Abstract
Background and Purpose —
Recent studies have shown that the cellular immune response in the development of vascular remodeling modulates the resulting pathological alterations. We show that hypoxia-inducible factor 1 (Hif-1) (specifically expressed in T cells) is involved in the immune response to vascular remodeling that accompanies arteriosclerosis.
Methods and Results —
To study the role of T cells in the development of vascular remodeling, femoral arterial injury induced by an external vascular polyethylene cuff was examined in mice lacking Hif-1 (specifically in T cells). We found that cuff placement caused prominent neointimal hyperplasia of the femoral artery in Hif-1-(T-cell)-deficient mice compared with that in control mice and that infiltration of inflammatory cells at the adventitia was markedly increased in the mutant mice. Studies to clarify the mechanism of augmented vascular remodeling in the mutant mice showed enhanced production of cytokines by activated T cells and augmented antibody production in response to a T-dependent antigen in the mutant mice.
Conclusions —
The results of this study revealed that Hif-1 α in T cells plays a crucial role in vascular inflammation and remodeling in response to cuff injury as a negative regulator of T cell-mediated immune response. Potential new therapeutic strategies that target Hif-1 α are described.
Keywords: Arteriosclerosis, T cells, Hif-1 α Hypoxia, Vascular remodeling
The vascular response to mechanical arterial injury involved in arteriosclerosis or in-stent restenosis leads to neointimal formation and inward remodeling. Recent studies have shown that the immune system plays an important role in the development of vascular remodeling in response to arterial injury.1 Studies2–4 in mice have shown that arterial injury is associated with local accumulation of antibodies, and mice lacking functional T and B cells exhibit increased neointima formation, indicating that adaptive immune responses to neoantigens in the damaged tissue modulate the vascular remodeling. During the development of vascular remodeling, a hypoxic microenvironment accompanying arteriosclerosis or stent-mediated overdistention in the injured vascular region is thought to be one of the factors modulating proliferation of myofibroblasts and increased matrix synthesis in the adventitial region.5 It has also been reported that hypoxia accelerates the progression of atherosclerosis6,7 and modulates vascular remodeling after arterial injury.8 Several studies10,11 have shown evidence of hypoxia within the arterial wall in atherosclerosis in an animal model9 and in human disease. Adaptation to low oxygen tension in local tissues is important for activities of immune cells, because immune cells are often exposed to different oxygen tensions that markedly affect cellular metabolism as they survey different tissue microenvironments.12 These findings indicate that adaptation of immune cells to a hypoxic condition in vascular remodeling modulates the pathogenesis. However, there are many unanswered questions as to how the immune response is regulated in vascular remodeling and how it may be targeted for therapeutic intervention.13 Little is known about the functions of T cells under the condition of progression of vascular remodeling.
Hypoxia-inducible factor 1 (Hif-1) is a transcription factor that regulates gene expression in response to hypoxia; it is composed of heterodimers of an oxygen-sensitive α subunit and a constitutively expressed β subunit (also known as arylhydrocarbon receptor nuclear translocator). Hif-1α regulates the expressions of genes in response to hypoxia to maintain physiological oxygen homeostasis.14 In addition to hypoxic stabilization of Hif-1α, resulting in upregulation of Hif-1α functions, several factors relevant to inflammation and oxidative stress, including nitric oxide, tumor necrosis factor α,15 angiotensin II,16 interleukin (IL) 1, insulin, and insulinlike growth factors,17,18 have been shown to induce nonhypoxic Hif-1α stabilization. Recently, it has also been shown that hypoxia alone is not sufficient and that T-cell receptor (TCR)-mediated signaling is required for the accumulation of Hif-1α in human peripheral T cells. TCR engagement does not influence hypoxia-dependent stabilization; however, it stimulates synthesis of Hif-1α, probably via the phosphatidylinositol 3-kinase/mammalian target of the rapamycin pathway.19 Therefore, it is plausible that Hif-1α in T cells is involved in T-cell activation for immune responses in vivo.
In the present study, we showed that cuff placement at the femoral artery caused prominent neointimal hyperplasia in Hif-1α-T-cell-deficient mice compared with that in control mice. This finding of augmentation of arterial hyperplasia in the mutant mice and the results of several experiments on T-cell-mediated immune responses in vivo and in vitro suggested that Hif-1α in T cells plays a crucial role in vascular inflammation and remodeling in response to cuff injury as a negative regulator of T-cell-mediated immune response. These results are consistent with the results of recent studies showing that Hif-1α is a negative regulator of T-cell response in vitro and in vivo in models of acute inflammation.20
Methods
A detailed description of all methods is available in the supplemental materials (available at: http://atvb.aha.journals.org).
