Supplemental Digital Content is available in the text.
Keywords: biomarkers, brain ischemic, hemostasis, prognosis, systematic review
Abstract
Objectives—
The prediction of patients at risk for poor clinical outcome after acute ischemic stroke remains challenging. An imbalance of coagulation factors may play an important role in progression and prognosis of these patients. In this systematic review, we assessed the current literature on hemostasis biomarkers and the association with poor clinical outcome in acute ischemic stroke.
Approach and Results—
A systematic search of Embase, Medline, Cochrane Library, Web of Science, and Google Scholar was performed on studies reporting on hemostasis biomarkers and clinical outcome after acute ischemic stroke. Studies were considered eligible if blood samples were collected within 72 hours after symptom onset. Additionally, clinical outcome should be assessed using a disability score (Barthel Index or modified Rankin scale). Methodological quality of included studies was assessed with an adapted version of the Quality Assessment of Diagnostic Accuracy Studies questionnaire. A total of 80 articles were read full text, and 41 studies were considered eligible for inclusion, reporting on 37 different hemostasis biomarkers. No single biomarker appeared to be effective in predicting poor clinical outcome in acute ischemic stroke patients.
Conclusions—
Based on current literature, no clear recommendations can be provided on which hemostasis biomarkers are a predictor of clinical outcome after acute ischemic stroke. However, some biomarkers show promising results and need to be further investigated and validated in large populations with clear defined study designs.
Highlights.
The prediction of patients at risk for poor clinical outcome after acute ischemic stroke remains challenging.
Hemostasis biomarkers might render the possibility to differentiate which patients are at risk of poor clinical outcome.
Based on current literature, no clear recommendations can be provided on which hemostasis biomarkers are a predictor of clinical outcome after acute ischemic stroke.
The vast majority of ischemic strokes are the consequence of thrombotic or thromboembolic occlusion of one or more cerebral arteries, although in some patients small vessel occlusion, vasculopathy, or hemodynamic factors may play a role. The formation and lysis of an obstructing clot and perhaps the patency of the microvascularature in the ischemic area may in part be determined by coagulation and fibrinolytic activity in the circulating blood. An imbalance of coagulation factors may play an important role in progression and outcome of ischemic stroke. Many previous studies investigated the association between hemostasis blood biomarkers and the risk of arterial thrombosis, including ischemic stroke.1,2 Increased levels of specific biomarkers, including VWF (von Willebrand Factor), fibrinogen, and D-dimer, have shown to be risk factors for acute ischemic stroke.1,3,4
The ability to predict clinical outcome after ischemic stroke may help to improve the selection of the most appropriate therapy (systemic thrombolytic, antithrombotic, and/or intraarterial interventions) already in the acute phase in the individual patient. Currently, clinicians are unable to predict the effect of reperfusion therapy and thereby clinical outcome after ischemic stroke. Since the coagulation system plays an important role in stroke pathogenesis, blood biomarkers of coagulation might render the possibility to differentiate which patients are at risk of poor clinical outcome.
Therefore, the aim of this systematic review was to assess the available literature on data regarding the predictive value of hemostasis biomarkers in acute ischemic stroke in relation to poor clinical outcome.
Methods
This systematic review was prepared in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.5
Article Search
We systematically searched the following databases: Embase, Medline, Cochrane Library, Web of Science, and Google Scholar. A search strategy was constructed in collaboration with a biomedical information specialist of the Erasmus Medical Centre Medical Library (online-only Data Supplement). There were no restrictions regarding year of publication. The search was performed on September 22, 2017, and repeated on June 20, 2018.
Study Selection
For this systematic review, we included case–control studies and cohort studies, as well as prospective and retrospective studies. Studies were considered eligible when they met the following criteria: (1) involving patients with acute ischemic stroke; (2) patients ≥18 years of age; (3) computed tomography or magnetic resonance imaging should be performed to exclude hemorrhage and thus confirm the clinical diagnosis of ischemic stroke; (4) a venous blood biomarker of hemostasis should be assessed within 72 hours after symptom onset, and the study should report on the relationship between biomarker level and clinical outcome; (5) clinical outcome should be assessed with the use of a disability or handicap scale (modified Rankin scale [mRS] or Barthel Index). We excluded reviews, abstracts from congresses, letters, editorials, and case reports. Studies written in languages other than English or Dutch were excluded. Duplicates were removed using Endnote database. During the first phase of the review process, 2 authors (S.J. Donkel and B. Benaddi) independently selected relevant studies based on title and abstract. Studies included during the first phase were read in full text, after which studies meeting the eligibility criteria were included. Disagreements were resolved by consensus.
Data Extraction
Data were extracted by 2 authors (S.J. Donkel and B. Benaddi) with the use of standardized forms. The following data were collected: first author, publication year, study design, sample size, type of biomarker, assay used to measure the biomarker, time of blood collection, National Institutes of Health Stroke Scale (NIHSS) on admission, mean or median age of study population, proportion of male patients, time of outcome assessment, type of disability scale used to define clinical outcome, definition of poor outcome reported in the study, and level of biomarker in patients with poor and good outcome. For studies including both ischemic and hemorrhagic stroke, isolated data for ischemic stroke were retracted. For studies reporting on more than one blood biomarker, each biomarker is reported separately. When levels of biomarkers were measured at more than one time point, the first biomarker measurement was used. If clinical outcome was assessed at more than one time point, the time point nearest to 3 months was used for this review. Studies that did not report on definition of outcome, poor outcome was defined as mRS score >2 or Barthel Index <85. When possible, we stratified for type of reperfusion therapy (intravenous r-TPA [recombinant tissue-type plasminogen activator] or mechanical thrombectomy).
Quality Assessment
The quality of individual studies was assessed with an adapted version of the Quality Assessment of Diagnostic Accuracy Studies questionnaire (Figure in the online-only Data Supplement).6 This checklist contains 15 items which can be scored as yes (+), no (−), or unclear (?), for a maximum score of 15 points. All studies that met the inclusion criteria were included in this systematic review, irrespective of the quality score.
Results
Study Selection
The search yielded a total of 6404 articles. After elimination of duplicates, 4044 articles were screened based on title and abstract (Figure). After the initial selection process, 80 articles were read full text. Finally, a total of 41 articles were considered eligible for inclusion.
Figure.
