Abstract
Objective
To investigate the independent associations between age-specific annual weight gain from birth to age 4 years and fat deposition in metabolically distinct compartments at age 4.5 years in a South Asian longitudinal birth cohort.
Methods
Volumetric abdominal MRI with comprehensive segmentation of deep (DSAT) and superficial subcutaneous (SSAT), and visceral adipose tissues (VAT) was performed in 316 children (150 boys, and 166 girls in three ethnic groups: 158 Chinese, 94 Malay, and 64 Indian) aged 4.5 years. Associations between fat volumes and annual relative weight gain conditional on past growth were assessed overall and stratified by sex and ethnicity.
Results
Conditional relative weight gain had stronger associations with greater SAT and VAT at age 4.5 years in girls than boys, and in Indians compared with Malay and Chinese. Overall, the magnitude of association was the largest during 2-3 years for SAT, and 1-2 years for VAT. Despite similar body weight, Indian children, and girls had the highest DSAT and SSAT volumes at age 4.5 years (all p-interaction<0.05). No significant sex or ethnic differences were observed in VAT. With increasing BMI, Indian children had the highest tendency to accumulate VAT, and girls accumulated more fat than boys in all depots (all p-interaction<0.001).
Conclusions
Indian ethnicity and female sex predisposed children to accumulate more fat in the visceral adipose depot with increasing conditional relative weight gain in the second year of life. Thus, 1-2 years age may be a critical window for interventions to reduce visceral fat accumulation.
Keywords: abdominal fat partitioning, conditional relative weight gain, magnetic resonance imaging (MRI), childhood obesity, sex and ethnic differences
Introduction
Differential partitioning of fat has different metabolic consequences, with strong evidence of associations in adulthood (1–4). Within the abdominal fat depots, superficial subcutaneous adipose tissue (SSAT), deep subcutaneous adipose tissue (DSAT) and visceral adipose tissue (VAT)) have different functional characteristics and associations with metabolic risk (2, 3). SSAT, along with lower body fat depots like gluteofemoral fat, has been proposed as a protective depot that can safely sequester fat, and is associated with better glycemic control and a lower cardiovascular risk profile (1, 4). In contrast, DSAT and VAT have greater expression of lipolytic and pro-inflammatory genes and are strongly linked with insulin resistance and higher cardiometabolic risk (5–7). Many of the adverse effects of obesity on cardio-metabolic risk factors during childhood are strongly associated with increased accumulation of abdominal fat (8). Rapid weight gain in early life, childhood and adolescence has been shown to be positively associated with abdominal fat accumulation in adulthood (9–11). However, whether these associations begin in early childhood, and whether differential associations may partially explain sex and ethnic differences in abdominal fat accumulation is unknown.
Sex and ethnic differences in abdominal fat partitioning have been reported in adolescents and adults (12–17), with multiple studies showing that men have higher levels of VAT than women, which may partly explain their increased predisposition to metabolic diseases (12, 13, 18). The timing of this sexual dimorphism is not clear, with some reports indicating that it emerges even before puberty (19). South Asians have a strong predisposition to accumulate abdominal fat, particularly in the DSAT compartment, compared to other ethnic groups, accompanied by higher risk of cardiovascular and metabolic diseases (15, 20).
In this study, we evaluated the associations of sex and ethnicity with abdominal fat accumulation in early childhood in an Asian cohort. We also assessed whether sex and ethnicity modified the associations of adiposity, and rapid weight gain during infancy and early childhood with abdominal fat volumes at age 4.5 years. Early identification of such at-risk groups could help in designing interventions to prevent adverse metabolic outcomes. Previous studies on abdominal fat in children have often focused on pre-adolescents and adolescents (16, 21), with a few based on neonates (22–24). Most studies of abdominal fat quantification in children have used proxy measures of abdominal fat, such as waist circumference or waist-to-hip ratio, or have assumed that fat partitioning at a single abdominal magnetic resonance (MR) / computed tomography (CT) image slice (usually L4-L5 vertebrae or at the umbilicus) is representative of fat partitioning in the entire abdomen (16, 25). Abdominal fat area estimated from a single image slice has differential associations with fat volume and metabolic risk factors based on the slice location between L1 and L5 vertebrae, thereby limiting the use of single slice measurements for abdominal fat depots (26, 27). In this study, we used volumetric MRI with comprehensive image segmentation to measure DSAT, SSAT and VAT volumes.