Animal Experiments
All experimental procedures conformed to the guidelines for animal experimentation administered by the University of Tokushima, Tokushima, Japan. Mice with either a Hif-1a-disrupted allele (Hif-1aD) or Hif-1a-floxed allele (Hif-1aflox) were generated as described previously. The Hif-1a+/D mice were mated with Lck-Cre transgenic mice, and their offspring carrying both the lck promoter-driven Cre transgene and the Hif-1a-disruped gene (Lck-Cre;Hif-1a+/D) were mated with Hif-1aflox/flox mice to produce Lck-Cre;Hif-1aflox/D mice. Femoral cuff placement was performed in male Lck-Cre;Hif-1αflox/Δ or Lck-Cre;Hif-1αflox/+ mice (aged 8–12 weeks), as previously described.21 Vascular remodeling of the mice was characterized six weeks after femoral arterial injury induced by an external vascular polyethylene cuff model. Polyethylene tubing (inside diameter, 0.58 mm; Becton Dickinson, Franklin Lakes, New Jersey) was used as the cuff. Animals were anesthetized by the injection of 2,2,2-tribromoethanol (Sigma-Al-drich Co, St Louis, Mo) into the peritoneal cavity.
Detection of Hypoxia With Hypoxyprobe
Hypoxyprobe (Hypoxyprobe Inc, Burlington, Mass) is a pimonidazole that forms covalent protein adducts in viable hypoxic cells in a manner that is inversely proportional to oxygen concentration in the physiological range.22 Briefly, mice were injected with 60 mg/kg body weight of the pimonidazole agent and then euthanized two hours after injection to characterize the distribution of pimonidazole-protein adducts via in situ immunohistochemical analysis.
Results
Detection of Hypoxia With Pimonidazole Around Vascular Walls in a Cuff-Injured Artery
We examined whether hypoxia is present in or around a cuff-injured femoral artery by binding of pimonidazole, a hypoxia detection agent that binds to regions of hypoxia in a manner that is inversely proportional to oxygen concentration.22 The cuff-injured artery demonstrated binding of this hypoxia-sensitive compound in the region around vascular walls from day 1 after cuff placement (Figure 1A). Consistent with the pimonidazole-positive regions around cuffed vascular walls, expression levels of Hif-1α around cuffed vascular walls were higher (Figure 1A). On day 14 after cuff placement, CD3-positive T cells were detected around the cuffed vascular walls, and some parts of these signals (green) were merged with Hif-1α-positive signals (red), as well as with those of pimonidazole (Figure 1A and Supplementary Figure S1). The formation of neointimal hyperplasia in the development of cuff-induced vascular remodeling was detected on day 42 after cuff placement, not on day 14, when infiltration of CD3-positive T cells around the cuffed vascular walls started (Figure 1B). These results suggest that there are at least two events before the progression of cuff-induced neointimal formation: (1) binding of pimonidazole and Hif-1α expression around cuffed vascular walls and (2) infiltration of Hif-1α-expressing T cells around cuffed vascular walls.
Figure 1.
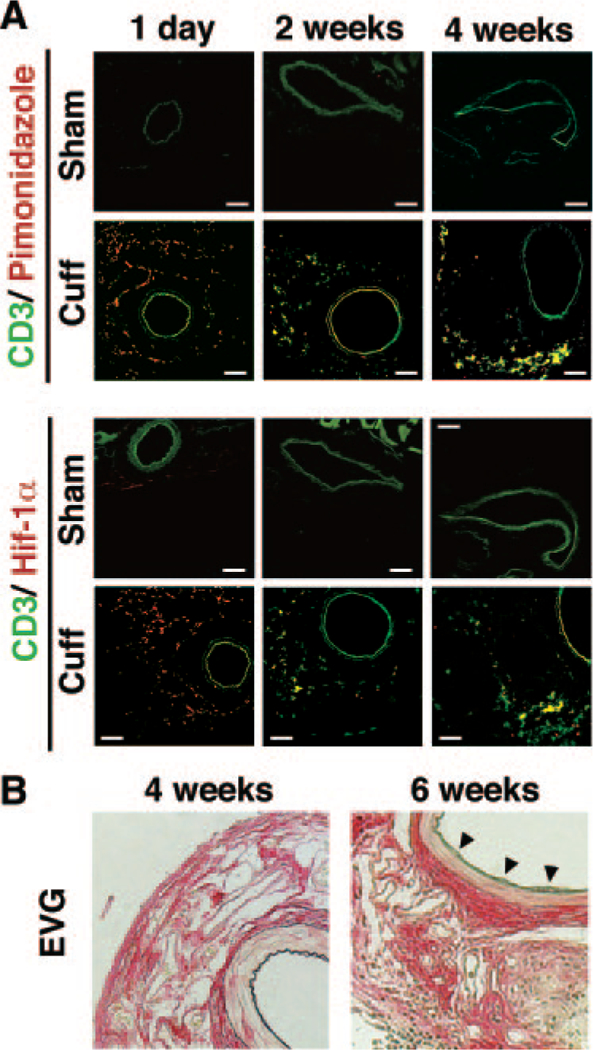
Development of vascular remodeling of cuff-injured artery in mice. A, Immunofluorescence analysis of cuff-injured femoral arteries. Representative cross sections for CD3 (green), pimonidazole-protein adducts (red, upper panel), and hypoxiainducible factor (Hif) 1α (red, lower panel) at 1 and 14 days after cuff placement. Green signals of intima, media, and other muscle tissues around vessels are nonspecific signals, as proved in Supplementary Figure S1. The bar indicates 200 μm. B, Representative cross sections with hematoxylin-eosin staining of the cuffed femoral artery at 14 and 42 days after cuff placement. Elastica van Gieson staining (EVG) indicates.
