Abstract
Background
Photosensitisation is a clinical condition occurring in both humans and animals that causes significant injury to affected individuals. In livestock, outbreaks of photosensitisation caused by ingestion of toxic plants are relatively common and can be associated with significant economic loss.
Objectives
The agents that are most commonly implicated in outbreaks of photosensitisation have not been formally investigated on a global scale. To address this question, a systematic review of the literature was undertaken to determine the most common causative agents implicated in outbreaks of photosensitisation in livestock in Australia and globally, as well as the prevalence and morbidity of such outbreaks.
Methods
A systematic database search was conducted to identify peer-reviewed case reports of photosensitisation in livestock published worldwide between 1900 and April 2018. Only case reports with a full abstract in English were included. Non peer-reviewed reports from Australia were also investigated. Case reports were then sorted by plant and animal species, type of photosensitisation by diagnosis, location, morbidity and mortality rate and tabulated for further analysis.
Results
One hundred and sixty-six reports qualified for inclusion in this study. Outbreaks were reported in 20 countries. Australia (20), Brazil (20) and the United States (11) showed the highest number of peer-reviewed photosensitisation case reports from this analysis. Hepatogenous (Type III) photosensitisation was the most frequently reported diagnosis (68.5%) and resulted in higher morbidity. Panicum spp., Brachiaria spp. and Tribulus terrestris were identified as the most common causes of hepatogenous photosensitisation globally.
Conclusions
Hepatogenous photosensitisation in livestock represents a significant risk to livestock production, particularly in Australia, Brazil, and the United States. Management of toxic pastures and common pasture weeds may reduce the economic impact of photosensitisation both at a national and global level.
Introduction
Photosensitisation is a global health issue affecting domestic livestock production with numerous underlying aetiological causes [1]. Clinical photosensitisation occurs when photodynamic compounds accumulate in the skin, cornea, and/or mucoid membranes [2]. Any portion of the animal exposed to sunlight and lacking protective fleece, hair or pigmentation can develop lesions within minutes to hours of exposure [3]. Severity can range from mild erythema and oedema to severe necrosis and skin sloughing [2].
Photosensitisation can be classified into three major categories based on aetiology: primary (Type I), congenital (Type II), and hepatogenous or secondary (Type III) [4]. Primary (Type I) photosensitisation occurs when photocytotoxic compounds, or their photoactive metabolites, are present within peripheral tissues following ingestion or via local percutaneous absorption following direct dermal contact [5]. Congenital (Type II) photosensitisation is rare and caused by abnormal heme synthesis resulting in accumulation of photodynamic metabolites, including uroporphyrin, coproporphyrin, and protoporphyrin derivatives in the skin [2,6]. Hepatogenous (Type III) photosensitisation is by far the most common in animals [2] and is caused by accumulation of phytoporphyrin (also known as phylloerythrin) in dermal tissues [7]. Any aetiological agent that impairs hepatobiliary excretion, either by damage to the hepatocytes directly (hepatotoxicity), or by damage to the functionality of the bile ducts themselves (cholestasis), can cause accumulation of phytoporphyrin resulting in clinical signs of photosensitisation [8].
Many outbreaks of photosensitisation are sporadic or transient [2]. Incidence and prevalence of photosensitisation varies depending on location, distribution of causative plants or fungi [9,10], the nature of the farming system [11], and environmental conditions [12], and the resistance/susceptibility of the species and individual animals within the flock or herd [12–20]. In livestock, the vast majority of photosensitisation outbreaks are associated with ingestion of plants [4,9].
Some cases are complex with synergistic or additive effects caused by different aetiological factors, a hypothesis supported by the sporadic pattern of certain outbreaks and the difficulty of experimentally recreating photosensitisation outbreaks associated with some plant species or other causal agents in their own right [2,21–23]. For example, sporidesmin, a mycotoxin contained in the spores of the saprophytic fungus Pithomyces chartarum, is capable of causing hepatogenous photosensitisation [12,24], especially in New Zealand where approximately 95% P. chartarum are toxigenic [25]. Sporadic outbreaks involving P. chartarum have suggested that sporidesmin may accelerate the progress of the liver damage in animals [2], when neither the presence of liver damage or the fungus would have been sufficient individually to cause photosensitisation [12,25]. The broad range of plant species implicated in outbreaks of secondary photosensitisation, the possibility of synergistic or interactive effects with mycotoxins, or other toxic entities, and the relative dearth of information on their biochemical profile commonly makes confirmation of a definitive causal agent problematic.
Several reviews of the incidence and prevalence photosensitisation in livestock have been published [1,4,26–31]. However, these have either focused on summarisation of diagnostic criteria [1,8,27,31,32], emphasised particular aetiological agents or been restricted to certain geographical regions [3,4,26,28,33]. As such, although providing useful information to the clinician or epidemiologist, they are generally limited in their ability to determine outbreak patterns, the prevalence of the causative agents, their relationship to morbidity, and the mortality on a larger scale. The lack of a holistic approach to the study of photosensitisation therefore limits our understanding of its impact on livestock globally.
The objective of this study was, therefore, to review the global presentation of cases of photosensitisation in the scientific literature, published in the English language, and to determine potential trends in which causal agents worldwide that are most commonly associated with outbreaks of photosensitisation in livestock. A review of the literature, including both peer-reviewed and non peer-reviewed sources, was undertaken for a better understanding of photosensitisation across a number of key countries globally where a significant body of published case information could be obtained.
Methods
Database analysis, search, and selection criteria: Peer-reviewed articles
Eleven electronic databases [Pubmed, Web of Science, CAB Abstracts, Proquest, Sciquest, Trove, EthOS, BASE, Open Access and Dissertations, NDLTD, and the DART Europe E Theses Portal] were mined for peer-reviewed articles relating to cases of photosensitisation in livestock. All case reports were extracted regardless of year or language of publication. Searches included the period from 1900 to April 2018. Search keywords included ‘photosensitisation’ and / or ‘photosensitization’, ‘photodermatitis’, ‘facial eczema’, ‘geeldikkop’, ‘dikoor’, ‘plochteach’ or ‘alveld’ in the title. The Boolean operator “OR” was used to join terms. Language restriction was applied; articles published in languages other than English, or did not provide an abstract in English, were excluded from the search result. Reference lists of retrieved articles were reviewed to identify all relevant case studies or reports to maximise article retrieval. The PRISMA checklist is attached as Supporting Checklist.
The following selection criteria were then applied; the publication should: 1) be an outbreak report with photosensitisation as the main clinical sign or differential diagnosis; 2) relate to any species of livestock, including alpaca, camel, cattle, deer, donkey, goat, horse, llama, mule, pig, reindeer, sheep, and water buffalo; and 3) contain clinicopathological findings that confirm a diagnosis of photosensitisation. Quality assessment of individual peer-reviewed reports was not performed. In particular, an assumption made with respect to the peer reviewed articles was that the causal agent responsible for all reported outbreaks were correctly identified in the associated publication.
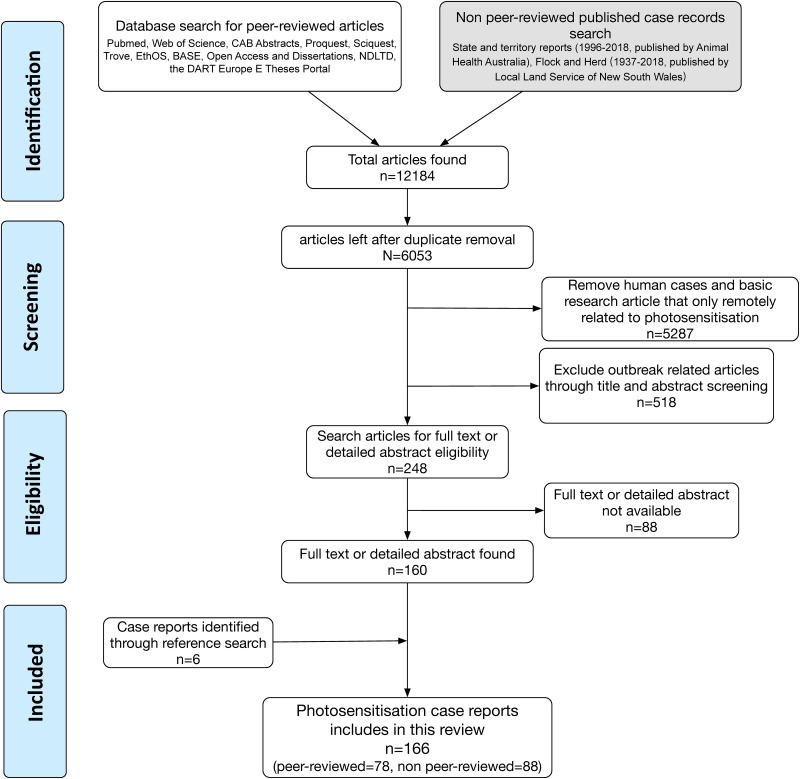
Exclusion criteria included: duplications of published articles; species other than domestic livestock; publications in which the full text or a detailed abstract was not available; and publications in which the diagnosis of photosensitisation could not be established over other differentials. Search and selection methodology for this study is summarised in Fig 1.
Fig 1. Literature search criteria and the number of articles included and excluded in this review.
Database analysis, search, and selection criteria: Non peer-reviewed articles
Although the reliability of the diagnosis and the utility of information in non-peer reviewed reports can sometimes be questionable, it was considered that non peer-reviewed reports could be useful as supplemental information to peer-reviewed data by providing careful data extraction and thus review of the evidence in each report was undertaken. Australia was selected as the sole country for analysis of non peer- reviewed information due to its well-established case reporting systems via Australian Government Administrative or Government-Accredited Animal Health Officers (District Veterinarians, Regional Veterinary Officers, and Biosecurity Officers). Reports from these sources are published either in Animal Health Surveillance Quarterly Report (AHSQR, http://www.sciquest.org.nz/ahsq) that cover outbreaks nationally in Australia, or in the ‘Flock and Herd’ case note series that cover outbreaks in New South Wales (NSW) (F&H, http://www.flockandherd.net.au, site maintained by Local Land Services in NSW). Non peer-reviewed case material was searched up to and including April 2018. Again, the same selection and exclusion criteria were applied as described above.
Extracted information from non peer-reviewed case reports included, but was not limited to, species; age; quantitative and qualitative information regarding proportion of the herd or flock affected; geographical and chronologic information; type of photosensitisation and clinical description; causative agents confirmed or suspected. The accuracy of the diagnosis and entirety of the report was reviewed by the authors to ensure consistency, and reports, where clinicopathological data or causality did not appear consistent with a diagnosis of photosensitisation, were excluded from the analysis. However, due to the lack of peer-reviewed process, the accuracy and reliability of non peer-reviewed reports were considered to represent a lower level of evidence than peer-reviewed ones.
Results
Following review of the peer-reviewed scientific literature, 78 reports presenting with a full text or detailed abstract were analysed (Fig 1). Data on causal plant species or organisms; geographical location; type of photosensitisation (primary, hepatogenous, congenital, unknown); outbreak years; animal species; size of flock or herd, percentage of morbidity and mortality were extracted and tabulated. A summary of information presented in the peer-reviewed literature is shown in Table 1.
