Abstract
Background
High-quality chest compressions are imperative for Cardio-Pulmonary-Resuscitation (CPR). International CPR guidelines advocate, that chest compressions should not be interrupted for ventilation once a patient’s trachea is intubated or a supraglottic-airway-device positioned. Supraglottic-airway-devices offer limited protection against pulmonary aspiration. Simultaneous chest compressions and positive pressure ventilation both increase intrathoracic pressure and potentially enhances the risk of pulmonary aspiration. The hypothesis was, that regurgitation and pulmonary aspiration is more common during continuous versus interrupted chest compressions in human cadavers ventilated with a laryngeal tube airway.
Methods
Twenty suitable cadavers were included, and were positioned supine, the stomach was emptied, 500 ml of methylene-blue-solution instilled and laryngeal tube inserted. Cadavers were randomly assigned to: 1) continuous chest compressions; or, 2) interrupted chest compressions for ventilation breaths. After 14 minutes of the initial designated CPR strategy, pulmonary aspiration was assessed with a flexible bronchoscope. The methylene-blue-solution was replaced by 500 ml barium-sulfate radiopaque suspension. 14 minutes of CPR with the second designated ventilation strategy was performed. Pulmonary aspiration was then assessed with a conventional chest X-ray.
Results
Two cadavers were excluded for technical reasons, leaving 18 cadavers for statistical analysis. Pulmonary aspiration was observed in 9 (50%) cadavers with continuous chest compressions, and 7 (39%) with interrupted chest compressions (P = 0.75).
Conclusion
Our pilot study indicate, that incidence of pulmonary aspiration is generally high in patients undergoing CPR when a laryngeal tube is used for ventilation. Our study was not powered to identify potentially important differences in regurgitation or aspiration between ongoing vs. interrupted chest compression. Our results nonetheless suggest that interrupted chest compressions might better protect against pulmonary aspiration when a laryngeal tube is used for ventilation.
Background
More than 350,000 people suffer out-of-hospital cardiac arrests annually in the United States.[1] Early initiation of advanced cardiopulmonary resuscitation (CPR) is key to favorable outcomes. Airway management is also crucial, and adequate ventilation and oxygenation is essential.[2]
Endotracheal intubation is generally considered the optimal method of managing the airway during CPR.[3, 4] An endotracheal tube promotes effective ventilation and oxygenation, minimizes gastric insufflation, and consequently reduces the risk of regurgitation and pulmonary aspiration.[5, 6] However, endotracheal intubation is challenging and requires considerable experience and skills, along with regular retraining.[7–9] Supraglottic airway devices are less invasive, much easier to insert than endotracheal intubation, and consequently reduce the time needed for successful intubation.[10–14] Supraglottic airway devices, including the laryngeal tube are thus increasingly used, especially by less experienced providers including paramedics.[15] A limitation common to all supraglottic airway devices is that they provide little protection against regurgitation and pulmonary aspiration[5, 16, 17]—and aspiration is a major clinical concern since it promotes pneumonia and acute respiratory distress syndrome.[18]
High quality chest compressions are the most important component during CPR, and even small interruptions much reduce systemic blood flow.[19] Consequently, international CPR guidelines stress that interruptions of chest compression should be limited to the extent possible; for example, even ventilation should be restricted to a 30:2 chest compression/ ventilation ratio until the airway is secured. They further specify that once a patient’s trachea has been intubated or a supraglottic airway device has been inserted, chest compression should not be interrupted for ventilation of the lungs.[4, 6, 20]
Simultaneous chest compressions and positive pressure ventilation, both increase intrathoracic pressure and potentially enhances the risk of regurgitation and pulmonary aspiration. However, it remains unknown whether continuing CPR during ventilation promotes regurgitation and pulmonary aspiration in patients ventilated through supraglottic airway devices. The aim of this pilot, cross-over study was therefore to evaluate the effect of continuing or interrupting chest compressions during ventilation on regurgitation and pulmonary aspiration. In particular, we considered the hypothesis that pulmonary aspiration is more common during continuous versus interrupted chest compressions in human cadavers ventilated with a laryngeal tube airway.
