Abstract
Background
Visceral leishmaniasis is a neglected parasitic disease with no vaccine available and its pharmacological treatment is reduced to a limited number of unsafe drugs. The scarce readiness of new antileishmanial drugs is even more alarming when relapses appear or the occurrence of hard-to-treat resistant strains is detected. In addition, there is a gap between the initial and late stages of drug development, which greatly delays the selection of leads for subsequent studies.
Methodology/Principal findings
In order to address these issues, we have generated a red-shifted luminescent Leishmania infantum strain that enables long-term monitoring of parasite burden in individual animals with an in vivo limit of detection of 106 intracellular amastigotes 48 h postinfection. For this purpose, we have injected intravenously different infective doses (104—5x108) of metacyclic parasites in susceptible mouse models and the disease was monitored from initial times to 21 weeks postinfection. The emission of light from the target organs demonstrated the sequential parasite colonization of liver, spleen and bone marrow. When miltefosine was used as proof-of-concept, spleen weight parasite burden and bioluminescence values decreased significantly.
Conclusions
In vivo bioimaging using a red-shifted modified Leishmania infantum strain allows the appraisal of acute and chronic stage of infection, being a powerful tool for accelerating drug development against visceral leishmaniasis during both stages and helping to bridge the gap between early discovery process and subsequent drug development.
Author summary
Visceral leishmaniasis is a neglected disease that poses a significant threat to impoverished human populations of low-income countries. Due to the unavailability of vaccines, pharmacological treatment is the only approach to control the disease that otherwise can be lethal. To date, drug management in endemic regions is based on combinations of a handful of mostly unsafe drugs, where the emergence of resistant strains is an additional problem. To accelerate the discovery of new drug entities, several gaps from the early discovery of a compound to its public use, should be filled. One of these gaps is the need of a rapid go/no-go testing system for compounds based on robust preclinical models. Here, we propose a new long-term model of murine visceral leishmaniasis using in vivo bioluminescent imaging. For this purpose, a red-shifted bioluminescent Leishmania infantum strain was engineered. This strain has allowed the appraisal of the disease in individual animals and the monitoring of parasite colonization in liver, spleen and bone marrow. As proof of concept of this platform, mice were infected with the transgenic L. infantum strain treated with a standard schedule of miltefosine, the only oral drug available against Leishmania parasites. Bioluminescence and parasite load in the target organs were compared showing a good correlation. Our findings provide a robust and reproducible tool for drug discovery in a chronic model of murine visceral leishmaniasis.
Introduction
Leishmaniasis is a complex of neglected parasitic diseases affecting the poorest people in 98 countries, particularly those with weak or non-existent health systems. [1]. There are at least three different forms of clinical presentations; cutaneous, mucocutaneous and visceral leishmaniasis, the latter being fatal if left untreated [2]. Visceral leishmaniasis (VL) is estimated to produce 300.000 new cases and between 20.000–40.000 deaths every year. Most of the cases are localized in three geographical regions; South Asia and East Africa where the disease is caused by Leishmania donovani and the transmission is mostly anthroponotic. By its part, in Brazil, where the disease is produced by L. infantum chagasi, the transmission is zoonotic and occurs mainly from infected dogs [3].
Nowadays, therapeutic or prophylactic human vaccines are still lacking, and the cure of patients is based on chemotherapy [4, 5]. Treatment of VL was mainly based on painful intramuscular injections of pentavalent antimonials, such as sodium stibogluconate (SSG). SSG has been the first-line antileishmanial drug in India, although its clinical efficacy in some areas of North Bihar State has gradually declined, due to the emergence of fully resistant L. donovani strains. SSG is being substituted by liposomal amphotericin B (AmBisome) as first-line treatment, despite slow intravenous administration of the drug is needed [6–8]. In East Africa, SSG was the first-line regimen for decades, but due to its toxicity and following WHO recommendations in 2010, SSG + paromomycin combination therapy became the treatment of choice [9]. However, the administration of this drug combination is painful and requires patient hospitalization, and therefore, more friendly alternatives were implemented. These include single dose of AmBisome plus 10 consecutive days of SSG, single dose of AmBisome plus 10 days of miltefosine or miltefosine alone for 28 days. However, none of these combinations improved the results of the treatment of choice in Phase II clinical trials [10].
Miltefosine is the last drug successfully introduced against VL. It is also the only drug that has a good oral bioavailability. However, an increase in relapse rates has been reported in India and Nepal, probably associated with low drug exposure [11, 12]. In addition, miltefosine is potentially embryotoxic and fetotoxic in experimental animals and thereby, its administration is not recommended in women during pregnancy [13].
For all these reasons, there is an unmet need to fill the antileishmanial drug discovery pipeline with safer drugs that display new mechanisms of action, likely allowing combination therapy in order to prevent the emergence of resistant strains [14]. During this process, and once compounds have shown high in vitro potency, selectivity, specificity, low toxicity and good predictable pharmacokinetic/pharmacodynamic properties, a proof of concept that undoubtedly shows the in vivo efficacy of lead compounds, is required. Both mice and hamsters are used as models of acute and chronic VL, respectively, during the evaluation of the proof of concept [15, 16]. The most frequent technique to evaluate the infection course after drug treatment has been microscopic counting of amastigotes in liver, spleen and bone marrow smears stained with Giemsa dye. However, these are labour-intensive techniques that require specific skill training and present low sensitivity when parasite burdens are low after treatment [17], therefore, they are limited by tissue sampling biases, which require large animal cohorts.
In vivo real-time imaging combined with modified parasites expressing bioluminescent or fluorescent reporters may accelerate the initial stage of drug discovery at the preclinical level. Fluorescent reporters in the near infrared wavelength avoid interference with haemoglobin and do not require the addition of substrate. However, and despite the number of near infrared proteins currently available [18, 19] further reporters with longer emission wavelengths are still required in order to increase sensitivity. In this regard, red-shifted bioluminescent reporters [20] are currently allowing the appraisal of different infections produced by Trypanosomatids in vivo in real-time without the need to kill animals. This tool allows to run longitudinal studies with a reduced number of animals since they are not sacrificed, and in addition each animal is its own control, therefore the variability of experimental outcomes is limited [21–25]. In summary, in vivo real-time imaging allows to develop the proof of concept in a record time, accelerating the drug discovery process.