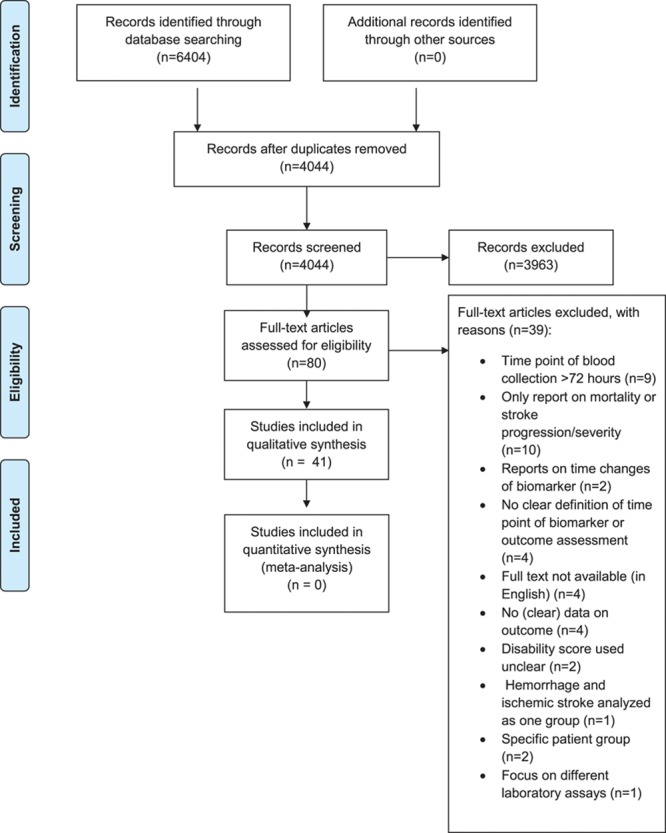
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of study selection.
Study Characteristics
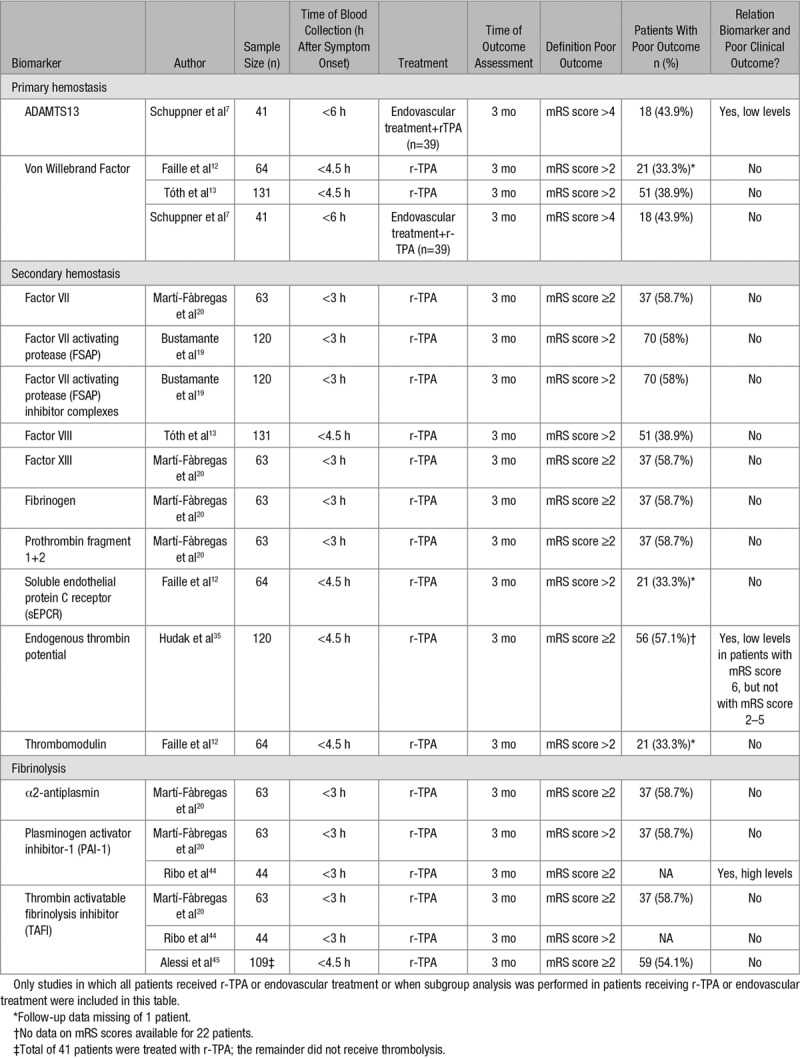
Forty-one studies reported a total of 37 different hemostatic biomarkers in 13 569 patients (Table 1), with a mean age ranging from 54.7 to 76 years; 8434 patients (62%) were male. The sample size of individual studies ranged from 41 to 3212 patients. Nineteen studies reported on more than one biomarker. The time point of blood collection ranged from 3 to 72 hours after symptom onset. All studies reported on clinical outcome using a disability score for outcome assessment; most studies used the mRS. Definition of poor outcome varied between individual studies. However, most studies defined poor outcome as mRS score >2. Nine studies reported on clinical outcome at discharge, 10 studies assessed clinical outcome at 1 month, and 22 studies assessed clinical outcome at 3 months from stroke onset or more. Eight studies measured biomarker levels before the initiation of r-TPA or endovascular treatment (Table 2).
Table 1.
Overview of Studies Included in Systematic Review
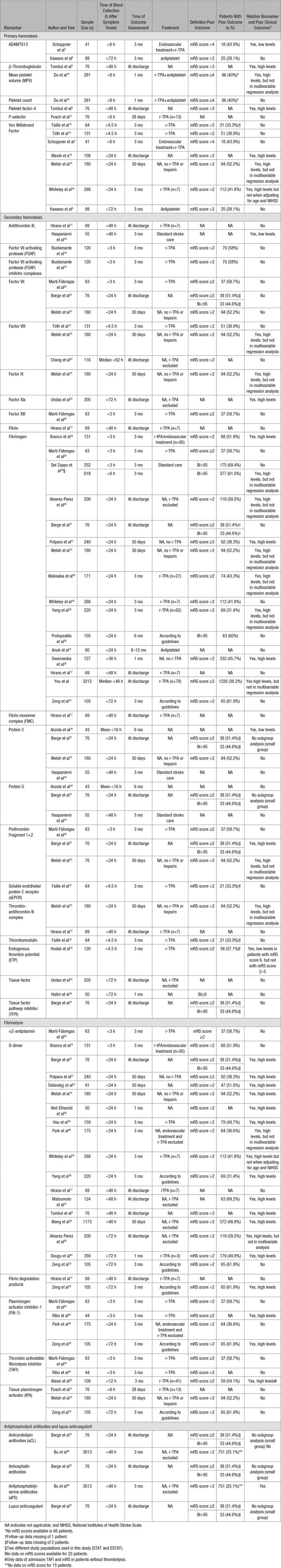
Table 2.
