Abstract
Background
BM32 is a grass pollen allergy vaccine based on recombinant fusion proteins consisting of nonallergenic peptides from the IgE-binding sites of the 4 major grass pollen allergens and the hepatitis B preS protein.
Objective
We sought to study the safety and clinical efficacy of immunotherapy (allergen immunotherapy) with BM32 in patients with grass pollen–induced rhinitis and controlled asthma.
Methods
A double-blind, placebo-controlled, multicenter allergen immunotherapy field study was conducted for 2 grass pollen seasons. After a baseline season, subjects (n = 181) were randomized and received 3 preseasonal injections of either placebo (n = 58) or a low dose (80 μg, n = 60) or high dose (160 μg, n = 63) of BM32 in year 1, respectively, followed by a booster injection in autumn. In the second year, all actively treated subjects received 3 preseasonal injections of the BM32 low dose, and placebo-treated subjects continued with placebo. Clinical efficacy was assessed by using combined symptom medication scores, visual analog scales, Rhinoconjunctivitis Quality of Life Questionnaires, and asthma symptom scores. Adverse events were graded according to the European Academy of Allergy and Clinical Immunology. Allergen-specific antibodies were determined by using ELISA, ImmunoCAP, and ImmunoCAP ISAC.
Results
Although statistical significance regarding the primary end point was not reached, BM32-treated subjects, when compared with placebo-treated subjects, showed an improvement regarding symptom medication, visual analog scale, Rhinoconjunctivitis Quality of Life Questionnaire, and asthma symptom scores in both treatment years. This was accompanied by an induction of allergen-specific IgG without induction of allergen-specific IgE and a reduction in the seasonally induced increase in allergen-specific IgE levels in year 2. In the first year, more grade 2 reactions were observed in the active (n = 6) versus placebo (n = 1) groups, whereas there was almost no difference in the second year.
Conclusions
Injections of BM32 induced allergen-specific IgG, improved clinical symptoms of seasonal grass pollen allergy, and were well tolerated.
Keywords: Allergy, grass pollen allergy, allergen, allergen immunotherapy, recombinant allergen, B-cell epitope–based immunotherapy, efficacy, hypoallergenic, clinical trial, safety
Allergen-specific immunotherapy is the only disease-modifying treatment for allergy and has long-lasting effects, even after discontinuation.1–5
It has been shown that allergen immunotherapy (AIT) is more cost-effective than pharmacotherapy.6 However, there are several aspects of current allergen extract–based AIT that can be improved, such as safety and convenience.7 There is a need for safe AIT forms requiring only few administrations. Recently, a grass pollen allergy vaccine (BM32) has been developed that is based on recombinant fusion proteins consisting of nonallergenic peptides derived from the IgE-binding sites of the 4 major timothy grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Phl p 6) and the preS protein derived from the large surface antigen of the hepatitis B virus (HBV).8 Allergen-specific T-cell epitopes were reduced in the recombinant fusion proteins of BM32. Therefore preS was selected to serve as an immunologic carrier protein providing T-cell help for the production of blocking allergen-specific IgG antibodies. The immunologic characterization of BM32 showed a lack of IgE reactivity and allergenic activity, and at the same time, the vaccine induced allergen-specific IgG in animals, which blocked allergic patients’ IgE binding to the grass pollen allergens and inhibited allergen-induced basophil degranulation.8
In a subsequent clinical skin test study in human subjects, it was demonstrated that BM32 induced neither immediate type skin reactions nor T cell–mediated late-phase reactions, as evaluated by atopy patch testing.9 This confirmed the lack of allergenic activity and demonstrated that allergen-specific T-cell epitopes, which in previous synthetic allergy vaccines gave rise to systemic late-phase side effects,10–13 have indeed been eliminated to a large extent in BM32.
A subsequent safety and dose-finding study conducted as a double-blind, placebo-controlled study in an allergen exposure chamber setting showed that 3 monthly injections of BM32 led to a significant reduction of total nasal symptom scores during a 6-hour grass pollen exposure in patients treated with 80 and 160 μg of BM32, which was accompanied by a reduction of the total ocular symptom score and immediate-type skin sensitivity, as determined by using titrated skin prick tests (SPTs).14 The clinical effects were associated with an induction of allergen-specific IgG (IgG1 = IgG4 > IgG2) production and a reduction in allergen-specific T-cell proliferation by inhibition of IgE-facilitated allergen presentation through allergen-specific IgG antibodies.14
Here we report the first double-blind, placebo-controlled, multicenter field trial, which investigated the clinical efficacy and immunogenic effects, as well as tolerability, of BM32.
Methods
Study subjects
To be eligible for the study, subjects had to be aged 18 to 60 years and of either sex, with a positive history of grass pollen allergy confirmed by a positive SPT response (wheal >3 mm) to grass pollen extract and allergen-specific IgE levels (measured by using ImmunoCAP; Thermo Fisher Scientific, Uppsala, Sweden) of at least 3.5 kUA/L to both grass pollen extract and rPhl p 1/rPhl p 5 at screening or within 12 months before inclusion. They also had to show moderate-to-severe symptoms of grass pollen allergy during the grass pollen season (GPS) of the screening year 2012. Major criteria for exclusion were symptomatic perennial or seasonal coallergies during the GPS, severe ongoing atopic dermatitis, uncontrolled asthma specified by an FEV1 of less than 70% of predicted value, nasal polyposis, sensitization to Phl p 7 with allergen-specific IgE levels of greater than 0.35 kUA/L, and participation in a grass pollen–specific immunotherapy trial or use of marketed grass pollen–specific immunotherapy in the 2 years before study start. The complete list of inclusion and exclusion criteria can be found in the study protocol in the Study Protocol and Table E1 in this article’s Online Repository at www.jacionline.org. Table I shows that subjects were evenly distributed regarding age, sex, symptoms, and immunologic characteristics regarding the treatment groups.
Table I. Demographic, clinical, and serologic characterization of the SA population.
| BM32 low | BM32 high | Placebo | Total | |
|---|---|---|---|---|
| No. of subjects | 53 | 60 | 53 | 166 |
| Age (V1) | ||||
| Mean | 28.7 | 29.8 | 29.1 | 29.2 |
| Median | 26 | 28 | 25 | 26 |
| Range | 18-53 | 18-52 | 18-58 | 18-58 |
| Sex (V1) | ||||
| Male (%) | 34 (64.2) | 36 (60.0) | 31 (58.5) | 101 (60.8) |
| Ethnic group (V1) | ||||
| White (%) | 53 (100.0) | 59 (98.3) | 52 (98.1) | 164 (98.8) |
| Asian (%) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (0.6) |
| African (%) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 1 (0.6) |
| Severity of grass pollen allergy (V1) | ||||
| Moderate (%) | 34 (64.1) | 39 (65.0) | 37 (69.8) | 110 (66.3) |
| Severe (%) | 19 (35.9) | 21 (35.0) | 16 (30.2) | 56 (33.7) |
| Subjects with a history of grass pollen–associated asthma (V1) | ||||
| No. (%) | 20 (38.5) | 12 (20.0) | 14 (26.4) | 46 (27.7) |
| Total IgE (kU/L) (V5) | ||||
| Mean | 273 | 179 | 194 | 214 |
| Median | 118 | 117 | 129 | 119 |
| Range | 20.6-1235 | 10.4-2217 | 8.8-1121 | 8.8-2217 |
| sIgE, Timothy grass (kUA/L [V1]) | ||||
| Mean | 35.0 | 29.0 | 33.3 | 32.3 |
| Median | 21.0 | 21.6 | 19.9 | 21.0 |
| Range | 3.45-100 | 3.48-100 | 2.63-100 | 2.63-100 |
| sIgE, Timothy grass (kUA/L [V5]) | ||||
| Mean | 29.9 | 21.9 | 26.0 | 25.8 |
| Median | 17.0 | 17.3 | 17.4 | 17.3 |
| Range | 2.07-100 | 2.95-76.3 | 2.20-100 | 2.07-100 |
| sIgE, Phl p 1 (kUA/L [V5]) | ||||
| Mean | 17.5 | 12.4 | 15.9 | 15.2 |
| Median | 8.56 | 9.82 | 8.46 | 8.67 |
| Range | 1.13-95.4 | 0.10-41.8 | 0.66-81.4 | 0.10-95.4 |
| sIgE Phl p 2 (kUA/L [V5]) | ||||
| Mean | 4.04 | 2.47 | 5.11 | 3.82 |
| Median | 1.92 | 1.67 | 2.27 | 1.76 |
| Range | 0.10-20.8 | 0.10-18.0 | 0.10-32.7 | 0.10-32.7 |
| sIgE, Phl p 5 (kUA/L [V5]) | ||||
| Mean | 20.0 | 14.1 | 14.5 | 16.1 |
| Median | 11.0 | 9.12 | 6.12 | 9.34 |
| Range | 0.10-100 | 0.10-100 | 0.10-100 | 0.10-100 |
| sIgE, Phl p 6 (kUA/L [V5]) | ||||
| Mean | 7.96 | 6.31 | 5.11 | 6.45 |
| Median | 3.71 | 2.66 | 2.05 | 2.99 |
| Range | 0.10-67.3 | 0.10-67.9 | 0.10-50.2 | 0.10-67.9 |
| SPT, grass pollen (mm2 [V5]) | ||||
| Mean | 67.7 | 77.3 | 77.0 | 74.2 |
| Median | 51.3 | 76.2 | 68.0 | 65.2 |
| Range | 8.17-241 | 7.72-190 | 0.75-443 | 0.75-443 |
V1 and V5 denote visits 1 and 5 before treatment, respectively.
Study design
This study was a multicenter, double-blind, placebo-controlled, parallel-group, prospective study to investigate the safety and efficacy of 2 years of treatment with BM32 in patients with grass pollen allergy with allergic rhinitis, mild asthma, or both. The trial has been registered under EudraCT no. 2012-000442-35 and ClinicalTrial.gov Identifier NCT01538979. The Study Protocol is available in this article’s Online Repository. This study was carried out in 11 centers in 5 European countries (5 sites in Germany, 2 sites in Austria, and 1 site each in Belgium, Denmark, The Netherlands, and Slovenia) and was conducted in accordance with the Declaration of Helsinki and in compliance with Good Clinical Practice guidelines. The study protocol was approved by the ethics committees and competent legal authorities in each participating country, and all subjects provided written informed consent.