Nowadays, the mouse is used as acute preclinical model of VL, being liver the main affected organ when experimental treatments are initiated at early times postinfection. On the contrary, hamster is a more stringent and relevant model to recreate human VL [26, 27]. Generally speaking, during chronic infections the persistence of pathogens yields a state of T cell dysfunction known as exhaustion that is characterized by the loss of effector functions, low recall response and suboptimal T cell proliferation [28]. This is a hallmark feature shared by mice, dogs and humans and it is associated with disease progression [29–31].
Here, we describe a chronic murine model of VL that combines in vivo real-time image with stably modified strain expressing red-shifted luciferase (luc) aiming to track the presence of parasites in target organs during a long-time course of infection that can be used for preclinical drug-discovery. Miltefosine was used as proof of concept to assess the suitability of this technique during drug discovery.
Methods
Ethics statement
The animal research described in this manuscript complies with Spanish Act (RD 53/2013) and European Union Legislation (2010/63/UE). The protocols were approved by the Animal Care Committee of the Centro de Biología Molecular Severo Ochoa (CBMSO, Madrid, Spain), project licence number JMJ/bb. Animals were maintained under specific pathogen-free conditions in individually ventilated cages. They experienced a 12 h light/dark cycle and had access to food and water ad libitum.
Methods, mice and parasites
Seven to eight weeks-old female Balb/c mice were obtained from Janvier Labs (St Berthevin Cedex, France) and housed in specific pathogen-free facilities in the P2-facility of CBMSO for this study. L. infantum (strain MCAN/ES/96/BCN 150) promastigotes (previously obtained from infected dogs) were a gift from J.M. Requena (CBMSO, Madrid, Spain). Parasites were routinely cultured at 26 °C in M199 medium supplemented with 25 mM HEPES pH 6.9, 10 mM glutamine, 7.6 mM hemin, 0.1 mM adenosine, 0.01 mM folic acid, 1x RPMI 1640 vitamin mix (Sigma-Aldrich), 10% (v/v) heat-inactivated fetal calf serum (FCS) and antibiotic cocktail (50 U/ml penicillin, 50 μg/ml streptomycin).
Generation of red-shifted luc L. infantum strain
The 1647-bp PpyRE9h coding region was amplified by PCR from pGEX-6P-2 HCO RE9h vector, a kind gift from Dr. Bruce Branchini, (Department of Chemistry, Connecticut College, CT, USA). The oligonucleotides used as primers (RBF919 and RBF920 in Table 1) introduced NcoI-NotI as restriction sites for cloning into pLEXSY-PAC vector (Jena Bioscience) and XhoI restriction site in the forward primer for cloning into pSK II vector. The 1647-bp PCR amplified fragment containing the PpyRE9h coding region was digested with XhoI and NotI and cloned first into pSK II vector previously cut with the same restriction enzymes to yield pSK-PpyRE9h plasmid. Then, this plasmid was cut with NcoI-NotI and the PpyRE9h ORF was cloned into pLEXSY-PAC vector to yield the pLEXSY-PAC-PpyRE9h construct. Parasites expressing red-shifted luc were obtained after electroporation of L. infantum BCN150 promastigotes with the linear SwaI-targeting fragment obtained from pLEXSY-PAC-PpyRE9h vector. Transfections were performed by electroporation (Gene Pulser X cell System, Biorad) using 10 μg of DNA fragments under the following conditions: 25 μF, 1500 v, 9 ms in 4 mm gap cuvettes. Subsequent plating on semisolid media containing 200 μg/mL puromycin as selection antibiotic, allowed the isolation of individual colonies that were subcultured in liquid media under antibiotic pressure. The correct integration of each fragment into the 18S rRNA locus of the resulting clones (PpyRE9h+L. infantum) was confirmed by PCR amplification analysis, using appropriate primers (Table 1).
Table 1. Oligonucleotides used in this work.
| Oligo No. | Sequencea,b | Purposedc | Orientation |
|---|---|---|---|
| RBF919 | ccgCTCGAGCCATGGCCACCATGGAGGACGCCAAGAACATCAA | PpyRE9h | Forward |
| RBF920 | aaggaaaaaaGCGGCCGCTCAGATCTTGCCGCCCTTCTTGG | PpyRE9h | Reverse |
| RBF 630 | CTTGTTTCAAGGACTTAGCCATG | 5′integration | Forward |
| RBF 637 | TATTCGTTGTCAGATGGCGCAC | 5′integration | Reverse |
| RBF 644 | CATGTGCAGCTCCTCCCTTTC | 3′integration | Forward |
| RBF 645 | CCTTGTTACGACTTTTGCTTC | 3′integration | Reverse |
aUnderlined sequence indicates restriction site.
bBold sequence indicates optimized translation initiation sequence.
cOrientation of primers: F, forward; R, reverse
In vitro luciferase assay and microscopy
The Luciferase Assay System (Promega) was used to assess luc expression in PAC-isolated clones from semisolid in vitro cultures. Briefly, 100×106 parasites were washed off with PBS and then lysed with 1 ml of Cell Culture Lysis Reagent provided by the manufacturer. Cell lysate was serially diluted in 96-well plate and then, ten microlitres of each cell lysate dilution were mixed with 90 μL of luciferin substrate. Luminescence was measured immediately using a Synergy HT microplate reader (BioTek).