Overview of Biomarkers Measured Before Start of r-TPA or Endovascular Treatment
Quality Assessment
The overall quality of the included studies was moderate (median 9 points of a maximum of 15 points, ranging from 7 to 13 points). The quality of the study design, as marked by the quality score, did not directly influence the results found by individual studies. However, only a few studies reported on blinding of measurement of individual biomarkers (12/42) and clinical data collection (6/42), and only one study performed a sample size calculation to justify the number of included patients (Table in the online-only Data Supplement). None of the studies used a predefined biomarker cutoff value for poor clinical outcome.
Primary Hemostasis
A total of 7 different biomarkers of primary hemostasis were measured in 10 studies, encompassing a total of 1314 patients of whom 690 (52.5%) were male and with a mean age ranging from 64.7 to 74.4 years.7–16 Platelet count was measured in 281 patients <8 hours after symptom onset and showed no significant association with poor outcome.10 In the same study, mean platelet volume was significantly higher in patients with poor outcome compared to patients with a good prognosis.10 After adjustment for stroke severity at baseline (measured with the NIHSS), no significant association was detected. Additionally, in this study, poor outcome was defined as an mRS score ≥4 which is relatively high as compared with other studies. When focusing on markers of platelet activation, P-selectin levels, measured <6 hours after symptom onset, were investigated in one study with a total of 76 patients and showed no association with poor outcome.11 β-Thromboglobulin and platelet factor-4 levels measured <48 hours after symptom onset were shown to increase with ascending mRS score in one study conducted in 76 patients.9
A total of 7 studies, including 881 patients, reported on VWF:Ag (Von Willebrand Factor antigen) levels and outcome after stroke.7,8,12–16 Most studies used an ELISA for measuring VWF:Ag levels in plasma.7,12,15,16 Two studies determined VWF:Ag levels with immunoturbidimetric assays.13,14 Latex agglutination assays were used in one study.8 Significantly higher levels of VWF:Ag at admission in patients with a poor clinical outcome were found in 3 studies.14–16 However, this association remained significant in only one study after adjustment for possible confounders including age, sex, diabetes mellitus, and hypertension.14 Three studies collected blood samples before the infusion of r-TPA (Table 2).7,12,13 Additionally, in one of these studies, endovascular treatment was performed after infusion of r-TPA.7 VWF:Ag levels did not show to be associated with clinical outcome after 3 months when measured before reperfusion treatment.7,12,13
Large VWF multimers are cleaved into smaller and less active forms by ADAMTS13 (A Disintegrin And Metalloprotease with ThromboSpondin motif repeats 13). Two studies reporting on ADAMTS13 and clinical outcome were included in this review.7,8 ADAMTS13 activity showed no significant association with stroke outcome in one study performed in 89 patients when measured <72 hours after symptom onset.8 This is in contrast to a prospective study conducted in 41 acute ischemic stroke patients with a large vessel occlusion treated with r-TPA followed by endovascular treatment.7 The authors reported that low ADAMTS13 activity before the start of treatment was independently associated with unfavorable outcome. Unfavorable outcome was defined as mRS score 5 to 6, which is higher than the common cutoff value of 2.
Secondary Hemostasis
Twenty biomarkers of secondary hemostasis were assessed in 27 different studies, with a total of 7645 patients.13,15–33,47 Mean age of patients ranged from 53 to 75.9 years, and 4688 (61.3%) were male. Soluble tissue factor, the initiator of secondary hemostasis, and tissue factor pathway inhibitor did not show a significant association with clinical outcome.21,23,36 A total of 5 coagulation factors were identified in this review. FVII (Factor VII), FVII-activating protease, and FVII-activating protease inhibitor complexes were not associated with stroke outcome.15,19–21 One out of 3 studies on factor VIII and stroke outcome found that high factor VIII levels, measured <24 hours after symptom onset, were related to poor outcome after 30 days.15 However, there was no association when adjusting for demographic and clinical variables. The same study found similar results for high levels of factor IX and poor outcome. Levels of activated FXIa (factor XI) measured <72 hours after symptom onset and stroke outcome at discharge were described in one study, which found a significantly lower Barthel Index and higher mRS scores in patients with the presence of circulating FXIa (respectively, P=0.023 and P=0.037).23 FXIII showed no relationship with clinical outcome.20
Four studies evaluated protein C levels in patients with ischemic stroke, with a total of 354 patients.15,18,21,34 Only one study in 43 patients showed that patients with low protein C concentration were more impaired on the clinical severity scores and had higher mortality rates at 6 months when compared with patients with normal levels of protein C.34 Studies on protein S levels did not show an association with ischemic stroke outcome.18,21,34
Two out of 3 studies evaluating prothrombin fragment F1+2 showed a positive association with poor outcome at discharge and after 30 days.15,21 Prothrombin F1+2 measured before reperfusion treatment showed no association with 3-month clinical outcome.20 Thrombin generation assays were used to assess thrombin formation in plasma of ischemic stroke patients before the infusion of r-TPA in one study including 120 patients.35 The authors found that a decrease in the total amount of thrombin generated (endogenous thrombin potential) from patients’ plasma was an independent predictor for mortality (mRS score 6) after 3 months. These results were explained by ongoing activation of coagulation, resulting in consumption of coagulation factors, thereby decreasing thrombin generation. Two studies measured antithrombin levels in plasma within 48 hours after symptom onset.17,18 One study found a weak to moderate correlation (r=0.3, P=0.02) between antithrombin and outcome after 3 months as assessed with Barthel Index. However, there was no significant correlation when outcome was assessed using mRS (r=0.3, P=0.06).18 Furthermore, one study including 180 patients found significantly higher levels of thrombin-antithrombin complex, measured <24 hours after symptom onset in patients with mRS score >2 compared to patients with good outcome after 30 days (respectively, 4.5 and 3.7 μg/L; P=0.04).15 No significant association between high levels of thrombin-antithrombin complex and poor outcome was found in multivariable regression analysis.