Methods
Human cadavers were provided by the Department of Anatomy of the Cleveland Clinic Lerner College of Medicine. At the Cleveland Clinic the IRB has determined that since the cadavers used in research studies are donated through the Cleveland Clinic Body Donation Program the Director of that program, Dr. Richard Drake, will provide final approval for all studies using cadavers. There does not need to be a separate IRB approval.
We used human cadavers with a body mass index <45 kg/m2 without rigor mortis. All retained lifelike tissue characteristics and anatomical structures, as determined by an attending anatomist. Cadavers were warmed to ambient temperature before study.
Exclusion criteria’s included known pathologies of the upper airway or upper alimentary tract including oral cavity, pharynx, larynx, oesophagus and stomach. We also excluded cadavers that had previous surgery or radiation to the head, neck, chest, oesophagus, or stomach. And finally, we excluded cadavers that had a current or previous tracheostomy, or previous known regurgitation or aspiration of gastric content.
Protocol
Suitable human cadavers were positioned supine on an autopsy table. The face, mouth, teeth, and upper aerodigestive tract were inspected to identify any injuries or lesions, along with evidence of previous regurgitation of gastric contents.
The belt of a CPR board Lucas (Physio Control, Redmond, WA) was properly mounted on the torso of the cadaver. The Lucas device uses a piston and suction cup to deliver chest compressions with active recoil. The system was correctly positioned by an experienced investigator, closely following the manufacturer’s instructions. Chest compression depth was set to 5 cm.
A conventional gastric tube was inserted orally and the stomach was emptied. Thereafter, 500 ml of 0.01% methylene-blue-solution (Merck Chemicals, Darmstadt, Germany) was instilled into the stomach and the tube removed. We selected 500 ml because larger volumes increase the risk of aspiration.[21] Fibreoptic bronchoscopy confirmed that there was no unwanted methylene-blue staining of the pharynx or larynx. The cadaver was then intubated using an appropriately sized laryngeal tube (King LTS-D, Ambu, Noblesville, IN, USA). Correct positioning, an acceptable seal, and good ventilation were confirmed by giving a single breath delivered with a bag-valve mask.
