Abstract
Background
Chikungunya is a mosquito-borne virus transmitted by mosquitoes from the Aedes genus. The virus, endemic to parts of Asia and Africa, has recently undergone an emergence in other parts of the world where it was previously not found including Indian Ocean Islands, Europe, the Western Pacific and the Americas. There is no vaccine against chikungunya virus, which means that prevention and mitigation rely on personal protective measures and community level interventions including vector control.
Methodology/Principal findings
A systematic review (SR) was conducted to summarize the literature on individual and community mitigation and control measures and their effectiveness. From a scoping review of the global literature on chikungunya, there were 91 articles that investigated mitigation or control strategies identified at the individual or community level. Of these, 81 were confirmed as relevant and included in this SR. The majority of the research was published since 2010 (76.5%) and was conducted in Asia (39.5%). Cross sectional studies were the most common study design (36.6%). Mitigation measures were placed into six categories: behavioural protective measures, insecticide use, public education, control of blood and blood products, biological vector control and quarantine of infected individuals. The effectiveness of various mitigation measures was rarely evaluated and outcomes were rarely quantitative, making it difficult to summarize results across studies and between mitigation strategies. Meta-analysis of the proportion of individuals engaging in various mitigation measures indicates habitat removal is the most common measure used, which may demonstrate the effectiveness of public education campaigns aimed at reducing standing water.
Conclusions/Significance
Further research with appropriate and consistent outcome measurements are required in order to determine which mitigation measures, or combination of mitigation measures, are the most effective at protecting against exposure to chikungunya virus.
Introduction
Chikungunya virus (CHIKV) is a mosquito-borne virus that is transmitted to humans by mosquitoes from the Aedes genus, most commonly Ae. aegypti and Ae. albopictus. While CHIKV has been endemic in many parts of Africa and Asia for decades, it has recently re-emerged and spread to new areas including the pacific islands, South America, and the Caribbean where it was not previously recorded [1]. The large outbreak of CHIKV in the Indian Ocean Islands beginning in 2005 is believed to be due to a mutation in the East/Central/South Africa (ECSA) strain of the virus that has improved transmission by Ae. albopictus over Ae. aegypti [2]. An outbreak on Réunion Island in 2005–06 had an attack rate of 35%. Autochthonous transmission in Italy (2007), France (2010), the Caribbean islands (2013) and South America (2014) [3–6] has made CHIKV a global public health issue, as the range of affected areas continues to increase and non-endemic countries are experiencing increases in travel-related CHIKV infections [7–9]. There have also been several instances of viremic travellers importing CHIKV into regions where Ae. albopictus is present [10], which resulted in local outbreaks of CHIKV. These travel related outbreaks demonstrate that CHIKV can be imported to new areas where Ae. albopictus and Ae. aegypti are already present, which includes a high proportion of the U.S. and Europe [11, 12].
Although present in some of the same areas as dengue and malaria, CHIKV has historically received far less attention due to the self-limiting symptoms and a low risk of death. Symptoms are nonspecific and include febrile arthralgia, myalgia, headache, and rash which typically resolve within a few weeks [13], however, in a proportion of infected individuals the arthralgia is incapacitating and may lead to a chronic condition [14]. The 2005 outbreak on Réunion Island was well recorded and provides the basis for our understanding of the impact of the current CHIKV strain, including complications such as encephalitis [15], and in utero transmission of CHIKV [16]. The reported case fatality rate was approximately 1/1000 [17], whereas prior to the outbreak the virus was not known to cause mortality.
There is no available vaccine or antiviral treatment for chikungunya. Therefore, prevention relies primarily on individual personal protective measures and community level interventions including vector control measures. Recommendations by the Unites States Center for Disease Control and Prevention (CDC) include controlling mosquito breeding by removing stagnant water, the use of insecticides and repellents, and wearing protective clothing [18]. However, it is unclear which strategy or combination of strategies is most effective in the prevention of CHIKV.
A systematic review (SR) and meta-analysis was conducted in order to summarize which individual and community level prevention and control strategies have been investigated and which are most effective to prevent or reduce transmission of CHIKV. The results of this systematic review will highlight the consistencies and generalizability of the findings and will be useful for guiding future research and further development of educational and vector control measures to assist in reducing the risk of local transmission of CHIKV.
Methods
Research question, team and protocol
This SR was conducted following standard SR methodology endorsed by the Cochrane collaboration and is reported in accordance with the PRISMA guidelines [19].
The research question for this review is “what individual and community level prevention and control strategies have been investigated and which have been the most effective at reducing or preventing transmission of CHIKV”. The PICO components of this question included studies conducted on CHIKV affected human populations, examining interventions to prevent exposure to CHIKV (or exposure to mosquitoes in a CHIKV affected area) in humans, with outcomes related to the frequency of use and magnitude of effect of the mitigation strategy being examined. All controls were considered for inclusion. The review team expertise included epidemiology, public health, microbiology, vector-borne diseases, zoonotic diseases, knowledge synthesis and meta-analysis.
This SR was prioritized from a scoping review that characterized the global knowledge on CHIKV conducted by the Public Health Agency of Canada [20]. The protocol was developed a priori to ensure that this SR was objective, reproducible and updateable. The protocol includes the research question, definitions, inclusion criteria, and pretested tools (screening form, risk of bias tool, and the data extraction form). The protocol is available in the supplementary material (S1 Appendix).
Scoping review search strategy, eligibility criteria and study characterisation
The scoping review search was conducted to identify all primary research related to chikungunya in English, French, Spanish, or Portuguese in seven databases: Scopus, PubMed, CINAHL, CAB, LILACS, Agricola and Cochrane. The search was conducted on May 27, 2015 and updated January 6, 2017 using the search algorithm: (Chikungunya OR CHIK OR CHIKV) OR (alphavirus AND mosquito* AND control). No date limits were applied. The scoping review included a grey literature search and an extensive search verification procedure to evaluate that relevant studies had been captured [20]. All studies on any aspect of chikungunya and its vectors were included and characterized by topic. The scoping review characterized 91 studies as examining mitigation measures at the individual or community level to prevent/control CHIKV. These 91 studies were considered for inclusion in the SR. Details of the scoping review search strategy and flow of articles are available in the supplementary material (S2 and S3 Appendices).
Relevance screening
Primary research on the review topic in English, French, Spanish or Portuguese was eligible for inclusion. Articles were excluded if they did not contain pertinent information on mitigation or control measures. Although there are studies on the development of a CHIKV vaccine, there is currently no vaccine commercially available, and therefore studies could not be conducted on the application of a CHIKV vaccine in a community and its impact on decreasing the burden of CHIKV. For this reason articles pertaining to vaccine development were excluded. Only studies that included humans as the host species were included in this SR. Each potentially relevant study identified in the scoping review was confirmed for relevance against these eligibility criteria prior to proceeding with risk of bias assessment and data extraction. The studies included in this SR are available in the supplementary material (S4 Appendix).
Risk of bias assessment and data extraction
The risk of bias assessment form was adapted from the tools endorsed by the Cochrane Collaboration and aimed to determine the internal validity of each study [21]. Each study was rated as having a low, high, or unclear risk of bias based on 10 criteria that appraised the study design, reporting of methodology and data exclusions. The data extraction form captured characteristics such as type and details of the intervention and data on all relevant outcomes. A low risk of bias indicates the study was done well with no concerns for biased results based on the reporting of the study, a study considered to have an unclear risk of bias indicates that one or more criteria could not be assessed due to a lack of reporting. Studies with a high risk of bias are considered to have important methodological flaws such as incomplete reporting, missing or excluded observations, inappropriate outcomes, or a lack of investigation into possible confounding variables that are likely to bias the results. All stages of the SR, relevance screening, risk of bias assessment and data extraction were completed by two independent reviewers for each study and conflicts between reviewers were resolved by consensus.
Study management and data analysis
All stages of the scoping review and SR were conducted using the web-based management software DistillerSR (DistillerSR, Evidence Partners, Ottawa, Canada) to facilitate reviewing. Extracted data were exported to MS excel (Microsoft Corporation, Redmond, WA, USA) for data cleaning and descriptive summary. The extracted data is available in the supplementary material (S5 Appendix).
Meta-analysis was conducted using the metaprop command in STATA version 13 (StataCorp, College Station, Texas, USA). Random effects meta-analysis using the DerSimonian and Laird weighting method [22] was conducted for those mitigation measures where the proportion of the population using an intervention (prevalence) was reported. To stabilize the variances a Freeman-Tukey Double Arcsine Transformation was used when conducting the meta-analysis [23]. If the prevalence of a particular mitigation measure was reported more than once in the same study, such as during different years, they were treated as separate lines of data in the meta-analysis. The assumption of independence was not considered to be violated among these studies given these observational data represent a new sampling frame at each time period [24].
Heterogeneity was measured using I2, which describes the proportion of total variation in study estimates that is due to heterogeneity, and it was considered high if I2>50% [25]. Sub-group analysis was conducted to determine whether any of the heterogeneity between studies could be explained. The proportion of the population employing various control measures were grouped by the control method used. These groups included the following personal protective measures: use of personal repellent, room repellents, unspecified repellent use, physical barriers, habitat removal, insecticide use in or around the home, and mosquito avoidance, as well as insecticide use as a community level intervention. Within each of these subgroups we also examined whether outbreak status, whether or not an outbreak was occurring at the time of study, could be used to explain any variation in the proportion of the population using various mitigation methods.
Results
Descriptive statistics
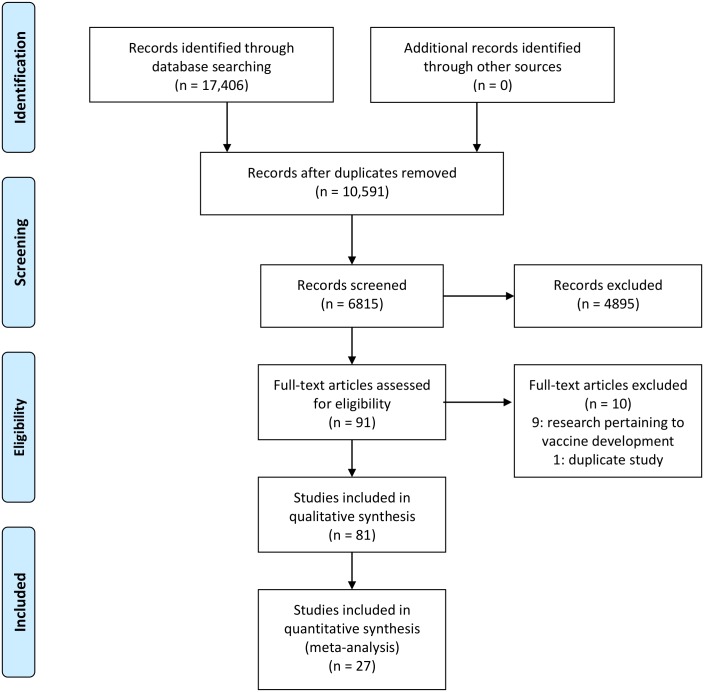
There were 1920 articles characterized in the scoping review, 91 of which were identified as evaluating individual or community mitigation measures against CHIKV. Eighty-one of these studies were relevant to this SR review question, Fig 1. Of the ten excluded articles, one was excluded as a duplicate study and nine were on vaccine development for humans. The effectiveness or prevalence of the use of a vaccine in the prevention of CHIKV in a community setting was not evaluated in any study. Of the 91 included studies, 8 were disease transmission models. These studies are included in the summaries for reference as they simulate effectiveness of individual and community level control methods. However, they were not included in any further analysis as they are not considered primary research.