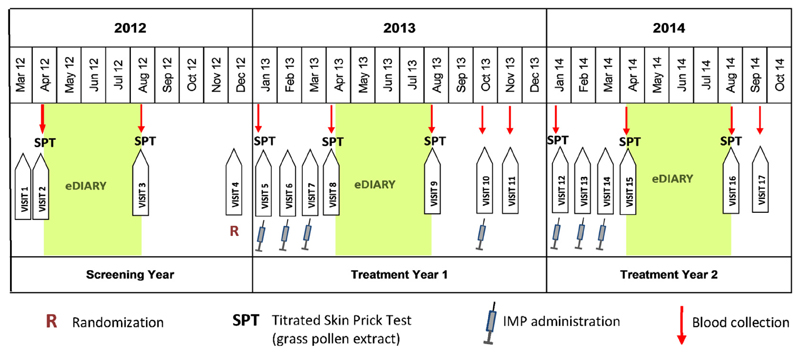
The study was conducted over 3 GPSs (see Table E2 in this article’s Online Repository at www.jacionline.org) from May 2012 (first patient in) until October 2014 (last patient out). A screening year (2012) was followed by 2 treatment years (2013 and 2014) with an interim analysis performed by an independent data monitoring committee (IDMC) in December 2013 (Figs 1 and 2).
Fig 1.
Study design. Patients recruited during the screening year (2012) were randomized at visit 4 (R) and treated 3 times preseasonally during each of the 2 treatment years (2013 and 2014) and once in October 2013 (investigational medicinal product [IMP] administration). Symptoms, medication, VAS, and quality-of-life data were obtained by using eDiaries from mid-April until mid-August (green background). Titrated SPTs were performed at 8 time points, and blood samples were taken at 11 time points (red arrows).
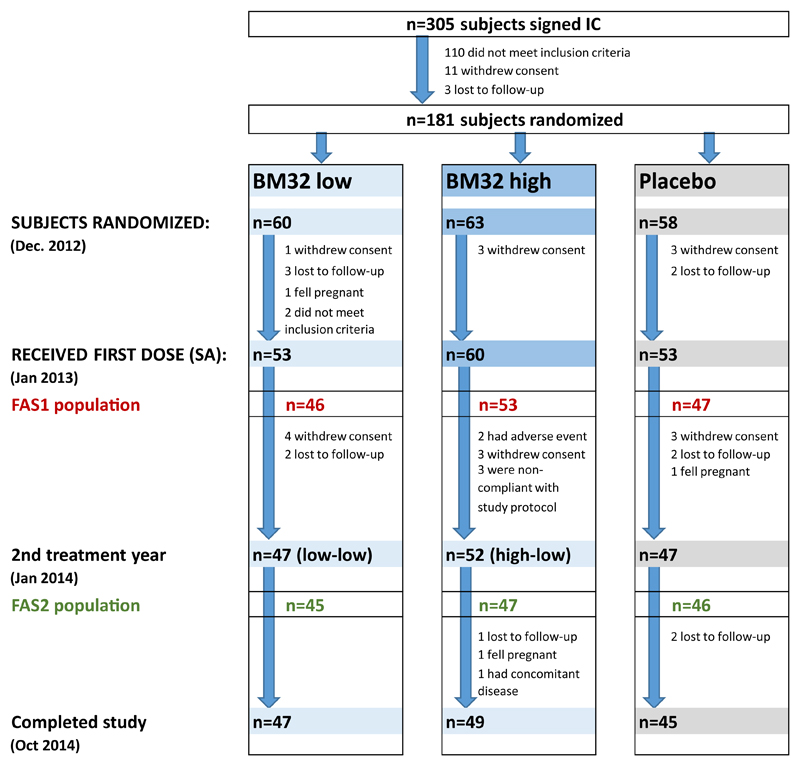
Fig 2.
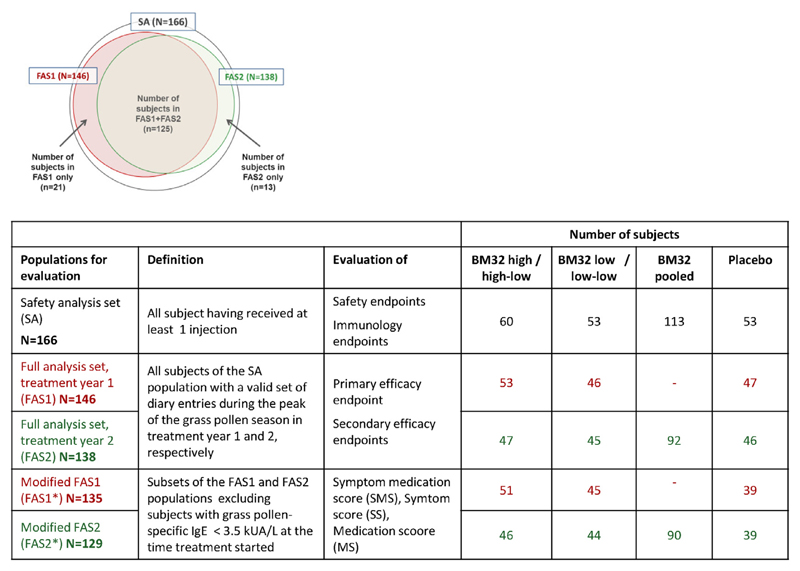
Study populations, enrollment, and randomization. Numbers of subjects screened and signing informed consent (IC) forms, randomized, receiving the first injection (SA population), entering treatment year 2, and completing the study are shown. FAS1 (red numbers) and FAS2 (green numbers) are displayed. Discontinuations (numbers and reasons) are indicated.
We intended to recruit all subjects before or during the GPS of 2012 and collect data on their allergy symptoms and intake of allergy medication through an electronic diary. However, the recruitment period had to be extended until January 2013. Therefore detailed data collected during the 2012 GPS were available for less than half of the subjects. All other subjects completed a paper version of the diary retrospectively once to summarize their allergy symptoms and need for allergy medication in the 2012 GPS.
These data, as well as serologic data (allergen-specific IgE levels) and results from the titrated SPTs performed after the 2012 GPS (visit 3), were evaluated by an IDMC. The aim of this data review was (1) to identify and exclude subjects not eligible with respect to the inclusion and exclusion criteria (in particular subjects with coallergies interfering with study end points and subjects with only mild symptoms during the GPS) and (2) to assign the 181 eligible subjects (Fig 2) to one of 2 groups according to the severity of their sensitization to grass pollen (group 1, moderate; group 2, severe). To be allocated to group 2, subjects had to have grass pollen–specific IgE levels of greater than 17.5 kUA/L and a positive SPT response with grass pollen extract at a dilution of 1:128 by using SPT end point titration. Eligible subjects were randomized in a 1:1:1 ratio to treatment with 20 μg of each of the 4 fusion proteins (ie, 80 μg of antigen in total, which was termed BM32 low), with 40 μg of the 4 fusion proteins (ie, 160 μg of antigen in total, which was termed BM32 high), or with placebo. Randomization was carried out by the contract research organization as block randomization stratified for centers and severity to ensure even distribution of the 3 study arms over all participating centers and even distribution of subjects with severe symptoms over the 3 study arms. Subjects received 3 preseasonal injections and an additional booster injection after the GPS of treatment year 1 (ie, 2013) and 3 preseasonal injections in treatment year 2 (ie, 2014; Fig 1). Electronic diaries were used for daily documentation of the subjects’ well-being by using a visual analog scale (VAS),15 grass pollen allergy–related symptoms, and intake of allergy medication (standby medication) during the GPS of the 2 treatment years. The study schedule indicating time points (visits) for injections, blood sampling, and titrated SPTs is shown in Fig 1.
As a result of a blinded interim analysis conducted at the end of the first treatment year (ie, 2013) and after a subsequent study protocol amendment, all subjects of the 2 actively treated groups received the low dose of BM32 in treatment year 2 and were pooled for statistical analysis of the results of year 2 (ie, BM32 pooled) to increase the power of the study.
Study medication
BM32 vaccine and placebo
BM32 is an equimolar mix of 4 recombinant fusion proteins consisting of hypoallergenic peptides derived from B-cell epitopes of the 4 major allergens from grass pollen, Phl p 1, Phl p 2, Phl p 5, and Phl p 6, which are fused to hepatitis B–derived preS.8 Details of the peptides, including a depiction of their sequences and arrangement, are presented in detail in a study by Zieglmayer et al.14 These 4 active pharmaceutical ingredients (APIs) are adsorbed onto aluminum hydroxide in a physiologic buffer containing 0.9% NaCl. Two different BM32 doses (BM32 low dose: 20 μg of each API, 80 μg of antigen in total; BM32 high dose: 40 μg of each API, 160 μg of antigen in total) were selected for the study based on the results of a previous phase IIa safety and dose-finding study conducted in a pollen exposure chamber.14 The study medication was manufactured by Biomay AG (Vienna, Austria) and Polymun Scientific GmbH (Klosterneuburg, Austria). It was provided as suspension “ready for injection” containing either 0.2 mg/mL BM32 APIs and 1.5 mg/mL of Al3+ (BM32 low dose) or 0.4 mg/mL BM32 APIs and 3.0 mg/mL Al3+ (BM32 high dose) in a physiologic buffer containing 0.9% NaCl. The placebo preparation contained 3.0 mg/mL Al3+ in the same buffer. The vaccine was administered as a subcutaneous injection. The dose volume was 400 μL.
Concomitant antiallergic medication
All study subjects had access to predefined concomitant antiallergic medication (topical antihistamine, oral antihistamine, nasal corticosteroid, and oral corticosteroid) to be used in a stepwise manner during the GPS when needed.
Assessment of clinical efficacy parameters
The primary end points of the study were the mean daily combined symptom medication scores (SMSs) calculated for the peak of the GPS in treatment years 1 and 2 (see the Study Protocol in this article’s Online Repository). The SMS was calculated as the sum of the symptom score (SS) and medication score (MS). Secondary end points were the mean daily SMS in both years during the whole GPS, as well as the mean daily SS and MS during the pollen peak and the whole GPS. Six symptoms of allergic rhinoconjunctivitis (AR/C; ie, runny nose, blocked nose, sneezing, itchy nose, gritty/red/itchy eyes, and watery eyes) in accordance with the European Academy of Allergy and Clinical Immunology (EAACI) position paper16 and 3 asthma symptoms (cough, wheezing, and tightness of chest/shortness of breath) were recorded once daily in an electronic diary. Diary entries were to be made in the evening, reflecting the past 24 hours, from approximately 4 weeks before until approximately 4 weeks after the GPS. The SS was calculated as the sum of scores for the 6 symptoms of AR/C measured as follows: 0, no symptoms; 1, mild symptoms; 2, moderate symptoms; and 3, severe symptoms.16,17 An asthma SS was formed from the sum of scores from the 3 asthma symptoms using the same scale (0-3), as described for the SS.