Each cadaver was evaluated under two conditions: 1) continuous chest compressions at a rate of 100/min with ventilation at a rate of 10 breaths/min (a breath every six seconds without pausing chest compressions); and, 2) interrupted chest compressions with ventilation at a rate of 30:2, with compressions paused as little as practical. Randomization (1:1) was based on computer-generated codes that were maintained in opaque envelopes until after intubation. Ventilation complied with current recommendations, with each breath lasting about 1 sec and having sufficient volume to slightly raise the chest.[22]
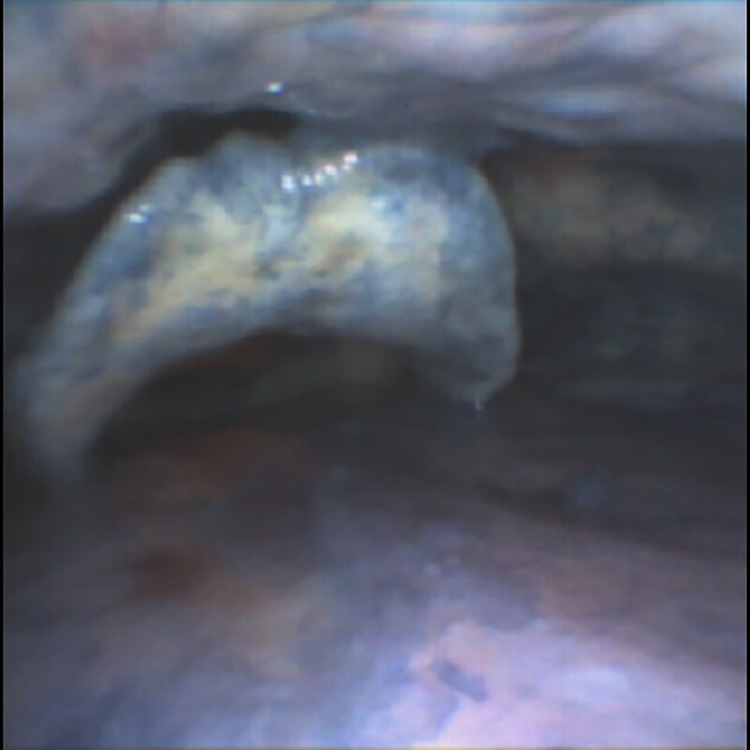
After 14 minutes of the initial designated CPR and ventilation, both were stopped and the amount of regurgitation and pulmonary aspiration were assessed using a conventional flexible bronchoscope (Fig 1). The dye solution was aspirated from the stomach and replaced with 500 ml of Liquid E-Z Paque, barium sulfate radiopaque suspension (Bracco Diagnostics Inc., Monroe Twp., NJ). Again, fiberoptic bronchoscopy was used to confirm that there was no contamination of the pharynx or larynx. CPR was then restarted with the designated ventilation strategy and maintained for another 14 minutes. Thereafter regurgitation and pulmonary aspiration was assessed with a conventional chest X-ray (Fig 2).
Fig 1. Regurgitation of methylene-blue solution, assessed by flexible bronchoscopy.
Fig 2. Pulmonary aspiration of barium sulfate radiopaque suspension, assessed by chest x-ray.
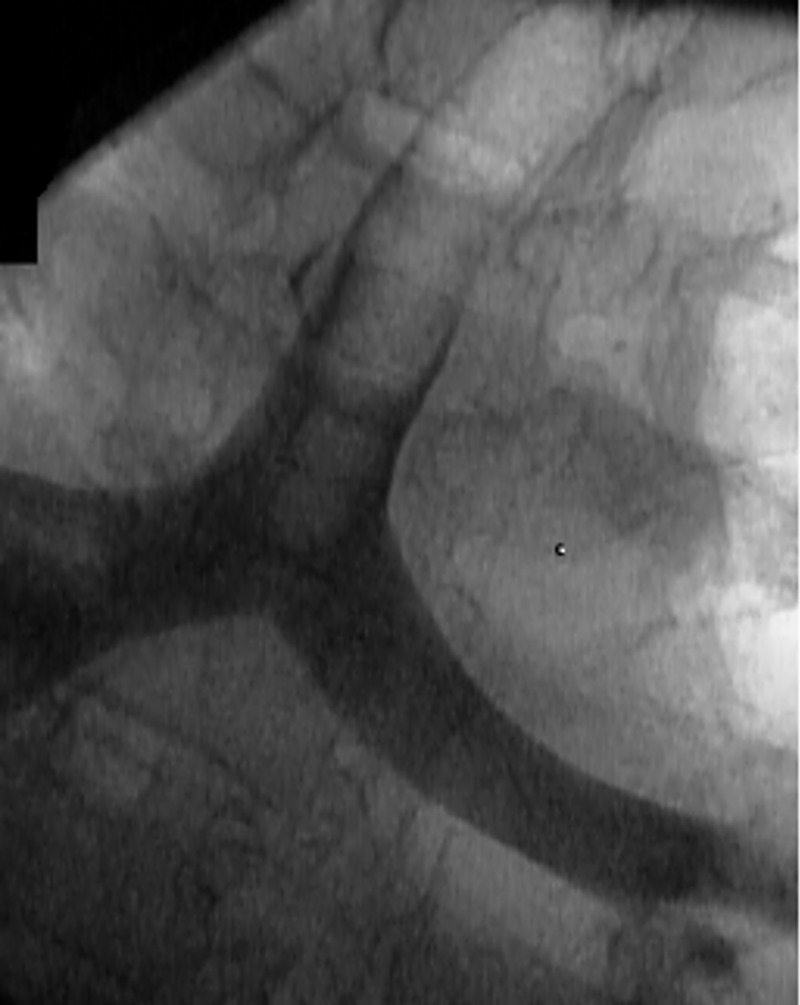
Oesophageal regurgitation was defined when methylene blue or barium sulfate was detected either by bronchoscopy or radiograph in the upper oesophagus, and/or larynx.[5] Pulmonary aspiration was defined by methylene blue or barium sulfate within the trachea below the vocal cords.[5]
Statistical analysis
Given the a priori pilot nature of the project, we did not conduct a formal sample-size estimate. Instead, we planned to evaluate 20 cadavers because we expected that many to be available over a reasonable data-acquisition period. Our primary goal was to roughly estimate the incidence of pulmonary aspiration and regurgitation with each CPR and ventilation approach, thus providing information to guide the sample-size estimate of a potential future trial.
