To the Editor
The determination of allergen-specific IgE had been introduced into allergy diagnosis 50 years ago.1 In addition to clinical anamnesis and provocation testing, it represents one of the cornerstones in the diagnosis of IgE-associated allergy.2 The detailed characterization of the disease-causing allergen molecules through molecular cloning technologies3,4 has brought about new forms of molecular allergy diagnosis that allow measuring IgE specific to a large number of micro-arrayed allergen molecules comprising the most relevant allergen sources by chip technology.5,6 Molecular allergy diagnosis is extremely useful for the resolution of complicated sensitization patterns especially in children and allows selection of personalized forms of treatment such as allergen avoidance, diet, and/or allergen-specific immunotherapy.7 An important advantage to traditional forms of allergen extract–based quantitative IgE testing is that allergen microarrays allow simultaneous determination of IgE and IgG levels specific for multiple allergens with small volumes of serum and the disease-causing allergens can be precisely defined.1,6 However, not all allergists have direct access to the new forms of molecular allergy diagnosis and therefore would need to send blood samples to specialized laboratories. The shipment of serum or blood samples to laboratories performing molecular analysis requires sophisticated packaging and cooling, is often restricted by complex safety and transportation rules (eg, following rules of airline safety), and therefore is very expensive or even impossible.
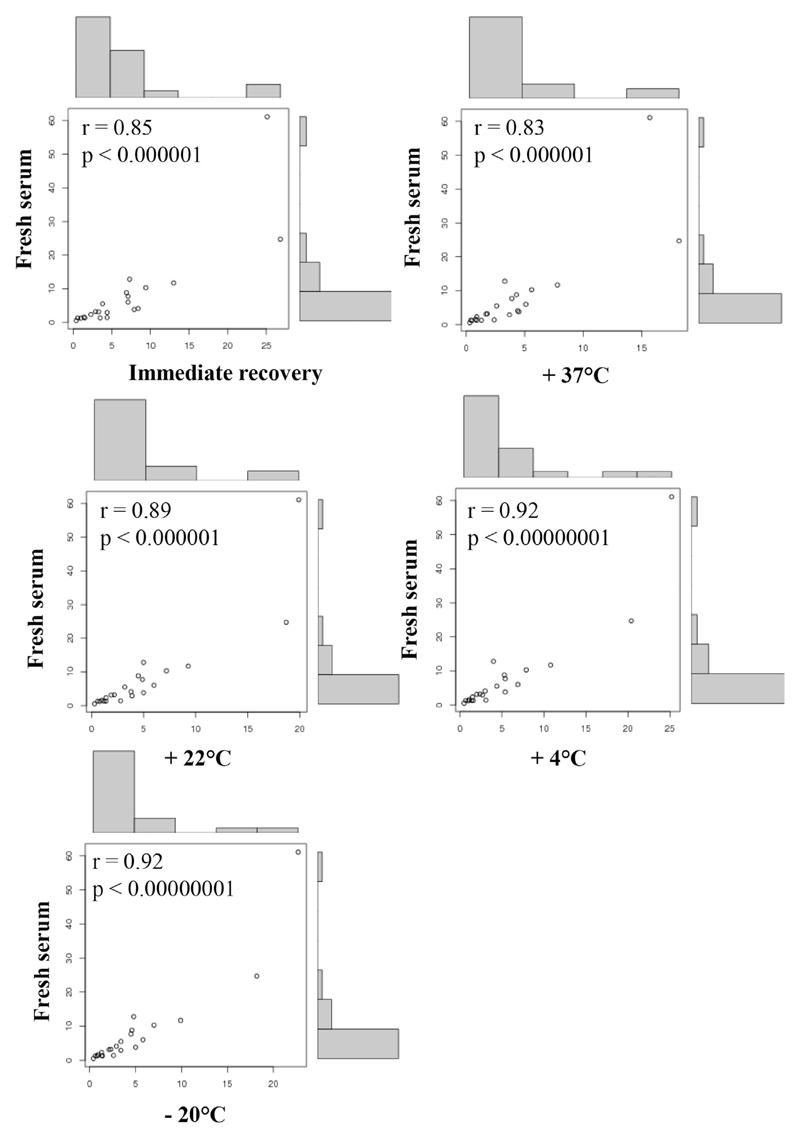
To overcome these limitations, we investigated whether it is possible to simplify the collection, packaging, and sending of blood samples for micro-array analysis. For this purpose, we first performed a series of pilot experiments: Anticoagulated whole-blood samples or serum from allergic patients was immobilized on different types of paper (ie, filter paper, nitrocellulose), air-dried, and eluates obtained with different buffers (ie, PBS, sample diluent) were tested for IgE reactivity to 175 micro-arrayed allergen molecules using ImmunoCAP ISAC technology (see Table E1 and Fig E1 in this article’s Online Repository at www.jacionline.org). Elution with PBS allowed recovering allergen-specific IgE from paper-dried blood spots better than from sample diluent (Fig E1). When we compared the effects of eluting 1 punched piece of paper with elution of 3 pieces of punched paper in 50 mL of PBS, elution from 3 pieces gave the best results and hence was used in all experiments shown. Paper-dried serum and blood spots could be stored at different temperatures (ie, +37°C, +22°C, +4°C, −20°C), giving similar results regarding allergen-specific IgE levels as compared with immediate recovery after drying (see Figs E2 and E3 in this article’s