Table 1. Extracted photosensitisation outbreak data from the peer-reviewed literature by aetiological agent.
Percentage morbidity and mortality are included where reported.
| Aetiological agent | Country | Type of photosensitisation | Year | Species | Flock/Herd size | Morbidity (%) | Mortality (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Alfalfa hay (predominantly Medicago sativa) | US | Primary | 2013 | Horse | 116 | 6.9 | N/A | [34] |
| Alfalfa hay (predominantly M. sativa) | US | Primary | 2004 | Horse | 70 | 100.0 | 1.4 | [34] |
| Alfalfa hay (predominantly M. sativa) | US | Primary | 2008 | Horse | N/A | N/A | N/A | [34] |
| Alternanthera philoxeroides (Alligator weed) | AU | Hepatogenous | 1998 | Cattle | 70 | 82.9 | N/A | [35] |
| Ammi majus (Bishop’s weed) | US | primary | 1978 | Sheep | N/A | N/A | N/A | [36] |
| Biserrula pelecinus vars. Casbah and Mauro (Biserrula) | AU | Primary | 2015 | Sheep | 167 | 100 | N/A | [37] |
| B. pelecinus cv Casbah (Biserrula) | AU | Primary | 2013 | Sheep | 120 | 25.0 | N/A | [38] |
| Brachiaria brizantha (Palisade grass) | BR | Hepatogenous | 2010 | Sheep | 80 | 16.3 | 12.5 | [39] |
| B. brizantha (Palisade grass) | BR | Hepatogenous | 2010 | Sheep | 113 | 43.4 | 35.4 | [39] |
| Brachiaria decumbens (Signal grass) | CO | Hepatogenous | 2015 | Cattle | N/A | N/A | N/A | [20] |
| B. decumbens (Signal grass) | MY | Hepatogenous | 1985 | Goat | 12 | 25.0 | N/A | [40] |
| B. decumbens (Signal grass) | BR | Hepatogenous | 2003 | Goat | 118 | 14.4 | N/A | [41] |
| B. decumbens (Signal grass) | BR | Hepatogenous | 2003 | llama | 1 | N/A | N/A | [42] |
| B. decumbens (Signal grass) | BR | Hepatogenous | 2009 | Sheep | 24 | 45.8 | N/A | [21] |
| B. decumbens (Signal grass) | NG | Hepatogenous | 1982 | Sheep | 36 | N/A | 38.9 | [43] |
| B. decumbens (Signal grass) | BR | Hepatogenous | 2009 | Buffalo | 17 | 52.9 | N/A | [44] |
| B. decumbens (Signal grass) | BR | Hepatogenous | 2003 | Sheep | 28 | 25.0 | 21.4 | [45] |
| Brassica rapa (Turnip) | NZ | Hepatogenous | 2014 | Cattle | N/A | N/A | N/A | [46] |
| Copper | BR | Hepatogenous | 2006 | Buffalo | 4 | 100.0 | N/A | [47] |
| Dicrocoelium dendriticum | UK | Hepatogenous | 2011 | Sheep | 65 | 49.2 | 3.1 | [48] |
| Enterolobium contortisiliquum (Pacara earpod tree) | BR | Hepatogenous | 2014 | Cattle | 62 | 22.6 | 3.2 | [49] |
| E. contortisiliquum (Pacara earpod tree) | BR | Hepatogenous | 2002 | Cattle | N/A | N/A | N/A | [50] |
| Flood damaged alfalfa hay (predominantly Medicago sativa) | US | Hepatogenous | 1957 | Cattle | 40 | N/A | N/A | [51] |
| Foxtail-or- chardgrass mixture cut hay | US | Hepatogenous | 1991 | Cattle | 8 | 100.0 | 12.5 | [16] |
| Froelichia humboldtiana (Ervanço) | BR | Primary | 2014 | Cattle | 70 | 38.6 | N/A | [52] |
| F. humboldtiana (Ervanço) | BR | Primary | 2014 | Donkey | N/A | N/A | N/A | [53] |
| F. humboldtiana (Ervanço) | BR | Primary | 2014 | Goat | 15 | 100.0 | N/A | [54] |
| F. humboldtiana (Ervanço) | BR | Primary | 2014 | Mule | N/A | N/A | N/A | [53] |
| F. humboldtiana (Ervanço) | BR | Primary | 2006 | Sheep | 5 | 100.0 | N/A | [55] |
| F. humboldtiana (Ervanço) | BR | Primary | 2014 | Horse | N/A | N/A | N/A | [23] [53] |
| Heliotropium europaeum (Common heliotrope) | AU | Hepatogenous | 1985 | Sheep | 120 | 4.2 | N/A | [13] |
| Heracleum sphondylium (Hogweed) | UK | Primary | 2010 | Horse | N/A | N/A | N/A | [56] |
| Hypericum erectum (St. John’s wort) | JP | Primary | 1980 | Cattle | 5 | 100.0 | N/A | [57] |
| H. erectum (St. John’s wort) | TN | Primary | 1999 | Horse | 34 | N/A | N/A | [58] |
| Jamesdicksonia dactylidis | AU | Hepatogenous | 2017 | Cattle | 678 | 24.3 | 2.8 | [59] |
| Liver fluke | AT | Hepatogenous | 2003 | Cattle | N/A | N/A | N/A | [60] |
| Lotus corniculatus (Birdsfoot trefoil) | NZ | Primary | 1992 | Sheep | 40 | 7.5 | N/A | [61] |
| L. corniculatus (Birdsfoot trefoil) | NZ | Primary | 1993 | Sheep | 56 | 26.8 | N/A | [61] |
| L. corniculatus (Birdsfoot trefoil) | NZ | Primary | 1991 | Sheep | 30 | 33.3 | N/A | [61] |
| Malachra fasciata (Malachra) | BR | Primary | 2016 | Sheep | 3 | 100 | N/A | [62] |
| Medicago sativa (Lucerne, alfafa) | ES | Primary | 2004 | Sheep | 1850 | 24.3 | N/A | [63] |
| Microcystis aeruginosa | SA | Hepatogenous | 1993 | Cattle | N/A | N/A | N/A | [64] |
| Myoporum insulare (Common boobialla) | AU | Hepatogenous | 1980 | Cattle | 177 | 14.1 | 6.2 | [65] |
| Narthecium ossifragum (Bog asphodel) | NO | Hepatogenous | 1999 | Sheep | 165 | 9.7 | N/A | [66] |
| N. ossifragum (Bog asphodel) | NO | Hepatogenous | 1990 | Sheep | 28 | 17.9 | N/A | [18] |
| Nodularia spumigena | SA | Hepatogenous | 1993 | Cattle | N/A | N/A | N/A | [64] |
| N. spumigena | SA | Hepatogenous | 1993 | Sheep | N/A | N/A | N/A | [64] |
| Pangola grass | TW | Hepatogenous | 1978 | Cattle | 8428 | 4.9 | 1.4 | [67] |
| Panicum coloratum (Klein grass) | US | Hepatogenous | 1987 | Sheep | 24 | 100.0 | N/A | [68] |
| P. coloratum (Klein grass) | AU | Hepatogenous | 1989 | Sheep | 2000 | N/A | N/A | [69] |
| Panicum dichotomiflorum (Fall panicum) | BR | Hepatogenous | 2009 | Sheep | 365 | 22.2 | 10.7 | [70] |
| P. dichotomiflorum (Fall panicum) | US | Hepatogenous | 2006 | Horse | 14 | 100.0 | 35.7 | [71] |
| Panicum miliaceum (Proso millet) | IR | Hepatogenous | 2008 | Sheep | 10 | 10.0 | N/A | [72] |
| P. miliaceum (Proso millet) | IR | Hepatogenous | 2008 | Sheep | 253 | 32.8 | 16.2 | [73] |
| Panicum schinzii (Sweet grass) | AU | Hepatogenous | 1986 | Sheep | 200 | 30.0 | 20.0 | [74] |
| P. schinzii (Sweet grass) | AU | Hepatogenous | 1991 | Sheep | 70 | 28.6 | 21.4 | [75] |
| P. schinzii (Sweet grass) | AU | Hepatogenous | 1986 | Sheep | 200 | 25.0 | 15.0 | [74] |
| Panicum virgatum (Switch grass) | US | Hepatogenous | 1991 | Sheep | 104 | 16.4 | N/A | [76] |
| Persicaria lapathifolia (Pale knotweed) and P. orientalis | AU | Hepatogenous | 2009 | Cattle | 50 | 4.0 | 20.0 | [15] |
| Petroselinum crispum (Parsley) | UK | Hepatogenous | 1997 | Pig | 18 | 88.9 | N/A | [77] |
| Phytolacca octandra (Inkweed) | NZ | Hepatogenous | 2006 | Cattle | 400 | 5.0 | N/A | [78] |
| Pithomyces chartarum | NZ | Hepatogenous | 1997 | Fallow deer | 20 | 60.0 | 30.0 | [79] |
| P. chartarum | AU | Hepatogenous | 1985 | Sheep | 200 | 15.0 | N/A | [80] |
| P. chartarum | SA | Hepatogenous | 1970 | Sheep | N/A | N/A | N/A | [81] |
| P. chartarum | TR | Hepatogenous | 2005 | Sheep | 1000 | 2.2 | N/A | [82] |
| P. chartarum | US | Hepatogenous | 1994 | Sheep | N/A | N/A | N/A | [83] |
| P. chartarum | AU | Hepatogenous | 1978 | Sheep | 22698 | 10.7 | 4.1 | [84] |
| Polygonum lapathifolium (Pale persicaria) | AU | Hepatogenous | 1986 | Cattle | 380 | N/A | 1.6 | [85] |
| Porphyrins | UK | CEP | 2008 | Cattle | N/A | N/A | N/A | [86] |
| Porphyrins | UK | CEP | 1956 | Cattle | N/A | N/A | N/A | [87] |
| Protoporphyrin | FR | CEPP | 1991 | Cattle | N/A | N/A | N/A | [88] |
| Protoporphyrin | IE | CEPP | 2015 | Cattle | 20 | 5.0 | N/A | [89] |
| Protoporphyrin | NZ | CEPP | 2011 | Cattle | N/A | N/A | N/A | [90] |
| Protoporphyrin | UK | CEPP | 2000 | Cattle | 20 | 5.0 | N/A | [91] |
| Protoporphyrin | UK | CEPP | 2013 | Cattle | 26 | 7.7 | N/A | [92] |
| Protoporphyrin | US | CEPP | 1999 | Cattle | 70 | 1.4 | N/A | [93] |
| Senecio brasiliensis (Flor-das-almas) | BR | Hepatogenous | 2013 | Cattle | 162 | 51.2 | N/A | [94] |
| Senecio spp | BR | Hepatogenous | 2014 | Sheep | 860 | 0.9 | 1.2 | [95] |
| Tribulus terrestris (Goat’s-head, puncture vine) | AU | Hepatogenous | 1983 | Goat | 35 | 17.1 | 5.7 | [96] |
| T. terrestris (Goat’s-head, puncture vine) | AU | Hepatogenous | 1982 | Sheep | 1200 | 20.8 | 14.7 | [11] |
| T. terrestris (Goat’s-head, puncture vine) | IR | Hepatogenous | 1998 | Sheep | 11 | 100.0 | N/A | [22] |
| T. terrestris (Goat’s-head, puncture vine) | IR | Hepatogenous | 1975 | Sheep | 700 | 8.5 | 4.3 | [97] |
| T. terrestris (Goat’s-head, puncture vine) | TR | Hepatogenous | 2013 | Sheep | 24 | 100.0 | N/A | [98] |
| T. terrestris (Goat’s-head, puncture vine) | AU | Hepatogenous | 1982 | Sheep | 190 | 36.8 | 24.2 | [11] |
| Trifolium alexandrinum (Berseem) | IN | Hepatogenous | 2013 | Cattle | N/A | N/A | N/A | [99] |
| Unidentified | MY | Hepatogenous | 2012 | Cattle | N/A | N/A | N/A | [100] |
| Unidentified | AU | Hepatogenous | 1985 | Sheep | 35 | 42.9 | 28.6 | [74] |
| Unidentified | AU | Hepatogenous | 1986 | Sheep | 100 | 7.0 | N/A | [74] |
| Unidentified (White clover, phalaris and rye grass) | AU | Hepatogenous | 1964 | Sheep | 100 | 20.0 | N/A | [101] |
N/A, not available; CEPP, Congenital Erythropoietic Protoporphyria; CEP, Congenital Erythropoietic Protoporphyria; AU, Australia; AT, Austria; BR, Brazil; CO, Columbia; FR, France; IN, India; IR, Iran; IRE, Ireland; JP, Japan; MY, Malaysia; NZ, New Zealand; NG, Nigeria; NO, Norway; SA, South Africa; ES, Spain; TW, Taiwan; TN, Tunisia; TR, Turkey; UK, United Kingdom; US, United States
Following review of the Australian non peer-reviewed scientific literature, 88 non peer-reviewed Australian case reports with a full text or detailed abstract were identified for further review. Data on causal plant species or organism, country of outbreak, type of outbreak related to type of photosensitisation (primary, hepatogenous, congenital, unknown), animal species, size of flock or herd and percentage morbidity and mortality was extracted. Data contained in these reports was often less comprehensive than comparative peer-reviewed articles and information on the above criteria were extracted where available. A summary of information presented in Australian non peer-reviewed literature is shown in Table 2.