In order to recover the infectivity of PpyRE9h+L. infantum strain after cloning, 108 metacyclic promastigotes were inoculated intravenously (IV) in the tail vein. Mice were sacrificed 4 weeks later and spleens were used to recover infective amastigotes. Amastigotes were isolated from the spleen by passing the tissue through a wire mesh. Then, splenocytes were disrupted by passing sequentially through 27G1/2 and 30G1/2 needles. Finally, cell debris was retained by passing successively through polycarbonate membrane filters with pore sizes of 8 μm, 5 μm and 3 μm (Isopore, Millipore). Released amastigotes (free of host cells), were washed twice with PBS (4000 x g for 20 min at 4°C) and counted by direct microscopy. To assess luciferase expression in intracellular infections, phorbol 12-myristate 13-acetate (PMA)-differentiated THP1 human monocytic leukemia cells, were grown on 8-well chambered coverslips (IBIDI) and incubated with infective amastigotes recuperated from mice in a ratio of 1:10 for further 4 hours. Extracellular parasites were removed by extensive washing with warm PBS and processed for immunofluorescence analysis 5 days after infection. Briefly, coverslips were fixed with 2% (v/v) paraformaldehyde in PBS and incubated with 0.1% (v/v) Triton X-100 in PBS for 10 min at room temperature in order to permeabilize the cells. Slides were then probed with 1:1000 red firefly luciferase polyclonal antibody (ThermoFisher); followed by 1:500 DyLight 633-conjugated goat anti-rabbit IgG secondary antibody for 30 min at room temperature. DNA was labelled using 1 μM Hoechst 33342 before mounting with Vectashield mounting medium. Images were acquired on a Zeiss LSM800 microscope with airyscan at 60X magnification.
In vivo mice infection with PpyRE9h+L. infantum parasites. Bio-luminescent imaging (BLI)
Different infective doses of stationary phase PpyREh9+L. infantum promastigotes (ranging from 5x104 to 5x108) were IV injected to 6–8 weeks-old female Balb/c mice. Every week animals were placed in a Charge-Coupled Device (CCD) IVIS 100 Xenogen system (Caliper Life Science) for BLI analysis and images were acquired 10–20 min after intraperitoneal D-luciferin injection (150 mg/kg). Briefly, the animals were lightly anesthetized with 2.5% isofluorane (then reduced to 1.5%), before being placed on the camera. To standardize image capture and in order to allow comparison between mice, the images presented in the figures correspond to an acquisition time of 1 min duration, taken once luminescence plateaued.
To estimate the parasite burden in living mice, Regions Of Interest (ROIs) around liver and spleen in ventral and lateral animals’ positions were drawn using Living Image v.4.3 to quantify BLI expressed as radiance (p/s/cm2/sr). The detection threshold for in vivo imaging was estimated using uninfected mice placed in different positions (ventral and lateral), using ROIs of whole animals (n = 16).
At the end of some experiments, animals were euthanized and dissected, to confirm parasite burden by more conventional methods. Briefly, spleens and livers were reimaged ex vivo and used to quantify parasite load by Limit Dilution Assay (LDA). LDA was calculated as the geometric mean of the titer obtained from quadruplicate cultures x reciprocal fraction of the homogenized organ added to the first well. The titer was the reciprocal value of the last dilution in which parasites were observed [32].
Statistic analysis
Individual animal values were used as the unit of analysis of in vivo and ex vivo experiments. Statistical differences between groups were evaluated using t-student test using SigmaPlot v.14.0. Differences of P < 0.05 were considered significant.
Results
Highly sensitive in vitro imaging of L infantum expressing red-shifted luc
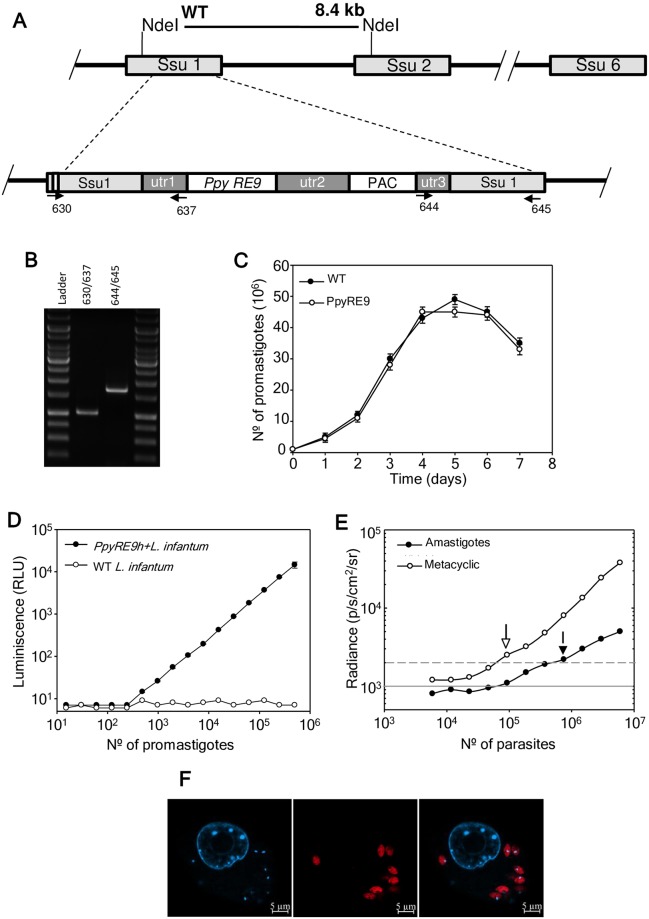
PpyRE9h was stably integrated into the 18S rRNA promoter using pLEXSY vector (Fig 1A). Correct integration of reporter gene into the resulting clones (PpyRE9h+L. infantum) was confirmed by PCR amplification analyses using the primers of Table 1 (Fig 1B). The PCR-confirmed clones were screened for luciferase activity and those having higher activity were selected for in vivo experiments. Promastigote cultures of PpyRE9h+L.infantum grew at the same rate as wild-type parasites (Fig 1C). There was a linear relationship between the luciferase activity in vitro and the number of parasites independently of the parasite stage (logaritmic, metacyclic or freshly isolated amastigotes) and independently of the instrument for measuring (luminometer or IVIS camera). Fig 1D shows the relationship between luciferase activity and the number of logaritmic promastigotes (PpyRE9h+L.infantum and wild-type strains). Cell lysates from promastigotes were serially diluted into 96-well plate, D-luciferin was added and luciferase activity was measured using a luminometer (103–106 cells range, r2 = 0.999), being the detection limit of 103 promastigotes/well. The PCR-clone with the highest luciferase activity was selected for recovering infectivity through mouse (see Materials and Methods). Fig 1E shows the outcomes using metacyclic promastigotes and free amastigotes in the IVIS camera. In this experiment 6x106 parasites (metacyclic and amastigotes) were serially diluted in a 96-well plate, 100 μl D-luciferin were added and luciferase activity was measured in the IVIS camera (4.68x104-6x106 cells range, r2 = 0.987 for metacyclic and r2 = 0.994 for amastigotes). The detection limit was 9.37x104 metacyclic/well and 7.5x105 amastigotes/well. This suggests that more amastigotes than metacyclic promastigotes are required for their detection by the IVIS camera.