Fig 1. PRISMA flow diagram of articles through the scoping and systematic review processes.
The 81 included studies were mainly published since 2010 (76.5%) and the largest proportion of the research originated in Asia (39.5%), while the least amount originated in Australasia (3.7%). The studies were divided into six major categories of mitigation and control measures, Table 1. The most common mitigation measures were behavioural protective measures, which were described in 67.9% of the studies. Quarantine of infected individuals was the least common measure, described in 6.2% of the studies, Table 1. Twenty-nine studies (35.8%) in this review reported on the result of mitigation measures, the majority of which (17.3%) reported on the number of chikungunya cases. Based on the risk of bias evaluation, the majority of studies scored low (51.9%) or unclear risk of bias (40.7%), whereas 8.6% were considered to have a high risk of bias, Table 1. Studies with a high risk of bias are considered to have one or more serious flaws in their conduct or reporting that may bias the reported study outcomes in an unknown direction and magnitude.
Table 1. General characteristics of 81 primary research publications outlining community and individual level mitigation factors for chikungunya virus.
| Category | Count (percentage) | |
|---|---|---|
| Continent of Study 1,2 | ||
| Asia | 32 (39.5%) | |
| Europe | 13 (16.0%) | |
| Indian Ocean Islands | 13 (16.0%) | |
| Central/ South America/Caribbean | 13 (16.0%) | |
| North America | 6 (7.4%) | |
| Africa | 4 (4.9%) | |
| Australasia | 3 (3.7%) | |
| Document Language | ||
| English | 77 (95.1%) | |
| French | 2 (2.5%) | |
| Spanish | 2 (2.5%) | |
| Date of Publication | ||
| 1971–1980 | 1 (1.2%) | |
| 2001–2010 | 19 (23.5%) | |
| 2011–2016 | 62 (76.5%) | |
| Risk of Bias | ||
| Low risk of bias | 42 (51.9%) | |
| Unclear risk of bias | 33 (40.7%) | |
| High risk of bias | 7 (8.6% | |
| Study Design1 | ||
| Observational | ||
| Cross sectional | 30 (36.6%) | |
| Outbreak investigation | 14 (17.1%) | |
| Case study/series | 6 (7.3%) | |
| Prevalence survey | 5 (6.1%) | |
| Cohort | 4 (4.9%) | |
| Surveillance/monitoring program | 4 (4.9%) | |
| Case control | 1 (1.2%) | |
| Experimental | ||
| Quasi-experiment | 8 (9.8%) | |
| Controlled trial | 1 (1.2%) | |
| Disease transmission model | 8 (9.6%) | |
| Descriptive | 7 (8.5%) | |
| Risk assessment | 2 (2.5%) | |
| Mixed methods | 1 (1.2) | |
| Mitigation measure(s) described1 | ||
| Behavioural protective measures | 55 (67.9%) | |
| Use of insecticides | 48 (59.3%) | |
| Public education | 31 (38.3%) | |
| Control/treatment of blood products | 9 (11.1%) | |
| Biologic mosquito control | 6 (7.4%) | |
| Quarantine of infected individual | 5 (6.2%) | |
| Outcome measurement3 | ||
| Number of CHIKV cases | 14 (17.3%) | |
| Density of vector population | 11 (13.6%) | |
| Presence of breeding habitat | 4 (4.9%) | |
| Level of knowledge | 4 (4.9%) | |
| Presence of antibody (Ab) response to Ae. albopictus salivary proteins | 2 (2.5%) | |
1 Total number sums to >81 as studies can fall into more than one category.
2 Total percentages do not equal 100 due to rounding.
3 Total number sums to <81 as not all studies reported outcomes.
Behavioural protective measures
Behavioural protective measures were investigated in 55 studies. These include personal protective measures that individuals can apply to themselves or a living space to prevent mosquito bites such as the use of repellents (applied to the skin and in the form of diffusers), physical barriers such as long clothing, bed nets and screens, removal of vector breeding habitat, and mosquito avoidance, Table 2. Of the 55 studies, four used behavioural protective measures in disease transmission models to predict the effects of different mitigation measures [26–29].
Table 2. Frequency of use and effectiveness of behavioural protective measures for the prevention of chikungunya virus infection in humans.
| Ref | Publication year/location | Proportion and description of protective measure | Sample size (cases/controls)1 | Outcome |
|---|---|---|---|---|
| Removal of breeding habitat | ||||
| South/Central America | ||||
| [39] | 2014/Colombia | 81% removal of standing water | 171 | n/r2 |
| [56] | 2016/French Guiana | 32.3% remove stagnant water | 1462 | n/r |
| [58] | 2016/Nicaragua | 73.1% eliminate breeding sites | 848 | n/r |
| 56.8% cover water containers | 848 | n/r | ||
| 38.3% clean water containers | 848 | n/r | ||
| Europe | ||||
| [33] | 2012/France | 17.7% eliminate standing water | 1506 | n/r |
| [42] | 2013/Spain | Avoid stagnant water on property 58.4% (2008), 57.6% (2009), 68.7% (2010) | 428 (2008), 245 (2009), 147 (2010) | n/r |
| Asia | ||||
| [51] | 2007/India | n/r | n/r | Reduced incidence of cases and larval densities3 |
| [61] | 2011/India | 78% cover water storage containers | 50 | n/r |
| [46] | 2011/India | Changing stored water frequently: cases 44%, controls 87% | 600 (150/450) | A greater proportion of CHIKV negative controls change standing water and turn over empty containers than CHIKV cases (p<0.001) |
| [43] | 2012/China | n/r | n/r | Decreased Breteau and mosq-ovitrap indices 2 weeks following application3 |
| Indian Ocean Islands | ||||
| [38] | 2008/Reunion Island | 57% often destroy habitat, 27% never destroy habitat | 1035 | Increased odds of CHIKV with habitat destruction (OR 1.12, p<0.05) |
| [49] | 2009/Maldives | n/r | n/r | Decreased Breteau Index3 |
| [35] | 2009/Mayotte Island | 82.7% eliminate artificial breeding sites | 888 | The prevalence of CHIKV was higher among individuals that did not eliminate artificial breeding sites from their property (p<0.05) |
| 78.3% empty water from receptacles | 888 | n/r | ||
| 80.4% cover or turn over storage containers | 888 | n/r | ||
| [62] | 2010/Reunion Island | 78% implemented individual protections against mosquito bites or preventive measures against breeding places | 74 | n/r |
| [37] | 2014/Reunion Island | 97% eliminate standing water | 1029 | n/r |
| [53] | 2014/Reunion Island | 49.5% prevent breeding sites on their property | 850,804 | n/r |
| Repellents Applied to a Person | ||||
| North America | ||||
| [66] | 2016/US Virgin Islands | 87% do not wear repellent treated clothing | 433 | n/r |
| 56% use skin repellents | 440 | n/r | ||
| [67] | 2016/USA | 16% use permethrin on clothing | 149 | n/r |
| Europe | ||||
| [33] | 2012/France | 17.4% applied repellents to skin | 1506 | n/r |
| Asia | ||||
| [34] | 2010/India | 17.7% use repellent applied to the skin | 857 | n/r |
| [63] | 2014/India | 10% use repellent creams | 81 | n/r |
| Indian Ocean Islands | ||||
| [38] | 2008/Reunion Island | 35.8% use repellent creams and sprays | 1035 | Lack of repellent use is associated with contraction of CHIKV (OR 1.4, p<0.001) |
| [65] | 2008/Reunion Island | 69.7% use skin repellent | 221 cases | No significant difference in repellent use among CHIKV cases (p = 0.08) |
| [64] | 2009/Reunion Island | 79% of parents applied a repellent product more than once per day on the skin of their child | 277 | n/r |
| [37] | 2014/Reunion Island | 2.25% use repellent bracelets | 1024 | n/r |
| 0.10% use anti-mosquito patch | 1024 | n/r | ||
| 22.85% use essential oils | 1024 | n/r | ||
| 36.82% use anti-mosquito body sprays/creams | 1024 | n/r | ||
| Australasia | ||||
| [68] | 2016/Australia | Use of insecticide treated clothing: cases 37.2%, controls 37.3% | 102 (43/59)4 | No difference in CHIKV infection for use of treated clothing (p = 0.99) |
| Space Repellents | ||||
| North America | ||||
| [72] | 2016/Mexico | Use of citronella candles: cases 9.5%, controls 19.9% | 250 (74/176) | Fewer CHIKV cases when Citronella is used (OR 0.37, 95%CI 0.12–0.99). |
| Use of mosquito coils: cases 24.3%, controls 28.4% | 250 (74/176) | No effect of mosquito coil use (OR 0.84, 95% CI 0.4–1.75) | ||
| Asia | ||||
| [69] | 2008/India | 55.1% use mosquito coils | 300 | n/r |
| 45.5% fumigated with plant based materials | 300 | n/r | ||
| [34] | 2010/India | 46.6% use fumes as a repellent | 857 | n/r |
| 15.1% use mosquito coils | 857 | n/r | ||
| 4% use a mosquito mat or liquidator | 857 | n/r | ||
| [70] | 2011/India | 5.7% use liquidator or mat | 528 | n/r |
| [63] | 2014/India | 60% use liquid vaporizers | 81 | n/r |
| 24.4% use coils | 81 | n/r | ||
| 17.8% use repellent mats | 81 | n/r | ||
| [71] | 2016/Bangladesh | 64% use mosquito coils | 1933 | Mosquito coils had no impact on transmission risk (OR 1.0, 95% CI 0.8–1.2) |
| Indian Ocean Islands | ||||
| [38] | 2008/Reunion Island | 45.5% use diffusers | 1035 | Lack of repellent use is associated with contraction of CHIKV (OR 1.4, p<0.001) |
| [37] | 2014/Reunion Island | 69.04% use mosquito coils | 1024 | n/r |
| 52.73% space sprays | 1024 | n/r | ||
| 38.09% use non-electric diffusers | 1024 | n/r | ||
| 19.43% use rechargeable electric vaporizers/diffusers | 1024 | n/r | ||
| 0.29% use ultra-sound devices | 1024 | n/r | ||
| 18.55% use plants (citronella, geranium) | 1024 | n/r | ||
| Unspecified Repellent Use | ||||
| North America | ||||
| [67] | 2016/USA | 59% use repellents in travel to CHIKV risk areas | 149 | n/r |
| [72] | 2016/Mexico | Use of repellent in the last month: cases 31.1%, controls 34.1% | 250 (74/176) | No effect of repellent use in the last month (OR 1.06, 95% CI 0.58–1.94) |
| South/Central America | ||||
| [58] | 2016/Nicaragua | 44.1% use repellents | 848 | n/r |
| Europe | ||||
| [48] | 2010/Italy | n/r | 325 | Fewer cases of CHIKV among those using repellents (OR 0.35, 95% CI 0.15–0.77) |
| [42] | 2013/Spain | Use repellents 45.8% (2008), 46.9% (2009), 15.0% (2010) | 428 (2008), 245 (2009), 147 (2010) | n/r |