Methods
Study population
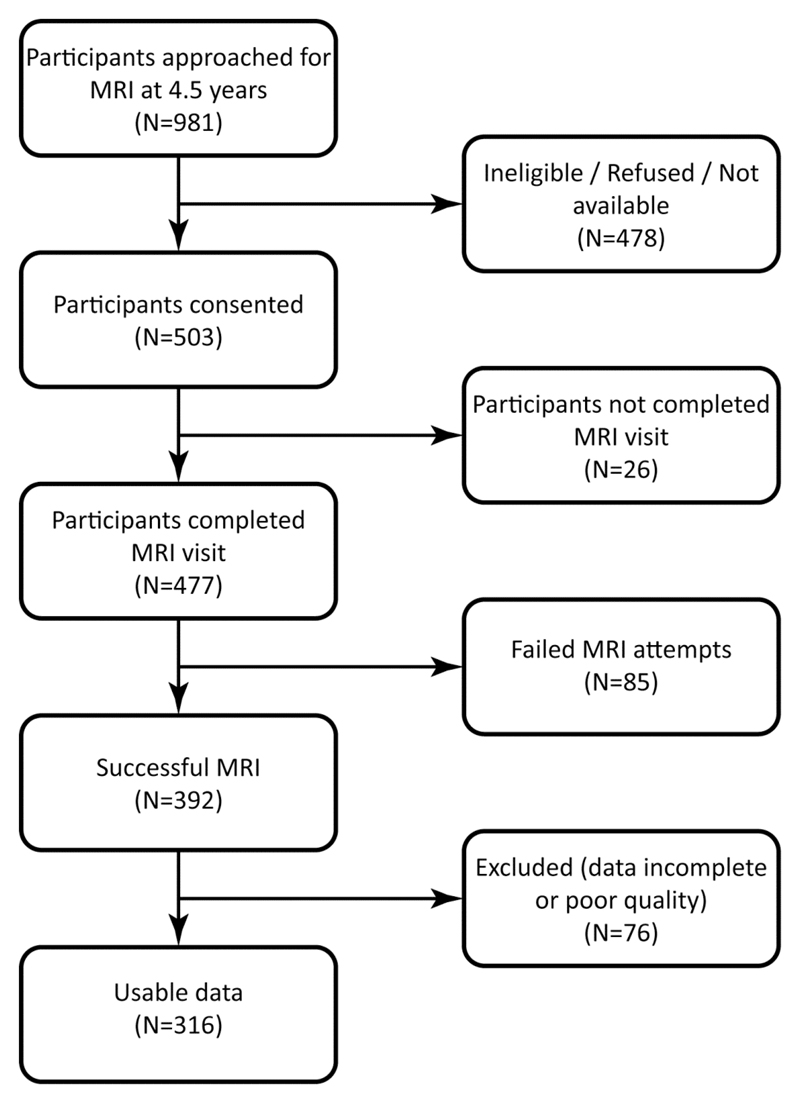
The participants were recruited from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study, a prospective birth cohort (28). Briefly, 1247 pregnant women aged 18 years and above were recruited in their first trimester from KK Women’s and Children’s Hospital and the National University Hospital between June 2009 and September 2010. A total of 1176 babies were born. Of the 981 children approached for MRI at 4.5 years of age, 503 consented (Figure 1). Successful MRI was performed on 392 children, 76 (19%) were excluded due to poor image quality, leaving a final sample of 316 participants: 150 boys and 166 girls across three ethnic groups (158 Chinese, 94 Malay, and 64 Indian). Written informed consent was obtained from the women and the study was approved by the National Healthcare Group Domain Specific Review Board and the SingHealth Centralized Institutional Review Board.
Figure 1.
Flow chart of the GUSTO study population at age 4.5 years.
Maternal data
Demographics (age, ethnicity and education level) and maternal prepregnancy weight were collected through questionnaires at study enrolment in the first trimester. Gestational age was determined by an ultrasound scan at 7-12 weeks. During the study visit in the second trimester (26-28 weeks), maternal height and weight were measured and an oral glucose tolerance test was administered to determine fasting and 2-h plasma glucose levels. Maternal prepregnancy BMI was calculated as self-reported prepregnancy weight (kg) divided by the squared height (m2). Serial measurements (every 1 to 2 months) of maternal weight throughout pregnancy were extracted from clinical records. Gestational weight gain per week between 15 and 35 weeks of gestation was determined from the linear weight trajectory obtained using a linear mixed-effects model with the best linear unbiased predictor (29).
Offspring anthropometric measures
Weight and length of the infant at birth were abstracted from hospital medical records. Infant weight at 12 months was measured to the nearest g using a calibrated SECA 334 weighing scale. At 24, 36, and 48 months, child’s weight was measured to the nearest g using SECA 803 weighing scales. Recumbent length of the infant/child at birth, 3, 6, 9, 12, 15, 18, and 24 months was measured from the crown of the head to the soles of the feet using an infant mat (SECA 210 Mobile Measuring Mat) to the nearest 0.1 cm. Standing height at 36 and 48 months were measured using a SECA 213 stadiometer. Skinfold thicknesses (triceps, biceps, subscapular and supra-iliac) were measured in triplicate using Holtain skinfold calipers (Holtain Ltd.) on the right side of the body, recorded to the nearest 0.2 mm. Abdominal circumference was measured at the level of the iliac crest using an inelastic measuring tape. The observers underwent anthropometric training and standardization sessions every 3 months to be equipped to obtain anthropometric measurements that, on average, were closest to the values measured by the trainer. The inter-observer reliability was assessed by technical error of measurement and coefficient of variation and the error values were found to be low (30).
MRI protocol and image analysis
The children underwent MRI of the abdomen without sedation using the Siemens Skyra 3T MR scanner. Sixty axial slices with 5-mm slice thickness and in-plane resolution of 0.94 × 0.94 mm were acquired using a water-suppressed HASTE sequence (repetition time (TR)=1000 ms, echo time (TE)=95 ms) and body matrix coil after anatomical localization. The images were acquired in the abdominal region using the liver dome and upper sacrum as anatomical references. The HASTE sequence was chosen over the commonly used Dixon sequence for abdominal imaging because of its fast imaging capability and lesser susceptibility to breathing artifacts (31). The breath-hold requirement for the Dixon sequence makes it unsuitable for pediatric population.
The SAT and VAT depots were segmented from the abdominal MR images using a fully automated graph theoretic segmentation algorithm (32). The first step of the algorithm used intensity thresholding to remove non-fat from fat tissues. Connected component analysis was then used to remove the upper limbs and create a fat mask. In the second step, the fat mask was classified into SAT and VAT compartments by removing the narrow connections between them using graph cuts. The output image was then manually edited to remove bowel and other misclassified structures. The SAT compartment was subclassified into DSAT and SSAT by manually drawing a boundary along the fascial plane. The volumes of each fat depot were computed by summing the voxels and multiplying by the image resolution.