Table 2. Photosensitisation outbreaks in livestock in Australia extracted from two non peer-reviewed publication series.
Percentage morbidity and mortality are included where available.
| Aetiological agent | State | Type | Species | Flock/Herd size | Morbidity (%) | Mortality (%) | Reference |
|---|---|---|---|---|---|---|---|
| B. decumbens (Signal grass) | WA | Hepatogenous | Sheep | 300 | 20.0 | N/A | AHSQR 17: 1 |
| Biserrula casbah (Biserrula) | WA | Primary | Sheep | 500 | 40.0 | N/A | AHSQR 7: 3 |
| Biserrula or clover | WA | Primary | Sheep | N/A | N/A | N/A | AHSQR 9: 4 |
| Biserrula spp. (Biserrula) | WA | Primary | Cattle | N/A | N/A | N/A | AHSQR 16: 2 |
| Brassica napus (Rape, canola) | VIC | Hepatogenous | Cattle | 200 | 3.0 | N/A | AHSQR 14: 1 |
| Brassica spp. | VIC | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 4: 1 |
| Cynosurus echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 9 | N/A | 22.2 | AHSQR 19: 2 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 25 | 24.0 | N/A | AHSQR 11: 2 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 35 | 28.6 | N/A | AHSQR 11: 2 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 6: 2 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 510 | 32.4 | 10.0 | AHSQR 7: 2 |
| C. echinatus (Rough dog’s tail grass) | WA | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 7: 3 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 270 | 11.9 | 0.7 | AHSQR 8: 2 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 11 | 100.0 | N/A | AHSQR 15: 2 |
| C. echinatus (Rough dog’s tail grass) | VIC | Hepatogenous | Cattle | 150 | 53.3 | N/A | AHSQR 18: 2 |
| Echinochloa utilis (Japanese barnyard millet) | VIC | Hepatogenous | Sheep | 300 | 10.0 | 6.7 | AHSQR 14: 4 |
| Heliotrope spp. | VIC | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 6: 3 |
| Heliotropium europaeum (Common heliotrope) | NSW | Hepatogenous | Cattle | 60 | 100.0 | 66.7 | F&H Sep, 2015 |
| H. europaeum (Common heliotrope) | NSW | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 19: 4 |
| Hypericum perforatum (St John’s wort) | NSW | Primary | Sheep | 550 | 33.6 | N/A | F&H Mar, 2012 |
| H. perforatum (St John’s wort) | NSW | Primary | Sheep | 300 | 50.0 | 3.3 | AHSQR 13: 4 |
| Lantana camara (Lantana) | QLD | Hepatogenous | Cattle | 100 | N/A | 3.0 | AHSQR 11: 1 |
| L. camara (Lantana) | QLD | Hepatogenous | Cattle | 35 | 5.7 | 2.8 | AHSQR 12: 1 |
| L. camara (Lantana) | NSW | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 14: 3 |
| L. camara (Lantana) | QLD | Hepatogenous | Cattle | 250 | N/A | 3.2 | AHSQR 16: 3 |
| L. camara (Lantana) | QLD | Hepatogenous | Cattle | 250 | 0.8 | N/A | AHSQR 16: 3 |
| L. camara (Lantana) | QLD | Hepatogenous | Cattle | 20 | 50.0 | N/A | AHSQR 16: 3 |
| L. camara (Lantana) | QLD | Hepatogenous | Cattle | 80 | N/A | 7.5 | AHSQR 21: 1 |
| L. camara (Lantana) | NSW | Hepatogenous | Cattle | 47 | N/A | 19.2 | AHSQR 8: 1 |
| L. camara (Lantana) | QLD | Hepatogenous | Cattle | 120 | N/A | 2.5 | AHSQR 10: 2 |
| Lantana spp. | NSW | Hepatogenous | Cattle | 47 | N/A | N/A | AHSQR 11: 4 |
| Lantana spp. | QLD | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 12: 4 |
| Lantana spp. | QLD | Hepatogenous | Cattle | 220 | 5.9 | 1.4 | AHSQR 4: 2 |
| Lantana spp. | QLD | Hepatogenous | Cattle | 800 | 18.8 | N/A | AHSQR 7: 2 |
| Lantana spp. | QLD | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 7: 3 |
| Lantana spp. | QLD | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 9: 2 |
| Lolium perenne (Perennial ryegrass) | VIC | Primary | Cattle | 120 | 25.0 | N/A | AHSQR 12: 2 |
| Mentha pulegium (pennyroyal) and Lotus uliginosus (big trefoil) | TAS | Primary | Sheep | N/A | N/A | N/A | AHSQR 12: 1 |
| P. chartarum | VIC | Hepatogenous | Cattle | 23 | 69.6 | 30.4 | AHSQR 6: 1 |
| P. chartarum | WA | Hepatogenous | Cattle | 750 | 40.0 | N/A | AHSQR 8: 2 |
| P. chartarum | NSW | Hepatogenous | Cattle | 10 | 70.0 | N/A | F&H Dec, 2011 |
| P. chartarum | VIC | Hepatogenous | Cattle | N/A | 50.0 | ‘significant’ | AHSQR 4: 2 |
| P. chartarum | SA | Hepatogenous | Cattle | N/A | N/A | N/A | AHSQR 5: 2 |
| P. chartarum | TAS | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 20:2 |
| P. chartarum | VIC | Hepatogenous | Sheep & Cattle | 114 | 5–50 | 1–30 | AHSQR 16: 2 |
| P. chartarum | WA | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 9: 4 |
| P. chartarum | NSW | Hepatogenous | Sheep | 1000 | N/A | N/A | F&H Nov, 2015 |
| P. effusum (Hairy panic), Brassica napus (Rape, canola) and Heliotropium europaeum (Common heliotrope) | VIC | Hepatogenous | Sheep | 48 | 4.2 | N/A | AHSQR 14: 1 |
| P. effusum (Hairy panic), T. terrestris (goat’s-head, puncture vine) | NSW | Hepatogenous | Sheep | 230 | 100.0 | N/A | AHSQR 14: 1 |
| P. gilvum (Sweet panic) and Brassica | NSW | Hepatogenous | Sheep | 500 | 8.4 | N/A | AHSQR 18: 1 |
| P. coloratum (Klein grass) | NSW | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 13: 1 |
| Panicum effusum (Hairy panic) | VIC | Hepatogenous | Sheep | 350 | 14.3 | 8.6 | AHSQR 15: 1 |
| Panicum gilvum (Sweet panic) | NSW | Hepatogenous | Sheep | 520 | 7.7 | 1.2 | F&H Jul, 2013 |
| Panicum hillmanii (Hillmann’s panic) | VIC | Hepatogenous | Sheep | 400 | 6.3 | N/A | AHSQR 14: 1 |
| Panicum miliaceum (Proso millet) | WA | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 11: 3 |
| Panicum spp. | NSW | Hepatogenous | Sheep | many | N/A | N/A | F&H, 1981 |
| Panicum spp. | NSW | Hepatogenous | Sheep | N/A | N/A | 10 | AHSQR 1: 2 |
| Panicum spp. | VIC | Hepatogenous | Sheep | 2170 | N/A | 1.4 | AHSQR 20: 1 |
| Panicum spp. | VIC | Hepatogenous | Sheep | 200 | N/A | 10.0 | AHSQR 9: 1 |