Stepwise standby medication (ie, concomitant antiallergic medication) use was scored as follows: oral antihistamine (2 mg of ceterizine) was defined as 2 points per tablet; topical antihistamine (0.5 mg/mL azelastine ophtalmic solution or 1 mg/mL azelastine nasal spray) was defined as 0.5 points per drop or puff; topical corticosteroid (50 μg of mometasone furoate nasal spray) was defined as 1 point per puff; and oral corticosteroid (40-mg methyl prednisolone tablet) was defined as 18 points per tablet.
As additional secondary efficacy end points, the patient’s well-being was assessed by 2 independent measures: mean daily VAS15 and mean weekly scores obtained with the standardized Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ),18 both of which were calculated during the pollen peak, as well as during the whole GPS. Overall AR/C symptom–related well-being was reported by patients daily by indicating a point on a 10-cm-long line between the left (feeling good) and right (feeling very bad) on a VAS presented in the electronic diary. The RQLQ, consisting of 28 questions addressing 7 different aspects of quality of life that are impaired by AR/C on a 7-point scale from 0 (not impaired at all) to 6 (severely impaired) was completed once weekly in the electronic diary.
The GPS was defined to start with the first day with a pollen count of greater than 25 grains/m3 per 24 hours and to end on the last day with a pollen count of greater than 25 grains/m3 per 24 hours. The grass pollen peak season was defined as the 15 consecutive days during the GPS with the highest cumulative pollen count for each site. Centers were excluded from the evaluation of the SMS if more than 50% of days in the peak season had a pollen count of 25 grains/m3 or less. Pollen counts were purchased from the Research Unit of Aerobiology and Pollen Information at the Medical University of Vienna, Vienna, Austria (https://www.pollenwarndienst.at/en/current-data/daily-load.html).
Statistical analysis
The safety analysis (SA) set (n = 166 patients) included all subjects who had received at least 1 injection (see Fig E1 in this article’s Online Repository at www.jacionline.org). Subjects of the SA population with a valid set of diary entries during the peak of the GPS in treatment years 1 and 2 were defined as full analysis set (FAS) 1 (n = 146) and FAS2 (n = 138), respectively (see Fig E1). Efficacy was evaluated in the FAS (Fig 2 and see Fig E1). A post hoc analysis was performed in a subset of the FAS population (FAS1*, n = 135; FAS2*, n = 129; see Fig E1), excluding subjects with grass pollen–specific IgE levels of less than 3.5 kUA/L at the time when treatment started (ie, visit 5) because these subjects had grass pollen–specific IgE levels of greater than the 3.5 kUA/L cutoff only in the 12 months before the study but not at visit 5. The sample size for the study was calculated by assuming a power of 80% and a 2-sided significance level of 2.5% to account for multiple testing of 6 elementary hypotheses within the prespecified closed testing procedure,19 as described below. However, the procedure needed at most only half from the 5% 2-sided significance level (ie, 2.5%) for controlling the type I error for multiple testing in the strong sense for a test of an elementary hypothesis and for a test of a seventh null hypothesis (ie, the intersection of the first and second elementary null hypothesis) within the second year. Assumptions regarding effect size and variances were based on data from a previous subcutaneous immunotherapy study20 with a recombinant birch pollen allergen and a similar symptom and medication scoring system as in the present study, with the exemption that the SS and MS were evaluated separately. For the purpose of sample size estimation, the sum of the SS and MS, as well as the related pooled SDs, were calculated, resulting in a reduction by 54% (placebo, 9.8; verum, 4.5; pooled σ, 5.3) in year 1 and by 47% (placebo, 6.6; verum, 3.5; pooled σ, 4.1) in year 2. The parameters of year 2 (which led to the more conservative sample size), together with a total dropout rate of 25%, were assumed for the present study and resulted in a required sample size of 60 per treatment group (180 patients in total). Evaluation of the primary end point, key secondary end points, and well-being by using the VAS was performed by using ANOVAs with the factors treatment, allergy severity (moderate/severe), and study center as covariates. For the primary end point (SMS), a closed testing procedure was used to adjust for multiplicity when testing differences between the 3 treatment groups (low, high, and placebo) at 2 years (years 1 and 2). Therefore the closed testing procedure extends the testing strategy of Bonferroni adjustment for 2 years and hierarchically compares treatment groups within a year. The hierarchical preferences were first priority of high versus placebo, second priority of low versus placebo, and third priority of high versus low in year 1 and first priority of pooled high-low/low-low versus placebo, second priority of high-low versus placebo and low-low versus placebo, and third priority of high-low versus low-low in year 2. Absolute differences to placebo were calculated based on least-squares (LS) means (ANOVA). Percentage differences between treatment groups were computed based on ratio of geometric means. For all other efficacy end points, descriptive summary statistics were used (eg, mean, mean difference from placebo, and 95% CI for mean difference from placebo), and the Wilcoxon 2-sample test was used to compare the groups of BM32- and placebo-treated subjects. A first or second priority test in a given year had also priority before a third priority test in the other year. Specific futility boundaries and stopping rules were defined for the prespecified interim analysis (details are provided in this article’s Online Repository at www.jacionline.org).
The software used for statistical analysis was SAS (version 9.3; SAS Institute, Cary, NC). The statistical analysis plan is provided as supplementary information in this article’s Online Repository at www.jacionline.org. The post hoc analysis of the FAS1* and FAS2* subpopulations was done with GraphPad Prism software (version 6.00; GraphPad Software, La Jolla, Calif) by using an unpaired t test to compare the groups. Welch correction was applied in cases of unequal variances.
Assessment of safety
The major safety end point was the frequency of adverse events (AEs) with regard to the occurrence, seriousness, intensity, and relatedness to the study drug. AEs known to be related to specific immunotherapy (SIT) were analyzed separately, grouped into local and systemic reactions, and graded according to the grading system suggested by the EAACI and the World Allergy Organization.17,21 Further safety variables included vital signs, physical examinations, and safety laboratory assessments (see the Study Protocol in this article’s Online Repository).
Safety end points were evaluated in the SA set, which includes all subjects who have been randomized and received at least 1 injection. All safety data obtained in this study were presented with descriptive statistics. Comparisons between the treatment groups were done with the χ2 or Fisher exact tests.
Assessment of immunologic parameters
Blood samples were drawn at a number of time points throughout the study (Fig 1) and analyzed for changes in levels of IgE and IgG (IgG1 and IgG4) against the grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6 and measured as described by Zieglmayer et al.14 Immunologic end points were evaluated in the SA set. Changes in antibody levels were compared between treatment groups by using the Wilcoxon 2-sample test and the Wilcoxon signed-rank test for comparisons between visits within treatment groups.
Results
Preseasonal treatment with 3 injections of BM32 improves the SMS during the GPS
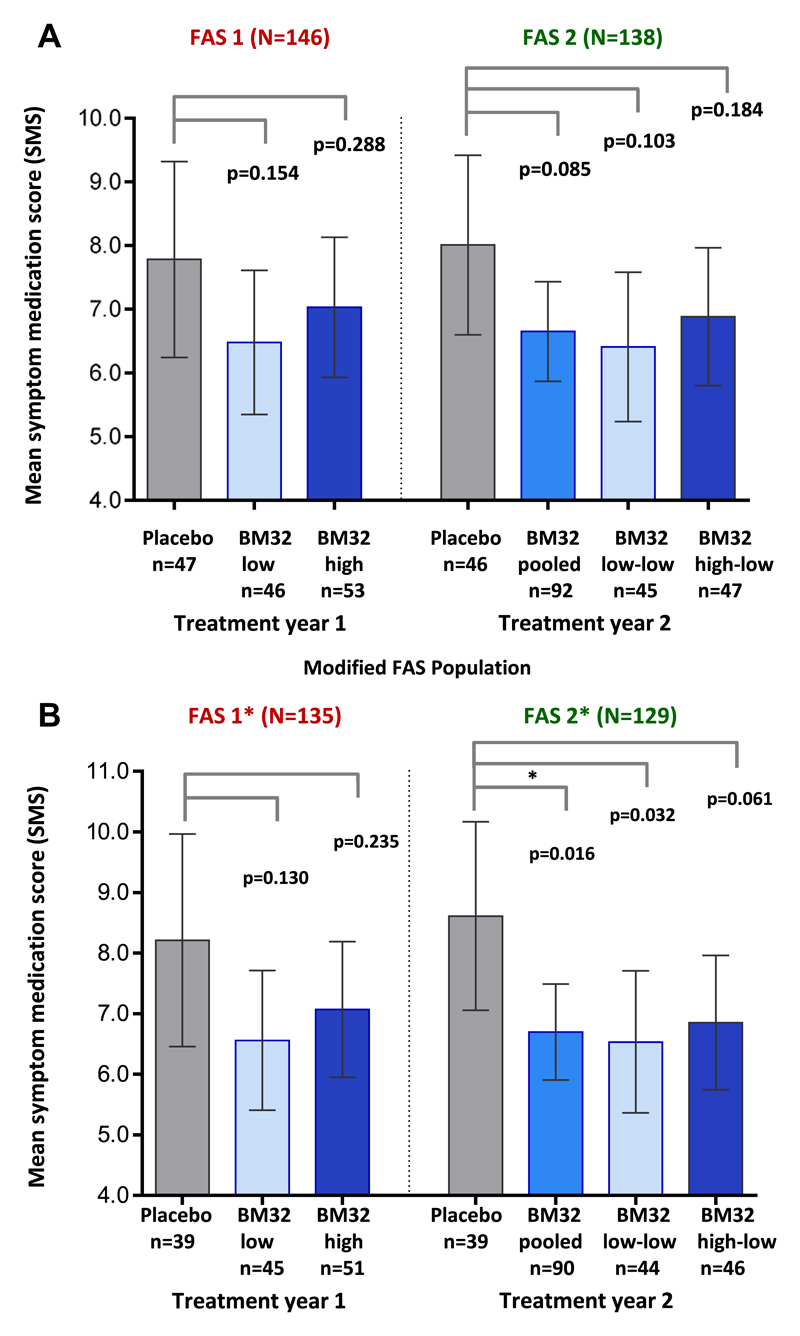
Mean SMSs observed in the different study groups during the peak pollen season of years 1 and 2 with P values for the difference in LS means between the groups are shown in Fig 3, A. A reduction of the mean SMS during the pollen peak season of 17.9% (95% CI, 41.2% to −14.7%; P = .245) and 10.2% (95% CI, 34.5% to −23.1%; P = .500) compared with the placebo group was observed for the BM32 low-dose and BM32 high-dose group in year 1, respectively. The reduction in SMS was more pronounced in year 2, reaching 22.0% (95% CI, 39.8% to −0.98%; P = .059) for the BM32 pooled group, although still missing statistical significance in the primary end point (P = .085 for difference in LS means between the BM32 pooled group and the placebo group). The 2 subsets of the BM32 pooled group consisted of subjects having received either BM32 low dose or BM32 high dose in treatment year 1 were evaluated separately. These subgroups are referred to as “BM32 low-low” and “BM32 high-low,” respectively. The reduction in the SMS compared with placebo was 24.8% (95% CI, 43.5% to −0.21%; P = .052) for the BM32 low-low group and 19.3% (95% CI, 40.0% to −8.6%; P = .155) for the BM32 high-low group (ie, not significant).