The manual editing procedure was performed by a trained MR reader who was blinded to all the participant information. The quality of the manual editing was validated by repeating the procedure twice on randomly selected 30 data sets and measuring the intra-rater reliability. The mean Dice similarity indices for intra-rater reliability for DSAT, SSAT and VAT were 0.95, 0.98 and 0.99 respectively.
Estimation of conditional relative weight gain
Conditional relative weight gain was computed as the standardized residual of current weight regressed on current height and all previous weight and height measures (9, 33). The conditional relative weight gain denotes the child’s deviation from the expected weight gain for a specific interval, given the child’s current height and past growth measures. A child with a positive conditional weight gain in an interval indicates that he/she experienced a faster growth in this interval, than expected for his/her current height and prior size. We determined the conditional relative weight gain in annual intervals between birth and 4 years and in 3-month intervals in the first 18 months of life. These conditional relative weight gain measures are uncorrelated with each other and thus eliminate the statistical problems associated with modeling highly correlated weight measures. For example, conditional relative weight gain can be used to evaluate the effect of growth between 1 and 2 years on childhood abdominal fat, independent of growth between birth and 1 year.
Statistical analysis
Statistical analyses were performed using SPSS 23.0 software. We used Pearson’s chi-squared test to evaluate if there were sex differences across the ethnic groups. Analysis of variance (ANOVA) was used to compare the means of abdominal fat depot volumes and anthropometric measures between the ethnic groups and sexes. To assess ethnic and sex differences in the association between childhood adiposity and abdominal fat partitioning at age 4.5 years, we used analysis of covariance (ANCOVA) with the abdominal fat depots (SAT, VAT, DSAT and SSAT) as dependent variables, ethnicity or sex as the covariate, BMI at 48 months and the interaction terms ‘BMI × ethnicity’ or ‘BMI × sex’ as independent variables in separate analyses.
Multiple linear regression models were used to examine the association of abdominal fat depot volumes (SAT, DSAT, SSAT, and VAT) with conditional relative weight gain during infancy and early childhood, overall, and stratified by sex and ethnicity. Multiplicative interaction terms ‘conditional relative weight gain × sex’ or ‘conditional relative weight gain × ethnicity’ were added as independent variables to assess the sex and ethnic modifications of associations for every growth period (birth-1 year, 1-2 years, 2-3 years, and 3-4 years) in separate analyses. Beta coefficients and their 95% confidence intervals reflecting the association between the fat depot volumes and conditional relative weight gain measures and the interaction p-values were calculated. The analyses were adjusted for sex or ethnicity, maternal prepregnancy BMI, maternal antenatal fasting glucose, gestational weight gain per week, gestational age at delivery, and maternal education.
Results
Pearson’s chi-squared testing showed no relationship between sex and ethnicity (p=0.612). Table 1 shows the demographics and clinical characteristics of the study participants, stratified by ethnicity and sex. No difference in mean birth weight was observed between the three ethnic groups within the studied samples. Indian children were the tallest at age 4.5 years (107.7 cm, p<0.001), followed by Chinese (105.4 cm) and Malay children (104.3 cm), but no differences were observed in weight. No differences were seen among the ethnic groups for BMI, abdominal circumference or sum of skinfold thicknesses. Nonetheless, significant differences in abdominal subcutaneous fat distribution were observed between the ethnic groups; Indian children had the highest SAT (both DSAT and SSAT), followed by Malay and Chinese children (504.5 ml, 588.3 ml, and 668.6 ml for Chinese, Malay and Indian children respectively, p=0.013). The ethnic difference in VAT was small and non-significant.