We estimated the incidences of pulmonary aspiration and regurgitation with 95% bootstrap confidence intervals for each group and the overall study population. Bootstrap confidence intervals were used instead of binomial confidence intervals to account for within-pair correlation. Additionally, we roughly estimated the effect of continuous (versus interrupted) compressions on pulmonary aspiration using a McNemar test for paired data.
Results
We studied 20 cadavers, but two were excluded for technical reasons, one assigned to each initial treatment. Therefore 18 cavers were included in the statistical analyses, 9 starting with continuous chest compressions and 9 starting with interrupted chest compressions S1 Table. The characteristics of cadavers was summarized in Table 1. Overall, 7 (39%) were female; the mean of age was 66 (SD = 12) and the mean of BMI was 26 (SD = 6).
Table 1. Characteristics of cadavers.
| Overall | Randomization schedule | ||
|---|---|---|---|
| Factor | (N = 18) | Continuous then interrupted (N = 9) |
Interrupted then continuous (N = 9) |
| Age—years | 66 ± 12 | 71 ± 12 | 62 ± 9 |
| Gender—female % | 7 (39%) | 3 (33%) | 4 (44%) |
| Body Mass Index–kg/m2 | 26 ± 6 | 27 ± 6 | 26 ± 6 |
Continuous variables were summarized as mean ±SD. Categorical variable was summarized as n (%).
Regurgitation was detected in all cadavers during continous compression, and 15 (83%) with interupted compression. Pulmonary aspiration was observed in 9 (50%) cadavers with continuous chest compressions, and 7 (39%) with interupted chest compression (P = 0.75). None of the differences was statistically significant (Table 2).
Table 2. Rate of regurgitation and aspiration.
| Chest compression | Rate (%) | 95% CI* | |
|---|---|---|---|
| Aspiration | Continuous | 9 (50%) | (28%, 72%) |
| Interrupted | 7 (39%) | (17%, 61%) | |
| Regurgitation | Continuous | 18 (100%) | (100%, 100%) |
| Interrupted | 15 (83%) | (67%, 100%) |
* 95% confidence intervals (CIs) were estimated from 1000 times bootstrapping.