| Asia | ||||
| [73] | 2010/India | 58% of houses used mosquito repellent in the last month | 1301 | n/r |
| [46] | 2011/India | Mosquito repellent use: cases 47%, controls 71% | 600 (150/450) | Greater proportion of those negative for CHIKV used repellents than CHIKV positive cases (p<0.001) |
| [45] | 2011/India | 88.4% use repellents | 354 | n/r |
| [75] | 2015/India | 52.5% use repellents | 135 | n/r |
| [74] | 2016/India | 57.5% use repellents | 247 | n/r |
| [59] | 2016/Suriname | Mean repellent use over 3 surveys 47.4% | 1637, 1583, 1622 | For 2 of 3 surveys a greater proportion of self-reported CHIKV positive cases used repellent than did CHIKV negative cases (p = 0.026, p = 0.01), no difference in third survey (p = 0.45) |
| Australasia | ||||
| [68] | 2016/Australia | Use of repellents: cases 93%, controls 94.9% | 102 (43/59)4 | No difference in CHIKV infection for use of repellent (p = 0.69) |
| Physical Barrier—Space | ||||
| North America | ||||
| [66] | 2016/Us Virgin Islands | 92% do not use a mosquito net | 433 | n/r |
| 75% stayed in screened or air conditioned rooms | 433 | n/r | ||
| [67] | 2016/USA | 5% use mosquito nets in CHIKV risk areas | 149 | n/r |
| [72] | 2016/Mexico | Use of screens: cases 62.2%, controls 55.7% | 250 (74/176) | No impact on CHIKV transmission (OR 1.07, 95% CI 0.57–2.01) |
| Open windows: cases 90.5%, controls 78.4% | 250 (74/176) | No impact on CHIKV transmission (OR 1.49, 95% CI 0.63–3.53) | ||
| South/Central America | ||||
| [56] | 2016/French Guiana | 32.9% close windows | 1462 | n/r |
| [58] | 2016/Nicaragua | 48.5% use mosquito nets | 848 | n/r |
| 18.8% use window screens | 848 | n/r | ||
| Europe | ||||
| [48] | 2010/Italy | n/r | 325 | Lower prevalence of CHIKV among those who use window screens (OR 0.43, 95% CI 0.21–0.89) |
| Asia | ||||
| [69] | 2008/India | 30% use mosquito nets | 300 | n/r |
| [34] | 2010/India | 2.6% use mosquito nets | 857 | n/r |
| [46] | 2011/India | Use of mosquito nets: cases 33%, controls 56% | 600 (150/450) | Individuals with CHIKV were less likely to use mosquito nets (p<0.001) |
| Use of screens: cases 13%, controls 43% | 600 (150/450) | Individuals with CHIKV were less likely to use screens (p<0.01) | ||
| [45] | 2011/India | 8.2% use mosquito nets | 354 | n/r |
| 8.2% use window screens | 354 | n/r | ||
| [63] | 2014/India | 2.2% use mosquito nets | 81 | n/r |
| [75] | 2015/India | 28.9% use mosquito nets | 135 | n/r |
| [74] | 2016/India | 14.2% use screens | 247 | n/r |
| 30.8% use mosquito nets | 247 | n/r | ||
| Indian Ocean Islands | ||||
| [38] | 2008/Reunion Island | 43.2% use mosquito nets | 1035 | n/r |
| [64] | 2009/Reunion Island | Use of a mosquito net to protect infants under 30 months of age: overall 70%, by age <6 months 78%, 6–12 months 70%, 12–24 months 62% and >2 years 57% | 277 | n/r |
| [37] | 2014/Reunion Island | 14.26% use mosquito nets | 1024 | n/r |
| Australasia | ||||
| [68] | 2016/Australia | Use of door screens: cases 0%, controls 0.03% | 102 (43/59)4 | No difference in CHIKV infection for use of door screens (p = 0.51) |
| Use of windows screens: cases 0%, controls 0.08% | 102 (43/59)4 | No difference in CHIKV infection for use of window screens (p = 0.07) | ||
| Use of mosquito nets: cases 95.3%, controls 96.6% | 102 (43/59)4 | No difference in CHIKV infection for use of mosquito nets (p = 0.57) | ||
| Africa | ||||
| [77] | 2016/Gabon | Mosquito net use: 79–96% | 162 | No correlation between bed net use and CHIKV infection3 |
| Physical Barrier—Long Clothing | ||||
| North America | ||||
| [66] | 2016/US Virgin Islands | 67% do not wear long clothing | 432 | n/r |
| Asia | ||||
| [46] | 2011/India | Wearing long clothing: cases 0%, controls 80% | 600 (150/450) | Individuals with CHIKV were less likely to wear long dresses (p<0.001) |
| [75] | 2015/India | 13.3% use protective clothing | 135 | n/r |
| Indian Ocean Islands | ||||
| [35] | 2009/Mayotte Island | 17.1% wear long clothing | 888 | n/r |
| Australasia | ||||
| [68] | 2016/Australia | Use of protective clothing: cases 55.8%, controls 62.7% | 102(43/59) | No difference in CHIKV infection for use of protective clothing (p = 0.42) |
| Unspecified Barrier | ||||
| Europe | ||||
| [42] | 2013/Spain | Use of physical barriers 20.8% (2008), 15.5% (2009), 6.9% (2010) | 428 (2008), 245 (2009), 147 (2010) | n/r |
| Avoidance | ||||
| [35] | 2009/Mayotte Island | 28.9% reduce outdoor activities | 888 | n/r |
| 50.8% avoid mosquito infested areas | 888 | n/r | ||
1 Cases/controls = number of individual who were positive for CHIKV/ negative for CHIKV in the study.
2 Indicates the measure was not reported.
3 Extractable data was not provided in the article.
4 Individuals were cases if they were CHIKV or dengue positive.
Removal of vector habitat was the most commonly reported behavioural measure (33 studies) [30–62]. The proportion of the population engaging in this behaviour was reported in 12 studies [33, 35, 37–39, 42, 46, 53, 56, 58, 61, 62] and ranged from 17.7% [33] to 97% [37], Table 2. Several studies mentioned that breeding habitat removal occurred without providing any further details [30–32, 45, 48, 55, 57, 60]. Other studies mentioned removal of vector habitat such as solid waste [42], removal of bushes and grasses [52], and yard sanitation [53] or provided no details on the type of vector habitat that was removed [40, 41, 47, 50].
The effectiveness of breeding habitat removal was reported in six studies. On Mayotte Island in 2009 the prevalence of CHIKV was found to be greater in individuals who did not remove breeding habitat (chi squared p<0.05) [35], and a study in India from 2011 reported that CHIKV negative participants had an odds ratio of 6.68 (95% CI 4.16–10.74) for reporting changing stored water frequently and an odds ratio of 10.34 (95% CI 6.33–16.91) for reporting that they turn empty containers upside down [46]. Another study from India reported a decrease in the incidence of cases following removal of breeding habitat [51]. In contrast, one study reported slightly greater odds, 1.12 (p<0.05), of contracting CHIKV among individuals who reported destroying breeding sites around their home [38]. Removal of breeding habitat was also associated with a decrease in larval densities in three studies [43, 49, 51].
Mosquito repellents include synthetic and natural substances that deter mosquitoes from approaching or landing. They can be applied to an individual through the use of creams or sprays or treated clothing, or they can be used to exclude mosquitoes from a space through the use of diffusers, smoke, or ultra-sound devices. The use of mosquito repellents was reported in 30 studies. The proportion of the population using individual repellents was reported in 10 studies [33, 34, 37, 38, 63–68] and ranged from 0.1% [37] to 79% [64], Table 2. The proportion of the population using space repellents was reported in eight studies [34, 37, 38, 63, 69–72] and ranged from 0.3% using an ultra-sound device to 69% using a mosquito coil [37], Table 2. The proportion of the population using an unspecified repellent was reported in 11 studies [42, 45, 46, 58, 59, 67, 68, 72–75]. Five studies mentioned that repellents were used without providing any further details [40, 52, 56, 61, 62]. One study determined the effectiveness of various repellents by comparing the length of time they kept Ae. aegypti at bay [76].
The effectiveness of skin repellent or repellent impregnated clothing was evaluated in two studies, both of which reported no effect on the rate CHIKV infections [65, 68]. Among observational studies the lack of repellent use (unspecified type) resulted in greater odds of contracting CHIKV, OR 1.4 (p<0.001) [38], the lack of space repellent use had a greater odds of contracting CHIKV, OR 3.45 (95% CI 2.34–5.09) [46] and OR 2.85 (95% CI 0.15–0.77) [48], however two other studies found no significant association between the use of space repellents and CHIKV infection [71, 72]. Citronella use had a marginally significant protective association with CHIKV infection, OR 0.37 (95% CI 0.12–0.99) whereas there was no association between CHIKV infection and the use of mosquito coils [72].
Physical barriers were described in 26 studies and can be divided into individual barriers such as long clothing, or space barriers such as bed nets, and screens. The proportion of the population using individual physical barriers was reported in five studies [35, 46, 66, 73, 75] and ranged from 0% to 80% [46], Table 2. Six studies reported the use of mosquito nets without any further details [33, 40, 52, 54, 55, 70]. Only two studies reported outcomes on the use of individual barriers. One study found that individuals with CHIKV were less likely to wear long clothing [46], while the other found no association between CHIKV infection and wearing long clothing [68]. The proportion of the population using space barriers was reported in 17 studies [34, 37, 38, 45, 46, 56, 58, 63, 64, 66–69, 72, 74, 75, 77] and ranged from 2.2% [63] to 96.6% [68]. Most of the studies that examined outcomes of space barriers found a positive effect. One study from India in 2011 reported that CHIKV negative participants had 4.51 (95% CI 2.94–6.93) greater odds of reporting mosquito net use and 7.4 (95% CI 3.18–17.22) greater odds of using window/door screens in their homes [46]. A study from Italy in 2010 found that individuals who did not use window screens had 2.32 (95% CI 1.12–4.76) greater odds of contracting CHIKV [48]. Two studies found no association between the use of a space barrier and CHIKV infection [68, 77].