Increase in Number of T Cells in T-Cell-Specific Hif-1 𝛂-Deficient Lymph Nodes
To investigate the biological function of Hif-1α in T cells, the Hif-1α gene was specifically inactivated in mouse T cells using the Cre-loxP system. Mice with either an Hif-1α-disrupted allele (Hif-1αΔ) or an Hif-1α-floxed allele (Hif-1αflox) were generated as previously described23 (supplementary text and Supplementary Figure S2). We examined whether Hif-1α deficiency in T-lineage cells affects T-cell development and distribution in lymphoid organs. The total nucleated lymphoid cell numbers in lymphoid organs from Hif-1α-deficient mice were comparable with those of the control mice (Figure 2A and B). In addition, the numbers of CD4- and CD8-single positive T cells in lymph nodes from mutant mice were significantly increased compared with those in lymph nodes from control mice, although the number of B220-positive B cells was not decreased (Figure 2A and B). As shown in Figure 2C, the structure of reticular fibroblasts constructed with ER-TR7-positive mesenchymal cells and the distribution of CD3-positive T cells and B220-positive B cells were normal in the mutant mouse lymph nodes. These results indicated that T-cell-specific Hif-1α-deficient T cells were normally generated in the thymus and that their distribution in the spleen and lymph nodes was unimpaired.
Figure 2.
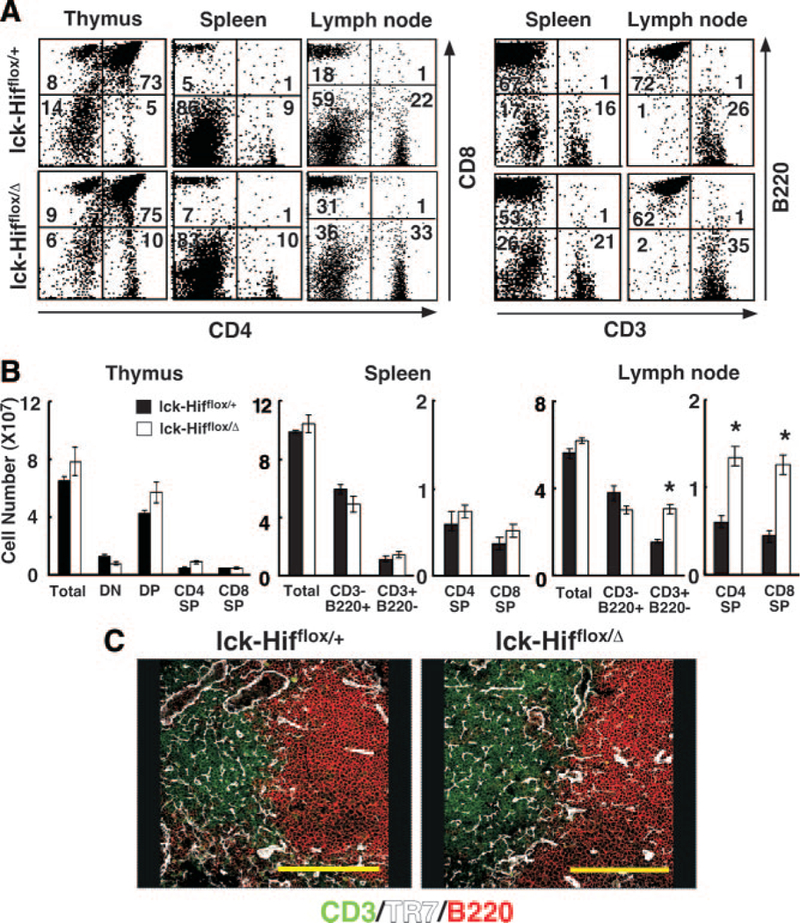
Flow-cytometric analysis of lymphoid cells from Lck-Cre;Hif-1aflox/+ (lck-Hifflox/+) and Lck-Cre;Hif-1aflox/D (lck-Hifflox/D) mice. A, Lymphoid cells from thymus, lymph node, and spleen samples of 5-week-old mice with the indicated genetic characteristics were two-color stained with fluorescein iso-thiocyanate-labeled antibody (x-axis) and phosphatidylethanolamine-labeled antibody (y-axis) with the indicated specificity. The data are representative dot blots. Numbers within each box indicate the frequency of cells within the box. B, Data in bar graphs are mean±SE of lymphoid cell numbers in Lck-Cre;Hif-1αflox/+ or Lck-Cre;Hif-1αflox/Δ mice. DN indicates; DP, ; and SP, . *P<0.05. C, Three-color immunofluorescence analysis of adult lymph node sections for CD3 (green), B220 (red), and ER-TR7 (white).