Discussion
Interruption of chest compressions decrease coronary perfusion pressure, reduce rate of return of spontaneous circulation (ROSC), diminish defibrillation success, and unsurprisingly are associated with poor outcome.[23] Consequently, interruptions should be kept as short as necessary and avoided when practical.[4, 6, 20] Several observational studies reported significant increases in survival rates among patients having CPR, at least in patients with a shockable rhythm.[24–26] although by far the largest prospective trial investigating the impact of continuous versus interrupted chest compression, failed to demonstrate any increase of survival and favorable neurologic function in patients suffering from out-of-hospital CPR.[27] Nonetheless, current CPR guidelines advocate continuous chest compressions once the airway is secured by an endotracheal tube or a supraglottic airway device.[4, 20]
Our study steamed from the suspicion that continuous chest compression might promote aspiration because of the simultaneous increases in intrathoracic pressure from chest compression and positive pressure ventilation, especially in patients ventilated through supraglottic airway devices. In our human cadaver pilot study, the strategy of continuous chest compressions and reducing the time without chest compressions, was associated with an absolute 10% increase in the incidence of pulmonary aspiration, although the difference was statistically not significant.
Passive regurgitation and pulmonary aspiration of gastric content is normally prevented by pressure from the distal esophagus sphincter and the muscle tonus of the esophagus. Both appear to be much reduced during CPR as suggested by the high incidence of pulmonary aspiration in the peri-CPR setting.
Aspiration was first described by Mendelsohn in 1946 and is defined as the inhalation of gastric contents (or oro-pharyngeal secretions) into the larynx and into the lower respiratory tract.[28] Clinical consequences of pulmonary aspiration varies and may often remain unrecognized, and without any significant clinical consequences. However, clinical deterioration mostly depends on the origin of the aspirate, and may be caused by chemical pneumonitis, infectious pneumonia, exacerbation of a pre-existing asthmatic disease, acute lung injury or even acute respiratory distress syndrome.[29] The incidence of pulmonary aspiration during CPR is extremely difficult to assess and is currently mostly unknown unclear, but estimates range from 20 to 65%.[18, 30, 31]
Perbet and colleagues report that 65% of patients given out-of-hospital CPR suffered from early-onset pneumonia,[30] although some pneumonia may have resulted from systemic inflammatory response syndrome or lung contusion.[30] In routine medico-legal autopsies, aspiration of gastric contents are observed in up to 25% of all cases (independent of the cause of death).[31] Furthermore, the timing of pulmonary aspiration in outpatients given CPR ranges from before CPR is initiated, during airway intervention (e.g. by a possible vomiting stimulus), during CPR after airway intervention (as in our study), or even thereafter during hospitalization.
The incidence of regurgitation is about 12% in patients who have in-hospital CPR, and 20% for out-of-hospital CPR.[16, 32] But in a previous human cadaver study, the incidence of regurgitation during CPR ranged between 0 and 80% depending on the airway device used.[5] In our current study, every human cadaver showed evidence of regurgitation during continuous chest compressions and nearly all (83%) did during interrupted chest compressions. The higher incidence in our human cadavers might be based on a relatively large 500-ml gastric volume and the prolonged CPR duration of 14 minutes. Regurgitation is concerning because it is required for aspiration but is not itself of any substantive clinical importance. In contrast, mortality from pulmonary aspiration ranges from 3.5 to 12%.[29]
We studied cadavers because they well approximate the anatomy and regurgitation risks in humans having CPR.[5] Consequently, cadavers are widely used in CPR studies and their findings are generally considered reliable. Animal or manikin models are almost surely less realistic.[33] A clinical trial would of course provide the best evidence, but it seems unlikely that one will address our question any time soon. To provide a close resemblance to live tissues, our cadavers were not preserved and each cadaver was prewarmed to ambient temperature. A limitation is that like most cadavers donated for medical research, ours came from elderly subjects and were at the lower end of the BMI spectrum. Results may have differed had we used cadavers with other characteristics, but it seems likely that the general sense of our finding would remain similar.
We did not measure the extent of pulmonary aspiration. However, clinical consequences mostly depend on the origin of the aspirate, instead of the extent. [29]
Another limitation of our trial is that we did not measure any ventilation minute volume and instead gave clinically reasonable breaths. Both hypoventilation and hyperventilation are associated with poor outcome after CPR.[34] Presumably aspiration risk increases as a function of both tidal volume and peak airway pressure.