Avoidance of mosquitoes was mentioned in three studies [33, 35, 56]. Only one of the studies mentioned the proportion of the population avoiding mosquitoes by reducing outdoor activities (28.9%) or avoiding mosquito infested areas (50.8%) [35], Table 2. The others provided no further information [32, 56] and none evaluated effectiveness.
Use of insecticides
Thirty-one studies investigated the use of insecticides as a control measure. Insecticide use consisted of space spraying with adulticides in and around homes (22 studies) or the addition of larvicides to water sources (9 studies), Table 3. Of the 31 studies, five were disease transmission models that simulated the effects of insecticide use in controlling an outbreak [26, 28, 29, 78, 79]. Another 17 studies reported on the use of insecticides without providing any further details [31, 32, 34, 36, 40, 45, 47, 50, 53, 57, 60, 75, 80–84].
Table 3. Frequency and effectiveness of insecticide use for the prevention and control of chikungunya virus infection in humans.
| Ref | Publication year/location | Insecticide used | Prevalence of use | Sample size (cases/controls)1 | Outcome |
|---|---|---|---|---|---|
| Adulticide | |||||
| Europe | |||||
| [33] | 2012/France | n/r2 | 20.2% of survey respondents | 1506 | n/r |
| [42] | 2013/Spain | Alfacipermetrin | 16.4% (2008), 17.1% (2009), 6.1% (2010) | 428 (2008), 245 (2009), 147 (2010) | n/r |
| [30] | 2015/France | Deltamethrin | n/r | n/r | Fogging reduced the mosquito population by 97% 48hrs after treatment |
| South America | |||||
| [56] | 2016/French Guiana | n/r | 34.7% use indoor insecticide sprays | 1462 | n/r |
| Asia | |||||
| [85] | 1975/Burma | Pyrethrum | n/r | n/r | Decreased house index3 |
| [73] | 2010/India | n/r | 42.4% of households | 1301 | n/r |
| [46] | 2011/India | n/r | CHIKV cases 87%, controls 97% | 600 (150/450) | Greater use of insecticides among CHIKV negative controls than among CHIKV cases (p<0.001) |
| [70] | 2011/India | n/r | 16.9% of homes | 528 | n/r |
| [81] | 2011/Singapore | n/r | n/r | n/r | Median larval densities in clusters dropped from 380 in 2008 to 100 in 2009, (p = 0.011) |
| [86] | 2012/India | Pyrethroid | n/r | n/r | Decreased number of suspected cases3 |
| [43] | 2012/China | n/r | n/r | n/r | Decrease in Breteau and Mosq-ovitrap indices 2 weeks following application |
| [63] | 2014/India | Sprays with pyrethrum and its related compounds | 32.2% of survey respondents | 81 | n/r |
| [87] | 2015/India | Pyrethrum | n/r | n/r | Decline in CHIKV cases 3 weeks following application3 |
| [74] | 2016/India | n/r | 6.07% use insecticidal sprays | 247 | n/r |
| Indian Ocean Islands | |||||
| [38] | 2008/Reunion Island | n/r | Sprays: never 32.5%, sometimes 18.1%, often 35.9% | 1035 | Decreased odds of contracting CHIKV when using household insecticide OR 0.83 (p<0.05) |
| Diffusers: never 10.5%, sometimes 18.1%, often 71.4% | 1035 | Decreased odds of contracting CHIKV when using household insecticide OR 0.83 (p<0.05) | |||
| [49] | 2009/Maldives | n/r | n/r | n/r | Decreased Breteau index3 |
| [44] | 2012/Reunion Island | Naled and Pyrethrum | n/r | n/r | Reduction in the risk index (number of receptacles containing Ae. albopictus larvae per 100 households)3 |
| [37] | 2014/Reunion Island | n/r | 0.39% of households | 1024 | n/r |
| [88] | 2014/Reunion Island | Deltamethrin | n/r | 162 (week 1), 55 (week 2), 65 (week 4), 49 (week 6) | Human antibody response to Ae. albopictus salivary proteins decreased over a 6 week period |
| [55] | 2016/Reunion Island | Deltamethrin | n/r | n/r | Human Ab response to Ae. albopictus salivary proteins decreased3 Decreased house and Breteau indices3 |
| Australasia | |||||
| [41] | 2014/Australia | Pyrethroid | n/r | n/r | Decrease in Ae. albopictus3 |
| [68] | 2016/Australia | n/r | Cases 0.05%, controls 0.08% | 102 (43/59)4 | No difference in CHIKV infection rates between those using insecticide and those that don’t (p = 0.7) |
| Larvicide | |||||
| Europe | |||||
| [42] | 2013/Spain | Diflubenzuron | 16.4% (2008), 17.1% (2009), 6.1% (2010) | 428 (2008), 245 (2009), 147 (2010) | n/r |
| Asia | |||||
| [85] | 1975/Burma | Abate | n/r | n/r | Reduced house index3 |
| [51] | 2007/India | Abate | n/r | n/r | Reduced incidence of CHIKV cases3 |
| [73] | 2010/India | Abate | 67.5% of households | 1301 | n/r |
| [89] | 2011/Singapore | n/r | n/r | n/r | Median larval densities in clusters dropped from 380 in 2008 to 100 in 2009, (p = 0.011) |
| [46] | 2011/India | Abate | CHIKV cases 0%, controls 60% | 600 (150/450) | Greater use of insecticides among CHIKV negative controls than among cases (p<0.001) |
| [86] | 2012/India | Temephos | n/r | n/r | Decreased number of suspected cases3 |
| [87] | 2015/India | Temephos | n/r | n/r | Decline in CHIKV cases 3 weeks following application3 |
| Indian Ocean Islands | |||||
| [44] | 2012/Reunion Island | Pyriproxyphen and Spinosad | n/r | n/r | Reduction in the risk index (number of recepticles containing Ae. albopictus larvae per 100 households).3 |
| Unspecified Use | |||||
| Europe | |||||
| [48] | 2010/Italy | n/r | n/r | 325 | No effect of pest control measures on risk of CHIKV infection OR 0.58 (95% CI 0.28–1.20, p = 0.14) |
| South America | |||||
| [39] | 2014/Colombia | n/r | 84% use chemical control | 171 | n/r |
| Indian Ocean Island | |||||
| [65] | 2008/Reunion Island | n/r | n/r | n/r | Use of insecticides in the home did not decrease the number of CHIKV infected individuals (p = 0.41) |
1 Cases/controls = number of individual who were positive for CHIKV/ negative for CHIKV in the study.
2 Indicates that the measure was not reported.
3 Extractable data was not provided in the article.
4 Individuals were cases if they were CHIKV or dengue positive.
The proportion of the population using adulticides was reported in 11 studies [33, 37, 38, 42, 46, 56, 63, 68, 70, 73, 74] and ranged from 0.39% [37] to 97% [46], Table 3. Outcomes of adulticide use were reported in 13 studies, Table 3. Decreases in the vector population were reported in eight studies as a reduction in a larval index [43, 44, 49, 55, 85], a decreased catch rate of adult mosquitoes [41, 62], and a decrease in median larval densities [81]. Five studies looked at rates of infection and found that there was a decrease in the number of chikungunya cases following adulticide fogging [86, 87], in two studies households not using adulticides had greater odds of contracting CHIKV, OR 1.2 (p<0.05) [36] and 3.22 (95%CI 2.27–4.55) [46]. The remaining study found no difference in infection rates between households using adulticides and those who did not (p = 0.7) [68]. A decrease in human antibody response (Ab) to Ae. albopictus salivary proteins was also reported in two studies, indicating a decrease in mosquito bites with adulticide use [55, 88].
The proportion of the population using larvicides was reported in three studies [42, 46, 73] and ranged from 0% [46] to 67.5% [73], Table 3. Outcomes of larvicide use were reported in seven studies, Table 3. Three of these studies reported a reduction in the vector population [44, 85, 89], and four reported a reduction in the number of chikungunya cases [46, 51, 86, 87].
Unspecified insecticide use was described in three studies, Table 3. One study reported that 84% of the study population was using chemical control [39]. The other two studies found that use of insecticides in the home did not decrease the number of CHIKV infected individuals [65] and there was no significant association between the use of pest control measures and CHIKV infection, OR 0.58 (95% CI 0.28–1.20) [48].
Although there was significant heterogeneity within each of the subgroups analysed, there were trends indicating that habitat removal was the most commonly employed mitigation measure. Random effects meta-analysis estimated the overall proportion of individuals that practice habitat removal is 65% (95% CI 55%-74%, I2 = 99.61%). Compared to the least used protective measure, physical barriers, which was estimated to be used by 25% (95% CI 17%-35%, I2 = 99.14%) of the study sample across studies. Insecticide was used by 30% (95% CI 17%-44%, I2 = 99.6%) of the sample population. The groups of studies examining habitat removal, physical barriers and insecticides had high heterogeneity between studies. Subgrouping by outbreak status did not explain a significant amount of heterogeneity, although there was a trend towards increased use of each mitigation measure during an outbreak compared to when no outbreak was occurring, Table 4.
Table 4. Meta-analysis results for the frequency of use of various mitigation and control measures for chikungunya virus, subgrouped by outbreak status.
| Mitigation measure | Reference | Ongoing outbreak | n1 | Pooled prevalence | 95% CI (%) | I2 |
|---|---|---|---|---|---|---|
| Room repellent | [34] [37] [38] [69–72] | Yes | 11 | 41% | 27–55 | 99.44% |
| [63] | No | 3 | 34% | 12–60 | 94.48% | |
| Personal repellent | [34] [37] [38] [64] [65] [68] | Yes | 9 | 30% | 13–49 | 99.62% |
| [33] [63] [66] [67] | No | 5 | 30% | 12–52 | 98.77% | |
| Unspecified repellent | [45] [58] [59] [68] [73] | Yes | 5 | 69% | 55–82 | 99.12% |
| [42] [74] [75] | No | 5 | 43% | 31–57 | 95.16% | |
| Physical barrier | [34] [35] [37] [38] [45] [58] [64] [69] [72] | Yes | 10 | 29% | 16–43 | 99.34% |
| [42] [56] [63] [66] [67] [74] [75] | No | 13 | 23% | 12–37 | 98.96% | |
| Insecticide use | [33] [44] [45] | Yes | 10 | 29% | 16–43 | 99.34% |
| [32] [34] [46] [48] | No | 13 | 23% | 12–37 | 98.96% | |
| Habitat removal | [33] [37] [38] [39] [53] [58] [61] [62] | Yes | 12 | 72% | 60–83 | 99.65% |
| [33] [42] [56] | No | 5 | 46% | 29–64 | 99.09% |
1The sample size may be larger than the number of references as there may be more than one data point extracted per study
Biological mosquito control
Six studies mentioned the use of biological mosquito control [28, 39, 42, 61, 85, 90]. Of the six studies, one modeled the effects of biological mosquito control in a disease transmission model [28]. In 2013 a study in Spain investigated the introduction of Bacillus thuringiensis to water storage containers for larval control, but did not report a measure of effectiveness for the intervention [42]. A pilot study in 1975 in Burma added the larvivorous fish Lebistes reticulatus to water storage containers, however the water storage practices of the participants did not favour the survival of the fish for more than 24 hours [85]. In India (2011) the introduction of larvivorous fish to water storage containers and a complementary education campaign resulted in a significant reduction in Aedes larvae, OR 0.51 (p<0.001) [61]. In 2014 a study in Colombia reported that 73% (125/171) of the survey population was using biological mosquito control but did not provide any further details [39]. A study in Puerto Rico in 2016 reported on the use of autocidal gravid ovitraps to capture adult female Ae. aegypti and found that the proportion of chikungunya virus IgG antibody among participants from the two intervention communities was one half that of participants from control communities (risk ratio = 0.52, 95% CI 0.38–0.71) [90].