Fig 1. Generation of a L. infantum stably expressing PpyRE9h red-shifted luciferase.
A) Scheme of the structure of the 18S rRNA locus on wild type and planned integration of PpyRE9h gene. Key: utr1: non-translated region of aprt gene; utr2: 1.4 kb intergenic region from cam operon; and utr3: UTR of dhfr-ts gene; PAC; puromycin resistance cassette. B) PCR confirmation of successful integration of the reporter cassette. Primers 630/637 and 644/645 together confirm the correct integration of the reporter cassette into genome sequences. (see Table 1 for sequences). C) Growth rate of wild-type (black circle) and stably-modified promastigotes PpyRE9h+L.infantum (white circle). Parasites were counted using a Coulter counter. D) In vitro luciferase activity assay of diluted lysates from wild-type (WT) and PpyRE9h+L.infantum promastigotes after PCR confirmation. E) Minimal metacyclic promastigote and infective amastigote number detectable by the IVIS camera. Parasites were loaded in 96-well plates, D-luciferin added and the BLI signal was detected in the IVIS camera. Grey lines indicate detection thresholds determined as the mean (solid line) and mean +2SDs (dashed line) of background luminescence of wells with PBS free-parasites. F) Microscopic image of intracellular PpyRE9h+L.infantum amastigotes infecting PMA-differentiated THP-1 cell line. Bioluminescent amastigotes stained with anti-luciferase IgG (αLuc, red) and Hoechst 33342 DNA stain (H33342, cyan).
Spleen-isolated amastigotes were used to infect PMA differentiated THP-1 macrophages. Four hours later the non-phagocytosed parasites were gently washed off with warm PBS and left for further 96 h. The infection was stained using anti-firefly luciferase antibody and its localization was confirmed to be cytosolic by confocal microscopy (Fig 1F).
Threshold sensitivity of bioluminescence PpyRE9h+L. infantum strain in vivo
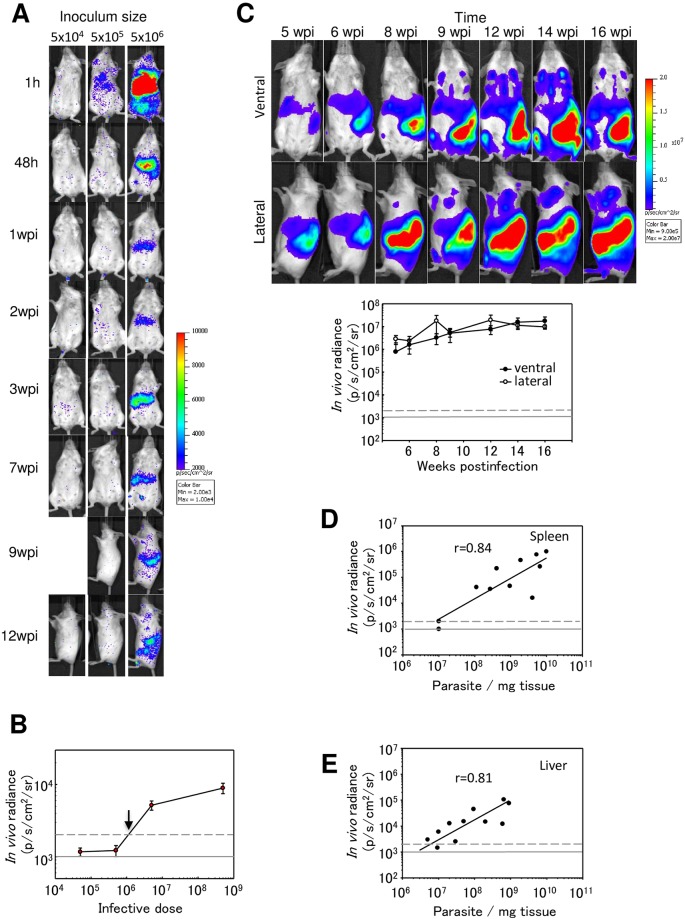
The infectivity of the selected clone was enhanced by passing through Balb/c mice that were successively infected with 108 promastigotes by IV route until spleen weight increased up to 0.7–1 g (4–5 passages through mice). To establish the in vivo sensitivity of the PpyRE9h+L. infantum strain, Balb/c mice were IV injected with different doses of infective metacyclic promastigotes (5x104-5x106) and photographed 1 h postinfection. At this time, the bioluminescent signal was detected in the liver but only with the highest doses (5x105 and 5x106). Forty-eight hours later when most promastigotes have transformed into amastigotes; BLI signal was only detected from mice infected with 5x106 parasites (Fig 2A). The appraisal of the infection showed that BLI signal in the liver peaked ~3 weeks post-infection (acute phase), then disappeared slowly from this organ and increased in the spleen (chronic phase) (Fig 2A). To estimate the in vivo limit of detection with PpyRE9h+L. infantum parasites, we used BLI signal expressed as radiance (p/s/cm2/sr). The limit of detection in vivo was estimated to be above 1x106 parasites at 48 h postinfection, when metacyclic parasites have transformed into intracellular amastigotes (Fig 2B).
Fig 2. Evaluation of L. infantum infection in Balb/c mice by in vivo BLI.