A total of 16 studies encompassing 6697 patients evaluated fibrinogen in relation to stroke outcome.15–17,20,21,24–33,47 Nine (56%) studies with a total of 5699 patients reported that high fibrinogen levels were related to poor outcome.15,24–29,32,47 Fibrinogen levels were measured according to the Clauss method in most studies.15,20,25,26,31,32 One study found that fibrinogen levels of >3.7 g/L were associated with poor clinical outcome at 30 days after ischemic stroke with a sensitivity of 79.3% and a specificity of 80.4%.27 Most studies adjusted for age and sex in regression models. Only a few studies reported adjustments for acute inflammation (C-reactive protein), stroke severity, and the use of thrombolytic therapy. Treatment regimens used in individual studies and whether blood samples were drawn before start of treatment were not mentioned in most studies. Six studies did not find a significant association between fibrinogen levels and stroke outcome after multivariable analysis.15,25,26,28,29,47 Fibrinogen levels were measured before the infusion of r-TPA in one study and showed no association with clinical outcome 3 months after ischemic stroke.20 One study measured fibrinogen levels within 6 hours after stroke onset in the placebo group of 2 different study cohorts.25 The authors found a significantly higher proportion of patients with lower fibrinogen levels (<450 mg/dL) who had a good outcome after 3 months in one of the cohorts. However, these results could not be demonstrated in the other cohort. One large multicenter study including 3212 acute ischemic stroke patients found a significant association between high fibrinogen levels (>4.0 g/L) and poor outcome when adjusting for age and sex (odds ratio [OR], 1.42; 95% confidence interval [CI], 1.16–1.74; P=0.001).47 When more confounding factors were added to the model (eg, NIHSS, medical history, smoking status), this association was no longer significant (OR, 1.15; 95% CI, 0.86–1.53; P=0.338). The authors found a 1.76-fold increase in the risk of in-hospital mortality (hazard ratio, 1.76; 95% CI, 1.10–2.81; P=0.019) in patients with high fibrinogen levels.
Fibrinolysis
A total of 6 biomarkers of fibrinolysis were measured in 21 different studies with a total of 3988 patients.9,11,15–17,20,21,24,26,27,29,33,37–45 Seventeen studies including 3696 patients reported on D-dimer and clinical outcome after ischemic stroke.9,15–17,21,24,26,27,29,33,37–43 Eight studies determined D-dimer levels with (latex enhanced) immunoturbidimetric assays.17,24,26,29,38,39,41,42 ELISAs were used in 6 studies9,15,16,21,33,40 and one study measured D-dimer with Enzyme-linked Fluorescence Assays.27 Except for 3 studies, all studies reported an association between high D-dimer levels and poor outcome with a high sensitivity of 77% to 87% and a moderate specificity of 40% to 79.7% (cutoff values ranging from 0.1 to 1.99 mg/L).15,27,29,38 Studies using multivariable regression analysis were mostly adjusted for age and sex. Adjustments for stroke severity at admission were made in 6 studies.15,16,26,29,39,40 The association between high D-dimer levels and poor outcome did not remain significant after adjustment for possible confounders in 3 studies.16,26,40 Since D-dimer is a degradation product of fibrin and levels increase as a result of thrombolytic therapy, treatment regimens used in individual patients and the precise moment of blood collection are important factors to consider; individual studies are heterogeneous in reporting on these important confounders. Two studies reported patients receiving thrombolytic therapy were excluded.40,41 On the other hand, one study only included patients with an available blood sample for D-dimer level measurement after the initiation of thrombolytic therapy.39 Another study reported 3 patients were treated with intravenous r-TPA after blood samples were collected.43 It remained unclear whether the authors adjusted for thrombolytic therapy. Two other studies specifically mentioned that patients did not receive any thrombolytic agents.15,27 Thrombolytic therapy was included in regression models in one study.29 Blood samples were collected before any kind of reperfusion treatment was started in 3 studies.16,24,37 The majority of studies did not describe what treatment patients received or if blood samples were obtained before treatment was initiated. Fibrin degradation products were evaluated in 2 studies of which one study found significantly higher levels of fibrin degradation products, measured <72 hours after symptom onset, in patients with poor outcome compared with patients with good outcome (respectively, 1.60 mg/L [0.90–5.60 mg/L] and 0.90 mg/L [0.60–2.10 mg/L]; P=0.033).33 The authors determined sensitivity and specificity for fibrin degradation products with a cutoff point of 0.95 mg/L using receiver operator characteristic curve (ROC) analysis and found a sensitivity of 72.0% and a specificity of 56.7%. The same study observed similar results with plasminogen activator inhibitor-1 (PAI-1), an inhibitor of fibrinolysis. PAI-1 levels >21.65 ng/mL predicted poor outcome, with 83.3% sensitivity and 66.7% specificity. Another study that measured PAI-1 levels before the initiation of reperfusion therapy also found significantly higher levels in patients with poor outcomes.44 High levels of TAFI (thrombin activatable fibrinolysis inhibitor) were associated with poor outcome in patients without thrombolysis in one study including 109 patients.45 In the same study, TAFI levels of patients that did receive r-TPA after blood samples were collected were not associated with poor clinical outcome. Two other studies with a total of 107 patients did not find any relation between TAFI and stroke outcome.20,44 TPA (tissue-type plasminogen activator) antigen and α2-antiplasmin were not associated with stroke outcome.11,15,20,33
Antiphospholipid Antibodies and Lupus Anticoagulant
Two studies measured 4 different antiphospholipid antibodies in a total of 3089 patients.21,46 Data on the association with stroke outcome and anticardiolipin antibodies, anticephalin antibodies, and lupus anticoagulant in one of these studies were lacking because of small number of patients with positive antibodies (6 positive patients for every antibody).21 The presence of antiphosphatidylserine antibodies in plasma of ischemic stroke patients showed a significant association with mortality and major disability at 3 months after adjusting for age, sex, NIHSS, and cardiovascular risk factors (OR, 1.10; 95% CI, 1.01–1.21; P=0.03) in one study.46
Discussion
Despite the availability of substantial clinical research data on hemostasis biomarkers in relation to clinical outcome after ischemic stroke, the value of such biomarkers as independent predictors is still unproven. Although this general conclusion may point to an inability of hemostasis markers in the acute phase to predict a complex pathophysiological process in the long run, several methodological issues of the studies included in this review are also point of discussion. Furthermore, we included studies written in Dutch or English; therefore studies written in different languages are potentially excluded. The overall quality of included studies was moderate. However, the items on the quality assessment tool used in this systematic review did not assess methodological aspects important for this systematic review, for instance time point of blood collection. Furthermore, there was a lot of clinical and methodological heterogeneity between studies included in this systematic review, for instance, regarding the inclusion of patients with all different ischemic stroke subtypes. For example, previous studies found that VWF levels differed between stroke subtypes, and highest levels of VWF were found in patients with large artery atherosclerosis and cardioembolic strokes.48,49 Therefore, it might be that the underlying stroke pathology drives the concentration of certain biomarkers, such that the potential predictive value for clinical outcome is obscured in the acute phase. Additionally, bias may have been introduced by the fact that different assays were used for measuring biomarker levels. For example, for D-dimer, it is known that there is a lot of interassay variability because of different specificity of monoclonal antibodies depending on the fibrin fragments used as the immunogen as well as the material used to calibrate the assay.50
Most studies did not report on the type of treatment that was applied in the included patients and whether blood samples were collected before or after the start of treatment. Therefore, it is unclear whether high levels of biomarkers reflect the effect of thrombolytic therapy rather than a causal, patient-related risk factor. For instance, significantly higher D-dimer levels are found in patients receiving r-TPA compared to healthy controls and patients receiving heparin.51,52 One study, where part of the included patients were treated with r-TPA, reported that high levels of D-dimer predicted poor clinical outcome, with a sensitivity of 81.2% and a specificity of 79.7% with a cutoff value of 1.99 mL/L.29
Another critical factor is the time interval between stroke onset and blood sampling; most studies collected blood within 24 h after stroke onset, which is a fairly wide time interval. Ideally, a hemostasis biomarker of acute stroke should be assessed in the emergency setting before the start of any kind of antithrombotic treatment. In this systematic review, only 13 studies measured biomarker levels within 4.5 to 6 hours after symptom onset and before any kind of reperfusion therapy (fibrinolysis or endovascular treatment) was started. When taking all these studies together, we found that most hemostatic biomarkers were not related to clinical outcome 3 months after the event. Only 3 biomarkers (ADAMTS13, endogenous thrombin potential, and PAI-1) measured in 3 studies did show a relationship.7,35,44 However, sample sizes were small. Despite promising results in the acute phase, current literature on these biomarkers is still in its infancies and need further investigation.