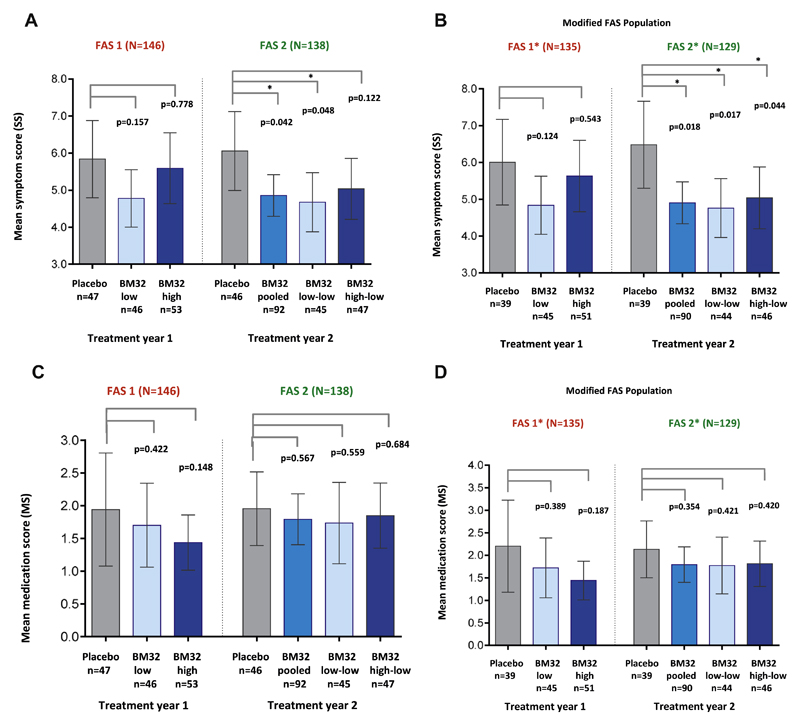
Fig 3.
Comparison of SMSs in treatment groups. Shown are mean SMSs with 95% CIs (y-axes) during the peak pollen seasons of treatment years 1 (placebo, BM32 low, and BM32 high groups) and 2 (placebo, BM32 pooled, BM32 low-low, and BM32 high-low groups) for the FAS population (A) and the modified FAS population (B), which excluded subjects with grass pollen–specific IgE levels of less than 3.5 kUA/L when treatment started. P values for differences of LS means between groups are indicated.
The analysis of the SS produced similar results as the SMS. In treatment year 1, the mean SS reduction compared with placebo was stronger in the BM32 low-dose than in the BM32 high-dose group and was more pronounced in year 2: BM32 pooled group, 24.5% (95% CI, 41.1% to 3,30%; P = .026); BM32 low-low group, 27.6% (95% CI, 45.3% to 4.13%; P = .025); and BM32 high-low group, 21.4% (95% CI, 40.4% to −3.66%; P = .087 [not significant]). Mean SSs and P values for differences in LS means between the groups are shown in Fig E2, A, in this article’s Online Repository at www.jacionline.org.
The MS was reduced in all actively treated subjects compared with the placebo group in treatment years 1 and 2 (see Fig E2, C).
Improvement of the SMS is best in subjects with high levels of grass pollen–specific IgE
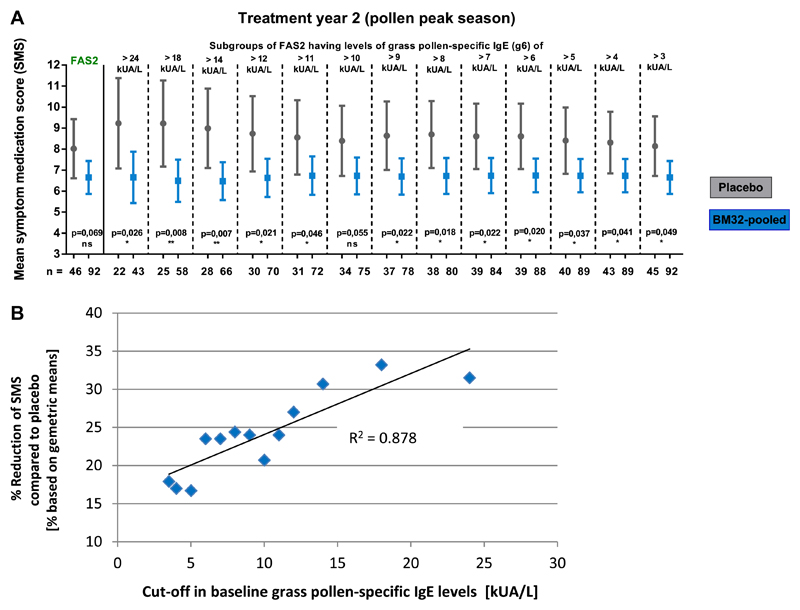
A post hoc analysis for exploratory purposes showed that 11 of the 146 FAS1 subjects and 9 of the 138 FAS2 subjects (see Fig E1) had timothy grass pollen allergen–specific IgE levels at visit 5 of less than 3.5 kUA/L (Table I). Also, these subjects had low skin sensitivity to grass pollen allergens at visit 5 (data not shown). A grass pollen allergen–specific IgE level of greater than 3.5 kUA/L was defined as an inclusion criterion for the screening visit (see Table E1). When these subjects were excluded (ie, FAS*), we found a more pronounced reduction of the mean SMS compared with placebo in all groups. In year 2 the BM32 pooled and low-low groups were reduced by 24.4% and 25.2%, with nominal P values for the difference in LS means of 0.016 and 0.036, respectively. Mean SMSs for all groups in both treatment years and nominal P values for differences in LS means between the groups are shown in Fig 3, B.
Therefore we analyzed whether the grass pollen allergen–specific IgE levels at screening and clinical treatment effects are associated. Fig E3 in this article’s Online Repository at www.jacionline.org shows that the reduction in SMSs increases with grass pollen allergen–specific IgE levels and that the reduction in SMSs compared with placebo correlated highly (R2 = 0.878) with grass pollen allergen–specific IgE levels.
Also for the mean SS and MS, the improvement was more pronounced in the FAS* populations after removal of subjects with timothy grass pollen–specific IgE levels of less than 3.5 kUA/L, reaching a 28.8% reduction of the SS to placebo (P = .017 for the difference in LS means) for the low-low group and a reduction of 26.7% (P = .018 for difference in LS means) of the pooled group in year 2 (see Fig E2, B and D).
Preseasonal treatment with BM32 improves well-being and rhinoconjunctivitis-related quality of life during the GPS
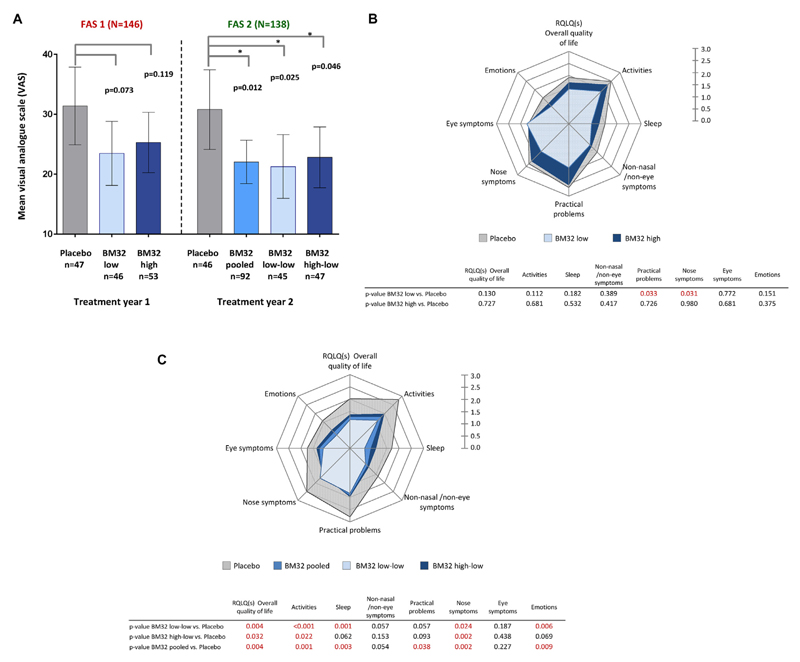
Assessment of the level of well-being during the peak pollen season by using a VAS showed that well-being in the first year was 19.5% better in the BM32 high-dose group compared with placebo and 25.2% better in the BM32 low-dose group than in the placebo group (ie, not significant). In treatment year 2, the difference in well-being between the actively treated and placebo groups was increased further (BM32 pooled, 28.6%; BM32 low-low, 31.2%; BM32 high-low, 26%; P < .05 for differences in LS means; Fig 4, A).
Fig 4.
Well-being measured by using the VAS and RQLQ in treatment groups. A, Shown are mean VAS scores with 95% CIs (y-axes) during the peak pollen seasons of treatment years 1 (placebo, BM32 low, and BM32 high group) and 2 (placebo, BM32 pooled, BM32 low-low, and BM32 high-low groups) for the FAS. P values for differences in LS means between groups are indicated. B and C, Rhinoconjunctivitis-related quality of life parameters recorded for the indicated groups during the pollen peak seasons during year 1 (Fig 4, B) and year 2 (Fig 4, C) are displayed as mean scores in spider plot diagrams. P values for differences in LS means between groups are listed underneath the spider plots for each of the parameters.
Likewise, we found an improvement regarding RQLQ parameters in year 1 (pollen peak season) for the actively treated subjects, as shown in the spider plot diagram (Fig 4, B). In year 2 we observed a consistent and strong improvement regarding all RQLQ parameters for each of the actively treated groups compared with the placebo group, reaching small P values for most of the RQLQ parameters and for the RQLQ overall quality of life (Fig 4, C).