| Panicum spp. | NSW | Hepatogenous | Sheep | 450 | 5.1 | N/A | F&H Sep, 2015 |
| Panicum spp., B. decumbens (Signal grass),Chloris gayana (Rhodes grass), Echium plantagineum (Paterson’s curse) | WA | Hepatogenous | Cattle | 500 | 2.0 | N/A | AHSQR 15: 1 |
| Persicaria spp. | NSW | Hepatogenous | Cattle | 50 | 4.0 | N/A | F&H Apr, 2013 |
| Pithomyces chartarum | TAS | Hepatogenous | Cattle | 290 | 20.7 | 0.3 | AHSQR 11: 1 |
| Pithomyces spp. | NSW | Hepatogenous | Sheep | 14 | 14.3 | N/A | AHSQR 16: 4 |
| Pithomyces spp. | WA | Hepatogenous | Sheep | 1000 | 5.0 | N/A | AHSQR 18: 2 |
| Polygonum sp. | NSW | Primary | Cattle | 130 | 3.9 | N/A | AHSQR 14: 4 |
| T. terrestris (Goat’s-head, puncture vine) | WA | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 13: 1 |
| T. terrestris (Goat’s-head, puncture vine) | WA | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 16: 3 |
| T. terrestris (Goat’s-head, puncture vine) | SA | Hepatogenous | Sheep | 100 | N/A | N/A | AHSQR 9: 1 |
| Tribulus terrestris (Goat’s-head, puncture vine) | NSW | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 13: 1 |
| Trifolium resupinatum (Shaftal clover) | NSW | Hepatogenous | Sheep | N/A | N/A | N/A | F&H 1987 |
| Unidentified | TAS | Primary | Cattle | 150 | 3.3 | N/A | AHSQR 12: 3 |
| Unidentified | VIC | Hepatogenous | Cattle | 300 | 6.7 | N/A | AHSQR 15: 2 |
| Unidentified | VIC | Hepatogenous | Cattle | 250 | 20.0 | N/A | AHSQR 15: 2 |
| Unidentified | SA | Hepatogenous | Cattle | 350 | 25.1 | 5.1 | AHSQR 15: 2 |
| Unidentified | VIC | Primary | Cattle | N/A | N/A | N/A | AHSQR 15: 3 |
| Unidentified | TAS | Hepatogenous | Cattle | 100 | 13.0 | 3.0 | AHSQR 19: 4 |
| Unidentified | TAS | Hepatogenous | Cattle | 900 | 25.0 | 0.7 | AHSQR 19: 4 |
| Unidentified | TAS | Hepatogenous | Cattle | 160 | 18.8 | N/A | AHSQR 4: 2 |
| Unidentified | WA | Primary | Sheep | N/A | N/A | N/A | AHSQR 16: 3 |
| Unidentified | NSW | Primary | Sheep | N/A | 50–100 | N/A | AHSQR 20: 2 |
| Unidentified | NSW | Primary | Sheep | 8 flocks | 50–100 | 5–15 | F&H Nov, 2015 |
| Unidentified (Aphid-infested thistles) | NSW | Hepatogenous | Cattle | 36 | 100.0 | 41.7 | F&H 1956 |
| Unidentified (Aphids) | WA | Primary | Sheep | 4200 | N/A | N/A | AHSQR 12: 3 |
| Unidentified (Pyrrolizidine alkaloids) | QLD | Hepatogenous | Horse | 20 | 100.0 | 65.0 | AHSQR 15: 2 |
| Unidentified (Pyrrolizidine alkaloids) | SA | Hepatogenous | Sheep | N/A | N/A | N/A | AHSQR 13: 3 |
| Unidentified (Ryegrass, silage, oats, straw, grape Marc and pasture hay) | VIC | Hepatogenous | Cattle | 270 | 3.7 | N/A | AHSQR 13: 1 |
| Unidentified (Ryegrass) | VIC | Hepatogenous | Cattle | 160 | 6.3 | 1.3 | AHSQR 12: 2 |
N/A, not available; F&H, Flock and Herd, AHSQR, Animal Health Surveillance Quarterly; NSW, New South Wales; SA, Southern Australia; QLD, Queensland; TAS, Tasmania; VIC, Victoria; WA, Western Australia
Geographical distribution and species differentiation of photosensitisation outbreaks reported in the peer-reviewed literature
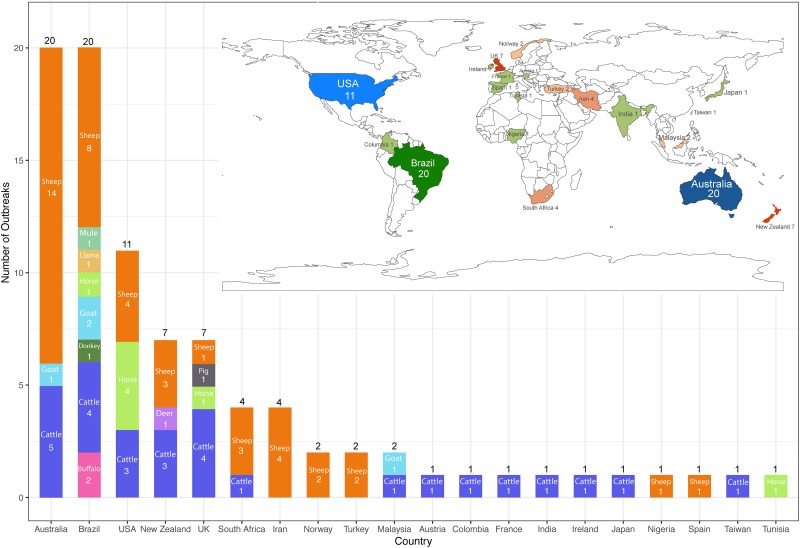
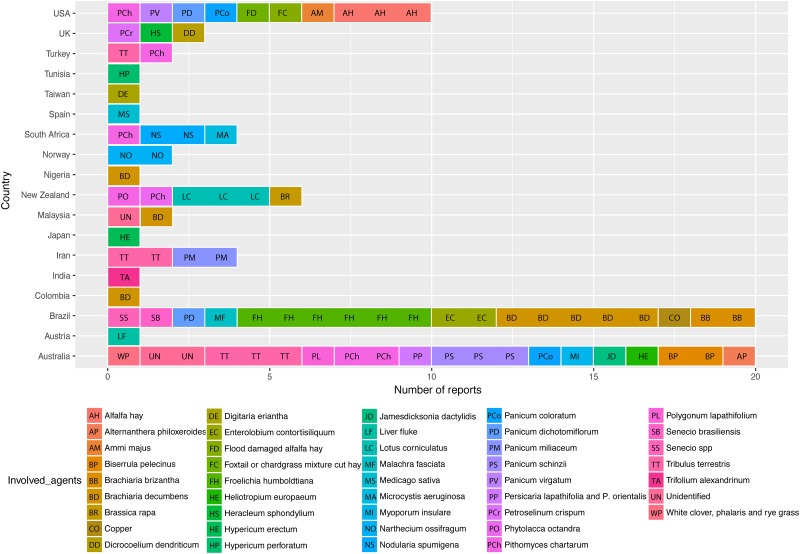
Photosensitisation outbreak reports were identified from 20 different countries in the peer-reviewed literature (Fig 2). The greatest number of reports were from Australia (20), Brazil (20), and the United States (11), followed by New Zealand (7), United Kingdom (7), South Africa (4), Iran (4), Norway (2), Turkey (2) and Malaysia (2), and 10 other countries with only one outbreak report each.
Fig 2. Global geographic distribution and species differentiation of peer-reviewed case reports of clinical photosensitisation in domestic livestock.
A prominent species predisposition was identified in these reports. Sheep were the most reported species in photosensitisation outbreaks globally, with 47.2% (42/89) reported outbreaks, followed by cattle (32.6%, 29/89) and horses (7.9%, 7/89). Other reported livestock species included the goat (4.5%, 4/89), buffalo (2.2%, 2/89), and one case each in the donkey, deer, mule, llama and pig (all 1.1%, 1/89).
Geographical distribution and species differentiation of photosensitisation outbreaks reported in Australia
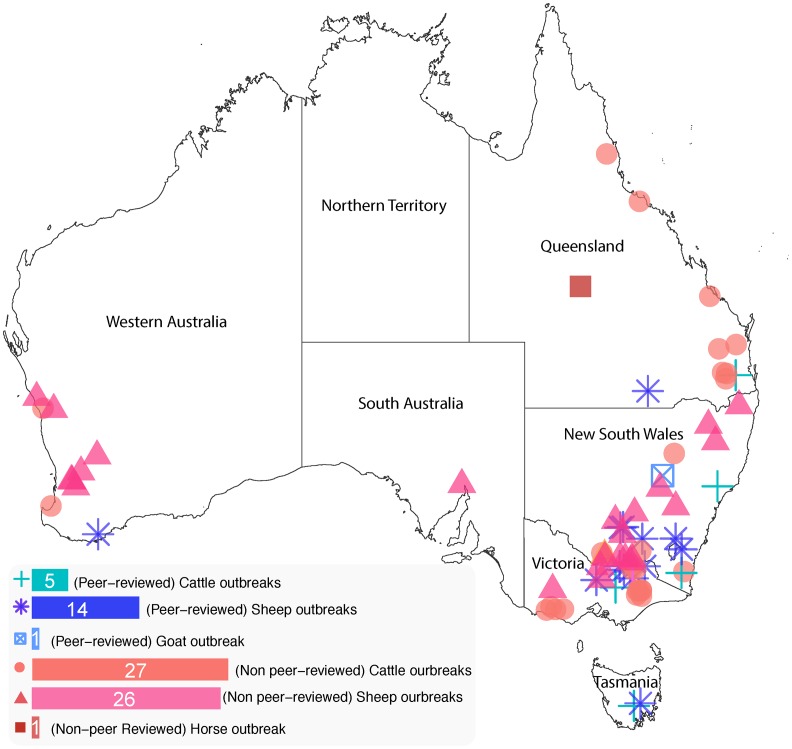
The geographical distribution of case data in Australia was further examined by state. When peer-reviewed and non peer-reviewed reports were considered together, the highest number of outbreaks was observed in New South Wales and Victoria (33 reports each), followed by Queensland (15 reports), Western Australia (15 reports), Tasmania (9 reports), and South Australia (4 reports) (Fig 3). There was no photosensitisation case report from the Northern Territory.
Fig 3. The number of combined peer-reviewed and non peer-reviewed photosensitisation case reports identified by geographical location and species in different states in Australia.
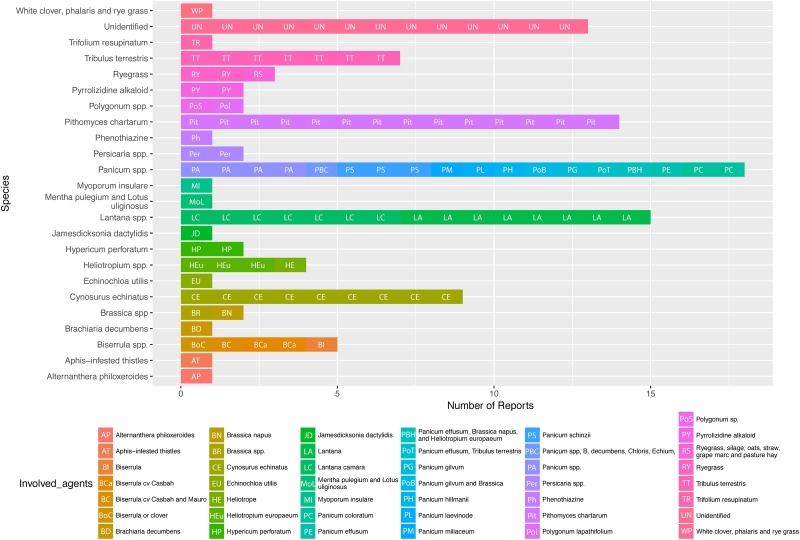
Panicum spp., P. chartarum and T. terrestris were clearly represented as causal agents with 12, 4, and 4 cases reported respectively. However, Lantana spp. (15 reports) and Cynosurus echinatus (9 reports) are also prevalent within specific geographical locations (Lantana spp in Queensland and C. echinatus in Victoria, Table 2).
As observed in global cases, a similar species predisposition, or reporting bias, was observed in reports from Australia. Sheep and cattle presented as the most frequently reported livestock species in photosensitisation outbreaks in Australia. Over 54% (40/74) of reported outbreaks concerned sheep, 43.2% (32/74) cattle, with the remaining outbreaks horse and goat (1 case only in each species).