Online Repository at www.jacionline.org). Fig 1 shows that there is an excellent correlation (r >0.83; P < .000001) between IgE levels measured in fresh serum of 1 patient to the 21 recognized allergens and results obtained after immediate recovery or after storage for 1 week at +37°C, +22°C, +4°C, and −20°C. In a next set of experiments we show that there is an excellent correlation (r > 0.87; P < .001) between allergen-specific IgE levels measured in fresh blood versus immediately recovered whole-blood samples from 9 patients to 8 of the most frequently recognized allergens (see Fig E4 in this article’s Online Repository at www.jacionline.org). To further investigate whether the results are reliable and reproducible, we performed a detailed analysis of sera from 17 patients for whom we compared immediate recovery and recovery from paper-dried serum samples stored for 2 weeks at 37°C for the 8 most frequently detected allergens. The demographic and clinical characterization of the 17 patients (ie, #1-#18) is presented in Table E2 in this article’s Online Repository at www.jacionline.org.
Fig. 1.
Correlations of allergen-specific IgE levels measured in fresh serum of an allergic patient (#18, Table E2) with IgE levels measured in serum samples recovered immediately and after 1 week storage at +37°C, +22°C, +4°C, and −20°C. Correlations are shown for the 21 recognized allergens in scatter plots with r and P values.
Thus, 136 analyses were performed in triplicates for each condition (ie, immediate recovery and recovery after 2 weeks of storage at 37°C) (see Fig E5 in this article’s Online Repository at www.jacionline.org). The statistical analysis showed again that there is an excellent correlation between results obtained with the fresh sera versus immediate recovery as well as for the fresh sera versus recovered samples obtained after 2 weeks at 37°C (Fig E5).
To analyze potential sensitivity loss we have investigated all allergens recognized by the fresh serum samples from the 17 patients tested in Fig E5. The 17 patients had shown positive IgE reactivity of 0.3 ISU-IgE or greater to 182 allergen molecules. After immediate recovery we lost 13 positive reactions (ie, 7.1%) if the cutoff for the recovered samples was set at greater than or equal to 0.3 ISU-IgE and in 2 (ie, 1%) when the cutoff was set at greater than or equal to 0.1 ISU-IgE. For recovery after 2 weeks at 37°C, we lost 26 (ie, 14.8%) when the cutoff in the recovered samples was set at greater than or equal to 0.3 ISU-IgE and in 8 (ie, 4.3%) when the cutoff was set at greater than or equal to 0.1 ISU-IgE. The analysis of the loss of sensitivity showed that it occurred only for serum samples with very low allergen-specific IgE levels (ie, <1.5 ISU-IgE) for a given allergen. Thus, IgE titers after recovery were lower than in fresh samples, which may lead to a slight loss of sensitivity.