Enhancement of T-Cell Activation, Proliferation, and Thymus-Dependent Antibody Production in T-Cell-Specific Hif-1α-Deficient Mice
To determine how Hif-1α in T cells may participate in the immune response, the TCR responsiveness of Hif-1α-deficient T cells in vitro was examined. As shown in Figure 3A, T cells from the Lck-Cre;Hif1αflox/Δ mice exhibited normal increases in CD25 (IL-2Rα) and CD69 on stimulation with either the combination of anti-CD3 and anti-CD28 antibodies or concanavalin A (Con A), indicating that T cells lacking Hif-1α normally transmit TCR signals that provoke early cellular responses. Interestingly, IL-2 production on stimulation with the combination of anti-CD3 and anti-CD28 antibodies was significantly increased in the mutant T cells (Figure 3B). Consistent with this result, augmentation of the proliferation of mutant T cells on stimulation with anti-CD3 and anti-CD28 antibodies or Con A was also observed (Figure 3C). This enhanced IL-2 production in Hif-1α-deficient T cells may be relevant to increased T-cell numbers in peripheral lymphoid organs in the Lck-Cre;Hif1αflox/Δ mice, as shown in Figure 2B. Furthermore, messenger RNA transcripts encoding interferon (IFN) γ were significantly increased in Hif-1α-deficient T cells after TCR stimulation (Figure 3D). In addition to enhanced T-cell activation in the Lck-Cre;Hif1αflox/Δ mice, lymphocytes from these mice were unimpaired in an allogeneic mixed lymphocyte reaction, similar to that for healthy mice (Figure 3E). These results suggest that Hif-1α in T cells may modulate T-cell activation via TCR signaling, including changes in production levels of inflammatory cytokines.
Figure 3.
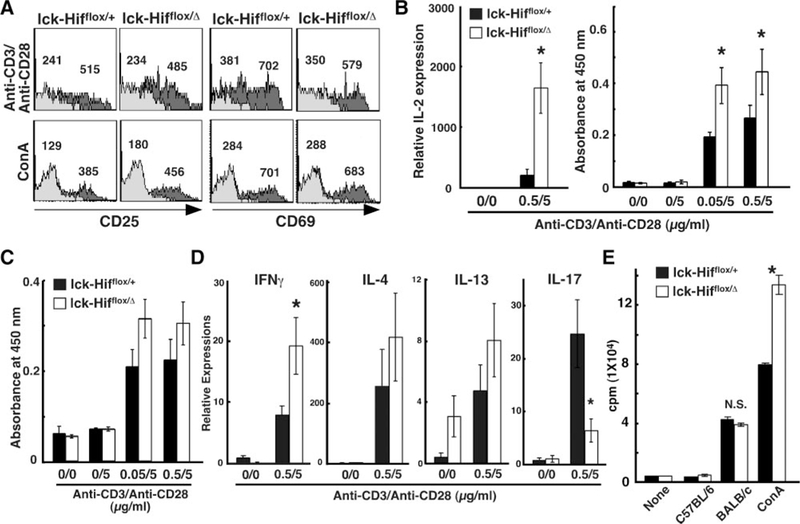
In vitro experiments on activation, proliferation, and function of Hif-1α–deficient T cells. A, Initiation of TCR signals provoking early cellular responses. After 16 hours in culture with either the combination of anti-CD3 and anti-CD28 antibodies or Con A (2.5μg/mL), lymphocytes from mandibular lymph nodes were two-color stained with phosphatidylethanolamine-labeled anti-CD4 antibody and fluorescein isothiocyanate–labeled anti-CD25 or anti-CD69 antibody or normal IgG. The profiles of CD25 and CD69 expression are shown by electrically gated CD4+ cells, in the presence of stimulants (dark gray area) or the absence of stimulants (gray area). Numbers indicate the mean fluorescence intensity of CD25 and CD69 within the cells. B, Lymphocytes from lymph nodes after 16 hours inculture with the combination of anti-CD3 and anti-CD28 antibodies were used for measurement of interleukin (IL) 2 transcripts. The culture supernatants were used for measurement of IL-2 proteins by enzyme-linked immunosorbent assay. The values are shown as absorbance at 450 nm. C, On day 2 of culture, cultured wells were tested for cell proliferation by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The values are shown as absorbance at 450 nm. Values are expressed as mean_SD. *P<0.001. D, Lymphocytes from lymph nodes after 12 hours in culture with the combination of anti-CD3 and anti-CD28 antibodies were used for measurement of transcripts for interferon (IFN) γ, IL-4, IL-13, and IL-17. E, Thymidine-incorporation assay of proliferating allogeneic BALB/c lymph node lymphocytes (5.0×105 cells) incubated with an equal amount of lymphocytes derived from Lck-Cre;Hif-1αflox/+ or Lck-Cre;Hif-1αflox/Δ mice. *P<0.05.