Prevalence of category of photosensitisation in livestock and aetiological agent, a global analysis
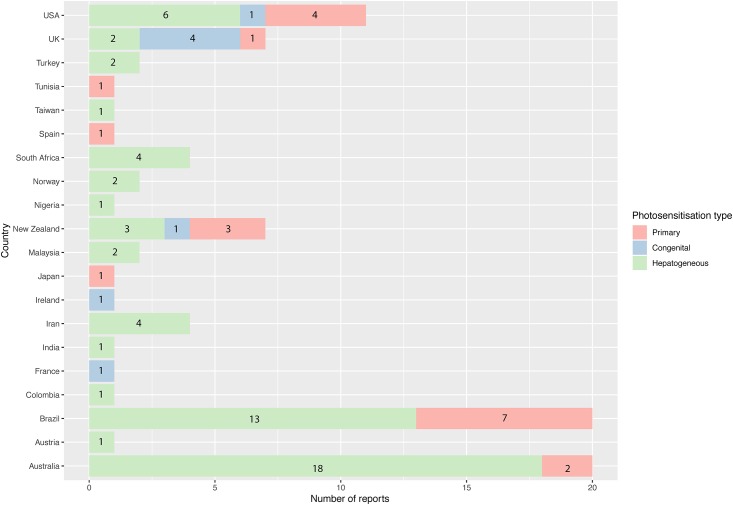
In all peer-reviewed articles examined, 68.5% (61/89) reported cases were suspected or confirmed to be cases of hepatogenous (Type III) photosensitisation (Table 1). In comparison, only 22.5% (20/89) reported cases, were diagnosed or suspected to be primary (Type I) in nature. Only 9.0% (8/89) reported cases of outbreaks in the US, UK, New Zealand, Ireland, and France, were diagnosed or suspected as congenital (Type II) photosensitisation (Fig 4).
Fig 4. Numbers of peer-reviewed case reports by country by type of photosensitisation: Primary, congenital or hepatogenous.
Common aetiological agents were reported in multiple locations globally (Fig 5). For example, Brachiaria decumbens has been reported as a causal agent in Brazil, Colombia, and Nigeria; various species of the Panicum genus of grasses have been identified as causal agents in outbreaks of photosensitisation in Australia, Brazil, Iran, and the United States; P. chartarum, a fungus that produces a specific mycotoxin called sporidesmin, was reported as an aetiological cause of hepatogenous photosensitisation in Australia, New Zealand, South Africa, Turkey, and the United States; Tribulus terrestris was reported as a causal species in Australia, Iran, and Turkey. Certain species were found to have a more contained geographical distribution, for example, F. humboldtiana, a primary photosensitising plant, was identified in six cases solely occurring in Brazil. Biserrula spp., an annual legume from the Mediterranean [102], was reported as a causative agent of primary photosensitisation only in Australia (Table 1, Fig 5).
Fig 5. Causative agents identified in peer-reviewed photosensitisation case reports worldwide.
Congenital cases of photosensitisation accounted for the least number of outbreaks which were restricted to the United Kingdom (4), France (1), Ireland (1), New Zealand (1), and the United States (1) (Table 1).
Causative agents of photosensitisation in Australia
Analysis of published photosensitisation case reports in Australia alone, taking both peer-reviewed and non peer-reviewed reports together, identified that outbreaks of photosensitisation in livestock related to ingestion of the Panicum genus of grasses were the most commonly reported aetiology in this region (18/94, 19.1%, Fig 6). The second most common confirmed aetiological agent was Lantana spp. (15/94, 16.0%). The soil-borne ubiquitous fungus P. chartarum also accounted for a significant proportion of analysed case reports (14/94, 14.9%). These three agents represented the confirmed aetiological causes of the majority of photosensitisation cases reported in Australia (47/94, 50%).
Fig 6. Plant species and other causative agents identified in published cases of photosensitisation in livestock in Australia.
Livestock morbidity related to photosensitisation type
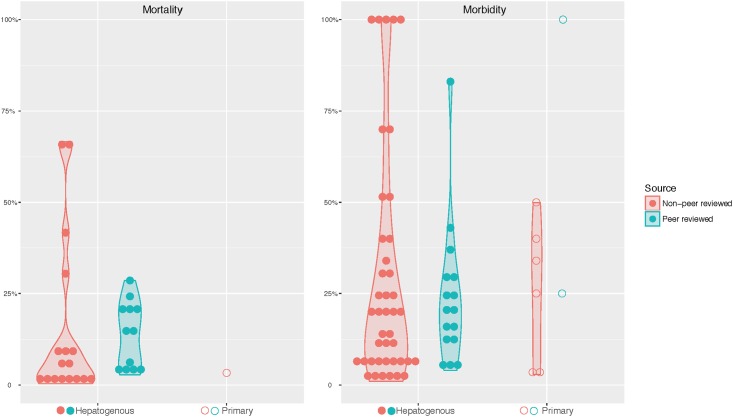
Calculation of the morbidity (affected / flock number) and mortality (deaths / flock number) was compared between peer-reviewed reports (78 reports in total, Table 1) or non-peer reviewed reports (88 reports in total, Table 2) where the aetiological agent had been specified. The extracted data was visualised using a violin plot (Fig 7). This analysis showed a wide variation in morbidity and mortality between the two publication modalities with the peer reviewed publications showing higher figures for morbidity than those in non-peer reviewed publications. No photosensitisation related deaths were in outbreaks of congenital photosensitisation, and analysis showed that hepatogenous photosensitisation exhibited wider variation in morbidity and mortality than outbreaks of primary photosensitisation (Fig 7). Specifically, of the 26 peer-reviewed reports that described mortality related to photosensitisation, 25 were hepatogenous in nature. Only one mortality was associated with an outbreak of primary photosensitisation whilst none were associated with congenital cases (Fig 7a). Morbidity varied greatly ranging from 5–100% (Fig 7b).
Fig 7. Mortality (a) and morbidity (b) in photosensitisation case reports published in the peer-reviewed and non peer-reviewed literature in Australia.
Outbreaks reports that failed to provide an absolute number of the dead animal, the affected animal, or the flock size are not included.
Discussion
Photosensitisation outbreaks are commonly reported in Australasia, Brazil and the United States
Reporting bias was implicit in the method used for report extraction and data compilation in this study as only reports with a full text or abstract in English were selected for review and this fact explains, in part, the high numbers of case reports identified in Australia and the United States specifically. Each of these countries has a strong track record in scientific publication in the veterinary field, produces large numbers of sheep and cattle, also the most frequently reported livestock species in photosensitisation outbreaks (Table 1, Fig 2), and relies significantly on livestock production for export.
The number of reported cases from Europe as a whole was relatively low with only 12 articles found in the literature for this region. This finding suggests that photosensitisation is less of an issue for European farming systems compared to those of Australia, New Zealand, Brazil, and the US. Differences in climate [13–17], farming practices [12], availability of non-toxic pasture species and native or invasive weed species [103,104] associated with primary or secondary photosensitisation are likely to be playing a key role in this finding. However, the study selection criteria also would have been biased for publications from a large number of European countries as well. It possible that some photosensitisation outbreaks have been reported in native language publications, which would not have been selected due to by the filtering process. Nevertheless, the large number of case reports published in Brazil, identifying a diverse range of plant species as causally related to outbreaks of photosensitisation (Table 1, Fig 2), suggests that publication language barrier is not necessarily an obstacle to presentation of case reports in the literature, where English is the common language of scientific publication.
Hepatogenous photosensitisation is common in domestic livestock
This report identifies hepatogenous photosensitisation to be the most common presentation of clinical photosensitisation in livestock (Fig 4), a finding in agreement with the general scientific literature [2]. Although inter-species and intra-species variation in sensitivity to photosensitising agents exists, toxic plants and organisms capable of causing outbreaks in domestic livestock should also be considered a potential risk to all grazing herbivores. This is particularly the case for native wildlife where plant-related outbreaks of hepatogenous photosensitisation have been reported in kangaroos and wombats [105–107].
Congenital (Type II) photosensitisation was rarely identified in the published literature (8/89 reports) in this study. Congenital erythropoietic protoporphyria has only been reported in cattle, and only in a small number of countries in Europe as well as the United States and New Zealand. This likely reflects the common ancestry of many cattle herds in aforementioned two countries with animals largely exported to these regions from the United Kingdom, and therefore the reliance on a small genetic pool of beef cattle in particular [90,93]. Interestingly, despite presenting with the highest number of peer-reviewed case reports, no congenital photosensitisation cases have yet been reported in Australia. This is likely a reflection of the selectivity of Australian livestock import systems and reliance on a highly conserved gene pool present in the cattle imported into this jurisdiction.
Photosensitisation shows highly variable morbidity and mortality worldwide
A high degree of variability was reported for both morbidity and mortality in outbreaks of both primary and hepatogenous photosensitisation (Tables 1 & 2, Fig 7). This further suggests the difficulty of attributing an overall economic impact of photosensitisation to the global livestock industry, since the severity and magnitude of each outbreak is multifactorial and can differ significantly. An additional confounding factor is that higher mortality rates were recorded in hepatogenous photosensitisation found in peer-reviewed case reports compared to those reported in non-peer reviewed publications (Fig 7a), suggesting that only the most severe photosensitisation outbreaks were selected to be published by attending clinicians. This supports the author’s anecdotal findings that the majority of photosensitisation outbreaks are either submitted for publication in non peer-reviewed publications, or are not reported in print at all.
Prevalence of photosensitisation case reports in Australia
Outbreak reports in this analysis represent a bias towards Australia. Certain countries, such as New Zealand, are also known anecdotally to have a high incidence of photosensitisation. The incidence of New Zealand may be underrepresented in this review as the available reporting systems for these outbreaks are not as apparent as Australia, where there is a strong network of government veterinarians and good mechanisms for the presentation of non peer-reviewed case reports. It is also widely acknowledged that the more common a disease, the less a producer is likely to request the services of a veterinarian for diagnoses, and the less a veterinarian is likely to report the outbreak formally.
Australia has experienced outbreaks of primary photosensitisation that are specific and unique to this region. One examples is photosensitisation caused by the pasture legume Biserrula pelecinus, although a native of the southern Mediterranean, it is exclusively used as a livestock fodder only in Australia [102] where this pasture legume is now clearly identified as causing outbreaks of primary photosensitisation [37,38]. Outbreaks that have never been recorded in its native domains where it grows as a native only in mixed swards. The specific need to identify drought tolerant, hardy annuals to fill the summer feed gap in Australian livestock production systems therefore resulted in introduction of pasture species that has selectively caused livestock toxicity [108]. This situation is not unique to Australia but this is the first clearly correlated example of an introduced species being propagated for pasture fodder which then gives rise to consistent outbreaks of primary photosensitisation in production livestock.
The majority of reports of photosensitisation cases in Australia reported in this review were located were in two states: New South Wales and Victoria (Fig 3). The disproportionate representation of these two states in this dataset is a reflection of a) the relatively high number of primary beef and lamb producers operating in these two states in Australia, and b) reporting bias due to availability of non-peer reviewed case reports presented in producer publications such as ‘Flock and Herd’ (a NSW publication). However, this relative over-reporting in our view does not rule out our findings that under-reporting of clinical outbreaks is still occurring in Australia due to the perception that photosensitisation is ’common’ in production flocks and herds (Y. Chen, survey of Australian veterinarians, unpublished data). Both states also showed a higher prevalence of causes related to Panicum genus of grasses and P. chartarum. Outbreaks in Queensland, by comparison, were mainly related to ingestion of the toxic weed species Lantana spp. These different causal species profiles suggest that environmental adaptation of such invasive plants is critical to their establishment and therefore the prevalence of toxicity associated with them [12,109].