In summary, our human cadaver pilot study was not powered to identify potentially important difference in regurgitation or aspiration. The results nonetheless suggest that interrupted chest compression might better protect against pulmonary aspiration when a laryngeal tube is used for ventilation. A well-powered trial is needed to confirm our observations.
Supporting information
(PDF)
Acknowledgments
Received from the Department of Outcomes Research, Quantitative Health Sciences, and General Anesthesiology, Cleveland Clinic, Cleveland Ohio, United States of America. Physio Control (Lund, Sweden) provided the Lucas 3 CPR board, and Ambu (Noblesville, IN) provided the laryngeal tubes for the study. Both companies were not involved in design of the study, data collection, analysis, or interpretation of the data. The manuscript was written by the investigators and was not shared with the companies in advance. Preliminary data of this trial have been presented as a poster during Scientific Sessions of the American Heart Association in Anaheim, CA in October 2017 and Anesthesiology 2018, October 15th, San Francisco, USA.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study project was funded by a grant by the Research Program Committee of the Cleveland Clinic Foundation and the Instituto Salud Carlos III (BA17/00032 to ER). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McCarthy JJ, Carr B, Sasson C, Bobrow BJ, Callaway CW, Neumar RW, et al. Out-of-Hospital Cardiac Arrest Resuscitation Systems of Care: A Scientific Statement From the American Heart Association. Circulation. 2018. 10.1161/CIR.0000000000000557 . [DOI] [PubMed] [Google Scholar]
- 2.Genbrugge C, Meex I, Boer W, Jans F, Heylen R, Ferdinande B, et al. Increase in cerebral oxygenation during advanced life support in out-of-hospital patients is associated with return of spontaneous circulation. Critical care (London, England). 2015;19:112 Epub 2015/04/19. 10.1186/s13054-015-0837-5 ; PubMed Central PMCID: PMCPmc4377035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit JL, Gerecht RB, Steuerwald MT, McMullan JT. Endotracheal intubation versus supraglottic airway placement in out-of-hospital cardiac arrest: A meta-analysis. Resuscitation. 2015;93:20–6. Epub 2015/05/27. 10.1016/j.resuscitation.2015.05.007 . [DOI] [PubMed] [Google Scholar]
- 4.Soar J, Nolan JP, Bottiger BW, Perkins GD, Lott C, Carli P, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–47. 10.1016/j.resuscitation.2015.07.016 . [DOI] [PubMed] [Google Scholar]
- 5.Piegeler T, Roessler B, Goliasch G, Fischer H, Schlaepfer M, Lang S, et al. Evaluation of six different airway devices regarding regurgitation and pulmonary aspiration during cardio-pulmonary resuscitation (CPR)—A human cadaver pilot study. Resuscitation. 2016;102:70–4. Epub 2016/02/28. 10.1016/j.resuscitation.2016.02.017 . [DOI] [PubMed] [Google Scholar]
- 6.Perkins GD, Travers AH, Berg RA, Castren M, Considine J, Escalante R, et al. Part 3: Adult basic life support and automated external defibrillation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. 2015;95:e43–69. 10.1016/j.resuscitation.2015.07.041 . [DOI] [PubMed] [Google Scholar]
- 7.Mulcaster JT, Mills J, Hung OR, MacQuarrie K, Law JA, Pytka S, et al. Laryngoscopic intubation: learning and performance. Anesthesiology. 2003;98(1):23–7. . [DOI] [PubMed] [Google Scholar]