A) Representative images of Balb/c mice infected via IV with 5x104—5x106 metacyclic PpyRE9h luciferase-expressing promastigotes. Pseudocolour heat-maps indicate intensity of bioluminescence from low (blue) to high (red). All images use the same log10 scale heat-map, minimum and maximum radiance values are indicated. Animals at 9 and 12 weeks postinfection are in lateral position where most of the BLI signal was detected B) Quantification of whole animal total bioluminescence for mice in the experiment illustrated in A) at 48 h postinfection. C) Time-appraisal of Balb/c mice after IV injection with 5x108 red-shifted bioluminescent parasites (PpyRE9h+L.infantum) in ventral and lateral positions. Quantification of ventral and lateral bioluminescence from the experiment represented by the images in C). D-E) Correlation between in vivo bioluminescence values and parasite burdens in the liver and the spleen. Bioluminescence was measured in vivo in ROIs around liver and spleen and parasite numbers were quantified by limiting dilution assay after animals were sacrificed. Grey lines indicate detection thresholds determined as the mean (solid line) and mean +2SDs (dashed line) of background luminescence of control uninfected animals.
We were interested in developing a chronic model of infection to use as proof of concept for well-established infections in spleen and bone marrow. In order to evaluate the stability of bioluminescent signal through time in chronic infections, 5x108 metacyclic promastigotes were IV injected and animals were photographed in ventral and lateral positions from 5 to 16 weeks post-infection. The spleen infection was detected independently of the animal positions. BLI signal was increasing from week 5 to reach the maximum radiance 12 weeks after infection (Fig 2C). Moreover, bone marrow radiance was detected only in ventral images from 8 to 16 weeks, and the bioluminescent signal was increasing during this time (Fig 2C).
To establish a correlation between BLI signal detected in vivo and the parasite burden in liver and spleen, mice (n = 12, one animal died before the end of the experiment) were infected with parasite dose ranging from 5x106-5x108. Nine weeks postinfection, animals were imaged and the luminescence was recorded in vivo in the regions of interest (ROI) previously drawn around the spleen and liver. Animals were euthanized and the liver and spleen processed to determine parasite burden. Both organs showed a good correlation with the in vivo recorded BLI signal (Fig 2D and 2E).
Validation of the luciferase model with miltefosine
PpyRE9h+L. infantum infected mice were treated with miltefosine as a proof-of-concept to validate this model in a long-term follow-up infection.
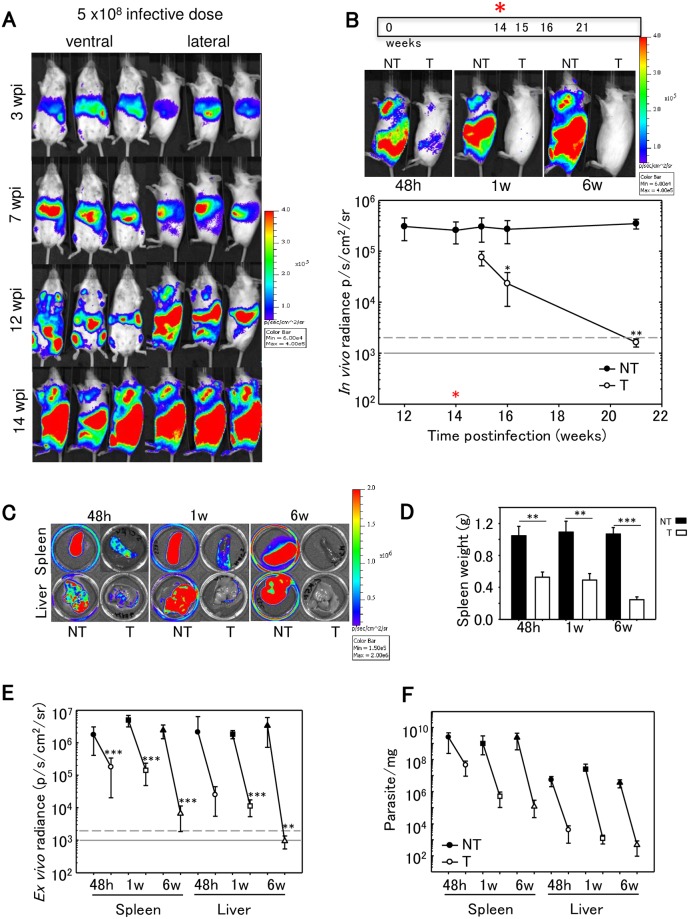
Mice (n = 30) were IV infected with 5x108 metacyclic promastigotes and imaged for BLI after 3, 7, 12 and 14 wpi confirming that infection was established (Fig 3A). Animals were divided in groups and half of them were treated with miltefosine 40 mg/kg/day for 5 days by oral gavage. Once miltefosine treatment was ended, animals were imaged and sacrificed at different times (48 h, 1 and 6 weeks post-treatment that corresponded to 15, 16 and 21 wpi). Fig 3B (top panel) shows that BLI signal in whole animals was almost undetectable after miltefosine treatment (48 h post-treatment) in a chronic infection, and that the BLI reduction persisted for 6 weeks after the end of the treatment. Quantification of the BLI signal revealed that radiance in untreated animals (3x105 p/s/cm2/sr) decreased significantly to 2.32x104 and 1.61x103; one and six weeks, respectively after the end of the treatment, reaching BLI values similar to non-infected animals (Fig 3B; bottom panel P<0.05).
Fig 3. Chronic L. infantum infection in space and time.
A) Representative ventral and lateral view images of Balb/c mice taken at sequential time points over the course of 14 weeks after IV infection with 5 x 108 PpyRE9h luciferase-expressing L.infantum metacyclic promastigotes (representative of n = 30). In the images corresponding to 14 wpi only lateral views are shown because most of BLI signal was detected in this position. Heat-maps are on log10 scales indicate intensity of bioluminescence from low (blue) to high (red); the minimum and maximum radiances for the pseudocolour scale are indicated. B) Animals (n = 15) were treated with 40 mg/kg/d miltefosine via oral for 5 days. Treated and untreated animals were photographed, sacrificed and the spleen and liver imaged at 48 h, 1 week and 6 weeks after the end of miltefosine treatment (15, 16 and 21 wpi). Quantification of lateral bioluminescence for mice shown in B. The red asterisk indicates the start of miltefosine treatment. In vivo radiance from untreated (black circle) and treated (white circle) animals is represented. Black asterisks indicate P-values for t-student test (B,D,E). Comparisons between miltefosine treated groups and untreated control groups (*P < 0.05; **P<0.01; ***P<0.001). Grey line indicates detection thresholds determined as the mean (solid line) and mean +2SDs (dashed line) of background luminescence of control uninfected animals. C) Ex vivo imaging (spleen and liver) in untreated and treated animals at 48h, 1w and 6 w after the end of the treatment (BLI signal results from the D-luciferin injected ex vivo). D) Spleen weights from untreated (black) and treated (white) animals at 48h, 1w and 6 w after the end of the treatment. Each point is the mean ± SD, n = 5 per group. E) Ex vivo bioluminescence signal from spleens and livers obtained from untreated (black symbols) and treated animals (white symbols) at 48 h (circle), 1w (square) and 6w (triangle) after the end of miltefosine treatment. F) Parasite burdens in untreated and treated mice determined by limited dilution assay on livers and spleens.