An interesting finding that emerged from analysis of the non peer-reviewed Australian literature in this study was the identification of C. echinatus (rough dog’s tail) as a putative causative agent in outbreaks of hepatogenous photosensitisation from eight separate reports in Victoria and Western Australia (Table 2). Despite its common appearance in the non peer-reviewed literature, this plant has not been formally confirmed to be associated with an outbreak of photosensitisation [110] where controlled feeding trials have been unable to confirm C. echinatus as causing hepatotoxic damage sufficient to cause secondary clinical photosensitivity [111]. This contradictory evidence further suggests that anecdotal case reports should be viewed cautiously until true causality has been proven, as many epidemiological investigations focus on commonalities rather than specifics in outbreak patterns. In cases of photosensitisation a causal relationship between the clinical signs and the suspected agent(s) cannot be assumed to be proven until a direct or evidence-based causation can be established.
Objective measurement of clinical signs of photosensitisation in domestic species
Morbidity in outbreaks of photosensitisation, of all underlying aetiological causes, was found to be highly variable in the literature. This variation is likely to be caused by reporting error by the producer or veterinarian based on inconsistent identification of affected animals. One issue that may have hampered accurate identification of the number of animals affected in outbreaks is the fact that mild cases are commonly overlooked by both the producer and veterinarian, therefore under-reporting of morbidity is likely to occur. Previously, no objective photosensitisation scoring protocol had been defined in literature resulting in prevalence and severity of affected animals to be hard to compare between outbreaks. Recently, a semi-objective photosensitisation grading system has been developed for sheep to address this issue [37]. The use of such a grading system in future outbreaks will allow better correlation between access to potentially photosensitising pastures or feedstuffs with more accurate determination of the timing of onset of outbreaks, as well as a more definitive and consistent identification of the severity of the condition. Furthermore, a mechanism for de novo case reporting related to known causal agents, not just those that are novel or unusual, (such as seen with the publication ’Flock and Herd’ or via an incidence register) would better document the prevalence, and therefore economic impact, of photosensitisation globally.
Conclusions
Photosensitisation is a common, but likely underreported, entity in the literature. Hepatogenous photosensitisation is by far the most common presentation. Some species, the Panicum genus of grasses and Pithomyces chartarum in particular, consistently were reported in photosensitisation cases. Novel species are also implicated in outbreaks of photosensitisation, including pasture legumes Biserrula pelecinus, but primary photosensitisation is a rare occurrence in general. Significant variation in both morbidity, mortality and severity was observed in both peer-reviewed and non-peer reviewed reports. Variations in reported outbreaks may reflect true differences in morbidity rates between aetiological agents, but may also be partly due to the fact that mild presentations are overlooked, and lesions are not consistently graded by an unified standard. Together, our findings help identify the aetiology and geographical patterns, the plant and animal species implicated, and morbidity and mortality patterns of photosensitisation in livestock globally. This suggests that control of pasture species or weeds known to cause toxic outbreaks would have a significant impact on the prevalence of the condition in livestock globally, but also particularly in Australia, Brazil, and the United States where these outbreaks appear to be more common.
Supporting information
(DOC)
Acknowledgments
The authors acknowledge financial support from the Graham Centre for Agricultural Innovation and School of Animal and Veterinary Science (Charles Sturt University). The authors would like to thank Dr. Paul Weston for consultation on the data presentation methods from the statistical perspective. The authors would also like to thank Animal Health Australia (www.animalhealthaustralia.com.au) for providing access to archived reports of Animal Health Surveillance Quarterly.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Yuchi Chen is supported by a PhD scholarship funded by Graham Centre for Agricultural Innovation and CSU School of Animal and Veterinary Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rowe LD. Photosensitization problems in livestock. Vet Clin of Nor Am: Fo Ani Prac. 1989;5: 301–323. 10.1016/S0749-0720(15)30978-6 [DOI] [PubMed] [Google Scholar]
- 2.Mauldin EA, Peters-Kennedy J. Integumentary System In: Maxie MG, editor. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6 ed St. Louis; 2016. pp. 577–580. [Google Scholar]
- 3.Fu PP, Xia Q, Zhao Y, Wang S, Yu H, Chiang HM. Phototoxicity of herbal plants and herbal products. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2013;31: 213–255. 10.1080/10590501.2013.824206 [DOI] [PubMed] [Google Scholar]
- 4.Quinn JC, Kessell A, Weston LA. Secondary plant products causing photosensitization in grazing herbivores: Their structure, activity and regulation. Int J Mol Sci. 2014;15: 1441–1465. 10.3390/ijms15011441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrington GM. Integumentary System In: Kahn CM, editor. The Merck Veterinary Manual. 10 ed London, United Kingdom: Wiley; 2010. pp. 665–800. [Google Scholar]
- 6.Doyle KA, Gordon H. Merck Veterinary Manual. Blackwell Publishing Ltd; 2008. pp. 278–278. 10.1111/j.1751-0813.1993.tb08058.x [DOI] [Google Scholar]
- 7.Kellerman TS, Coetzer JA. Hepatogenous photosensitivity diseases in South Africa. Onderstepoort J Vet Res. 1985;52: 157–173. [PubMed] [Google Scholar]
- 8.Flåøyen A. Plant-associated hepatogenous photosensitization diseases In: Tu AT, Gaffield W, editors. Natural and Selected Synthetic Toxins. 1999. pp. 204–219. [Google Scholar]
- 9.McKenzie R. Australia’s Poisonous Plants, Fungi and Cyanobacteria. CSIRO Publishing; 2012. 10.1111/j.1751-0813.2012.01001.x [DOI] [Google Scholar]
- 10.Kingsbury JM. Plants poisonous to livestock. A review. J Dairy Sci. 1958;41: 875–907. 10.3168/jds.S0022-0302(58)91020-8 [DOI] [Google Scholar]
- 11.Glastonbury JRW, Doughty FR, Whitaker SJ, Sergant E. A syndrome of hepatogenous photosensitisation, resembling geeldikkop, in sheep grazing Tribulus terrestris. Aust Vet J. 1984;61: 314–316. 10.1111/j.1751-0813.1984.tb07135.x [DOI] [PubMed] [Google Scholar]
- 12.Pollock ML, Wishart H, Holland JP, Malone FE, Waterhouse A. Photosensitisation of livestock grazing Narthecium ossifragum: Current knowledge and future directions. Vet J. 2015;206: 275–283. 10.1016/j.tvjl.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 13.Peterson JE, Payne A, Culvenor CC. Heliotropium europaeum poisoning of sheep with low liver copper concentrations and the preventive efficacy of cobalt and antimethanogen. Aust Vet J. 1992;69: 51–56. [DOI] [PubMed] [Google Scholar]
- 14.Morton JM, Campbell PH. Disease signs reported in south-eastern Australian dairy cattle while grazing Brassica species. Aust Vet J. 1997;75: 109–113. [DOI] [PubMed] [Google Scholar]
- 15.Lugton IW, Woolacott J. Liver necrosis and photosensitisation in cattle after eating Persicaria lapathifolia (pale knotweed) and Persicaria orientalis (Prince’s feather). Aust Vet J. 2014;92: 62–64. 10.1111/avj.12148 [DOI] [PubMed] [Google Scholar]
- 16.Witte ST, Curry SL. Hepatogenous photosensitization in cattle fed a grass hay. J Vet Diagn Invest. 1993;5: 133–136. 10.1177/104063879300500135 [DOI] [PubMed] [Google Scholar]
- 17.Gleen BL, Panciera RJ, Monlux AW. A hepatogenous photosensitivity disease of cattle: II. Histopathology and pathogenesis of the hepatic lesions. Vet Path. 1965;2: 49–67. 10.1177/030098586500200104 [DOI] [PubMed] [Google Scholar]
- 18.Flåøyen A. A difference in susceptibility of two breeds of sheep to the “Alveld toxin”. Vet Res Commun. 1991;15: 455–457. 10.1007/BF00346543 [DOI] [PubMed] [Google Scholar]
- 19.Aslani MR, Movassaghi AR, Mohri M, Ebrahim-pour V, Mohebi AN. Experimental Tribulus terrestris poisoning in goats. Small Rumin Res. 2004;51: 261–267. 10.1016/S0921-4488(03)00195-0 [DOI] [Google Scholar]
- 20.Cardona-Álvarez J, Vargas-Vilória M, Paredes-Herbach E. Clinical and histopathological study of the phototoxic dermatitis in Zebu calves in grazing of Brachiaria decumbens. Revista MVZ Cordoba. 2016;21: 5366–5380. [Google Scholar]
- 21.Saturnino KC, Mariani TM, Barbosa-Ferreira M, Brum KB, Santos Fernandes dos CE, Lemos RAA. Experimental poisoning by Brachiaria decumbens in feedlot sheep. Pesq Vet Bras. 2010;30: 195–202. 10.1590/S0100-736X2010000300002 [DOI] [Google Scholar]
- 22.Aslani MR, Movassaghi AR, Mohri M, Pedram M, Abavisani A. Experimental Tribulus terrestris poisoning in sheep: Clinical, laboratory and pathological findings. Vet Res Commun. 2003;27: 53–62. 10.1023/A:1022010707704 [DOI] [PubMed] [Google Scholar]
- 23.Medeiros RMT, Bezerra VKD, Riet-Correa F. Experimental poisoning by Froelichia humboldtiana in horses. Ciência Rural. 2014;44: 1837–1840. 10.1590/0103-8478cr20131417 [DOI] [Google Scholar]