To further investigate the effect of the mutant T cells on B-cell functions through activation and proliferation of T cells, we examined the levels of antigen-specific antibodies elicited by T-cell responses in vivo. Mice were immunized with the thymus-dependent antigen trinitrophenyl (TNP)-keyhole limpet hemocyanin, given in incomplete Freund adjuvant, and the titers of induced antibodies against TNP in the serum were determined. As shown in Figure 4A, nonimmunized Lck-Cre;Hif1αflox/Δ mice exhibited higher serum levels of total IgM and IgG2b among the isotypes examined than did control Lck-Cre;HIF-1αflox/+ mice. This experiment included initial immunization followed by a boost with TNP-keyhole limpet hemocyanin in phosphate-buffered saline on day 30. IgM and IgG3 TNP-specific antibody responses in the mutant mice were comparable to those in the control mice, whereas IgG1 and IgG2b anti-TNP antibody responses were significantly augmented (Figure 4B). These results suggest that Hif-1α-deficient T cells can support an antigen-specific antibody response mediated by B cells in vivo but that Hif-1α in T cells is necessary for regulation of antibody response to a thymus-dependent antigen.
Figure 4.
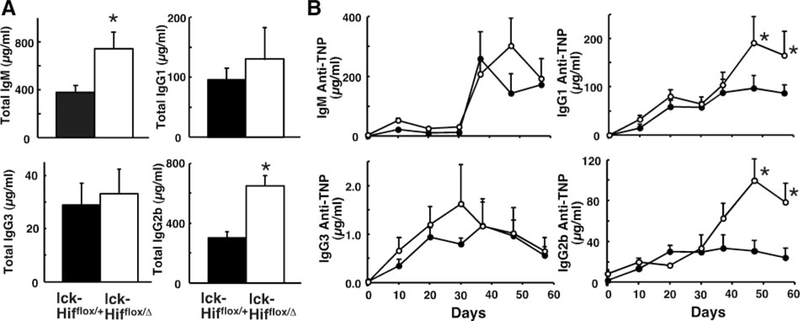
Thymus-dependent antibody responses in Lck-Cre;Hif-1αflox/+ and Lck-Cre;Hif-1αflox/Δ mice. A, Preimmune serum immunoglobulin levels in Lck-Cre;Hif-1αflox/+ (shaded bars) or Lck-Cre;Hif-1αflox/Δ (unshaded bars) mice as measured by enzyme-linked immunosorbent assay. *P_0.05. B, Antigen-specific immune responses in Lck-Cre;Hif-1αflox/+ (shaded circles) or Lck-Cre;Hif-1αflox/Δ (unshaded circles) mice immunized with trinitrophenyl (TNP)–keyhole limpet hemocyanin (KLH). Eight-week-old mice were immunized with TNP-KLH in incomplete Freund adjuvant on day 0 and boosted with the antigen in phosphate-buffered saline on day 30. Values are expressed as meanαSE. Data represent the serum antibody titers to TNP for five individual mice. *P¼0.05.
Unimpaired Chemotactic Activity of Hif-1 α-Deficient T Cells Toward Stroma Cell-Derived Factor 1 in an In Vitro Experiment
Hif-1α has been reported to be involved in neointima formation after vascular injury and to mediate the upregu-lation of stroma cell-derived factor 1 (SDF-1).24 To investigate the role of Hif-1α in T cells in chemokine-mediated migration of T cells, we next examined the chemotactic activity of Hif-1α-deficient T cells to SDF-1, which is expressed in the vascular region.24 We did not find distinct responses of the Hif-1α-deficient T cells or control cells toward SDF-1 under a hypoxic condition (Figure 5), suggesting that the Hif-1α deficiency in T cells does not alter the function of T cells in SDF-1-mediated migration under a hypoxic condition.
Figure 5.
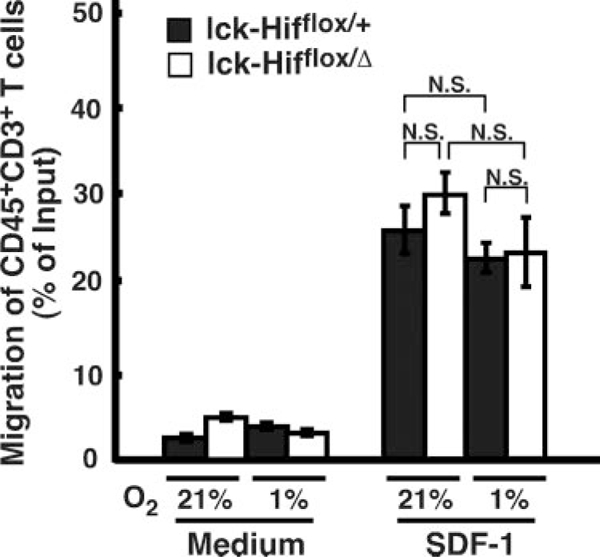
Transwell chemotaxis of Hif-1α-deficient T cells to stroma cell-derived factor (SDF) 1. Freshly isolated splenocytes were examined for chemotactic responses to recombinant SDF-1 (250 ng/mL) after 2 hours of incubation. The responses are expressed as percentage of migrated CD45+CD3+ T cells within input total cells. Migrated cells were stained for CD45 and CD3 to identify the subset of T-cell subpopulations in total splenocytes. Values are expressed as meanαSE. NS indicates nonsignificant.