- 8.Piegeler T, Neth P, Schlaepfer M, Sulser S, Albrecht R, Seifert B, et al. Advanced airway management in an anaesthesiologist-staffed Helicopter Emergency Medical Service (HEMS): A retrospective analysis of 1047 out-of-hospital intubations. Resuscitation. 2016;105:66–9. Epub 2016/06/01. 10.1016/j.resuscitation.2016.04.020 . [DOI] [PubMed] [Google Scholar]
- 9.Thoeni N, Piegeler T, Brueesch M, Sulser S, Haas T, Mueller SM, et al. Incidence of difficult airway situations during prehospital airway management by emergency physicians—a retrospective analysis of 692 consecutive patients. Resuscitation. 2015;90:42–5. 10.1016/j.resuscitation.2015.02.010 . [DOI] [PubMed] [Google Scholar]
- 10.Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305–52. 10.1016/j.resuscitation.2010.08.017 . [DOI] [PubMed] [Google Scholar]
- 11.Bein B, Carstensen S, Gleim M, Claus L, Tonner PH, Steinfath M, et al. A comparison of the proseal laryngeal mask airway, the laryngeal tube S and the oesophageal-tracheal combitube during routine surgical procedures. Eur J Anaesthesiol. 2005;22(5):341–6. Epub 2005/05/28. . [DOI] [PubMed] [Google Scholar]
- 12.Bollig G, Lovhaug SW, Sagen O, Svendsen MV, Steen PA, Wik L. Airway management by paramedics using endotracheal intubation with a laryngoscope versus the oesophageal tracheal Combitube and EasyTube on manikins: a randomised experimental trial. Resuscitation. 2006;71(1):107–11. Epub 2006/09/01. S0300-9572(06)00099-2 [pii] 10.1016/j.resuscitation.2006.02.016 . [DOI] [PubMed] [Google Scholar]
- 13.Rabitsch W, Schellongowski P, Staudinger T, Hofbauer R, Dufek V, Eder B, et al. Comparison of a conventional tracheal airway with the Combitube in an urban emergency medical services system run by physicians. Resuscitation. 2003;57(1):27–32. Epub 2003/04/02. S0300957202004355 [pii]. . [DOI] [PubMed] [Google Scholar]
- 14.Goliasch G, Ruetzler A, Fischer H, Frass M, Sessler DI, Ruetzler K. Evaluation of advanced airway management in absolutely inexperienced hands: a randomized manikin trial. European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2013;20(5):310–4. Epub 2012/08/24. 10.1097/MEJ.0b013e328358455e . [DOI] [PubMed] [Google Scholar]
- 15.Wang HE, Schmicker RH, Daya MR, Stephens SW, Idris AH, Carlson JN, et al. Effect of a Strategy of Initial Laryngeal Tube Insertion vs Endotracheal Intubation on 72-Hour Survival in Adults With Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. Jama. 2018;320(8):769–78. Epub 2018/09/01. 10.1001/jama.2018.7044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone BJ, Chantler PJ, Baskett PJ. The incidence of regurgitation during cardiopulmonary resuscitation: a comparison between the bag valve mask and laryngeal mask airway. Resuscitation. 1998;38(1):3–6. . [DOI] [PubMed] [Google Scholar]
- 17.Wenzel V, Idris AH, Dorges V, Nolan JP, Parr MJ, Gabrielli A, et al. The respiratory system during resuscitation: a review of the history, risk of infection during assisted ventilation, respiratory mechanics, and ventilation strategies for patients with an unprotected airway. Resuscitation. 2001;49(2):123–34. . [DOI] [PubMed] [Google Scholar]
- 18.Radu RR, Kaserer A, Seifert B, Simmen HP, Ruetzler K, Spahn DR, et al. Prevalence and in-hospital outcome of aspiration in out-of-hospital intubated trauma patients. Eur J Emerg Med. 2017. 10.1097/MEJ.0000000000000465 . [DOI] [PubMed] [Google Scholar]
- 19.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–70. . [DOI] [PubMed] [Google Scholar]