Animals were sacrificed at different times after the end of treatment (48h, 1 week and 6 weeks posttreatment) and the organs (spleen and liver) were photographed after injecting D-luciferin ex vivo (Fig 3C). There was a significant marked reduction in the weight of the spleen, which reached values similar to those of the uninfected animals at 6 weeks after the end of the treatment (Fig 3D P<0.001). Ex vivo bioluminescent values recorded from treated and untreated animals over the time were plotted (Fig 3E) confirming the BLI reduction seen in vivo. The BLI decrease was significant at all analysed times in both organs (P<0.001) with the exception of the liver at 48h posttreatment that was not significant and liver at 6wpi (P<0.01). Both organs showed logarithmic reduction of BLI from the end of the treatment to 6 weeks later. Ex vivo parasite burden was estimated using limiting dilution assay confirming parasite load reductions of 98%, 99,9%, and 99,9%, at 48h, 1 week and 6 weeks postinfection (Fig 3F).
Discussion
The introduction of new medicines against VL from the initial concept to public release is a time-consuming and expensive process. Moreover, the clinical recurrences after treatment failure and the emergence of resistances are worsened by the shortage of new clinical entities and the long period needed to release a new medicine [33]. To bridge the gap between early drug identification and in vivo preclinical studies, new bioimaging tools have recently been introduced to accelerate the drug discovery process while drastically reducing the number of animals used.
To develop robust preclinical in vivo platforms, several aspects related to the genetic modifications introduced in the pathogen and the suitability of the animal model should be addressed before their validation with a proof of concept [34, 35]. In such a way, we present here the generation of the strain PpyRE9h+L. infantum and its utility to quantify the parasite load in vivo in infected mice in real time. As the virulence of the modified strain can be lost after genetic manipulation and passage in culture, as soon as the correct integration of the construct was confirmed, the selected clone was passed through mice to recover its infectivity [36, 37, 38].
Once D-luciferin was administered, the light detected by CCD camera and transformed to pseudocolor images, enabled parasite traceability in the whole body and the estimation of parasite burden in a murine model of chronic VL, reducing the number of animals to be analysed in longitudinal studies. During in vivo infections amastigotes enter into a semi-quiescent physiological stage in which major energetic processes are specifically repressed [39], explaining the differences in light emission between metacyclic promastigotes and freshly isolated amastigotes. However, our results show that parasites emitted light enough to provide accurate and rapid radiance that allow the appraisal up to 21 wpi. In this study, light could be detected in Balb/c mice in the liver during the acute phase of infection and later in the spleen and bone marrow during the chronic phase, allowing a continuous and long-term follow-up of the infection. Under these conditions, light detected in vivo–that corresponded to ROIs drawn around liver and spleen—correlated well with parasite burden calculated from LDA, which it would allow to estimate the parasite burden without the sacrifice of animals.
The location of parasites (peripheral or deeper tissues) within the mammalian host has been pointed as a key factor affecting the limit of parasite detection in vivo [40]. The light emitted by freshly isolated amastigotes from splenic lesions in our system showed a detection threshold similar to the previously reported by other authors [22].
In experimental VL, the hamster is considered the best experimental model since it reproduces many clinicopathological features of the human disease and can be fatal in the absence of treatment [41]. High-dose murine models of VL develop hallmarks of progressive human, primate, and canine disease with loss of gp38 stromal cells [42], remodelling of splenic marginal zone region [43], altered migration of DCs [42] and loss of follicular germinal centers [44]. For this reason, Balb/c mice have been proposed as an adequate model of chronic VL [45–46]. In addition, during chronic infections the persistence of pathogens yields a state of T cell dysfunction known as exhaustion that is characterized by the loss of effector functions, low recall response and suboptimal T cell proliferation [28]. In VL, this stage of T cell exhaustion is associated with disease progression in mouse, dogs and human infections [29–31]. For this reason and in order to have a murine BLI model of chronic VL, the inoculum size was increased to 5x108 metacyclic parasites per mouse.
In previous studies we have used the same L. infantum/Balb/c model showing hallmarks of progressive infection [47]. PpyRE9h+L. infantum strain allowed a continuous monitoring of parasite load from the beginning of the infection up to animal’s sacrifice, detecting both acute and chronic infections. In view of these results, PpyRE9h+L. infantum constitutes an ideal tool for the appraisal of drug efficacy in in vivo preclinical models.
The assay was validated by the treatment with miltefosine, starting 14 weeks post-infection and extended for long-term appraisal (6 weeks after drug withdrawal). In rodents miltefosine is known to produce significant parasite burden reduction (90–99% depending on parasite strain) in liver and spleen, along with no-sterile cure (when the treatment is initiated at 7–21 days postinfection [48–50]. These no-sterile curative results were confirmed later in hamster models of chronic VL treated with high-dose miltefosine (20 mg/kg/10 days), started 40 days post infection, although it resulted in 100% survival measured 20 wpi [23]. In our study, both radiance and parasite burden values dropped immediately after the end of treatment and they remained decreasing during the long-term follow up, although sterile cure was never achieved. A possible disease recurrence due to incomplete parasite suppression was expected. However, and despite the long-term follow up, much beyond the half-life of miltefosine [51], no recurrence was seen.