Enhancement of Intimal Hyperplasia in T-Cell-Specific Hif-1α-Deficient Mice by Arterial Cuff Injury
To clarify the function of Hif-1α in T cells in vascular remodeling, we examined morphological changes of the injured artery six weeks after polyethylene tube cuff placement using T-cell-specific Hif-1α-deficient mice. Morphological changes were estimated by changes in thickening of the vascular wall after cuff injury. No morphological differences were observed in uninjured femoral arteries between the control and Lck-Cre;Hif1αflox/Δ mice. Cuff-injured femoral arteries in the mutant mice showed a prominent increase in the intimal area (indicated by arrowheads in Figure 6A) but not in the medial area compared with those in the control mice (Figure 6A). As shown in Figure 6B, the ratios of intima to media and intima to lumen in the mutant mice that had been subjected to cuff injury were also significantly increased compared with those in the controls. Furthermore, immuno-histological studies showed that the numbers of CD3-positive T cells and CD79a-positive B cells were increased at the inflamed adventitia in the mutant mice, although increases in these cells were not found at the intima and media (Figure 6C). These results indicate that Hif-1α deficiency in T cells augments the intimal hyperplasia developed by cuff-injured femoral arteries, accompanying infiltration of inflammatory cells.
Figure 6.
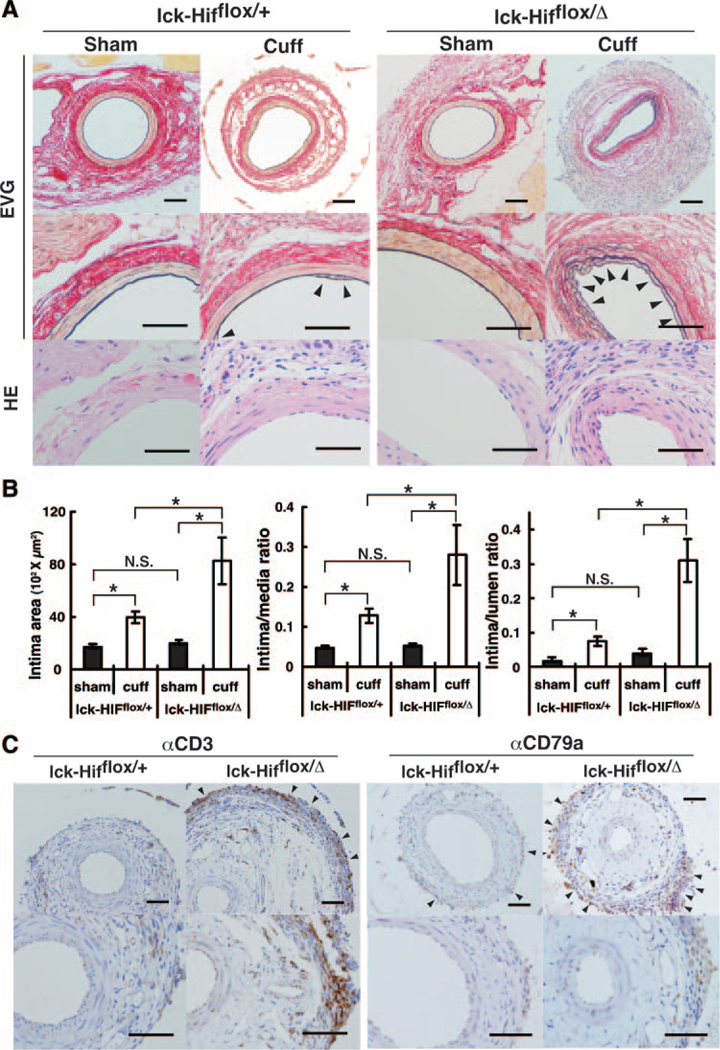
Neointimal formation after cuff placement in Lck-Cre;Hif-1αflox/+ and Lck-Cre;Hif-1αflox/Δ mice. A, Histologica analyses of uninjured and cuff-injured femoral arteries. Representative cross sections with hematoxylin-eosin (HE) and (EVG) staining of the cuffed femoral artery at 42 days after cuff placement. Arrowheads indicate neointima formation. B, Quantitative analysis of arterial intimal area, ratio of intimal area to medial area, and ratio of intimal area to luminal area in uninjured and cuff-injured femoral arteries. Values are expressed as meanαSD. *P<0.05. NS indicates nonsignificant. C, Immunohistochemical analyses for αCD3 and αCD79a in uninjured and cuff-injured femoral arteries. Arrowheads indicate infiltration of immune cells. The bar indicates 200 μm.
Discussion
In this study, we investigated the role of Hif-1α expressed in T cells in vascular remodeling by using an arterial cuff injury model, because characteristics of the cellular immune response to vascular remodeling by arterial injury accompanied by a hypoxic microenvironment during the inflammatory progression are likely to influence the resulting pathological alterations.6–11 We first showed that hypoxia was present at the vascular wall around the cuff-injured artery and that Hif-1α-expressing T cells infiltrated around the hypoxic vascular region. These results suggest that the function of infiltrating T cells around cuff-injured vessels in the development of vascular remodeling might be modulated by Hif-1α in a hypoxic microenvironment, even though there are many other modulators of Hif-1α in addition to hypoxia in the vascular wall, such as inflammation and oxidative stress (ie, nitric oxide, tumor necrosis factor α,15 angiotensin II,16 IL-1, insulin, and insulinlike growth factors).17,18
The results of our in vitro experiments showed that chemotactic activity of Hif-1α-deficient T cells toward SDF-1 was not impaired. These results are consistent with other results of the present study showing that migration of even Hif-1α-deficient T cells to areas around cuff-injured vessels was not impaired in mice. These results suggest that aggravation of vascular inflammation and remodeling in response to cuff injury may not result from dysfunction of chemotactic activity of T cells in Hif-1α-deficient mice and that another T cell-mediated aberrant immunologic response may be involved in its pathogenesis.