An alternative approach may be to consider the value of biomarkers taken at later time points, which may still offer helpful information for secondary antithrombotic medication after initial reperfusion therapy. For instance, in the study of Tóth et al. VWF and FVIII levels assessed before start of thrombolytic therapy were not associated with poor clinical outcome.13 However, when these biomarkers were measured immediately after thrombolytic therapy, they found that high levels were associated with poor clinical outcome 3 months after stroke (respectively, OR, 6.31; 95% CI, 1.83–21.73 and OR, 7.10; 95% CI, 1.77–28.38). The optimal time point of blood collection also depends on the research question. For instance, for the choice of secondary treatment, one would want to measure a biomarker associated with clinical outcome immediately after or 24 hours after reperfusion therapy. On the other hand, to select the optimal reperfusion treatment, the biomarker measurement should be performed as soon as the patients arrive in the hospital. Therefore, it might be that more than one sampling moment is interesting depending on the research question.
Furthermore, we found that the time of clinical outcome assessment varied among studies. Some studies evaluated outcome at discharge; however, the authors did not mention the precise time from symptom onset to discharge. Currently, the best time point of clinical outcome assessment is point of discussion.53,54 The mRS, the most widely used disability score in stroke patients, has a moderate overall reliability and is generally assessed 3 to 6 months after stroke.55 When assessing outcome at discharge, the context of hospitalization makes it difficult to score activities of daily living, leading to higher mRS scores. Generally, an mRS score of >2 is considered poor clinical outcome. However, some studies used slightly lower or higher cutoff points, resulting in an under- or overestimation of the proportion of patients with poor clinical outcome. Furthermore, clinical outcome depends on several factors, including hemorrhagic transformation following thrombolysis, poor recanalization, or post-stroke infections.56 Most studies did not report on these confounding factors.
From the results obtained from current literature, it is premature to conclude that any hemostasis biomarker can be used in the clinical setting to predict which patients are at risk of poor clinical outcome after ischemic stroke. However, some biomarkers, including ADAMTS13, endogenous thrombin potential, and PAI-1 when assessed before the start of reperfusion treatment, may be promising candidates. Further adequately powered studies, with clear defined inclusion and exclusion criteria, with serial biomarker measurements at different time points during the acute phase, and with adequate statistical adjustments for possible confounders, are required to determine which hemostatic biomarkers can be used in the clinical setting. Currently, 3 randomized clinical trials investigating the effectiveness and safety of different means of acute treatment for ischemic stroke in a total of 2500 patients (MR CLEAN NO-IV57, MR CLEAN MED58 and MR CLEAN LATE59) are ongoing and involve the collection of blood samples at 3 time points in the acute phase (before, immediately after, and 24 h after reperfusion therapy) and during follow-up (1–6 months). The results of these studies will enable us to gain more knowledge and even provide new biomarkers, as well as more insight in the optimal time point for blood biomarker measurement for the prediction of clinical outcome in ischemic stroke patients.
Conclusion
Based on current literature, no clear recommendations can be provided on which hemostasis biomarkers are a predictor of clinical outcome after acute ischemic stroke. However, some biomarkers show promising results in the acute phase of ischemic stroke. Prospective studies with a homogenous study design with clearly defined time points of blood collection and outcome assessment are needed to establish or refute the role of selected and novel biomarkers in the prediction of clinical outcome.
Acknowlegdments
Additionally, we thank G. Jonge for her excellent help with the literature search used to make this systematic review.
Sources of Funding
We acknowledge the support of the Netherlands Cardiovascular Research Initiative which is supported by the Dutch Heart Foundation (CVON2015-01: CONTRAST), the support of the Brain Foundation Netherlands (HA2015.01.06), the support of Health Holland, Top Sector Life Sciences & Health (LSHM17016), and of Medtronic, Inc. The collaboration project is additionally financed by the Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships.
Disclosures
D.W.J. Dippel reports grants from Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organisation for Health Research and Development and unrestricted grants from AngioCare BV, unrestricted grants from Medtronic/Covidien/EV3, unrestricted grants from MEDAC Gmbh/LAMEPRO, unrestricted grants from Penumbra Inc., unrestricted grants from Top Medical/Concentric, unrestricted grants from Stryker and Stryker European Operations BV, all outside the submitted work. HtC is chairman of the board of the Dutch Federation of Anticoagulation Clinics and consultant to Stago. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ADAMTS13
- A Disintegrin and Metalloprotease with ThromboSpondin Motif Repeats 13
- BI
- Barthel Index
- CI
- confidence interval
- FVII
- Factor VII
- mRS
- modified Rankin scale
- NIHSS
- National Institutes of Health Stroke Scale
- OR
- odds ratio
- PAI-1
- plasminogen activator inhibitor-1
- r-TPA
- recombinant tissue-type plasminogen activator
- TAFI
- thrombin activatable fibrinolysis inhibitor
- TPA
- tissue-type plasminogen activator
- VWF
- Von Willebrand Factor
- VWF:Ag
- Von Willebrand Factor antigen
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.118.312102.