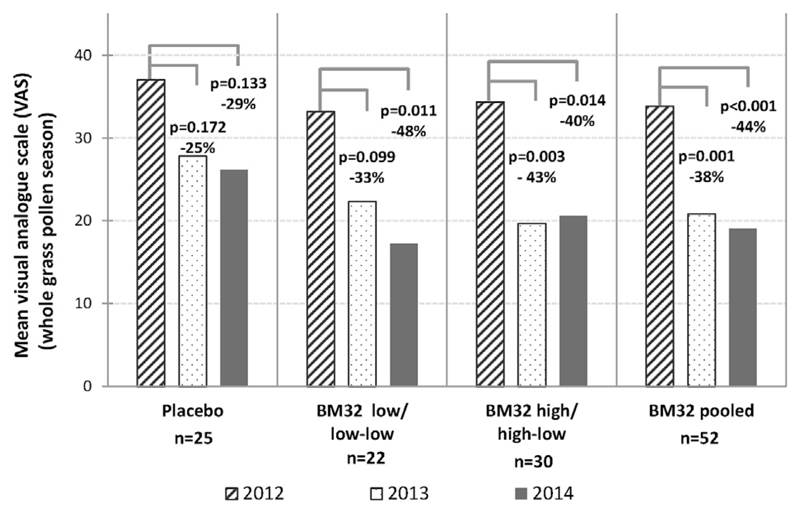
For patients who kept an electronic diary in the baseline year (2012), we compared the improvement of well-being determined by using a VAS in the subsequent 2 treatment years with the baseline year (see Fig E4 in this article’s Online Repository at www.jacionline.org). We noted an improvement in well-being also in the placebo group in treatment years 1 and 2. An at least 40% improvement in well-being in all actively treated groups (BM32 low-low, BM32 high-low, and BM32 pooled) was found in year 2 compared with the baseline year (see Fig E4).
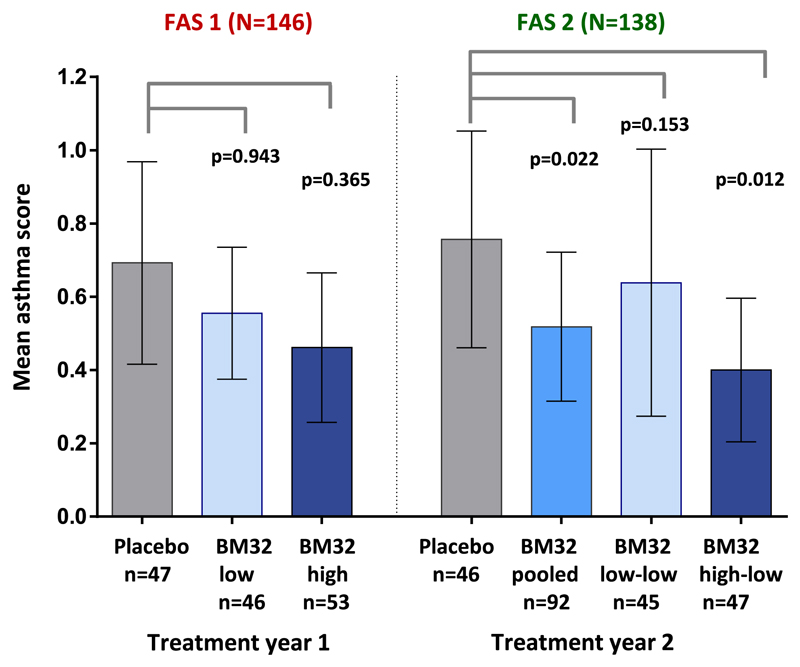
Treatment with BM32 improves grass pollen–related asthma symptoms
The study population included also subjects with grass pollen allergy with a history of grass pollen–associated asthma (BM32 low-dose group, n = 20; BM32 high-dose group, n = 12; placebo group, n = 14; Table I). Therefore we also analyzed the effects of BM32 AIT on asthma symptoms. The post hoc analysis of the asthma SS showed that there was a 24.3% and 39.2% reduction in the asthma SS in the BM32 low-dose and BM32 high-dose groups, respectively, when compared with the placebo group in treatment year 1 (see Fig E5 in this article’s Online Repository at www.jacionline.org; not significant). In treatment year 2, the reduction in asthma SS compared with the placebo group in the BM32 pooled and the BM32 high-low group was 31.9% (P = .022 for difference in LS means) and 48.7% (P = .012 for difference in LS means), respectively. In the BM32 low-low group the reduction in asthma SS in year 2 was 17.1% (P = .153 for difference in LS means; not significant). Mean asthma scores for all groups in both years and P values for differences in LS means are shown in Fig E5.
Vaccination with BM32 induces significant increases in allergen-specific IgG, IgG1, and IgG4 responses
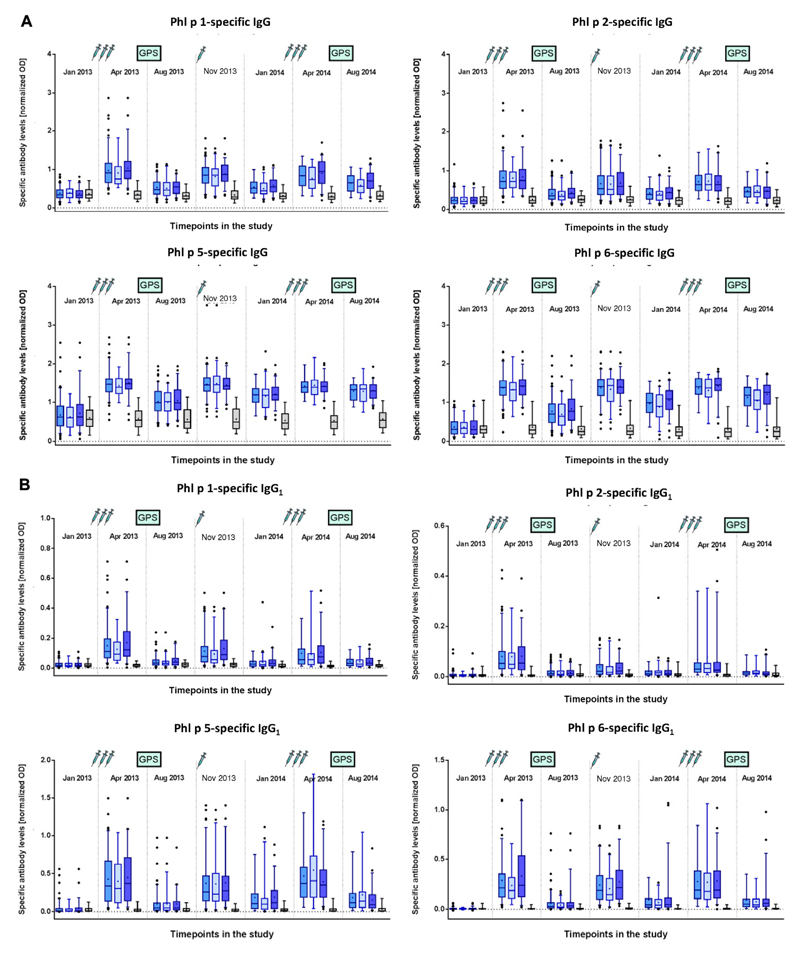
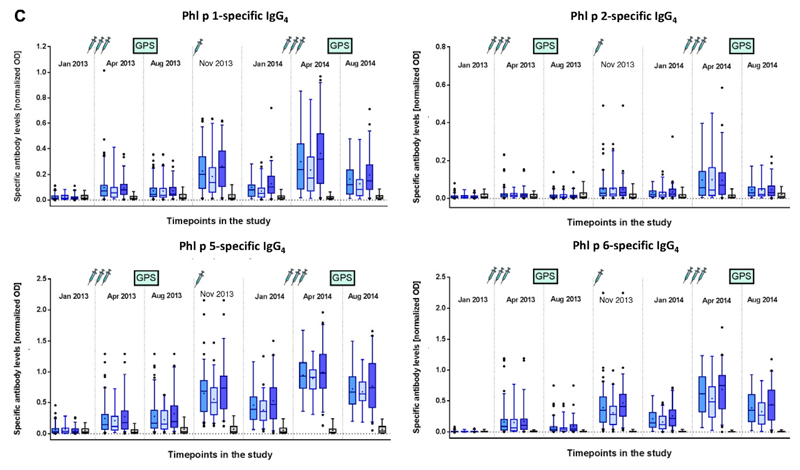
We found that the first course of 3 subcutaneous injections of BM32 given in monthly intervals induced strong increases in levels of IgG antibodies specific for the 4 major grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6, whereas no such increases were found in the placebo-treated subjects (Fig 5, A). No relevant difference regarding induction of allergen-specific IgG levels was noted when comparing the BM32 low-dose and BM32 high-dose groups. Allergen-specific IgG levels decreased between visit 8 (April 2013) and visit 9 (August 2013) but did not completely return to baseline levels (Fig 5, A). One injection in October 2013 (visit 10) was sufficient to boost allergen-specific IgG levels up to those induced by the preseasonal course of 3 injections. A decrease in allergen-specific IgG levels was noted also between visit 10 (October 2013) and visit 12 (January 2014), which could be boosted again by the 3 preseasonal injections and remained at higher levels after the second GPS in August 2014 compared with treatment after the season in year 1 (Fig 5, A). When we analyzed allergen-specific IgG1 and IgG4 subclass responses, we noted an interesting difference between allergen-specific IgG1 and IgG4 responses (Fig 5, B and C). Allergen-specific IgG1 responses increased after each course of injections to a similar level in year 1 as in year 2 and after the injections decreased always to similar levels (Fig 5, B). By contrast, allergen-specific IgG4 levels continued to increase after each of the booster injections so that levels obtained after the boost in October 2013 were greater than after the first course of injections in April 2013, and levels obtained after the third course of injections in April 2014 were greater than after the October booster injection (Fig 5, C). Thus it seemed that continuous treatment with BM32 builds up a continuously increasing allergen-specific IgG4 response.
Fig 5.
Development of allergen-specific IgG and IgG subclass responses. Shown are mean IgG (A), IgG1 (B), and IgG4 (C) levels (y-axes, OD values) specific for the 4 major grass pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Phl p 6) for the different treatment groups (left to right: BM32 pooled, BM32 low/low-low, BM32 high/high-low, and placebo groups) for 7 time points during the study. Injections and GPSs are indicated.