The present study also demonstrated that Hif-1α in T cells was a negative regulator of T cell-mediated immune response to vascular remodeling, which includes regulation of the production of cytokines and specific antibodies. Thus, Hif-1α in T cells is required for regulation of the adaptive immune response, including both cellular and humoral immunity. These results were relevant to a recent article20 showing that a defect of Hif-1α in T cells improved septic pathogenesis via their functional inhibition in a genetic mouse model; therefore, Hif-1α is a negative regulator of T-cell response in vivo in models of acute inflammation. The present study further demonstrated that the function of Hif-1α in T cells extends to the adaptive immune system, including humoral immune responses.
Recent studies2–4 have shown that T and B cells contribute to the modulation of vascular remodeling by arterial injury. The results of these studies have suggested that the adaptive immune system evoked by arterial tissue injury serves to limit the extent of the subsequent vascular repair process and that the mechanisms involved in restriction of the vascular repair process might include inhibition of smooth muscle cell growth by T cell-derived IFN-γ and antibody-mediated removal of proinflammatory debris. The present study showed that aberrant production of IFN-γ and antibodies in mice lacking the Hif-1α gene in T cells result in enhancement of arterial injury-mediated vascular remodeling (including neointima formation), indicating that immune response by Hif-1α in T cells is strictly regulated during vascular remodeling.
Previous studies showed that T helper type1 (Th1) cytokines, especially IFN-γ, might be key factors for inducing vascular intimal hyperplastic lesions as well as more typical vascular remodeling.25 This is supported by the results of the present study showing enhanced IFN-γ production in activated T cells lacking expression of the Hif-1α gene. On the other hand, production of transcripts encoding IL-4 and IL-13, T helper type2 (Th2)-related cytokines, was also not impaired, suggesting possible roles for Hif-1 as a negative regulator in T-cell activation. Furthermore, enhanced IL-2 production in Hif-1α-deficient T cells may result in increased T-cell numbers in peripheral lymphoid organs in the mutant mice. Taken together, these results suggest that mechanisms involved in excess vascular remodeling resulting from Hif-1α deficiency in T cells include aberrant production of cytokines in injured arterial tissues.
However, it remains to be elucidated how Hif-1α regulates TCR-mediated T-cell activation as a suppressive modulator in Th1 and Th2 cytokine production. The mechanisms involved in aberrant cytokine production in Hif-1α- deficient T cells remain to be elucidated; they may include additional transactivation of the IL-2 gene by crosstalk between the arylhydrocarbon receptor and Hif-1α/β signaling pathways. Indeed, previous studies revealed that the IL-2 promoter region contains novel distal regulatory elements for the arylhydrocarbon receptor, another competitive heterodimeric partner of Hif-1β, which can induce IL-2 and can cooperate in modulation of the proximal promoter.26 This finding suggests that the deficiency in Hif-1α allows the arylhydrocarbon receptor to recruit more Hif-1β, resulting in activation of arylhydrocarbon receptor/Hif-1β signaling and resultant IL-2 production in T cells.
Regarding autoreactive inflammation in the development of vascular remodeling by arterial injury, the present study showed an increased production of antibodies against specific antigens in Hif-1α-deficient mice, indicating possible roles of Hif-1α in enhancement of autoreactivity. Also, autoimmunity and inflammatory tissue damage were found in chimeric mice with complete Hif-1 α deletion in T and B cells.27 However, it remains to be elucidated how Hif-1α in T cells modulates autoreactivity against injured artery tissue. To examine autoimmunity in Hif-1α-deficient T cells in vivo, we characterized morphological phenotypes in the kidney and liver and determined the number of regulatory suppressor T cells in the thymus. However, no significant difference was found in the morphology of these organs, even though the amounts of antibodies in serum were increased, or in the number of Foxp3-positive cells, a marker of regulatory suppressor T cells, which are localized in thymic medullar regions (data not shown). Thus, the autoreactive phenotype observed in the previous study may result from a lack of normal B-cell functions by deficiency of Hif-1α in B cells in Lck-Cre;Hif1αflox/Δ mice.
Overall, the modulations of initiation and progression of vascular remodeling in response to arterial injury by Hif-1α in T cells may suppress immune responses in hypoxic regions in the body and represent a protective mechanism against unnecessary and excess immune responses, such as in autoimmune and allergy diseases. This implies that control of the hypoxic microenvironment by modulation of Hif-1α activity in arteriosclerosis or in-stent restenosis remains a possible therapeutic target for local intervention.