References
- 1.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, Rasmussen ML, Wu KK. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100:736–742. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 2.Sonneveld MA, de Maat MP, Portegies ML, Kavousi M, Hofman A, Turecek PL, Rottensteiner H, Scheiflinger F, Koudstaal PJ, Ikram MA, Leebeek FW. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. 2015;126:2739–2746. doi: 10.1182/blood-2015-05-643338. doi: 10.1182/blood-2015-05-643338. [DOI] [PubMed] [Google Scholar]
- 3.van Schie MC, DE Maat MP, Dippel DW, de Groot PG, Lenting PJ, Leebeek FW, Hollestelle MJ. von Willebrand factor propeptide and the occurrence of a first ischemic stroke. J Thromb Haemost. 2010;8:1424–1426. doi: 10.1111/j.1538-7836.2010.03863.x. doi: 10.1111/j.1538-7836.2010.03863.x. [DOI] [PubMed] [Google Scholar]
- 4.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115:2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Berrocoso T, Giralt D, Bustamante A, Etgen T, Jensen JK, Sharma JC, Shibazaki K, Saritas A, Chen X, Whiteley WN, Montaner J. B-type natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology. 2013;81:1976–1985. doi: 10.1212/01.wnl.0000436937.32410.32. doi: 10.1212/01.wnl.0000436937.32410.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuppner R, Dirks M, Grosse GM, Böckmann M, Goetz F, Pasedag T, Bode-Böger SM, Martens-Lobenhoffer J, Budde U, Lanfermann H, Lichtinghagen R, Weissenborn K, Worthmann H. ADAMTS-13 Activity Predicts Outcome in Acute Ischaemic Stroke Patients Undergoing Endovascular Treatment. Thromb Haemost. 2018;118:758–767. doi: 10.1055/s-0038-1637732. doi: 10.1055/s-0038-1637732. [DOI] [PubMed] [Google Scholar]
- 8.Kawano T, Miyashita K, Takeuchi M, Nagakane Y, Yamamoto Y, Kamiyama K, Manabe Y, Todo K, Metoki N, Akaiwa Y, Toyoda K, Nagatsuka K. Blood biomarkers associated with neurological deterioration in patients with acute penetrating artery territory infarction: a multicenter prospective observational study. Int J Stroke. 2018;13:207–216. doi: 10.1177/1747493016677982. doi: 10.1177/1747493016677982. [DOI] [PubMed] [Google Scholar]
- 9.Tombul T, Atbas C, Anlar O. Hemostatic markers and platelet aggregation factors as predictive markers for type of stroke and neurological disability following cerebral infarction. J Clin Neurosci. 2005;12:429–434. doi: 10.1016/j.jocn.2004.06.013. doi: 10.1016/j.jocn.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Wang Q, He B, Liu P, Chen JY, Quan H, Ma X. Association of mean platelet volume and platelet count with the development and prognosis of ischemic and hemorrhagic stroke. Int J Lab Hematol. 2016;38:233–239. doi: 10.1111/ijlh.12474. doi: 10.1111/ijlh.12474. [DOI] [PubMed] [Google Scholar]
- 11.Pusch G, Debrabant B, Molnar T, Feher G, Papp V, Banati M, Kovacs N, Szapary L, Illes Z. Early dynamics of P-selectin and interleukin 6 predicts outcomes in ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:1938–1947. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.005. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Faille D, Labreuche J, Meseguer E, Huisse MG, Ajzenberg N, Mazighi M. Endothelial markers are associated with thrombolysis resistance in acute stroke patients. Eur J Neurol. 2014;21:643–647. doi: 10.1111/ene.12369. doi: 10.1111/ene.12369. [DOI] [PubMed] [Google Scholar]
- 13.Tóth NK, Székely EG, Czuriga-Kovács KR, Sarkady F, Nagy O, Lánczi LI, Berényi E, Fekete K, Fekete I, Csiba L, Bagoly Z. Elevated factor viii and von willebrand factor levels predict unfavorable outcome in stroke patients treated with intravenous thrombolysis. Front Neurol. 2018;8:721. doi: 10.3389/fneur.2017.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menih M, Križmarić M, Hojs Fabjan T. Clinical role of von Willebrand factor in acute ischemic stroke. Wien Klin Wochenschr. 2017;129:491–496. doi: 10.1007/s00508-017-1200-4. doi: 10.1007/s00508-017-1200-4. [DOI] [PubMed] [Google Scholar]
- 15.Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27:247–253. doi: 10.1159/000196823. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- 16.Whiteley W, Wardlaw J, Dennis M, Lowe G, Rumley A, Sattar N, Welsh P, Green A, Andrews M, Sandercock P. The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke. 2012;43:86–91. doi: 10.1161/STROKEAHA.111.634089. doi: 10.1161/STROKEAHA.111.634089. [DOI] [PubMed] [Google Scholar]
- 17.Hirano K, Takashima S, Dougu N, Taguchi Y, Nukui T, Konishi H, Toyoda S, Kitajima I, Tanaka K. Study of hemostatic biomarkers in acute ischemic stroke by clinical subtype. J Stroke Cerebrovasc Dis. 2012;21:404–410. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.013. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Haapaniemi E, Tatlisumak T, Soinne L, Syrjälä M, Kaste M. Natural anticoagulants (antithrombin III, protein C, and protein S) in patients with mild to moderate ischemic stroke. Acta Neurol Scand. 2002;105:107–114. doi: 10.1034/j.1600-0404.2002.1o112.x. [DOI] [PubMed] [Google Scholar]
- 19.Bustamante A, Díaz-Fernández B, Giralt D, Boned S, Pagola J, Molina CA, García-Berrocoso T, Kanse SM, Montaner J. Factor seven activating protease (FSAP) predicts response to intravenous thrombolysis in acute ischemic stroke. Int J Stroke. 2016;11:646–655. doi: 10.1177/1747493016641949. doi: 10.1177/1747493016641949. [DOI] [PubMed] [Google Scholar]
- 20.Martí-Fàbregas J, Borrell M, Cocho D, Belvís R, Castellanos M, Montaner J, Pagonabarraga J, Aleu A, Molina-Porcel L, Díaz-Manera J, Bravo Y, Alvarez-Sabín J, Dávalos A, Fontcuberta J, Martí-Vilalta JL. Hemostatic markers of recanalization in patients with ischemic stroke treated with rt-PA. Neurology. 2005;65:366–370. doi: 10.1212/01.wnl.0000171704.50395.ba. doi: 10.1212/01.wnl.0000171704.50395.ba. [DOI] [PubMed] [Google Scholar]