Table 1. Demographic and clinical characteristics of the study participants.
| Ethnicity | Sex | ||||||
|---|---|---|---|---|---|---|---|
| Chinese, n=158 | Malay, n=94 | Indian, n=64 | P value | Boys, n=150 | Girls, n=166 | P value | |
| Maternal data | |||||||
| Prepregnancy BMI (kg/m2) | 21.7 ± 3.1 | 25.2 ± 5.5 | 23.3 ± 4.6 | <0.001 | 23.0 ± 4.3 | 23.6 ± 4.8 | 0.230 |
| Antenatal fasting glucose at 26 weeks (mmol/L) | 4.3 ± 0.4 | 4.2 ± 0.5 | 4.5 ± 0.5 | <0.001 | 4.3 ± 0.4 | 4.3 ± 0.5 | 0.849 |
| Gestational weight gain per week, (g/week) | 468.0 ± 117.7 | 462.6 ± 149.3 | 434.7 ± 121.8 | 0.223 | 446.5 ± 114.3 | 471.8 ± 140.3 | 0.086 |
| Gestational age at delivery (weeks) | 38.8 ± 1.4 | 38.1 ± 1.9 | 38.7 ± 1.8 | 0.021 | 38.4 ± 1.7 | 38.7 ± 1.9 | 0.136 |
| Maternal education, n | <0.001 | 0.524 | |||||
| < 12 years | 61 | 70 | 22 | 71 | 82 | ||
| ≥ 12 years | 95 | 24 | 40 | 76 | 83 | ||
| Weight (kg) | |||||||
| Birth | 3.1 ± 0.4 | 3.0 ± 0.5 | 3.0 ± 0.6 | 0.543 | 3.1 ± 0.4 | 3.0 ± 0.5 | 0.181 |
| 1 year | 9.2 ± 1.1 | 9.3 ± 1.2 | 9.4 ±1.1 | 0.623 | 9.6 ± 1.1 | 9.0 ± 1.1 | <0.001 |
| 2 year | 11.8 ± 1.5 | 11.9 ± 1.6 | 12.1 ± 1.9 | 0.676 | 12.2 ± 1.6 | 11.6 ± 1.6 | 0.002 |
| 3 year | 14.0 ± 2.0 | 14.4 ± 2.1 | 14.6 ± 2.7 | 0.157 | 14.4 ± 2.2 | 14.0 ± 2.2 | 0.118 |
| 4 year | 16.0 ± 2.2 | 16.5 ± 2.9 | 17.1 ± 3.9 | 0.051 | 16.5 ± 2.6 | 16.3 ± 3.1 | 0.662 |
| 4.5 years | 17.3 ± 2.6 | 17.5 ± 3.0 | 18.3 ± 4.1 | 0.108 | 17.7 ± 3.1 | 17.4 ± 3.1 | 0.366 |
| Height at 4.5 years (cm) | 105.4 ± 4.2 | 104.3 ± 3.6 | 107.7 ± 4.5 | <0.001 | 105.6 ± 4.4 | 105.4 ± 4.2 | 0.649 |
| BMI at 4.5 years (kg/m2) | 15.5 ± 1.6 | 16.0 ± 1.9 | 15.6 ± 2.5 | 0.114 | 15.8 ± 1.9 | 15.6 ± 1.9 | 0.322 |
| Abdominal circumference at 4.5 years (cm) | 51.4 ± 4.0 | 52.1 ± 5.2 | 52.7 ± 6.7 | 0.219 | 51.8 ± 4.9 | 51.9 ± 5.1 | 0.954 |
| Sum of skinfold thicknesses at 4.5 years (mm) | 30.0 ± 9.1 | 32.5 ± 12.3 | 33.0 ± 16.0 | 0.110 | 28.2 ± 9.9 | 34.2 ± 12.6 | <0.001 |
| Abdominal fat at 4.5 years (ml) | |||||||
| Total SAT | 504.5 ± 258.9 | 588.3 ± 443.1 | 668.6 ± 538.5 | 0.013 | 494.9 ± 346.6 | 623.9 ± 420.4 | 0.003 |
| DSAT | 128.6 ± 105.3 | 171.3 ± 191.6 | 205.9 ± 222.7 | 0.004 | 129.2 ± 142.8 | 182.0 ± 179.6 | 0.004 |
| SSAT | 375.9 ± 158.3 | 417.0 ± 254.3 | 462.7 ± 317.8 | 0.033 | 365.8 ± 205.7 | 441.8 ± 245.1 | 0.003 |
| VAT | 195.6 ± 61.3 | 186.5 ± 70.2 | 184.1 ± 114.5 | 0.501 | 195.4 ± 72.1 | 186.2 ± 81.7 | 0.295 |
Statistically significant (p<0.05) p values are highlighted in bold.
SAT, subcutaneous adipose tissue; DSAT, deep subcutaneous adipose tissue; SSAT, superficial subcutaneous adipose tissue; VAT, visceral adipose tissue.
Body weight was similar in boys and girls at birth, and at age 4.5 years, as were height, BMI and abdominal circumference at age 4.5 years. The sum of skinfold thicknesses was higher in girls than boys (34.2 mm vs. 28.2 mm, p<0.001). Girls also had larger volumes of DSAT (182.0 ml vs. 129.2 ml, p=0.004) and SSAT (441.8 ml vs. 365.8 ml, p=0.003) than boys, while VAT volumes were similar.
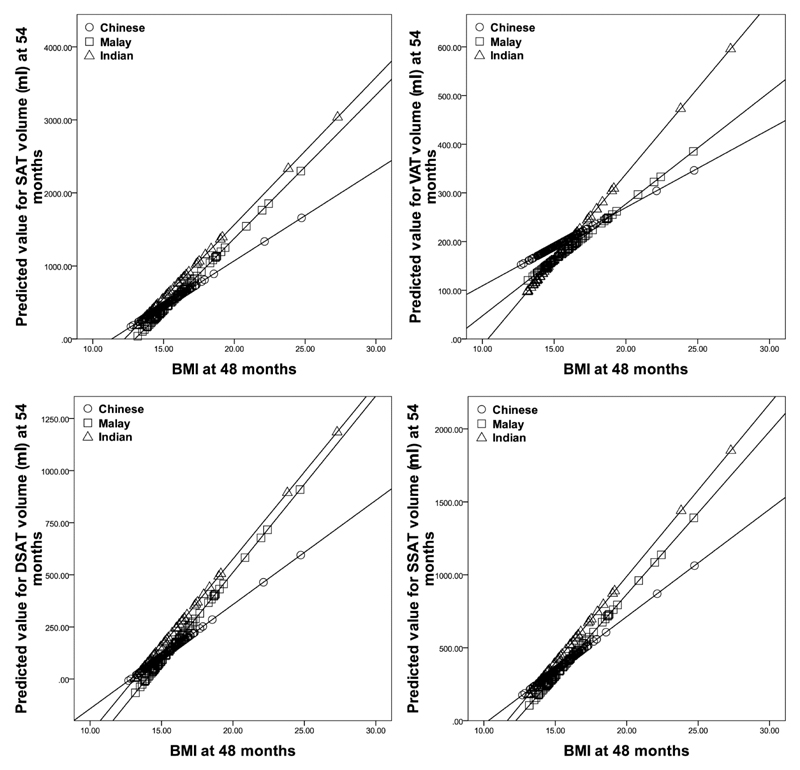
Figure 2 shows how ethnicity modified the relationships between BMI and abdominal adiposity. With increasing BMI, Chinese children had the least tendency to accumulate fat in all abdominal depots. Indian and Malay children tended to accumulate DSAT and SSAT to similar degrees, while Indian children had the strongest tendency to accumulate VAT. Figure 3 shows sex differences in the magnitude of the association between BMI and abdominal adiposity. With increasing BMI, girls had a stronger tendency than boys to accumulate fat in all depots.