Public education
Public education measures were described in 30 studies and covered topics such as how to avoid mosquito bites [30, 32, 36, 42, 57, 60, 80, 83, 86, 89, 91–94], how to recognise and remove vector breeding habitat [30, 32, 42, 44, 51, 53, 57, 59, 61, 78, 82, 83, 85, 86, 89, 91, 92, 94, 95], how to recognise chikungunya symptoms [53, 60, 61, 80, 92, 93], and general chikungunya information [53, 61, 73, 80, 83, 92, 96, 97]. Educational material was delivered through print media, either by newspaper or pamphlets [30–32, 44, 51, 57, 73, 80–82, 86, 95], in person to groups or individuals [36, 42, 51, 53, 60, 61, 83, 92–96], through social media [31], a website [93] or through television and radio [44, 80].
The impact of public health education campaigns was evaluated for the topics of vector habitat removal [32, 42, 44, 51, 57, 59, 85, 89, 93] and general chikungunya information [61, 92, 96, 97], Table 5. The public education campaigns that focused on recognising and removing vector breeding habitat resulted in a decrease in the number of breeding sites [92, 95], a reduction in the incidence of chikungunya cases [32, 51], a reduction in larval indices [44, 85, 89], prevention of local transmission following imported cases [57], and an increase in the number of individuals removing stagnant water [42]. For the public education campaigns that provided general chikungunya information, the impact of the campaign was measured as an increase in knowledge determined through the use of questionnaires [61, 92, 96, 97].
Table 5. Topic, delivery method, and effectiveness for public education campaigns aimed at reducing or preventing chikungunya transmission.
| Ref | Publication year/location | Delivery method | Outcome |
|---|---|---|---|
| Recognising and removing vector breeding habitat | |||
| North America | |||
| [95] | 2014/USA | In person and print media. Door to door and public spaces | 22.6% reduction in container habitats in the communities being educated compared to a 32.3% increase in the sites not receiving education (p = 0.004) |
| [57] | 2016/USA | Print media | No local transmission following nine imported cases |
| Europe | |||
| [42] | 2013/Spain | Delivered in person door to door | Increase over three years in the number of individuals who remove stagnant water on their property (chi squared p<0.05) |
| Asia | |||
| [85] | 1975/Burma | n/r1 | Reduced House Index from 50% to 15% |
| [92] | 2006/India | In person to school children who then replayed it to family members | Decrease in the number of mosquito breeding sites (Z = 7.82, p = 0) |
| [51] | 2007/India | In person and print delivered door to door and in public spaces | Reduced incidence of CHIKV cases |
| [89] | 2011/Singapore | n/r | Median larval densities in clusters dropped from 380 in 2008 to 100 in 2009 |
| [32] | 2013/India | Print media delivered door to door | Reduced incidence of CHIKV cases |
| Indian Ocean Islands | |||
| [44] | 2012/Reunion Island | High media coverage | Reduction in the risk index (number of receptacles containing Ae. albopictus larvae per 100 households) |
| General chikungunya information | |||
| South America | |||
| [96] | 2014/Colombia | Delivered to medical students and professionals at a conference | Increase in the proportion of correct answers on a questionnaire after the intervention |
| Asia | |||
| [92] | 2006/India | Delivered in person to school children who then replayed it to family members | Change in knowledge scores on a questionnaire |
| [61] | 2011/India | Delivered in person in public spaces | Increase in new knowledge determined through questionnaire administration |
| [97] | 2012/India | Informational session delivered in the workplace | Increased knowledge regarding mosquitoes and control measures |
1Indicates the measure was not reported
Other topics of public education included avoiding mosquito bites [30, 32, 36, 42, 53, 80, 83, 86, 89, 92, 94] and information on insecticide fogging [30], however quantification of the impact of the campaigns were not reported. One study [84] described mitigation measures implemented in various countries and did not provide any further details on the use or effectiveness of these measures.
Control of blood products
Nine studies reported the control or treatment of blood and blood products in order to lessen the risk of transmission through blood transfusions. The most common action was a ban on donations or temporary deferral of blood donors during a defined risk of being viremic period, Table 6. During an outbreak in Italy in 2007, officials implemented a temporary ban on blood donation for individuals living in the area of the outbreak, donation deferral for those who had visited the area during the outbreak, and a quarantine period for blood donated by individuals who had visited the area prior to the outbreak [98]. The Netherlands implemented a donation deferral from travellers returning from Thailand in 2013 and estimated the risk of a viremic traveller donating blood to be very low at 0.068 travellers per year [99]. Martinique quarantined blood donations in 2014 [100], as did Thailand [101], Table 6. One study completed in Singapore in 2011 mentioned that mass screening occurred, but provided no further details [52]. One study developed a screening method capable of detecting asymptomatic donors [102].
Table 6. Blood and blood product control methods used to prevent transfusion transmission of chikungunya virus and estimated risk of transfusion related infection.
| Ref | Publication year/location | Screening method | Donation deferral period | Length of quarantine | Risk of infection |
|---|---|---|---|---|---|
| Donation ban | |||||
| [98] | 2008/Italy | Pre donation questionnaire | 21 days | 5 days | Highest weekly estimated risk of yielding one viremic unit from an asymptomatic viremic donor was 1:3801 |
| Donation deferral | |||||
| [99] | 2013/ Netherlands |
n/r1 | n/r | n/a2 | Modeled number of potentially infected donors returning from Thailand during a chikungunya outbreak was 0.068 infected donors / year |
| Blood product quarantine | |||||
| [100] | 2014/ Martinique |
Serology, post donation reporting of febrile symptoms | n/a | 72-hour post donation quarantine | n/a |
| [101] | 2014/Thailand | Serology, pre-donation questionnaire, enhanced post donation report | n/a | 7 days | n/a |
1Indicates the measure was not reported
2Indicates the measure was not applicable to that study
In areas where an active outbreak of CHIKV is occurring the risk of a viremic individual donating blood is significantly higher. For example, during an outbreak in Italy [98] the risk of blood from an asymptomatic viremic donor was estimated to be 1 in 3801 (95% CI not reported) and in Thailand in 2009, the same risk was 1 in 2429 (0.04%, 95% CI 0.02%–0.06%) [101]. As a follow up to the Thailand study, the authors created a disease transmission model to estimate the mean number of transfusion transmitted CHIKV cases that would have occurred in the absence of blood safety implementation measures. The model indicated that screening measures implemented during the 2009 outbreak effectively reduced the risk of transfusion transmission [103].
Treatment of blood products to reduce the risk of transmission was evaluated in two studies. One study successfully used amotosalen photochemical treatment (PCT) or riboflavin pathogen reduction treatment (PRT) to significantly reduce the number of plaque forming units per millilitre [104]. Another study used Theraflex UV-platelet system for pathogen inactivation which resulted in a significant log reduction in post treatment titres [105].
Quarantine of infected individuals
Quarantine and isolation of infected individuals was described in five studies. Of these five studies, three were disease transmission models that used quarantine to predict the effects of different mitigation measures [26, 28, 78]. Other outbreak responses involved the rapid detection and isolation of infectious individuals together with vector control measures [89, 106].
Discussion
This SR aimed to identify and summarize all of the research on the use and effectiveness of individual and community level mitigation and control measures for CHIKV. The results indicate that a variety of mitigation and control methods for chikungunya have been used worldwide, that a great deal of variability exists in the frequency of use of these mitigation measures, and that there is a lack of consistency among studies when reporting outcomes. While this review was able to identify some possible trends in the frequency of use of mitigation measures, it has done more to highlight the current gaps in knowledge and/ or reporting that prevent summarization of how effective mitigation measures are at the prevention or control of chikungunya.
Removal of breeding habitat was used by the greatest proportion of respondents across studies, and was also the most commonly reported of the personal protective measures (33/55). This could possibly demonstrate the effectiveness of public education in altering behaviour, as habitat removal was the most commonly reported topic of the education campaigns. However, only five studies described both public education and personal protective measures [32, 42, 44, 53, 61] and only three measured the effectiveness of education in terms of reductions in vector breeding habitat [42, 92, 95], which limits the evidence on how effective public education campaigns may be at affecting behaviour change.
Public education campaigns that focused on general chikungunya knowledge used pre and post questionnaires in order to evaluate how effective the campaign was in improving knowledge, however, this study design cannot measure or make inference to behaviour change and long term adoption of mitigation measures [107]. Methods of measuring behaviour change and impact are needed to fully evaluate the impact of public education campaigns on vector habitat removal or the use of personal protective measures.
Adulticide and larvicide studies examined effectiveness of use inside and outside the home. Outcomes ranged from vector density measurements at different intervals post application to the risk of CHIKV infection in humans based upon data collected through primary research and surveillance. For most mosquito related outcomes authors reported a significant decrease in mosquito or larvae populations. Similarly, outcomes of human infection reported either a decline in CHIKV cases following insecticide application or a protective effect of using insecticides in the home for most studies where there was a high prevalence of exposure to CHIKV. Despite the demonstrated effectiveness of insecticides in controlling vector populations, there are concerns associated with its use, such as the development of insecticide resistance as well as the potential effects on environmental and human health [108]. There are also economic considerations since the greatest cost-benefit of insecticide application in public spaces is likely to be achieved in small to medium sized towns, whereas in larger urban centres the cost may overcome the public health benefits [109].
Quantitative data was available for only 60% of the studies examining the use and effectiveness of personal and community interventions for the prevention of CHIKV. Studies that reported a measure of the effectiveness of a mitigation strategy typically had a pre- and post-intervention evaluation where the outcome measure was taken at two or more time points prior to and after intervention implementation. There was a lack of consistency in the reported outcome measures across studies, this prohibited meta-analysis and direct comparisons of the effectiveness of interventions captured in this SR. The insecticide category is a good example of this heterogeneity, where several studies did not specify whether an adulticide or larvicide was used, and each study had a different outcome measurement including rates of CHIKV infection, vector density, and larval counts. Although each of these outcome measures can evaluate whether or not an insecticide had an impact, they cannot be summarized together to evaluate the consistency of insecticide impact across studies.