- 24.Di Menna ME, Flåøyen A, Ulvund MJ. Fungi on Narthecium ossifragum leaves and their possible involvement in alveld disease of Norwegian lambs. Vet Res Commun. 1992;16: 117–124. [DOI] [PubMed] [Google Scholar]
- 25.Pilsworth RC, Knottenbelt D. Photosensitisation and sunburn. Eq Vet Edu. 2010;19: 32–33. 10.1111/j.2042-3292.2007.tb00549.x [DOI] [Google Scholar]
- 26.Baber R. Photosensitisation: A note of caution in the use of Brachiaria pastures-A review. Trop Anim Health Prod. 1989;21: 277–280. 10.1007/BF02261108 [DOI] [PubMed] [Google Scholar]
- 27.Galitzer SJ, Oehme FW. Photosensitizaton: A literature review. Vet Res Commun. 1978;2: 217–230. 10.1007/BF02291451 [DOI] [Google Scholar]
- 28.Casteel SW, Bailey EM, Reagor JC, Rowe LD. Photosensitization: An investigation and review of the problem in cattle of south Texas. Vet Hum Toxicol. 1986;28: 251–254. [PubMed] [Google Scholar]
- 29.Sandler IL. Photosensitizing agents: a brief review of the literature. J Am Med Assoc. American Medical Association; 1939;112: 2411–2413. 10.1001/jama.1939.62800230002012b [DOI] [Google Scholar]
- 30.Barnes J, Anderson LA, Phillipson JD. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. Journal of Pharmacy and Pharmacology. Blackwell Publishing Ltd; 2001;53: 583–600. 10.1211/0022357011775910 [DOI] [PubMed] [Google Scholar]
- 31.Hussain SM, Herling VR, Rodrigues PHM, Naz I, Khan H, Khan MT. Mini review on photosensitization by plants in grazing herbivores. Trop Anim Health Prod. 2018;36: 1–11. 10.1007/s11250-018-1583-x [DOI] [PubMed] [Google Scholar]
- 32.Laustriat G. Molecular mechanisms of photosensitization. Biochimie. 1986;68: 771–778. 10.1016/S0300-9084(86)80092-X [DOI] [PubMed] [Google Scholar]
- 33.Knupp SNR, Knupp LS, Riet-Correa F, Lucena RB. Plants that cause photosensitivity in ruminants in Brazil. Semina:Ciencias Agrarias. 2016;37: 2009–2020. 10.5433/1679-0359.2016v37n4p2009 [DOI] [Google Scholar]
- 34.Puschner B, Chen X, Read D, Affolter VK. Alfalfa hay induced primary photosensitization in horses. Vet J. 2016;211: 32–38. 10.1016/j.tvjl.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 35.Bourke CA, Rayward D. Photosensitisation in dairy cattle grazing alligator weed (Alternanthera philoxeroides) infested pastures. Aust Vet J. 2003;81: 361–362. 10.1111/j.1751-0813.2003.tb11515.x [DOI] [PubMed] [Google Scholar]
- 36.Witzel DA, Dollahite JW, Jones LP. Photosensitization in sheep fed Ammi majus (Bishop’s weed) seed. Am J Vet Res. 1978;39: 319–320. [PubMed] [Google Scholar]
- 37.Quinn JC, Chen Y, Hackney B, Tufail MS, Weston LA, Loukopoulos P. Acute-onset high-morbidity primary photosensitisation in sheep associated with consumption of the Casbah and Mauro cultivars of the pasture legume Biserrula. BMC Vet Res. 2018;14: 11 10.1186/s12917-017-1318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessell AE, Ladmore GE, Quinn JC. An outbreak of primary photosensitisation in lambs secondary to consumption of Biserrula pelecinus (biserrula). Aust Vet J. 2015;93: 174–178. 10.1111/avj.12318 [DOI] [PubMed] [Google Scholar]
- 39.Albernaz TT, da Silveira JAS, e Silva NDS, Oliveira CHS, Belo Reis ADS, Oliveira CMC, et al. Photosensitization of sheep kept on Brachiaria brizantha pasture in the state of Pará. Pesq Vet Bras. 2010;30: 741–748. 10.1590/S0100-736X2010000900006 [DOI] [Google Scholar]
- 40.Mazni OA, Sharif H, Khushry MYM, Vance HN. Photosensitization in goats grazed on Brachiria decumbens. Mardi Res Bull. 1985; 203–206. [Google Scholar]
- 41.de Lemos RAA, Nakazato L, Herrero Junior GO, Silveira ACD, Porfírio LC. Fotossensibilização e colangiopatia associada a cristais em caprinos mantidos sob pastagens de Brachiaria decumbens no Mato Grosso do Sul. Ciência Rural. 1998;28: 507–510. 10.1590/S0103-84781998000300026 [DOI] [Google Scholar]
- 42.Birgel Junior EH, Santos dos MC, de Ramos JAC, Pogliani FC, Birgel DB, Libera AMMPD, et al. Secondary hepatogenous photosensitization in a llama (Lama glama) bred in the state of Sáo Paulo, Brazil. Can Vet J. 2007;48: 323–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opasina BA. Photosensitisation jaundice syndrome in West African Dwarf sheep and goats grazed on Brachiaria decumbens. Trop grassl. 1985;19: 120–123. [Google Scholar]
- 44.De Oliveira CHS, Barbosa JD, Oliveira CMC, Bastianetto E, Melo MM, Haraguchi M, et al. Hepatic photosensitization in buffaloes intoxicated by Brachiaria decumbens in Minas Gerais state, Brazil. Toxicon. 2013;73: 121–129. 10.1016/j.toxicon.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 45.Brum KB, Haraguchi M, Lemos R. Crystal-associated cholangiopathy in sheep grazing Brachiaria decumbens containing the saponin protodioscin. Pesq Vet Bras. 2007;27 10.1590/s0100-736x2007000100007 [DOI] [Google Scholar]
- 46.Collett MG. Bile duct lesions associated with turnip (Brassica rapa) photosensitization compared with those due to sporidesmin toxicosis in dairy cows. Vet Path. 2014;51: 986–991. 10.1177/0300985813513042 [DOI] [PubMed] [Google Scholar]
- 47.Minervino AHH, Júnior RAB, Rodrigues FAML, Ferreira RNF, Reis LF, Headley SA, et al. Hepatogenous photosensitization associated with liver copper accumulation in buffalos. Res Vet Sci. 2010;88: 519–522. 10.1016/j.rvsc.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 48.Sargison ND, Baird GJ, Sotiraki S, Gilleard JS, Busin V. Hepatogenous photosensitisation in Scottish sheep casued by Dicrocoelium dendriticum. Vet Parasitol. 2012;189: 233–237. 10.1016/j.vetpar.2012.04.018 [DOI] [PubMed] [Google Scholar]
- 49.Olinda RG, Medeiros RMT, Dantas AFM, De Lemos RAA, Riet-Correa F. Poisoning by Enterolobium contortisiliquum in cattle in Northeastern Brazil. Pesq Vet Bras. 2015;35: 44–48. 10.1590/S0100-736X2015000100010 [DOI] [Google Scholar]
- 50.Grecco FB, Dantas AFM, Riet-Correa F, Leite CGD, Raposo JB. Cattle intoxication from Enterolobium contortisiliquum pods. Vet Hum Toxicol. 2002;44: 160–162. [PubMed] [Google Scholar]
- 51.Gleen BL, Monlux AW, Panciera RJ. A hepatogenous photosensitivity disease of cattle: I. Experimental production and clinical aspects of the disease. Pathol Vet. 2016;1: 469–484. 10.1177/030098586400100601 [DOI] [PubMed] [Google Scholar]
- 52.Souza PEC, Oliveira SS, Aguiar-Filho CR, Cunha ALB, Albuquerque RF, Evêncio-Neto J, et al. Primary photosensitization in cattle caused by Froelichia humboldtiana. Res Vet Sci. 2012;93: 1337–1340. 10.1016/j.rvsc.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 53.Knupp SNR, Borburema CC, de Oliveira Neto TS, de Medeiros R, Knupp LS, Riet-Correa F, et al. Outbreaks of primary photosensitization in equidae caused by froelichia humboldtiana. Pesq Vet Bras. 2014;34: 1191–1195. 10.1590/S0100-736X2014001200008 [DOI] [Google Scholar]
- 54.Santos DS, Silva CCB, Araújo VO, de Fátima Souza M, Lacerda-Lucena PB, Simões SVD, et al. Primary photosensitization caused by ingestion of Froelichia humboldtiana by dairy goats. Toxicon. 2017;125: 65–69. 10.1016/j.toxicon.2016.11.258 [DOI] [PubMed] [Google Scholar]
- 55.Pimentel LA, Riet-Correa F, Guedes KMR, Macêdo JTSA, Medeiros RMT, Dantas AFM. Primary photosensitization in equidae and ruminants in the Brazilian semi-arid caused by Froelichia humboldtiana (Amaranthaceae). Pesq Vet Bras. 2007;27: 23–28. 10.1590/S0100-736X2007000100005 [DOI] [Google Scholar]
- 56.Ivens P. Horses: Hogweed suspected of causing primary photosensitisation in a horse. Vet Rec. 2011;169: 81–82. 10.1136/vr.d4472 [DOI] [PubMed] [Google Scholar]
- 57.Kawada H. Photesthesis in cattle fed the grass, Hypericum erectum. J Jpn Vet Med Assoc. 1980;33: 372–375. [Google Scholar]
- 58.Chabchoub A, Landolsi F, Lasfar F, Amira A, Bousrih A. Photosensitization and keratitis in the Arabian thoroughbred horse. Rev Med Vet. 1999;150: 617–624. [Google Scholar]
- 59.Golder HM, Moss N, Rogers G, Jackson B, Gannon N, Wong P, et al. Acute photosensitisation and mortality in a herd of dairy cattle in Tasmania. N Z Vet J. 2017;65: 39–45. 10.1080/00480169.2016.1232181 [DOI] [PubMed] [Google Scholar]
- 60.Flöck M, Baumgartner M, Bago Z, Schilcher F. Photosensitivity due to liver fluke disease in cattle. Tier Prax. 2003;31: 143–149. [Google Scholar]
- 61.Stafford KJ, West DM, Alley MR, Waghorn GC. Suspected photosensitisation in lambs grazing birdsfoot trefoil (Lotus corniculatus). N Z Vet J. 1995;43: 114–117. 10.1080/00480169.1995.35866 [DOI] [PubMed] [Google Scholar]
- 62.de Araújo VO, Oliveira Neto TS, Simões SVD, da Silva TKF, Riet-Correa F, Lucena RB. Primary photosensitization and contact dermatitis caused by Malachra fasciata Jacq. N.V. (Malvaceae) in sheep. Toxicon. 2017;138: 184–187. 10.1016/j.toxicon.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 63.Ferrer LM, Ortín A, Loste A, Fernández A, Verde MT, Ramos JJ. Photosensitisation in sheep grazing alfalfa infested with aphids and ladybirds. Vet Rec. 2007;161: 312–314. 10.1136/vr.161.9.312 [DOI] [PubMed] [Google Scholar]
- 64.Van Halderen A, Harding WR, Wessels JC, Schneider DJ, Heine EW, Van der Merwe J, et al. Cyanobacterial (blue-green algae) poisoning of livestock in the western Cape Province of South Africa. J S Afr Vet Assoc. 1995;66: 260–264. [PubMed] [Google Scholar]
- 65.Jerrett IV, Chinnock RJ. Outbreaks of photosensitisation and deaths in cattle due to Myoporum aff. Insulare R. Br. toxicity. Aust Vet J. 1983;60: 183–186. [DOI] [PubMed] [Google Scholar]
- 66.Wisløff H, Wilkins AL, Scheie E, Flåøyen A. Accumulation of sapogenin conjugates and histological changes in the liver and kidneys of lambs suffering from alveld, a hepatogenous photosensitization disease of sheep grazing Narthecium ossifragum. Vet Res Commun. 2002;26: 381–396. 10.1023/A:1016298929610 [DOI] [PubMed] [Google Scholar]