Figure 2.
Effect of ethnicity on the association between BMI at 48 months and abdominal adipose depot volumes at 54 months (p<0.001 for interaction).
Figure 3.
Effect of sex on the association between BMI at 48 months and abdominal adipose depot volumes at 54 months (p<0.001 for interaction).
Associations between conditional relative weight gain in early childhood and abdominal adiposity at age 4.5 years stratified by ethnicity and sex are presented in Table 2 and Table 3 respectively. Overall, SAT and VAT were positively associated with conditional relative weight gain at all annual intervals in all three ethnic groups. The associations for DSAT and SSAT followed the trend similar to that observed for SAT (data not shown). Significant differences in the strength of association were observed among the three ethnic groups, with Indian children showing a stronger association than Malay and Chinese children at all time points (p-interaction <0.01).
Table 2. Estimated regression coefficients of the association between conditional relative weight gain and abdominal adiposity (SAT and IAT) at age 4.5 years stratified by ethnicity.
| Conditional relative weight gain | Chinese |
Malay |
Indian |
‘Ethnicity × conditional relative weight gain’ interaction p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |||||
| SAT | |||||||||||||
| 0-1 year | 105.65 | 57.58 | 153.71 | <0.001 | 92.01 | -8.15 | 192.17 | 0.071 | 318.04 | 156.82 | 479.26 | <0.001 | 0.003 |
| 1-2 years | 116.30 | 65.20 | 167.41 | <0.001 | 152.07 | 56.63 | 247.52 | 0.002 | 399.61 | 265.60 | 533.62 | <0.001 | <0.001 |
| 2-3 years | 109.04 | 65.13 | 152.95 | <0.001 | 255.53 | 190.72 | 320.34 | <0.001 | 268.57 | 128.33 | 408.82 | 0.001 | 0.001 |
| 3-4 years | 1.99 | -54.61 | 58.59 | 0.944 | 171.34 | 72.87 | 269.82 | 0.001 | 358.85 | 235.12 | 482.59 | <0.001 | <0.001 |
| VAT | |||||||||||||
| 0-1 year | 10.26 | -2.61 | 23.12 | 0.117 | 12.81 | -5.50 | 31.13 | 0.167 | 56.63 | 23.12 | 90.15 | 0.002 | 0.007 |
| 1-2 years | 34.86 | 22.63 | 47.09 | <0.001 | 18.74 | 1.32 | 36.16 | 0.035 | 85.79 | 61.59 | 109.99 | <0.001 | <0.001 |
| 2-3 years | 9.34 | -2.73 | 21.42 | 0.128 | 29.83 | 15.39 | 44.28 | <0.001 | 41.40 | 10.16 | 72.65 | 0.011 | 0.026 |
| 3-4 years | -10.43 | -24.96 | 4.11 | 0.158 | 17.66 | -0.80 | 36.12 | 0.060 | 69.36 | 40.51 | 98.22 | <0.001 | <0.001 |
All regressions were adjusted for sex, maternal education, maternal prepregnancy BMI, maternal antenatal fasting glucose, gestational weight gain per week, and gestational age at delivery. Statistically significant (p<0.05) p values are highlighted in bold.
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Table 3. Estimated regression coefficients of the association between conditional relative weight gain and abdominal adiposity (SAT and IAT) at age 4.5 years, overall, and stratified by sex.
| Conditional relative weight gain | Total1 |
Boys2 |
Girls2 |
‘Sex × conditional relative weight gain’ interaction p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |||||
| SAT | |||||||||||||
| 0-1 year | 145.23 | 98.43 | 192.02 | <0.001 | 90.49 | 29.14 | 151.84 | 0.004 | 201.19 | 130.80 | 271.58 | <0.001 | 0.025 |
| 1-2 years | 185.44 | 140.17 | 230.71 | <0.001 | 193.98 | 131.47 | 256.49 | <0.001 | 177.45 | 112.49 | 242.41 | <0.001 | 0.690 |
| 2-3 years | 192.18 | 152.41 | 231.95 | <0.001 | 145.30 | 94.61 | 195.99 | <0.001 | 233.41 | 168.70 | 298.11 | <0.001 | 0.038 |
| 3-4 years | 154.11 | 103.95 | 204.28 | <0.001 | 76.80 | 7.60 | 146.00 | 0.030 | 196.73 | 127.45 | 266.01 | <0.001 | 0.009 |
| VAT | |||||||||||||
| 0-1 year | 21.15 | 10.86 | 31.45 | <0.001 | 9.41 | -3.83 | 22.64 | 0.162 | 30.24 | 14.41 | 46.07 | <0.001 | 0.094 |
| 1-2 years | 37.65 | 28.05 | 47.26 | <0.001 | 25.94 | 11.62 | 40.25 | 0.001 | 46.24 | 33.64 | 58.85 | <0.001 | 0.039 |
| 2-3 years | 22.01 | 12.41 | 31.60 | <0.001 | 12.23 | 0.355 | 24.112 | 0.044 | 30.54 | 15.06 | 46.01 | <0.001 | 0.094 |
| 3-4 years | 20.83 | 9.58 | 32.09 | <0.001 | 2.36 | -12.52 | 17.24 | 0.753 | 30.30 | 14.60 | 46.01 | <0.001 | 0.005 |
Regressions were adjusted for sex, ethnicity, maternal education, maternal prepregnancy BMI, maternal antenatal fasting glucose, gestational weight gain per week, and gestational age at delivery.
Regressions were adjusted for ethnicity, maternal education, maternal prepregnancy BMI, maternal antenatal fasting glucose, gestational weight gain per week, and gestational age at delivery.