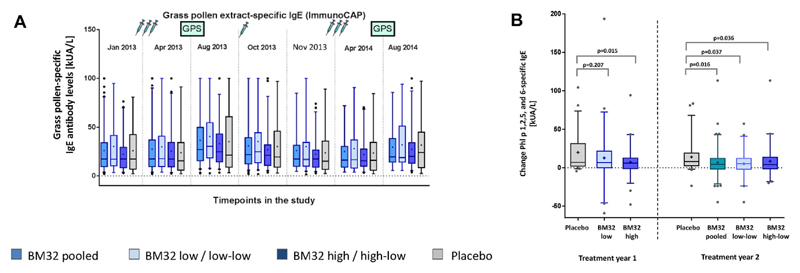
Vaccination with BM32 does not induce increases in grass pollen allergen–specific IgE levels and prevents seasonally induced boosts of grass pollen–specific IgE production
We also studied the effects of vaccination with BM32 on grass pollen allergen–specific IgE levels. Fig 6, A, shows that vaccination with both low-dose and high-dose BM32 did not induce relevant increases of grass pollen allergen–specific IgE levels. No relevant changes were observed after each of the 3 injection courses (visit 5, January 2013 vs visit 8, April 2013; visit 9, August 2013 vs visit 11, November 2013; and visit 12, January 2014 vs visit 15, April 2014). Increases in grass pollen allergen–specific IgE levels were noted in all groups only after seasonal grass pollen exposure in August 2013 and August 2014 (Fig 6, A). We compared the increases in grass pollen allergen–specific IgE levels after each of the 2 pollen seasons in the actively and placebo-treated patients (Fig 6, B) and found that in treatment year 2 the increases in the sum of Phl p 1–, 2–, 5–, and 6–specific IgE levels were lower in all actively treated groups compared with the placebo group (BM32 pooled: P = .016; BM32 low-low: P = .037; and BM32 high-low: P = .036; Fig 6, B). In treatment year 1, the increase in the mean allergen-specific IgE level in the verum groups was also lower than in the placebo-treated subjects, with a reduction in the BM32 high-dose group (P = .015; Fig 6, B).
Fig 6.
Effects of immunotherapy with BM32 on grass pollen allergen–specific IgE levels. A, Mean timothy grass pollen allergen–specific IgE levels (y-axes, kUA/L) for the different treatment groups (left to right: BM32 pooled, BM32 low/low-low, BM32 high/high-low, and placebo groups) for 7 time points during the study. Injections and GPSs are indicated. B, Changes in mean levels of IgE specific for the 4 major grass pollen allergens (y-axes: kUA/L) between visits 8 and 9 (year 1) and visits 15 and 16 (year 2) for the different treatment groups (year 1: placebo, BM32 low, and BM32 high groups; year 2: placebo, BM32 pooled, BM32 low-low, and BM32 high-low groups). P values indicate differences between the placebo and actively treated groups.
Treatment with BM32 is safe and well tolerated
Treatment with BM32 was safe and well tolerated. No adrenaline was needed during the complete study. The majority of side effects were late-phase local reactions (Table II and see Table E3 in this article’s Online Repository at www.jacionline.org). Tables II and III represent a summary of the treatment-associated AEs (Table II) and the grading of systemic AEs (Table III). Systemic AEs are further specified according to MeDRA terms in Table E3. Local reactions were late-phase reactions, which were more frequent and more intense in actively treated subjects than in placebo-treated subjects (Table II). Systemic reactions were late-phase reactions. They were more frequent in the first treatment year and more frequent in the BM32 low-dose (ie, 23 events) and BM32 high-dose (ie, 18 events) groups than in the placebo group (ie, 6 events). According to the EAACI grading, the systemic reactions observed in year 1 were graded 0 to 2, whereas no grade 3 or grade 4 reactions were observed (Table II). Two severe AEs were noted in the first year. In 1 subject a chronic inflammatory central nervous system disease was diagnosed after the patient reported paraesthesia, but this patient had reported neurologic problems already before the study. A second subject who was also allergic to birch pollen and associated pathogenesis-related protein 10 allergen–containing food experienced an episode of angioedema several hours after treatment and after consumption of a nut-containing cereal bar.
Table II. Overview of the number of local and systemic reactions during treatment with BM32.
| 2013 (year 1) | 2014 (year 2) | |||||
|---|---|---|---|---|---|---|
| BM32 low (n = 53) | BM32 high (n = 60) | Placebo (n = 53) | BM32 pooled (n = 113) | Placebo (n = 53) | ||
| No. of injections | 194 | 220 | 197 | 309 | 147 | |
| No. of severe AEs (%) | 0 | 2 (3.3%) | 0 | 1 (1.0%) | 0 | |
| No. of systemic reactions | 23 (28.3%) | 18 (21.7%) | 6 (9.4%) | 6 (4.4%) | 3 (5.6%) | |
| No. of local reactions | 313 (88.7%) | 417 (86.7%) | 197 (73.6%) | 393 (55.8%) | 124 (52.8%) | |
Numbers in parentheses indicate the percentage of subjects within the respective group in whom an event occurred.
Table III. Grading of systemic reactions according to the EAACI grading system.
| 2013 (year 1) | 2014 (year 2) | |||||
|---|---|---|---|---|---|---|
| BM32 low (n = 53) | BM32 high (n = 60) | Placebo (n = 53) | BM32 pooled (n = 113) | Placebo (n = 53) | ||
| Grade 0 | 7 (5.7%) | 2 (3.3%) | 3 (5.7%) | 1 (0.9%) | 2 (3.8%) | |
| Grade 1 | 13 (17.0%) | 11 (13.3%) | 2 (1.9%) | 4 (3.5%) | 1 (1.9%) | |
| Grade 2 | 3 (5.7%) | 5 (5.0%) | 1 (1.9%) | 0 | 0 | |
| Grade 3 | 0 | 0 | 0 | 1 (0.9%) | 0 | |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | |
Numbers in parentheses indicate the percentage of subjects within the respective group in whom an event occurred. Not graded refers to reactions that are not typical for allergic reactions.
In treatment year 2, only a few systemic side effects were observed, and they were equally frequent in the active and placebo groups (BM32 pooled group, 6 events/113 subjects; placebo group, 3 events/53 subjects). All but 1 of these reactions were late-phase reactions of grade 0 or 1 according to the EAACI classification. One severe AE was observed in 1 patient of the BM32 low-low group who experienced an immediate flush after injection associated with an increase in blood pressure, which then returned to a normal levels. After consultation with 2 independent international experts, this reaction was classified as a grade 3 reaction.
Discussion
We report the first multicenter, double-blind, placebo-controlled phase IIb field study conducted with the recombinant B cell epitope–containing grass pollen allergy vaccine BM32 in patients with grass pollen allergy for a period of 2 years. BM32 is different from other recombinant and synthetic allergy vaccines being evaluated in clinical trials. It consists of nonallergenic peptides of the 4 major grass pollen allergens Phl p 1, Phl p 2, Phl p 5, and Phl p 6, which are expressed as fusion proteins attached to the hepatitis B–derived preS protein.8,14,22 Whereas previously described recombinant hypoallergenic vaccines, such as rBet v 1 fragments,23 rBet v 1 trimer,24 a Bet v 1 folding variant,25 and contiguous Bet v 1–derived overlapping synthetic peptides,26 as well as synthetic peptides derived from the major cat allergen Fel d 1,27 contain allergen-specific T-cell epitopes, the presence of allergen-specific T-cell epitopes in BM32 has been reduced, and hepatitis B–derived preS is used as an immunologic carrier protein to induce allergen-specific blocking IgG antibody responses.8 We found that only 3 injections of BM32 given before the pollen season reduced SMSs, SSs, and MSs (not significant) compared with placebo treatment in the first year, and this effect was much more pronounced in the second year. Although we failed to reach the primary end point (ie, daily combined SMS calculated for the peak of the GPS in treatment years 1 and 2), in the FAS population our study shows that treatment with BM32 improves symptoms of grass pollen allergy. Of note, in addition to the improvement in SMSs, SSs, and MSs, we noted that patients also substantially improved regarding VAS and RQLQ scores.
After a blinded interim analysis conducted at the end of the first treatment year, an IDMC suggested to continue the study and to switch the 40-μg dose (ie, 160 μg of the 4 BM32 components) to 20 μg (ie, 80 μg of the 4 BM32 components) to increase the power of the study. In fact, it also appeared that the improvement in SMSs was lower at the 40-μg dose. The latter can be explained by the fact that low-dose antigen favors antibody affinity maturation.28–30
In the second year we observed a substantial improvement in the actively treated patients over placebo-treated patients. The improvement in symptoms in the second treatment year was accompanied by a continuous increase in levels of allergen-specific IgG4 antibodies, as was also observed for allergen extract–based subcutaneous forms of AIT earlier.30,31 We observed that the allergen-specific IgG antibodies induced by BM32 were strongly boosted already by 1 booster injection administered in autumn after the pollen season and further increased after another 3 injections given monthly before the pollen season of treatment year 2. This study also highlighted another possible advantage of BM32 because unlike other forms of AIT (eg, sublingual immunotherapy), which induce only low levels of allergen-specific IgG antibodies but high boosts of allergen-specific IgE levels,32 treatment with BM32 did not increase grass pollen allergen–specific IgE production.
There are several possible reasons for the observed preferential IgG induction. First, preS, the carrier protein, does not induce TH2 immune responses but rather TH1 responses.33 Second, the vaccine did not activate or only mildly activated grass pollen allergen–specific TH2 responses. Third, removal of large parts of the grass pollen allergen sequences from the vaccine makes it less allergenic compared with the full allergen. Fourth, because of the low allergenic activity of BM32, high doses of the vaccine can be injected. High-dose immunization is known to favor IgG induction, whereas low-dose immunization favors IgE induction.34 Moreover, we noted that treatment with BM32 reduced significantly the boosts of allergen-specific IgE production caused by seasonal allergen exposure (ie, blunting effect) similar to what has been noted for allergen extract–based vaccines35 and other recombinant and synthetic vaccines.10,36 A gradual decrease in allergen-specific IgE levels caused by such a mechanism might likely be one of the responsible mechanisms underlying the long-term effect of AIT after discontinuation of treatment. Therefore we plan to investigate the long-term effects of BM32 in follow-up studies.
Major advantages of BM32 are that only a few injections were needed to achieve clinical improvement, whereas other forms of AIT require daily administrations (eg, sublingual immunotherapy) or multiple injections (ie, allergen extract–based subcutaneous AIT).7 Another major advantage of BM32 is that the vaccine was very well tolerated by the patients, although extremely high doses (ie, 80 or 160 μg) were administered without any updosing.14
In a recently published phase IIb field study conducted with contiguous overlapping synthetic peptides of the major birch pollen allergen Bet v 1 in which 50 and 100 μg was injected, systemic side effects were observed in more than 64% of the actively treated subjects,13 whereas in our study 22% of the patients had systemic side effects in year 1 and only 5% in year 2. In the study with synthetic Bet v 1 peptides, 2 patients required systemic epinephrine, whereas in our study systemic side effects were mild, and no epinephrine was required. Because of the excellent safety profile and due to the fact that BM32 did not boost allergen-specific IgE responses, the vaccine might be well suited for therapy of children. AIT in children is indeed an important future research need because it becomes increasingly clear that early intervention can prevent allergic sensitization and/or progression of IgE sensitization to symptomatic disease and then to severe disease manifestations.37–39
Of note, patients with grass pollen–induced asthma tolerated the BM32 vaccine very well and showed an improvement in asthma symptoms. Therefore BM32 can be considered also for the treatment of patients with grass pollen–induced asthma.