In a pilot experiment we were also able to show that IgE recovered from dried serum after 1 and 7 weeks of storage at +37°C was still functional and when loaded to basophils triggered dose-dependent mediator release (see Fig E6 in this article’s Online Repository at www.jacionline.org).
These results indicate that shipment of paper-dried blood and serum spots can be performed simply by mail without need for cooling or express delivery at different seasons and in different climate zones and allows reliable and reproducible detection even of low levels of allergen-specific IgE (Fig 1; see Figs E1-E5). Interestingly, there seemed to be a greater loss of IgE signals for high allergen-specific IgE levels as compared with lower ones (eg, Bet v 1 and Phl p 5: Fig E1). We therefore studied the general loss of protein during the recovery procedure. Table E3 in this article’s Online Repository at www.jacionline.org presents for sera from 9 allergic patients the protein contents and recovery of protein after storage for 2 weeks at +37°C, +22°C, +4°C, −20°C. The range of recovery for the 9 sera at the 4 tested conditions was 9% to 18% with the elution procedure applied in our experiments (ie, dried serum, filter paper, 3 punched pieces, 50 μL PBS).
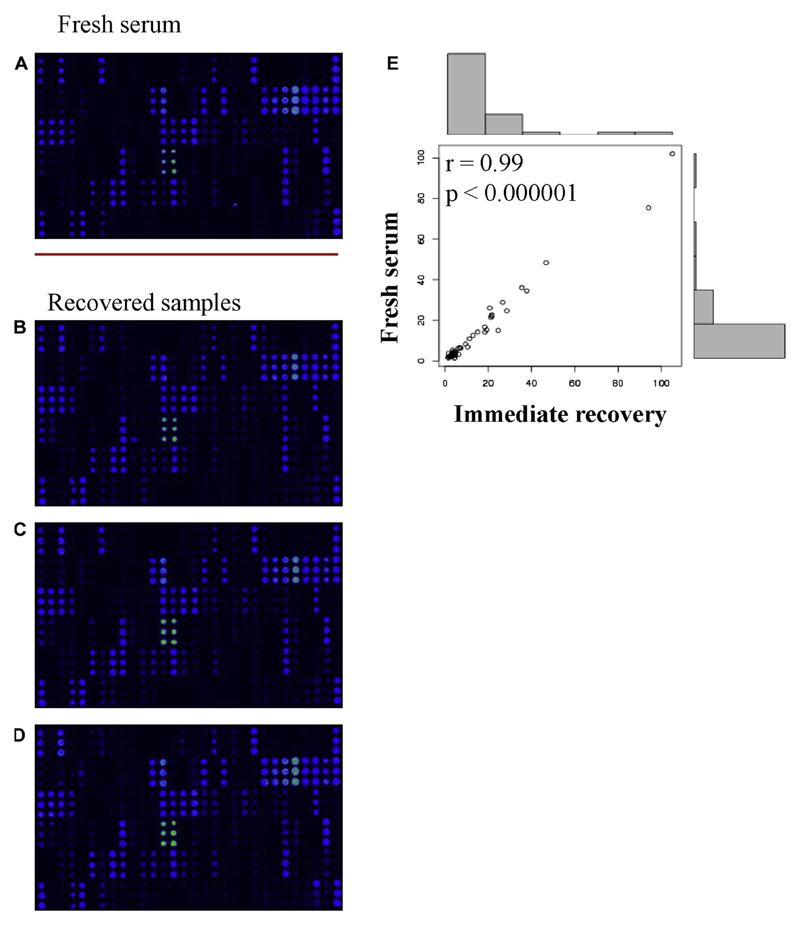
Because measurement of allergen-specific IgG to micro-arrayed allergens is also important to study clinically relevant questions such as the induction of allergen-specific IgG in the course of allergen-specific immunotherapy8 or the occurrence of allergen-specific IgG as a sign of natural allergen exposure9 we studied whether allergen-specific IgG can be also recovered from paper-dried serum spots. Fig 2 shows a comparison of specific IgG reactivity to the micro-arrayed allergens in a fresh, 1:50 diluted serum sample and in 3 identical immediately recovered eluates that were 15-fold less diluted to compensate for the protein loss occurring during the immobilization and elution procedure. There was an excellent correlation of allergen-specific IgG levels measured in the fresh serum and the recovered samples (r = 0.99; P < .000001) to the recognized allergens (Fig 2, E; see Table E4 in this article’s Online Repository at www.jacionline.org). The rate of recovery of specific IgG was comparable when eluates were obtained immediately after drying or after storage at different temperatures (−20°C-+37°C) (data not shown).