Statistically significant (p<0.05) p values are highlighted in bold.
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
The highest predicted increase in SAT volume per s.d. increase in conditional relative weight gain z-score in Chinese children was observed for the weight gain from 1 to 2 years (β=116.30 ml, 95% CI: 65.20, 167.41, p<0.001) and in the Malay children from 2 to 3 years (β=255.53 ml, 95% CI: 190.72, 320.34, p<0.001). Indian children had the strongest association in the 1-2 year time interval (β=399.61 ml, 95% CI: 265.60, 533.62, p<0.001). The 1-2 year age period in the Chinese children also showed the strongest association with VAT (β=34.86, 95% CI: 22.63, 47.09, p<0.001). The magnitude of association with VAT was highest between 2-3 year period and 1-2 year period in the Malay children and Indian children respectively. Indian children also showed the strongest positive association between conditional relative weight gain measures in shorter time intervals in the first 18 months and abdominal fat at 4.5 years, compared with Malay and Chinese. (data not shown).
Girls had stronger associations between conditional relative weight gain and abdominal adiposity at all growth intervals, except for SAT at the 1-2 year interval, where the association for boys was marginally but non-significantly higher than that of girls. The conditional relative weight gain between 1-2 years showed the strongest positive association with VAT for both boys and girls. One s.d. increase in conditional relative weight gain z-score was associated with 46.24 ml increase in VAT volume in girls compared to 25.94 ml observed in boys. The association with SAT was stronger in the 1-2 year period for boys and 2-3 year period for girls. Girls had stronger associations compared to boys across all the shorter time intervals, but the differences were significant only across selected time intervals (data not shown).
Discussion
We observed positive associations between conditional relative weight gain in early life (annual intervals between birth and 4 years) and abdominal adiposity at age 4.5 years and these associations appeared to be sex-, ethnicity- and time-dependent. Rapid weight gain during early life is associated with obesity in later childhood and adolescence (34, 35). Very few studies have investigated its association with adult abdominal adiposity (9–11). To our knowledge, ours is the first study to assess the association of conditional relative weight gain in infancy and early childhood with comprehensive abdominal fat measures at age 4.5 years. In our study, among all three ethnic groups we found that higher conditional relative weight gain in infancy and early childhood was strongly associated with greater abdominal adiposity at age 4.5 years. However, the association was significantly stronger for Indian children than for Chinese and Malay children, suggesting that rapid weight gain during early childhood might be more detrimental to metabolic health in Indian children. We also found certain periods of growth to contribute more to higher abdominal fat accumulation. Overall, 2-3 year period had the highest magnitude of association for SAT and 1-2 year period had the strongest association for VAT. The temporal changes in these associations varied between the ethnic groups indicating ethnic-specific sensitive periods of early growth that could have stronger influence on childhood obesity.
Faster weight gain in infancy and early childhood had a stronger effect on both SAT and VAT accumulation in girls than in boys. Our results suggest that weight gain in boys during this period may be linked to higher fat-free mass accumulation, rather than fat mass. This is in agreement with the results of longitudinal studies showing higher fat-free mass in boys than girls from birth, and higher fat mass for girls than boys after 18 months of life (36).
We observed sex and ethnic differences in abdominal fat partitioning in GUSTO children at age 4.5 years. Among adults, studies have reported higher VAT in Caucasians compared to African-Americans (14). Indian adults have been reported to accumulate more visceral fat even at low BMI values compared to other ethnic groups (37). These ethnic differences have been shown to be present even at birth (22, 23). In the GUSTO cohort, we previously reported that Indian and Malay neonates had a larger DSAT volume than Chinese neonates, with no differences in SSAT or VAT volumes (23). At 4.5 years, we continue to find that abdominal fat partitions differently among the three ethnic groups for all the subcutaneous fat depots (total SAT, DSAT and SSAT), but not for VAT. The differential associations observed between early life weight gain and abdominal fat could possibly partially explain the sex and ethnic differences in the abdominal fat partitioning.
Ethnicity can also modify the associations between abdominal fat depots and metabolic risk factors. For instance, while VAT is strongly linked to insulin resistance, African-Americans are more insulin resistant than Caucasians despite their lower VAT (14, 21). South Asians accumulate higher body fat than Caucasians, even at low BMI, and also show a stronger association between abdominal adiposity and hyperinsulinemia (38). Ethnic differences in abdominal fat partitioning have also been reported in Asian neonates, within 3 weeks of birth (22, 23). In our current study, Indian children had the lowest levels of VAT among lean children, yet showed the strongest tendency to accumulate VAT with increasing BMI. Between the sexes, girls had higher DSAT and SSAT volumes than boys, but no significant differences in VAT volumes. Our results agree with previous studies reporting higher SAT in girls, but no significant differences in VAT (23, 39). The association we observed between BMI and abdominal adiposity in early childhood was stronger in girls than boys.
VAT is more detrimental to metabolic health than SAT. Though this has been well studied in adults (3, 6), the results are inconclusive in the pediatric population. Increased VAT has been shown to be associated with adverse metabolic profile in children with obesity (40, 41), while some studies have shown SAT to be a stronger predictor of metabolic risk factors than VAT (8, 40). Further research is required to ascertain the role of SAT and VAT in metabolic health in children. Besides its impact on metabolic health, increased visceral obesity in childhood leads to faster maturation and polycystic ovary syndrome in girls (42). Thus, the increased predisposition to accumulate fat in the abdominal depots could possibly have several adverse effects of obesity later in life.