- 21.Berge E, Friis P, Sandset PM. Hemostatic activation in acute ischemic stroke. Thromb Res. 2001;101:13–21. doi: 10.1016/s0049-3848(00)00380-7. [DOI] [PubMed] [Google Scholar]
- 22.Chang TR, Albright KC, Boehme AK, Dorsey A, Sartor EA, Kruse-Jarres R, Leissinger C, Martin-Schild S. Factor VIII in the setting of acute ischemic stroke among patients with suspected hypercoagulable state. Clin Appl Thromb Hemost. 2014;20:124–128. doi: 10.1177/1076029613488936. doi: 10.1177/1076029613488936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Undas A, Slowik A, Gissel M, Mann KG, Butenas S. Active tissue factor and activated factor XI in patients with acute ischemic cerebrovascular events. Eur J Clin Invest. 2012;42:123–129. doi: 10.1111/j.1365-2362.2011.02565.x. doi: 10.1111/j.1365-2362.2011.02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Branco JP, Oliveira S, Sargento-Freitas J, Santos Costa J, Cordeiro G, Cunha L, Freire Gonçalves A, Pinheiro J. S100β protein as a predictor of poststroke functional outcome: a prospective study. J Stroke Cerebrovasc Dis. 2018;27:1890–1896. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.046. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 25.del Zoppo GJ, Levy DE, Wasiewski WW, Pancioli AM, Demchuk AM, Trammel J, Demaerschalk BM, Kaste M, Albers GW, Ringelstein EB. Hyperfibrinogenemia and functional outcome from acute ischemic stroke. Stroke. 2009;40:1687–1691. doi: 10.1161/STROKEAHA.108.527804. doi: 10.1161/STROKEAHA.108.527804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Perez FJ, Castelo-Branco M, Alvarez-Sabin J. Albumin level and stroke. Potential association between lower albumin level and cardioembolic aetiology. Int J Neurosci. 2011;121:25–32. doi: 10.3109/00207454.2010.523134. doi: 10.3109/00207454.2010.523134. [DOI] [PubMed] [Google Scholar]
- 27.Potpara TS, Polovina MM, Djikic D, Marinkovic JM, Kocev N, Lip GY. The association of CHA2DS2-VASc score and blood biomarkers with ischemic stroke outcomes: the Belgrade stroke study. PLoS One. 2014;9:e106439. doi: 10.1371/journal.pone.0106439. doi: 10.1371/journal.pone.0106439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakisaka Y, Ago T, Kamouchi M, Kuroda J, Matsuo R, Hata J, Gotoh S, Isomura T, Awano H, Suzuki K, Fukuda K, Okada Y, Kiyohara Y, Ooboshi H, Kitazono T REBIOS Investigators. Plasma S100A12 is associated with functional outcome after ischemic stroke: Research for Biomarkers in Ischemic Stroke. J Neurol Sci. 2014;340:75–79. doi: 10.1016/j.jns.2014.02.031. doi: 10.1016/j.jns.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Yang XY, Gao S, Ding J, Chen Y, Zhou XS, Wang JE. Plasma d-dimer predicts short-term poor outcome after acute ischemic stroke. PLoS ONE. 2014;9:e89756. doi: 10.1371/journal.pone.0089756. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Protopsaltis J, Kokkoris S, Korantzopoulos P, Milionis HJ, Karzi E, Anastasopoulou A, Filioti K, Antonopoulos S, Melidonis A, Giannoulis G. Prediction of long-term functional outcome in patients with acute ischemic non-embolic stroke. Atherosclerosis. 2009;203:228–235. doi: 10.1016/j.atherosclerosis.2008.05.042. doi: 10.1016/j.atherosclerosis.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Anuk T, Assayag EB, Rotstein R, Fusman R, Zeltser D, Berliner S, Avitzour D, Shapira I, Arber N, Bornstein NM. Prognostic implications of admission inflammatory profile in acute ischemic neurological events. Acta Neurol Scand. 2002;106:196–199. doi: 10.1034/j.1600-0404.2002.01224.x. [DOI] [PubMed] [Google Scholar]
- 32.Swarowska M, Ferens A, Pera J, Slowik A, Dziedzic T. Can prediction of functional outcome after stroke be improved by adding fibrinogen to prognostic model? J Stroke Cerebrovasc Dis. 2016;25:2752–2755. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.029. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Zeng L, Liu J, Wang Y, Wang L, Weng S, Chen S, Yang GY. Cocktail blood biomarkers: prediction of clinical outcomes in patients with acute ischemic stroke. Eur Neurol. 2013;69:68–75. doi: 10.1159/000342896. doi: 10.1159/000342896. [DOI] [PubMed] [Google Scholar]
- 34.Anzola GP, Magoni M, Ascari E, Maffi V. Early prognostic factors in ischemic stroke. The role of protein C and protein S. Stroke. 1993;24:1496–1500. doi: 10.1161/01.str.24.10.1496. [DOI] [PubMed] [Google Scholar]
- 35.Hudák R, Székely EG, Kovács KR, Nagy A, Hofgárt G, Berényi E, Csiba L, Kappelmayer J, Bagoly Z. Low thrombin generation predicts poor prognosis in ischemic stroke patients after thrombolysis. PLoS One. 2017;12:e0180477. doi: 10.1371/journal.pone.0180477. doi: 10.1371/journal.pone.0180477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halim AG, Hamidon BB, Cheong SK, Raymond AA. The prognostic value of tissue factor levels in acute ischaemic stroke. Singapore Med J. 2006;47:400–403. [PubMed] [Google Scholar]
- 37.Üstündağ M, Orak M, Güloglu C, Tamam Y, Sayhan MB. Plasma d-dimer levels in acute ischemic stroke: association with mortality, stroke type and prognosis. Nobel Med. 2010;6:37–42. [Google Scholar]
- 38.Abd-Elhamid YA, Tork MA, Abdulghani MO. Prognostic value of d-dimer measurement in patients with acute ischemic stroke. Egypt J Neurol Psychiatr Neurosurg. 2016;53:146–150. [Google Scholar]