The frequency at which various mitigation strategies were used within a population was extracted from 33.7% of studies. These were usually point in time cross-sectional surveys that could not evaluate how effective the intervention was at decreasing the mosquito density or preventing cases of CHIKV. The analysis on the proportion of individuals using various interventions was highly heterogeneous and as a result, the findings in Table 4 should be interpreted with caution. There was not enough data to conduct meta-regression or explore subgroups beyond outbreak status in order to try and explain some of the heterogeneity. It is possible that location and date of study may account for some of the variation between subgroups as there may be different preferences for certain mitigation or control measures in different locations, at different times and by different populations [110].
Much of the literature described more than one mitigation measure, with particular overlap occurring between behavioural measures, insecticide use, and public education (Tables 2, 3 and 5). This overlap makes it difficult to determine whether the measured impact in the study was due to one particular intervention or the combination of interventions. Research on similar vector-borne diseases suggests that a combination of interventions is likely more effective than a single intervention on its own [110, 111]. Thus, future research should consider study designs that can evaluate single strategy vs. multifaceted interventions. Data on the potential impact of a variety of options will facilitate future development of the most effective community level mitigation strategies.
A lack of reporting on the effectiveness of interventions for emerging diseases has been noted elsewhere [112] and this SR resulted in similar findings. With the exception of one randomized control trial [95] (public education) and one case control study [46] (insecticide use), the rest of the studies were prevalence surveys or cross-sectional studies, which can only provide point in time data on the sampled population. The latter can investigate associations between outcome measures and risk factors such as the use of an intervention. However, cross-sectional surveys cannot provide chronological evidence that the intervention preceded the outcome (e.g. CHIKV infection) and thus limits interpretation of the association. Thus the data available from the studies identified in this SR is limited and highlights the need for controlled trials that may provide cause-effect evidence while controlling for confounding variables, and for improved consistency in the selection of outcome measures.
The use of personal protection and behavioural mitigation measures captured in this SR tended to be greater during times of CHIKV outbreaks, suggesting that there may need to be a greater emphasis on mitigation and control measures during non-outbreak periods in order to decrease the risk of an outbreak occurring or the magnitude of an outbreak by reducing the likelihood of local transmission [113]. This observation may be transferable to other mosquito-borne diseases as well, although this review only examined personal protective and behavioural mitigation measures during CHIKV outbreaks compared to periods when no outbreak was occurring. At an individual level, the motivation to practice protective behaviours is likely higher during an outbreak due to perceived risk of infection and normalization of protective behaviours by public education and media. The fact that habitat removal is the most prevalent form of mitigation, both during and not during an outbreak, could possibly be due to the fact that public education measures tend to focus on habitat and breeding site removal (Table 5). More research is needed to determine whether there is a relationship between the two, which would suggest that public education campaigns are successful in altering behaviour.
While there are some limitations to this SR, such as possible language bias due to the exclusion of articles in languages other than English, French, Spanish and Portuguese, or the possibility that research was missed by the scoping review search or not properly classified, every effort was made to minimize potential biases by developing the protocol and all tools used in the scoping review and this SR a priori and pre-testing them with the review team.
The results of this review could have implications for the mitigation of other mosquito borne viruses. Aedes aegypti and Ae. albopictus are also vectors for dengue and zika viruses, therefore any mitigation measure that is effective for CHIKV is likely to be effective in controlling local transmission of those viruses, and vice versa. As these viruses are undergoing rapid emergence in various parts of the world [114–116], identifying those measures that can prevent local transmission should be considered of public health importance.
Conclusions
This SR summarized the knowledge on community level mitigation and control methods for CHIKV and highlighted current research gaps. There was a lack of research that assessed the effectiveness of various mitigation strategies. This SR focused on the prevention of CHIKV infection in humans, however many of the identified interventions are designed to control the vector or prevent mosquito bites (e.g nets, coils, insecticide), we acknowledge that there is other research measuring the effectiveness of these vector control and disease prevention strategies applied to other mosquito species and outcomes (e.g. mosquito bites, vector abundance) that were outside the scope of this SR. With respect to CHIKV research, future studies should focus on consistent reporting of outcomes and the standardisation of how outcomes are measured in order to be able to summarize the effectiveness of individual and complex interventions across studies. Being able to determine which mitigation and control strategies are the most effective will aid public health officials in designing effective education and vector control programs in order to reduce the risk of local transmission of CHIKV.
Supporting information
(DOCX)
(DOCX)
(DOC)
(DOCX)
(XLSX)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Powers A, Logue C. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2011; 88(9):2363–2377 [DOI] [PubMed] [Google Scholar]
- 2.Schuffenecker I, Iteman I, Michault A, Murri S, Franqeul L, Vanev M, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006; 3:e263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renault P, Solet J, Sissoko D, Balleydier E, Larrieu S, Filleul C, et al. A major epidemic of Chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007; 77(4):727–731. [PubMed] [Google Scholar]
- 4.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli A, Panning M, et al. Infection with Chikungunya virus in Italy: An outbreak in a temperate region. Lancet. 2007; 370:1840–1846. 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- 5.Gould E, Gallian P, De Lamballerie X, Charrel R. First cases of autochthonous Dengue fever and Chikungunya fever in France: from bad dream to reality! Clin Microbiol Infect. 2010; 16(12):1702–1704. 10.1111/j.1469-0691.2010.03386.x [DOI] [PubMed] [Google Scholar]
- 6.Morrison T. Reemergence of Chikungunya virus. J Virol. 2014; 88(21):11644–11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harter KR, Bhatt S, Hyung TK, Mallon WK. Chikungunya fever in Los Angeles, California. West J Emerg Med. 2014; 15(7):841–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Garcia MD, Bangert M, de Ory F, Potente A, Hernandez L, Lasala F, et al. Chikungunya virus infections among travellers returning to Spain, 2008 to 2014. Euro Surveill. 2016; 21(36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therien C, Jourdan G, Holloway K, Tremblay C, Drebot M. First Imported Case of Chikungunya Virus Infection in a Travelling Canadian Returning from the Caribbean. Can J Infect Dis Med Microbiol. 2016; 10.1155/2016/2980297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caminade C, Medlock JM, Ducheyne K, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012; 9(75): 2708–2717 10.1098/rsif.2012.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vega-Rua A, Zouache K, Caro V, Diancourt L, Delaunay P, Grandadam M, et al. High efficiency of temperate Aedes albopictus to transmit chikungunya and dengue viruses in the Southeast of France. PloS One. 2013; 8(3)e59716 10.1371/journal.pone.0059716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vega-Rua A, Zouache K, Girod R, Failloux A, Lourenco-de-Oliveira R. High level vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J Virol. 2014; 88(11):6294–6306. 10.1128/JVI.00370-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir Res. 2013;99: 345–70 10.1016/j.antiviral.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilte C, Staikowsky F, Staikovsky F, Madec Y, Carpentier F, Kassab S, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7: e2137 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gérardin P, Couderc T, Bintner M, Tournebize P, Renouil M, Lémant J, et al. Chikungunya virus-associated encephalitis A cohort study on La Réunion Island, 2005–2009. Neurology 2016;86(1): 10.1212/WNL.0000000000002234 [DOI] [PubMed] [Google Scholar]
- 16.Ramful D, Carbonnier M, Pasquet M, Bouhmani B, Ghazouani J, Noormahomed T, et al. Mother-to-child transmission of Chikungunya virus. Pediatr Infect Dis J. 2007;26:811–15 10.1097/INF.0b013e3180616d4f [DOI] [PubMed] [Google Scholar]
- 17.Josseran L, Paquet C, Zehgnoun A, Caillere N, Le Tertre A, Solet JL, et al. Chikungunya disease outbreak, Reunion Island [letter]. Emerg Infect Dis. 2006;12(12):1994–95. 10.3201/eid1212.060710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Avoid mosquito bites. Atlanta, GA: US Department of Health and Human Services, CDC, 2016. http://www.cdc.gov/features/stopmosquitoes/. Retrieved October 14, 2016
- 19.Moher D, Liberati A, Tezlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- 20.Mascarenhas M, Garasia S, Berthiaume P, Corrin T, Greig J, Ng V, et al. (2018) A scoping review of published literature on chikungunya virus. PLoS ONE. 2018;13(11): 10.1371/journal.pone.0207554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions: Version 5.1.0. The Cochrane Collaboration 2011
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88 [DOI] [PubMed] [Google Scholar]
- 23.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Mat Stat. 1950;21(4):607–11 [Google Scholar]
- 24.Peters JL, Mengersen KL. Meta-analysis of repeated measures study design. J Eval Clin Pract. 2008;14(5):941–50 10.1111/j.1365-2753.2008.01010.x [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26.Dumont Y, Chiroleu F. Vector control for the Chikungunya disease. Math Biosci Eng. 2010;7(2):313–45 10.3934/mbe.2010.7.313 [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Stechlinski P. Application of control strategies to a seasonal model of chikungunya disease. Appl Math Model. 2015;39(12):3194–3220 [Google Scholar]
- 28.Moulay D, Aziz-Alaoui MA, Kwon HD. Optimal control of chikungunya disease: Larvae reduction, treatment and prevention. Math Biosci Eng. 2012;9(2):369–92 10.3934/mbe.2012.9.369 [DOI] [PubMed] [Google Scholar]
