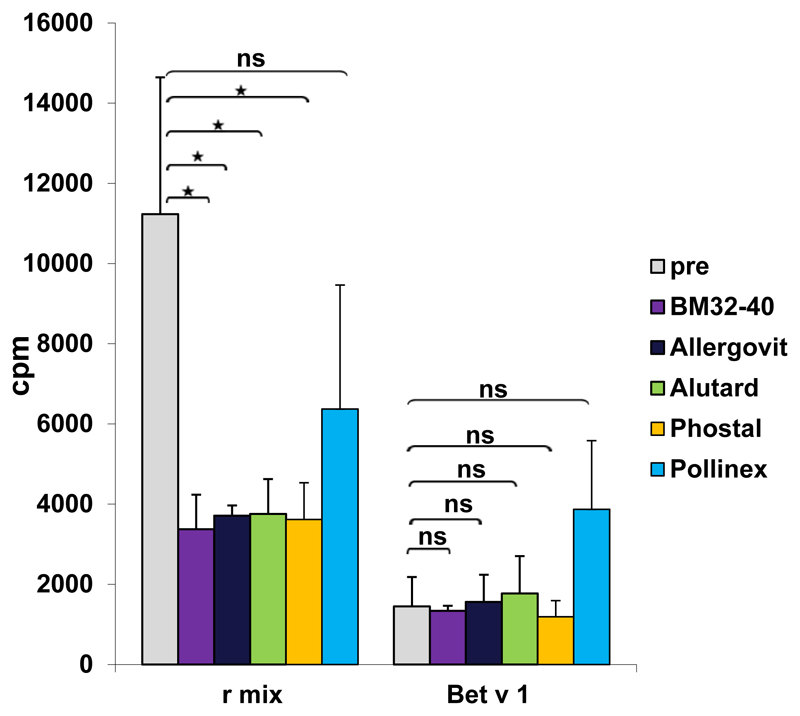
Fig E3.
Rabbit antibodies induced by means of vaccination with BM32 and registered allergen extract–based AIT vaccines inhibit allergen-specific T-cell proliferation. PBMCs from a patient with grass pollen allergy were exposed to a mix of grass pollen allergens or rBet v 1 that had been preincubated with rabbit anti-BM32 IgG antibodies (violet bars), anti-Allergovit IgG (black bars), anti-Alutard IgG (green bars), anti-Phostal IgG (orange bars), anti-Pollinex (blue bars), or IgG from normal rabbits without immunization (before: gray bars; x-axis). Lymphocyte proliferation responses after subtraction of medium background are shown as counts per minute (y-axis).
Table E1. Grass pollen allergen–specific IgE levels in sera from patients used for IgE inhibition studies.
| Patient no. | Phleum species extract (kUA/L) | Phl p 1 (kUA/L) | Phl p 2 (kUA/L) | Phl p 5 (kUA/L) | Phl p 6 (kUA/L) |
|---|---|---|---|---|---|
| 1 | 669.0 | 106.7 | 61.5 | 125.0 | 72.0 |
| 2 | 101.0 | 63.3 | ND | 81.3 | ND |
| 3 | 132.0 | 64.4 | 31.8 | 64.0 | 58.3 |
| 4 | 194.0 | 77.6 | 38.7 | 96.9 | 94.1 |
| 5 | 94.0 | 95.1 | 19.0 | 49.4 | 37.5 |
| 6 | 83.0 | 31.0 | 14.5 | 50.7 | 31.2 |
| 7 | 48.0 | 22.3 | 11.5 | 31.5 | 28.0 |
| 8 | 235.0 | 90.4 | 14.2 | 93.2 | 83.4 |
ND, Not determined.
Acknowledgments
We acknowledge the technical assistance of Patricia Vegh from the Christian Doppler Laboratory of Allergy Research, Department of Pathophysiology and Allergy Research, Medical University of Vienna. We thank Dr Christian Lupinek, Department of Pathophysiology and Allergy Research, Medical University of Vienna, for help regarding statistical evaluations.
Supported by research grants from the Christian Doppler Research Association, Vienna, Austria; Biomay AG, Vienna, Austria; the Austrian Science Fund (FWF; projects F4604, F4605, and F4611); and the Austrian Research Promotion Agency (FFG; projects 830432 and 835415).
Footnotes
Disclosure of potential conflict of interest: B. Linhart has received a grant from the European Union FP7 (FAST). A. Neubauer has received grants from the Austrian Research Promotion Agency (project nos. 830432 and 835415) and is employed by and has received stock/stock options from Biomay. R. Henning is employed by and has received stock/stock options from Biomay. R. Valenta has received grants from Biomay AG, Austrian Science Fund, and Christian Doppler Research Association; has consultant arrangements with Biomay AG, Fresenius Medical Care, and Viravaxx; and has received payment for development of educational presentations from Thermo Fisher. M. Focke-Tejkl has received a grant from the Austrian Science Fund (FWF P29991). The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Cox L, Calderόn M, Pfaar O. Subcutaneous allergen immunotherapy for allergic disease: examining efficacy, safety and cost-effectiveness of current and novel formulations. Immunotherapy. 2012;4:601–16. doi: 10.2217/imt.12.36. [DOI] [PubMed] [Google Scholar]
- 2.Keil MA, Röder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Mölken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–60. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–17, e1-11. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immuno-therapy of grass pollen allergy. EBioMedicine. 2016;11:43–57. doi: 10.1016/j.ebiom.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–7. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 6.Cornelius C, Schöneweis K, Georgi F, Weber M, Niederberger V, Zieglmayer P, et al. Immunotherapy with the PreS-based grass pollen allergy vaccine BM32 induces antibody responses protecting against hepatitis B Infection. EBioMedicine. 2016;11:58–67. doi: 10.1016/j.ebiom.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larchò M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 8.Rosewich M, Schulze J, Eickmeier O, Telles T, Rose MA, Schubert R, et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010;160:403–10. doi: 10.1111/j.1365-2249.2010.04106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
References
- E1.Marth K, Focke M, Flicker S, Valenta R. Human monoclonal antibody-based quantifications of group 2 grass pollen allergens. J Allergy Clin Immunol. 2004;113:470–4. doi: 10.1016/j.jaci.2003.11.042. [DOI] [PubMed] [Google Scholar]
- E2.Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498–505. doi: 10.1034/j.1398-9995.2001.056006498.x. [DOI] [PubMed] [Google Scholar]
- E3.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–17, e1–11. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175:377–85. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- E7.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–68. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- E8.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- E9.Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014;133:612–9. doi: 10.1016/j.jaci.2014.01.007. [DOI] [PubMed] [Google Scholar]
- E10.Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101–3. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Paul WE, Katz DH, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J Exp Med. 1970;132:283–99. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]