Fig. 2.
Screen-shots of IgG reactivity to micro-arrayed allergens in (A) a fresh serum sample and in (B-D) immediately recovered eluates from 3 identically prepared paper-dried blood spots. E, Scatter plot of the correlations of allergen-specific IgG levels with r and P values for the 48 recognized allergens (Table E4).
Our results thus indicate that it is not only possible to recover from a paper-dried blood or serum spot sufficient IgE and IgG to test for reactivity to more than 170 different allergens by micro-array technology but it is also reliable and reproducible. Because the paper-dried blood spots can be stored at different temperatures (−20°C-+37°C) without losing activity, it should be possible to send them sealed in plastic by envelopes via simple mail from all over the world to laboratories performing micro-array analysis. The test results can then be sent by mail or email back to the allergist to aid in the management of complicated cases. We thus provide a simple procedure for making molecular allergy diagnosis from paper-dried blood samples available worldwide.
Acknowledgments
This study was supported by the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung [FWF]) via grant number F4605 and the FWF-funded PhD programs Inflammation and Immunity (IAI) and Molecular, Cellular and Clinical Allergology (MCCA). R.V. is recipient of a megagrant of the Government of the Russian Federation (grant no. 14.W03.31.0024).
Footnotes
Disclosure of potential conflict of interest: R. Valenta has received research grants from Biomay AG, Vienna, Austria, and Viravaxx, Vienna, Austria, and serves as a consultant for these companies. F. Gastager is an employee of HVD Vertriebs Ges.m.b.H, Vienna, Austria. The rest of the authors declare that they have no relevant conflicts of interest.
We thank Prof Ryosuke Nakamura for kindly providing the huRBL cell line.
References
- 1.van Hage M, Hamsten C, Valenta R. ImmunoCAP assays: pros and cons in allergology. J Allergy Clin Immunol. 2017;140:974–97. doi: 10.1016/j.jaci.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. WAO - ARIA -GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6:1–17. doi: 10.1186/1939-4551-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas WR. The advent of recombinant allergens and allergen cloning. J Allergy Clin Immunol. 2011;127:855–9. doi: 10.1016/j.jaci.2010.12.1084. [DOI] [PubMed] [Google Scholar]
- 4.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 5.Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol. 2016;27:1–250. doi: 10.1111/pai.12563. [DOI] [PubMed] [Google Scholar]
- 7.Fedenko E, Elisyutina O, Shtyrbul O, Pampura A, Valenta R, Lupinek C, et al. Microarray-based IgE serology improves management of severe atopic dermatitis in two children. Pediatr Allergy Immunol. 2016;27:645–9. doi: 10.1111/pai.12572. [DOI] [PubMed] [Google Scholar]
- 8.Wollmann E, Lupinek C, Kundi M, Selb R, Niederberger V, Valenta R. Reduction in allergen-specific IgE binding as measured by microarray: a possible surrogate marker for effects of specific immunotherapy. J Allergy Clin Immunol. 2015;136:806–9. doi: 10.1016/j.jaci.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siroux V, Lupinek C, Resch Y, Curin M, Just J, Keil T, et al. Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: the EGEA study. J Allergy Clin Immunol. 2017;139:643–54. doi: 10.1016/j.jaci.2016.05.023. [DOI] [PubMed] [Google Scholar]