- 67.Lin SC, Wu YH, Tsai YS, Chang TW, Tsai KR. Studies on the hepatogenous photosensitization of cattle in Taiwan. TW J Vet Med & Ani Hus; 1981;30: 159–170. 10.1155/2017/5276106 [DOI] [Google Scholar]
- 68.Bridges CH, Camp BJ, Livingston CW, Bailey EM. Kleingrass (Panicum coloratum L.) poisoning in sheep. Vet Path. 1987;24: 525–531. 10.1177/030098588702400609 [DOI] [PubMed] [Google Scholar]
- 69.Regnault T. Secondary photosensitisation of sheep grazing bambatsi grass (Panicum coloratum var makarikariense). Aust Vet J. 1990;67: 419–419. 10.1111/j.1751-0813.1990.tb03040.x [DOI] [PubMed] [Google Scholar]
- 70.Riet-Correa F, Haraguchi M, Dantas AFM, Burakovas RG, Yokosuka A, Mimaki Y, et al. Sheep poisoning by Panicum dichotomiflorum in northeastern Brazil. Pesq Vet Bras. 2009;29: 94–98. 10.1590/S0100-736X2009000100015 [DOI] [Google Scholar]
- 71.Johnson AL, Divers TJ, Freckleton ML, McKenzie HC, Mitchell E, Cullen JM, et al. Fall Panicum (Panicum dichotomiflorum) Hepatotoxicosis in Horses and Sheep. J Vet Int Med. 2006;20: 1414–1421. 10.1111/j.1939-1676.2006.tb00760.x [DOI] [PubMed] [Google Scholar]
- 72.Badiei K, Mostaghni K, Nazifi S, Khodakaram Tafti A, Ghane M, Momeni SA. Experimental Panicum miliaceum poisoning in sheep. Small Rumin Res. 2009;82: 99–104. 10.1016/j.smallrumres.2009.02.002 [DOI] [Google Scholar]
- 73.Nazifi S, Ghane M, Fazeli M, Ghafari N, Azizi S, Mansourian M. Proso millet (Panicum miliaceum) poisoning in Iranian fat-tailed sheep. Comp Clin Pathol. 2009;18: 249–253. 10.1007/s00580-008-0784-5 [DOI] [Google Scholar]
- 74.Button C, Paynter DI, Shiel MJ, Colson AR, Paterson PJ, Lyford RL. Crystal-associated cholangiohepatopathy and photosensitisation in lambs. Aust Vet J. 1987;64: 176–180. 10.1111/j.1751-0813.1987.tb09677.x [DOI] [PubMed] [Google Scholar]
- 75.Lancaster MJ, Vit I, Lyford RL. Analysis of bile crystals from sheep grazing Panicum schinzii (sweet grass). Aust Vet J. 1991;68: 281 [DOI] [PubMed] [Google Scholar]
- 76.Puoli JR, Reid RL, Belesky DP. Photosensitization in lambs grazing switchgrass. Agron J. 1992;84: 1077–1080. 10.2134/agronj1992.00021962008400060033x [DOI] [Google Scholar]
- 77.Griffiths IB, Douglas RG. Phytophotodermatitis in pigs exposed to parsley (Petroselinum crispum). Vet Rec. 2000;146: 73–74. 10.1136/vr.146.3.73 [DOI] [PubMed] [Google Scholar]
- 78.Collett MG, Thompson KG, Christie RJ. Photosensitisation, crystal-associated cholangiohepatopathy, and acute renal tubular necrosis in calves following ingestion of Phytolacca octandra (inkweed). N Z Vet J. 2011;59: 147–152. 10.1080/00480169.2011.567966 [DOI] [PubMed] [Google Scholar]
- 79.Smith BL, Asher GW, Thompson KG, Hoggard GK. Hepatogenous photosensitisation in fallow deer (Dama dama) in New Zealand. N Z Vet J. 1997;45: 88–92. 10.1080/00480169.1997.36001 [DOI] [PubMed] [Google Scholar]
- 80.Greenwood PE, Williamson GN. An outbreak of facial eczema in sheep. Aust Vet J. 1985;62: 65–66. 10.1111/j.1751-0813.1985.tb14241.x [DOI] [PubMed] [Google Scholar]
- 81.Marasas WF, Adelaar TF, Kellerman TS, Minne JA, Van Rensburg IB, Burroughs GW. First report of facial eczema in sheep in South Africa. Onderstepoort J Vet Res. 1972;39: 107–112. [PubMed] [Google Scholar]
- 82.Ozmen O, Sahinduran S, Haligur M, Albay MK. Clinicopathological studies on facial eczema outbreak in sheep in Southwest Turkey. Trop Anim Health Prod. 2008;40: 545–551. 10.1007/s11250-008-9132-7 [DOI] [PubMed] [Google Scholar]
- 83.Hansen DE, McCoy RD, Hedstrom OR, Snyder SP, Ballerstedt PB. Photosensitization associated with exposure to Pithomyces chartarum in lambs. J Am Vet Med Assoc. 1994;204: 1668–1671. [PubMed] [Google Scholar]
- 84.Edwards JR, Richards RB, Gwynn RVR, Love RA. A facial eczema outbreak in sheep. Aust Vet J. 1981;57: 392–394. 10.1111/j.1751-0813.1981.tb00534.x [DOI] [PubMed] [Google Scholar]
- 85.McKenzie RA, Dunster PJ, Burchill JC. Smartweeds (Polygonum spp) and photosensitisation of cattle. Aust Vet J. 1988;65: 128. [DOI] [PubMed] [Google Scholar]
- 86.Huxley JN, Lloyd EL, Parker CS, Woolf JR, Strugnell BW. Congenital erythropoietic porphyria in a longhorn calf. Vet Rec. 2009;165: 694–695. 10.1136/vr.165.23.694 [DOI] [PubMed] [Google Scholar]
- 87.Amoroso EC, Loosmore RM, Rimington C, Tooth BE. Congenital porphyria in bovines: first living case in Britain. Nat. Nature; 1957;180: 230–231. [DOI] [PubMed] [Google Scholar]
- 88.Schelcher F, Delverdier M, Bezille P, Cabanie P, Espinasse J. Observation on bovine congenital erythrocytic protoporphyria in the blonde d’Aquitaine breed. Vet Rec. 1991;129: 403–407. 10.1136/vr.129.18.403 [DOI] [PubMed] [Google Scholar]
- 89.McAloon CG, Doherty ML, O’Neill H, Badminton M, Ryan EG. Bovine congenital erythropoietic protoporphyria in a crossbred limousin heifer in Ireland. Ir Vet J. 2015;68: 15 10.1186/s13620-015-0044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Black A, Thomsen A. Congenital protoporphyria in limousin calves. Surv. 2011;38: 11. [Google Scholar]
- 91.Armstrong SC, Jonsson NN, Barrett DC. Bovine congenital erythrocytic protoporphyria in a Limousin calf bred in the UK. Vet Rec. 2002;150: 608–610. 10.1136/vr.150.19.608 [DOI] [PubMed] [Google Scholar]
- 92.Truyers I, Ellis K. Case report: bovine congenital erythropoietic protoporphyria in a pedigree Limousin herd. Livestock. 2013;18: 30–33. 10.12968/live.2013.18.2.30 [DOI] [Google Scholar]
- 93.Pence ME, Liggett AD. Congenital erythropoietic protoporphyria in a Limousin calf. J Am Vet Med Assoc. 2002;221: 277–9– 240 10.2460/javma.2002.221.277 [DOI] [PubMed] [Google Scholar]
- 94.Giaretta PR, Panziera W, Galiza GJA, Brum JS, Bianchi RM, Hammerschmitt ME, et al. Seneciosis in cattle associated with photosensitization. Pesq Vet Bras. 2014;34: 427–432. 10.1590/S0100-736X2014000500007 [DOI] [Google Scholar]
- 95.Giaretta PR, Panziera W, Hammerschmitt ME, Bianchi RM, Galiza GJN, Wiethan IS, et al. Clinical and pathological aspects of chronic Senecio spp. poisoning in sheep. Pesq Vet Bras. Colégio Brasileiro de Patologia Animal; 2014;34: 967–973. 10.1590/S0100-736X2014001000008 [DOI] [Google Scholar]
- 96.Glastonbury JR, Boal GK. Geeldikkop in goats. Aust Vet J. 1985;62: 62–63. [DOI] [PubMed] [Google Scholar]
- 97.Amjadi AR, Ahourai P, Baharsefat M. First report of geeldikkop in sheep in Iran. Arch Inst Razi; 1977. pp. 71–78. [Google Scholar]
- 98.Naci Haydardedeoğlu O. Hepatogenous photosensitization in Akkaraman lambs: special emphasis to oxidative stress and thrombocytopenia. Ankara Üniv Vet Fak Derg. 2013;60: 116–122. 10.1501/Vetfak_0000002564 [DOI] [Google Scholar]
- 99.Thawait VK, Dixit AA, Maiti SK, Gupta R. Berseem induced photosensitization and its therapeutic management in cattle. Int Poli. Intas Pharmaceuticals Ltd; 2013;14: 228–229. [Google Scholar]
- 100.Jesse FF, Ramanoon SZ. Hepatogenous photosensitization in cattle—A case report. Vet World. 2012;5: 764–766. 10.5455/vetworld.2012.764-766 [DOI] [Google Scholar]
- 101.Dent CHR, Rofe JC. A condition resembling facial eczema in sheep in New South Wales. Aust Vet J. 1967;43: 71–71. 10.1111/j.1751-0813.1967.tb15072.x [DOI] [Google Scholar]
- 102.Ghamkhar K, Revell C, Erskine W. Biserrula pelecinus L.—genetic diversity in a promising pasture legume for the future. Crop Pasture Sci. 2012;63: 833 10.1071/CP12126 [DOI] [Google Scholar]
- 103.Riet-Correa F, Medeiros RMT, Schild AL. A review of poisonous plants that cause reproductive failure and malformations in the ruminants of Brazil. J Appl Toxicol. 2012;32: 245–254. 10.1002/jat.1754 [DOI] [PubMed] [Google Scholar]
- 104.Abera D, Jibat T, Sori T, Feyisa A, Beyene T. Assessment of plant and chemical poisoning in livestock in central Ethiopia. J Environ Anal Toxicol. 2014;4: 1–5. 10.4172/2161-0525.1000215 [DOI] [Google Scholar]
- 105.Johnson JH, Jensen JM. Hepatotoxicity and secondary photosensitization in a red kangaroo (Megaleia rufus) due to ingestion of Lantana camara. J Zoo Wildl Med. 1998;29: 203–207. [PubMed] [Google Scholar]
- 106.Woolford L, Fletcher MT, Boardman WSJ. Suspected pyrrolizidine alkaloid hepatotoxicosis in wild southern hairy-nosed wombats (Lasiorhinus latifrons). 2014. pp. 7413–7418. 10.1021/jf405811n [DOI] [PubMed] [Google Scholar]
- 107.Steventon CA, Raidal SR, Quinn JC, Peters A. Steroidal saponin toxicity in eastern grey kangaroos (Macropus giganteus): A novel clinicopathologic presentation of hepatogenous photosensitization. J Wildl Dis. 2018;54: 491–502. 10.7589/2017-03-066 [DOI] [PubMed] [Google Scholar]
- 108.Salam KP, Murray-Prior R, Bowran D. A ’Dream‘pasture and its comparison with two existing annual pasture legumes for Western Australia: a farmers’ eye view. In: Livestock Research for Rural Development [Internet]. 2010. [cited 4 Nov 2015]. http://www.lrrd.org/lrrd22/9/sala22167.htm [Google Scholar]
- 109.Towers NR. Facial eczema—problems and successes in control. Proc N Z Grassl Assoc. 1986; 121–127. [Google Scholar]
- 110.Read E, Edwards J, Deseo M, Rawlin G, Rochfort S. Current understanding of acute bovine liver disease in Australia. Toxins (Basel). 2017;9: 8 10.3390/toxins9010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lancaster MJ, Jubb TF, Pascoe IG. Lack of toxicity of rough dog’s tail grass (Cynosurus echinatus) and the fungus Drechslera biseptata for cattle. Aust Vet J. 2006;84: 98–100. 10.1111/j.1751-0813.2006.tb12238.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.