Several studies with AmBisome and stibogluconate in mice have shown that the infection status influenced treatment outcome, so that treatments were less effective in the chronic infection model than in acute infection models [52, 53]. The changes that occur in liver and spleen structure and function during early and late stages on infection might be the cause [46]. The mouse model proposed in this work would provide accurate information about potential drugs and their efficacy on the later stages of infection when it has been described that the efficacy of several drugs might be more compromised.
In conclusion, the gold standard methods used to evaluate the efficacy of antileishmanial drugs based on LDA or microscopic examination are laborious and time-consuming, have intrinsic variability, require intensive use of animals and cannot be monitored in real time. However, novel in vivo bioimaging models based on bioluminescent L. infantum parasites are highly sensitive, easily traceable, and yet provide statistically valuable outcomes with the use of far fewer animals than traditional methods. This technology is reproducible; less expensive because it reduces the number of animals needed, it is barely distressing for animals and can be easily adapted to different experimental models being thereby suitable to accelerate drug development.
Acknowledgments
The authors would like to thank Imanol Peña, M. Pilar Manzano, Juan Cantizani, Jose M. Fiandor and J. Julio Martin from Tres Cantos OpenLab Foundation (Madrid-Spain) for helpful discussions during the performance of the research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
RAV received a postdoctoral fellowship from Tres Cantos Open Lab Foundation CBM-ULe_TC178 TCOLF (http://www.openlabfoundation.org). MCGC received a predoctoral fellowship from Consejería de Educación de la Junta de Castilla y León (http://www.educa.jcyl.es/es). RBF was granted by Ministerio de Economía y Competitividad del Gobierno de España, AGL2016-79813-C2-1R (http://www.mineco.gob.es/portal/site/mineco/) and Consejería de Educación de la Junta de Castilla y León LE020P17 (http://www.educa.jcyl.es/es). MFE was granted by Tres Cantos Open Lab Foundation CBM-ULe_TC178 TCOLF (http://www.openlabfoundation.org). RRT was funded by Ministerio de Economía y Competitividad del Gobierno de España, SAF2017-83575-R. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012;7:e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pace D. Leishmaniasis. J Infect. 2014;69 Suppl 1:S10–18. [DOI] [PubMed] [Google Scholar]
- 3.Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–154. 10.2147/CLEP.S44267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundar S, Chakravarty J. An update on pharmacotherapy for leishmaniasis. Expert Opin Pharmacother. 2015;16:237–252. 10.1517/14656566.2015.973850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGwire BS, Satoskar AR. Leishmaniasis: clinical syndromes and treatment. QJM. 2014;107:7–14. 10.1093/qjmed/hct116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Control of the leishmaniases; World Health Organ Tech Rep Ser. 2010;xii-xiii:1–186. [PubMed] [Google Scholar]
- 7.Burza S, Mahajan R, Sinha PK, van Griensven J, Pandey K, Lima MA, et al. Visceral leishmaniasis and HIV co-infection in Bihar, India: long-term effectiveness and treatment outcomes with liposomal amphotericin B (AmBisome). PLoS Negl Trop Dis. 2014;8:e3053 10.1371/journal.pntd.0003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burza S, Sinha PK, Mahajan R, Lima MA, Mitra G, Verma N, et al. Five-year field results and long-term effectiveness of 20 mg/kg liposomal amphotericin B (Ambisome) for visceral leishmaniasis in Bihar, India. PLoS Negl Trop Dis. 2014;8:e2603 10.1371/journal.pntd.0002603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis. 2012;6:e1674 10.1371/journal.pntd.0001674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasunna M, Njenga S, Balasegaram M, Alexander N, Omollo R, Edwards T, et al. Efficacy and safety of AmBisome in combination with sodium stibogluconate or miltefosine and miltefosine monotherapy for African visceral leishmaniasis: phase II randomized trial. PLoS Negl Trop Dis. 2016;10:e0004880 10.1371/journal.pntd.0004880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B. et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012;55:543–550. 10.1093/cid/cis474 [DOI] [PubMed] [Google Scholar]
- 12.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis. 2013;56:1530–1538. 10.1093/cid/cit102 [DOI] [PubMed] [Google Scholar]
- 13.Sindermann H, Engel J. Development of miltefosine as an oral treatment for leishmaniasis. Trans R Soc Trop Med Hyg. 2006;100 Suppl 1:S17–20. [DOI] [PubMed] [Google Scholar]
- 14.van Griensven J, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet 2011;377:443–444. 10.1016/S0140-6736(10)62237-4 [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Nishi. Visceral leishmaniasis: Experimental models for drug discovery. Indian J Med Res. 2011;133:27–29. [PMC free article] [PubMed] [Google Scholar]
- 16.Mears ER, Modabber F, Don R, Johnson GE. A review: the current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Negl Trop Dis. 2015;9:e0003889 10.1371/journal.pntd.0003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretagne S, Durand R, Olivi M, Garin JF, Sulahian A, Rivollet D, Vidaud M, Deniau M. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin Diagn Lab Immunol. 2001;8:828–31. 10.1128/CDLI.8.4.828-831.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu X, Royant A, Lin MZ, Aguilera TA, Lev-Ram V, Steinbach PA, et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science 2009;324:804–807. 10.1126/science.1168683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filonov GS, Piatkevich KD, Ting LM, Zhang J, Kim K, Verkhusha VV. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol. 2012;29:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branchini BR, Ablamsky DM, Davis AL, Southworth TL, Butler B, Fan F, et al. Red-emitting luciferases for bioluminescence reporter and imaging applications. Anal Biochem. 2010;396:290–297. 10.1016/j.ab.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 21.Michel G, Ferrua B, Lang T, Maddugoda MP, Munro P, Pomares C. et al. Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, in vivo, ex vivo and in vitro. PLoS Negl Trop Dis. 2011;5:e1323 10.1371/journal.pntd.0001323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouault E, Lecoeur H, Meriem AB, Minoprio P, Goyard S, Lang T. Imaging visceral leishmaniasis in real time with golden hamster model: Monitoring the parasite burden and hamster transcripts to further characterize the immunological responses of the host. Parasitol Int. 2017;66:933–939. 