Another unexpected advantage of BM32 was that it induced not only allergen-specific IgG antibodies but also antibodies specific for the hepatitis B surface antigen preS, which were found to inhibit in vitro infection of liver cells by HBV.22 This was the first demonstration that vaccination with preS alone without S antigen can induce HBV-specific antibody responses in human subjects. Therefore it is possible that BM32-vaccinated subjects are also protected against HBV infections.40
Our study also has also limitations, such as low pollen exposure in certain study centers, leading to the exclusion of patients from the evaluation in line with predefined thresholds for pollen exposure, which affected the power of the study negatively (Table E2). Nevertheless, our study shows that AIT with BM32 improves symptoms of grass pollen allergy, is convenient in application, and is very well tolerated so that it will undergo phase III evaluation as a next step. The fact that BM32 does not boost allergen-specific IgE production indicates also that BM32 can be used in preventive vaccination studies to investigate whether the vaccine can be used to prevent the progression from rhinitis to asthma or eventually the progression from sensitization to allergic symptoms or even for prophylactic vaccination.
Extended Data
Fig E1.
Definition of analyzed subpopulations. Venn diagram showing the SA population, as well as the FAS1 and FAS2 populations, for treatment years 1 and 2, respectively (upper part). The table below defines the populations and shows subject numbers.
Fig E2.
Comparison of SSs and MSs in treatment groups. A and B, Mean SSs with 95% CIs (y-axes) during the peak pollen seasons of treatment years 1 (placebo, BM32 low, and BM32 high groups) and 2 (placebo, BM32 pooled, BM32 low-low, and BM32 high-low groups) for the FAS (Fig E2, A) and modified FAS (Fig E2, B) populations, which excluded subjects with grass pollen–specific IgE levels of less than 3.5 kUA/L when treatment started. C and D, Mean MSs with 95% CIs for the FAS (Fig E2, C) and modified FAS (Fig E2, D) populations are displayed in the same way. P values for differences of the LS means between groups are indicated.
Fig E3.
Association of grass pollen allergen–specific IgE levels at screening and treatment effects in years 2 and 1. A, Comparison of mean SMSs (y-axis, whiskers represent 5% to 95% of the CI) of FAS2 subjects (year 2) from the placebo group (gray symbols) and pooled treatment groups (blue symbols) and of subgroups thereof with different grass pollen allergen–specific IgE levels. B, Correlation between mean reductions in SMS (placebo vs treatment groups) and grass pollen allergen–specific IgE baseline levels (year 2).
Fig E4.
Changes of well-being as measured by using the VAS in the 2 treatment years compared with baseline values. Comparison of mean levels of well-being (y-axis) during the whole GPS at baseline (2012) with the 2 treatment years (2013 and 2014) in subjects with available baseline data from different groups (placebo, BM32 low/low-low, BM32 high/high-low, and BM32-pooled groups). P values denote differences of LS means between 2012 and the 2 treatment years.
Fig E5.
Comparison of asthma scores in treatment groups. Shown are mean asthma scores with 95% CIs (y-axes) during the peak pollen seasons of treatment years 1 (placebo, BM32 low-dose, and BM32 high-dose groups) and 2 (placebo, BM32 pooled, BM32 low-low, and BM32 high-low groups) for the FAS population. P values for comparisons of the placebo group with treatment groups are indicated.
Table E1. List of inclusion and exclusion criteria (as defined in the study protocol).
| Inclusion criteria | |
| 1 | Positive history of grass pollen allergy, positive SPT response to grass pollen extract, grass pollen allergen–specific IgE and rPhl p 1/rPhl p 5–specific IgE (≥3.5 kUA/L) at the screening visit or within 12 months before the screening visit |
| 2 | Moderate-to-severe symptoms of grass pollen allergy during peak pollen season in the baseline period (exact definition of this criterion is specified in the study reference manual [SRM]) |
| 3 | Age between 18 and 60 years (male/female) |
| 4 | Subjects must have standard health insurance |
| 5 | Subject must appear capable of understanding and complying with all relevant aspects of the study protocol |
| 6 | Subject must be available during the study period to complete all treatments and assessments |
| Exclusion criteria | |
| 1 | Symptomatic perennial allergies or symptomatic seasonal coallergies during the GPS |
| 2 | Atopic dermatitis |
| 3 | Pregnancy or breast-feeding |
| 4 | Women with childbearing potential who are not using a medically accepted birth control method |
| 5 | Autoimmune diseases and immune defects, including immunosuppression and immune complex–induced immunopathies |
| 6 | Contraindication for adrenaline |
| 7 | Severe general maladies, malignant diseases |
| 8 | Patients undergoing long-term treatment with systemic corticosteroids, immunosuppressive drugs, tranquilizers, or psychoactive drugs |
| 9 | Contraindications for SPTs, such as skin inflammation in the test area and urticaria facticia |
| 10 | Asthma not controlled by low-dose inhaled corticosteroids, meaning that patients with a history of concomitant asthma should have an FEV1 of greater than 70% at inclusion; patients without a history of asthma should have an FEV1 of grater then 70% or a peak expiratory flow of greater than 80% at inclusion |
| 11 | Chronic use of b-blockers |
| 12 | Participation in another clinical trial within 1 month before the study; however, participation during the previous month solely in the form of blood donation and/or without other interventions will be acceptable |
| 13 | Patients who participated in a pollen SIT trial or received marketed pollen SIT in 2 y before the study |
| 14 | Patients who had a previous grass pollen SIT or have participated in a clinical trial of grass pollen SIT |
| 15 | Risk of noncompliance with the study procedure and restrictions (eg with alcohol, drug, or medication abuse within the past year) |
| 16 | Use of prohibited medication before screening (visit 1) and throughout the study:
|
| 17 | Use of antihistamines 3 days before visits 1 or V2 |
| 18 | Patients with nasal polyposis |
| 19 | Patients sensitized to Phl p 7 (specific IgE to Phl p 7 and/or Bet v 4 >0.35 kUA/L) |
SIT, Specific immunotherapy.
Table E2. Pollen exposure during the peak grass pollen season in different study centers and numbers of evaluated subjects.
| Pollen exposure during grass pollen peak: Total cumulative pollen count (grains/m3/15 d]/no. of days >25 grains/m3/24 h [d]) | |||||
|---|---|---|---|---|---|
| Center no. | Center country | No. of subjects evaluated, FAS1/FAS2 | Screening, 2012 | Treatment, year 1, 2013 | Treatment, year 2, 2014 |
| 101 | Munich (Germany) | 6/6 | 566/10 | 756/13 | 798/12 |
| 102 | Berlin (Germany) | 21/18 | 635/11 | 1135/15 | 1219/12 |
| 103 | Wiesbaden (Germany) | 8/7 | NA* | 1441/13 | 1199/12 |
| 104 | Marburg (Germany) | 7/7 | 484/9 | 1495/14 | 830/13 |
| 105 | Bonn (Germany) | 3/3 | 778/9 | 1424/14 | 547/9 |
| 201 | Vienna (Austria) | 36/29 | 459/10 | 1172/14 | 707/9 |
| 202 | Graz (Austria) | 13 (0)†/12 | 327/5 | 399/6 | 514/10 |
| 301 | Ghent (Belgium) | 27/22 | 1211/14 | 1507/15 | 970/13 |
| 401 | Copenhagen (Denmark) | 13/11 | 1097/13 | 1055/15 | 1886/15 |
| 501 | Rotterdam (The Netherlands) | 2/2 | 754/11 | 937/11 | 848/11 |
| 601 | Golnik (Slovenia) | 23/22 | 674/14 | 1842/15 | 932/14 |
No pollen data from 2012 are available.
Center not evaluated in year 1 because of insufficient pollen exposure during the GPS.
Table E3. Detailed listing of systemic side effects.
| Treatment year 1: no. of subjects (%)/no. of events | |||
| System organ class | BM32 low (n = 53) | BM32 high (n = 60) | Placebo (n = 53) |
| Grade 0 | |||
| General disorders | 0 | 0 | 1 (1.9)/1 |
| Influenza-like illness | 0 | 0 | 1 (1.9)/1 |
| Skin and subcutaneous tissue disorder | 1 (1.9)/2 | 1 (1.7)/1 | 2 (3.8)/2 |
| Urticaria | 1 (1.9)/1 | 0 | 1 (1.9)/1 |
| Erythema | 1 (1.9)/1 | 0 | 0 |
| Pruritus, generalized | 0 | 0 | 1 (1.9)/1 |
| Rash, musculopapular | 0 | 1 (1.7)/1 | 0 |
| Psychiatric disorders | 1 (1.9)/2 | 1 (1.7)/1 | 0 |
| Insomnia | 0 | 1 (1.7)/1 | 0 |
| Restlessness | 1 (1.9)/1 | 0 | 0 |
| Nervous system disorders | 1 (1.9)/3 | 0 | 0 |
| Dysgeusia | 1 (1.9)/3 | 0 | 0 |
| Grade 1 | |||
| Skin and subcutaneous disorders | 3 (5.7)/5 | 3 (5.0)/3 | 0 |
| Rash | 2 (3.8)/2 | 0 | 0 |
| Urticaria | 1 (1.9)/1 | 1 (1.7)/1 | 0 |
| Cold sweat | 1 (1.9)/1 | 0 | 0 |
| Pruritus | 0 | 1 (1.7)/1 | 0 |
| Pruritus, generalized | 0 | 1 (1.7)/1 | 0 |
| Rash, generalized | 1 (1.9)/1 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 2 (3.8)/3 | 2 (3.3)/4 | 0 |
| Asthma | 1 (1.9)/1 | 0 | 0 |
| Nasal congestion | 0 | 1 (1.7)/1 | 0 |
| Nasal obstruction | 0 | 1 (1.7)/1 | 0 |
| Rhinorrhea | 1 (1.9)/1 | 0 | 0 |
| Throat irrigation | 0 | 1 (1.7)/1 | 0 |
| Throat tightness | 0 | 1 (1.7)/1 | 0 |
| General disorders and administration site conditions | 2 (3.8)/3 | 0 | 1 (1.9)/2 |
| Chills | 1 (1.9)/1 | 0 | 0 |
| Fatigue | 0 | 0 | 1 (1.9)/1 |
| Injections-site paresthesia | 1 (1.9)/1 | 0 | 0 |
| Pyrexia | 0 | 0 | 1 (1.9)/1 |
| Sense of oppression | 1 (1.9)/1 | 0 | 0 |
| Infections and infestations | 1 (1.9)/1 | 1 (1.7)/1 | 0 |
| Rhinitis | 1 (1.9)/1 | 1 (1.7)/1 | 0 |
| Ear and labyrinth disorders | 0 | 1 (1.7)/1 | 0 |
| Ear pruritus | 0 | 1 (1.7)/1 | 0 |
| Eye disorders | 0 | 1 (1.7)/2 | 0 |
| Eye pruritus | 0 | 1 (1.7)/2 | 0 |
| Gastrointestinal disorders | 1 (1.9)/1 | 0 | 0 |
| Oral pruritus | 1 (1.9)/1 | 0 | 0 |
| Grade 2 | |||
| Skin and subcutaneous tissue disorders | 1 (1.9)/1 | 2 (3.3)/3 | 1 (1.9)/1 |
| Urticaria | 1 (1.9)/1 | 2 (3.3)/2 | 1 (1.9)/1 |
| Angioedema | 0 | 1 (1.7)/1 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 2 (3.8)/2 | 1 (1.7)/2 | 0 |
| Dyspnea | 2 (3.8)/2 | 0 | 0 |
| Throat irritation | 0 | 1 (1.7)/1 | 0 |
| Throat tightness | 0 | 1 (1.7)/1 | 0 |
| Grade 3 | |||
| No adverse events observed | |||
| Grade 4 | |||
| No adverse events observed | |||
| Treatment year 2: no. of subjects (%)/no. of events | |||
| BM32 pooled (n = 113) | Placebo (n = 53) | ||
| Grade 0 | |||
| Skin and subcutaneous tissue disorder | 0 | 1 (1.9)/1 | |
| Urticaria | 0 | 1 (1.9)/1 | |
| Respiratory, thoracic, and mediastinal disorders | 0 | 1 (1.9)/1 | |
| Throat irritation | 0 | 1 (1.9)/1 | |
| Nervous system disorders | 1 (0.9)/1 | 0 | |
| Dysgeusia | 1 (0.9)/1 | 0 | |
| Grade 1 | |||
| Skin and subcutaneous tissue disorders | 1 (0.9)/2 | 0 | |
| Eczema | 1 (0.9)/2 | 0 | |
| General disorders and administration site | 1 (0.9)/1 | 1 (1.9)/1 | |
| Fatigue | 0 | 1 (1.9)/1 | |
| Pyrexia | 1 (0.9)/1 | 0 | |
| Nervous system disorders | 1 (0.9)/1 | 0 | |
| Headache | 1 (0.9)/1 | 0 | |
| Grade 2 | |||
| No adverse events observed | |||
| Grade 3 | |||
| Immune system disorders | 1 (0.9)/1 | 0 | |
| Flush, increase in blood pressure | 1 (0.9)/1 | 0 | |
| Grade 4 | |||
| No AEs observed | |||
Different categories of systemic AEs are listed in the left column according to MedDRA. Numbers of observed AEs and numbers of subjects in whom these events occurred are listed for the BM32 low-dose, BM32 high-dose, and placebo groups for 2003 (treatment year 1) and for the pooled treatment groups (low-low and high-low), as well as the placebo group, for 2003 (treatment year 2).