Strengths of our study include its longitudinal study design, participants from the three Asian ethnic groups comprising the majority of the Singaporean population, growth outcomes measured at multiple time points throughout infancy and childhood and MR-based abdominal fat partitioning determined at age 4.5 years. A limitation is the lack of blood samples obtained at this age, precluding us from assessing the metabolic syndrome from measurements of triglycerides, fasting glucose and insulin. These would have been useful in understanding the relationship between fat partitioning and the metabolic profile of these children. A further limitation is that, while maternal weight data was collected using validated electronic weighing scales in the antenatal clinics and infant anthropometry using standardized research protocols, there may still be residual measurement error as in any cohort study. In this study, we have focused on investigating the total effect of ethnicity on growth and abdominal fat partitioning among the Chinese, Malay and Indian children. However, in earlier studies within GUSTO, we did observe ethnic differences in infant feeding practices and dietary intake that could potentially mediate the effect of ethnicity on fat partitioning (43, 44). Measurements of physical activity were not available during early childhood. Since feeding practices and dietary intake are potential causal intermediates of the link between ethnicity and fat accumulation, adding them as covariates in a simple multiple regression model can lead to bias in the estimate of the total effect of ethnicity due to statistical over-adjustment (45). Decomposing the total effect of ethnicity on fat accumulation into different pathways (diet, feeding practices, physical activity, etc.) requires more sophisticated statistical approaches (structural equation model or marginal structural model), which is beyond the scope of the current work.
In summary, we observed ethnic and sex differences in abdominal fat partitioning in Asian children at age 4.5 years. We also found that sex, ethnicity, and time period modified the associations of rapid infancy weight gain and whole body adiposity with abdominal fat. In particular, we found that Indian ethnicity, female sex and the second year of life predisposed children with increasing adiposity and weight gain to accumulate more fat in the visceral adipose depot. Our study highlights the strengths of multi-ethnic cohorts for investigating the etiology of ethnic variation in cardiometabolic risk during early development. Identification of sex- and ethnicity-specific risk factors could help in developing targeted interventions to prevent future adverse metabolic outcomes. Previous studies have indicated that physical activity interventions in children can prevent excess fat mass accumulation in childhood (46, 47). Our findings may help in identifying children who are at higher risk of fat accumulation, and who may therefore be suitable targets for preventive interventions.
What is already known about this subject?
Early life weight gain is associated with adult abdominal fat.
No studies have investigated whether these associations begin in early childhood, and whether differential associations may partially explain sex and ethnic differences in fat accumulation.
What does this study add?
The study describes associations between age-specific conditional relative weight gains and fat deposition in metabolically distinct abdominal fat compartments in a South Asian longitudinal birth cohort.
We showed that Indian ethnicity and female sex predisposed children to accumulate more fat in the visceral adipose depot with increasing conditional relative weight gain. The magnitude of association was higher for the 1-2 year time period.
These findings aid early identification of children at higher risk of fat accumulation, and suggest that 1-2 years might be an important period for interventions to prevent adverse metabolic outcomes.
Acknowledgements
The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Kenneth Kwek, Krishnamoorthy Niduvaje, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Yin Bun Cheung, and Yiong Huak Chan.
Funding: This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore – NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore and Nestec.
Footnotes
Clinical Trial Registration: Clinicaltrials.gov identifier NCT01174875.
Disclosure: Keith M. Godfrey has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. Keith M. Godfrey and Peter D. Gluckman are part of an academic consortium that has received research funding Nestec. The other authors declared no conflicts of interest. Keith M. Godfrey is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (as a NIHR Senior Investigator (NF-SI-0515-10042) and through the NIHR Southampton Biomedical Research Centre) and the European Union's Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977.
References
- 1.Golan R, Shelef I, Rudich A, et al. Abdominal Superficial Subcutaneous Fat. Diabetes care. 2012;35(3):640–7. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. American Journal of Physiology-Endocrinology And Metabolism. 2000;278(5):E941–E8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 3.Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolopoulos K, Karpe F, Frayn K. Gluteofemoral body fat as a determinant of metabolic health. International journal of obesity. 2010;34(6):949. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 5.Palou M, Priego T, Sánchez J, et al. Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features. Cellular Physiology and Biochemistry. 2009;24(5–6):547–56. doi: 10.1159/000257511. [DOI] [PubMed] [Google Scholar]
- 6.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 7.Walker GE, Verti B, Marzullo P, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity. 2007;15(8):1933–43. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 8.González-Álvarez C, Ramos-Ibáñez N, Azprioz-Leehan J, Ortiz-Hernández L. Intra-abdominal and subcutaneous abdominal fat as predictors of cardiometabolic risk in a sample of Mexican children. European journal of clinical nutrition. 2017;71(9):1068. doi: 10.1038/ejcn.2017.28. [DOI] [PubMed] [Google Scholar]