- 20.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S444–64. Epub 2015/10/17. 10.1161/cir.0000000000000261 . [DOI] [PubMed] [Google Scholar]
- 21.McClave SA, DeMeo MT, DeLegge MH, DiSario JA, Heyland DK, Maloney JP, et al. North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN Journal of parenteral and enteral nutrition. 2002;26(6 Suppl):S80–5. Epub 2002/10/31. 10.1177/014860710202600613 . [DOI] [PubMed] [Google Scholar]
- 22.Greif R, Lockey AS, Conaghan P, Lippert A, De Vries W, Monsieurs KG. European Resuscitation Council Guidelines for Resuscitation 2015: Section 10. Education and implementation of resuscitation. Resuscitation. 2015;95:288–301. Epub 2015/10/20. 10.1016/j.resuscitation.2015.07.032 . [DOI] [PubMed] [Google Scholar]
- 23.Yeung J, Chilwan M, Field R, Davies R, Gao F, Perkins GD. The impact of airway management on quality of cardiopulmonary resuscitation: an observational study in patients during cardiac arrest. Resuscitation. 2014;85(7):898–904. 10.1016/j.resuscitation.2014.02.018 . [DOI] [PubMed] [Google Scholar]
- 24.Bobrow BJ, Ewy GA, Clark L, Chikani V, Berg RA, Sanders AB, et al. Passive oxygen insufflation is superior to bag-valve-mask ventilation for witnessed ventricular fibrillation out-of-hospital cardiac arrest. Ann Emerg Med. 2009;54(5):656–62 e1. 10.1016/j.annemergmed.2009.06.011 . [DOI] [PubMed] [Google Scholar]
- 25.Kellum MJ, Kennedy KW, Ewy GA. Cardiocerebral resuscitation improves survival of patients with out-of-hospital cardiac arrest. Am J Med. 2006;119(4):335–40. 10.1016/j.amjmed.2005.11.014 . [DOI] [PubMed] [Google Scholar]
- 26.Garza AG, Gratton MC, Salomone JA, Lindholm D, McElroy J, Archer R. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119(19):2597–605. 10.1161/CIRCULATIONAHA.108.815621 . [DOI] [PubMed] [Google Scholar]
- 27.Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, et al. Trial of Continuous or Interrupted Chest Compressions during CPR. N Engl J Med. 2015;373(23):2203–14. 10.1056/NEJMoa1509139 . [DOI] [PubMed] [Google Scholar]
- 28.Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205. . [DOI] [PubMed] [Google Scholar]
- 29.Beck-Schimmer B, Bonvini JM. Bronchoaspiration: incidence, consequences and management. Eur J Anaesthesiol. 2011;28(2):78–84. 10.1097/EJA.0b013e32834205a8 . [DOI] [PubMed] [Google Scholar]
- 30.Perbet S, Mongardon N, Dumas F, Bruel C, Lemiale V, Mourvillier B, et al. Early-onset pneumonia after cardiac arrest: characteristics, risk factors and influence on prognosis. Am J Respir Crit Care Med. 2011;184(9):1048–54. 10.1164/rccm.201102-0331OC . [DOI] [PubMed] [Google Scholar]
- 31.Knight BH. The significance of the postmortem discovery of gastric contents in the air passages. Forensic Sci. 1975;6(3):229–34. . [DOI] [PubMed] [Google Scholar]
- 32.Virkkunen I, Ryynanen S, Kujala S, Vuori A, Piilonen A, Kaaria JP, et al. Incidence of regurgitation and pulmonary aspiration of gastric contents in survivors from out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2007;51(2):202–5. 10.1111/j.1399-6576.2006.01229.x . [DOI] [PubMed] [Google Scholar]
- 33.Schebesta K, Hupfl M, Rossler B, Ringl H, Muller MP, Kimberger O. Degrees of Reality Airway Anatomy of High-fidelity Human Patient Simulators and Airway Trainers. Anesthesiology. 2012;116(6):1204–9. 10.1097/ALN.0b013e318254cf41 WOS:000304356500010. [DOI] [PubMed] [Google Scholar]
- 34.Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(9 Suppl):S345–51. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.