Supplementary Material
Acknowledgments
We thank Max Gassmann, DVM, PhD for kindly providing the antibody against mouse Hif-1α and Toshio Matsumoto, MD, PhD for helpful discussions.
Sources of Funding
This study was supported by grant 21590335 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Dr Tomita).
Footnotes
Disclosures
None.
References
- 1.Mitchell RN, Libby P. Vascular remodeling in transplant vasculopathy. Circ Res. 2007;100:967–978. [DOI] [PubMed] [Google Scholar]
- 2.Dimayuga PC, Li H, Chyu KY, Fredrikson GN, Nilsson J, Fishbein MC, Shah PK, Cercek B. T cell modulation of intimal thickening after vascular injury: the bimodal role of IFN-γ in immune deficiency. Arterioscler Thromb Vasc Biol. 2005;25:2528–2534. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Holm J, Holm S, Fotev Z, Hedrich HJ, Fingerle J. T lymphocytes inhibit the vascular response to injury. Proc Natl Acad Sci USA. 1991;88:10530–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remskar M, Li H, Chyu KY, Shah PK, Cercek B. Absence of CD40 signaling is associated with an increase in intimal thickening after arterial injury. Circ Res. 2001;88:390–394. [DOI] [PubMed] [Google Scholar]
- 5.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. [DOI] [PubMed] [Google Scholar]
- 6.Nakano D, Hayashi T, Tazawa N, Yamashita C, Inamoto S, Okuda N, Mori T, Sohmiya K, Kitaura Y, Okada Y, Matsumura Y. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E-knockout mice. Hypertens Res. 2005;28:837–845. [DOI] [PubMed] [Google Scholar]
- 7.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau AK, Chaufour X, McLachlan C, Leichtweis SB, Celermajer DS, Sullivan C, Stocker R. Intimal thickening after arterial balloon injury is increased by intermittent repetitive hypoxia, but intermittent repetitive hyperoxia is not protective. Atherosclerosis. 2006;185: 254–263. [DOI] [PubMed] [Google Scholar]
- 9.Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870 – 876. [DOI] [PubMed] [Google Scholar]
- 10.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51: 1258 −1265. [DOI] [PubMed] [Google Scholar]
- 11.Vink A, Schoneveld AH, Lamers D, Houben AJ, van der Groep P, van Diest PJ, Pasterkamp G. HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages [published online ahead of print July 2, 2007]. Atherosclerosis. 2007;195:e69–e75. [DOI] [PubMed] [Google Scholar]
- 12.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 α and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. [DOI] [PubMed] [Google Scholar]
- 14.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304 −4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandau KB, Zhou J, Kietzmann T, Brune B. Regulation of the hypoxiainducible factor 1α by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001;276:39805–39811. [DOI] [PubMed] [Google Scholar]
- 16.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. [DOI] [PubMed] [Google Scholar]
- 17.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Burgel T. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512:157–162. [DOI] [PubMed] [Google Scholar]
- 18.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277:27975–27981. [DOI] [PubMed] [Google Scholar]
- 19.Makino Y, Nakamura H, Ikeda E, Ohnuma K, Yamauchi K, Yabe Y, Poellinger L, Okada Y, Morimoto C, Tanaka H. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J Immunol. 2003; 171:6534 −6540. [DOI] [PubMed] [Google Scholar]
- 20.Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P, Ohta A, Lentsch AB, Lukashev D, Sitkovsky MV. Targeted deletion of HIF-1α gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE. 2007;2:e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aihara K, Azuma H, Akaike M, Ikeda Y, Sata M, Takamori N, Yagi S, Iwase T, Sumitomo Y, Kawano H, Yamada T, Fukuda T, Matsumoto T, Sekine K, Sato T, Nakamichi Y, Yamamoto Y, Yoshimura K, Watanabe T, Nakamura T, Oomizu A, Tsukada M, Hayashi H, Sudo T, Kato S. Strain-dependent embryonic lethality and exaggerated vascular remodeling in heparin cofactor II-deficient mice. J Clin Invest. 2007;117: 1514 −1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide]: analysis of drug adducts by fluorescent antibodies vs bound radioactivity. Br J Cancer. 1995;72:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, Gonzalez FJ, Takahama Y. Defective brain development in mice lacking the Hif-1α gene in neural cells. Mol Cell Biol. 2003;23:6739–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karshovska E, Zernecke A, Sevilmis G, Millet A, Hristov M, Cohen CD, Schmid H, Krotz F, Sohn HY, Klauss V, Weber C, Schober A. Expression of HIF-1α in injured arteries controls SDF-1alpha mediated neointima formation in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2540–2547. [DOI] [PubMed] [Google Scholar]
- 25.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E_/_ mice. Am J Pathol. 2000;157:1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon MS, Esser C. The murine IL-2 promoter contains distal regulatory elements responsive to the Ah receptor, a member of the evolutionarily conserved bHLH-PAS transcription factor family. J Immunol. 2000;165: 6975–6983. [DOI] [PubMed] [Google Scholar]
- 27.Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α-deficient chimeric mice.Proc Natl Acad Sci U S A. 2002;99:2170-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