- 9.De França GA, Rolfe EDL, Horta B, et al. Associations of birth weight, linear growth and relative weight gain throughout life with abdominal fat depots in adulthood: the 1982 Pelotas (Brazil) birth cohort study. International Journal of Obesity. 2016;40(1):14. doi: 10.1038/ijo.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demerath EW, Reed D, Choh AC, et al. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obesity. 2009;17(11):2060–6. doi: 10.1038/oby.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindblom JM, Lorentzon M, Hellqvist Å, et al. BMI changes during childhood and adolescence as predictors of amount of adult subcutaneous and visceral adipose tissue in men: the GOOD Study. Diabetes. 2009;58(4):867–74. doi: 10.2337/db08-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demerath EW, Sun SS, Rogers N, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity. 2007;15(12):2984–93. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues–the biology of pear shape. Biology of sex differences. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depot–specific adiposity in white and African American adults. The American journal of clinical nutrition. 2010;91(1):7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoo CM, Leow MK-S, Sadananthan SA, et al. Body fat partitioning does not explain the interethnic variation in insulin sensitivity among Asian ethnicity: the Singapore adults metabolism study. Diabetes. 2014;63(3):1093–102. doi: 10.2337/db13-1483. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity. 2008;16(5):1066–71. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 17.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2(6):e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender medicine. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Q, Horlick M, Thornton J, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. The Journal of Clinical Endocrinology & Metabolism. 2002;87(5):2164–70. doi: 10.1210/jcem.87.5.8452. [DOI] [PubMed] [Google Scholar]
- 20.Kohli S, Lear SA. Differences in subcutaneous abdominal adiposity regions in four ethnic groups. Obesity. 2013;21(11):2288–95. doi: 10.1002/oby.20102. [DOI] [PubMed] [Google Scholar]
- 21.Staiano A, Broyles S, Gupta A, Katzmarzyk P. Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity. 2013;21(6):1251–5. doi: 10.1002/oby.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modi N, Thomas EL, Uthaya SN, et al. Whole body magnetic resonance imaging of healthy newborn infants demonstrates increased central adiposity in Asian Indians. Pediatric research. 2009;65(5):584. doi: 10.1203/pdr.0b013e31819d98be. [DOI] [PubMed] [Google Scholar]
- 23.Tint MT, Fortier MV, Godfrey KM, et al. Abdominal adipose tissue compartments vary with ethnicity in Asian neonates: Growing Up in Singapore Toward Healthy Outcomes birth cohort study. The American journal of clinical nutrition. 2016;103(5):1311–7. doi: 10.3945/ajcn.115.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gale C, Logan KM, Jeffries S, et al. Sexual dimorphism in relation to adipose tissue and intrahepatocellular lipid deposition in early infancy. International journal of obesity (2005) 2015;39(4):629. doi: 10.1038/ijo.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster-Gandy J, Warren J, Henry C. Sexual dimorphism in fat patterning in a sample of 5 to 7-year-old children in Oxford. International journal of food sciences and nutrition. 2003;54(6):467–71. doi: 10.1080/09637480310001322323. [DOI] [PubMed] [Google Scholar]
- 26.Brown RE, Kuk JL, Lee S. Measurement site influences abdominal subcutaneous and visceral adipose tissue in obese adolescents before and after exercise. Pediatric obesity. 2015;10(2):98–104. doi: 10.1111/j.2047-6310.2014.224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis KJ, Grund B, Visnegarwala F, et al. Visceral and subcutaneous adiposity measurements in adults: influence of measurement site. Obesity. 2007;15(6):1441–7. doi: 10.1038/oby.2007.172. [DOI] [PubMed] [Google Scholar]
- 28.Soh S-E, Tint MT, Gluckman PD, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. International journal of epidemiology. 2013;43(5):1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 29.Cheung YB. Statistical analysis of human growth and development. CRC Press; 2013. [Google Scholar]
- 30.Aris IM, Bernard JY, Chen L-W, et al. Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. International journal of obesity. 2017;41(7):1011. doi: 10.1038/ijo.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semelka RC, Kelekis NL, Thomasson D, Brown MA, Laub GA. HASTE MR imaging: description of technique and preliminary results in the abdomen. Journal of Magnetic Resonance Imaging. 1996;6(4):698–9. doi: 10.1002/jmri.1880060420. [DOI] [PubMed] [Google Scholar]
- 32.Sadananthan SA, Prakash B, Leow MKS, et al. Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. Journal of Magnetic Resonance Imaging. 2015;41(4):924–34. doi: 10.1002/jmri.24655. [DOI] [PubMed] [Google Scholar]
- 33.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. The Lancet. 2013;382(9891):525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. Jama. 2009;301(21):2234–42. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 35.Taveras EM, Rifas-Shiman SL, Belfort MB, et al. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells JC. Sexual dimorphism of body composition. Best practice & research Clinical endocrinology & metabolism. 2007;21(3):415–30. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metabolic syndrome and related disorders. 2009;7(6):497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 38.Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of Adiposity on Insulin Resistance and Glycemia Markers Among UK Children of South Asian, Black African-Caribbean, and White European Origin. Diabetes Care. 2013;36(6):1712–9. doi: 10.2337/dc12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen W, Punyanitya M, Silva AM, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutrition & metabolism. 2009;6(1):17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caprio S, Hyman LD, McCarthy S, et al. Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot. The American journal of clinical nutrition. 1996;64(1):12–7. doi: 10.1093/ajcn/64.1.12. [DOI] [PubMed] [Google Scholar]
- 41.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. The Lancet. 2003;362(9388):951–7. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Zegher F, López-Bermejo A, Ibáñez L. Central Obesity, Faster Maturation, and ‘PCOS’in Girls. Trends in Endocrinology & Metabolism. 2018 doi: 10.1016/j.tem.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Pang WW, Aris IM, Fok D, et al. Determinants of breastfeeding practices and success in a multi-ethnic asian population. Birth. 2016;43(1):68–77. doi: 10.1111/birt.12206. [DOI] [PubMed] [Google Scholar]
- 44.Toh JY, Yip G, Han WM, et al. Infant feeding practices in a multi-ethnic Asian cohort: the GUSTO study. Nutrients. 2016;8(5):293. doi: 10.3390/nu8050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass) 2009;20(4):488. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutin B, Owens S. Role of exercise intervention in improving body fat distribution and risk profile in children. American Journal of Human Biology. 1999;11(2):237–47. doi: 10.1002/(SICI)1520-6300(1999)11:2<237::AID-AJHB11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Khan NA, Raine LB, Drollette ES, et al. Impact of the FITKids physical activity intervention on adiposity in prepubertal children. Pediatrics. 2014;133(4):e875–e83. doi: 10.1542/peds.2013-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]