10.1016/j.parint.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 23.Reimão JQ, Oliveira JC, Trinconi CT, Cotrim PC, Coelho AC, Uliana SR. Generation of luciferase-expressing Leishmania infantum chagasi and assessment of miltefosine efficacy in infected hamsters through bioimaging. PLoS Negl Trop Dis. 2015;9:e0003556 10.1371/journal.pntd.0003556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLatchie AP, Burrell-Saward H, Myburgh E, Lewis MD, Ward TH, Mottram JC, et al. Highly sensitive in vivo imaging of Trypanosoma brucei expressing "red-shifted" luciferase. PLoS Negl Trop Dis. 2013;7:e2571 10.1371/journal.pntd.0002571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis MD, Fortes Francisco A, Taylor MC, Burrell-Saward H, McLatchie AP, Miles MA, et al. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol. 2014;16:1285–1300. 10.1111/cmi.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrión J, Nieto A, Iborra S, Iniesta V, Soto M, Folgueira C, et al. Immunohistological features of visceral leishmaniasis in BALB/c mice. Parasite Immunol. 2006;28:173–183. 10.1111/j.1365-3024.2006.00817.x [DOI] [PubMed] [Google Scholar]
- 27.Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166:1912–1920. [DOI] [PubMed] [Google Scholar]
- 28.Gigley JP, Bhadra R, Moretto MM, Khan IA. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28:377–384. 10.1016/j.pt.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stäger S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431 10.1371/journal.ppat.1000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J Immunol. 2013;191:5542–5550. 10.4049/jimmunol.1301810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, et al. CD8 T cell exhaustion in human visceral leishmaniasis. J Infect Dis. 2014;209:290–299. 10.1093/infdis/jit401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. [DOI] [PubMed] [Google Scholar]
- 33.Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017;11:e0006052 10.1371/journal.pntd.0006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reguera RM, Calvo-Álvarez E, Alvarez-Velilla R, Balaña-Fouce R. Target-based vs. phenotypic screenings in Leishmania drug discovery: A marriage of convenience or a dialogue of the deaf? Int J Parasitol Drugs Drug Resist. 2014;4:355–357. 10.1016/j.ijpddr.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvo-Álvarez E, Álvarez-Velilla R, Fernández-Prada C, Balaña-Fouce R, Reguera RM. Trypanosomatids see the light: recent advances in bioimaging research. Drug Discov Today 2015;20:114–121. 10.1016/j.drudis.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 36.Pescher P, Blisnick T, Bastin P, Späth GF. Quantitative proteome profiling informs on phenotypic traits that adapt Leishmania donovani for axenic and intracellular proliferation. Cell Microbiol. 2011;13:978–991. 10.1111/j.1462-5822.2011.01593.x [DOI] [PubMed] [Google Scholar]
- 37.Moreira D, Santarém N, Loureiro I, Tavares J, Silva AM, et al. Impact of continuous axenic cultivation in Leishmania infantum virulence. PLoS Negl Trop Dis. 2012;6:e1469 10.1371/journal.pntd.0001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melo GD, Goyard S, Lecoeur H, Rouault E, Pescher P, Fiette L, et al. New insights into experimental visceral leishmaniasis: Real-time in vivo imaging of Leishmania donovani virulence. PLoS Negl Trop Dis. 2017; 11:e0005924 10.1371/journal.pntd.0005924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kloehn J, Saunders EC, O'Callaghan S, Dagley MJ, McConville MJ. Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling. PLoS Pathog. 2015;11:e1004683 10.1371/journal.ppat.1004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang T, Lecoeur H, Prina E. Imaging Leishmania development in their host cells. Trends Parasitol. 2009;25:464–473. 10.1016/j.pt.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 41.Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol. 2015;108:1–24. [DOI] [PubMed] [Google Scholar]
- 42.Ato M, Stäger S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol. 2002; 3:1185–1191. 10.1038/ni861 [DOI] [PubMed] [Google Scholar]
- 43.Engwerda CR, Ato M, Cotterell SE, Mynott TL, Tschannerl A, Gorak-Stolinska PM, et al. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am J Pathol. 2002;161:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smelt SC, Engwerda CR, McCrossen M, Kaye PM. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J Immunol. 1997;158:3813–3821. [PubMed] [Google Scholar]
- 45.Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, et al. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. 10.1111/j.0105-2896.2004.00188.x [DOI] [PubMed] [Google Scholar]
- 46.Kaye PM, Beattie L. Lessons from other diseases: granulomatous inflammation in leishmaniasis. Semin Immunopathol. 2016;38:249–260. 10.1007/s00281-015-0548-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calvo-Álvarez E, Stamatakis K, Punzón C, Álvarez-Velilla R, Tejería A, Escudero-Martínez JM, et al. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl Trop Dis. 2015;9:e0003666 10.1371/journal.pntd.0003666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother. 1992;36:1630–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escobar P, Yardley V, Croft SL. Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob Agents Chemother. 2001;45:1872–1875. 10.1128/AAC.45.6.1872-1875.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fortin A, Hendrickx S, Yardley V, Cos P, Jansen H, Maes L. Efficacy and tolerability of oleylphosphocholine (OlPC) in a laboratory model of visceral leishmaniasis. J Antimicrob Chemother. 2012;67:2707–2712. 10.1093/jac/dks273 [DOI] [PubMed] [Google Scholar]
- 51.Bresiser A, Kim DJ, Fleer EA, Damenz W, Drube A, et al. Distribution and metabolism of hexadecylphosphocholine in mice. Lipids 1987; 22:925–926. [DOI] [PubMed] [Google Scholar]
- 52.Mullen AB, Baillie AJ, Carter KC. Visceral leishmaniasis in the BALB/c mouse: a comparison of the efficacy of a nonionic surfactant formulation of sodium stibogluconate with those of three proprietary formulations of amphotericin B. Antimicrob Agents Chemother. 1998; 42:2722–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voak AA, Harris A, Qaiser Z, Croft SL, Seifert K. Pharmacodynamics and biodistribution of single-dose liposomal amphotericin B at different stages of experimental visceral leishmaniasis. Antimicrob Agents Chemother. 2017; 61. pii: e00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.