- 29.Ndeffo-Mbah ML, Durham DP, Skrip LA, Nsoesie EO, Brownstein JS, Fish D, et al. Evaluating the effectiveness of localized control strategies to curtail chikungunya. Sci Rep. 2016; 10.1038/srep23997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17): [DOI] [PubMed] [Google Scholar]
- 31.Ahmed S, Francis L, Ricketts RP, Christian T, Polson-Edwards K, Olowokure B. Chikungunya virus outbreak, Dominica, 2014. Emerg Infect Dis. 2015;21(5):909–11 10.3201/eid2105.141813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil SS, Patil SR, Durgawale PM, Patil AG. A study of the outbreak of Chikungunya fever. J Clin Diagn Res. 2013;7(6):1059–62 10.7860/JCDR/2013/5330.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raude J, Chinfatt K, Huang P, Betansedi CO, Katumba K, Vernazza N, et al. Public perceptions and behaviours related to the risk of infection with Aedes mosquito-borne diseases: a cross-sectional study in Southeastern France. BMJ Open. 2012;2(6): 10.1136/bmjopen-2012-002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijayakumar K, Anish TS, Streekala KN, Ramachandran R, Philip RR. Environmental factors of households in five districts of Kerala affected by the epidemic of chikungunya fever in 2007. Natl Med J India. 2010;23(2):82–4 [PubMed] [Google Scholar]
- 35.Raude J, Setbon M. The role of environmental and individual factors in the social epidemiology of chikungunya disease on Mayotte Island. Health Place. 2009;15(3):659–69 10.1016/j.healthplace.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa MJ, Tang CS, Nishibuchi M. Incidence of chikungunya fever in Singapore: implications of public health measures and transnational movements of people. Trop Med Health. 2010;38(1):39–45 [Google Scholar]
- 37.Thuilliez J, Bellia C, Dehecq JS, Reilhes O. Household-level expenditure on protective measures against mosquitoes on the island of La Réunion, France. PLoS Negl Trop Dis. 2014;8(1):e2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setbon M, Raude J, Pottratz D. Chickungunya on Reunion Island: social, environmental and behavioural factors in an epidemic context. Population. 2008;63(3):491–519 [Google Scholar]
- 39.Campo Carey AR, Benavides Ocampo M, Martinez Duran ME, Caro Nunez OA, Gomez Amaya JA, Nieto Sanchez DL, et al. [Chikungunya outbreak in the municipality of Mahates, Bolivar, 2014]. Informe Quincenal—Epidemiologico Nacional. 2014;19(21);342–67 Spanish [Google Scholar]
- 40.Nhan TX, Claverie A, Roche C, Teissier A, Colleuil M, Baudet JM, et al. Chikungunya virus imported into French Polynesia, 2014. Emerg Infec Dis. 2014;20(10):1773–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knope KE, Doggett SL, Kurucz N, Feldman R, Johansen CA, Nicholson J, et al. Arboviral diseases and malaria in Australia, 2011–12: annual report of the national arbovirus and malaria advisory committee. Commun Dis Intell Q Rep. 2014;38(2):E122–42. [PubMed] [Google Scholar]
- 42.Chebabi Abramides G, Roiz D, Guitart R, Quitana S, Gimenez N. Control of the Asian Tiger mosquito (Aedes albopictus) in a firmly established area in Spain: risk factors and people’s involvement. Trans R Soc Trop Med Hyg. 2013;107:706–15 10.1093/trstmh/trt093 [DOI] [PubMed] [Google Scholar]
- 43.Qiaoli Z, Jianfeng H, De W, Zijun W, Xinguang Z, Haojie Z, et al. Maiden outbreak of Chikungunya in Dongguan City, Guangdong Province, China: epidemiological characteristics. PLoS One. 2012;7(8): e42830 10.1371/journal.pone.0042830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flahault A, Aumont G, Boisson V, de Lamballerie X, Favier F, Fontenille D, et al. An interdisciplinary approach to controlling chikungunya outbreaks on French islands in the south-west Indian Ocean. Med Trop. 2011;72:66–71 [PubMed] [Google Scholar]
- 45.Balasubramaniam SM, Krishnakumar J, Stephen T, Gaur R, Appavoo N. Prevalence of Chikungunya in urban field practice area of a private medical college, Chennai. Indian J Communitty Med. 2011;36(2):124–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majra JP, Acharya D. Impact of knowledge and practices on prevention of chikungunya in an epidemic area in India. Ann Trop Med Public Health. 2011;4(1):3–6 [Google Scholar]
- 47.Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17(5):910–3 10.3201/eid1705.101873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moro ML, Gagliotti C, Silvi G, Angelini R, Sambri V, Rezza G, et al. Chikungunya virus in North-eastern Italy: a seroprevalence survey. Am J Trop Med Hyg. 2010;82(3):508–11 10.4269/ajtmh.2010.09-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoosuf AA, Shiham I, Mohamed AJ, Ali G, Luna JM, Pandav R, et al. First report of chikungunya from the Maldives. Trans R Soc Trop Med Hyg. 2009;103(2):192–6 10.1016/j.trstmh.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 50.Angelini P, Macini P, Finarelli AC, Po C, Venturelli C, Bellini R, et al. Chikungunya epidemic outbreak in Emilia-Romagna (Italy) during summer 2007. Parassitologia. 2008;50(1–2):97–8 [PubMed] [Google Scholar]
- 51.Selvavinayagam TS. Chikungunya fever outbreak in Vellore, South India. Indian J Communitty Med. 2007;32(4):286–7 [Google Scholar]
- 52.Surendran SN, Kannathasan S, Kajatheepan A, Jude PJ. Chikungunya-type fever outbreak: some aspects related to this new epidemic in Jaffna district, northern Sri Lanka. Trop Med and Health. 2007;35(3):249–52 [Google Scholar]
- 53.Boyer S, Foray C, DeHecq JS. Spatial and temporal heterogeneities of Aedes albopictus density in La Reunion Island: rise and weakness of entomological indices. PLoS One. 2014;9(3): e91170 10.1371/journal.pone.0091170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goorah S, Dewkurun MK, Ramchurn SK. Assessing the sustainability of individual behavior change against mosquitoes after the outbreak of vector-borne disease in Mauritius: a case study. Internet J of Medical Update. 2013;8(1):9–16 [Google Scholar]
- 55.Ndille EE, Doucoure S, Poinsignon A, Mouchet F, Cornelie S, D’Ortenzio E, et al. Human IgG Antibody Response to Aedes Nterm-34kDa Salivary Peptide, an Epidemiological Tool to Assess Vector Control in Chikungunya and Dengue Transmission Area. PLoS Negl Trop Dis. 2016;10(12):e0005109 10.1371/journal.pntd.0005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fritzell C, Raude J, Adde A, Dusfour I, Quenel P, Flamand C. Knowledge, Attitude and Practices of Vector-Borne Disease Prevention during the Emergence of a New Arbovirus: Implications for the Control of Chikungunya Virus in French Guiana. PLoS Negl Trop Dis. 2016;10(11):e0005081 10.1371/journal.pntd.0005081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goddard J, Varnado WC, Hand S, Meyer F. Chikungunya in Mississippi: The Health Department response to imported cases. J Miss State Med Assoc. 2016;57(5):138–41 [PubMed] [Google Scholar]
- 58.Kuan G, Ramirez S, Gresh L, Ojeda S, Melendez M, Sanchez N, et al. Seroprevalence of anti-chikungunya virus antibodies in children and adults in Managua, Nicaragua, after the first chikungunya epidemic, 2014–2015. PLoS Negl Trop Dis. 2016;10(6):e0004773 10.1371/journal.pntd.0004773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genderen FT, Krishnadath I, Sno R, Grunberg MG, Zijlmans W, Adhin MR. First chikungunya outbreak in Suriname; clinical and epidemiological features. PLoS Negl Trop Dis. 2016;10(4):e0004625 10.1371/journal.pntd.0004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liew C,Yung CF. First detection of chikungunya infection and transmission in Brunei Darussalam. Singpore Med J. 2012;53(4):e66–8 [PubMed] [Google Scholar]
- 61.Ghosh SK, Chakaravarthy P, Panch SR, Krishnappa P, Tiwari S, Ojha VP, et al. Comparative efficacy of two poeciliid fish in indoor cement tanks against chikungunya vector Aedes aegypti in villages in Karnataka, India. BMC Public Health. 2011; 10.1186/1471-2458-11-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilain P, Larrieu S, Renault P, Baville M, Filleul L. How to explain the re-emergence of chikungunya infection in Reunion Island in 2010? Acta Trop. 2012;123(2):85–90 10.1016/j.actatropica.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 63.Anand T, Kumar R, Saini V, Meena G, Ingle G. Knowledge and use of personal protective measures against mosquito borne diseases in a resettlement colony of Delhi. Ann Med Health Sci Res. 2014;4(2):227–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorge F, Tamburro M, de Pertat T. [Use and effects of insect repellents in infants in Reunion Island (France) during the 2005–2006 chikungunya epidemic: the 2009 INR study]. Bull Epidemiol Hebd (Paris). 2011;6:54–9. French [Google Scholar]
- 65.Staikowsky F, le Roux K, Schuffenecker I, Laurent P, Grivard P, Develay A, et al. Retrospective survey of Chikungunya disease in Reunion Island hospital staff. Epidemiol Infect. 2008;136(2):196–206 10.1017/S0950268807008424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cherry CC, Beer KD, Fulton C, Wong D, Buttke D, Staples JE, et al. Knowledge and use of prevention measures for chikungunya virus among visitors—Virgin Islands National Park, 2015. Travel Med Infect Dis. 2016;14(5):475–80. 10.1016/j.tmaid.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lalani T, Yun H, Tribble D, Ganesan A, Kunz A, Fairchok M, et al. A comparison of compliance rates with anti-vectorial protective measures during travel to regions with dengue or chikungunya activity, and regions endemic for Plasmodium falciparum malaria. J Travel Med. 2016;23(5): 10.1093/jtm/taw043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millman AJ, Esposito DH, Biggs HM, Decenteceo M, Klevos A, Hunsperger E, et al. Chikungunya and dengue virus infections among United States community service volunteers returning from the Dominican Republic, 2014. Am J Trop Med Hyg. 2016;94(6):1336–41 10.4269/ajtmh.15-0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aswathy S, Dinesh S, Kurien B, Johnson AJ, Leelamoni K. A post-epidemic study on awareness of vector habits of chikungunya and vector indices in a rural area of Kerala. J Commun Dis. 2011;43(3):209–15 [PubMed] [Google Scholar]
- 70.Anish T, Vijayakumar K, Leela IA. Domestic and Environmental Factors of Chikungunya-affected Families in Thiruvananthapuram (Rural) District of Kerala, India. J Glob Infect Dis. 2011;3(1):32–6 10.4103/0974-777X.77293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salje H, Lessler J, Paul KK, Azman AS, Rahman MW, Rahman M, et al. How social structures, space, and behaviors shape the spread of infectious diseases using chikungunya as a case study. Proc Natl Acad Sci USA. 2016;113(47):13420–25 10.1073/pnas.1611391113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bloch D, Roth NM, Caraballo EV, Munoz-Jordan J, Hunsperger E, Rivera A, et al. Use of household cluster investigations to identify factors associated with chikungunya virus infection and frequency of case reporting in Puerto Rico. PLoS Negl Trop Dis. 2016;10(10): 10.1371/journal.pntd.0005075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puwar T, Sheth JK, Kohli V, Yadav R. Prevalence of chikungunya in the city of Ahmedabad, India, during the 2006 outbreak: A community based study. Dengue Bull. 2010;34:40–5 [Google Scholar]