- 39.Hsu PJ, Chen CH, Yeh SJ, Tsai LK, Tang SC, Jeng JS. High plasma D-dimer indicates unfavorable outcome of acute ischemic stroke patients receiving intravenous thrombolysis. Cerebrovasc Dis. 2016;42:117–121. doi: 10.1159/000445037. doi: 10.1159/000445037. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Kim J, Kim OJ, Kim JK, Song J, Shin DA, Oh SH. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Crit Care. 2013;17:R45. doi: 10.1186/cc12564. doi: 10.1186/cc12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto M, Sakaguchi M, Okazaki S, Furukado S, Tagaya M, Etani H, Shimazu T, Yoshimine T, Mochizuki H, Kitagawa K. Relationship between plasma (D)-dimer level and cerebral infarction volume in patients with nonvalvular atrial fibrillation. Cerebrovasc Dis. 2013;35:64–72. doi: 10.1159/000345336. doi: 10.1159/000345336. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Ning R, Wang Y. Plasma D-dimer level, the promising prognostic biomarker for the acute cerebral infarction patients. J Stroke Cerebrovasc Dis. 2016;25:2011–2015. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.031. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Dougu N, Takashima S, Sasahara E, Taguchi Y, Toyoda S, Hirai T, Nozawa T, Tanaka K, Inoue H. Predictors of poor outcome in patients with acute cerebral infarction. J Clin Neurol. 2011;7:197–202. doi: 10.3988/jcn.2011.7.4.197. doi: 10.3988/jcn.2011.7.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribo M, Montaner J, Molina CA, Arenillas JF, Santamarina E, Alvarez-Sabín J. Admission fibrinolytic profile predicts clot lysis resistance in stroke patients treated with tissue plasminogen activator. Thromb Haemost. 2004;91:1146–1151. doi: 10.1160/TH04-02-0097. doi: 10.1160/TH04-02-0097. [DOI] [PubMed] [Google Scholar]
- 45.Alessi MC, Gaudin C, Grosjean P, et al. Changes in activated thrombin-activatable fibrinolysis inhibitor levels following thrombolytic therapy in ischemic stroke patients correlate with clinical outcome. Cerebrovasc Dis. 2016;42:404–414. doi: 10.1159/000447722. doi: 10.1159/000447722. [DOI] [PubMed] [Google Scholar]
- 46.Bu X, Peng H, Zhong C, et al. Antiphosphatidylserine antibodies and clinical outcomes in patients with acute ischemic stroke. Stroke. 2016;47:2742–2748. doi: 10.1161/STROKEAHA.116.013827. doi: 10.1161/STROKEAHA.116.013827. [DOI] [PubMed] [Google Scholar]
- 47.You S, Yin X, Liu H, Zheng D, Zhong C, Du H, Zhang Y, Zhao H, Qiu C, Fan L, Pei S, Ma Z, Cao Y, Liu CF. Hyperfibrinogenemia is significantly associated with an increased risk of in-hospital mortality in acute ischemic stroke patients. Curr Neurovasc Res. 2017;14:242–249. doi: 10.2174/1567202614666170621103604. doi: 10.2174/1567202614666170621103604. [DOI] [PubMed] [Google Scholar]
- 48.Hanson E, Jood K, Karlsson S, Nilsson S, Blomstrand C, Jern C. Plasma levels of von Willebrand factor in the etiologic subtypes of ischemic stroke. J Thromb Haemost. 2011;9:275–281. doi: 10.1111/j.1538-7836.2010.04134.x. doi: 10.1111/j.1538-7836.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 49.Sonneveld MA, van Dijk AC, van den Herik EG, van Loon JE, de Lau LM, van der Lugt A, Koudstaal PJ, de Maat MP, Leebeek FW. Relationship of Von Willebrand Factor with carotid artery and aortic arch calcification in ischemic stroke patients. Atherosclerosis. 2013;230:210–215. doi: 10.1016/j.atherosclerosis.2013.07.046. doi: 10.1016/j.atherosclerosis.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 50.Reber G, de Moerloose P. D-dimer assays for the exclusion of venous thromboembolism. Semin Thromb Hemost. 2000;26:619–624. doi: 10.1055/s-2000-13217. doi: 10.1055/s-2000-13217. [DOI] [PubMed] [Google Scholar]
- 51.Fassbender K, Dempfle CE, Mielke O, Schwartz A, Daffertshofer M, Eschenfelder C, Dollman M, Hennerici M. Changes in coagulation and fibrinolysis markers in acute ischemic stroke treated with recombinant tissue plasminogen activator. Stroke. 1999;30:2101–2104. [PubMed] [Google Scholar]
- 52.Zi WJ, Shuai J. Plasma D-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS One. 2014;9:e86465. doi: 10.1371/journal.pone.0086465. doi: 10.1371/journal.pone.0086465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Bugnicourt JM, Godefroy O. Day-7 or day-90 modified Rankin scale score: what is the best measure of outcome after thrombolysis in ischemic stroke? J Thromb Thrombolysis. 2013;36:316. doi: 10.1007/s11239-012-0820-9. doi: 10.1007/s11239-012-0820-9. [DOI] [PubMed] [Google Scholar]
- 54.Cappellari M, Moretto G, Bovi P. Day-7 modified Rankin Scale score as the best measure of the thrombolysis direct effect on stroke? J Thromb Thrombolysis. 2013;36:314–315. doi: 10.1007/s11239-012-0805-8. doi: 10.1007/s11239-012-0805-8. [DOI] [PubMed] [Google Scholar]
- 55.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009;40:3393–3395. doi: 10.1161/STROKEAHA.109.557256. doi: 10.1161/STROKEAHA.109.557256. [DOI] [PubMed] [Google Scholar]
- 56.Bustamante A, García-Berrocoso T, Rodriguez N, Llombart V, Ribó M, Molina C, Montaner J. Ischemic stroke outcome: a review of the influence of post-stroke complications within the different scenarios of stroke care. Eur J Intern Med. 2016;29:9–21. doi: 10.1016/j.ejim.2015.11.030. doi: 10.1016/j.ejim.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 57.Is Intravenous Alteplase Still of Added Benefit in Patients With Acute Ischaemic Stroke Who Undergo Intra-Arterial Treatment? ISRCTN 80619088. http://www.isrctn.com/ISRCTN80619088. Accessed October 29, 2018.
- 58.Endovascular Treatment for Acute Ischemic Stroke; the Use of Periprocedural Heparin or Antiplatelet Agents. ISRCTN 76741621. http://www.isrctn.com/ISRCTN76741621. Accessed October 29, 2018.
- 59.Endovascular Treatment of Acute Ischemic Stroke in the Netherlands for Late Arrivals. ISRCTN 19922220. http://www.isrctn.com/ISRCTN19922220. Accessed October 29, 2018.