Clinical implications.
This study shows that a recombinant B cell epitope–based grass pollen allergy vaccine requires only a few injections, is safe, and improves symptoms of grass pollen allergy.
Acknowledgments
Supported by Biomay AG, Vienna, Austria, and research grants F4605 and F4613 of the Austrian Science Fund (FWF). R.V. is recipient of a Megagrant of the Government of the Russian Federation, grant number 14.W03.31.0024.
Abbreviations used
- AE
Adverse event
- AIT
Allergen immunotherapy
- API
Active pharmaceutical ingredient
- AR/C
Allergic rhinoconjunctivitis
- EAACI
European Academy of Allergy and Clinical Immunology
- FAS
Full analysis set
- GPS
Grass pollen season
- HBV
Hepatitis B virus
- IDMC
Independent data monitoring committee
- LS
Least-squares
- MS
Medication score
- RQLQ
Rhinoconjunctivitis Quality of Life Questionnaire
- SA
Safety analysis
- SMS
Symptom medication score
- SPT
Skin prick test
- SS
Symptom score
- VAS
Visual analog scale
Footnotes
Disclosure of potential conflict of interest: A. Neubauer is employed by Biomay and is a minor stockholder. W. Aberer has received travel support from Biomay AG. O. Pfaar’s institution received grant funds from Biomay, and he has received consultant fees from HAL-Allergy Holding B.V./HAL-Allergie GmbH, Allergy Therapeutics/Bencard Allergie GmbH, Novartis Pharma, Laboratorios LETI/LETI Pharma, MEDA Pharma, ALK-Abelló, Anergis S.A., Biotech Tools S.A., Sanofi US Services, Mobile Chamber Experts (a GA2LEN partner), Pohl-Boskamp, Stallergenes-Greer, Lofarma, and Allergopharma; he has received grants from Stallergenes-Greer, HAL-Allergy Holding B.V./HAL-Allergie, Allergy Therapeutics/Bencard Allergie GmbH, Laboratorios LETI/LETI Pharma, and Anergis S.A.; his institution has received funds from these along with ALK-Abelló, Allergopharma, Lofarma, Nuvo, Circassia, and Biotech Tools S.A.; he has received lecture fees from HAL-Allergy Holding B.V./HAL-Allergie GmbH, Allergy Therapeutics/Bencard Allergie GmbH, Novartis Pharma, Laboratorios LETI/LETI Pharma, ALK-Abelló, Allergopharma, Lofarma, and StallergenesGreer; and he has received payment for educational presentations from Stallergenes-Greer. L. Klimek’s institution has received consultant fees from ALK-A-belló, Allergopharma, Bionorica, Boehringer Ingelheim, Lofarma, Novartis, MEDA Pharma, and GlaxoSmithKline; has grants with ALK-Abelló, Allergopharma, Bencard, Biomay, HAL, GlaxoSmithKline, LETI, Lofarma, Novartis, and Roxall; has received fees for lectures from ALK-Abelló, Allergopharma, Bionorica, Boehringer, GlaxoSmithKline, Lofarma, Novartis, and MEDA Pharma; receives fees for manuscript preparation from MEDA Pharma and Bionorica; and receives fees for Board membership from MEDA Pharma and Novartis. W. Pfützner and his institution have received clinical study fees from Biomay. He has received consultant fees from ALK-Abelló and lecture fees from ALK-Abelló and Novartis and has grants from ALK-Abelló and Biomay. U. Darsow’s institution has received funds as clinical study compensation. N. Novak’s institution contributed study patients and has received funding from ALK-Abelló; she received consultant fees from LETI Pharma, ALK-Abelló, and Stallergenes and for lectures from ALK-Abelló, LETI Pharma, Stallergenes, HAL Allergy, and Novartis. R. Gerth van Wijk’s institution has received funding for study participation and grant funds from Dutch Lung Foundation and STW, and he has received consultant and lecture fees from ALK-Abelló and Allergopharma and travel funding from the European Academy of Allergy and Clinical Immunology and UEMS. H.-H. Müller received fees from SynteractHCR and Deutschland GmbH for statistical analysis and his institution receives funding from St Jude Medical for statistical analysis not relevant to this work. J. Klinger’s institution has received fees for review activities from SynteractHCR. F. Stolz is employed by Biomay. N. Breit has received consulting fees and travel support from Biomay. R. Henning is employed by Biomay AG, holds stock, and has patents with the company. R. Valenta’s institution has grant funding from Biomay AG, Thermo Fisher, and Fresenius Medical Care, and he has received consultant fees from Biomay AG, Thermo Fisher, and Fresenius Medical Care. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 2.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 5.Jutel M, Agache I, Bonnini S, Burks AW, Calderon M, Canonica W, et al. International Consensus on Allergen Immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:356–68. doi: 10.1016/j.jaci.2015.12.1300. [DOI] [PubMed] [Google Scholar]
- 6.Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247–54. doi: 10.1097/MOO.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 7.Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351–7. doi: 10.1016/j.jaci.2015.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207–17. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101–3. doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101:14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 12.Spertini F, Perrin Y, Audran R, Pellaton C, Boudousquié C, Barbier N, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–40. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Spertini F, DellaCorte G, Kettner A, de Blay F, Jacobsen L, Jutel M, et al. Efficacy of 2 months of allergen-specific immunotherapy with Bet v 1-derived contiguous overlapping peptides in patients with allergic rhinoconjunctivitis: results of a phase IIb study. J Allergy Clin Immunol. 2016;138:162–8. doi: 10.1016/j.jaci.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, et al. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43–57. doi: 10.1016/j.ebiom.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Méchin H, Daures JP, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007;62:367–72. doi: 10.1111/j.1398-9995.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 16.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–67. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 17.Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317–24. doi: 10.1111/j.1398-9995.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999;104:364–9. doi: 10.1016/s0091-6749(99)70380-5. [DOI] [PubMed] [Google Scholar]
- 19.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–60. [Google Scholar]
- 20.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling H-J, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(suppl 82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x. [DOI] [PubMed] [Google Scholar]
- 22.Cornelius C, Schöoneweis K, Georgi F, Weber M, Niederberger V, Zieglmayer P, et al. Immunotherapy with the PreS-based grass pollen allergy vaccine BM32 induces antibody responses protecting against hepatitis B infection. EBioMedicine. 2016;11:58–67. doi: 10.1016/j.ebiom.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–81. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001;15:2045–7. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- 25.Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008;145:193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- 26.Pellaton C, Perrin Y, Boudousquié C, Barbier N, Wassenberg J, Corradin G, et al. Novel birch pollen specific immunotherapy formulation based on contiguous overlapping peptides. Clin Transl Allergy. 2013;3:17. doi: 10.1186/2045-7022-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidler P, Healey D, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Goidl EA, Paul WE, Siskind GW, Benacerraf B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J Immunol. 1968;100:371–5. [PubMed] [Google Scholar]
- 29.González-Fernández A, Milstein C. Low antigen dose favours selection of somatic mutants with hallmarks of antibody affinity maturation. Immunology. 1998;93:149–53. doi: 10.1046/j.1365-2567.1998.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–16. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 31.Shamji MH, Ljørring C, Francis JN, Calderon MA, Larché M, Kimber I, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–26. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 32.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 33.Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, et al. A nonallergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG toward IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013;190:3068–78. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constant SL, Bottomly K. Induction of Th1 and Th2 CD41 T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 35.Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 36.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 37.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–57. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristiansen M, Dhami S, Netuveli G, Halken S, Muraro A, Roberts G, et al. Allergen immunotherapy for the prevention of allergy: A systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18–29. doi: 10.1111/pai.12661. [DOI] [PubMed] [Google Scholar]
- 39.Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. 2015;135:1199–206. doi: 10.1016/j.jaci.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerlich WH, Glebe D. Development of an allergy immunotherapy leads to a new type of hepatitis B vaccine. EBioMedicine. 2016;11:5–6. doi: 10.1016/j.ebiom.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]