- 74.Tenglikar PV, Hussain M, Nigudgi SR, Ghooli S. Knowledge and practices regarding mosquito borne disease among people of an urban area in Kalaburgi, Karnataka. Ntl J Community Med. 2016;7(3):223–25 [Google Scholar]
- 75.Mehta D, Solanki H, Patel P, Umat P, Chauhan R, Shukla S, et al. A study on knowledge, attitude & practice regarding mosquito borne diseases in an urban area of Bhavnagar. Indian J Prev Soc Med. 2015;6(2):29–32 [Google Scholar]
- 76.Uc-Puc V,Herrera-Bojorquez J, Carmona-Carballo C, Che-Mendoza A, Medina-Barreiro A, Chable-Santos J, et al. [Effectiveness of commercial repellents against Aedes aegypti (L.) in Yucatan, México]. Salud Publica Mex. 2016;58(4):472–5. Spanish [PubMed] [Google Scholar]
- 77.Gabor JJ, Schwarz NG, Esen M, Kremsner PG, Grobusch MP. Dengue and chikungunya seroprevalence in Gabonese infants prior to major outbreaks in 2007 and 2010: A sero-epidemiological study. Travel Med Infect Dis. 2016;14(1):26–31 10.1016/j.tmaid.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 78.Mecoli M, De Angelis V, Brailsford SC. Using system dynamics to evaluate control strategies for mosquito-borne diseases spread by human travel. Comput Oper Res. 2013;40(9):2219–28 [Google Scholar]
- 79.Poletti P, Messeri G, Ajelli M, Vallorani R, Rizzo C, Merler S. Transmission potential of Chikungunya virus and control measures: the case of Italy. PLoS One. 2011;6(5): 10.1371/journal.pone.0018860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, Matheus S, et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill. 2014;19(13): [DOI] [PubMed] [Google Scholar]
- 81.Jain S, Kadri S, Venkatesh S, Lal S, Katyal R. Epidemiological investigation of an outbreak of chikungunya in hyderabad and nalgonda districts of andhra pradesh, India. Int J Health Sci. 2007;1(2):303–8 [PMC free article] [PubMed] [Google Scholar]
- 82.Gould LH, Osman MS, Farnon EC, Griffith KS, Godsey MS, Karch S, et al. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans R Soc Trop Med Hyg. 2008;102(12):1247–54 10.1016/j.trstmh.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 83.Jain R, Acharya AS, Khandekar J, Jais M. Entomo-epidemiological investigations of chikungunya outbreak in Delhi, India. Ann Trop Med Public Health. 2013;6:297–300 [Google Scholar]
- 84.Impoinvil DE, Ahmad S, Troyo A, Keating J, Githeko AK, Mbogo CM, et al. Comparison of mosquito control programs in seven urban sites in Africa, the Middle East, and the Americas. Health Policy. 2007;83(2):196–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thaung U, Ming CK, Thein M. Dengue haemorrhagic fever in Burma. Southeast Asian J Trop Med Public Health. 1975;6(4):580–91 [PubMed] [Google Scholar]
- 86.Seyler T, Sakdapolrak P, Prasad SS, Dhanraj R. A chikungunya outbreak in the metropolis of Chennai, India, 2006. J Environ Health. 2012;74(6):8–13 [PubMed] [Google Scholar]
- 87.Uthappa CK, Allam RR, Gunti D, Nalini C, Udaragudi PR, Tadi GP, et al. Chikungunya outbreak in Atmakur village, Medak district, Telangana State, India. Indian J Med Res. 2015;142(Suppl 1): S108–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doucoure S, Mouchet F, Cornelie S, Drame PM, D’Ortenzio E, DeHecq JS, et al. Human antibody response to Aedes albopictus salivary proteins: a potential biomarker to evaluate the efficacy of vector control in an area of Chikungunya and Dengue Virus transmission. Biomed Res Int. 2014; 10.1155/2014/746509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho K, Ang LW, Tan BH, Tang CS, Ooi PL, James L, et al. Epidemiology and control of chikungunya fever in Singapore. J Infect. 2011;62(4):263–70 10.1016/j.jinf.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 90.Lorenzi OD, Major C, Acevedo V, Perez-Padilla J, Rivera A, Biggerstaff BJ, et al. Reduced incidence of chikungunya virus infection in communities with ongoing Aedes aegypti mosquito trap intervention studies—Salinas and Guayama, Puerto Rico, November 2015-February 2016. MMWR Morb Mortal Wkly Rep. 2016;65(18):479–80 10.15585/mmwr.mm6518e3 [DOI] [PubMed] [Google Scholar]
- 91.Soulaphy C, Souliphone P, Phanthavong K, Phonekeo D, Phimmasine S, Khamphaphongphane B, et al. Emergence of chikungunya in Moonlapamok and Khong districts, Champassak Province, the Lao People’s Democratic Republic, May to September 2012. Western Pac Surveill Response J. 2013;4(1):46–50 10.5365/WPSAR.2012.3.4.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaidya V, Sawant S. A KAP study in Pune City involving school children as a strategy for effective vector control in chikungunya. Indian J Public Health and Dev. 2013;4(4):438–43 [Google Scholar]
- 93.Tsiodras S, Pervanidou D, Papadopoulou E, Kavatha D, Baka A, Koliopoulos G, et al. Imported chikungunya fever case in Greece in June 2014 and public health response. Pathog Glob Health. 2016;110(2):68–73 10.1080/20477724.2016.1176311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Ortenzio E, Grandadam M, Balleydier E, Jaffar-Bandjee MC, Michault A, Brottet E, et al. A226V strains of Chikungunya virus, Reunion Island, 2010. Emerg Infect Dis. 2011;17(2):309–11 10.3201/eid1702.101056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Healy K, Hamilton G, Crepeau T, Healy S, Unlu I, Farajollahi A, et al. Integrating the public in mosquito management: active education by community peers can lead to significant reduction in peridomestic container mosquito habitats. PLoS One. 2014;9(9):e108504 10.1371/journal.pone.0108504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bedoya-Arias JE, Murillo-García DR, Bolaños-Muñoz E, Hurtado-Hurtado N, Ramírez-Jaramillo V, Granados-Álvarez S, et al. Healthcare students and workers’ knowledge about epidemiology and symptoms of chikungunya fever in two cities of Colombia. J Infect Dev Ctries. 2015;9(3):330–32 10.3855/jidc.6445 [DOI] [PubMed] [Google Scholar]
- 97.Prajapati A, Parikh S, Fancy M, Bala DV. Impact of educational intervention regarding mosquito borne diseases and their control measures among the link workers of urban health centers (UHCs) of Ahmedabad city. Natl J Community Med. 2012;3(2):178–82 [Google Scholar]
- 98.Liumbruno GM, Calteri D, Petropulacos K, Mattivi A, Po C, Macini P, et al. The Chikungunya epidemic in Italy and its repercussion on the blood system. Blood Transfus. 2008;6(4):199–210 10.2450/2008.0016-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lieshout-Krikke RW, Zaaijer HL, Prinsze FJ. The yield of temporary exclusion of blood donors, exposed to emerging infections abroad. Vox Sang. 2013;104(1):12–18 10.1111/j.1423-0410.2012.01631.x [DOI] [PubMed] [Google Scholar]
- 100.Gallian P, de Lamballerie X, Salez N, Piorkowski G, Richard P, Paturel L, et al. Prospective detection of chikungunya virus in blood donors, Caribbean 2014. Blood. 2014;123(23):3679–81 10.1182/blood-2014-03-564880 [DOI] [PubMed] [Google Scholar]
- 101.Appassakij H, Promwong C, Rujirojindakul P, Wutthanarungsan R, Silpapojakul K. The risk of blood transfusion-associated Chikungunya fever during the 2009 epidemic in Songkhla Province, Thailand. Transfusion. 2014;54(8):1945–52 10.1111/trf.12575 [DOI] [PubMed] [Google Scholar]
- 102.Chiu CY, Bres V, Yu G, Krysztof D, Naccache SN, Lee D, et al. Genomic assays for identification of chikungunya virus in blood donors, Puerto Rico, 2014. Emerg Infect Dis. 2015;21(8):1409–13 10.3201/eid2108.150458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Appassakij H, Promwong C, Rujirojindakul P, Khuntikij P, Silpapojakul K. Risk of transfusion-transmitted chikungunya infection and efficacy of blood safety implementation measures: experience from the 2009 epidemic in Songkhla Province, Thailand. Transfusion. 2016;56(8):2100–7 10.1111/trf.13675 [DOI] [PubMed] [Google Scholar]
- 104.Tan LK, Lam S, Low SL, Tan FH, Ng LC, Teo D. Evaluation of pathogen reduction systems to inactivate dengue and chikungunya viruses in apheresis platelets suspended in plasma. Adv Infect Dis. 2013;3:1–9 [Google Scholar]
- 105.Faddy HM, Fryk JJ, Prow NA, Watterson D, Young PR, Hall RA, et al. Inactivation of dengue, chikungunya, and Ross River viruses in platelet concentrates after treatment with ultraviolet C light. Transfusion. 2016;56(6 Pt 2):1548–55 10.1111/trf.13519 [DOI] [PubMed] [Google Scholar]
- 106.Leo YS, Chow ALP, LiKiang T, Lye DC, Li L, Ng LC. Chikungunya outbreak, Singapore, 2008. Emerg Infect Dis. 2009;15(5):836–7 10.3201/eid1505.081390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bartlett-Healy K, Hamilton G, Healy S, Crepeau T, Unlu I, Farajollahi A, et al. Source reduction behavior as an independent measurement of the impact of a public health education campaign in an integrated vector management program for the Asian tiger mosquito. Int J Environ Res Public Health. 2011;8(5):1358–67 10.3390/ijerph8051358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van den Berg H, Zaim M, Yadav RS, Soares A, Ameneshewa B, Mnzava A, et al. Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect. 2012;120:577–82. 10.1289/ehp.1104340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guzzetta G, Trentini F, Poletti P, Baldacchino FA, Montarsi F, Capelli G, et al. Effectiveness and economic assessment of routine larviciding for prevention of chikungunya and dengue in temperate urban settings in Europe. PLoS Negl Trop Dis. 2017;11(9): 10.1371/journal.pntd.0005918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Atkinson J, Vallely A, Fitzgerald L, Whittaker M, Tanner M. The architecture and effect of participation: a systematic review of community participation for communicable disease control and elimination. Implications for malaria elimination. Malar J. 2011;10: 10.1186/1475-2875-10-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12(6):887–93 10.3201/10.3201/eid1206.051210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schiavo R, May Leung M, Brown M. Communicating risk and promoting disease mitigation measures in epidemics and emerging disease settings. Pathog Glob Health. 2014;108(2):76–94 10.1179/2047773214Y.0000000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nájera JA. Malaria control: achievements, problems and strategies. Parassitologia. 2001;43:1–89 [PubMed] [Google Scholar]
- 114.Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of chikungunya, dengue, and zika outbreaks in Europe. PLoS Negl Trop Dis. 2016;10(4): e0004664 10.1371/journal.pntd.0004664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Butterworth MK, Morin CW, Comrie AC. An analysis of the potential impact of climate change on dengue transmission in the Southeastern United States. Environ Health Perspect. 2016;125(4):579–84 10.1289/EHP218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ganushkina LA, Patraman IV, Rezza G, Migliorini L, Litvinov SK, Sergiev VP. Detection of Aedes aegypti, Aedes albopictus, and Aedes koreicus in the area of Sochi, Russia. Vector Borne Zoonotic Dis. 2016;16(1):58–60 10.1089/vbz.2014.1761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOC)
(DOCX)
(XLSX)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.